Abstract
Ligands with different properties and different selectivity are highly needed for in vitro and in vivo studies on the (patho)physiological influence of the chemical mediator histamine and its receptor subtypes. A selection of well-described ligands for the different receptor subtypes and different studies is shown with a particular focus on affinity and selectivity. In addition, compounds with radioactive or fluorescence elements will be presented with their beneficial use for other species or different investigations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fluorescence
- Histamine
- Histamine H1 receptor
- Histamine H2 receptor
- Histamine H3 receptor
- Histamine H4 receptor
- PET
- Radioligands
1 Introduction
1.1 Chemical Probes
Chemical probes (small-molecule tools) are well-characterized substances regarding their influence on a biological target or action mechanism. Depending on the target of interest, the assay setup, and the read-out mechanisms, different chemical probes or combinations of probes can be used due to their beneficial biochemical or pharmacological properties (Stark 2020). According to the clearly defined criteria, the chemical probe should have an affinity below 100 nM, >10-fold selectivity against off-targets, potency in concentration ranges below 10 μM in cell-based assays, and no pan-assay interference compounds (PAINS) elements (Arrowsmith et al. 2015). Chemical probes are powerful research tools that can lead to verification of the proposed mechanism of action as well as the development of novel drug-like candidates. Besides, chemical probes are necessary for drug-discovery processes as they serve for assay system validation. Without a valid assay system, results are not comparable or reliable and cannot be transferred for further evaluation (Bunnage et al. 2013).
In the case of the histamine receptor subtypes, these innovative tools can be used for different purposes, as in vitro examination of receptor affinities, in vivo estimation of receptor localization, and distribution or clinical trials for drug approval. Histamine receptors belong to the group of G-protein coupled receptors (GPCRs), and therefore chemical probes are defined as ligands. A ligand quality as a chemical probe depends on various characteristics as affinity, efficacy, binding properties (covalent or non-covalent), solubility, permeability, and especially the type of assay system (Bunnage et al. 2013). A general classification of the quality of a chemical cannot be made due to high assay versatility but rather an allocation of well-characterized ligands for particular assays (Frye 2010). Despite the numerous possibilities to modify histaminergic transmission by genetic or enzymatic intervention, this review focuses on ligand-based modification only.
1.2 Physiology of Histamine
Sir Henry Dale et al. described 2-(1H-imidazole-4-yl)ethanamine (histamine) in 1910. This biogenic amine was firstly extracted from human tissue in 1927 (Dale and Laidlaw 1910; Best et al. 1927). Histamine is ubiquitously distributed in the body of numerous species and involved in regulating various physiological functions of smooth muscles, gastrointestinal, cardiovascular, immune system, central and peripheral neurons. As a local mediator, histamine leads to increased acid production in the stomach, triggers local vasodilation in the skin and lungs, and acts as an inflammatory mediator of the immune system. As a neurotransmitter in the CNS, histamine influences temperature regulation, the sleep-wake rhythm, and learning and memory processes (Obara et al. 2019; Panula et al. 2015).
1.2.1 Biosynthesis and Metabolism
Due to the aromatic imidazole moiety, histamine has two tautomeric structures where either the nitrogen at 1-position (N1-H) or 3-position (N3-H) carries the hydrogen. Several studies in gas, liquid, and solid-state indicate that N1-H is the predominant tautomer (Ramírez et al. 2003). For a precise description of histamine and metabolites, Black and Ganellin introduced a specific nomenclature (Fig. 1) (Black and Ganellin 1974).
The aliphatic amine is referred to as Nα and the N1 of the imidazole ring is described as Nπ and N3 as Nϊ. With the aliphatic and aromatic amines, histamine contains two basic centers. The Nα has a pKa value of 9.4, and the imidazole nitrogen Nϊ is less basic with a pKa value of 5.8. Under physiological conditions (pH: 7.4), histamine is in the form of mono cation, protonated at the Nα-position.
The histamine biosynthesis is a one-step process, where l-histidine undergoes an oxidative decarboxylation, mainly catalyzed by the histidine decarboxylase (HDC) (Obara et al. 2019; Parsons and Ganellin 2006). Histamine is stored in vesicles by the vesicular monoamine-transporter VMAT-2 (Hu and Chen 2017). Various stimuli lead to a release of histamine through exocytosis. Once released, histamine does not undergo reuptake by specific transporters or channels like other neurotransmitters. Organic cation transporters (OCT-1, OCT-2, OCT-3, VMAT-1) are discussed for transport mechanisms (Slamet Soetanto et al. 2019; Ogasawara et al. 2006). Histamine is metabolized to inactive Nτ-methylhistamine via N-methyltransferase (HNMT) or diamine oxidase (DAO) to inactive imidazolyl acetic acid. Nτ-methylhistamine is further metabolized through monoamine oxidase B (MAO B) to Nτ-methylimidazoylacetic acid (Shahid et al. 2009; Akdis and Blaser 2003).
Histamine exerts its action through four receptor subtypes, histamine 1-4 receptors (H1-4R). All receptor subtypes belong to the class A G-protein coupled receptors (GPCRs) (Shahid et al. 2009).
1.2.2 Histamine Receptors
1.2.2.1 Histamine H1 Receptor
The histamine H1 receptor (H1R) is a Gq-coupled GPCR and is expressed ubiquitously in the body. Activation on histamine H1R stimulates phospholipase C (PLC) activation and further results in the inositol-1,4,5-trisphosphate (IP3) and diacetyl glycine (DAG) formation, increased intracellular Ca2+ concentration, and activation of protein kinase C (PKC), respectively. The increased Ca2+ concentration leads to vasoconstriction in lung smooth muscle cells and large blood vessels. On the other hand, in the small blood vessels, dilatation occurs due to the stimulation of nitric oxide (NO) formation. These processes lead to H1R-mediated allergic reactions, characterized by heavy breathing, skin redness, itching, and swelling of the nasal mucous membranes (Leurs et al. 2002). In addition to peripheral tissues such as the lungs, skin, cardiovascular system, and gastrointestinal tract (GIT), the receptor is also broadly distributed in the CNS (Panula et al. 2015; Shahid et al. 2009).
The H1R with high density in different brain areas influences attention, wakefulness, sleep-wake rhythm, and cognition in the hypothalamus, the thalamus, the amygdala, and the cortex (Sadek et al. 2016a). Activation of the H1R leads to reduced food intake (Díaz et al. 2019). Blockade of the H1R in the CNS can lead to significant weight gain, often observed as a side effect of antipsychotics or first generation of antiallergic H1R antagonists (Kaar et al. 2019).
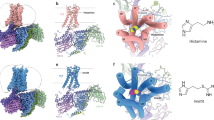
In 2011, the first X-ray crystal structure of a stabilized H1R with the antagonist doxepin was reported (Shimamura et al. 2011).
Currently, only H1R antagonists are used as pharmacological treatment options. The involvement of the H1R in allergic reactions qualifies H1R antihistamines as an essential substance class in their treatment. H1R antagonists are divided into two generations based on their central nervous side effect profiles. In treating allergic reactions, representatives of the first generation are less often used, as they penetrate the blood-brain barrier (BBB). This further results in side effects but can be used for drug repurposing. For instance, the sedative effect is exploited in sleep disorders, and H1R blockade in CNS leads to the use in the treatment of nausea and vomiting (Simons and Simons 2011).
The second generation of H1R antihistamines penetrates the BBB to a smaller extent and expresses fewer sedative effects. Active second-generation metabolites and newly designed compounds with higher selectivity rates and simultaneously reduced BBB penetration can therefore be used as a safer and more potent therapeutic antiallergic alternative.
1.2.2.2 Histamine H2 Receptor
The histamine H2 receptor (H2R) is a Gs-coupled receptor. Signalling through Gs leads to activation of the adenylate cyclase (AC), an increased cyclic adenosine monophosphate (cAMP) concentration, protein kinase A activation (PKA) followed by downstream processes (e.g., cAMP-responsive element-binding protein (CREB) cascade) (Obara et al. 2019; Panula et al. 2015; Sadek et al. 2016a).
The influence on gastric acid production is the well-described process associated with H2R. H2R stimulation leads to an increase in cAMP concentration and further in gastric acid secretion. Pharmacologically, gastric secretion is suppressed by H2R antagonists, which enabled the treatment of reflux diseases, dyspepsia, and gastric ulcers. However, this class was replaced by more efficient and safer proton pump inhibitors (PPIs) as omeprazole, esomeprazole, and pantoprazole (Panula et al. 2015).
H2R is expressed in the CNS and seems to influence cognitive processes as well as the sleep-wake rhythm (Haas et al. 2008), even though the exact relationship is still unclear. Besides, the H2R is involved in the glucose metabolism of food-intake control (Schneider et al. 2014). Also, the H2R is expressed in numerous types of cells, including smooth muscle cells, endothelial and epithelial cells, chondrocytes, neutrophils, eosinophils, monocytes, macrophages, dendritic cells, T and B cells (Jutel et al. 2009).
1.2.2.3 Histamine H3 Receptor
The Histamine H3 receptor (H3R) is the third histamine receptor subtype, discovered toward the end of the last century and first cloned in 1999 (Panula et al. 2015). The H3R is predominantly expressed in the CNS; however, expression in the periphery has also been detected and is increasingly being discussed on therapeutic aspects (Sander et al. 2008). High receptor density is found in the CNS in the cortex, the striatum, the nucleus accumbens, the amygdala, the palladium, and the hippocampus (Sadek et al. 2016a; Sander et al. 2008; Nieto-Alamilla et al. 2016). The H3R is predominantly expressed as a presynaptic receptor and acts as an autoreceptor controlling histamine synthesis and release via a negative feedback mechanism. As a heteroreceptor on non-histaminergic synapses, the H3R also regulates the release of dopamine (DA), acetylcholine (ACh), norepinephrine (NA), glutamate (Glu), serotonin (5-HT), γ-aminobutyric acid (GABA), substance P and other neurotransmitters (Sander et al. 2008; Nieto-Alamilla et al. 2016). Postsynaptic H3Rs were recently described (Ghamari et al. 2019). Presynaptic H3R is a Gi/o coupled GPCR (Sadek et al. 2016a; Nieto-Alamilla et al. 2016). The Gαi subunit inhibits the AC and thereby leads to a decreased cAMP concentration and reduced PKA activity. The reduced cAMP level reduces CREB-mediated gene transcription and lowers protein biosynthesis. HDC activity and histamine synthesis are reduced (Sander et al. 2008; Nieto-Alamilla et al. 2016; Obara et al. 2019).
The Gβγ subunit binds to and inhibits the P- and Q-type of voltage-gated calcium channels. The inhibition of these channels reduces Ca2+ influx into the presynapse and diminishes the depolarization. Activation of GIRK potassium channels leads to increased K+ efflux and hyperpolarization. Decreased Ca2+ influx and increased K+ efflux result in decreased neurotransmitter exocytosis (Nieto-Alamilla et al. 2016).
H3R antagonists suppress the H3R mediated inhibitory effect and consequently increase the histamine biosynthesis and release. Through H3R heteroreceptors, other neurotransmitters and mediators like ACh, NA, DA, 5-HT, Glu, and GABA are modulated (Nieto-Alamilla et al. 2016; Ghamari et al. 2019; Obara et al. 2019).
As GPCR with high constitutive activity, antagonists of the H3R can act as neutral antagonists or inverse agonists. Inverse agonists can decrease the basal activity of the receptor by stabilizing the inactive state (Latorraca et al. 2017; Berg and Clarke 2018).
An influence of H3R inverse agonists on the waking state, through increased histamine concentrations and postsynaptic H1R activation, led to the development of pitolisant, the first and so far, only H3R inverse agonist approved by the FDA (Food and Drug Administration) and the EMA (European Medicines Agency). Pitolisant (Wakix®) is indicated for treating narcolepsy with and without cataplexy (Ghamari et al. 2019; Romigi et al. 2018) and excessive daytime sleepiness in obstructive sleep apnea (Ozawade®) (Wang et al. 2021).
As described, the H3R mediates several neurotransmitter levels and targets diseases associated with CNS disorders. These include schizophrenia, epilepsy, attention deficit hyperactivity disorder (ADHD), autism, altered sleep-wake rhythms, Prader-Willi syndrome, and neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Ghamari et al. 2019; Reiner et al. 2020; Sadek and Stark 2016).
1.2.2.4 Histamine H4 Receptor
The histamine H4 receptor (H4R) is a Gi-coupled GPCR identified in 2000 as the last histamine receptor subtype. H4Rs are structurally related to H3Rs. However, the expression patterns of the receptors differ significantly, so that the H4R shows increased peripheral expression (Panula et al. 2015; Schneider and Seifert 2016).
The H4R is mainly expressed on hematopoietic cells, especially on eosinophilic granulocytes (Buckland et al. 2003). Besides, mast cells, natural killer cells as well as monocytes also express the H4R (Gschwandtner et al. 2013; Capelo et al. 2016). Strong evidence suggested H4R association with various functional inflammatory responses mediated by histamine, including chemotaxis and cell recruitment upregulation of adhesion molecule expression, and modulation of cytokine and chemokine release (Neumann et al. 2014; Hartwig et al. 2015). Preclinical and emerging clinical data supporting the association of H4R with pruritus and atopic skin inflammation (Werfel et al. 2016). For example, H4R agonists have been described to upregulate the TH2-associated and itch-inducing cytokine IL-31 (Gutzmer et al. 2009).
Other diseases discussed in context with the therapeutic H4R blockade include bronchial asthma and non-specific inflammatory reactions (Panula et al. 2015). Up to date, no H4R ligand has been approved by drug agencies.
2 In Vitro Assays
In the case of GPCRs, in vitro assays display test systems to evaluate ligand affinity, functionality, and efficacy in a cell-based system. In receptor binding assay, a ligand can be characterized by its affinity at the receptor of interest (on-target) and its selectivity over undesired receptors (off-targets).
Intrinsic activity and efficacy are determined in functional assays, and at least two types of chemical probes can be beneficial for in vitro assays conduction. Unlabelled or reference ligands display a large group of compounds. With well-defined receptor profiles and high on-target affinities, they are needed to standardize the results. In a validated assay, the reference ligand is the critical element to compare and transfer to other results and studies. Reference ligands are used in both binding studies and functional assays.
The second group is labelled ligands, subdivided into radioactive (radio-) and fluorescent-labelled ligands. These labelled ligands are used as measurable/countable values to determine affinities and receptor distributions. The labelled ligand should show high selectivity, especially for receptor distribution assays and assays with native or primary cells (containing more than one receptor).
2.1 Reference Ligands
Histamine is the endogenic ligand of histamine receptors (HR) that shows moderate to high affinities at all four receptor subtypes. Interestingly, the affinity at H1R and H2R is more than 1,000-fold lower compared to those at H3R and H4R. As an endogenous ligand, histamine displays unfavorable properties as a chemical probe. At H1R and H2R, the affinity is not high enough, and for H3R and H4R, the missing selectivity is an essential drawback. However, histamine is used as a reference in functional assays to calculate the intrinsic activity and efficacy compared to the endogenous ligand.
2.1.1 Histamine H1 Receptor
2.1.1.1 Agonists
First attempts to synthesize H1R agonists lead to imidazole-containing analogues as 2-thiazoylhistamine (2-TEA). Like histamine, the affinity and selectivity of 2-TEA are not suitable for use as a chemical probe. With an intrinsic activity of 27% compared to histamine, 2-TEA is a partial agonist with moderate efficacy (EC50: 440 nM) (Seifert et al. 2003). The introduction of a 3,3-diphenylpropyl group in position 2 of imidazole leads to a substance class named histaprodifens. Histaprodifens display partial agonists with an intrinsic activity of up to 77% (methylhistaprodifen) and 84% (suprahistaprodifen) (Elz et al. 2000). H1R affinity of methylhistaprodifen is moderate. However, it is still mostly used as an H1R agonist, with an acceptable selectivity over H2R (~100×) and H3R (~10×). Although histaprodifen and derivatives were synthesized already 20 years ago (Elz et al. 2000), meanwhile no remarkable progress was made in the field of H1R agonists. A full agonist with high and selective H1R affinity remained to be designed.
2.1.1.2 Antagonists
In contrast to agonists, a variety of H1R antagonists with high affinities were synthetized. A well-characterized representative of H1R antagonists is mepyramine. It belongs to the group of first-generation antihistamines, as it penetrates the blood-brain barrier. Due to its high affinity (pKi: 8.4) and selectivity over other HRs (~100×), mepyramine was used to synthesize radio and fluorescent-labelled analogues/derivatives (Rose et al. 2012). Doxepin, another first-generation antihistamine, showed increased affinity at H1R with a pKi value of 9.5. While the other HR’s selectivity is high, some off-target interaction to serotonin receptors (5-HTR) is reported (Mansbach 2008; Zhou et al. 2018). With a resolved doxepin-H1R crystal structure, doxepin is a promising chemical probe for clarifying structure activation relationships (Shimamura et al. 2011). Side effects of first-generation antihistamines due to central H1R blockade resulted in developing second generation of H1R antagonists. Well-described members of this group are the frequently used antiallergic drugs cetirizine and loratadine (Sadek and Stark 2016). Their highest benefit of chemical probes is the transferability from in vitro data to clinical outcomes. Desloratadine, an active metabolite of loratadine, was introduced to the market and showed increased affinity to H1R. Desloratadine as well as loratadine show affinities at 5-HT2R, in a comparable range like doxepin (pKi: 6.5–7.5). This should be kept in mind when analyzing the results of native cell experiments. Cetirizine points out the stereoselective binding at H1R; the racemic Cetirizine shows a pKi value of 8.2, where the pure R-enantiomer called levocetirizine has an affinity of pKi: 8.5, and the S-enantiomer has a lower affinity with a pKi value of 7.1 (Hair and Scot 2006). Due to its higher affinity, levocetirizine was brought to the market as a pure enantiomeric drug (Fig. 2). Histamine H1R ligands are summarized in Table 1.
2.1.2 Histamine H2 Receptor
2.1.2.1 Agonists
Compared with H1R agonists, the search for selective H2R agonists started with the imidazole ring variation. As a result, 4-methylhistamine was synthesized and is nowadays used as an H4R agonist with around 100-fold selectivity at H4R over H2R. The replacement of imidazole with aminothiazole leads to amthamine (Fig. 3). Even though amthamine acts as a full agonist at H2R, it shows a low affinity (pKi: 5.2) and no selectivity to other receptor subtypes. The partial agonist impromidine (intrinsic activity 79%) has an improved affinity to H2R (pKi:7.2) and selectivity over H1R (~100×) but retains its lack of selectivity over H3R (pKi:7.2) and H4R (pKi:7.9). According to the stereoselectivity of histamine receptors, sopromidine shows interesting behavior. Comparable to cetirizine at the H1R, the two stereoisomers of sopromidine show different binding affinities to H2R. Moreover, R-sopromidine acts as a full agonist, where S-sopromidine acts as a moderate antagonist (Elz et al. 1989). It is noteworthy that guanidine-based impromidine derivatives show affinity at H3R and H4R and were used as lead structures for selective H3R and H4R ligands (Venable and Thurmond 2012; Gbahou et al. 2012).
UR KAT471 is a potent partial agonist of the H2R and shows more than 100-fold selectivity over the other HR subtypes. The selectivity, high affinity (pKi of 7.2), and potency superior to histamine make UR KAT471 the favored H2R agonist for in vitro assays (Kraus et al. 2009).
2.1.2.2 Antagonists
Like H2R agonists, antagonists also show difficulties in terms of selectivity at H4R. Aminopotentidine is a member of cyano substituted guanidine with a nanomolar affinity (pKi: 7.4) and shows selectivity over H1R and H4R, but not over H3R. With an iodide substitution at the 3-position of the benzamide group, the affinity to H2R was significantly improved, resulting in pKi value of 9.4. In this way, a radiolabelled ligand is designed when using the radioactive isotope 125I (half-live of 59.5 days). Replacement of the substituted guanidino group by square acid resulted in more selective H2R antagonists. Tritium-labelled UR DE-257 shows high selectivity over the other HRs (>100×) and can be used as a radiolabelled ligand for H2R. Commercially available H2R antagonists also contain guanidino group (cimetidine-cyanoguanidine group, ranitidine-nitroethendiamine, and famotidine-sulfamoylamidine). Imidazole in cimetidine was replaced with bioisosteric heterocycles such as furan (ranitidine) and aminothiazole (famotidine) to prevent interaction with CYP450 enzyme complex. These drugs show affinities in a low micromolar to the nanomolar concentration range and are characterized as inverse agonists with an intrinsic activity of −88% (cimetidine and famotidine) and −100% (ranitidine). As in the case of approved H1R antagonists, the benefit of these drugs is not defined by their high affinity or selectivity but rather on the transferability of in vitro data to clinical outcomes (Fig. 3). Histamine H2R ligands are summarized in Table 2.
2.1.3 Histamine H3-Receptor
2.1.3.1 Agonists
With the discovery of H3Rs in 1983, numerous H1R and H2R ligands were examined due to their affinity to the H3R. Histamine shows high selectivity for H3R over H1R and H2R, and it was used as a lead structure. Slight variations with methylation of histamine resulted in R-α-methylhistamine and Nα-methylhistamine (Fig. 4). Both ligands are potent agonists with an intrinsic activity of 100% compared to that of histamine. Like histamine, they show selectivity over H1R and H2R of about 100-fold, with a high affinity at H3R. The following discovery of H4R disclosed for both ligands full H4R agonism (100%) with moderate affinities with pKi values of 6.5 and 6.6, respectively. Nα-Methylhistamine is a commercially available [3H]labelled ligand for H3R binding studies (Panula et al. 2015) leading to good comparability of assay results.
Replacement of the aliphatic alkyl amine by piperidine led to immepip and methylimmepip. Methylimmepip shows high affinity (pKi value of 9.0) and selectivity to H3R over H1R, H2R, and H4R (>1,000×) and intrinsic activity of 90%. These features make methylimmepip a valuable chemical probe for in vitro studies. Proxyfan shows an interesting efficacy behavior depending on the assay characteristics; as a selective ligand, it can act as an inverse agonist, antagonist, or (partial) agonist (Sadek and Stark 2016). The only non-imidazole-based agonist described so far, ZEL-H16 (1,3-bis(3-(piperidin-1-yl)propyl)-1H-indole), possesses high affinity (pKi value of 8.6) and selectivity to H3R. It is a partial agonist with an intrinsic activity of 60% compared with that of histamine (Shi et al. 2012).
2.1.3.2 Antagonists
The early antagonists of the H3R, like thioperamide, showed high selectivity over H1R and H2R but not over H4R. (hH3R pKi: 7.1, hH4R pKi: 7.3). Thioperamide was found to be an inverse agonist at H3R and an agonist at H4R. Examining antagonistic properties enabled classifying rat (r), mouse (m), or human (h) H3R isoforms. Thioperamide shows a slightly reduced affinity to hH3R compared to rH3R. Ciproxifan, an H3R selective inverse agonist, has a 100-fold higher affinity to the rH3R whereas clobenpropit shows nearly no difference. Clobenpropit shows sub-nanomolar affinity to hH3R, and even though it has a good selectivity over H4R, it served as a blueprint for the design and synthesis of H4R ligands due to its likewise nanomolar affinity to the H4R (Tiligada and Ennis 2020). Iodoproxyfan also shows a sub-nanomolar affinity and is used as a radiolabelled ([125I]) ligand, with high selectivity over H1R and H2R and slight selectivity over H4R.
Despite the general assumption that the imidazole moiety causes serious interactions due to the CYP450 enzyme class interactions, these compounds have been dosed in low concentration in numerous in vitro and in vivo experiments without any signs of interaction. The receptor selectivity might be a more important aspect concerning off-target affinities (e.g., H4R). The imidazole replacement by cyclic tertiary amines like N-substituted piperidine or pyrrolidine resulted in the next generation of H3R antagonists. With pitolisant, the affinity to hH3R as well as the selectivity over H4R was increased. The FDA and EMA approved the inverse agonist pitolisant for the treatment of narcolepsy with or without cataplexy (Wang et al. 2021). Based on the H3R pharmacophore of pitolisant, the potent fluorescent-labelled ligand bodilisant was synthesized. As the only H3R inverse agonist/antagonist on the drug market, pitolisant displays excellent properties as a reference substance. Several other inverse agonists/antagonists were synthesized and investigated in preclinical and clinical assays. ABT-288 showed high affinity and selectivity. In several assays, procognitive effects of ABT-288 were observed. Similar affinity to human and rat H3R (pKi: hH3R: 8.7, rH3R: 8.1) can simplify the analysis of different assay systems (Esbenshade et al. 2012). The steroidal alkaloid conessine shows structural differences to other H3R antagonists with comparable receptor affinity (pKi: 8.5) to pitolisant and ABT-288. The selectivity over other HRs (>1,000×) and other GPCR is high. It is a highly suitable second reference substance to reveal pattern associated unspecific binding in a new assay. An affinity of conessine to adrenergic α2 receptors should be noted concerning GPCR based assays (Zhao et al. 2008) (Fig. 4). Histamine H3R ligands are summarized in Table 3.
2.1.4 Histamine H4 Receptor
2.1.4.1 Agonists
Several former reported HR ligands showed affinity to H4R after it was discovered. The high similarity of H3R and H4R binding pockets led to some obstacles in finding selective ligands. Histamine and the radiolabelled [3H]histamine show similar high affinities to the receptors. However, it is commonly used as a radiolabelled ligand in H4R displacement studies. 4-Methylhistamine, synthesized initially as an H2R ligand, shows a high affinity to H4R with at least 100-fold selectivity over other HRs (Fig. 5). It is characterized as a full agonist with 100% intrinsic activity compared to histamine. Some other H2R agonists display H4R affinities like impromidine (pKi value of 7.6) and R-/S-sompromidine (pKi values of S: 5.5, R: 6.1). The partial agonist ST-1006 is described as the most potent H4R agonist (pEC50 value of 8.95) and shows high affinity and good selectivity over H1R, H2R, and H3R (Sander et al. 2009).
2.1.4.2 Antagonists
As mentioned, the H3R inverse agonist iodoproxifan was used as a lead structure for H4R antagonists. Other H3R antagonist iodophenpropit showed nearly the same affinity at H4R (pKi value of 7.9) and H3R (pKi value of 8.1) with agonistic and inverse agonistic activities, respectively, displaying functional selectivity. The 125I-labelled form of iodophenpropit is frequently used as a radiolabelled ligand for H4R in displacement studies. One of the best-studied and most used reference ligands in the field of H4R is JNJ-7777120. It possesses a high affinity (pKi value of 8.4) and selectivity (other HRs > 100×). Although the affinity of humans and rats (pKi value of 8.6) varies little, the intrinsic activity is different. In humans, JNJ-7777120 acts as an inverse agonist, whereas in rats, it acts as a partial agonist (51% activity compared to that of histamine (100%)).
Moreover, the behavior in humans can change because JNJ-7777120 shows agonist activity in β-arrestin recruitment pathways. It is therefore called a biased agonist (Rosethorne and Charlton 2011). As a chemical probe for in vitro characterizations, this might be acceptable or welcomed to study species differences, reducing the portability between in vitro and in vivo data. Nevertheless, JNJ-7777120 is a well-characterized tool for understanding the physiology of the H4R (Thurmond et al. 2004).
One of the well-described H4R ligands is adriforant (also known as PF-03893787 or ZPL-389), an inverse agonist at hH4R. Compared to JNJ-777712, adriforant shows species variations in affinity (pKi values hH4R: 8.2, hH4R: 8.2, and dog H4R: 5.8) and intrinsic activity as it acts as antagonists in dogs and partial agonist in rats. This species-dependent behavior arises from low amino acid sequence identities. The human H4R only shows around 70% amino acid homology to rats and dogs. Rats again possess only 64% of homology to dogs and even only 84% to mice (Jiang et al. 2008). Also, histamine as an endogenic ligand shows these species differences with pKi values of 7.8 and 6.9 at human and rat H4R isoforms, respectively (Fig. 5). Histamine H4R ligands are summarized in Table 4.
2.1.4.3 Multi-target Histamine Ligands
The involvement of histamine and its receptors in complex immunological and neurophysiological processes, and the beneficial effects of HR ligands in clinical trials, led to their participation in polyvalent ligands (Łażewska and Kieć-Kononowicz 2018). Such multi-target directed ligands (MTDLs) address more than one target and simultaneously impact pathophysiological conditions (Proschak et al. 2019). The design of such a ligand must differ from “dirty drugs.” The latter interacts with several off-targets, while the former has a designed polypharmacological profile. Rationally designed multi-target directed chemicals probes (MTCPs) need to be extremely well described regarding their targets and potential off-targets. MTCPs are necessary tools to study target combinations in vivo and as references in vitro. Compared to single target chemical probes, affinities should be more balanced, e.g., when combining receptor ligands with enzyme inhibitors (Khanfar et al. 2016).
The dual-acting ligand GSK-1004723 possesses a high affinity to its on targets H1R (pKi value of 10.2) and H3R (pKi value of 10.6) with an excellent selectivity to H2R and H4R (>10,000×). Therefore, it is an MTDL prototype because the on-target affinity arises from design and not due to coincidence. As an MTCP it is useful for validating assay systems with more than one HR subtype.
As dopamine plays an important role in neurodegenerative and neurological diseases, tools combining HR and dopamine receptor (DR) affinity are needed. As recently shown, the MTDL ST-2223 combines high H3R(pKi value of 8.3), D2R (pKi value of 7.7), and D3R (pKi value of 8.7) affinities with excellent selectivity to off-targets like H1R (pKi value of 7.0), D1R (pKi value of 6.3), and D5R (pKi value <5.5) (Eissa et al. 2021). ST-2223 and ST-718 showed beneficial effects in autism-like behavior. ST-718 possesses higher selectivity over HRs but lower selectivity and affinity at DRs (Venkatachalam et al. 2021). Contilisant combines H3R affinity (pKi value of 8.0) and sigma 1 receptor affinity (pKi value of 7.2) with inhibition of enzymes like MAO A (IC50 value of 145 nM), MAO B (IC50 value of 87 nM), acetylcholine esterase (IC50 value of 530 nM), and butyrylcholinesterase (IC50 value of 359). It can be used to demonstrate the possibility to combine a different kind of target interactions (enzyme inhibition and receptor binding) (Bautista-Aguilera et al. 2017, 2018). A multi-target ligand recently developed by Cao et al. which has high affinities to dopaminergic, serotoninergic, and histaminergic receptors, showed promising effects and good pharmacokinetics in rats (Cao et al. 2018).
2.2 Labelled Ligands
In contrast to reference ligands, labelled ligands are normally not used to compare or transfer data from one to the other experiment. Labelled ligands are necessary for the read-out mechanisms in different assays. As the data obtained is based on the read-out, labelled ligands significantly influence the entire assay. Displacement data can vary between a labelled agonist or antagonist due to their different affinity and binding characteristics. For each histamine receptor subtype, only a few labelled ligands were used in in vitro assays. Radiolabelled ligands contain one or more radioactive atoms, normally beta- or gamma-emitters. PET ligands are labelled with positron emitters. The chemical structure from unlabelled to labelled ligands is only minimally changed, and binding properties are retained. Fluorescent-labelled ligand requires a fluorophore, which is usually not incorporated in the primary ligand. Fluorophores like BODIPY were coupled to an HR pharmacophore. The development of fluorescent chemical probes is a rising research field, as newer techniques like BRET extensively take benefit of these ligands and many laboratories have regulative problems with the use of radioactive materials.
2.2.1 Radiolabelled Ligands
In vitro ligand, characteristics are usually not changed by inserting a radioactive atom in their chemical scaffold. As seen with histamine, binding affinities of radiolabelled ligands at all HR subtypes remain. [3H]histamine shows high affinities at H3R and H4R and selectivities over H1R and H2R. For H4R, it is a commonly used radiolabelled ligand in displacement studies. As an agonist, the binding mode can differ from the antagonist, and displacement assays can produce different results when a radiolabelled antagonist like [125I]iodophenpropit is used.
Also [125I]iodophenpropit shows high affinities at H3R and H4R and is often used as a radiolabelled ligand. For radioligand displacement assays, both radioligands resulted in similar pKi values for several H4R ligands, including, e.g., histamine (pKi ([125I]iodophenpropit) value of 7.6 ± 0.2, pKi ([3H]histamine) value of 7.8 ± 0.1).
For H1R, histamine’s affinity is unfavorable and thus the selective agonist mepyramine was radiolabelled, resulting in [3H]mepyramine. Due to its high affinity and selectivity, it is the mostly used and one of the most suitable radiolabelled ligand for H1R. It was used for early H1R visualization studies to determine H1R distribution in human brains (Martinez-Mir et al. 1990).
Iodination at 3-position of the selective H2R antagonist (over H1R and H4R) aminopotentidine (pKi value of 7.4) resulted in [125I]iodoaminopotentidine with 100-fold improved the affinity to H2R (pKi value of 9.4). The antagonist [3H]UR-DE257 displays the highest selectivity of the other HRs (>100×) and is, therefore, better useable for visualization of receptor expression studies.
In various radiolabelled H3R ligands, [3H]Nα-methylhistamine is the most used one. The affinity and selectivity are comparable, respectively, better than [125I]-iodoproxyfan and [125I]-iodophenpropit (Van Der Goot and Timmerman 2000).
As an H4R selective ligand, tritium labelled [3H]JNJ-7777120 was synthesized, showing the mentioned advantages and disadvantages of JNJ-777120. In H4R displacement assays [3H]-histamine is still the most used radiolabelled ligand, despite its low selectivity to H3R.
2.2.2 Fluorescent-Labelled Receptor Ligands
In the beginning, fluorescent ligands were mainly used as the histological stains for identifying the biogenic amines and their receptors in tissues (McGrath et al. 1996). In 2000 the GPCR was described and visualized using green fluorescent protein-tagged (GFP-tagged) GPCRs taking advantage of molecular biology techniques (Kallal and Benovic 2000). This was a starting point for the drug discovery to study the exact ligand–receptor interaction. For many years, radioligand assays have been conducted to examine receptor–ligand interactions, both in vitro and in vivo (Ma and Zemmel 2002). Fluorescent ligands gain even more attention with the development of new cutting-edge techniques, as Förster resonance energy transfer (FRET), fluorescence correlation spectroscopy (FCS), scanning confocal microscopy (SCM), and bioluminescence resonance energy transfer (BRET). These techniques enabled the development of pharmacological assays on live or fixed tissues, with small amounts of tissue, or on single cells, and the results can be obtained immediately (McGrath et al. 1996; Michalet et al. 2006; Al-Damluji et al. 1997).
Those methods are now widespread in pharmacology. The fluorescent-labelled ligands are used to study subcellular localization, internalization or dimerization, and ligand binding kinetics of receptors. Moreover, using fluorescent ligands enables the measurement of specific and non-specific receptor binding (Mackenzie et al. 2000).
As described in radiolabelled ligands, it is essential to define the fluorescent ligand affinity and selectivity. Other than radiolabelling, fluorescently labelled ligands show some variations of their chemical structure compared to the lead ligand. The fluorophore needs to be attached to the ligand to retain the affinity and selectivity of the starting compound. It is usually obtained by the fluorophore placement far away from the part of the pharmacophore. A variety of fluorophores (e.g., DANSyl, Sanger’s reagent, AlexaFluor®, or Bodipy®) could be linked to ligands with or without a linker, providing possibilities for the design and development of novel, potent, selective, and affine fluorescent ligands (Kuder and Kieć-Kononowicz 2014).
The coupling of BODIPY, boroazaindacene derivative, to mepyramine created a structure called mepyramine-BODIPY 630–650 (short: mepyramine-BY630). The remarkable properties of mepyramine were maintained, whereby the affinity was even slightly increased (Rose et al. 2012).
The beneficial squaric acid moiety of [3H]UR-DE 257 was used to synthesize a fluorescent-labelled ligand and resulted in UR-KAT 51. As mentioned above, also this ligand is coupled to a Bodipy® fluorophore. It is characterized as an antagonist with a pKi of 8.4 and used in nanoBRET assays, displaying great properties (Grätz et al. 2020).
Bodilisant, a piperidine-based hH3R ligand coupled with the BODIPY pharmacophore, shows ideal chemical probe characteristics as a fluorescent-labelled ligand for H3R. The high affinity (pKi value of 8.4) and high selectivity over other HRs was combined with an excellent quantum yield of Φ: 0.92. Bodilisant demonstrated its benefits in H3R labelling studies and a fluorescence-polarization experiment to determine receptor residence times of different H3R ligands (Tomasch et al. 2013; Reiner and Stark 2019).
A fluorescent-labelled ligand with high affinity and selectivity for the H4R has not yet been reported. The fluorescent-labelled ligand UR-DEBa 242 (PY-5 labelled) (Bartole et al. 2020) shows a high affinity to H4R (pKi value of 7.9) and selectivity over H1R and H2R, but not over H3R (pKi value of 8.6).
3 In Vivo Assays
In contrast to the precise criteria defined for the in vitro chemical probes examined (affinity below 100 nM, selectivity over 10-fold against related target), the demands for in vivo chemical probes are far more complex as the processes in living cells and tissues are much more complicated. Therefore, defining general criteria for chemical probes in animal studies is more challenging (Stark 2020) and depends on the experimental design.
It is not simple to set cut-off values in vivo and demonstrate on-target effects in animal models due to enormous differences in species. As chemical probes are not necessarily equal with preclinical candidates, it should be first determined if a chemical probe contains appropriate pharmacodynamic and pharmacokinetic characteristics to be examined in animal models. Moreover, their safety profile and tolerance should be examined in the species of interest. Different chemical probes are chosen based on animal models and targeted disease, and here will be explained which of them reached in vivo preclinical and clinical trials as well as highlight the best chemical probe for each receptor in different behavioral models.
3.1 Histamine H1 Receptor
Histamine H1R was the first described receptor subtype and is the most abundant histamine receptor. Hence, it has been cloned over three decades ago, it has been extensively studied, and various in vivo studies that target H1R have been conducted.
3.1.1 Agonists
H1R agonists have not raised specific interest in the scientific community, and so far, only a few compounds with agonistic properties have been described. As all histamine receptor ligands, H1R agonists were first developed with a slight modification of histamine as imidazole derivatives. However, bioisosteric replacement of carbon with sulfur led to H1R agonist 2-(thiazol-2-yl)ethanamine (2-TEA, 2). Substitution with bulky substituent leads to 2-(3-(trifluoromethyl)phenyl) histamine. These compounds are well tolerated and are used to mimic histamine effects in in vivo studies (Malmberg-Aiello et al. 2000). Further modification leads to histamine-trifluoromethyl-toluidine (HTMT) development, a more potent agonist than histamine, that also shows affinity to H2R and can be considered as a dual targeting ligand. HTMT is used in vivo studies to examine the role of histamine in immunomodulation, as shown in rabit (Tripathi et al. 2010) and mice (Lapilla et al. 2011). Later developed H1R agonists were histaprodifen and suprahistaprodifen, which still need to be examined in vivo. Suprahistaprodifen showed partial agonism at H4R, while histaprodifen derivatives acted as an inverse H4R agonist (Deml et al. 2009). It is assumed that suprahistaprodifen binds at the H1R in two different orientations. As the H1R showed itself a high level of agonist independent constitutive binding (Jongejan et al. 2005), the application of H1R in vivo models makes it even more complex. Therefore, there is an urge for further development and examination of H1R agonists.
3.1.2 Antagonists
In contrast to moderately described H1R agonists, H1R antagonists are a well-known class that reached blockbuster drugs’ status. These ligands are widely used for therapy of allergies, nausea, conjunctivitis, or rhinitis. In general, H1R antagonists can be divided into two classes.
First-generation includes H1R antagonists like doxepin, mepyramine, doxepin, and diphenhydramine. They are lipophilic enough to cross the BBB and therefore express sedative effects. Since they show affinity to other receptor subtypes (e.g., cholinergic), they cannot be considered optimal chemical probes. First-generation H1R antagonists showed reinforcing effects in monkeys as they increased rates of suppressed and non-suppressed behavior (Bergman and Spealman 1986). However, from a pharmacological point of view, this disadvantage was used in PET tracers’ design. As first-generation H1R antagonist cross the BBB and penetrate CNS, they are often radiolabelled and used to estimate receptor distribution and localization. [11C]mepyramine, and [3H]doxepin are commonly used as a PET tracer for H1R, both in humans and animals (e.g., guinea pig). However, [11C]doxepin has lower metabolic degradation and provides higher contrast images, and therefore is the most commonly used PET ligand to measure histamine occupancy in the brain in humans (Yanai et al. 1992a, b, 1995; Ishiwata et al. 2007; Zhou et al. 2002; Sato et al. 2013).
The later developed, the second generation of antihistaminic are more polar structures and are in the form of zwitterion at physiological pH. Therefore, they cross the BBB only up to a small extent, expressing none to light sedating effects.
Some of these H1R antagonists represent suitable substrates for P glycoprotein and thus are rapidly effluxed without crossing the BBB to a remarkable extent under physiological conditions. Second-generation antihistaminic includes commercially available drugs: loratadine, desloratadine, cetirizine, levocetirizine terfenadine, fexofenadine, which are well-examined in preclinical studies. Stereoselective separation has led to some enantiomers leading to a higher affinity at H1R (e.g., levocetirizine). Some of the second-generation antihistamine compounds (e.g., terfenadine) display severe cardiac side effects, prolonging QT interval that can lead to Torsades de Pointes arrhythmias due to hERG channel interaction (human Ether-a-go-go Related Gene for voltage-dependent potassium ion channel). In line with these results, terfenadine has been withdrawn from the market. Structural modifications of terfenadine lead to designing fexofenadine that does not prolong QT interval and has an improved safety profile. Fexofenadine does not cross the BBB and does not occupy histamine H1R in the central cortex, in contrast to cetirizine (Sato et al. 2013; Tashiro et al. 2002). For review on this therapeutic and safety aspects, see Cataldi et al. Holgate et al. (Cataldi et al. 2019; Holgate et al. 2003).
Bilastine possesses highly suitable characteristics due to a peripheral acting H1R antagonist for in vivo studies. The high selectivity over several other receptors (including dopamine, serotonin, histamine, adrenaline, acetylcholine, and others) is ideal for studying H1R antagonism or blocking the peripheral H1R. Doxepin and mepyramine can be used as brain penetrating H1R antagonists, although doxepin showed low bioavailability.
Both first- and second-generation antagonists are drugs used daily. However, their use should be controlled in children, as they have seizure-inducing potential (Miyata et al. 2011).
They are used as antiallergic drugs, and both antihistaminic first- and second-generation reduced 48r80-induced scratching behavior in mice (Sugimoto et al. 1998). On the other hand, histamine, H1R antagonists hydroxyzine and cetirizine, did not prevent the development of acute skin lesions in Maltese-beagle dogs (Bäumer et al. 2011).
To address the problems caused by antihistamines of the first and second generation and improve the treatment of histamine-related diseases, dual ligands have been designed. Dual H1R/H4R antagonism presents a promising therapeutic option for allergy treatment due to the involvement of both receptor subtypes in allergy pathology, as shown in the acute murine asthma model in mice. Independent administration of H4R selective antagonist, JNJ-7777120, and H1R antagonist, mepyramine resulted in low to moderate eosinophilia effects. However, when they were administered in combination, statistically significant synergic effects were observed, indicating that dual MTDLs can be a new route in developing potent antiallergic reagents (Deml et al. 2009).
Other dual ligands developed are H1R/H3R GSK-1004723 and GSK-835726, both currently in clinical trials for allergic rhinitis (Daley-Yates et al. 2012). GSK-1004723 has a high affinity to both receptor subtypes with a long duration of action, potency similar to azelastine. It does not penetrate CNS and therefore does not express sedative effects (Slack et al. 2011). Besides the pharmacodynamic, the pharmacokinetic is important for in vivo measurements (Table 5).
3.2 Histamine H2 Receptor
3.2.1 Agonists
H2R agonists do not have therapeutic use. However, due to lack of selectivity, they often served as blueprints for the design and synthesis of H3R and H4R ligands.
Amthamine and dimaprit were firstly developed by slight modification and bioisosteric replacement in the imidazole ring. Impromidine, developed initially as vasodilators, is used as a chemical probe. It is guanidine derivatives and showed affinity to H3R. Stereoselectivity of H2R ligand plays an important role. It has been demonstrated that the R-enantiomer of sopromidine is a potent H2R agonist, whereas the S-enantiomer is the moderate antagonist (Elz et al. 1989). Therefore, sopromidine can be considered a useful chemical probe to gain better insight into functional selectivity and signalling pathways. N-acetylation of guanidino group leads to the synthesis of UR-AK381, UR-AK480, UR-BIT82. They are full agonists that showed 4,000 times the potency of histamine at recombinant human and guinea pig H2R but still display affinity to other receptor subtypes.
3.2.2 Antagonists
As well as in the case of H1R, H2R antagonists are far more common and used than H2R agonists. H2R antagonists, such as cimetidine, ranitidine, nizatidine, famotidine, and zolantadine, reached the status of blockbuster drugs and were used in the therapy of gastric ulcers, dyspepsia, or GERD (Taha et al. 1996; Lauritsen et al. 1990). Nowadays, they are replaced with more efficient PPI. Burimamide was the first selective H2R antagonist with moderate efficacy (Wyllie et al. 1972). Cimetidine was the first imidazole-containing H2R antagonist that reached a blockbuster status, discovered by Sir James Black. It is a potent CYP3A4 inhibitor and can induce drug–drug interaction. To address this problem, new non-imidazole derivatives have been developed. Even though well studied, they are currently subjecting to repurposing. Zolantadine can cross the BBB and has over 30-fold selectivity to H2R over other receptor subtypes. It is equally potent in rats and guinea pigs. It was examined in rats for possible wake-promoting effects but showed no significant impact (Monti et al. 1990). It has been shown that zolantadine does not alter the in vivo histamine metabolism in the brain of male Sprague-Dawley albino rats (Hough et al. 1988). On the other hand, it has been confirmed that zolantidine reduced in force morphine-induced antinociception in rhesus monkeys, speculating that nociceptive morphine effects are associated with H2R agonism (Lindsay et al. 1990). The only developed PET H2R tracer is [11C]nizatidine. However, due to its poor BBB permeability, it failed in brain imaging studies. Pharmacokinetic properties of selected H2R ligands are summarized in Table 6.
3.2.3 COVID Effects
In line with the current COVID-19 situation, histamine receptor antagonists are investigated on repurposing strategies to prevent coronavirus infection (Ishola et al. 2021). Azelastine, an H1R antagonist, expressed a particular effect on dendritic and T cells interaction in vitro (Schumacher et al. 2014). This antihistaminic agent together with hydroxyzine and diphenhydramine expressed antiviral activity against SARS-CoV-2 in vitro and is currently studied as a potential tool for fighting COVID-19 (Reznikov et al. 2021). Famotidine and cimetidine have higher affinities to H2R, where famotidine show specificity for HCV (59.5%) and HHV (49.5%) (Ishola et al. 2021). Besides, it has been confirmed that patients treated with famotidine had decreased risk of developing severe SARS-CoV-2 symptoms (Freedberg et al. 2020). One plausible explanation is that famotidine binds a papain-like protease encoded by the SARS-CoV-2 genome. This protease is necessary for the entry of SARS-CoV-2 into the cell. Even though reported results were contradictory, reporting both beneficial and no significant effects of histamine and histamine H2R antagonist are extensively investigated as potential targets for fighting COVID 19 (Ennis and Tiligada 2021). Famotidine is currently in phase III of Multi-site Adaptive Trials for COVID 19, where the effect on the standard of care is being compared with the same therapy with high intravenous doses of famotidine (NCT04370262). These repurposing studies may not lead to a novel therapeutic indication for these compounds but may offer possibilities for the design of new ligands from a different lead structure.
3.3 Histamine H3 Receptor
In contrast to H1R and H2R, histamine is nowadays extensively investigated due to novel therapeutic options. The involvement of H3R in various neurological disorders as a sleep-wake disorder, AD, epilepsy, schizophrenia, and cognitive impairments makes this receptor subtype an interesting target for the design and synthesis of chemical probes and potential drug-like candidates.
3.3.1 Agonists
As with the other two receptor subtypes, slight modifications were made on the histamine core to develop imidazole-based derivatives. Therefore, R-α-methylhistamine that expresses high affinity to H3R was developed. Although it is not selective and shows affinity to other receptor subtypes, it has still been used for labelling in behavioral studies, for examining histamine function in the brain and its connection with other neurotransmitters (Sadek and Stark 2016). For instance, it was confirmed that R-α-methylhistamine induces water drinking in rodents (Clapham and Kilpatrick 1994; Ligneau et al. 1998; Faghih et al. 2002). Alternatively, this ligand provoked dose-dependent inhibition of noradrenaline release in Male albino or the Wistar rats (Di Carlo et al. 2000). The latter effect was abolished when thioperamide, an H3R antagonist, was administered. [3H]R-α- methylhistamine is fast metabolized in humans with a half-life time (t1/2) of 1.6 h. Like histamine, it is methylated by the HNMT, and thereby loses agonist properties at the H3R (Rouleau et al. 2000). To increase the half-life time the prodrug BP2-94 was invented. BP2-94 is non-enzymatically transformed to [3H]R-α-methylhistamine. Half-life time of BP2-94 shows a biphasic behavior with t1/2(1): 1 h and t1/2(2): >24 h (Rouleau et al. 1997). Orally administered neither [3H]R-α-MeHA nor BP2-94 reached the CNS (Rouleau et al. 2000). Later developed imetit and imepip have higher potency but are not selective, also showing high affinities at H4R. Imetit inhibited the binding of [3H]R-α-MeHA in the rat brain (Garbarg et al. 1992), while imepip decreased histamine release in rats (Jansen et al. 1998).
Methimmepip is a methylated derivative imepip derivative with increased selectivity, over 200-fold selectivity to H3R over H4R, and a promising chemical probe for H3R. Both imepip and methimmepip have gastroprotective effects, as shown in gastric lesions caused by gastric acid in rats (Coruzzi et al. 2011). Even though the reported result about histamine’s role in obesity is contradictory, they are very species-dependent (Díaz et al. 2019; Lecklin et al. 1998). H3R agonists have been extensively researched as potential targets in obesity therapy. Histamine H3R agonist imetit significantly decreases appetite at fat mass and plasma concentration of leptin and expresses anorexigenic effects in diet-induced obese (DiO) WT mice (Yoshimoto et al. 2006).
3.3.2 Antagonists
Although the role of histamine H3R is still not fully understood, several H3R ligands are currently in clinical trials for ADHD, sleep-wake disorder, AD, and cognitive impairments. As well agonists, firstly developed H3R antagonists were imidazole-based structures. Thioperamide was one of the first synthesized H3R imidazole-containing antagonists. It is a highly potent H3R and H4R agonist. In vivo thioperamide positively impacts cognitive function in male spontaneously hypertensive rat pups (SHR) shown (Komater et al. 2003). Besides, thioperamide did not cause a locomotor sensation as psychostimulants (e.g., amphetamine or methylphenidate) used in ADHD therapy.
Moreover, locomotor hyperactivity induced by amphetamine was inhibited by thioperamide also in male CRH mice (Clapham and Kilpatrick 1994). Therefore, thioperamide represents a possibly safer alternative than currently available therapy, as it also does not express abuse potential. Besides, it has been shown that thioperamide increases appetite and consequently body weight in DiO WT mice when administered twice per day (Yoshimoto et al. 2006). With another imidazole-based derivative clobenpropit, thioperamide reduced alcohol intake in alcohol-preferring rats and is currently investigated for their abuse potential (Panula 2020).
Besides these two, ciproxifan is another later developed imidazole-based derivative that showed higher affinity at H3R and is often used as a reference in behavioral studies in rodents, mainly due to its precognitive effects, in combination with good pharmacokinetic properties. For instance, ciproxifan decreased impulsivity and increased attention in adult, male hooded Lister rats (Ligneau et al. 1998; Day et al. 2007), showed precognitive effects in APPTg2576 male mice (Bardgett et al. 2011) and enhanced performance SHR pups (Fox et al. 2002).
To address problems caused by imidazole derivatives, as drug–drug interactions and high affinity to H4R, non-imidazole derivatives have been developed and extensively studied. These derivatives are not CYP450 substrates and therefore have a safer profile, indicating that they can be better chemical probes, which led to the design and development of preclinical and clinical H3R candidates.
Pitolisant is the only commercially available H3R inverse agonist/antagonist (Ligneau et al. 2007), approved by EMA in 2016 and by FDA in 2019 for treatment of narcolepsy with or without cataplexy (Schwartz 2011; Lamb and Pitolisant 2020). Its safe profile and non-abuse potential are confirmed in in vivo animal models and in human studies (Uguen et al. 2013). This led to its non-controlled drug status in the USA (Lamb and Pitolisant 2020).
It is dosed once per day and, due to its safety and low interaction potential, can be considered an ideal chemical probe in behavioral sleep-wake models (Syed 2016). Pitolisant reduced H3R brain activity in Swiss mice and has been in clinical trials for obstructive sleep apnea, schizophrenia, ADHD, and photosensitive epilepsy (Kuhne et al. 2011). GSK-189254 is a potent H3R antagonist that reduced narcoleptic episodes in Ox −/− mice (Guo et al. 2009). Another H3R selective antagonist, JNJ-5207852, can be considered a chemical probe in this model. Ex vivo studies demonstrated that JNJ-5207852 had a high affinity to rat and human receptors and highly occupied H3R (Kuhne et al. 2011). It expressed wake-promote in male Sprague-Dawley rats and mice in a time-dependent manner (Barbier et al. 2004). Also, it expresses resuscitating effects in Wistar rats suffering from hemorrhagic shock (Jochem et al. 2016), and its mechanism is yet to be explored.
Non-imidazole H3R antagonist ABT-239 was efficient in multiple behavioral studies to improve social memory and cognitive impairment and is commonly used as a reference. ABT-239 (1.0 mg/kg) attenuated methamphetamine-induced hyperactivity in mice and gating deficits in DBA/2 mice with schizophrenia (Fox et al. 2005). It showed promising effects on cognition deficits induced by prenatal ethanol exposure in male adult rats (Varaschin et al. 2010) and CD1 mice when the histaminergic system had complete integrity (Provensi et al. 2016). Moreover, this H3R antagonist showed neuroprotective and anticonvulsive effects in male Swiss albino mice (Bhowmik et al. 2014) and expressed attenuation tau hyper-phosphorylation (Bitner et al. 2011). In line with these results, ABT-239 can be considered an excellent chemical probe in cognitive-behavioral models. Another chemical probe that can be used in cognitive-behavioral studies is GSK-189254. This selective H3R antagonist showed 10.000-fold selectivity for human H3R and has been used in animal models for attention models as it showed memory improved cognitive performance in rats (Medhurst et al. 2007). It showed antinociceptive potential, as it was confirmed that it modulates neuropathic pain in rats (McGaraughty et al. 2012) and reduced narcoleptic episodes in orexin −/− mice (Guo et al. 2009; Tiligada et al. 2009). DL77 is another new histamine H3R antagonist that recently gained more interest for its procognitive potential and high in vitro and in vivo potency (EC50 2.1 mg/kg). It showed promising results in ASD-like behaviors in VPA-exposed animals, procognitive effects by significantly ameliorating memory deficits induced by MK-801 in male Wistar rats, and anticonvulsant effects in male Wistar rats (Eissa et al. 2018; Sadek et al. 2016b).
As well as agonist role, antagonist role in obesity therapy has been studied. A-331440 developed by Abbott is a non-imidazole H3R antagonist that reduced weight in higher doses (5 mg/kg and in 15 mg/kg) in C57BL/6 J mice, previously treated with a high-fat diet (Hancock et al. 2004). However, this compound showed some genotoxic potential in rats, and therefore analogues have been synthesized: A-417022 and A-423579. Both ligands reduced weight in mice in high dosage, and the latter caused statistically significant weight loss in weight-matched Sprague-Dawley female rats (Hancock et al. 2005). Furthermore, H3R antagonism reduced calorie intake in higher mammalian species of pigs and rhesus monkeys, leading to several ligands that showed promising effects in preclinical studies (Lecklin et al. 1998). SCH- 497079, another H3R antagonist, reached phase II clinical trial (NCT00642993).
JNJ-39220675 is a novel, potent, and selective H3R antagonist. PET ligand [11C]JNJ-39220675 showed the excellent BBB permeability and occupied around 90% of H3R in the female baboon’s brain (Logan et al. 2012). It reduced alcohol intake in male adult selectively bred alcohol-preferring rats (Galici et al. 2011) and male JAX®C57BL/6 mice (Nuutinen et al. 2016) and is considered a suitable chemical probe for alcohol-induced behavioral studies. ST1283 is another H3R antagonist that showed promising effects on reducing alcohol intake in Tuck-Ordinary “TO” mice (Bahi et al. 2013).
Samelisant (SUVN-G3031), an orally available H3R inverse agonist, shows highly promising results in several preclinical and clinical studies. It is effective for wake-promoting, precognitive, and enhancing cortical arousal in animal models. It possesses high selectivity for the H3R, with comparable binding affinities to rH3R and h3R and no affinities to typical off-targets like 5-HTR or sigma receptors (Nirogi et al. 2021). Furthermore, side effects like CYP interaction, phospholipidosis, QT-time prolongation, and hERG blockade were not observed. Samelisant successfully completed phase I and is under investigation in phase II clinical trail (Nirogi et al. 2019). Thus, it is a promising drug candidate and a useful tool for examining species independent H3R antagonism/invers agonism.
Different PET tracers have been developed to examine and better understand histamine H3R role in the brain. [3H]thioperamide, [3H]5-methylthioperamide, and [11C]methylthioperamide were the first PET tracers for H3R that was used in mice. Several later developed H3R PET tracers as [11C]-UCL-1829, [18F]-FUB-272, [18F]-VUF-5182, or [18F]-ST-889 failed due to low brain reuptake (Selivanova et al. 2012; Funke et al. 2013). [18F]-VUF 5000 has been developed to overcome this obstacle and evaluated in vivo but failed to penetrate the brain (Windhorst et al. 1999).
[18F]Fluoroproxyfan and [125I]iodoproxyfan showed very heterogeneous distribution in the brain, indicating non-specific binding (Funke et al. 2013). On the other hand, [125I]iodophenpropit is commonly used as an iodinated PET tracer in vivo and ex vivo (e.g., in male Swiss mice or Wistar rats) showing over 40-fold preference to H3R (Ligneau et al. 1994; Stark et al. 1996; Mochizuki et al. 1996; Jansen et al. 1994; Humbert-Claude et al. 2012; Morisset et al. 2000). Later developed [11C]JNJ-10181457 showed sufficient uptake in the rat brain, but the confirmation on the distribution in H3R−/− mice was failed as a chemical probe. As the most promising candidate for PET studies [11C]GSK189254 stood out. It crosses the BBB, has high uptake, and is metabolically stable. Its binding can be fully blocked with the H3R selective antagonist JNJ-39220675 or by ciproxifan, indicating a high level of specific binding in the baboon. It is so far the best chemical probe for visualizing these receptor subtypes and estimating receptor occupancy (Ashworth et al. 2010; Plisson et al. 2009; Rusjan et al. 2020). [11C]TASP-0410457 as another promising candidate, showed high brain uptake in rats and monkeys has been developed recently (Koga et al. 2016).
Pharmacokinetic properties of the selected H3R ligands are summarized in Table 7.
3.4 Histamine H4 Receptor
Histamine H4R is the most recently discovered histamine receptor, cloned almost 20 years ago. Despite some discussion on its CNS effects, it is confirmed that this receptor subtype is mainly peripherally distributed and involved in immune processes or chemotaxis. Its association with diseases like asthma, allergic rhinitis, pruritus, or inflammation processes, in general, has been confirmed. However, the design and synthesis of highly selective histamine H4R ligand remained to be an up-to-date challenge.
3.4.1 Agonists
Histamine H4R ligands were developed up to a great extent as modification of other histamine receptor subtypes ligands. One of the first ligands examined on H4R was H3R agonist R-α-methylhistamine. This enantiomer shows a 17-fold higher H4R affinity when compared to its S-enantiomer (distomer) and is often used in behavioral studies targeting H4R (Saravanan et al. 2011). 4-Methylhistamine, firstly described as H2R ligand, showed 100-fold selectivity to H4R over other receptor subtypes. ST-1006 is an up-to-date one of the most potent H4R agonist/partial agonist. It was shown to be a partial agonist in hH4R in [35S]-GTP-γS-binding assay, but a full, partial, and inverse agonist for hH4R, mH4R, and rH4R, respectively, in luciferase binding assaies (Adami et al. 2018).
VUF-8430 showed 30-fold selectivity over H3R rats and expressed lasting dose-dependent antinociceptive and anorexiant effect in male Swiss albino mice in doses 20–40 μg (Galeotti et al. 2013). A higher dose (40 μg) demonstrated an anxiolytic effect. VUF-8430 (10–40 μg) reduced neuropathic pain provoked by oxidative stress in male CD1 mice (Sanna et al. 2017). As for other promising derivatives, VUF- 6884 has an over 300-fold affinity to H4R, and VUF 10460 has a 50-fold selectivity to H4R. The latter showed an ulcerogenic effect in male Wistar rats and is used in gastric models (Coruzzi et al. 2011).
3.4.2 Antagonists
JNJ-7777120 identified via high throughput screening by Johnson and Johnson represents the most frequently used H4R ligand in behavioral studies. It showed over a 1,000-fold selectivity for the rat histamine H4R over the histamine H3R with comparable affinities to mouse and human isoform and very low affinities to other off target (Thurmond 2015). JNJ-7777120 reduces asthmatic symptoms in female Balb/c mice (Deml et al. 2009), improves lung function in mice, and reduces wheel reaction in dogs in concentration 1.5 μmol (Roßbach et al. 2009). A dose of 100 mg/kg reduces the macroscopic injury, increases the colonic myeloperoxidase as well as TNF-α levels (Varga et al. 2005), and expresses an inhibitory effect on vestibular neuron activity in Male Wistar rats (Desmadryl et al. 2012). It has been confirmed that JNJ-7777120 blocked histamine-induced chemotaxis in mice, expressed anti-inflammatory effects in the peritonitis model of mice, and ameliorated pruritus in mice (Thurmond et al. 2004; Dunford et al. 2007). However, results are very species-dependent, as it has been shown that JNJ-7777120 is a biased ligand. Besides signalling through Gαi, this ligand activates the β-arrestin 2 signalling cascades (Rosethorne and Charlton 2011). In high concentration, JNJ-7777120 causes ERK activation and can act as a full agonist (Seifert et al. 2011). Even though the obtained data supported antagonistic profile, the reported IC50 differed from another, which is characteristic of a partial agonist rather than an antagonist. Additionally, even if this compound is extensively used as a standard, it has a short half-life in vivo, further interfering with behavioral studies (Neumann et al. 2013). Therefore, special attention should be paid to different JNJ-7777120 effects in different species. JNJ-7777120 is metabolized to the N-des-methylpiperazine derivative, which possesses a comparable affinity to the H4R (pKi value of 7.6) and higher metabolic stability. The metabolization rate is fast in rodents, but slower in humans (Engelhardt et al. 2009).
JNJ 28307474 was another potent H4R antagonist developed by Johnson and Johnson that was efficient in the preclinical arthritis model in BALB/c mice in doses of 20–50 mg/kg (Cowden et al. 2014; Buckland 2013). In the same species, it showed an anti-inflammatory effect by inhibiting TH2-mediated inflammation (Cowden et al. 2010). Auspicious effects were shown in chronic atopic dermatitis model in female wt mice, indicating long-lasting action of this H4R antagonist (Rossbach et al. 2016).
JNJ-10191584 showed promising profiles: a dose-dependent reduction in macroscopic damage in colitis model male Wistar rats and an inhibitory effect on vestibular neuron activity in Wistar or Long-Evans rats (Desmadryl et al. 2012). VUF-10214 and VUF-10148 also expressed anti-inflammatory properties in the carrageenan-induced paw edema model in male Wistar rats (Smits et al. 2008). Another potential antinociceptive agent is INCB-38579 that expressed at least 80-fold selectivity over other receptor subtypes and antinociceptive effects on female CD-1 mice and Sprague-Dawley rats (Shin et al. 2012). Two other potential PET tracers have been developed: [11C]JNJ-77771220 that can cross the BBB and [11C]VUF-1058 that exerts its action only peripherally (Funke et al. 2013).
H4R is extensively researched, which resulted in several drug-like candidates reaching clinical trials. Adriforant has been tested in clinical phase for atopic dermatitis second improved inflammatory skin lesions and UR-633225 has been screened for seasonal allergic rhinitis (NCT01260753) (Werfel et al. 2019).
JNJ-39758979 is another selective H4R antagonist with an over 80-fold selectivity to other receptor subtypes. In ovalbumin-sensitized mice showed a dose-dependent anti-inflammatory effect. Good pharmacokinetic profile and safety in dose range 10–1,200 mg were proved in clinical phase I for asthma (Thurmond et al. 2014). On the other hand, phase II in Japanese patients with atopic dermatitis (100 and 300 mg) was terminated and did not meet the endpoint for safety reasons, as two patients developed neutropenia (Murata et al. 2015). Unfortunately, clinical phase II in asthmatic patients also failed to meet the primary endpoint due to the lack of efficacy (Kollmeier et al. 2018).
Toreforant (JNJ-38518168) was another H4R antagonist that entered into a clinical trial for rheumatoid arthritis, but failed in clinical phase IIb due to the lack of efficacy (Boyle et al. 2019; Thurmond et al. 2016).
SENS-111 or seliforant has a high affinity to both animal and human isoform H4R. It was efficient against nystagmus in Long-Evans rats and Wistar rats (10 mg/kg), whereby increasing doses (up to 20 mg/kg) resulted in the loss of effectivity. These data are consistent with data from a clinical trial (Petremann et al. 2020). SENS-111 does not have sedative effects and is well tolerated. In phase I clinical studies, it did not significantly impact nystagmus, but improved latency 10–30% and vertigo episodes duration (Venail et al. 2018). The studies are currently halted in phase II where efficacy and safety of two dose-regimens 100 and 200 mg has been examined (NCT03110458) (Dyhrfjeld-Johnsen and Attali 2019).
Pharmacokinetic properties of the selected H4R ligands are summarized in Table 8.
4 Conclusion
Chemical probes are a potential novel tool that can provide insight into ligands affinity, selectivity, and receptor functional selectivity. Rational design of chemical probes generate ligands which will selectively target the receptor of interest, resolve signalling cascades and reveal the involvement of various proteins and second messengers. Here, selected histamine receptor ligands for all four receptor subtypes have been summarized and reviewed as chemical probes, focusing on in vitro and in vivo studies. The definition of a chemical probe in in vivo studies is more complex, as in vivo compartments differ enormously from one another. Therefore, a chemical probe needs to be selected explicitly for unique behavioral models. Even though a chemical probe differs from the ligand, already from their design, several highly versatile chemical probes for investigating the effects of histamine receptor subtypes have been developed (Fig. 2). With the description of their advantages and disadvantages as chemical probes, the selection for biochemical and pharmacological studies should be made easier for beginners to experts in this area. Nevertheless, an additional effect of these probes (or their metabolites) on off-targets cannot be ruled out and may be considered to evaluate the experimental results.
Abbreviations
- 2-TEA:
-
2-Thiazoleamine
- 5HT:
-
Serotonin
- AC:
-
Adenylyl cyclase
- ACh:
-
Acetylcholine
- AD:
-
Alzheimer’s disease
- ADHD:
-
Attention deficit hyperactivity disorder
- BBB:
-
Blood-brain barrier
- BRET:
-
Bioluminescence resonance energy transfer
- cAMP:
-
Cyclic adenosine monophosphate
- CNS:
-
Central nervous system
- CREB:
-
cAMP-responsive element-binding protein
- DA:
-
Dopamine
- DAG:
-
Diacetyl glycine
- DAO:
-
Diamine oxidase
- DiO:
-
Diet-induced obese
- EMA:
-
European Medicines Agency
- FCS:
-
Fluorescence correlation spectroscopy
- FDA:
-
Food and Drug Administration
- FRET:
-
Förster resonance energy transfer
- GABA:
-
γ-Aminobutyric acid
- GPCR:
-
G-protein coupled receptor
- GIT:
-
Gastrointestinal tract
- Gly:
-
Glutamate
- HCV:
-
Hepatitis C virus
- HDC:
-
Histidine decarboxylase
- hERG:
-
Ether-a-go-go related gene for voltage-dependent potassium ion channel
- HHV:
-
Human herpes virus
- HNMT:
-
Histamine-N-methyltransferase
- HTMT:
-
Histamine-trifluoromethyl-toluidide
- HR:
-
Histamine receptors (H1-4R)
- PAINS:
-
Pan-assay interference compounds
- IP3:
-
Inositol-1,4,5-trisphosphate
- MAO B:
-
Monoamine oxidase B
- MTCP:
-
Multi-target directed chemicals probes
- MTDL:
-
Multi-target directed ligands
- NA:
-
Norepinephrine
- NO:
-
Nitric oxide
- PD:
-
Parkinson’s disease
- PKA:
-
Protein kinase A
- PKC:
-
Protein kinase C
- PLC:
-
Phospholipase C
- PPI:
-
Proton pump inhibitors
- SARS:
-
Severe acute respiratory syndrome
- SHR:
-
Spontaneously hypertensive rat
- SCM:
-
Scanning confocal microscopy
- VMAT:
-
Vesicular monoamine-transporter
- wt:
-
Wild type
References
Adami M, Micheloni C, Grandi D, Stark H (2018) Differential effects of functionally different histamine H4 receptor ligands on acute irritant dermatitis in mice. Naunyn Schmiedebergs Arch Pharmacol 391:1387–1397
Akdis CA, Blaser K (2003) Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol 112:15–22
Al-Damluji S, Porter D, Krsmanovic LZ, Knutson JR, Kopin IJ (1997) Visual detection of transport-P in peptidergic neurones. Br J Pharmacol 120:876–882
Alewijnse AE et al (1998) Constitutive activity and structural instability of the wild-type human H2 receptor. J Neurochem 71:799–807
Altenbach RJ et al (2008) Structure–activity studies on a series of a 2-aminopyrimidine-containing histamine H4 receptor ligands. J Med Chem 51(20):6571–6580
Andaloussi M et al (2013) A novel series of histamine H4 receptor antagonists based on the pyrido[3,2-d]pyrimidine scaffold: comparison of hERG binding and target residence time with PF-3893787. Bioorg Med Chem Lett 23:2663–2670
Angeli A, Ferraroni M, Supuran CT (2018) Famotidine, an antiulcer agent, strongly inhibits helicobacter pylori and human carbonic anhydrases. ACS Med Chem Lett 9:1035–1038
Arrowsmith CH et al (2015) The promise and peril of chemical probes. Nat Chem Biol 11:536–541
Ashworth S et al (2010) Evaluation of11C-GSK189254 as a novel radioligand for the H 3 receptor in humans using PET. J Nucl Med 51:1021–1029
Auerbach S. DrugMatrix/ToxFX. https://ntp.niehs.nih.gov/data/drugmatrix/. Assessed May 2021
Bahi A, Sadek B, Schwed SJ, Walter M, Stark H (2013) Influence of the novel histamine H3 receptor antagonist ST1283 on voluntary alcohol consumption and ethanol-induced place preference in mice. Psychopharmacology (Berl) 228:85–95
Barbier AJ et al (2004) Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H 3 antagonist. Br J Pharmacol 143:649–661
Bardgett ME, Davis NN, Schultheis PJ, Griffith MS (2011) Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APPTg2576 mouse model of Alzheimer’s disease. Neurobiol Learn Mem 95:64–72
Bartole E et al (2020) UR-DEBa242: a Py-5-Labeled fluorescent multipurpose probe for investigations on the histamine H3 and H4 receptors. J Med Chem 63:5297–5311
Baumeister P et al (2015) [3H]UR-DE257: development of a tritium-labeled squaramide-type selective histamine H2 receptor antagonist. ChemMedChem 10:83–93
Bäumer W et al (2011) Lack of preventing effect of systemically and topically administered histamine H 1 or H 4 receptor antagonists in a dog model of acute atopic dermatitis. Exp Dermatol 20:577–581
Bautista-Aguilera ÓM et al (2017) Multitarget-directed ligands combining cholinesterase and monoamine oxidase inhibition with histamine H3R antagonism for neurodegenerative diseases. Angew Chem Int Ed 56:12765–12769
Bautista-Aguilera ÓM et al (2018) Contilisant, a tetratarget small molecule for Alzheimer’s disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R Agonism profile. J Med Chem 61:6937–6943
Berg KA, Clarke WP (2018) Making sense of pharmacology: inverse agonism and functional selectivity. Int J Neuropsychopharmacol 21:962–977
Bergman J, Spealman RD (1986) Some behavioral effects of histamine H1 antagonists in squirrel monkeys. J Pharmacol Exp Ther 239:104–110
Best CH, Dale HH, Thorpe WV (1927) The nature of the vaso-dilator constituents of certain tissue extracts. J Physiol 62:397–417
Bhowmik M, Saini N, Vohora D (2014) Histamine H3 receptor antagonism by ABT-239 attenuates kainic acid induced excitotoxicity in mice. Brain Res 1581:129–140
Bitner RS, Markosyan S, Nikkel AL, Brioni JD (2011) In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer’s disease. Neuropharmacology 60:460–466
Black JW, Ganellin CR (1974) Naming of substituted histamines. Experientia 30:111–113
Bosma R, Mocking TAM, Leurs R, Vischer HF (2017) Ligand-binding kinetics on histamine receptors. https://doi.org/10.1007/978-1-4939-6843-5_5
Boyle DL et al (2019) Toreforant, an orally active histamine H 4 -receptor antagonist, in patients with active rheumatoid arthritis despite methotrexate: mechanism of action results from a phase 2, multicenter, randomized, double-blind, placebo-controlled synovial biopsy study. Inflamm Res 68:261–274
Buckland J (2013) Experimental arthritis: antihistamines as treatments for autoimmune disease? Nat Rev Rheumatol 9:696
Buckland KF, Williams TJ, Conroy DM (2003) Histamine induces cytoskeletal changes in human eosinophils via the H 4 receptor. Br J Pharmacol 140:1117–1127
Bunnage ME, Chekler ELP, Jones LH (2013) Target validation using chemical probes. Nat Chem Biol 9:195–199
Cao X et al (2018) Synthesis and biological evaluation of fused tricyclic heterocycle Piperazine (Piperidine) derivatives as potential multireceptor atypical antipsychotics. J Med Chem 61:10017–10039
Capelo R et al (2016) Cellular analysis of the histamine H4 receptor in human myeloid cells. Biochem Pharmacol 103:74–84
Cataldi M, Maurer M, Taglialatela M, Church MK (2019) Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin Exp Allergy 49:1615–1623
Clapham J, Kilpatrick GJ (1994) Thioperamide, the selective histamine H3 receptor antagonist, attenuates stimulant-induced locomotor activity in the mouse. Eur J Pharmacol 259:107–114
Corcóstegui R, Labeaga L, Innerárity A, Berisa A, Orjales A (2005) Preclinical pharmacology of bilastine, a new selective histamine H 1 receptor antagonist: receptor selectivity and in vitro antihistaminic activity. Drugs R D 6:371–384
Coruzzi G et al (2011) Selective histamine H 3 and H 4 receptor agonists exert opposite effects against the gastric lesions induced by HCl in the rat stomach. Eur J Pharmacol 669:121–127
Cowden JM, Zhang M, Dunford PJ, Thurmond RL (2010) The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J Invest Dermatol 130:1023–1033
Cowden JM et al (2014) The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann Rheum Dis 73:600–608
Dale HH, Laidlaw P (1910) The physiological actions of b-imidazol ethylamine. J Physiol 41:318–344
Daley-Yates P et al (2012) The efficacy and tolerability of two novel H 1/H 3 receptor antagonists in seasonal allergic rhinitis. Int Arch Allergy Immunol 158:84–98
Day M et al (2007) Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem Pharmacol 73:1123–1134
Deml KF, Beermann S, Neumann D, Strasser A, Seifert R (2009) Interactions of histamine H1-receptor agonists and antagonists with the human histamine H4-receptor. Mol Pharmacol 76:1019–1030
Desmadryl G et al (2012) Histamine H 4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability. Br J Pharmacol 167:905–916
Díaz NF, Flores-Herrera H, Molina-Hernández A (2019) Central histamine, the H3-receptor and obesity therapy. CNS Neurol Disord Drug Targets 18:516–522
Di Carlo G et al (2000) Effect of R-(-)-alpha-methylhistamine and thioperamide on in vivo release of norepinephrine in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 24:275–284
Dunford PJ et al (2007) Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J Allergy Clin Immunol 119:176–183
Dyhrfjeld-Johnsen J, Attali P (2019) Management of peripheral vertigo with antihistamines: new options on the horizon. Br J Clin Pharmacol 85:2255–2263
Eissa N et al (2018) The histamine H3 receptor antagonist DL77 ameliorates MK801-induced memory deficits in rats. Front Neurosci 12:1–11
Eissa N et al (2021) The multi-targeting ligand ST-2223 with histamine H 3 receptor and dopamine D 2/D 3 receptor antagonist properties mitigates autism-like repetitive behaviors and brain oxidative stress in mice. Int J Mol Sci 22:1947
Elz S, Gerhard G, Schunack W (1989) Histamine analogues. 32nd communication: synthesis and pharmacology of sopromidine, a potent and stereoselective isomer of the achiral H2-agonist impromidine. Eur J Med Chem 24:259–262
Elz S et al (2000) Histaprodifens: synthesis, pharmacological in vitro evaluation, and molecular modeling of a new class of highly active and selective histamine H1-receptor agonists. J Med Chem 43:1071–1084
Engelhardt H, Smits RA, Leurs R, Haaksma E, De Esch IJP (2009) A new generation of antihistamines: histamine H4 receptor antagonists on their way to the clinic. Curr Opin Drug Discov Devel 12:628–643
Ennis M, Tiligada K (2021) Histamine receptors and COVID-19. Inflamm Res 70:67–75
Esbenshade TA et al (2012) Pharmacological properties and procognitive effects of ABT-288, a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 343:233–245
Faghih R et al (2002) Structure-activity relationships of non-imidazole H3 receptor ligands. Part 2: binding preference for D-amino acids motifs. Bioorg Med Chem Lett 12:2035–2037
Fox GB et al (2002) Effects of histamine H3 receptor ligands GT-2331 and ciproxifan in a repeated acquisition avoidance response in the spontaneously hypertensive rat pup. Behav Brain Res 131:151–161
Fox GB et al (2003) Two novel and selective nonimidazole H3 receptor antagonists A-304121 and A-317920: II. In vivo behavioral and neurophysiological characterization. J Pharmacol Exp Ther 305:897–908
Fox GB et al (2005) Pharmacological properties of ABT-239 [4-(2-{2-[(2R)-2-methylpyrrolidinyl] ethyl}-benzofuran-5-yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 r. J Pharmacol Exp Ther 313:176–190
Freedberg DE et al (2020) Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology 159:1129–1131.e3
Frye SV (2010) The art of the chemical probe. Nat Chem Biol 6:159–161
Funke U et al (2013) 11C-labeled and 18F-labeled PET ligands for subtype-specific imaging of histamine receptors in the brain. J Label Compd Radiopharm 56:120–129
Galeotti N, Sanna MD, Ghelardini C (2013) Pleiotropic effect of histamine H4 receptor modulation in the central nervous system. Neuropharmacology 71:141–147
Galici R et al (2011) JNJ-39220675, a novel selective histamine H3 receptor antagonist, reduces the abuse-related effects of alcohol in rats. Psychopharmacology (Berl) 214:829–841
Garbarg M, Arrang J-M, Rouleau A, Ligneau X, Dam Trung Tuong M, Schwartz JC, Ganellin CR (1992) S-[2-(4-Imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agonist. J Pharmacol Exp Ther 263:304–310
Gbahou F, Rouleau A, Arrang J-M (2012) The histamine autoreceptor is a short isoform of the H3 receptor. Br J Pharmacol 166:1860–1871
Ghamari N et al (2019) Histamine H3 receptor antagonists/inverse agonists: where do they go? Pharmacol Ther 200:69–84
Grätz L et al (2020) NanoBRET binding assay for histamine H2 receptor ligands using live recombinant HEK293T cells. Sci Rep 10:1–10
Gschwandtner M, Stark H, Gutzmer R (2013) Profiling of histamine H 4 receptor agonists in native human monocytes. Br J Pharmacol 170:136–143
Guo RX et al (2009) Differential effects of acute and repeat dosing with the H 3 antagonist GSK189254 on the sleep-wake cycle and narcoleptic episodes in Ox−/− mice. Br J Pharmacol 157:104–117
Gutzmer R et al (2009) The histamine H4 receptor is functionally expressed on TH2 cells. J Allergy Clin Immunol 123:619–625
Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88:1183–1241
Hair P, Scot L (2006) Levocetirizine. Drugs 66
Hancock AA et al (2004) Antiobesity effects of A-331440, a novel non-imidazole histamine H 3 receptor antagonist. Eur J Pharmacol 487:183–197
Hancock AA et al (2005) Antiobesity evaluation of histamine H3 receptor (H3R) antagonist analogs of A-331440 with improved safety and efficacy. Inflamm Res 54:45–47
Hartwig C et al (2015) The histamine H 4 -receptor (H 4 R) regulates eosinophilic inflammation in ovalbumin-induced experimental allergic asthma in mice. Eur J Immunol 45:1129–1140
Hirschfeld J et al (1992) Iodoaminopotentidine and related compounds: a new class of ligands with high affinity and selectivity for the histamine H2 receptor. J Med Chem 35:2231–2238
Holgate ST et al (2003) Consensus group on new-generation antihistamines (CONGA): present status and recommendations. Clin Exp Allergy 33:1305–1324
Hough LB et al (1988) Actions of the brain-penetrating H2-antagonist zolantidine on histamine dynamics and metabolism in rat brain. Biochem Pharmacol 37:4707–4711
Hu W, Chen Z (2017) The roles of histamine and its receptor ligands in central nervous system disorders: an update. Pharmacol Ther 175:116–132
Humbert-Claude M, Davenas E, Gbahou F, Vincent L, Arrang JM (2012) Involvement of histamine receptors in the atypical antipsychotic profile of clozapine: a reassessment in vitro and in vivo. Psychopharmacology (Berl) 220:225–241
Igel P, Dove S, Buschauer A (2010) Histamine H4 receptor agonists. Bioorg Med Chem Lett 20:7191–7199
Ishiwata K, Kawamura K, Yanai K, Harry Hendrikse N (2007) In vivo evaluation of P-glycoprotein modulation of 8 PET radioligands used clinically. J Nucl Med 48:81–87
Ishola AA et al (2021) Molecular basis for the repurposing of histamine H2-receptor antagonist to treat COVID-19. J Biomol Struct Dyn:1–18
Istyastono E et al (2011) Molecular determinants of ligand binding modes in the histamine H4 receptor: linking ligand-based 3D-QSAR models to in silico guided receptor mutagenesis studies. J Med Chem 54:8136–8147
Jablonowski JA et al (2003) The first potent and selective non-imidazole human histamine H4 receptor antagonists. J Med Chem 46:3957–3960
Jansen FP et al (1994) Characterization of the binding of the first selective radiolabeled histamine H3-receptor antagonist, [125I]-iodophenpropit, to rat brain. Br J Pharmacol 113:355–362
Jansen FP, Mochizuki T, Yamamoto Y, Timmerman H, Yamatodani A (1998) In vivo modulation of rat hypothalamic histamine release by the histamine H3 receptor ligands, immepip and clobenpropit. Effects of intrahypothalamic and peripheral application. Eur J Pharmacol 362:149–155
Jáuregui I et al (2016) Bilastine: a new antihistamine with an optimal benefit-to-risk ratio for safety during driving. Expert Opin Drug Saf 15:89–98
Jiang W et al (2008) Cloning and pharmacological characterization of the dog histamine H4 receptor. Eur J Pharmacol 592:26–32
Jochem J, Mitera A, Izydorczyk M, Nowak D, Waliczek M (2016) Cardiovascular effects of histamine H3 receptor antagonist JNJ 5207852 in haemorrhagic shock in rats. Ann Acad Medicae Silesiensis 70:89–94
Jongejan A et al (2005) Linking agonist binding to histamine H1 receptor activation. Nat Chem Biol 1:98–103
Jutel M, Akdis M, Akdis CA (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39:1786–1800
Kaar SJ, Natesan S, Howes OD (2019) Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology:107704
Kallal L, Benovic JL (2000) Using green fluorescent proteins to study G-protein-coupled receptor localization and trafficking. Trends Pharmacol Sci 21:175–180
Khanfar MA et al (2016) Multiple targeting approaches on histamine H3 receptor antagonists. Front Neurosci 10:1–17
Kitbunnadaj R et al (2005) N-substituted piperidinyl alkyl imidazoles: discovery of methimepip as a potent and selective histamine H3 receptor agonist. J Med Chem 48:2100–2107
Koga K et al (2016) Development of TASP0410457 (TASP457), a novel dihydroquinolinone derivative as a PET radioligand for central histamine H3 receptors. EJNMMI Res 6:1–14
Kollmeier AP et al (2018) Phase 2a, randomized, double-blind, placebo-controlled, multicentre, parallel-group study of an H4R-antagonist (JNJ-39758979) in adults with uncontrolled asthma. Clin Exp Allergy 48:957–969
Komater VA et al (2003) H3 receptor blockade by thioperamide enhances cognition in rats without inducing locomotor sensitization. Psychopharmacology (Berl) 167(4):363–372. https://doi.org/10.1007/s00213-003-1431-0
Kraus A et al (2009) NG-acylated aminothiazolylpropylguanidines as potent and selective histamine H2 receptor agonists. ChemMedChem 4:232–240
Kuder K, Kieć-Kononowicz K (2014) Fluorescent GPCR ligands as new tools in pharmacology-update, years 2008- early 2014. Curr Med Chem 21:3962–3975
Kuhne S, Wijtmans M, Lim HD, Leurs R, De Esch IJP (2011) Several down, a few to go: histamine H3 receptor ligands making the final push towards the market? Expert Opin Investig Drugs 20:1629–1648
Lamb Y, Pitolisant N (2020) A review in narcolepsy with or without cataplexy. CNS Drugs 34:207–218
Lapilla M et al (2011) Histamine regulates autoreactive T cell activation and adhesiveness in inflamed brain microcirculation. J Leukoc Biol 89:259–267
Latorraca NR, Venkatakrishnan AJ, Dror RO (2017) GPCR dynamics: structures in motion. ACS Chem Rev 117:139–155
Lauritsen K, Laursen LS, Rask-Madsen J (1990) Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases (part I). Clin Pharmacokinet 19:11–31
Łażewska D, Kieć-Kononowicz K (2018) Progress in the development of histamine H3 receptor antagonists/inverse agonists: a patent review (2013-2017). Expert Opin Ther Pat 28:175–196
Łazewska D et al (2009) Diether derivatives of homo- or substituted piperidines as non-imidazole histamine H3 receptor ligands. Bioorg Med Chem 17:3037–3042
Lecklin A, Etu-Seppälä P, Stark H, Tuomisto L (1998) Effects of intracerebroventricularly infused histamine and selective H1, H2 and H3 agonists on food and water intake and urine flow in Wistar rats. Brain Res 793:279–288
Leurs R, Church MK, Taglialatela M (2002) H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy 32:489–498
Ligneau X, Garbarg M, Vizuete ML, Diaz J, Purand K, Stark H, Schunack W, Schwartz JC (1994) [1251]Iodoproxyfan, a new antagonist to label and visualize cerebral histamine H3 receptors. J Pharmacol Exp Ther 271(1):452–459
Ligneau X et al (1998) Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. J Pharmacol Exp Ther 287:658–666
Ligneau X et al (2007) BF2.649 [1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: preclinical pharmacology. J Pharmacol Exp Ther 320:365–375
Lim HD et al (2005) Evaluation of histamine H1-, H2-, and H 3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H 4 receptor agonist. J Pharmacol Exp Ther 314:1310–1321
Lin JH (1991) Pharmacokinetic and Pharmacodynamic properties of histamine H2-receptor antagonists: relationship between intrinsic potency and effective plasma concentrations. Clin Pharmacokinet 20:218–236
Lindsay BH, Nalwalk JW, Battles AH (1990) Zolantidine-induced attenuation of morphine antinociception in rhesus monkeys. Brain Res 526:153–155
Liu H et al (2008) Cis-4-(piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h] quinazolin-2-amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia. J Med Chem 51:7094–7098
Logan J et al (2012) Blockade of the brain histamine h3 receptor by jnj-39220675: preclinical pet studies with [11C]gsk189254 in anesthetized baboon. Psychopharmacology (Berl) 223:447–455
Ma P, Zemmel R (2002) Value of novelty? Nat Rev Drug Discov 1:571–572
Mackenzie JF, Daly CJ, Pediani JD, McGrath JC (2000) Quantitative imaging in live human cells reveals intracellular α1- adrenoceptor ligand-binding sites. J Pharmacol Exp Ther 294:434–443
Malmberg-Aiello P, Ipponi A, Bartolini A, Schunack W (2000) Antiamnesic effect of metoprine and of selective histamine H1 receptor agonists in a modified mouse passive avoidance test. Neurosci Lett 288:1–4
Mansbach R (2008) In vitro pharmacological profile of docepin, a sleep-promoting histamine H1 antagonist. Eur Neuropsychopharmacol 18:357–358
Martinez-Mir MI et al (1990) Three histamine receptors (H1, H2 and H3) visualized in the brain of human and non-human primates. Brain Res 526:322–327
McGaraughty S, Chu KL, Cowart MD, Brioni JD (2012) Antagonism of supraspinal histamine H3 receptors modulates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther 343:13–20
McGrath JC, Arribas S, Daly CJ (1996) Fluorescent ligands for the study of receptors. Trends Pharmacol Sci 17:393–399
Medhurst AD et al (2007) GSK189254, a novel H3 receptor antagonist that binds to histamine H3 receptors in Alzheimer’s disease brain and improves cognitive performance in preclinical models. J Pharmacol Exp Ther 321:1032–1045
Michalet X, Weiss S, Jäger M (2006) Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem Rev 106:1785–1813
Miyata I, Saegusa H, Sakurai M (2011) Seizure-modifying potential of histamine H 1 antagonists: a clinical observation. Pediatr Int 53:706–708
Mochizuki T et al (1996) Brain penetration of the histamine H3 receptor antagonists thioperamide and clobenpropit in rat and mouse, determined with ex vivo [125I]iodophenpropit binding. Brain Res 743:178–183
Monti JM, Orellana C, Boussard M, Jantos H, Olivera S (1990) Sleep variables are unaltered by zolantidine in rats: are histamine H2-receptors not involved in sleep regulation? Brain Res Bull 25:229–231
Morisset S, Traiffort E, Arrang JM, Schwartz JC (2000) Changes in histamine H3 receptor responsiveness in mouse brain. J Neurochem 74:339–346
Mowbray CE et al (2011) Challenges of drug discovery in novel target space. The discovery and evaluation of PF-3893787: a novel histamine H4 receptor antagonist. Bioorg Med Chem Lett 21:6596–6602
Murata Y et al (2015) Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J Dermatol 42:129–139
Neumann D et al (2013) The dual H3/4R antagonist thioperamide does not fully mimic the effects of the ‘standard’ H4R antagonist JNJ 7777120 in experimental murine asthma. Naunyn Schmiedebergs Arch Pharmacol 386:983–990
Neumann D, Schneider EH, Seifert R (2014) Analysis of histamine receptor knockout mice in models of inflammation. J Pharmacol Exp Ther 348:2–11
Nieto-Alamilla G, Marquez-Gomez R, Arias-Montano J-A (2016) The histamine H3 receptor: structure, pharmacology, and function. Mol Pharmacol 90:649–673
Nirogi R et al (2019) Discovery and development of N-[4-(1-Cyclobutylpiperidin-4-yloxy)phenyl]-2-(morpholin-4-yl)acetamide Dihydrochloride (SUVN-G3031): a novel, potent, selective, and orally active histamine H 3 receptor inverse agonist with robust wake-promoting activity. J Med Chem 62:1203–1217
Nirogi R et al (2021) Samelisant (SUVN-G3031), a potent, selective and orally active histamine H3 receptor inverse agonist for the potential treatment of narcolepsy: pharmacological and neurochemical characterisation. Psychopharmacology (Berl) 1
Nuutinen S et al (2016) Histamine H3 receptor antagonist decreases cue-induced alcohol reinstatement in mice. Neuropharmacology 106:156–163
Obara I et al (2019) Histamine, histamine receptors, and neuropathic pain relief. Br J Pharmacol 177:580–599
Ogasawara M et al (2006) Recent advances in molecular pharmacology of the histamine systems: organic cation transporters as a histamine transporter and histamine metabolism. J Pharmacol Sci 101:24–30
Panula P (2020) Histamine, histamine H3 receptor, and alcohol use disorder. Br J Pharmacol 177:634–641
Panula P et al (2015) International union of basic and clinical pharmacology. XCVIII histamine receptors. Pharmacol Rev 67:601–655
Parsons ME, Ganellin CR (2006) Histamine and its receptors. Br J Pharmacol 147:127–135
Petremann M, Gueguen C, Delgado Betancourt V, Wersinger E, Dyhrfjeld-Johnsen J (2020) Effect of the novel histamine H4 receptor antagonist SENS-111 on spontaneous nystagmus in a rat model of acute unilateral vestibular loss. Br J Pharmacol 177:623–633
Plisson C et al (2009) 11C-GSK189254: a selective radioligand for in vivo central nervous system imaging of histamine H3 receptors by PET. J Nucl Med 50:2064–2072
Proschak E, Stark H, Merk D (2019) Polypharmacology by design: a medicinal Chemist’s perspective on multitargeting compounds. J Med Chem 62:420–444
Provensi G, Costa A, Passani MB, Blandina P (2016) Donepezil, an acetylcholine esterase inhibitor, and ABT-239, a histamine H3 receptor antagonist/inverse agonist, require the integrity of brain histamine system to exert biochemical and procognitive effects in the mouse. Neuropharmacology 109:139–147
Ramírez FJ, Tuñón I, Collado JA, Silla E (2003) Structural and vibrational study of the tautomerism of histamine free-base in solution. J Am Chem Soc 125:2328–2340
Reiner D, Stark H (2019) Ligand binding kinetics at histamine H3 receptors by fluorescence-polarization with real-time monitoring. Eur J Pharmacol 848:112–120
Reiner D et al (2020) Epigenetics meets GPCR: inhibition of histone H3 methyltransferase (G9a) and histamine H3 receptor for Prader–Willi syndrome. Sci Rep 10:1–8
Reznikov LR et al (2021) Identification of antiviral antihistamines for COVID-19 repurposing. Biochem Biophys Res Commun 538:173–179
Riddy DM et al (2019) Drug-receptor kinetics and sigma-1 receptor affinity differentiate clinically evaluated histamine H 3 receptor antagonists. Neuropharmacology 144:244–255
Romigi A, Vitrani G, Franco V (2018) Profile of pitolisant in the management of narcolepsy: design, development, and place in therapy. Drug Des Devel Ther 12:2665–2675
Rose RH, Briddon SJ, Hill SJ (2012) A novel fluorescent histamine H 1 receptor antagonist demonstrates the advantage of using fluorescence correlation spectroscopy to study the binding of lipophilic ligands. Br J Pharmacol 165:1789–1800
Rosethorne EM, Charlton SJ (2011) Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol Pharmacol 79:749–757
Roßbach K et al (2009) The histamine H4 receptor as a new target for treatment of canine inflammatory skin diseases. Vet Dermatol 20:555–561
Rossbach K et al (2016) Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy Eur J Allergy Clin Immunol 71:189–197
Rouleau A et al (1997) Bioavailability, antinociceptive and antiinflammatory properties of BP 2-94, a histamine H 3 receptor agonist prodrug. J Pharmacol Exp Ther 281:1085–1094
Rouleau A, Stark H, Schunack W, Schwartz JC (2000) Anti-inflammatory and antinociceptive properties of BP 2-94, a histamine H3-receptor agonist prodrug. J Pharmacol Exp Ther 295
Rusjan P et al (2020) Exploring occupancy of the histamine H3 receptor by pitolisant in humans using PET. Br J Pharmacol 177:3464–3472
Sadek B, Stark H (2016) Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology 106:56–73
Sadek B et al (2014) Non-imidazole histamine H3 receptor ligands incorporating antiepileptic moieties. Eur J Med Chem 77:269–279
Sadek B et al (2016a) Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res 312:415–430
Sadek B et al (2016b) Anticonvulsant and procognitive properties of the non-imidazole histamine H3 receptor antagonist DL77 in male adult rats. Neuropharmacology 106:46–55
Sander K, Kottke T, Stark H (2008) Histamine H3 receptor antagonists go to clinics. Biol Pharm Bull 31:2163–2181
Sander K et al (2009) 2,4-Diaminopyrimidines as histamine H4 receptor ligands-scaffold optimization and pharmacological characterization. Bioorg Med Chem 17:7186–7196
Sanna MD et al (2017) Histamine H4 receptor agonist-induced relief from painful peripheral neuropathy is mediated by inhibition of spinal neuroinflammation and oxidative stress. Br J Pharmacol 174:28–40
Saravanan C, Bharti SK, Jaggi S, Singh SK (2011) Histamine H4 receptor: a novel target for inflammation therapy. Mini-Reviews Med Chem 11:143–158
Sato H et al (2013) Histamine H1 receptor occupancy by the new-generation antidepressants fluvoxamine and mirtazapine: a positron emission tomography study in healthy volunteers. Psychopharmacology (Berl) 230:227–234
Schneider EH, Seifert R (2016) The histamine H4-receptor and the central and peripheral nervous system: a critical analysis of the literature. Neuropharmacology 106:116–128
Schneider EH, Neumann D, Seifert R (2014) Modulation of behavior by the histaminergic system: lessons from H1R-and H2R-deficient mice. Neurosci Biobehav Rev 42:252–266
Schnell D et al (2011) Expression and functional properties of canine, rat, and murine histamine H4 receptors in Sf9 insect cells. Naunyn Schmiedebergs Arch Pharmacol 383:457–470
Schumacher S, Kietzmann M, Stark H, Bäumer W (2014) Unique immunomodulatory effects of azelastine on dendritic cells in vitro. Naunyn Schmiedebergs Arch Pharmacol 387:1091–1099
Schwartz JC (2011) The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol 163:713–721
Seifert R et al (2003) Multiple differences in agonist and antagonist pharmacology between human and guinea pig histamine H1-receptor. J Pharmacol Exp Ther 305:1104–1115
Seifert R, Schneider EH, Dove S, Brunskole I, Neumann D, Strasser A, Buschauer A (2011) Paradoxical stimulatory effects of the “Standard” histamine H4-receptor antagonist JNJ7777120: the H4 receptor joins the club of 7 transmembrane domain receptors exhibiting functional selectivity. Naunyn Schmiedebergs Arch Pharmacol 383:457–470
Selivanova SV et al (2012) Radiofluorinated histamine H 3 receptor antagonist as a potential probe for in vivo PET imaging: Radiosynthesis and pharmacological evaluation. Bioorg Med Chem 20:2889–2896
Shahid M et al (2009) Histamine, histamine receptors, and their role in immunomodulation: an updated systematic review. Open Immunol J 2:9–41
Shi Y et al (2012) Identification and characterization of ZEL-H16 as a novel agonist of the histamine H3 receptor. PLoS One 7
Shimamura T et al (2011) Structure of the human histamine H 1 receptor complex with doxepin. Nature 475:65–72
Shin N et al (2012) INCB38579, a novel and potent histamine H 4 receptor small molecule antagonist with anti-inflammatory pain and anti-pruritic functions. Eur J Pharmacol 675:47–56
Simons FER, Simons KJ (2011) Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol 128
Slack RJ et al (2011) Pharmacological characterization of GSK1004723, a novel, long-acting antagonist at histamine H 1 and H 3 receptors. Br J Pharmacol 164:1627–1641
Slamet Soetanto T et al (2019) Histamine uptake mediated by plasma membrane monoamine transporter and organic cation transporters in rat mast cell lines. Eur J Pharmacol 849:75–83
Smits RA et al (2008) Fragment based design of new H4 receptor-ligands with anti-inflammatory properties in vivo. J Med Chem 51:2457–2467
Stark H (2020) The chemical probe–scopes, limitations and challenges. Expert Opin Drug Discovery 15:1365–1367
Stark H et al (1996) [125I]lodoproxyfan and related compounds: a reversible radioligand and novel classes of antagonists with high affinity and selectivity for the histamine H3 receptor. J Med Chem 39:1220–1226
Sugimoto Y, Umakoshi K, Nojiri N, Kamei C (1998) Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol 351:1–5
Syed YY (2016) Pitolisant: first global approval. Drugs 76:1313–1318
Szczepańska K et al (2018) Synthesis and biological activity of novel tert-butyl and tert-pentylphenoxyalkyl piperazine derivatives as histamine H3R ligands. Eur J Med Chem 152:223–234
Taha AS et al (1996) Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med 334:1435–1439
Tashiro M et al (2002) Roles of histamine in regulation of arousal and cognition: functional neuroimaging of histamine H1 receptors in human brain. Life Sci 72:409–414
Thurmond RL (2015) The histamine H4 receptor: from orphan to the clinic. Front Pharmacol 6:1–11
Thurmond RL et al (2004) A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther 309:404–413
Thurmond RL et al (2014) Clinical and preclinical characterization of the histamine H4 receptor antagonist JNJ-39758979s. J Pharmacol Exp Ther 349:176–184
Thurmond RL et al (2016) Toreforant, a histamine H4 receptor antagonist, in patients with active rheumatoid arthritis despite methotrexate therapy: results of 2 phase II studies. J Rheumatol 43:1637–1642
Tichenor MS, Thurmond RL, Venable JD, Savall BM (2015) Functional profiling of 2-aminopyrimidine histamine H4 receptor modulators. J Med Chem 58:7119–7127
Tiligada E, Ennis M (2020) Histamine pharmacology: from Sir Henry Dale to the 21st century. Br J Pharmacol 177:469–489
Tiligada E, Zampeli E, Sander K, Stark H (2009) Histamine H3 and H4 receptors as novel drug targets. Expert Opin Investig Drugs 18:1519–1531
Tomasch M, Schwed JS, Stark H (2013) Bodilisant-a novel fluorescent, highly affine histamine h3 receptor ligand. ACS Med Chem Lett 4:269–273
Tripathi T et al (2010) In vivo comparative immunotoxic study of histamine receptors (H1R, H2R, H3R and H4R)-agonist. East J Med 15:48–56
Uguen M et al (2013) Preclinical evaluation of the abuse potential of Pitolisant, a histamine H3 receptor inverse agonist/antagonist compared with Modafinil. Br J Pharmacol 169:632–644
Van Der Goot H, Timmerman H (2000) Selective ligands as tools to study histamine receptors. Eur J Med Chem 35:5–20
Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, Savage DD (2010) Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. J Pharmacol Exp Ther 334:191–198
Varga C et al (2005) Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol 522:130–138
Venable J, Thurmond R (2012) Development and chemistry of histamine H4 receptor ligands as potential modulators of inflammatory and allergic responses. Antiinflamm Antiallergy Agents Med Chem 5:307–322
Venail F et al (2018) Safety, tolerability, pharmacokinetics and pharmacokinetic-pharmacodynamic modelling of the novel H4 receptor inhibitor SENS-111 using a modified caloric test in healthy subjects. Br J Clin Pharmacol 84:2836–2848
Venkatachalam K, Eissa N et al (2021) The histamine H3R and dopamine D2R/D3R antagonist ST-713 ameliorates autism-like behavioral features in BTBR T + tf/J mice by multiple actions. Biomed Pharmacother 138
Wagner E, Wittmann HJ, Elz S, Strasser A (2011) Mepyramine-JNJ7777120-hybrid compounds show high affinity to hH 1R, but low affinity to hH4R. Bioorg Med Chem Lett 21:6274–6280
Walter M et al (2010) Azole derivatives as histamine H3 receptor antagonists, part I: thiazol-2-yl ethers. Bioorg Med Chem Lett 20:5879–5882
Wang J et al (2021) Pitolisant versus placebo for excessive daytime sleepiness in narcolepsy and obstructive sleep apnea: a meta-analysis from randomized controlled trials. Pharmacol Res:105522. https://doi.org/10.1016/j.phrs.2021.105522
Werfel T et al (2016) Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 138:336–349
Werfel T et al (2019) Efficacy and safety of the histamine H 4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol 143
Windhorst AD et al (1999) Evaluation of [18F]VUF 5000 as a potential PET ligand for brain imaging of the histamine H3 receptor. Bioorg Med Chem 7:1761–1767
Wyllie JH, Hesselbo T, Black JW (1972) Effects in man of histamine H2-receptor blockade by burimamide. Lancet:1117–1120
Xie SX, Ghorai P, Ye QZ, Buschauer A, Seifert R (2006) Probing ligand-specific histamine H1- and H2-receptor conformations with NG-acylated imidazolylpropylguanidines. J Pharmacol Exp Ther 317:139–146
Yanai K et al (1992a) Mapping of histamine H1 receptors in the human brain using [11C]Pyrilamine and positron emission tomography. J Neurochem 59:128–136
Yanai K et al (1992b) Histamine H1 receptors in human brain visualized in vivo by [11C]doxepin and positron emission tomography. Neurosci Lett 137:145–148
Yanai K et al (1995) Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br J Pharmacol 116:1649–1655
Yanai K et al (2011) Positron emission tomography evaluation of sedative properties of antihistamines. Expert Opin Drug Saf 10:613–622
Yoshimoto R et al (2006) Therapeutic potential of histamine H3 receptor agonist for the treatment of obesity and diabetes mellitus. Proc Natl Acad Sci U S A 103:13866–13871
Zhang YF, Chen XY, Zhong DF, Dong YM (2003) Pharmacokinetics of loratadine and its active metabolite descarboethoxyloratadine in healthy Chinese subjects. Acta Pharmacol Sin 24:715–718. 727
Zhao C et al (2008) The alkaloid conessine and analogues as potent histamine H3 receptor antagonists. J Med Chem 51:5423–5430
Zhou Y, Offord S, Dogan AS, Crabb AH, Wong DF (2002) Evaluation of antihistamine drugs by human [11C]doxepin dynamic PET studies with a parametric image approach. IEEE Nucl Sci Symp Med Imaging Conf 2:1085–1091
Zhou Y et al (2018) Exploring halogen bonds in 5-hydroxytryptamine 2B receptor-ligand interactions. ACS Med Chem Lett 9:1019–1024
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Falkenstein, M., Elek, M., Stark, H. (2021). Chemical Probes for Histamine Receptor Subtypes. In: Yanai, K., Passani, M.B. (eds) The Functional Roles of Histamine Receptors. Current Topics in Behavioral Neurosciences, vol 59. Springer, Cham. https://doi.org/10.1007/7854_2021_254
Download citation
DOI: https://doi.org/10.1007/7854_2021_254
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16996-0
Online ISBN: 978-3-031-16997-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)