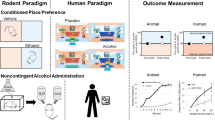
Abstract
The development of animal models that demonstrate excessive levels of alcohol consumption has played an important role in advancing our knowledge about neurobiological underpinnings and environmental circumstances that engender such maladaptive behavior. The use of these preclinical models has also provided valuable opportunities for discovering new and novel therapeutic targets that may be useful in the treatment of alcohol use disorder (AUD). While no single model can fully capture the complexities of AUD, the goal is to develop animal models that closely approximate characteristics of heavy alcohol drinking in humans to enhance their translational value and utility. A variety of experimental approaches have been employed to produce the desired phenotype of interest—robust and reliable excessive levels of alcohol drinking. Here we provide an updated review of five animal models that are commonly used. The models entail procedural manipulations of scheduled access to alcohol (time of day, duration, frequency), periods of time when access to alcohol is withheld, and history of alcohol exposure. Specially, the models involve (a) scheduled access to alcohol, (b) scheduled periods of alcohol deprivation, (c) scheduled intermittent access to alcohol, (d) scheduled-induced polydipsia, and (e) chronic alcohol (dependence) and withdrawal experience. Each of the animal models possesses unique experimental features that engender excessive levels of alcohol consumption. Both advantages and disadvantages of each model are described along with discussion of future work to be considered in developing more optimal models. Ultimately, the validity and utility of these models will lie in their ability to aid in the discovery of new and novel potential therapeutic targets as well as serve as a platform to evaluate treatment strategies that effectively reduce excessive levels of alcohol consumption associated with AUD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Alcohol use disorder (AUD) is a chronic relapsing disease that constitutes a significant public health problem. Heavy (excessive) levels of alcohol consumption over a prolonged period of time along with increased vulnerability to relapse represent hallmark features of AUD. The development of animal models that incorporate these key behavioral characteristics has played an important role in advancing our knowledge about biological underpinnings and environmental circumstances that engender such maladaptive behavior. These preclinical models are also crucial for identifying new potential therapeutic targets as well as providing a platform for evaluating the efficacy and safety of various treatment strategies.
We previously outlined numerous experimental approaches employed in developing rodent models of excessive alcohol self-administration (Becker 2013). As noted in that review, a major effort devoted to this endeavor has entailed devising experimental strategies that overcome the fact that rodents typically do not self-administer alcohol in sufficient amounts to produce overt signs of intoxication. Further, when given the opportunity to voluntarily drink alcohol, even under circumstances when access is unlimited, rodents rarely will consume alcohol in a manner that results in significant elevation in blood alcohol levels (above legal limits). In the past 2–3 decades, the field has progressed with the development of several new models and the resurrection and refinement of some older ones that demonstrate excessive and physiologically relevant levels of alcohol consumption. As detailed in our last review (Becker 2013), these models have generally entailed incorporating genetic manipulations (e.g., selective breeding for high alcohol drinking and preference), environmental manipulations that involve modifying scheduled access to alcohol and scheduled periods of alcohol deprivation, and linking drinking procedures with dependence models. Here we provide an updated review of these various models, again outlining procedural and translational advantages and disadvantages as well as addressing more contemporary work highlighting potential sex-related differences.
2 Models of Continuous Free-Choice Access to Alcohol
A common approach for studying voluntary alcohol consumption in rodents involves providing continuous (24-h) access to alcohol in a 2-bottle choice situation. Alcohol solutions of varying concentrations are presented in the home cage along with an alternative fluid (typically water) over a number of days. The main advantages of this approach are that it is relatively simple to implement, it enables relatively quick assessment of general avidity for alcohol, and it is a useful model for screening genetic determinants of the behavior (Ciccocioppo 2012; Crabbe et al. 2010). Indeed, unrestricted (24-h) daily access to alcohol in this 2-bottle choice model has been used to selectively breed lines of rats (e.g., P/NP, HAD/LAP, AA/ANA, and UChA/UChB) (McBride et al. 2014; Quintanille et al. 2006; Sommer et al. 2006) and mice (e.g., HAP/LAP) (Grahame et al. 1999) that display high vs. low alcohol preference and intake. The model has also been extensively employed to characterize alcohol consumption in unique recombinant inbred models of mice (e.g., BXD lines) (Gill et al. 1996; Phillips et al. 1994; Rodriguez et al. 1994, 1995) and different outbred strains of rats (Azarov and Woodward 2014a, b; Khanna et al. 1990; Priddy et al. 2017). The major disadvantage of this unlimited free-access model, however, is that it is difficult to determine whether alcohol intake reaches levels that are physiologically relevant (achieving significant blood alcohol levels that accompany behavioral signs of intoxication). Except for studies using selectively bred mice (Matson and Grahame 2013; Matson et al. 2014), most studies have not shown relevant levels of intoxication using this standard continuous access 2-bottle choice procedure. Also, since the main dependent variable is the cumulative amount of alcohol consumed each day (24-h period), the model does not allow for more refined analyses of temporal patterns and structure of drinking (e.g., bout frequency and duration). Restricting access to alcohol for shorter periods of time is a convenient way to relate alcohol consumption more precisely to resultant blood alcohol levels. Further, since rodents are nocturnal, providing scheduled access to alcohol during the dark phase of their circadian cycle (when eating, drinking, and general activity are at the highest levels) facilitates greater alcohol consumption.
3 Models Involving Scheduled Access to Alcohol
A mouse model that involves limited access to alcohol restricted to the dark phase of the circadian cycle was developed to model binge-like drinking (Crabbe et al. 2011a). The model, commonly referred to as “drinking-in-the-dark” (DID), was designed to yield a high level of alcohol intake over a defined period of time so as to produce blood alcohol concentrations above the US legal limit of intoxication (≥0.08 g/dL)—thereby satisfying the clinical criteria for binge-like drinking (NIAAA 2004). Typically, the procedure entails offering mice a single bottle of alcohol (20% v/v) for 2 h starting 3 h after the dark phase begins for 3 consecutive days. This is followed by a 4th day when access is extended to 4 h. This scheduled alcohol access produced significant consumption on the final day of this 4-day procedure in C57BL/6 mice, with resultant blood alcohol levels typically reaching ≥0.10 g/dL (Rhodes et al. 2005; Thiele et al. 2014; Thiele and Navarro 2014). Not surprisingly, alcohol intake in this model differed substantially across genotypes (inbred strains and recombinant mouse lines), and, importantly, drinking in C57BL/6 mice produced observable signs of intoxication as indexed by measures of motor incoordination (Rhodes et al. 2007). When the model incorporated a 2-bottle choice situation (water available as the alternative fluid), reduced alcohol intake and resultant blood alcohol levels have been reported (Phillips et al. 2010; Rhodes et al. 2007). However, another study with mice that had a history of drinking sucrose showed that alcohol intake in this 2-bottle choice situation (water as the alternative fluid) achieved significantly elevated blood alcohol levels (≥0.80 g/dL) (Giardino and Ryabinin 2013). Overall, the DID model has proven to reliably produce high levels of alcohol consumption in a short period of time, and this binge-like alcohol drinking does not appear related to motivation for obtaining calories contained in the alcohol (Lyons et al. 2008). In general, alcohol consumption in the DID model has been reported to be greater in female compared to male C57BL/6J mice (Levine et al. 2021; Younis et al. 2019).
A modified version of the 4-day DID model was employed by Crabbe and his colleagues to generate selectively bred lines of mice that drink substantial amounts of alcohol that produce behavioral signs of intoxication. In this work, mice from a genetically heterogeneous stock (HS/Npt) were tested in a 2-day single-bottle (20% alcohol) paradigm (2-h access the first day and then 4-h access the next day, both during the early part of the dark cycle). Over several generations, average blood alcohol levels registered immediately following the 4-h drinking session increased from an initial value of approximately 0.03 g/dL (prior to selective breeding) to 0.10 g/dL (Crabbe et al. 2009). This high drinking-in-the-dark (HDID-1) selected line also consumed significantly more alcohol than the control line from which they were selected, even though the selection was based on blood alcohol levels (not the amount of alcohol consumed). Interestingly, HDID-1 mice from the 13-17th selected generations consumed similar amounts of alcohol and other tastants (sucrose, saccharin, and quinine) as the control line when the solutions were presented under continuous (24-h) access conditions. However, greater intake was noted in the HDID-1 mice when preference testing was extended for several days under limited access conditions (Crabbe et al. 2011b). A second replicate line (HDID-2) displayed average blood alcohol levels that increased to 0.10 g/dL in 19 generations (Barkley-Levenson and Crabbe 2014). These HDID selected lines do not show differences in alcohol metabolism, but intake and blood alcohol levels were reduced when water was included as an alternative solution in the 4-day DID model (Barkley-Levenson and Crabbe 2014). A relatively modest preference for alcohol was displayed by male and female HDID lines when access was extended continuously (24-h/day) in the standard 2-bottle choice situation and intake remained relatively stable over several weeks (Crabbe et al. 2022). This suggests there are some distinct genetic influences that shape binge-like drinking when access is restricted versus those governing consumption when alcohol access is unlimited. Other studies have shown that HDID mice display increased sensitivity to some acute alcohol effects (locomotor stimulant effects) but reduced sensitivity to sedative/ataxia effects (Barkley-Levenson and Crabbe 2014). In studies using operant conditioning procedures, male and female HDID-2 mice (but not the HDID-1 line) showed increased oral self-administration (under both FR-1 and FR-3 schedules), but there was no apparent difference in progressive ratio responding (breakpoint) or cue-induced reinstatement of alcohol seeking behavior for either selected line compared to the progenitor line (Jensen et al. 2021; Savarese et al. 2021). These results align with findings indicating no differences in expression of conditioned place preference (Barkley-Levenson et al. 2015) and suggest some independence of genetic influences for motivational/rewarding effects of alcohol versus those underlying selection for binge-like drinking. On the other hand, the fact that both HDID lines show reduced alcohol-induced conditioned taste aversion suggests that reduced sensitivity to alcohol-related aversion may play a permissive role in elevated drinking in these mice (Barkley-Levenson et al. 2015).
Several studies have provided alcohol under limited access conditions (2-h/day) for an extended period of time. For example, presenting alcohol alone 2-h/day for 14 days produced faster rates of consumption (more drinking during the early portion of the drinking sessions) and tolerance to the ataxic effects of alcohol (Linsenbardt et al. 2011). This model has also been effectively used to study consequences of alcohol binge-like exposure in utero (Boehm et al. 2008) and during adolescence (Metten et al. 2011). Other studies have provided scheduled daily access to alcohol (2-h/day) over several weeks, but alcohol was presented along with water. Using this model, selectively bred high alcohol-preferring (HAP) lines of mice were shown to “front-load” (accelerate drinking during the early portion of the limited access sessions), and the emergence of this binge-like drinking pattern was associated with rewarding effects of alcohol (Ardinger et al. 2020). Using male and female C57BL/6 mice, studies have shown that 2–3 weeks of drinking alcohol under these conditions also lead to apparent habitual drinking, as defined by resistance to alcohol reward devaluation produced by adulteration of the alcohol solution with quinine (i.e., persistence of alcohol drinking in the face of aversion related to the bitter taste of quinine) (Bauer et al. 2021; Schuh et al. 2022; Sneddon et al. 2019, 2021). Another model of limited access drinking that entails simultaneously offering several concentrations of alcohol (5–40%) for 2 weeks was shown to demonstrate measures of negative affect related to alcohol withdrawal that varied depending on sex, age, and procedural conditions (Lee et al. 2017; Szumlinski et al. 2019).
Models involving scheduled access to alcohol have also been used in rats. Male rats selectively bred for high alcohol preference (P rats) consumed more alcohol in a 2-bottle free-choice situation (10% alcohol vs. water) when access was scheduled over four 1-h periods (each separated by 2 h) during the dark cycle compared to when the alcohol was available continuously for the equivalent 4-h period (Murphy et al. 1986). Building on these results, more recent studies have examined the effect of offering P rats concurrent access to three fluids (water vs. 15% alcohol vs. 30% alcohol) over three 1-h access periods during the dark phase of the circadian cycle. Over several weeks alcohol consumption in this model was shown to register significant blood alcohol levels (≥ 0.08 g/dL) as well as behavioral signs of intoxication (motor impairment), with effects more robust in females compared to males (Bell et al. 2011; McBride et al. 2010). Similar results were observed with selectively bred lines of high alcohol preference/drinking (P, HAD) rats when access to alcohol was limited to 2–3 h (Bell et al. 2014). Using a protocol that involved limited access (1-h/day) to multiple alcohol solutions in male Sardinian preferring (sP) rats, it was observed that randomizing the time of alcohol access each day produced higher levels of alcohol intake (and resultant blood alcohol levels) compared to consistently scheduled alcohol access (Colombo et al. 2014, 2015, 2017).
4 Models Involving Scheduled Alcohol Deprivation
Animals with a long history of daily access to alcohol display a transient yet robust increase in voluntary alcohol consumption and preference when alcohol is reintroduced after a period of deprivation. This alcohol deprivation effect was first formally described in rats (Sinclair and Senter 1968) but has also been demonstrated in mice (Salimov and Salimova 1993; Salimov et al. 2000; Tambour et al. 2008). Most studies have examined the phenomenon in rats using 2-bottle choice continuous access models. Increased alcohol drinking has been noted after relatively brief periods of deprivation (~24 h) as well as following longer (several weeks) deprivation intervals (Sinclair and Li 1989). The alcohol deprivation effect has also been demonstrated using limited access operant self-administration procedures in rats (Heyser et al. 1997; Holter et al. 1997) and mice (Sparta et al. 2009). However, there were no effects of deprivation on alcohol intake reported in a study using a modified (sipper-tube) self-administration procedure (Samson and Chappell 2001).
The alcohol deprivation effect has been demonstrated in outbred rat strains such as Wistar (Vengeliene et al. 2003) and Long-Evans (Sinclair and Tiihonen 1988). Similarly, an alcohol deprivation effect has been reported in rats selectively bred for high alcohol preference (P rats) under free-choice continuous access and limited access operant paradigms (McKinzie et al. 1998; Sinclair and Li 1989; Vengeliene et al. 2003). However, a robust increase in alcohol consumption following a period of deprivation has not been reliably observed in other rat lines selectively bred for high alcohol preference, including the Alko alcohol-accepting (AA) rats (Sinclair and Li 1989; Sinclair and Tiihonen 1988) and the Indiana high alcohol drinking (HAD) rats (Rodd-Henricks et al. 2000a). The Sardinian P (sP) rats, which were generated using the same selection criteria as the Indiana P rats, showed a fairly modest increase in alcohol intake that was very brief in duration (Agabio et al. 2000) or no increase after deprivation (Serra et al. 2003). Collectively, these data do not support a consistent relationship between selection for high alcohol preference/intake and expression of a robust alcohol deprivation effect.
Although the alcohol deprivation effect has been viewed as a model for alcohol relapse and craving, there are some drawbacks related to the model. One concern relates to the specificity of the phenomenon, since exaggerated intake of other rewarding tastants (e.g., sucrose and saccharin) can be demonstrated in rats following a period of deprivation (Avena et al. 2005; Wayner et al. 1972). As noted above, the increase in alcohol intake after short or long periods of deprivation is typically short-lived, with intake returning to baseline (pre-deprivation) levels in a few days. However, when P rats are given concurrent access to several alcohol concentrations (10, 20, and 30%) along with water, the alcohol deprivation effect was shown to be more robust and more durable (Rodd-Henricks et al. 2001). Further, this same experimental paradigm was reported to produce an alcohol deprivation effect in HAD rats even though these animals do not show such an effect when a single alcohol concentration is offered in a free-choice situation (Rodd et al. 2004).
While enhanced alcohol intake following a single deprivation period has been shown to be a transient effect, repeated deprivation experience produces longer lasting increases in alcohol consumption. For example, after long-term free access to several alcohol solutions, repeated “forced” abstinence periods resulted in progressively greater increases in alcohol intake, a shift in preference for higher alcohol concentrations, and longer lasting deprivation effects in Wistar rats (Spanagel and Holter 1999, 2000) and P rats (Rodd-Henricks et al. 2000b, 2001). Additionally, concurrent access to multiple concentrations of alcohol along with exposure to repeated cycles of deprivation produced significant increases in alcohol consumption in HAD rats, a genotype that does not readily exhibit an alcohol deprivation effect following a single period of deprivation (Rodd et al. 2009; Rodd-Henricks et al. 2000a). Using a similar experimental strategy involving multiple alcohol concentrations (0, 5, 10, and 15%) and several cycles of deprivation, increased alcohol consumption was demonstrated over repeated episodes of re-exposure to alcohol in rats selectively bred for low alcohol preference and drinking (NP and LAD rats) (Bell et al. 2004). This suggests that genetic selection for low alcohol preference/consumption can be overcome by experimental parameters that ordinarily engender expression of a more robust alcohol deprivation effect. Interestingly, offering several alcohol concentrations and repeated cycles of deprivation did not alter the magnitude or duration of a relatively brief and modest alcohol deprivation effect in sP rats (Serra et al. 2003).
In addition to enhancing the alcohol deprivation effect under 24-h free-choice conditions, repeated episodes of deprivation augmented and prolonged oral alcohol self-administration using operant conditioning procedures in Wistar, P, and HAD rats (Oster et al. 2006; Rodd et al. 2003; Spanagel and Holter 2000). Further, this effect was shown to be accompanied by an apparent enhancement of the reinforcing efficacy of alcohol, as indexed by higher breakpoint values under progressive ratio testing procedures (Oster et al. 2006; Rodd et al. 2003; Spanagel and Holter 2000). In a long-term drinking model involving several months of free-choice alcohol access and multiple episodes of deprivation, Wistar rats not only increased alcohol intake and demonstrated a progressive shift in preference for higher previously less preferred alcohol concentrations, but these rats also exhibited less sensitivity to the otherwise unfavorable adulteration of alcohol with quinine (Spanagel et al. 1996). This latter effect has been suggested to reflect more compulsive aspects of drinking that develops as a function of long-term access to alcohol with repeated intervening periods of abstinence (deprivation) (Spanagel 2009).
Fewer studies have systematically studied the alcohol deprivation model in mice (Vengeliene et al. 2014). In one study, the effect of repeated deprivation cycles on alcohol intake in a 2-bottle choice (10% alcohol vs. water) continuous access situation differed in substrains of C57BL/6 mice (Khisti et al. 2006). Repeated 4-day deprivation periods initially produced a robust alcohol deprivation effect in C57BL/6NCrl mice, but the transient increase in intake diminished in magnitude over successive deprivation cycles. In contrast, alcohol consumption did not significantly change following single or multiple cycles of deprivation in C57BL/6J mice. In a modified version of the alcohol deprivation effect, C57BL/6J mice showed increased alcohol intake following repeated weekly deprivation periods of 6 days (alcohol was reinstated 1 day each week). However, this effect was abolished with a longer (2-week) deprivation period (Melendez et al. 2006). Another study using male C57BL/6N mice also failed to show an effect of repeated alcohol deprivation periods (Vengeliene et al. 2014). In a recent study, male and female mice of the selectively bred HDID-1 and HDID-2 lines were given unlimited access to alcohol in their home cage continuously for 46 weeks. This was followed by five 2-week deprivation periods, with 2 weeks of resumed drinking after each deprivation period. Mice showed a stable pattern of intake over the initial 46 weeks that was not affected by repeated deprivation periods compared to mice that continued having uninterrupted access to alcohol (Crabbe et al. 2022).
Although relatively few studies have examined the alcohol deprivation effect in mice, single or multiple deprivation periods have not reliably produced enhanced alcohol drinking when alcohol is offered in the home cage under limited access conditions. A study in male C57BL/6J mice using the DID model examined alcohol intake over 6 weeks, with each of the 4-day drinking opportunities separated by 3 days off. A lickometer system was used in the study to show that mice gradually develop higher levels of intake during the first 15-min of access to the alcohol bottle (front-loading). In addition, increased alcohol intake was observed with repeated DID cycles (Wilcox et al. 2014). Another study using this procedure showed that repeated experience with weekly DID cycles (up to 10) did not result in significant signs of anxiety-like behavior but favored a subsequent increase in alcohol intake using a 24-h access protocol. However, mice did not show an increase in alcohol intake during the repeated cycles of DID (Cox et al. 2013).
5 Models Involving Scheduled Intermittent Alcohol Access
Another model that engenders a high level of alcohol consumption involves chronic intermittent access to alcohol. In this model, inherent in the scheduled intermittency of free access to alcohol are repeated periods of abstinence. Although the model is similar to the paradigm described above involving repeated periods of deprivation, in this case, the periods of alcohol access and deprivation are relatively short (days rather than weeks), thereby accelerating the pace at which excessive levels of alcohol intake can be established. This chronic intermittent access procedure was first described to produce increased drinking in rats when alcohol was provided on a continuous basis for 2 days with intervening 2-day abstinence periods (Wayner et al. 1972) or for 24-h every other day (Wise 1973). More recently, free access to 20% alcohol was offered in a 2-bottle choice situation (with water) for 24 h, 3 days a week (with no more than 2 days of abstinence between access days). Within 5–6 drinking sessions, alcohol consumption increased from baseline levels of about 2 g/kg/24-h to approximately 5–6 g/kg/24-h in Long-Evans rats (Simms et al. 2008). A similar outcome was reported in another study where Long-Evans rats exposed to the same procedure displayed progressively increased consumption and preference for 20% alcohol over 20 drinking sessions (Carnicella et al. 2009). This escalation of drinking along with an increased preference for alcohol was also demonstrated in Wistar rats (Simms et al. 2008), although another study using a 3-bottle choice situation (water vs. 5% vs. 20%) reported a two- to threefold difference in the change in alcohol intake and preference depending on the supplier of Wistar rats (Palm et al. 2011). The escalation of intake in Long-Evans and Wistar rats produced significantly elevated blood alcohol levels in samples taken after the first 30 min of the drinking sessions, with several subjects attaining levels above 0.08 g/dL (Carnicella et al. 2009; Simms et al. 2008). In another study, male Wistar rats that had 24-h access to alcohol in the home cage every other day not only displayed increased alcohol intake but also showed impaired working memory during acute (but not protracted) periods of abstinence (George et al. 2012). Increased alcohol consumption has also been noted in Sprague-Dawley rats following the 2-bottle (water vs. 20% alcohol) intermittent access paradigm (Bito-Onon et al. 2011), but the effect may only be observed in a portion of the animals (Moorman and Aston-Jones 2009).
In addition to home-cage drinking, this intermittent alcohol access model has also been extended to oral alcohol self-administration behavior using operant conditioning procedures. For example, Long-Evans rats were shown to vigorously respond to self-administered 20% alcohol when operant sessions scheduled every other day were gradually reduced from overnight to 30 min in duration (Simms et al. 2010). The increased amount of alcohol self-administered resulted in significant elevation of blood alcohol levels following the 30-min session, with average values of ~0.06 g/dL and several rats registering blood alcohol levels above 0.10 g/dL (Simms et al. 2010). In another study, prolonging the intermittent access schedule for several months not only increased home-cage alcohol drinking but also transferred to increased operant oral alcohol self-administration in Wistar rats (Hopf et al. 2010). Further, rats maintained on the intermittent access schedule to 20% alcohol for 3–4 months demonstrated resistance to quinine adulteration of alcohol in home-cage drinking and operant responding, but this effect was not observed in rats with a history of intermittent alcohol access for only 1.5 months (Hopf et al. 2010).
A few studies have examined drinking in this intermittent access model in rats selectively bred for high alcohol preference. For instance, P rats were shown to exhibit increased alcohol intake under conditions in which 24-h free-choice (20% alcohol vs. water) access was given every other day. However, this increase in alcohol consumption from an average baseline level of 4–5 g/kg/24-h) to 6–7 g/kg/24-h over 20 drinking sessions was relatively modest compared to the escalation of intake exhibited in Long-Evans and Wistar rats reported in the same study (Simms et al. 2008). In contrast, using a similar 2-bottle choice (20% alcohol vs. water), every other day scheduled access paradigm, the Sardinian P (sP) rats showed robust escalation of drinking (nearly a twofold increase in alcohol intake over 20 drinking sessions (Loi et al. 2010). This increase was also noted during the first hour of access during the dark phase, with intake rising from baseline levels of ~0.5 to 1.5–2.0 g/kg. Alcohol consumption in sP rats given intermittent access significantly exceeded intake registered in sP rats that were given the same alcohol solution (20% vs. water), but in a continuous access pattern. After 10 drinking sessions, consumption in the intermittent access group produced behavioral signs of intoxication, as measured by motor impairment in a rotarod task. Additionally, these rats exhibited resistance to the effects of quinine adulteration of alcohol as well as competing effects of concurrent access to saccharin (Loi et al. 2010). It is interesting that sP rats are very responsive to this chronic intermittent access procedure in which relatively short periods of access and abstinence (deprivation) are repeatedly alternated, while the Indiana P rats (but not sP rats) display robust escalation of drinking in a model of repeated deprivations where access and deprivation periods are longer in duration (Rodd-Henricks et al. 2001; Serra et al. 2003). An explanation for this discrepancy is not readily apparent at present (Loi et al. 2010).
Recent studies have suggested that sex and environmental factors (e.g., housing conditions) may modulate escalation of drinking in this model. Female Wistar rats that were pair housed (separated by a divider) did not show an increase in drinking compared to single-housed females or males independent of housing conditions (Moench and Logrip 2021). In another study, female Long-Evans rats showed significantly higher levels of intake compared to males and a gradual increase in alcohol preference, but neither males nor females showed an increase in alcohol intake over 7 weeks of intermittent access to 20% alcohol (vs. water) (Pirino et al. 2022). Intermittent access to alcohol in the home cage has also been used to “prime” rats before training them to respond to alcohol in an operant self-administration protocol. In these studies, it was observed that male P, Lister Hooded, and Long-Evans rats showed the expected gradual increase in alcohol intake over days of intermittent access while Wistar rats did not (Hernandez and Moorman 2020; McCane et al. 2021; Smeets et al. 2022). Overall, the emergence of escalated alcohol intake in the rats (and mice; see below) using this model depends on various factors including sex, genotype, and housing conditions (Carnicella et al. 2014; Spear 2020).
Similar studies have been conducted in mice. For example, Melendez (2011) reported that adult male C57BL/6J mice provided 24-h access to alcohol in a 2-bottle choice situation (15% alcohol vs. water) consumed significantly more alcohol when it was presented every other day in comparison to mice that received continuous access to alcohol every day. Specifically, initial alcohol intake (6–7 g/kg/24-h) increased to 14–15 g/kg/24-h over 7 drinking sessions in the intermittent access group while intake increased to 8–9 g/kg/24-h in the continuous access group. A large portion of the alcohol consumed occurred within the first 6 h when it was presented during the dark phase of the circadian cycle, and the increased level of drinking in the intermittent group reverted to lower (baseline) levels of intake when a continuous access schedule was implemented (Melendez 2011). In another study, C57BL/6J mice were first acclimated to increasing concentrations of alcohol and then maintained on a 24-h 2-bottle choice (20% alcohol vs. water) regimen, with access scheduled either every other day or continuously every day. Over the course of 4 weeks, alcohol consumption increased to ~20 g/kg/24-h in the intermittent access group compared to ~16 g/kg/24-h for the continuous access group (Hwa et al. 2011). This increase in alcohol consumption as a function of intermittent access was more robust in female (30 g/kg/day) compared to male (20 g/kg/day) C57BL/6J mice, and extending intermittent access for 16 weeks in the male subjects resulted in mild expression of withdrawal-related hyperexcitability. Also, intake over the first 2-h in a single-bottle test with 20% alcohol was greater in mice with intermittent compared to continuous access, and this greater intake resulted in higher blood alcohol levels (Hwa et al. 2011). However, using similar procedures, others have not observed this large a difference in intake between mice offered alcohol in an intermittent versus continuous fashion (Crabbe et al. 2012). The intermittent access model has been used with a variety of mouse genotypes including HDID, C3H/Hej, and C57BL/6J, among others). Although increased alcohol intake was observed in all the genotypes, it was not related to previous baseline levels of intake under continuous access conditions or subsequent withdrawal symptoms (Rosenwasser et al. 2013). Other recent studies have shown that female but not male C57BL/6J mice show escalation of alcohol intake in this model (Bloch et al. 2020; Cannady et al. 2020). Thus, in both rats and mice, females appear more likely to demonstrate elevated alcohol consumption when it is presented in an intermittent fashion. An explanation for this possible sex-related difference awaits further investigation.
6 Models Involving Schedule-Induced Polydipsia
Animals have been shown to engage in excessive drinking behavior when delivery of food reinforcement is scheduled in an intermittent fashion (typically a fixed time interval) that is not under the animal’s control (Falk 1961). This adjunctive behavior (excessive drinking) is displayed as a consequence of and in relation to another behavior that is evoked by environmental change (eating small amounts of food delivered in a scheduled manner that is not determined by the animal). The term schedule-induced polydipsia refers to the excessive nature of adjunctive drinking under these conditions, which greatly exceeds the fluid intake that would occur if the same total amount of food was presented all at once.
When an alcohol solution is the available fluid, this schedule-induced polydipsia results in excessive levels of alcohol consumption (10–14 g/kg/24-h) in rats that leads to dependence, as evidenced by overt signs of withdrawal when the alcohol is removed (Falk and Samson 1975; Falk et al. 1972). Alcohol consumption during daily 3-h sessions over several months was reported to be sufficient to produce dependence (Tang and Falk 1983). In a more recent study, a schedule-induced polydipsia procedure was used to assess alcohol consumption in rats selectively bred for high and low alcohol preference (Gilpin et al. 2008a). Across a number of alcohol concentrations, P rats and one of the replicate lines of HAD rats showed greater water and alcohol intake compared to their non-preferring counterparts (NP and LAD-2 rats). In all cases, blood alcohol levels were positively correlated with alcohol intake after the 1-h sessions, with many rats registering levels >0.08 g/dL (Gilpin et al. 2008a). This procedure has also been used to induce high levels of alcohol intake in adolescent male and female Sprague-Dawley rats, with intake (10% alcohol mixed with chocolate Boost®) yielding blood alcohol levels exceeding 0.08 g/dL in 30 min in males (0.086 ± 0.013 g/dL) and females (0.075 ± 0.010 g/dL) (Hosova and Spear 2017).
Schedule-induced polydipsia procedures have also been used to examine alcohol consumption in mice. In an early study involving outbred (ICR-DUB) female mice, four daily 1-h sessions (each separated by 6-h) produced high levels of drinking in mice given access to 6% alcohol (14–20 g/kg/day) or 10% alcohol (17–25 g/kg/day). In both cases, this level of intake over 7 days was not sufficient to produce significant signs of withdrawal following the scheduled access phase of the study (Ogata et al. 1972). Over 20 daily 1-h sessions, the alcohol-preferring C57BL/6J inbred strain consumed a substantial amount of 5% alcohol (~5 g/kg) relative to their initial intake (~1 g/kg). In contrast, the non-preferring DBA/2J inbred strain showed only a very modest increase in alcohol consumption under the same schedule conditions (Mittleman et al. 2003). However, in another study using a fixed-time schedule of food delivery (as opposed to a variable schedule used in the Mittelman et al. (2003) study, both C57BL/6J and DBA/2J mouse strains were shown to exhibit high levels of alcohol intake and signs of intoxication (Ford et al. 2013). These results highlight the importance of environmental factors (e.g., schedule of food presentation) interacting with genotype in governing alcohol consumption in this model.
Advantages of this model are that animals consume large quantities of alcohol orally and on a voluntary basis (Falk and Tang 1988). Disadvantages of this approach include lack of specificity of the effect since polydipsia can be seen when other fluids are made available (including water) and the fact that animals are typically maintained on a food-restricted diet. This latter issue raises concern about whether motivation to drink alcohol is related to its pharmacological effects or its caloric content. Of note, challenging the non-specific nature of this polydipsia model, a recent study showed that a subset of male Sprague-Dawley rats exhibited heightened alcohol consumption but no change in water intake under the same schedule-induced polydipsia experimental parameters (Fouyssac et al. 2021).
Another shortcoming of this model to consider is that when the schedule of intermittent reinforcement is relaxed, alcohol consumption reverts to control levels in rats (Tang et al. 1982). That is, elevated alcohol drinking does not endure under free-choice conditions even though the animals consumed large amounts of alcohol when it was available under intermittent schedules of food reinforcement (Ford 2014). However, inasmuch as such schedules that induce adjunctive behaviors are stressful (Falk 1971; Lopez-Grancha et al. 2006), it may be that studies in rodents have not utilized experimental parameters that are optimal for establishing the negative reinforcing effects of alcohol. That is, while schedule-induced polydipsia procedures are effective in establishing the positive reinforcing effects of alcohol (Meisch 1975), experimental conditions that facilitate association of alcohol consumption with stress relief (escape from the onerous nature of the intermittent, response non-contingent schedule of food delivery) may be required for producing long-lasting elevated drinking. A study conducted with high- and low-drinking mouse strains (C57BL/6J and DBA2/J, respectively) found that schedule-induced polydipsia results in high levels of alcohol intake and intoxication in both strains. Increases in blood alcohol levels were also associated with elevations in circulating levels of corticosterone due to the schedule restrictions (Ford et al. 2013; see also studies with monkeys in Jimenez et al. 2017). A more recent study conducted with male Sprague-Dawley identified a subpopulation of subjects that failed to drink a high level of water but consumed a high level of alcohol under the same schedule that induced polydipsia. This subpopulation was identified as “alcohol copers” for their avidity to drink alcohol to cope with the stress of this schedule. This same group of “alcohol coper” rats showed higher resistance to reduce drinking when the alcohol solution contained quinine (Fouyssac et al. 2021).
While this chapter mainly focuses on rodent models of excessive alcohol drinking, it is noteworthy that the schedule-induced polydipsia paradigm has been effectively used in nonhuman primates to demonstrate sustained high levels of alcohol intake (Grant et al. 2008). Further, the pattern of drinking during the induction phase of this model was shown to predict the degree of heavy drinking once the schedule-induced polydipsia regimen was relaxed. That is, cynomolgus monkeys that reached levels of intake that produced blood alcohol levels above 0.08 g/dL during the induction phase were classified as “gulpers” (as opposed to “sippers”) and showed higher levels of alcohol consumption during a subsequent 12-month continuous free-choice access period. Excessive alcohol consumption during this free-access period produced behavioral signs of intoxication in many of the subjects. Additionally, extending the open-access period to more than 2 years along with intervening periods of abstinence not only produced sustained excessive levels of alcohol consumption but also resulted in functional (synaptic) and morphological adaptations in the brain (putamen) (Cuzon Carlson et al. 2011). In separate studies, the level of aggressive temperament displayed by male and female rhesus macaques during late adolescence was shown to predict the level of alcohol intake in the schedule-induced polydipsia model; i.e., subjects displaying higher aggressive behavior also showed greater levels of alcohol intake and resultant blood alcohol levels (McClintick and Grant 2016). In another study, dominance hierarchy (dominant or subordinate status) did not relate to the levels of intake under schedule-induced polydipsia in adult male cynomolgus monkeys. However, after the induction phase of the study, when the schedule was relaxed, subordinate monkeys showed higher levels of alcohol intake than dominants (Galbo et al. 2022). Recently, Grant and her colleagues have shown that alcohol intake following induction in the schedule-induced polydipsia paradigm can be modulated by chemogenetic inhibition of the putamen in male and female rhesus monkeys. Specifically, inhibiting this area associated with habitual responding produced higher water and alcohol drinking (no sex-related differences were observed). However, during subsequent sessions alcohol intake reverted to baseline levels and water drinking was reduced to below baseline levels (Grant et al. 2022). Thus, the schedule-induced polydipsia procedure has proven to be effective and integral to this monkey model of heavy drinking that captures many of the features of alcohol use disorder.
7 Models Involving Alcohol Dependence and Withdrawal
Over the past two decades, rodent models involving chronic alcohol exposure producing dependence have been successfully linked with self-administration procedures to demonstrate excessive levels of alcohol intake (Becker 2008, 2014; Becker and Lopez 2016; Becker and Ron 2014). Indeed, numerous studies involving mice and rats have demonstrated escalated alcohol consumption using home-cage free-choice models and operant conditioning procedures (Griffin 2014; Lopez and Becker 2014; Vendruscolo and Roberts 2014). In most cases, dependence has been induced by administering alcohol vapor via inhalation chambers. For example, rats exposed to chronic alcohol vapor treatment consumed significantly more alcohol than non-dependent controls under free-choice unlimited (24 h/day) access conditions (Rimondini et al. 2002, 2003; Sommer et al. 2008). Similar results have been reported in mice following chronic alcohol vapor exposure, with voluntary alcohol consumption assessed using a limited access (2 h/day) schedule (Becker and Lopez 2004; Dhaher et al. 2008; Finn et al. 2007; Huitron-Resendiz et al. 2018; Lopez and Becker 2005; Lopez et al. 2017). Additionally, studies using operant conditioning procedures have demonstrated increased alcohol self-administration in mice (Chu et al. 2007; Lopez et al. 2014) and rats (C. K. Funk and Koob 2007; Gilpin et al. 2008b, c, 2009; Meinhardt and Sommer 2015; O'Dell et al. 2004; Richardson et al. 2008; Roberts et al. 1996, 2000) with a history of chronic alcohol vapor experience.
A key feature of this model that yields robust and reliable escalated alcohol responding/intake is the delivery of chronic alcohol exposure in an intermittent pattern such that multiple withdrawal episodes are experienced (Lopez and Becker 2005; O'Dell et al. 2004). This point highlights the importance of establishing the negative reinforcing effects of alcohol in driving enhanced motivation to imbibe (Becker 2014; Koob 2021, 2022). Additionally, the intensity of repeated chronic intermittent ethanol (CIE) exposure cycles (producing high and sustained blood alcohol levels) was shown to be critical in favoring escalation of alcohol consumption in the model (Griffin et al. 2009a). Further, the effect appears specific to alcohol because repeated cycles of CIE exposure did not produce alterations in water intake or consumption of highly palatable fluids such as sucrose and saccharin (Becker and Lopez 2004; Lopez et al. 2012). This suggests that the increase in alcohol consumption is not a non-specific effect related to a general need to hydrate with fluids or increase caloric intake.
Using this approach, enhanced alcohol responding/intake has been shown to be durable, evident in dependent animals well beyond acute withdrawal. Indeed, with an increased number of CIE exposure cycles, upregulated alcohol intake was shown to be not only further augmented but also sustained for a longer period of time (several weeks) following final withdrawal compared to intake in a separate group of non-dependent mice (Lopez and Becker 2005). Further, analysis of the temporal pattern of alcohol consumption revealed that dependent mice not only consumed more alcohol than non-dependent animals over the entire 2-h access period, but the rate of consumption was faster and progressively increased over successive withdrawal test periods (Griffin et al. 2009b; see also Robinson and McCool 2015 with rats).
In both mice and rats, escalation of alcohol self-administration following repeated cycles of CIE exposure was reported to be associated with significantly higher resultant blood alcohol levels compared to that achieved by more modest and stable levels of intake in non-dependent animals (Becker and Lopez 2004; Roberts et al. 2000). Additionally, the faster rate of alcohol intake and greater overall amount consumed exhibited by dependent mice have been shown to result in significantly higher peak and more sustained alcohol concentrations measured in the brain compared to levels achieved from consumption of alcohol in non-dependent animals (Griffin et al. 2009b). Moreover, greater voluntary alcohol consumption in dependent mice produced brain alcohol concentrations that approximated those levels experienced during chronic intermittent alcohol exposure that rendered the subjects dependent in the first place. While it is tempting to speculate that CIE-exposed animals display increased voluntary alcohol drinking behavior to attain blood and brain alcohol levels in a range consistent with sustaining dependence, the extent to which resultant brain alcohol concentrations play a role in driving as well as perpetuating enhanced alcohol drinking in dependent animals remains to be determined.
Despite the growing and convergent body of evidence indicating that rodent models of dependence involving CIE exposure produce robust escalation of voluntary alcohol consumption, the mechanisms underlying enhanced motivation to imbibe in the context of dependence are not fully understood. As noted above, mechanisms that govern the regulation of drinking behavior involve complex and dynamic processes (Koob and Le Moal 2008; Koob and Volkow 2016). An interplay among numerous biological and environmental factors influence the motivational effects of alcohol, and these may change as the subject gains more experience with the drug (Cunningham et al. 2000). Alcohol dependence may be characterized as an allostatic state fueled by progressive dysregulation of motivational processes and neural circuitry controlling intake (Becker 2008; Heilig et al. 2010; Koob 2003; Koob and Le Moal 2008; Koob and Schulkin 2019). Such neuroadaptations may play a role in enhancing the rewarding effects of alcohol, thereby fostering the transition from regulated alcohol use to uncontrolled, excessive levels of drinking. Additionally, the potential for alcohol to alleviate negative affect and other symptoms of withdrawal serves as a powerful motivational force that likely promotes and sustains high levels of drinking (Becker 2008; Heilig et al. 2010; Koob 2021, 2022).
Studies involving CIE exposure have provided evidence for enhanced rewarding effects of alcohol. For example, studies employing operant self-administration procedures have demonstrated augmented motivation to self-administer alcohol (increased responding and consumption) in alcohol-dependent mice (Chu et al. 2007; Lopez et al. 2014) and rats (Gilpin et al. 2008c, 2009; O'Dell et al. 2004; Roberts et al. 1996, 2000). Further, employing progressive ratio schedules, it was demonstrated that the amount of work rats were willing to expend in order to receive alcohol reinforcement was significantly increased following repeated cycles of CIE exposure (Brown et al. 1998). Another study reported that CIE-exposed rats displayed greater resistance to extinction of responding to alcohol reward, perhaps reflecting greater persistence in alcohol seeking behavior despite the fact that alcohol was no longer available (Gass et al. 2017). Also, animals with a history of CIE exposure were shown to exhibit exaggerated sensitivity to events that trigger alcohol relapse, i.e., presentation of alcohol-related cues and stress exposure (Funk et al. 2019; Gehlert et al. 2007; Liu and Weiss 2002; Sommer et al. 2008). These findings suggest that the reinforcing value of alcohol may be enhanced, and subjects may be rendered more vulnerable to relapse as a consequence of experiencing repeated opportunities to self-administer alcohol in the context of chronic intermittent exposure to the drug.
At the same time, another factor that could contribute to excessive drinking is the development of tolerance to the aversive effects of alcohol. Tolerance has long been viewed as playing an important role in the regulation of alcohol self-administration behavior (Deitrich et al. 1996; Elvig et al. 2021; Kalant 1996, 1998; Rigter and Crabbe 1980; Suwaki et al. 2001). In this vein, the development of tolerance to the aversive effects of alcohol (which ordinarily temper the amount consumed) may serve as a permissive factor, enabling higher levels of drinking. Recent evidence indicates that repeated cycles of CIE exposure in mice not only produces escalation of voluntary drinking but also reduced sensitivity (tolerance) to the aversive effects of alcohol in the same subjects, as determined by a conditioned taste aversion procedure (Lopez et al. 2012). This reduced sensitivity to alcohol-induced conditioned taste aversion could not be attributed to pharmacokinetic factors, and it could not simply be explained by a general learning deficit since both dependent and non-dependent mice exhibited a similar learned aversion to a non-alcohol noxious stimulus (lithium chloride). In another study, rats with a history of repeated cycles of CIE exposure were reported to exhibit long-lasting tolerance to the sedative/hypnotic effects of alcohol (Rimondini et al. 2008). Additionally, using operant discrimination procedures, it was found that the ability to detect (perceive) the subjective cues associated with alcohol intoxication was diminished during withdrawal from chronic alcohol exposure, and this tolerance effect was greater in mice that experienced multiple withdrawals during the course of the chronic alcohol treatment (Becker and Baros 2006). Thus, reduced sensitivity to feedback about the intoxicating effects of alcohol along with reduced sensitivity to the aversive effects of the drug may serve a permissive role in enabling greater alcohol consumption associated with dependence.
Another important mechanism to consider is the shift from goal-directed to habitual responding/drinking that could underlie higher levels of consumption in alcohol-dependent subjects (Vandaele and Janak 2018). Several studies have evaluated this possibility using different procedures. In some studies, alcohol reward was devalued using contingency degradation (Barker et al. 2020), satiation (Renteria et al. 2020), or associating alcohol reward with an aversive unconditioned stimulus (Lopez et al. 2014). Using these diverse strategies, it has been shown that alcohol-dependent mice are more resistant to the devaluation of alcohol reward, as indicated by persistence in working (responding) to obtain the drug. Other studies have evaluated the habitual or “compulsive” nature of alcohol drinking by adding quinine to the alcohol solution (den Hartog et al. 2016; Gioia and Woodward 2021; Russo et al. 2018) or by contingently delivering foot shock along with alcohol as a reinforcer (Radke et al. 2017). In these studies, mice that experienced repeated CIE exposure were more resistant to these manipulations demonstrating more habitual alcohol seeking and drinking. Collectively, these data support the notion that with prolonged alcohol exposure, the relative balance between rewarding/reinforcing and aversive properties of alcohol is shifted away from aversion in favor of reward/reinforcement. Thus, the combination of enhanced rewarding effects (through both positive and negative reinforcement) along with reduced sensitivity (tolerance) to the aversive qualities of alcohol intoxication may, in large part, drive excessive drinking associated with dependence. Elucidating neurobiological mechanisms underlying changes in sensitivity to both the rewarding and the aversive effects of alcohol is key to understanding motivational processes that are critical for regulating and controlling alcohol consumption, as well as adaptations in such processes that mediate transition to uncontrolled, harmful levels of drinking characteristic of dependence.
Finally, compromised cognitive function may be an important contributing factor that promotes increased vulnerability to relapse and impaired ability to exert control over drinking (Le Berre et al. 2017). Indeed, repeated cycles of chronic alcohol exposure and withdrawal experience have been shown to produce significant cognitive deficits. For example, CIE-exposed mice that displayed elevated alcohol consumption also exhibited deficits in performance in attention set-shifting and novel object recognition tasks (Hu et al. 2015; Pradhan et al. 2018). Similarly, studies conducted in mice (Badanich et al. 2011) and rats (Meinhardt et al. 2021) indicate that CIE exposure leads to deficits in behaviors mediated by the prefrontal cortex such as reversal learning and delay discounting. These studies open future avenues of investigation that probe mechanisms and circuits that link alcohol-induced alterations in cognition and motivation, which ultimately govern decision-making and behavioral control regarding alcohol consumption.
8 Summary and Future Challenges
This chapter reviews a number of animal models that have been established to study excessive alcohol consumption in rodents. In all cases, the common experimental strategy has been focused on utilizing procedures that effectively overcome the natural tendency of rodents to either avoid alcohol or consume it in limited amounts that typically do not produce overt signs of intoxication. A corollary to this is the increased recognition that recording blood alcohol levels achieved following alcohol consumption in each of the models is critical for validation of their physiological (and clinical) relevance. This point cannot be overstated. In many instances, experimental manipulations have been shown to produce statistically significant changes in drinking behavior, but physiological and behavioral relevance can best be realized when consumption yields significant changes in circulating alcohol concentrations.
The six models described in this chapter incorporate several procedural variables that engender excessive levels of alcohol intake along with resultant blood alcohol concentrations that exceed the legal limit of intoxication (0.08 g/dL) (Table 1). This includes manipulating scheduled access to alcohol (time of day, duration, frequency), periods of time when access to alcohol is withheld, and history of alcohol exposure. As detailed above, each model possesses unique experimental characteristics that confer both advantages and disadvantages. Of course, no single approach can claim to capture all the complexities that define problem drinking in humans. Nevertheless, development of these rodent models of excessive alcohol drinking has proven to be extremely valuable in advancing our knowledge about the biological and environmental contingencies that bear on this complex behavior.
At the same time, there is the opportunity and a need to further optimize the translational impact of these animal models. In updating this body of work since our last review (Becker 2013), we have not only emphasized findings from more recent studies but also highlight aspects of the studies that bear on relevance to drinking in humans. For example, most studies described in this chapter focused on experimental manipulations that minimize variance in study outcomes, with reliance on group averages for alcohol intake. This is understandable from the standpoint of wanting to utilize a model that produces an overall robust and reliable phenotype to probe mechanisms with sophisticated neurobiological tools and approaches. However, this is done at the expense of highlighting factors that contribute to individual differences in drinking (amount and pattern). Given the known heterogeneity of AUD and the burgeoning area of personalized medicine in relation to treatment strategies, this point has been more greatly appreciated in recent years. This is a subject that is deserving of more attention in studies on animal models of excessive alcohol drinking.
A related issue regards the influence of genetic factors. It is well known that genetic background has a significant effect on alcohol consumption in various rodent models. As described above, genotype (species, strain) and genetic factors related to selective breeding procedures have been shown to influence alcohol intake under some conditions. Additional investigations into the modulating effects of genetic factors on regulation of alcohol drinking in these models are certainly warranted. Extending this work to focus on genetically based risk factors that are predictive of excessive drinking phenotypes in these models has important clinical relevance. The emerging interest and evidence of epigenetic factors that influence alcohol consumption may be of importance to this area of research.
Most studies described in this chapter have been predominantly conducted using male subjects. In recent years, a growing body of evidence indicates sex-related differences in alcohol consumption and the mechanisms that regulate this behavior. Thus, there is a critical need for these models of excessive drinking to incorporate females in the study designs. Filling this relative void in information will enhance our understanding of potential sex-related differences in mechanisms that govern the amount and pattern of drinking. This, in turn, may have important implications for tailored treatment strategies for tempering excessive levels of alcohol intake in males and females.
In the quest to further improve the relevance of these preclinical models in relation to the human condition, other contributing factors that deserve more consideration in these models include the role of initial sensitivity to alcohol as well as acute and chronic tolerance (i.e., changes in response to alcohol as the subject gains more experience and exposure to the drug). This includes procedures that enable assessment of changes in the positive reinforcing (rewarding) effects of alcohol as well as the emergence of motivation to drink to alleviate a negative emotional state associated with chronic alcohol exposure (negative reinforcing effects of alcohol). Further, additional work is needed to probe cognitive (learning/memory) factors that guide decisions about initiating and terminating drinking behavior, as well as studies focused on distinguishing circumstances in which environmental factors such as cues, stress, and timing and predictability of access exert different effects on propensity to drink. Through increased refinement and more detailed characterization of procedures and factors that engender excessive alcohol drinking, the overall goal in developing these animal models is to advance our understanding of biological underpinnings and environmental influences that drive increased motivation for alcohol seeking and consumption. This enhances the translational value of this preclinical work and facilitates the ability of the models to better inform the clinical condition (AUD). Ultimately, the validity and utility of these models will lie in their ability to aid in the discovery of new and novel potential therapeutic targets as well as serve as a platform to evaluate treatment strategies that effectively reduce excessive levels of alcohol consumption.
References
Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G et al (2000) Development of short-lasting alcohol deprivation effect in Sardinian alcohol-preferring rats. Alcohol 21(1):59–62. https://doi.org/10.1016/s0741-8329(00)00072-0
Ardinger CE, Grahame NJ, Lapish CC, Linsenbardt DN (2020) High alcohol-preferring mice show reaction to loss of ethanol reward following repeated binge drinking. Alcohol Clin Exp Res 44(9):1717–1727. https://doi.org/10.1111/acer.14419
Avena NM, Long KA, Hoebel BG (2005) Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav 84(3):359–362. https://doi.org/10.1016/j.physbeh.2004.12.016
Azarov AV, Woodward DJ (2014a) Early ethanol and water consumption: accumulating experience differentially regulates drinking pattern and bout parameters in male alcohol preferring (P) vs. Wistar and Sprague Dawley rats. Physiol Behav 123:20–32. https://doi.org/10.1016/j.physbeh.2013.09.015
Azarov AV, Woodward DJ (2014b) Early ethanol and water intake: choice mechanism and total fluid regulation operate in parallel in male alcohol preferring (P) and both Wistar and Sprague Dawley rats. Physiol Behav 123:11–19. https://doi.org/10.1016/j.physbeh.2013.09.011
Badanich KA, Becker HC, Woodward JJ (2011) Effects of chronic intermittent ethanol expsoure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci 125(6):879–891. https://doi.org/10.1037/a0025922
Barker JM, Bryant KG, Montiel-Ramos A, Goldwasser B, Chandler LJ (2020) Selective deficits in contingency-driven ethanol seeking following chronic ethanol exposure in male mice. Alcohol Clin Exp Res 44(9):1896–1905. https://doi.org/10.1111/acer.14418
Barkley-Levenson AM, Crabbe JC (2014) High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol 48(3):217–223. https://doi.org/10.1016/j.alcohol.2013.10.007
Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC (2015) Rewarding and aversive effects of ethanol in high drinking in the dark selectively bred mice. Addict Biol 20(1):80–90. https://doi.org/10.1111/adb.12079
Bauer MR, McVey MM, Boehm SL 2nd (2021) Three weeks of binge alcohol drinking generates increased alcohol front-loading and robust compulsive-like alcohol drinking in male and female C57BL/6J mice. Alcohol Clin Exp Res 45(3):650–660. https://doi.org/10.1111/acer.14563
Becker HC (2008) Alcohol dependence, withdrawal, and relapse. Alcohol Res Health 31(4):348–361. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23584009
Becker HC (2013) Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci 13:355–377. https://doi.org/10.1007/7854_2012_203
Becker HC (2014) Alcohol dependence, withdrawal, and relapse. In: Noronha A, Cui C, Harris RA, Crabbe JC (eds) Neurobiology of alcohol dependence. Academic Press (Elsevier), Waltham, MA, pp 377–410
Becker HC, Baros AM (2006) Effect of duration and pattern of chronic ethanol exposure on tolerance to the discriminative stimulus effects of ethanol in C57BL/6J mice. J Pharmacol Exp Ther 319(2):871–878. https://doi.org/10.1124/jpet.106.108795
Becker HC, Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28(12):1829–1838. https://doi.org/10.1097/01.alc.0000149977.95306.3a
Becker HC, Lopez MF (2016) An animal model of alcohol dependence to screen medications for treating alcoholism. Int Rev Neurobiol 126:157–177. https://doi.org/10.1016/bs.irn.2016.02.006
Becker HC, Ron D (2014) Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol 48(3):205–208. https://doi.org/10.1016/j.alcohol.2014.04.001
Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM et al (2004) Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcohol Clin Exp Res 28(12):1867–1874. https://doi.org/10.1097/01.alc.0000148101.20547.0a
Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ (2011) Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav 100(1):90–97. https://doi.org/10.1016/j.pbb.2011.07.017
Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ (2014) Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol 48(3):225–234. https://doi.org/10.1016/j.alcohol.2013.10.004
Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE (2011) Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol 16(3):440–449. https://doi.org/10.1111/j.1369-1600.2010.00309.x
Bloch S, Rinker JA, Marcus MM, Mulholland PJ (2020) Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol 88:91–99. https://doi.org/10.1016/j.alcohol.2020.07.011
Boehm SL 2nd, Moore EM, Walsh CD, Gross CD, Cavelli AM, Gigante E, Linsenbardt DN (2008) Using drinking in the dark to model prenatal binge-like exposure to ethanol in C57BL/6J mice. Dev Psychobiol 50(6):566–578. https://doi.org/10.1002/dev.20320
Brown G, Jackson A, Stephens DN (1998) Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behav Pharmacol 9(2):149–161. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10065934
Cannady R, Nimitvilai-Roberts S, Jennings SD, Woodward JJ, Mulholland PJ (2020) Distinct region- and time-dependent functional cortical adaptations in C57BL/6J mice after short and prolonged alcohol drinking. eNeuro 7(3). https://doi.org/10.1523/ENEURO.0077-20.2020
Carnicella S, Amamoto R, Ron D (2009) Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol 43(1):35–43. https://doi.org/10.1016/j.alcohol.2008.12.001
Carnicella S, Ron D, Barak S (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48(3):243–252. https://doi.org/10.1016/j.alcohol.2014.01.006
Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ (2007) Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav 86(4):813–821. https://doi.org/10.1016/j.pbb.2007.03.009
Ciccocioppo R (2012) Genetically selected alcohol preferring rats to model human alcoholism. In: Sommer W, Spanagel R (eds) Behavioral neurobiology of alcohol addiction. Current topics in behavioral neurosciences, vol 13. Springer, Berlin, pp 251–269
Colombo G, Maccioni P, Acciaro C, Lobina C, Loi B, Zaru A et al (2014) Binge drinking in alcohol-preferring sP rats at the end of the nocturnal period. Alcohol 48(3):301–311. https://doi.org/10.1016/j.alcohol.2014.02.001
Colombo G, Lobina C, Maccioni P, Carai MA, Lorrai I, Zaru A et al (2015) Anxiety-like behaviors at the end of the nocturnal period in sP rats with a “history” of unpredictable, limited access to alcohol. Alcohol 49(7):707–712. https://doi.org/10.1016/j.alcohol.2015.04.010
Colombo G, Lobina C, Lorrai I, Acciaro C, Maccioni P, Gessa GL (2017) Binge drinking and anxiety at the end of the nocturnal period in alcohol-preferring sP rats. Alcohol 63:27–32. https://doi.org/10.1016/j.alcohol.2017.04.002
Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M et al (2013) Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res 37(10):1688–1695. https://doi.org/10.1111/acer.12145
Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA (2009) A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry 65(8):662–670. https://doi.org/10.1016/j.biopsych.2008.11.002
Crabbe JC, Phillips TJ, Belknap JK (2010) The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet 40(6):737–750. https://doi.org/10.1007/s10519-010-9371-z
Crabbe JC, Harris RA, Koob GF (2011a) Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci 1216:24–40. https://doi.org/10.1111/j.1749-6632.2010.05895.x
Crabbe JC, Spence SE, Brown LL, Metten P (2011b) Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol 45(5):427–440. https://doi.org/10.1016/j.alcohol.2010.12.001
Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P (2012) Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol Alcohol 47(5):509–517. https://doi.org/10.1093/alcalc/ags067
Crabbe JC, Hack WR, Ozburn AR, Savarese AM, Metten P (2022) Long-term alcohol drinking in high drinking in the dark mice is stable for many months and does not show alcohol deprivation effects. Addict Biol 27(1):e13074. https://doi.org/10.1111/adb.13074
Cunningham CL, Fidler TL, Hill KG (2000) Animal models of alcohol’s motivational effects. Alcohol Res Health 24(2):85–92. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11199282
Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR et al (2011) Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36(12):2513–2528. https://doi.org/10.1038/npp.2011.140
Deitrich RA, Radcliffe R, Erwin VG (1996) Pharmacological effects in the development of physiological tolerance and physical dependence. In: Begleiter H, Kissin B (eds) The pharmacology of alcohol and alcohol dependence. Oxford University Press, New York, pp 431–476
den Hartog C, Zamudio-Bulcock P, Nimitvilai S, Gilstrap M, Eaton B, Fedarovich H et al (2016) Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology 107:451–459. https://doi.org/10.1016/j.neuropharm.2016.03.031
Dhaher R, Finn D, Snelling C, Hitzemann R (2008) Lesions of the extended amygdala in C57BL/6J mice do not block the intermittent ethanol vapor-induced increase in ethanol consumption. Alcohol Clin Exp Res 32(2):197–208. https://doi.org/10.1111/j.1530-0277.2007.00566.x
Elvig SK, McGinn MA, Smith C, Arends MA, Koob GF, Vendruscolo LF (2021) Tolerance to alcohol: a critical yet understudied factor in alcohol addiction. Pharmacol Biochem Behav 204:173155. https://doi.org/10.1016/j.pbb.2021.173155
Falk JL (1961) Production of polydipsia in normal rats by an intermittent food schedule. Science 133(3447):195–196. https://doi.org/10.1126/science.133.3447.195
Falk JL (1971) The nature and determinants of adjunctive behavior. Physiol Behav 6(5):577–588. https://doi.org/10.1016/0031-9384(71)90209-5
Falk JL, Samson HH (1975) Schedule-induced physical dependence on ethanol. Pharmacol Rev 27(4):449–464. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/772699
Falk JL, Tang M (1988) What schedule-induced polydipsia can tell us about alcoholism. Alcohol Clin Exp Res 12(5):577–585. https://doi.org/10.1111/j.1530-0277.1988.tb00246.x
Falk JL, Samson HH, Winger G (1972) Behavioral maintenance of high concentrations of blood ethanol and physical dependence in the rat. Science 177(4051):811–813. https://doi.org/10.1126/science.177.4051.811
Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M et al (2007) Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41). Alcohol Clin Exp Res 31(6):939–949. https://doi.org/10.1111/j.1530-0277.2007.00379.x
Ford MM (2014) Applications of schedule-induced polydipsia in rodents for the study of an excessive ethanol intake phenotype. Alcohol 48(3):265–276. https://doi.org/10.1016/j.alcohol.2014.01.005
Ford MM, Steele AM, McCracken AD, Finn DA, Grant KA (2013) The relationship between adjunctive drinking, blood ethanol concentration and plasma corticosterone across fixed-time intervals of food delivery in two inbred mouse strains. Psychoneuroendocrinology 38(11):2598–2610. https://doi.org/10.1016/j.psyneuen.2013.06.011
Fouyssac M, Puaud M, Ducret E, Marti-Prats L, Vanhille N, Ansquer S et al (2021) Environment-dependent behavioral traits and experiential factors shape addiction vulnerability. Eur J Neurosci 53(6):1794–1808. https://doi.org/10.1111/ejn.15087
Funk CK, Koob GF (2007) A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res 1155:172–178. https://doi.org/10.1016/j.brainres.2007.04.009
Funk D, Coen K, Tamadon S, Le AD (2019) Effect of chronic alcohol vapor exposure on reinstatement of alcohol seeking induced by U50,488. Neuropharmacology 148:210–219. https://doi.org/10.1016/j.neuropharm.2019.01.017
Galbo LK, Davenport AT, Epperly PM, Daunais JB, Stinson BT, Czoty PW (2022) Social dominance in monkeys: lack of effect on ethanol self-administration during schedule induction. Alcohol 98:1–7. https://doi.org/10.1016/j.alcohol.2021.10.001
Gass JT, McGonigal JT, Chandler LJ (2017) Deficits in the extinction of ethanol-seeking behavior following chronic intermittent ethanol exposure are attenuated with positive allosteric modulation of mGlu5. Neuropharmacology 113(Pt A):198–205. https://doi.org/10.1016/j.neuropharm.2016.10.005
Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C et al (2007) 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci 27(10):2718–2726. https://doi.org/10.1523/JNEUROSCI.4985-06.2007
George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF (2012) Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A 109(44):18156–18161. https://doi.org/10.1073/pnas.1116523109
Giardino WJ, Ryabinin AE (2013) CRF1 receptor signaling regulates food and fluid intake in the drinking-in-the-dark model of binge alcohol consumption. Alcohol Clin Exp Res 37(7):1161–1170. https://doi.org/10.1111/acer.12076
Gill K, Liu Y, Deitrich RA (1996) Voluntary alcohol consumption in BXD recombinant inbred mice: relationship to alcohol metabolism. Alcohol Clin Exp Res 20(1):185–190. https://doi.org/10.1111/j.1530-0277.1996.tb01063.x
Gilpin NW, Badia-Elder NE, Elder RL, Stewart RB (2008a) Schedule-induced polydipsia in lines of rats selectively bred for high and low ethanol preference. Behav Genet 38(5):515–524. https://doi.org/10.1007/s10519-008-9224-1
Gilpin NW, Richardson HN, Koob GF (2008b) Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res 32(9):1535–1542. https://doi.org/10.1111/j.1530-0277.2008.00745.x
Gilpin NW, Richardson HN, Lumeng L, Koob GF (2008c) Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res 32(9):1688–1696. https://doi.org/10.1111/j.1530-0277.2008.00678.x
Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN (2009) Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res 33(12):2113–2123. https://doi.org/10.1111/j.1530-0277.2009.01051.x
Gioia DA, Woodward JJ (2021) Altered activity of lateral orbitofrontal cortex neurons in mice following chronic intermittent ethanol exposure. eNeuro 8(2). https://doi.org/10.1523/ENEURO.0503-20.2021
Grahame NJ, Li TK, Lumeng L (1999) Selective breeding for high and low alcohol preference in mice. Behav Genet 29(1):47–57. https://doi.org/10.1023/a:1021489922751
Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32(10):1824–1838. https://doi.org/10.1111/j.1530-0277.2008.00765.x
Grant KA, Newman NN, Gonzales SW, Cuzon Carlson VC (2022) Impact of putamen inhibition by DREADDs on schedule-induced drinking in rhesus monkeys. J Exp Anal Behav 117(3):493–504. https://doi.org/10.1002/jeab.761
Griffin WC 3rd, Lopez MF, Becker HC (2009a) Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res 33(11):1893–1900. https://doi.org/10.1111/j.1530-0277.2009.01027.x
Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC (2009b) Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201(4):569–580. https://doi.org/10.1007/s00213-008-1324-3
Griffin WC 3rd. (2014) Alcohol dependence and free-choice drinking in mice. Alcohol 48(3):287–293. https://doi.org/10.1016/j.alcohol.2013.11.006
Heilig M, Egli M, Crabbe JC, Becker HC (2010) Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 15(2):169–184. https://doi.org/10.1111/j.1369-1600.2009.00194.x
Hernandez JS, Moorman DE (2020) Orbitofrontal cortex encodes preference for alcohol. eNeuro 7(4). https://doi.org/10.1523/ENEURO.0402-19.2020. ENEURO.0402
Heyser CJ, Schulteis G, Koob GF (1997) Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res 21(5):784–791. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9267526
Holter SM, Landgraf R, Zieglgansberger W, Spanagel R (1997) Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res 21(5):862–868. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9267536
Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A (2010) Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res 34(9):1565–1573. https://doi.org/10.1111/j.1530-0277.2010.01241.x
Hosova D, Spear LP (2017) Voluntary binge consumption of ethanol in a sweetened, chocolate-flavored solution by male and female adolescent Sprague Dawley rats. Alcohol Clin Exp Res 41(3):541–550. https://doi.org/10.1111/acer.13315
Hu W, Morris B, Carrasco A, Kroener S (2015) Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin Exp Res 39(6):953–961. https://doi.org/10.1111/acer.12722
Huitron-Resendiz S, Nadav T, Krause S, Cates-Gatto C, Polis I, Roberts AJ (2018) Effects of withdrawal from chronic intermittent ethanol exposure on sleep characteristics of female and male mice. Alcohol Clin Exp Res 42(3):540–550. https://doi.org/10.1111/acer.13584
Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35(11):1938–1947. https://doi.org/10.1111/j.1530-0277.2011.01545.x
Jensen BE, Townsley KG, Grigsby KB, Metten P, Chand M, Uzoekwe M et al (2021) Ethanol-related behaviors in mouse lines selectively bred for drinking to intoxication. Brain Sci 11(2). https://doi.org/10.3390/brainsci11020189
Jimenez VA, Porcu P, Morrow AL, Grant KA (2017) Adaptations in basal and hypothalamic-pituitary-adrenal-activated deoxycorticosterone responses following ethanol self-administration in Cynomolgus monkeys. Front Endocrinol (Lausanne) 8:19. https://doi.org/10.3389/fendo.2017.00019
Kalant H (1996) Current state of knowledge about the mechanisms of alcohol tolerance. Addict Biol 1(2):133–141. https://doi.org/10.1080/1355621961000124756
Kalant H (1998) Research on tolerance: what can we learn from history? Alcohol Clin Exp Res 22(1):67–76. https://doi.org/10.1111/j.1530-0277.1998.tb03618.x
Khanna JM, Kalant H, Shah G, Sharma H (1990) Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol 7(5):429–434. https://doi.org/10.1016/0741-8329(90)90027-a
Khisti RT, Wolstenholme J, Shelton KL, Miles MF (2006) Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 40(2):119–126. https://doi.org/10.1016/j.alcohol.2006.12.003
Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27(2):232–243. https://doi.org/10.1097/01.ALC.0000057122.36127.C2
Koob GF (2021) Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev 73(1):163–201. https://doi.org/10.1124/pharmrev.120.000083
Koob GF (2022) Anhedonia, Hyperkatifeia, and negative reinforcement in substance use disorders. Curr Top Behav Neurosci 58:147–165. https://doi.org/10.1007/7854_2021_288
Koob GF, Le Moal M (2008) Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363(1507):3113–3123. https://doi.org/10.1098/rstb.2008.0094
Koob GF, Schulkin J (2019) Addiction and stress: an allostatic view. Neurosci Biobehav Rev 106:245–262. https://doi.org/10.1016/j.neubiorev.2018.09.008
Koob GF, Volkow ND (2016) Neurobiology of addition: a neurocircuitry analysis. Lancet Psychiatry 3(8):760–773. https://doi.org/10.1016/S2215-0366(16)00104-8
Le Berre A-P, Farna R, Sullivan EV (2017) Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res 41(8):1432–1443. https://doi.org/10.1111/acer.13431
Lee KM, Coehlo MA, Solton NR, Szumlinski KK (2017) Negative affect and excessive alcohol intake incubate during protracted withdrawal from binge-drinking in adolescent, but not adult, mice. Front Psychol 8:1128. https://doi.org/10.3389/fpsyg.2017.01128
Levine OB, Skelly MJ, Miller JD, Rivera-Irizarry JK, Rowson SA, DiBerto JF et al (2021) The paraventricular thalamus provides a polysynaptic brake on limbic CRF neurons to sex-dependently blunt binge alcohol drinking and avoidance behavior in mice. Nat Commun 12(1):5080. https://doi.org/10.1038/s41467-021-25368-y
Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL 2nd (2011) Tolerance to ethanol's ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcohol Clin Exp Res 35(7):1246–1255. https://doi.org/10.1111/j.1530-0277.2011.01459.x
Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22(18):7856–7861. https://doi.org/10.1523/JNEUROSCI.22-18-07856.2002
Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G (2010) Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res 34(12):2147–2154. https://doi.org/10.1111/j.1530-0277.2010.01311.x
Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181(4):688–696. https://doi.org/10.1007/s00213-005-0026-3
Lopez MF, Becker HC (2014) Operant ethanol self-administration in ethanol dependent mice. Alcohol 48(3):295–299. https://doi.org/10.1016/j.alcohol.2014.02.002
Lopez MF, Griffin WC 3rd, Melendez RI, Becker HC (2012) Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcohol Clin Exp Res 36(7):1180–1187. https://doi.org/10.1111/j.1530-0277.2011.01717.x
Lopez MF, Becker HC, Chandler LJ (2014) Repeated episodes of chronic intermittent ethanol promote insensitivity to devaluation of the reinforcing effect of ethanol. Alcohol 48(7):639–645. https://doi.org/10.1016/j.alcohol.2014.09.002
Lopez MF, Miles MF, Williams RW, Becker HC (2017) Variable effects of chronic intermittent ethanol exposure on ethanol drinking in a genetically diverse mouse cohort. Alcohol 58:73–82. https://doi.org/10.1016/j.alcohol.2016.09.003
Lopez-Grancha M, Lopez-Crespo G, Venero C, Canadas F, Sanchez-Santed F, Sandi C, Flores P (2006) Differences in corticosterone level due to inter-food interval length: implications for schedule-induced polydipsia. Horm Behav 49(2):166–172. https://doi.org/10.1016/j.yhbeh.2005.05.019
Lyons AM, Lowery EG, Sparta DR, Thiele TE (2008) Effects of food availability and administration of orexigenic and anorectic agents on elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res 32(11):1962–1968. https://doi.org/10.1111/j.1530-0277.2008.00784.x
Matson LM, Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol 18(6):921–929. https://doi.org/10.1111/j.1369-1600.2011.00412.x
Matson LM, Kasten CR, Boehm SL 2nd, Grahame NJ (2014) Selectively bred crossed high-alcohol-preferring mice drink to intoxication and develop functional tolerance, but not locomotor sensitization during free-choice ethanol access. Alcohol Clin Exp Res 38(1):267–274. https://doi.org/10.1111/acer.12216
McBride WJ, Kimpel MW, Schultz JA, McClintick JN, Edenberg HJ, Bell RL (2010) Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol 44(2):171–183. https://doi.org/10.1016/j.alcohol.2009.12.001
McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK (2014) The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol 48(3):209–215. https://doi.org/10.1016/j.alcohol.2013.09.044
McCane AM, Auterson CD, DeLory MJ, Lapish CC, Czachowski CL (2021) Differential effects of quinine adulteration of alcohol on seeking and drinking. Alcohol 92:73–80. https://doi.org/10.1016/j.alcohol.2021.01.003
McClintick MN, Grant KA (2016) Aggressive temperament predicts ethanol self-administration in late adolescent male and female rhesus macaques. Psychopharmacology (Berl) 233(23–24):3965–3976. https://doi.org/10.1007/s00213-016-4427-2
McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK (1998) The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res 22(5):1170–1176. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9726292
Meinhardt M, Sommer WH (2015) Postdependent state in rats as a model for medication development in alcoholism. Addict Biol 20(1):1–21. https://doi.org/10.1111/adb.12187
Meinhardt MW, Pfarr S, Fouquet G, Rohleder C, Meinhardt ML, Barroso-Flores J, Hoffman R, Jeanblanc J, Paul E, Wagner K, Hansson AC, Kohr G, Meier N, Halbach OB, Bell RL, Endepols H, Neumaier B, Schlonig K, Bartsch D, Naassila M, Spanagel R, Sommer WH (2021) Sci Adv 7(47):eabh2399. https://doi.org/10.1126/sciadv.abh2399
Meisch RA (1975) The function of schedule-induced polydipsia in establishing ethanol as a positive reinforcer. Pharmacol Rev 27(4):465–473. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1223913
Melendez RI (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35(4):652–658. https://doi.org/10.1111/j.1530-0277.2010.01383.x
Melendez RI, Middaugh LD, Kalivas PW (2006) Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res 30(12):2017–2025. https://doi.org/10.1111/j.1530-0277.2006.00248.x
Metten P, Brown LL, Crabbe JC (2011) Limited access ethanol drinking in the dark in adolescent and adult mice. Pharmacol Biochem Behav 98(2):279–285. https://doi.org/10.1016/j.pbb.2011.01.003
Mittleman G, Van Brunt CL, Matthews DB (2003) Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res 27(6):918–925. https://doi.org/10.1097/01.ALC.0000071930.48632.AE
Moench KM, Logrip ML (2021) Housing condition differentially impacts escalation of alcohol intake, relapse-like drinking, anxiety-like behavior, and stress history effects by sex. Alcohol Clin Exp Res 45(2):480–489. https://doi.org/10.1111/acer.14540
Moorman DE, Aston-Jones G (2009) Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol 43(5):379–386. https://doi.org/10.1016/j.alcohol.2009.07.002
Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK (1986) Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol 3(5):331–336. https://doi.org/10.1016/0741-8329(86)90010-8
NIAAA (2004) National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking. Winter 2004. NIAAA Newslett:3
O'Dell LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28(11):1676–1682. https://doi.org/10.1097/01.alc.0000145781.11923.4e
Ogata H, Ogato F, Mendelson JH, Mello NK (1972) A comparison of techniques to induce alcohol dependence and tolerance in the mouse. J Pharmacol Exp Ther 180(2):216–230. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/5062298
Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L et al (2006) Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol 38(3):155–164. https://doi.org/10.1016/j.alcohol.2006.06.001
Palm S, Roman E, Nylander I (2011) Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol 45(6):607–614. https://doi.org/10.1016/j.alcohol.2010.11.005
Phillips TJ, Crabbe JC, Metten P, Belknap JK (1994) Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res 18(4):931–941. https://doi.org/10.1111/j.1530-0277.1994.tb00062.x
Phillips TJ, Reed C, Burkhart-Kasch S, Li N, Hitzemann R, Yu CH et al (2010) A method for mapping intralocus interactions influencing excessive alcohol drinking. Mamm Genome 21(1–2):39–51. https://doi.org/10.1007/s00335-009-9239-9
Pirino BE, Martin CR, Carpenter BA, Curtis GR, Curran-Alfaro CM, Samels SB et al (2022) Sex-related differences in pattern of ethanol drinking under the intermittent-access model and its impact on exploratory and anxiety-like behavior in Long-Evans rats. Alcohol Clin Exp Res 46(7):1282–1293. https://doi.org/10.1111/acer.14853
Pradhan G, Melugin PR, Wu F, Fang HM, Weber R, Kroener S (2018) Calcium chloride mimics the effects of acamprosate on cognitive deficits in chronic alcohol-exposed mice. Psychopharmacology (Berl) 235(7):2027–2040. https://doi.org/10.1007/s00213-018-4900-1
Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. https://doi.org/10.1016/j.pbb.2016.08.001
Quintanille ME, Israel Y, Sapag A, Tampier L (2006) The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol 11(3–4):310–323. https://doi.org/10.1111/j.1369-1600.2006.00030.x
Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE et al (2017) Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict Biol 22(2):423–434. https://doi.org/10.1111/adb.12342
Renteria R, Cazares C, Gremel CM (2020) Habitual ethanol seeking and licking microstructure of enhanced ethanol self-administration in ethanol-dependent mice. Alcohol Clin Exp Res 44(4):880–891. https://doi.org/10.1111/acer.14302
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84(1):53–63. https://doi.org/10.1016/j.physbeh.2004.10.007
Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr, Crabbe JC (2007) Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav 6(1):1–18. https://doi.org/10.1111/j.1601-183X.2006.00210.x
Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL (2008) Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci 28(8):1641–1653. https://doi.org/10.1111/j.1460-9568.2008.06455.x
Rigter H, Crabbe JC (1980) Alcohol tolerance and dependence. Elsevier/North-Holland, New York, NY
Rimondini R, Arlinde C, Sommer W, Heilig M (2002) Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J 16(1):27–35. https://doi.org/10.1096/fj.01-0593com
Rimondini R, Sommer W, Heilig M (2003) A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol 64(4):445–449. https://doi.org/10.15288/jsa.2003.64.445
Rimondini R, Sommer WH, Dall'Olio R, Heilig M (2008) Long-lasting tolerance to alcohol following a history of dependence. Addict Biol 13(1):26–30. https://doi.org/10.1111/j.1369-1600.2007.00079.x
Roberts AJ, Cole M, Koob GF (1996) Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20(7):1289–1298. https://doi.org/10.1111/j.1530-0277.1996.tb01125.x
Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF (2000) Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22(6):581–594. https://doi.org/10.1016/S0893-133X(99)00167-0
Robinson SL, McCool BA (2015) Microstructural analysis of rat ethanol and water drinking patterns using a modified operant self-administration model. Physiol Behav 149:119–130. https://doi.org/10.1016/j.physbeh.2015.05.034
Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ (2003) Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology 28(9):1614–1621. https://doi.org/10.1038/sj.npp.1300214
Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ (2004) Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79(3):439–450. https://doi.org/10.1016/j.pbb.2004.08.018
Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, McBride WJ (2009) Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of high-alcohol-drinking (HAD) rats. Addict Biol 14(2):152–164. https://doi.org/10.1111/j.1369-1600.2008.00140.x
Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK (2000a) The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res 24(6):747–753. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10888060
Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK (2000b) Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res 24(1):8–16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10656186
Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2001) Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res 25(8):1140–1150. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11505045
Rodriguez LA, Plomin R, Blizard DA, Jones BC, McClearn GE (1994) Alcohol acceptance, preference, and sensitivity in mice. I. Quantitative genetic analysis using BXD recombinant inbred strains. Alcohol Clin Exp Res 18(6):1416–1422. https://doi.org/10.1111/j.1530-0277.1994.tb01444.x
Rodriguez LA, Plomin R, Blizard DA, Jones BC, McClearn GE (1995) Alcohol acceptance, preference, and sensitivity in mice. II. Quantitative trait loci mapping analysis using BXD recombinant inbred strains. Alcohol Clin Exp Res 19(2):367–373. https://doi.org/10.1111/j.1530-0277.1995.tb01517.x
Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S (2013) Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol 18(3):496–507. https://doi.org/10.1111/j.1369-1600.2012.00481.x
Russo M, Funk D, Loughlin A, Coen K, Le AD (2018) Effects of alcohol dependence on discrete choice between alcohol and saccharin. Neuropsychopharmacology 43(9):1859–1866. https://doi.org/10.1038/s41386-018-0101-1
Salimov RM, Salimova NB (1993) The alcohol-deprivation effect in hybrid mice. Drug Alcohol Depend 32(2):187–191. https://doi.org/10.1016/0376-8716(93)80012-4
Salimov RM, Salimova NB, Shvets LN, Maisky AI (2000) Haloperidol administered subchronically reduces the alcohol-deprivation effect in mice. Alcohol 20(1):61–68. https://doi.org/10.1016/s0741-8329(99)00057-9
Samson HH, Chappell A (2001) Effects of alcohol deprivation on alcohol consumption using a sipper-tube procedure. Alcohol Clin Exp Res 25(5):680–686. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11371717
Savarese AM, Ozburn AR, Barkley-Levenson AM, Metten P, Crabbe JC (2021) The impact of drinking in the dark (DID) procedural manipulations on ethanol intake in high drinking in the dark (HDID) mice. Alcohol 93:45–56. https://doi.org/10.1016/j.alcohol.2021.02.001
Schuh KM, Sneddon EA, Nader AM, Muench MA, Radke AK (2022) Orbitofrontal cortex subregion inhibition during binge-like and aversion-resistant alcohol drinking. Alcohol 99:1–8. https://doi.org/10.1016/j.alcohol.2021.11.004
Serra S, Brunetti G, Vacca G, Lobina C, Carai MA, Gessa GL, Colombo G (2003) Stable preference for high ethanol concentrations after ethanol deprivation in Sardinian alcohol-preferring (sP) rats. Alcohol 29(2):101–108. https://doi.org/10.1016/s0741-8329(03)00003-x
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32(10):1816–1823. https://doi.org/10.1111/j.1530-0277.2008.00753.x
Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE (2010) Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology 35(7):1453–1463. https://doi.org/10.1038/npp.2010.15
Sinclair JD, Li TK (1989) Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol 6(6):505–509. https://doi.org/10.1016/0741-8329(89)90059-1
Sinclair JD, Senter RJ (1968) Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol 29(4):863–867. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/5705408
Sinclair JD, Tiihonen K (1988) Lack of alcohol-deprivation effect in AA rats. Alcohol 5(1):85–87. https://doi.org/10.1016/0741-8329(88)90048-1
Smeets JAS, Minnaard AM, Ramakers GMJ, Adan RAH, Vanderschuren L, Lesscher HMB (2022) On the interrelation between alcohol addiction-like behaviors in rats. Psychopharmacology (Berl) 239(4):1115–1128. https://doi.org/10.1007/s00213-021-06059-4
Sneddon EA, White RD, Radke AK (2019) Sex differences in binge-like and aversion-resistant alcohol drinking in C57BL/6J mice. Alcohol Clin Exp Res 43(2):243–249. https://doi.org/10.1111/acer.13923
Sneddon EA, Schuh KM, Frankel JW, Radke AK (2021) The contribution of medium spiny neuron subtypes in the nucleus accumbens core to compulsive-like ethanol drinking. Neuropharmacology 187:108497. https://doi.org/10.1016/j.neuropharm.2021.108497
Sommer W, Hyytia P, Kiianmaa K (2006) The alcohol-preferring AA and alcohol avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol 11(3–4):289–309. https://doi.org/10.1111/j.1369-1600.2006.00037.x
Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA (2008) Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry 63(2):139–145. https://doi.org/10.1016/j.biopsych.2007.01.010
Spanagel R (2009) Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev 89(2):649–705. https://doi.org/10.1152/physrev.00013.2008
Spanagel R, Holter SM (1999) Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol 34(2):231–243. https://doi.org/10.1093/alcalc/34.2.231
Spanagel R, Holter SM (2000) Pharmacological validation of a new animal model of alcoholism. J Neural Transm (Vienna) 107(6):669–680. https://doi.org/10.1007/s007020070068
Spanagel R, Holter SM, Allingham K, Landgraf R, Zieglgansberger W (1996) Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 305(1–3):39–44. https://doi.org/10.1016/0014-2999(96)00174-4
Sparta DR, Ferraro FM 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE (2009) The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res 33(1):31–42. https://doi.org/10.1111/j.1530-0277.2008.00808.x
Spear LP (2020) Timing eclipses amount: the critical importance of intermittency in alcohol exposure effects. Alcohol Clin Exp Res 44(4):806–813. https://doi.org/10.1111/acer.14307
Suwaki H, Kalant H, Higuchi S, Crabbe JC, Ohkuma S, Katsura M et al (2001) Recent research on alcohol tolerance and dependence. Alcohol Clin Exp Res 25(5 Suppl ISBRA):189S–196S. https://doi.org/10.1097/00000374-200105051-00031
Szumlinski KK, Coelho MA, Lee KM, Tran T, Sern KR, Bernal A, Kippin TE (2019) DID it or DIDn't it? Exploration of a failure to replicate binge-like alcohol-drinking in C57BL/6J mice. Pharmacol Biochem Behav 178:3–18. https://doi.org/10.1016/j.pbb.2018.12.002
Tambour S, Brown LL, Crabbe JC (2008) Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res 32(12):2100–2106. https://doi.org/10.1111/j.1530-0277.2008.00798.x
Tang M, Falk JL (1983) Production of physical dependence on ethanol by a short drinking episode each day. Pharmacol Biochem Behav 19(1):53–55. https://doi.org/10.1016/0091-3057(83)90311-8
Tang M, Brown C, Falk JL (1982) Complete reversal of chronic ethanol polydipsia by schedule withdrawal. Pharmacol Biochem Behav 16(1):155–158. https://doi.org/10.1016/0091-3057(82)90028-4
Thiele TE, Navarro M (2014) “Drinking in the dark” (DID) procedures: a model of binge-like ethanol drinking in non-dependent mice. Alcohol 48(3):235–241. https://doi.org/10.1016/j.alcohol.2013.08.005
Thiele TE, Crabbe JC, Boehm SL 2nd (2014) “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68., 9 49 41-49 49 12. https://doi.org/10.1002/0471142301.ns0949s68
Vandaele Y, Janak PH (2018) Defining the place of habit in substance use disorders. Prog Neuropsychopharmacol Biol Psychiatry 87(Pt A):22–32. https://doi.org/10.1016/j.pnpbp.2017.06.029
Vendruscolo LF, Roberts AJ (2014) Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48(3):277–286. https://doi.org/10.1016/j.alcohol.2013.08.006
Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R (2003) A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res 27(7):1048–1054. https://doi.org/10.1097/01.ALC.0000075829.81211.0C
Vengeliene V, Bilbao A, Spanagel R (2014) The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol 48(3):313–320. https://doi.org/10.1016/j.alcohol.2014.03.002
Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A (1972) A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol Behav 8(2):345–362. https://doi.org/10.1016/0031-9384(72)90383-6
Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE et al (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39(3):579–594. https://doi.org/10.1038/npp.2013.230
Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29(3):203–210. https://doi.org/10.1007/BF00414034
Younis RM, Wolstenholme JT, Bagdas D, Bettinger JC, Miles MF, Damaj MI (2019) Adolescent but not adult ethanol binge drinking modulates ethanol behavioral effects in mice later in life. Pharmacol Biochem Behav 184:172740. https://doi.org/10.1016/j.pbb.2019.172740
Acknowledgments
Supported by NIH grants P50 AA010761 (HCB), R01 AA026536 (HCB), U01 AA014095 (HCB), and U24 AA020929 (MFL) and a grant from the Department of Veterans Affairs (BLRD BX000813) (HCB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Becker, H.C., Lopez, M.F. (2024). Animal Models of Excessive Alcohol Consumption in Rodents. In: Current Topics in Behavioral Neurosciences. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2024_461
Download citation
DOI: https://doi.org/10.1007/7854_2024_461
Published:
Publisher Name: Springer, Berlin, Heidelberg