Abstract
The vertebrate antigen receptors are anticipatory in their antigen recognition and display a vast diversity. Antigen receptors are assembled through V(D)J recombination, in which one of each Variable, (Diverse), and Joining gene segment are randomly utilized and recombined. Both gene rearrangement and mutational insertion are generated through randomness; therefore, the process of antigen receptors generation requires a rigorous testing system to select every receptor which is useful to recognize foreign antigens, but which would cause no harm to self cells. In the case of T cell receptors (TCR), such a quality control responsibility rests in thymic positive and negative selection. In this review, we focus on the critical involvement of self-peptides in the generation of a T cell repertoire, discuss the role of T cell thymic development in shaping the specificity of TCR repertoire, and directing function fitness of mature T cells in periphery. Here, we consider thymic positive selection to be not merely a one-time maturing experience for an individual T cell, but a life-long imprinting which influences the function of each individual T cell in periphery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Double Positive
- Homeostatic Proliferation
- Positive Selection Signal
- Double Positive Thymocyte
- Negative Selection Signal
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Role of Self-Peptides in Positive Selection
1.1 Differential Strength of TCR Interaction with Positively Selecting Self-Peptides/MHC Instructs the Cell Fate of Double Positive Thymocytes
Classical αβ T cells differentiate from bone marrow-derived early thymic progenitors, through two developmental checkpoints: β selection at the double negative stage (DN), and positive and negative selections at double positive (DP) stage (Jameson et al. 1995; Moran and Hogquist 2012; Morris and Allen 2012). Thymocyte survival and lineage commitment require a TCR on a DP thymocyte to interact with peptide–MHC ligand on epithelial cells in the cortex. Only a weak interaction may support DP thymocytes to complete positive selection to become mature T cells, while the interaction either too strong or too weak would lead to negative selection or death by neglect, respectively. How can the same TCR initiate either a transcriptional program of survival and differentiation or that of death? The profiles of Ca2+ responses and ERK activation are critical for these outcomes. A transient, high-intensity burst of Ca2+ influx and ERK activation leads to negative selection, whereas positive selection requires sustained Ca2+ and ERK signaling (Mariathasan et al. 2001; McNeil et al. 2005; Werlen et al. 2000) (also see reviews in Moran and Hogquist 2012; Morris and Allen 2012).
The TCR and self-peptide-MHC interaction can occur over a wide range of affinities, raising the question as to how positive selection signals can trigger distinct genetic programs to initiate DP thymocytes to commit to one specific development pathway rather than another. Such a “decision” is taken according to the strength of positive selection signals, in conjunction with signals provided by the coreceptors. Among the affinity range of TCR:peptide MHC interaction, positive selection of CD4+ T cells requires a stronger interaction than the selection of CD8+ T cells. Strong and sustained TCR signals promote CD4+ differentiation, whereas weaker and shorter signals generate CD8+ T cells (Moran and Hogquist 2012). An even stronger signal than the one for CD4+ selection may induce the development of regulatory T cells or innate-like T cells, such as natural killer T cells, and CD8αα intestinal intraepithelial T cells (Stritesky et al. 2012).
After receiving positive selection signals, preselection DP cells downregulate CD8 coreceptors. If a DP cell recognizes MHC class II molecules, a stronger TCR signal promotes CD4+ T cell development regardless of the downregulation of coreceptor CD8. However, if a preselection DP T cell recognizes MHC class I, the downregulation of coreceptor CD8 would cause TCR signaling to cease, so that CD8 coreceptor could not provide a signal strong enough to initiate a CD4+ T cell differentiation, but instead advocate co-receptor reversal to support the development of CD8+ T cells. Thus, the development of CD4+ and CD8+ T cells exhibits temporal difference that CD4+ T cells can undergo rapid selection within 48 h, while CD8+ T cells appear 4 days or more later (Saini et al. 2010). Several signaling molecules and transcriptional factors would lead to exclusive differentiation of either CD4+ or CD8+ T cell lineage (Rothenberg et al. 2008; Singer et al. 2008; Wang and Bosselut 2009). For example, ThPOK, or GATA-3, selectively promotes CD4+ T cell differentiation, whereas Runx3 or Runx1 selectively induces CD8+ T cells differentiation. Also, CD4 coreceptors have higher affinity for Lck than does CD8, endowing CD4 coreceptors to serve as better recruiters of Lck than CD8 (Alarcon and van Santen 2010; Hernandez-Hoyos et al. 2000; Legname et al. 2000; Schmedt et al. 1998; Wiest et al. 1993).
1.2 Preselection DP Thymocytes are More Sensitive to Positively Selecting Self-Peptides/MHC than Mature T Cells
Preselection DP thymocytes are more sensitive to activation than mature T cells (Davey et al. 1998; Eck et al. 2006; Sebzda et al. 1996; Stephen et al. 2009), despite the average number of TCRs being 10-fold lower than mature T cells (Bluestone et al. 1987; Havran et al. 1987). Positively selecting ligands can induce Ca2+ flux and upregulate CD69 expression on preselection DP thymocytes but not extrathymic mature T cells (Davey et al. 1998; Eck et al. 2006; Lo et al. 2009, 2012; Sebzda et al. 1996; Stephen et al. 2009). Preselection DP thymocytes are more responsive to low affinity positively selecting ligands, because of the lower expression of inhibitory coreceptors such as CD5 and CD45, lower expression of negative regulators in signaling pathways (such as tyrosine phosphatase SHP-1 and Cbl-b), and altered glycosylation of cell surface receptors (Azzam et al. 1998; McNeill et al. 2007; Plas et al. 1999; Chiang et al. 2000; Naramura et al. 1998; Davey et al. 1998). Moreover, preselection DP thymocytes also express stage-specific molecules that endow the sensitivity of DP thymocytes, including Tespa1 (which recruits PLC-γ1 and Grb2) (Wang et al. 2012), voltage-gated Na+ channels (VGSC; composed of an a pore unit and a b regulatory subunit) (Lo et al. 2012), and miR181a (Ebert et al. 2009, 2010; Li et al. 2007).
The VGSC is composed of a pore-forming SCN5A subunit, and a regulatory SCN4B subunit. The expression of both subunits are tightly regulated that only DN3 (ready for β selection) and DP thymocytes (ready for positive selection) express Scn5a and Scn4b, but not mature T cells. Also, only positive selection signals can maintain the expression of Scn5a and Scn4b transcripts up to 7 h, in that negative selection signals downregulate both transcripts within 1 h. Blocking the SCN5A pore activity with a specific inhibitor tetrodotoxin diminished gp250-induced Ca2+ influx and has little effect on MCC-induced Ca2+ responses. In reaggregate culture, tetrodotoxin inhibited positive selection of AND CD4+ T cells; in lentiviral bone marrow reconstitution experiments, shRNA knockdown of Scn5a inhibited thymic selection of CD4+ T cells, but not CD8+ T cells. The same CD4+ selection specific defect was observed in in vitro reaggregate culture system. Peripheral AND CD4+ T cells transfected with human SCN5A and SCN4B, which they normally do not express, gained the ability to upregulate CD69 expression in response to positively selecting ligands gp250, directly demonstrating that expression of VGSC contributes the increased sensitivity of DP thymocytes to weak positively selecting signals.
miR-181a is an microRNA that can enhance DP thymocyte sensitivity (Li et al. 2007) (Ebert et al. 2009, 2010). Like Scn5a and Scn4b, the expression of miR-181a precisely correlates with two selection events in thymocyte development: DN3 (β-selection) and DP (positive selection). Moreover, the expression of miR-181a is regulated by the strength of TCR signals, that the stronger the TCR signals (such as negative selection signals), the fewer miR-181a remains expressed after positive selection. miR-181a increases DP thymocyte sensitivity by inhibiting several phosphatases that negatively regulate the TCR signaling cascade, including Ptpn22 (which inhibits the phosphorylation of ZAP70 and Lck), Shp2 (a tyrosine kinase phosphatase), DUSP5, and DUSP6 (which inhibits ERK phosphorylation) (Ebert et al. 2009, 2010; Li et al. 2007). By negatively regulating the inhibitors in the TCR proximal signaling and ERK activation pathways, miR181-a therefore can enhance the sensitivity of DP thymocytes. Indeed, overexpression of miR-181a in mature T cells endows them to respond to ligands that were normally too weak to stimulate a response. Interestingly, similar to inhibition of VGSC pore function, inhibition of miR-181a in thymic cultures impaired Gag-Pol ability to positively select 5C.C7 CD4+ T cells; however, miR-181a deficiency also disrupts the central tolerance that more self-reactive T cells were positively selected.
SCN5A-SCN4B composed VGSC and miR-181a share many similar features: the expression of both are very developmental stage-specific (only at DN3 and DP stages) and quickly regulated by the strength of TCR signaling; both enhance the sensitivity of preselection DP thymocytes to respond to weak ligands, and while ectopic expression in mature T cells, mature T cells acquire the ability to respond to the ligands that they normally do not respond; both play roles in the Ca2+ signaling pathways. Together both components endow preselection DP thymocytes to translate low affinity positively selecting ligand interactions to a series of strong, sustained Ca2+ flux and ERK activation to deliver a selection signal whose strength and duration is “just right” to initiate genetic programs required for T cell maturation but not to induce programmed cell death.
1.3 Self-Peptides Presented by Cortical Thymic Epithelial Cells are Essential for Positive Selection
Self-peptides presented by MHC molecules on cortical thymic epithelial cells (cTECs) in the thymus are a sine qua non for positive selection. The issue if thymic epithelial cells present a unique repertoire of self-peptides has been an active area of investigation for several decades (Lorenz and Allen 1988; Marrack et al. 1993), but little progress had been made in recent years until the unique protein degradation machinery was identified in cTEC. Importantly, cTECs uniquely express β5t-containing thymoproteasomes to process antigens for MHC class I-restricted presentation (see review in Takahama et al. 2010). The unique β5t subunit in the thymoproteasome favors the production of peptides that are less stably bound to MHC class I molecules, because β5t subunit does not efficiently cleave substrates at hydrophobic residues to generate optimal MHC class I binding peptides (Murata et al. 2007). This feature of β5t may be parallel to the observation that short-lived TCR engagement would promote the positive selection of CD8+ T cell (Nitta et al. 2010). The deficiency of β5t subunit affected positive selection of CD8+ T cells of MHC class I-restricted TCR transgenic mouse strains to different extents. The β5t deficiency decreased positive selection of HY TCR transgenic CD8+ T cells strongly, but had no effect on OT-I TCR transgenic CD8+ T cells (Nitta et al. 2010). These data suggested that some of the self-peptides displayed by cTEC are unique, while some of the self-peptides overlapped to those presented by peripheral antigen-presenting cells (APCs). The thymoproteasome-specific self-peptides are critical for the positive selection of most CD8+ T cells (Nitta et al. 2010), and essential for the generation of an immunocompetent repertoire of CD8+ T cells. However, thymoproteasome is not the sole component on cTEC to generate self-peptides for MHC class I-restricted positive selection.
As for peptide generation for MHC class II, cTECs express lysosomal cysteine proteases cathepsin L, but not cathepsin S as in B cells and dendritic cells (Honey and Rudensky 2003). Cathepsin L-deficient mice have impaired CD4+ T cell selection, suggesting its critical role in generating self-peptides presented by MHC class II molecules (Honey et al. 2002; Nakagawa et al. 1998). However, cathepsin L expression is not exclusively restricted to cTECs, as macrophages also express cathepsin L (Hsieh et al. 2002; Nakagawa et al. 1998). While engineering a fibroblast cell line to express either cathepsin S or cathepsin L, and using mass spectrometry to analyze the study showed that self-peptides eluted from the MHC class II molecules of the fibroblasts, the overlap between the two self-peptide repertoires was substantial (Hsieh et al. 2002). Therefore, despite the expression of cathepsin L by cTECs and cathepsin S by B cells and dendritic cells, the universe of self-peptides presented by MHC class II on cTEC greatly overlaps with that presented by peripheral antigen-presenting cells. Thus, although cTECs appear to express some unique protein processing components, this has not been shown to result in the presentation of a truly unique repertoire of peptides.
2 The Specificity of Positively Selecting Self-Peptide Recognition
2.1 One Positively Selecting Self-peptide has to Select More than One TCR
Positively selecting self-peptides may directly influence the post-selection repertoire of mature T cells. For example, altered positively selecting self-peptides because of the β5t deficiency would affect the post-selection repertoire of mature T cells (Nitta et al. 2010). Such observations indicate DP thymocytes recognize positively selecting ligands with a certain degree of specificity, and raise two questions: first, to what degree of specificity do DP thymocytes recognize positively selecting ligands? Second, how does the specificity of positively selecting self-peptides affect the repertoire of mature T cells?
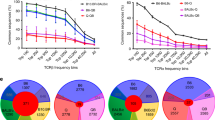
To examine the relationship between TCRs and a positively selecting self-peptide/MHC, a simple calculation reveals that it is not a monogamous relationship. A single positively selecting self-peptide must select multiple TCRs. For MHC class II-associated peptides, a B cell line expressing I-Ag7 MHC class II molecules was used to isolate endogenously processed peptides. Mass spectrometry analysis estimated an individual APC could display roughly 2,000 different peptide families (a set of peptides with the same core P1–P9 residues but containing variable numbers of extensions at amino- and carboxy-terminus is defined as a peptide family) (Suri et al. 2002). Also, MHC I-associated peptides from EL4 thymoma cell lines were analyzed by mass spectrometry, compared with those from β2m-deficient EL4 mutant cells (Fortier et al. 2008). The data suggested thousands of peptides were present in low copy numbers per cell (Fortier et al. 2008). Given that a mouse is estimated to have a total of 30 million different T cells (Casrouge et al. 2000), one single peptide family must select several thousand T cells to generate a complete T cell repertoire (Fig. 1a). Even if we have underestimated the number of peptide families presented by cortical thymic epithelia cells by 10 or 100 fold due to the presence of many low-abundant self-peptides not detectable by current mass spectrometry techniques, one self-peptide-MHC still has to positively select more than one TCR.
a Illustration of the relation between self-peptide repertoire and T cell repertoire. b Illustration of the relation between positively selecting ligands and the TCRs. One individual TCR can be positively selected by more than one positively selecting ligand; however, for some TCRs, a dominant positively selecting ligand may exist, if there is a particular ligand that can provide the most optimal strength of pMHC/TCR interaction. Also, each selecting ligand has its own spectrum of positively selecting capability. One positively selecting ligand can select many different TCRs, but not all of the post-selection T cells are positively selected by the optimal strength of pMHC/TCR interaction
2.2 Recognition of Positively Selecting Ligand by a Given TCR Exhibits a High Degree of Specificity
To directly examine the relationship between positively selecting self-peptides and post-selection TCR repertoire, we and others have identified naturally occurring positively selecting self-peptides for individual TCRs in the universe of self-peptides in vitro. The first naturally occurring positive selecting self-peptides identified were the MHC class I-restricted Cappa1 peptide and β-catenin peptide for OT-I TCRs (Hogquist et al. 1997; Santori et al. 2002). The approach used was to screen self-peptides that were eluted from purified Kb molecules from EL4 T cell thymoma, LB27.4 B cell lymphoma cells, and thymi from C57BL/6 mice. These eluted self-peptides were fractionized by reverse phase HPLC and used in a coreceptor downregulation assay with TAP-I deficient OT-I TCR transgenic thymocytes. In 80 fractions, only two self-peptides, Cappa1 and β-catenin, were able to positively select OT-I TCR transgenic DP thymocytes, and none were able to positively select a closely related 2C TCR DP thymocytes (Hogquist et al. 1997; Santori et al. 2002).
For MHC class II, a panel of 95 I-Ek self-peptides eluted from CH27 mouse B cell lines has been screened for the ability of individual peptide to induce positive selection of five I-Ek-restricted TCR transgenic mouse lines: AND, 2.102, N3L2, A1, and 5C.C7 (Ebert et al. 2009; Lo et al. 2009). Among the 95 tested, only one peptide, gp250, was confirmed for its ability to positively select AND TCR (Lo et al. 2009), whereas for 5C.C7, six peptides were found in which Gag-Pol was the most potent one (Ebert et al. 2009). Among the same pool of 95 peptides, none were found to be able to positively select 2.102, N3L2, or A1 TCR (Lo et al. 2009). Such a frequency and low success rate in identifying a naturally occurring positively selecting ligand for MHCI- and MCHII-restricted TCRs imply that the recognition of positively selecting ligand has a certain degree of specificity. The recognition of positively selecting ligand with a high degree of specificity is further supported by experiments that a single amino acid substitution of gp250 disrupts its ability to positively select AND TCR. Moreover, the positive selection of the two highly similar TCRs, AND, and 5C.C7, are mediated by two mutually exclusive self-peptides. Both TCRs recognize agonist peptide MCC, uses the same TCR α and β segments (Vα11 and Vβ3), and only differ by four amino acids in CDR3α regions (Malherbe et al. 2004). But positively selecting peptide gp250 for AND TCR did not positively select 5C.C7 TCR, and selecting ligand Gag-Pol for 5C.C7 was unable to positively select AND TCR. Therefore, in vitro, preselection DP thymocytes recognize positively selecting ligands with a high degree of specificity. Whether recognition of positively selecting self-peptides in vivo requires a similarly high degree of specificity would require further studies.
2.3 Positively Selecting Ligand Shapes the Antigen Specificity of Post-Selection TCR Repertoire
One positively selecting self-peptide has to select at least several thousands of TCRs, and TCR recognition of positively selecting self-peptide/MHC complexes has a high degree of specificity in vitro. Does such a specific recognition during positive selection affect peptide specificity of the peripheral T cell repertoire? Several studies examined the question by starting with a defined positively selecting self-peptide, and examined the question that how many and what TCRs were positively selected by a single selecting ligand. Initial studies were performed on mouse strains engineered to express MHC class II complexes loaded with a single peptide, by either generating a H-2M deficient mouse line or introducing a transgene that covalently linked MHC to a specific peptide.
In H-2M deficient mice, the peptide exchange machinery is disrupted and MHC class II molecules are predominately bound by a single peptide species, CLIP. Approximately 30–50 % of normal numbers of CD4+ T cells developed in H-2M deficient mice, and these post-selection CD4+ T cells expressed full range of Vβ segments (Grubin et al. 1997; Surh et al. 1997). A similar phenotype was observed in Eα52-68/I-Ab single chain mice, in which a transgene encoding the Eα52-68 peptide covalently bound to the I-Aβb chain was introduced to invariant chain and endogenous I-Aβb deficient background (Ignatowicz et al. 1996, 1997). In Eα52-68/I-Ab single chain mice, the percentage of mature CD4+ T cells decreased to around 20 % of wide type, and contained a full range of TCRβ usage (Ignatowicz et al. 1996, 1997). Both H-2M deficient and Eα52-68/I-Ab mice are capable of responding to immunization with multiple peptide antigens (Grubin et al. 1997; Ignatowicz et al. 1996, 1997; Surh et al. 1997). These studies demonstrated that a single predominant MHC bound peptide can positively select a very large and diverse repertoire of TCRs. However, these single peptide mice have skewed the amino acid frequency in the TCRα CDR3 loop and TCR Vα usage in positively selected T cells, and failed to positively select several defined clones of TCRs when introducing various transgenic TCRs onto these single peptide mice (Chmielowski et al. 2000; Fukui et al. 1997, 1998; Gapin et al. 1998; Grubin et al. 1997; Ignatowicz et al. 1996, 1997; Surh et al. 1997). Further studies showed that other low abundance self-peptides, not just the engineered dominant peptide, probably contributed to the generation of majority of post-selection TCR repertoire (Barton and Rudensky 1999). The study used mice expressing a human invariant chain (Ii) transgene in which CLIP region of human Ii was replaced with the Eα52-68 peptide (Ii-Eα mice). The Ii-Eα mice successfully restored the MHC class II expression to wild-type levels, but only 95 % of MHC class II molecules were bound with Eα52-68 peptide. These 5 % non-Eα52-68 peptides were dependent on H-2M molecules. When the H-2M deficiency was introduced to Ii-Eα mice, the number of CD4+ T cells decreased to 30 % of that seen in H-2M sufficient Ii-Eα mice (Barton and Rudensky 1999). The data suggested both high and low-abundant self-peptides may contribute to positive selection of T cells. With regard to the positive selection of CD8+ T cells, it has also been shown that a single peptide-MHC complex positively selects a diverse and specific CD8 T cell repertoire (Wang et al. 2009). Further evidence about the relationship between positive selection ligands and specificities of post-selection T cells comes from two transgenic mouse lines that each expresses a different single peptide–MHC class I complex (OVA and VSVp). Each mouse line exhibits exclusive TCR repertoires with unique peptide specificities that do not exist in the other mouse line, while the two lines do share some overlapping peptide specificities. From this piece of evidence, we learn that the positive selection in CD8+ T cells is very peptide specific. Each positively selecting ligand may exhibit a different spectrum of positive selection capability, resulting in a unique post-selection repertoire. However, two different positively selecting peptides may both be capable of positively selecting same TCRs. Taken together, positive selection requires the specific recognition of self-peptides to generate a complete T cell repertoire.
A question remains that whether the specific recognition of positively selecting self-peptides may influence the peptide specificities of post-selection T cell repertoire. To answer the question, four Ii-peptide transgenic mouse lines were generated, including Eα, CLIP, CD22, and Rab5a (Barton et al. 2002). The mature CD4+ T cells in these four Ii-peptide transgenic mouse lines showed different degrees of proliferative responses in mixed lymphocyte cultures. The CLIP- and Rab5a-selected CD4+ T cells proliferated most strongly, Eα-selected CD4+ T cells proliferated moderately, and CD22-selected CD4+ T cells proliferated weakly (Barton et al. 2002). The study convincingly showed that T cells selected by one peptide have different specificities compared with T cells selected by a second peptide (Barton et al. 2002). Similar studies examined the positive selection of T cells that are specific for MCC responses (Liu et al. 1997; Nakano et al. 1997). The CD4+ T cells were positively selected by MCC peptide, MCC variants, or unrelated Hb peptide (Nakano et al. 1997). The sequence of engineered self-peptides directly influenced the post-selection T cell’s capability of responding to the MCC peptide, and TCR usage (Liu et al. 1997; Nakano et al. 1997).
Thus, taken together, the recognition of positively selecting peptides has a certain degree of specificity. Even though a single peptide can induce positive selection of a large population of T cells (one peptide is capable of selecting 105 distinct TCR) (Gapin et al. 1998), but no single peptide is capable of selecting a full repertoire (Fig. 1b) (Barton et al. 2002; Barton and Rudensky 1999). When multiple peptides can select a T cell population responsive to the same antigen, the different peptides can select repertoire varying in patterns of fine antigen specificity and TCR usage.
3 The Role of Self-Peptides in Periphery
3.1 Positively Selecting Self-Peptides May Maintain Homeostatic Proliferation and Survival of Peripheral T Cells
In periphery, T cells require continuous low-level TCR interaction with self-peptides to survive (Brocker 1997; Davis et al. 2007; Min and Paul 2005; Morris and Allen 2012). Homeostatic proliferation is driven by a low-affinity interaction with self-peptide/MHC (Sprent et al. 2008; Surh and Sprent 2000, 2008). It remains unknown whether T cell reactivity to self-peptides in periphery relates to the affinity threshold established during positive selection. Positively selecting self-peptides may not only rescue DP thymocytes from programed cell death, but also program the long-term competitive fitness of post-selection T cells in periphery to influence its survival, proliferation, and functional alertness (Ernst et al. 1999; Goldrath and Bevan 1999; Viret et al. 1999) (Fig. 2).
Do positively selecting ligands contribute to naive T cell homeostatic proliferation in periphery? Is the ligand that mediate homeostatic proliferation the same as the positively selecting ligand in thymus? The first evidence came from the homeostatic proliferation experiments by using H-2M-deficient mice (Goldrath and Bevan 1999; Viret et al. 1999). The MHC class II molecules of H-2M-deficient mice are almost exclusively loaded with class II invariant chain peptides (CLIPs) (Fung-Leung et al. 1996; Martin et al. 1996; Miyazaki et al. 1996), so that the CLIP peptide was highly expressed in both thymus, and in periphery. Naive CD4+ cells from B6 mice failed to proliferate in T cell-depleted H-2M-deficient hosts, whereas naive CD4+ cells from H-2M-deficient hosts proliferate strongly (Goldrath and Bevan 1999; Viret et al. 1999). The difference in proliferative responses may result from the possibility that homeostatic proliferation is driven by the positively selecting ligands. H-2M deficient CD4+ T cells were positively selected by high abundant CLIP in the thymus, and therefore were capable of homeostatic proliferation while adoptively transferred to H-2M deficient hosts. On the other hand, wide-type B6 CD4+ T cells were positively selected by a normal self-peptide repertoire. Given the normal self-peptide repertoire is absent in H-2M deficient hosts because the peptide exchange machinery is disrupted, wide-type B6 CD4+ T cells failed to homeostatically proliferate. Additionally, in H-2M-deficient mice, recognition of self-peptide/MHC may maintain the survival and repertoire of mature CD4SP T cells (Kieper et al. 2004; Moses et al. 2003). Similar evidence comes from studies of CD8+ T cells using TAP-deficient mice. OVA-specific CD8 T cells failed to proliferate when transferred into TAP-deficient mice, but they would proliferate when transferred into transgenic mice expressing positively selecting altered peptide transgenes of OVA (Goldrath and Bevan 1999).
More recently, using the naturally occurring self-peptides for CD4+ T cell selection (gp250 peptide for positively selecting AND TCR and Gag-Pol peptide for 5C.C7 TCR) (Lo et al. 2009; Ebert et al. 2009), the relationship of the ligand that positively select and that to mediate homeostatic proliferation was tested. CFSE-labeled naive AND CD4+ T cells were adoptively transferred to chronic lymphopenic B6.Rag1 -/-H-2k recipients, and mice were injected intraperitoneally with additional positively selecting ligand gp250 or non-selecting control peptide Hb. Two- to ninefold more AND CD4+ T cells were recovered from the gp250-injected mice (Lo et al. 2009). A single mutation of the gp250 TCR contact residues disrupt gp250 ability to enhance the survival of AND CD4+ T cells, suggesting the enhanced survival is gp250-peptide specific. Interestingly, the recognition of self-peptides in periphery has the identical high degree of specificity to that observed for positive selection (Lo et al. 2009). In the case of positively selecting self-peptide Gag-Pol and 5C.C7 T cells, Gag-Pol peptide can also drive the homeostatic proliferation of 5C.C7 CD4+ T cells (Singh et al. 2012; Walker 2012), supporting the hypothesis that the crucial role of positively selecting ligands extends beyond the thymus into periphery.
3.2 Positively Selecting Self-Peptides May Function as Co-agonists to Augment Functional Sensitivity of Peripheral T Cells
In addition to homeostatic proliferation and maintenance of peripheral T cells, positively selecting ligands can also directly contribute to the functional alertness and responsiveness of naive T cells to cognate antigens. The first notion of such a possibility was suggested by the observation that self-peptides were co-localized with agonist peptides at the immunological synapse (Wulfing et al. 2002). The co-localization of self-peptides with agonist peptides was shown to participate in T cell activation by acting as a co-agonist in both MHC class I and class II systems (Irvine et al. 2002; Krogsgaard et al. 2005). The pseudodimer model suggests CD4 coreceptor allows certain self-peptide/MHC complexes to contribute to T cell activation, thus functioning as a coagonist. With 5C.C7 T cells (Irvine et al. 2002; Krogsgaard et al. 2005), three out of seven self-peptides were demonstrated to act as co-agonists and enhance T cell activation when presented in conjunction with small amounts of agonist peptide. Despite the unlikelihood of the CD8 co-receptor forming a similar pseudodimer, self-peptides have been shown to also function as coagonists in class I-restricted responses (Yachi et al. 2005). These observations raise the possibility that positively selecting peptides encountered in the thymus may continue to have profound effects on T cell responses in the periphery. In the gp250/AND system, the positively selecting self-peptide gp250 can enhance naive AND T cell activation to lower concentration of agonist MCC (Lo et al. 2009). Similarly, in the case of 5C.C7, the positively selecting ligand Gag-Pol can enhance naive 5C.C7 T cell activation in response to agonist MCC peptide, while other nonrelated peptides lacked such capability (Juang et al. 2010). The validity of the co-agonist model is still debated, and resolution of this issue awaits a determination of the precise number of TCRs (one or more than one) in the initial T cell recognition with limiting number (physiological numbers) of peptide/MHC complexes.
3.3 Positive Selection Signals May Program the Long-Term Survival and Function of Peripheral T Cells
So how does the positive selection “experience” become a critical determinant to influence peripheral T cell function and survival? Two studies in CD8+ T cells have related positive selecting signaling events to T cell maintenance and functional capability in periphery by using CD8+ TCR transgenic mouse lines which have a different affinity for self-peptide/MHC complexes (Cho et al. 2010; Sinclair et al. 2011), with OT-I CD8+ T cells having the highest sensitivity, followed by 2C CD8+ T cells, where HY CD8+ T cells have very low or undetectable sensitivity to homeostatic proliferation. The CD8+ T cell homeostatic proliferation relies on TCR and IL-7 signaling. The propensity for T cells to undergo homeostatic proliferation correlates with their intrinsic TCR affinity for self MHC ligands. Thus, for CD8+ T cells, naive cells from OT-1 and 2C TCR transgenic mouse lines have relatively high affinity for self-peptide/MHC ligands, while the HY CD8+ T cells have the relatively low self reactivity, such that they fail to homeostatically proliferate. The strength of positively selecting signals dynamically regulate IL-7Rα abundance and CD5 surface expression (Sinclair et al. 2011). IL-7 receptor signals are critical for all peripheral T cell subsets (Sinclair et al. 2011), and CD5 constitutively associates with SHP-1 to negative regulate TCR signals (Azzam et al. 1998). The stronger the positive selection signals, the higher the expression of IL-7R and CD5 express on the cell surface on mature T cells (Cho et al. 2010; Sinclair et al. 2011). The variation in IL-7R and CD5 expression may determine the capability of homeostatic survival and proliferation of these three TCR transgenic CD8+ T cell clones. Therefore, positive selection programs IL-7Rα and CD5 expression on mature T cells to influence the ability of new generated T cells to be maintained within the peripheral T cell repertoire (Fig. 3).
TCR affinity for self-peptide-MHC complexes not only influences their potential for homeostatic proliferation, but also tunes T cell activation threshold in response to cognate antigen. Our laboratory generated two TCR transgenic mice, LLO56 and LLO118, specific for an immunodominant Listeria epitope (listeriolysin 190–205) and only differing by 15 amino acids in their TCR sequences (Weber et al. 2012). These cells differed only in their CD5 levels, and showed dramatically different in vivo responses against Listeria infection. LLO56, with higher CD5 expression, has a significantly stronger recall response, whereas LLO118, with lower CD5 surface expression, mediated a better primary response. More interestingly, while sorting out LLO118 cells that express similar CD5 levels as LLO56 cells, CD5hi LLO118 cells became good recall responders as well (Fig. 3). This observation directly correlates CD5 expression level and peripheral function. While positive selection signals program CD5 surface expression, the competitive fitness and peripheral function are also imprinted in mature T cells.
4 Conclusion
Accumulated evidence strongly suggests that the recognition of a positively selecting ligand is not promiscuous, or simply a passive rescue process to prevent cell death by neglect or by negative selection. Instead, individual positively selecting ligands may enrich unique repertoires of mature T cells. One positively selecting self-peptide has to select more than several thousand T cells, and the TCR recognition of positively selecting self-peptides has a high degree of specificity. The specific interaction between individual TCRs and self-peptide/MHC complexes may determine the expression levels of signaling molecules and cytokine receptors, such as CD5 and IL-7Rα, therefore affecting functional sensitivity and competitive fitness of peripheral T cells. Similarly, one positively selecting ligand may differ with the other ligands in terms of the capability of positive selection, that some self-peptides may be better selectors to positively select a broader TCR repertoire, and some might select a narrower repertoire.
References
Alarcon B, and van Santen HM (2010) Two receptors, two kinases, and T cell lineage determination. Sci Signal 3:pe11
Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE (1998) CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188:2301–2311
Barton GM, Rudensky AY (1999) Requirement for diverse, low-abundance peptides in positive selection of T cells. Science 283:67–70
Barton GM, Beers C, deRoos P, Eastman SR, Gomez ME, Forbush KA, Rudensky AY (2002) Positive selection of self-MHC-reactive T cells by individual peptide-MHC class II complexes. Proc Natl Acad Sci U S A 99:6937–6942
Bluestone JA, Pardoll D, Sharrow SO, Fowlkes BJ (1987) Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature 326:82–84
Brocker T (1997) Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med 186:1223–1232
Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P (2000) Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol 164:5782–5787
Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H (2000) Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403:216–220
Chmielowski B, Muranski P, Kisielow P, Ignatowicz L (2000) On the role of high- and low-abundance class II MHC-peptide complexes in the thymic positive selection of CD4(+) T cells. Int Immunol 12:67–72
Cho JH, Kim HO, Surh CD, Sprent J (2010) T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity 32:214–226
Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA (1998) Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med 188:1867–1874
Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ (2007) T cells as a self-referential, sensory organ. Annu Rev Immunol 25:681–695
Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM (2009) An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol 10:1162–1169
Ebert PJ, Li QJ, Huppa JB, Davis MM (2010) Functional development of the T cell receptor for antigen. Prog Mol Biol Transl Sci 92:65–100
Eck SC, Zhu P, Pepper M, Bensinger SJ, Freedman BD, Laufer TM (2006) Developmental alterations in thymocyte sensitivity are actively regulated by MHC class II expression in the thymic medulla. J Immunol 176:2229–2237
Ernst B, Lee DS, Chang JM, Sprent J, Surh CD (1999) The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity 11:173–181
Fortier MH, Caron E, Hardy MP, Voisin G, Lemieux S, Perreault C, Thibault P (2008) The MHC class I peptide repertoire is molded by the transcriptome. J Exp Med 205:595–610
Fukui Y, Ishimoto T, Utsuyama M, Gyotoku T, Koga T, Nakao K, Hirokawa K, Katsuki M, Sasazuki T (1997) Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity 6:401–410
Fukui Y, Hashimoto O, Inayoshi A, Gyotoku T, Sano T, Koga T, Gushima T, Sasazuki T (1998) Highly restricted T cell repertoire shaped by a single major histocompatibility complex-peptide ligand in the presence of a single rearranged T cell receptor beta chain. J Exp Med 188:897–907
Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L (1996) Antigen presentation and T cell development in H2-M-deficient mice. Science 271:1278–1281
Gapin L, Fukui Y, Kanellopoulos J, Sano T, Casrouge A, Malier V, Beaudoing E, Gautheret D, Claverie JM, Sasazuki T et al (1998) Quantitative analysis of the T cell repertoire selected by a single peptide-major histocompatibility complex. J Exp Med 187:1871–1883
Goldrath AW, Bevan MJ (1999) Selecting and maintaining a diverse T-cell repertoire. Nature 402:255–262
Grubin CE, Kovats S, deRoos P, Rudensky AY (1997) Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity 7:197–208
Havran WL, Poenie M, Kimura J, Tsien R, Weiss A, Allison JP (1987) Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature 330:170–173
Hernandez-Hoyos G, Sohn SJ, Rothenberg EV, Alberola-Ila J (2000) Lck activity controls CD4/CD8 T cell lineage commitment. Immunity 12:313–322
Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, Jameson SC (1997) Identification of a naturally occurring ligand for thymic positive selection. Immunity 6:389–399
Honey K, Rudensky AY (2003) Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol 3:472–482
Honey K, Nakagawa T, Peters C, Rudensky A (2002) Cathepsin L regulates CD4+T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. J Exp Med 195:1349–1358
Hsieh CS, deRoos P, Honey K, Beers C, Rudensky AY (2002) A role for cathepsin L and cathepsin S in peptide generation for MHC class II presentation. J Immunol 168:2618–2625
Ignatowicz L, Kappler J, Marrack P (1996) The repertoire of T cells shaped by a single MHC/peptide ligand. Cell 84:521–529
Ignatowicz L, Rees W, Pacholczyk R, Ignatowicz H, Kushnir E, Kappler J, Marrack P (1997) T cells can be activated by peptides that are unrelated in sequence to their selecting peptide. Immunity 7:179–186
Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM (2002) Direct observation of ligand recognition by T cells. Nature 419:845–849
Jameson SC, Hogquist KA, Bevan MJ (1995) Positive selection of thymocytes. Annu Rev Immunol 13:93–126
Juang J, Ebert PJ, Feng D, Garcia KC, Krogsgaard M, Davis MM (2010) Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J Exp Med 207:1223–1234
Kieper WC, Burghardt JT, Surh CD (2004) A role for TCR affinity in regulating naive T cell homeostasis. J Immunol 172:40–44
Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM (2005) Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature 434:238–243
Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R (2000) Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity 12:537–546
Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P et al (2007) miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129:147–161
Liu CP, Parker D, Kappler J, Marrack P (1997) Selection of antigen-specific T cells by a single IEk peptide combination. J Exp Med 186:1441–1450
Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM (2009) An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+T cells. Nat Immunol 10:1155–1161
Lo WL, Donermeyer DL, Allen PM (2012) A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol 13:880–887
Lorenz RG, Allen PM (1988) Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A 85:5220–5223
Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG (2004) Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity 21:669–679
Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS (2001) Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol 167:4966–4973
Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH (1993) Comparison of peptides bound to spleen and thymus class II. J Exp Med 178:2173–2183
Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L (1996) H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell 84:543–550
McNeil LK, Starr TK, Hogquist KA (2005) A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A 102:13574–13579
McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR (2007) The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity 27:425–437
Min B, Paul WE (2005) Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Semin Immunol 17:201–207
Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D (1996) Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell 84:531–541
Moran AE, Hogquist KA (2012) T-cell receptor affinity in thymic development. Immunology 135:261–267
Morris GP, Allen PM (2012) How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol 13:121–128
Moses CT, Thorstenson KM, Jameson SC, Khoruts A (2003) Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci U S A 100:1185–1190
Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K (2007) Regulation of CD8 + T cell development by thymus-specific proteasomes. Science 316:1349–1353
Nakagawa T, Roth W, Wong P, Nelson A, Farr A, Deussing J, Villadangos JA, Ploegh H, Peters C, Rudensky AY (1998) Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280:450–453
Nakano N, Rooke R, Benoist C, Mathis D (1997) Positive selection of T cells induced by viral delivery of neopeptides to the thymus. Science 275:678–683
Naramura M, Kole HK, Hu RJ, Gu H (1998) Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc Natl Acad Sci U S A 95:15547–15552
Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y (2010) Thymoproteasome shapes immunocompetent repertoire of CD8+T cells. Immunity 32:29–40
Plas DR, Williams CB, Kersh GJ, White LS, White JM, Paust S, Ulyanova T, Allen PM, Thomas ML (1999) Cutting edge: the tyrosine phosphatase SHP-1 regulates thymocyte positive selection. J Immunol 162:5680–5684
Rothenberg EV, Moore JE, Yui MA (2008) Launching the T-cell-lineage developmental programme. Nat Rev Immunol 8:9–21
Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B (2010) Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal 3:ra23
Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, Naylor S, Vukmanovic S, Hogquist KA, Jameson SC (2002) Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity 17:131–142
Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A (1998) Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature 394:901–904
Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak SY, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PS (1996) Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med 183:1093–1104
Sinclair C, Saini M, van der Loeff IS, Sakaguchi S, Seddon B (2011) The long-term survival potential of mature T lymphocytes is programmed during development in the thymus. Sci Signal 4:ra77
Singer A, Adoro S, Park JH (2008) Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol 8:788–801
Singh NJ, Bando JK, Schwartz RH (2012) Subsets of nonclonal neighboring CD4+T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity 37:735–746
Sprent J, Cho JH, Boyman O, Surh CD (2008) T cell homeostasis. Immunol Cell Biol 86:312–319
Stephen TL, Tikhonova A, Riberdy JM, Laufer TM (2009) The activation threshold of CD4+T cells is defined by TCR/peptide-MHC class II interactions in the thymic medulla. J Immunol 183:5554–5562
Stritesky GL, Jameson SC, Hogquist KA (2012) Selection of self-reactive T cells in the thymus. Annu Rev Immunol 30:95–114
Surh CD, Sprent J (2000) Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J Exp Med 192:F9–F14
Surh CD, Sprent J (2008) Homeostasis of naive and memory T cells. Immunity 29:848–862
Surh CD, Lee DS, Fung-Leung WP, Karlsson L, Sprent J (1997) Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+T cells. Immunity 7:209–219
Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER (2002) In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J Immunol 168:1235–1243
Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K (2010) Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol 22:287–293
Viret C, Wong FS, Janeway CA Jr (1999) Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity 10:559–568
Walker LS (2012) Maintaining a competitive edge: new rules for peripheral T cell homeostasis. Immunity 37:598–600
Wang L, Bosselut R (2009) CD4-CD8 lineage differentiation: Thpok-ing into the nucleus. J Immunol 183:2903–2910
Wang B, Primeau TM, Myers N, Rohrs HW, Gross ML, Lybarger L, Hansen TH, Connolly JM (2009) A single peptide-MHC complex positively selects a diverse and specific CD8 T cell repertoire. Science 326:871–874
Wang D, Zheng M, Lei L, Ji J, Yao Y, Qiu Y, Ma L, Lou J, Ouyang C, Zhang X et al (2012) Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat Immunol 13:560–568
Weber KS, Li QJ, Persaud SP, Campbell JD, Davis MM, Allen PM (2012) Distinct CD4+helper T cells involved in primary and secondary responses to infection. Proc Natl Acad Sci U S A 109:9511–9516
Werlen G, Hausmann B, Palmer E (2000) A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature 406:422–426
Wiest DL, Yuan L, Jefferson J, Benveniste P, Tsokos M, Klausner RD, Glimcher LH, Samelson LE, Singer A (1993) Regulation of T cell receptor expression in immature CD4+CD8+ thymocytes by p56lck tyrosine kinase: basis for differential signaling by CD4 and CD8 in immature thymocytes expressing both coreceptor molecules. J Exp Med 178:1701–1712
Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM (2002) Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol 3:42–47
Yachi PP, Ampudia J, Gascoigne NR, Zal T (2005) Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol 6:785–792
Acknowledgments
We thank G. Morris and D. Donermeyer for their critical reading of the manuscript and comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lo, WL., Allen, P.M. (2013). Self-Peptides in TCR Repertoire Selection and Peripheral T Cell Function. In: Boehm, T., Takahama, Y. (eds) Thymic Development and Selection of T Lymphocytes. Current Topics in Microbiology and Immunology, vol 373. Springer, Berlin, Heidelberg. https://doi.org/10.1007/82_2013_319
Download citation
DOI: https://doi.org/10.1007/82_2013_319
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40251-7
Online ISBN: 978-3-642-40252-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)