Abstract
The biological bases of bipolar disorder include aspects related, among others, to neurohormonal pathways, neurotransmission, signal transduction, regulation of gene expression, oxidative stress, neuroplasticity, and changes in the immune system. There is still a gap in understanding its complex neurobiology and, consequently, developing new treatments. Multiple factors probably interact in this complex equation of pathophysiology of bipolar disorder, such as genetic, biochemical, psychosocial, and environmental stress events, correlating with the development and severity of the bipolar disorder. These mechanisms can interact to exacerbate inflammation, impair neurogenesis, and increase oxidative stress damage, cellular mitochondrial dysfunction, changes in neurotrophins and in epigenetic mechanisms, neuroendocrine dysfunction, activation of neuronal death pathways, and dysfunction in neurotransmission systems. In this review, we explore the up-to-date knowledge of the neurobiological underpinnings of bipolar disorders. The difficulty in developing new drugs for bipolar disorder is very much associated with the lack of knowledge about the precise pathophysiology of this disorder. Pharmacological treatment for bipolar patients is vital; to progress to effective medications, it is essential to understand the neurobiology in bipolar patients better and identify novel therapeutic targets.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Although current therapeutic agents for bipolar disorder are valuable, long-term response for most of the bipolar patients remains low (Gitlin et al. 1995). In general, treatment for bipolar disorder, can be challenging to manage, as treatments that act on manic/hypomanic episodes can lead to a depressive episode and the use of antidepressants can cause manic or hypomanic mood episodes (Geddes and Miklowitz 2013). Lithium is considered the gold standard drug for all phases of bipolar disorder (López-Muñoz et al. 2018).
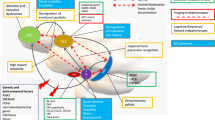
Lithium, so far, is the only one with benefit in both poles and efficacy also for suicidal behavior (Geddes and Miklowitz 2013). Therefore, the investigation of potential new therapeutic goals is a priority. In this review, we explore the up-to-date knowledge of the neurobiology bases of bipolar disorders and the neurobiological mechanisms of dysfunction in a wide range of pathways, including: glutamatergic dysfunction, oxidative stress, mitochondrial dysfunction, impaired neurogenesis, increased inflammation, and apoptosis. The difficulty in the development of new drugs for bipolar patients is associated with a lack of knowledge about the precise pathophysiology of this disorder (Lund et al. 2012). Investigating these new pathophysiology systems purportedly related to the complexity of the neuronal systems in bipolar patients could improve our knowledge of the complex mechanisms related to the action of psychotropic drugs, as well as the neurobiology in bipolar patients (Fig. 1).
The diagram provides a useful model for understanding the factors that contribute to planning clinical management. Therefore, a broad range of treatments is usually needed to treat bipolar disorders satisfactorily, adapted from Malhi et al. (2015). Illustrations are courtesy of Romayne Gadelrab
2 Hypothalamic-Pituitary-Adrenal Axis (HPA)
It is believed that disturbance of the hormonal stress system (the hypothalamic-pituitary-adrenal axis) can cause cognitive problems and worsen depressive symptoms in bipolar disorder (Watson et al. 2004) and can predict an inadequate clinical response to pharmacological treatments (Juruena et al. 2009a). One example of this is suggested in animal models, where a dysregulation in the HPA axis has an impact on serotonergic medications (Gartside et al. 2003).
The end product of the HPA axis is cortisol, which mediates its effects via two main receptors, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) (Juruena 2014). MR receptors modulate hormone release and complex behavior, such as emotion, memory, and sleep. In humans, the role of MR and GR receptors in the neurobiology of severe mental disorders has not been sufficiently characterized. However, recent research findings raise possibilities for new pharmacotherapies via modulation of the function of these receptors to normalize effects of cortisol and restore HPA axis balance (Geddes and Miklowitz 2013).
2.1 Treatments Targeting the HPA Axis
The effects of exogenous steroids on mood, including the potential to both elevate and lower mood, and to exacerbate mood disorders, are widely known. From a therapeutic standpoint, previous studies have shown that spironolactone, a mineralocorticoid receptor antagonist, can have antidepressant effects and can also maintain or improve cognition and memory (Murck et al. 2012). Hendler (1978) first described the therapeutic effects of spironolactone for prophylaxis in a case series of bipolar patients: after replacing lithium with spironolactone, five of the six patients were well maintained for at least 1 year (Hendler 1978). Some studies have demonstrated the therapeutic impact of spironolactone on affective symptoms in women with premenstrual syndrome (O’Brien et al. 1979; Wang et al. 1995) and eating disorders (Wernze 2000) and bipolar patients without remission, decreasing the sensitivity to stressful life events (Juruena et al. 2009).
Mifepristone is an antagonist of the glucocorticoid receptor and preliminary evidence suggested potential cognitive enhancing properties (Young et al. 2004). A larger RCT of adjunctive mifepristone failed to demonstrate a significant improvement in depressive symptoms in bipolar depression, although mifepristone was associated with improvement in cognitive function – and specifically spatial working memory (Young et al. 2004; Watson et al. 2012).
Ketoconazole and metyrapone, inhibitors of glucocorticoid synthesis, have also been investigated in bipolar disorder. In a trial with a very small sample in bipolar depression, with a treatment-resistant history, ketoconazole led to significant improvement in the severity of depression, but not mania (Brown et al. 2001). However, no RCTs have been performed.
A Cochrane review summarized the findings from antiglucocorticoid treatments for mood disorders (Gallagher et al. 2008). This comprised nine studies (three in psychotic major depression, five in non-psychotic major depression, and one in patients with bipolar depression); the authors described a significant reduction in the severity of depressive symptoms with antiglucocorticoid treatments.
3 Mitochondrial Dysfunction and Oxidative Stress in the Neurobiology of Bipolar
There is increasing data in the literature describing oxidative stress in the neurobiology of bipolar disorder. Corroborating the involvement of mitochondrial dysfunction in the pathophysiology of bipolar disorders, a study demonstrated that bipolar patients have an imbalance between the processes of mitochondrial fusion and fission (Scaini et al. 2017) observed by increased protein levels of fission protein (Fis-1) and decreased levels of fusion proteins (Mfn-2 and Opa-1), suggesting that the process of mitochondrial dynamics in patients with bipolar disorder is dysfunctional, which may increase mitochondrial fragmentation (Scaini et al. 2017). In addition, the same study indicated that the changes observed peripherally were directly correlated with functional decline in bipolar patients (Scaini et al. 2017). Effects of lithium in redox systems may have this effect in psychopathological disorders (Khairova et al. 2012).
3.1 Oxidative Stress and Pharmacological Approaches
Several clinical and preclinical studies have suggested the involvement of oxidative stress in bipolar disorders (Frey et al. 2006). Mitochondrial dysfunction has been described as the main triggering agent of this system with a consequent impairment in cellular energy metabolism (Berk et al. 2011b). An abnormal cellular energy state can lead to loss of neuronal function and plasticity and cognitive and behavioral changes characteristic of bipolar disorders (Kato 2007).
Several studies have found elevated levels of malondialdehyde (MDA) and carbonyl groups in the blood of patients with bipolar disorders (Descamps-Latscha et al. 2001). Postmortem studies have shown high levels of carbonyl, 4-HNE, and 8-ISSO groups in bipolar patients (Imai and Nakagawa 2003; Wang et al. 2009). In addition, the antioxidant defense system also appears to be altered in bipolar patients, even in the early stages of the disorder. A study detected an increase in glutathione (GST) in bipolar patients in the early stages of the disorder (Andreazza et al. 2009). The superoxide dismutase (SOD) enzyme has also been found to be increased in the blood of bipolar patients during episodes of mania and depression (Andreazza et al. 2010).
3.2 Alternative and Experimental Treatments Targeting Oxidative Stress
N-acetylcysteine (NAC) has been investigated in the treatment of acute and maintenance treatment of bipolar disorder. NAC is an immediate precursor to glutathione, an antioxidant protein with a significant role in the removal of numerous reactive oxygen species, levels of which are reduced in bipolar disorder (Rosa et al. 2014). In the first placebo-controlled RCT, adjunctive NAC treatment resulted in a significant improvement in depressive symptoms over placebo in bipolar patients at the maintenance treatment stage (Berk et al. 2008). Studying a subgroup analysis of bipolar patients in a depressive phase at baseline, the NAC treatment group showed significant improvements in depressive symptoms (eight of ten patients on NAC had treatment response at endpoint), alongside measures of executive function and QoL (Magalhaes et al. 2011). A further open-label study of 149 individuals with bipolar depression again showed adjunctive NAC treatment led to significant and robust reductions in depression scores and improvements in functioning and quality of life (Berk et al. 2011a). However, more recently, a large RCT found no difference between adjunctive NAC and placebo in bipolar depression in terms of reduction of depressive symptoms at the 16-week endpoint (Berk et al. 2019).
Creatine is the precursor of phosphocreatine (PCr), which has an important role in brain energy homeostasis through the phosphocreatine circuit and has been shown to have antioxidant properties (Pereira et al. 2018). In a small sample of patients with unipolar and bipolar treatment-resistant depression, open label adjunctive treatment with creatine monohydrate led to significantly improved depressive symptoms; however, in both bipolar disorder patients, there was a momentary switch to elevated mood (Roitman et al. 2007).
In a placebo-controlled RCT evaluating creatine monohydrate as an added treatment in bipolar disorder in a depressive episode, the patients did not show improvement in depressive symptom scores. However, the study did find significant superiority of creatine augmentation over placebo using remission criteria (Toniolo et al. 2018).
Cytidine is a pyrimidine with the main function on phospholipid homeostasis and membrane balance, which potentially alleviates mitochondrial dysfunction and regulates dysfunctional glia-neuronal glutamatergic cycling in bipolar disorder. In a 12-week RCT of 35 individuals with bipolar depression, adjunctive cytidine resulted in an earlier decrease in depressive severity alongside a decrease in glutamate/glutamine levels as measured by proton magnetic resonance spectroscopy (1H-MRS) (Yoon et al. 2009).
4 Immune System in Bipolar Disorder
It is known that multiple factors related to the immune system may be involved in the pathophysiology of bipolar disorder, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL). These mechanisms, in turn, promote deleterious effects that contribute to exaggerated inflammation (Carvalho et al. 2013). Studies have suggested that the pathophysiology of bipolar disorder may be related to changes in the immune system, with mood episodes being characterized as pro-inflammatory states. A pro-inflammatory increase in cytokines has been described in patients with bipolar disorders, mainly TNF-α. In addition, IL-2, IL-1β, IL-4, IL-6, and IL-10 concentrations are elevated in bipolar patients (Modabbernia et al. 2013). Investigations about inflammation in bipolar disorder have significantly increased in the last years, showing the importance of the immune system in the neurobiology of bipolar disorder, but with variable results regarding the type of episode, the effect of treatments, and the progression of the disorder (Leboyer et al. 2012).
Horrobin and Lieb (1981) were the first to link immunological disorders with bipolar disorder; the authors hypothesize that relapses of mood episodes in bipolar patients would be driven by the immune system as well as in other inflammatory diseases such as multiple sclerosis (Maes 2011; Drexhage et al. 2010).
Specifically, alterations in the arachidonic acid (AA) signalling pathway and regulation of cyclooxygenase (COX)-generated metabolites including prostaglandins and thromboxanes have been implicated in the neurobiology of bipolar patients, and targeting AA pathway may be a potential therapeutic approach (Rao and Rapoport 2009). A postmortem brain study of bipolar patients showed changes in the AA metabolism cascade (Rao et al. 2010). As well as a significant increase in pro-inflammatory and anti-inflammatory cytokines, they have been consistently reported in patients with bipolar disorder (Modabbernia et al. 2013). The study suggests that pro-inflammatory cytokines are increased regardless of the mood episode of patients with bipolar disorder, as well as the occurrence of a reduction in anti-inflammatory cytokines (Barbosa et al. 2014).
Studies have highlighted TNF-α as an important marker of bipolar progression, with a significant increase in patients with a chronic history when compared to first-episode bipolar patients (Tatay-Manteiga et al. 2017). These studies reinforce the presence of changes in inflammatory biomarkers in bipolar patients (Modabbernia et al. 2013).
4.1 Inflammation and Treatment in Bipolar Disorders
The AA cascade has been the subject of some studies, and it may be the common route of action of several medications used to treat bipolar patients, for example, anticonvulsants (sodium valproate and carbamazepine) and lithium (Rao et al. 2008). Possible effectiveness of adjuvant celecoxib treatment in both poles of bipolar patients has also been reported, suggesting an essential impact of the inflammation pathway (Arabzadeh et al. 2015; Mousavi et al. 2017).
Reports of lithium’s anti-inflammatory properties may contribute to its therapeutic efficacy. However, data demonstrated in the literature are contradictory about the effects of lithium on pro- or anti-inflammatory markers (Rapaport and Manji 2001). In pre-clinical studies, chronic lithium treatment reduced levels of motor impulsivity, plasma levels of IL-1β, IL-10 and RANTES (CCL-5) were reduced selectively within the orbitofrontal cortex of lithium-treated rats (Adams et al. 2020). These findings demonstrate that lithium may improve impulse control deficits in clinical populations decreasing the pro-inflammatory signalling on neuronal activity, particularly within the orbitofrontal cortex (Adams et al. 2020). Studies evaluated the action of the medications utilized in bipolar patients and suggest that the AA cascade, as well as the decreased activity of cyclooxygenase 2 (COX-2) and prostaglandin E2 (PGE2), can be a common target in the mood-stabilizing action of these drugs (Bosetti et al. 2002). A further study demonstrated that bipolar patients who showed a poor response to lithium had increased levels of TNF-α compared to those with a good response (Guloksuz et al. 2012) (Fig. 2).
Importance of early treatment: the impact on neuroprogression, Psycho-Neuro-Immune- Endocrine cascade. Adapted from Moylan et al. (2013). Illustrations courtesy of Romayne Gadelrab
4.2 Alternative and Experimental Treatments and Inflammation
Among non-steroidal anti-inflammatories (NSAIDs), there is the class of those specific inhibitors of COX-2; among these drugs is celecoxib. COX isoenzymes have a role as precursors of prostaglandin, thromboxanes, and prostacyclins. Prostaglandins are autacoid mediators with influence in most of the neurophysiological or pathophysiological progressions through the membrane receptors connected to the G-protein (Fitzpatrick 2004).
A meta-analysis examining the antidepressant effects of adjunctive anti-inflammatory agents in mood disorders, including MDD and bipolar disorder, found a moderate to large effect size (Husain et al. 2017). Interestingly, this meta-analysis also showed a significant reduction in manic symptoms when pooling the results of adjunctive anti-inflammatories (NSAIDs and NAC) in bipolar disorder.
The use of omega-3 fatty acids (naturally occurring anti-inflammatory molecules) in bipolar depression is restricted with one meta-analysis of five RCTs indicating a significant outcome in favor of omega-3 over placebo with moderate effect size (Sarris et al. 2012).
In a RCT with bipolar patients using celecoxib controlled with placebo, the authors found a significant decrease in TNF-α levels. However, in this study, there was no significant difference in the levels of IL-1β, IL-6, and high-sensitivity C-reactive protein (Kargar et al. 2014). This study evaluated inflammatory markers in the use of celecoxib. Although several studies are correlating bipolar disorder progression with inflammatory markers, the data are still controversial, highlighting the importance of new research in this area (Giridharan et al. 2019). Overall, these studies demonstrate strong evidence of inflammation in bipolar disorder leading to new therapeutic targets, such as the use of anti-inflammatory drugs, highlighting celecoxib and the AA pathway involved in the action of this drug (Husain et al. 2016).
A meta-analysis including three RCTs (n = 121) showed a significant effect on Young Mania Rating Scale scores from patients with bipolar disorder receiving adjunctive celecoxib treatment compared with placebo (Bavaresco et al. 2019). An RCT of adjunctive celecoxib in treatment-resistant bipolar depression found higher response and remission rates in the celecoxib than in the placebo arm (Halaris et al. 2020).
Minocycline is a second-generation tetracycline with anti-inflammatory properties. The effects of minocycline in combination with aspirin have also been examined in an RCT in bipolar depression that showed a significantly higher sustained clinical response rate in the minocycline and aspirin-treated group compared with placebo (Savitz et al. 2018).
A study investigating minocycline for bipolar depression patients demonstrated a significant effect size in reducing depressive symptoms (Soczynska et al. 2017). Remarkably, the clinical trials indicate that this second-generation tetracycline has more significant effects in bipolar disorder subjects with higher inflammatory indicators which highlights the need for inflammatory analysis +/− stratification in forthcoming RCTs of minocycline in bipolar patients. However, the largest and most recent RCT (n-266) in bipolar I and II and unipolar depression did not find a significant effect comparing placebo with celecoxib or minocycline (Husain et al. 2020). Husain et al. (2020) conclude that a possible therapeutic effect should not be considered in bipolar depression. Notwithstanding this negative finding, anti-inflammatory treatments are becoming a promising alternative for use in psychiatry.
5 Neurotrophins and Bipolar
In the middle of the twentieth century, the first neurotrophin, the nerve growth factor (NGF), was identified. This discovery broadened the horizon of neurobiology for the identification and elucidation of cellular functions. Almost 30 years after the identification of NGF, the prototype of neurotrophins for neurons of the autonomic nervous system was isolated, a homologue of NGF, which was called brain-derived neurotrophic factor (BDNF) (Lessmann et al. 2003).
Recent studies indicate that impairment of neuroplasticity and neuronal survival are the main events involved in the pathogenesis of bipolar disorders. These events are influenced by several factors, such as the harmonic action of neurotransmitters, hormones, neurotrophins, and inflammatory mediators, such as cytokines (Brietzke and Kapczinski 2008).
It has been shown that high levels of cortisol, released due to stress, can cause damage to the membranes of neuronal mitochondria, especially in patients with bipolar disorder, leading to the release of toxic compounds and culminating in changes in the structure of the DNA molecule in nucleus of these cells. All of this transformation triggers apoptosis mechanisms (Berk et al. 2011b).
5.1 Neurotrophins and Bipolar Treatment
The family of tyrosine kinase (Trk) receptors is composed of three receptors that can be activated by one or more neurotrophins: NGF, BDNF, NT-3, and NT-4/5. In studies on depression models, antidepressants increase TrkB signaling, which is dependent on the concentration of BDNF (Saarelainen et al. 2003). Antidepressants and mood stabilizers can increase serum BDNF levels (Frey et al. 2006). Chronic administration of antidepressants increases the expression of BDNF in the hippocampus, as well as in the CPF (Duman et al. 2000). Chronic treatment with lithium or sodium valproate demonstrated higher BDNF expression in preclinical studies (Fukumoto et al. 2001).
Several studies have suggested that BDNF/TrkB induction is one of the mechanisms responsible for the therapeutic effects of antipsychotics, mood stabilizers, and antidepressants (Coyle and Duman 2003; Nibuya et al. 1995). For example, it has been shown that the use of lithium modulates the phosphorylation of the TrkB and CREB receptors (Rantamaki et al. 2006). Studies show that the neuroprotective characteristics of lithium or sodium valproate may be responsible for its therapeutic effects, and one of the mechanisms involved would be the release of neurotrophins (Rosa et al. 2006; Laeng et al. 2004).
It has been proposed that the augmented expression of BDNF can trigger the neuroprotective properties of lithium and valproate (de Sousa et al. 2011; Hu et al. 2010).
Chronic treatment with lithium or sodium valproate produces protective effects against excitotoxicity and cell death induced by glutamate (Shao et al. 2005). Regarding BDNF, there is much evidence regarding its long-term role in synaptic plasticity in the hippocampus and neocortex. The application of exogenous BDNF enhances presynaptic efficacy by increasing the release of glutamate in excitatory synapses (Lessmann et al. 2003).
6 Bipolar Disorder and the Glutamatergic System
For a long time, the monoaminergic hypothesis explained both the neurobiology of bipolar disorders and the mechanisms of action of psychotropic drugs. Glutamate is related to a diversity of crucial functions including synaptic plasticity, learning, and memory (Riedel et al. 2003). This neurotransmitter acts on two classes of receptors: ionotropic and metabotropic. There are three types of ionotropic glutamate receptors: α-amino acid-3-hydroxy-5-methylisoxazol-4-proprionic (AMPA), N-methyl-D-aspartate (NMDA), and kainate. These three proteins are ion channels that, when activated, generate postsynaptic excitatory potential (Kew and Kemp 2005). Metabotropic receptors are not ion channels and are not exclusively located in the synapse region. When they bind to glutamate, they activate the G-protein, which is responsible for sending the message into the cell (Baudry et al. 2012).
Although all glutamate receptors respond to the same neurotransmitter, they perform very different functions (Jun et al. 2014). In physiological situations, ionotropic and metabotropic receptors regulate neurotransmission through excitatory synapses and modulate various neurophysiological functions in the brain, which include synaptic plasticity, mood, learning, and memory (Kew and Kemp 2005).
Several researchers have shown changes in glutamate receptors in the brain of patients with bipolar disorders, with decreased expression of NMDA receptor in the hippocampus and the prefrontal cortex of bipolar patients (McCullumsmith et al. 2007; Beneyto and Meador-Woodruff 2008). These functionally different receptors can be co-expressed in individual synapses, allowing precise temporal modulation of postsynaptic excitability and plasticity (Scheefhals and MacGillavry 2018). Recently, the NMDA receptor has been associated with the sociability and pathogenesis of autism spectrum disorder, which encourages further studies for the therapeutic exploration of the modulation of this receptor (Burket and Deutsch 2019).
6.1 Glutamate Neurotransmission and Bipolar Treatment
Several pharmacological approaches used in the treatment of psychiatric disorders act on the glutamatergic system. Significant evidence for the role of glutamate in bipolar disorder emerged from pharmacological interventions; for example, lamotrigine, an anticonvulsant also used in bipolar depression pharmacotherapy, indirectly inhibits the release of glutamate (Leach et al. 1991). Lithium is the gold standard mood stabilizer; it leads to an acute increase in the concentration of glutamate in the synapse and the chronic upregulation of transporter activity; its chronic use seems to promote the stabilization of excitatory neurotransmission (Li et al. 2002). Lithium attenuates the release of Ca2+ after acting on mGluR1 and mGluR5 receptors (Machado-Vieira et al. 2012; Sourial-Bassillious et al. 2009). As previously described, mitochondrial dynamics involve not only the processes of fission and fusion but also the movement of mitochondria through neurons, a process influenced by the concentration of ATP and Ca2+, with lithium’s influence (Curtis et al. 2011). In addition to changes in ATP levels, as already mentioned, studies have pointed out that bipolar patients show changes in intracellular Ca2+ signaling, causing an increase in cytosolic Ca2+ concentrations, related to excitotoxicity, decreased mitochondrial viability, and, ultimately, cell death (Mehta et al. 2013). The neuroprotective effects of lithium against the excitotoxicity of glutamate influences BDNF. Antipsychotics and anticonvulsants reduce glutamate in the central nervous system (Sitges et al. 2007; Juruena et al. 2009b; McLoughlin et al. 2009; Paraskevas et al. 2006; de la Fuente-Sandoval et al. 2013).
6.2 Alternative and Experimental Treatments Targeting the Glutamatergic System
6.2.1 Ketamine
Several studies have shown that glutamate-mediated synaptic plasticity is involved in bipolar pharmacotherapy. In addition, ketamine (an NMDA antagonist) has attracted attention for its rapid antidepressant activity, including in patients with bipolar depression (Zarate et al. 2012)
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist with significant evidence to treat both unipolar and bipolar depression, including in treatment-resistant cases (Coyle and Laws 2015). In an RCT crossover add-on study of 18 individuals with treatment-resistant bipolar depression (continued on lithium or valproate), following a single ketamine infusion (0.5 mg/kg over 40 min), depressive symptoms significantly decreased within 40 min compared with placebo, the effect lasting up to day 3 (Diazgranados et al. 2010). There was a notable response rate, with approximately 70% of subjects responding to ketamine. Acute dissociative symptoms were the most common side effect; otherwise ketamine at this dose was generally well tolerated. These findings were replicated in a similar clinical trial with treatment-resistant bipolar depression, which again showed a single ketamine infusion led to rapid, robust antidepressant effects, alongside significant improvements in suicidal ideation (Zarate et al. 2012). Although the antidepressant effects of ketamine are transient, the response may be maintained with repeated infusions (Diamond et al. 2014). Other forms of administration (oral, sublingual, intranasal, intramuscular, subcutaneous) may demonstrate more viable alternatives for repeated dosing. Nevertheless, additional research is required to fully assess and compare the safety and effectiveness of repeated dosing across different routes.
6.2.2 Memantine
Memantine is a non-competitive NMDA antagonist which, unlike ketamine, does not produce dissociative side effects at therapeutic doses. In a small RCT of memantine augmentation of lamotrigine in bipolar depression, although a primary benefit of memantine over placebo was demonstrated at 4 weeks, this effect was not maintained at the 8-week primary endpoint (Anand et al. 2012). A subsequent larger RCT exploring memantine augmentation to valproate in bipolar II depression again found no significant benefit over placebo in terms of response in the severity of depression (Lee et al. 2014). Of interest, this study found that TNF-α levels were decreased in the memantine arm, suggesting a potential anti-inflammatory mechanism. An open-label pilot trial in individuals with acute mania associated with bipolar I disorder found memantine monotherapy to have anti-manic effects at 3 weeks (Keck et al. 2009); however, larger RCTs are required to explore this further.
6.2.3 Riluzole
Riluzole is an NDMA modulator that is licensed for amyotrophic lateral sclerosis (ALS) treatment, acting to increase glutamatergic reuptake and enhance the production of neural growth factors, including BDNF (Bellingham 2011). Preliminary results in acute bipolar depression, in an open trial with riluzole added to lithium, found a significant treatment effect at 4 weeks with no switch observed and good tolerability (Zarate et al. 2005). However, in a subsequent RCT of 19 subjects with acute bipolar depression, there were no significant differences in depressive symptoms between riluzole monotherapy and placebo groups (Park et al. 2017).
7 Bipolar Disorder and the Cholinergic System
The cholinergic-adrenergic hypothesis of bipolar disorder was first proposed many decades ago (Janowsky et al. 1972) and suggested that mania was associated with an adrenergic predominance, while depression was associated with a central cholinergic predominance. Since this initial hypothesis, converging evidence has supported cholinergic dysfunction in bipolar disorder. A positron emission tomography (PET) study demonstrated decreased muscarinic type 2 (M2) binding in bipolar patients (Cannon et al. 2006); another study has reported reduced muscarinic receptor binding (M2 and M3) in the prefrontal cortex in bipolar patients (Gibbons et al. 2009).
7.1 Treatments Targeting the Cholinergic System
The short-acting acetylcholinesterase inhibitor physostigmine has been shown to lead to rapid but temporary reductions in manic symptoms in a few small case series (Davis et al. 1978). Augmentation with donepezil, a long-acting acetylcholinesterase inhibitor, was also shown to lead to marked reductions in mania in a small case series (Burt et al. 1999). However, a subsequent small RCT failed to show any significant benefit of adjunctive donepezil over placebo in treatment for refractory manic symptoms (Eden Evins et al. 2006).
Scopolamine, a muscarinic receptor antagonist and NMDA modulator, has also been examined for potential treatment of bipolar patients. In a double-blind RCT, including subjects with both major depressive disorder and bipolar depression, scopolamine administration led to a significant rapid and robust antidepressant response (Furey and Drevets 2006). An RCT (SCOPE-BD) is studying scopolamine in bipolar patients in a depressive episode (Hallahan n.d., ClinicalTrials.gov ID: NCT04211961).
8 Bipolar Disorder and the Melatonergic System
Melatonin is a vital hormone in the brain that is responsible for the regulation of circadian rhythms but also has essential roles in metabolic, immune, antioxidative, and mitochondrial functioning (Claustrat et al. 2005). Melatonin exerts its effects primarily through the MT1 and MT2 G-protein-coupled receptors.
There is accumulating evidence on the role of the melatonin system in bipolar disorder, with studies reporting changes in patters on melatonin secretion and supersensitivity of light-induced melatonin suppression (Lanfumey et al. 2013).
8.1 Treatments Targeting the Melatonergic System
Agomelatine is a potent MT1 and MT2 agonist and 5HT-2C antagonist and also acts to increase dopamine and noradrenaline levels (Guardiola-Lemaitre et al. 2014). A clinical trial in bipolar depression with agomelatine found that 81% of patients showed greater than 50% response in HAM-D score after 6 weeks (Calabrese et al. 2007); however, a larger RCT subsequently did not find a difference in depressive severity between agomelatine or placebo adjunctive therapy (Yatham et al. 2016).
Ramelteon is an MT1 and MT2 agonist, which has been shown to improve depressive symptoms in bipolar disorder with sleep problems (McElroy et al. 2011). Further work has shown ramelteon to be effective in maintaining mood stability in euthymic bipolar disorder individuals with sleep disturbances (Norris et al. 2013). Most recently a phase 3 RCT examining the efficacy and safety of adjunctive ramelteon as a maintenance treatment in bipolar disorder failed to show the efficacy of ramelteon in preventing relapse in bipolar subjects (Mahableshwarkar et al. 2017).
9 Conclusion
In the face of the complex symptomatology of bipolar disorder, there is still a hiatus in the understanding of its more complex neurobiology and, accordingly, in the treatment of bipolar disorder. Understanding new biological pathways will provide data with potential for clinical translation. This update of the neurobiological bases shows multiple factors probably interact in this complex equation of pathophysiology of bipolar disorder, such as genetic, biochemical, psychosocial, and environmental stress events, correlating with the development and severity of the bipolar disorder. Developing new pharmacological treatment for bipolar patients is crucial; however, new effective medications can only be developed when we understand better the neurobiology of bipolar disorder and find novel targets such as those described in this chapter.
References
Adams WK, Levesque DL, Cocker PJ, Kaur S, Bodnar TS, Young AH, Winstanley CA (2020) Decreased motor impulsivity following chronic lithium treatment in male rats is associated with reduced levels of pro-inflammatory cytokines in the orbitofrontal cortex. Brain Behav Immun 89:339–349
Anand A, Gunn AD, Barkay G, Karne HS, Nurnberger JI, Mathew SJ, Ghosh S (2012) Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord 14:64–70
Andreazza AC, Kapczinski F, Kauer-Sant’Anna M, Walz JC, Bond DJ, Gonçalves CA et al (2009) 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci 34(4):263–271
Andreazza AC, Shao L, Wang JF, Young LT (2010) Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry 67(4):360–368
Arabzadeh S, Ameli N, Zeinoddini A, Rezaei F, Farokhnia M, Mohammadinejad P, Ghaleiha A, Akhondzadeh S (2015) Celecoxib adjunctive therapy for acute bipolar mania: a randomized, double-blind, placebo-controlled trial. Bipolar Disord 17(6):606–614
Barbosa IG, Bauer ME, Machado-Vieira R, Teixeira AL (2014) Cytokines in bipolar disorder: paving the way for neuroprogression. Neural Plast 2014:360481
Baudry M, Greget R, Pernot F, Bouteiller JM, Xiaoning B (2012) Roles of group I metabotropic glutamate receptors under physiological conditions and in neurodegeneration. WIREs Membr Transp Signal 1(4):523–532
Bavaresco DV, Colonetti T, Grande AJ, Colom F, Valvassori SS, Quevedo J, da Rosa MI (2019) Efficacy of celecoxib adjunct treatment on bipolar disorder: systematic review and meta-analysis. CNS Neurol Disord Drug Targets 18:19–28
Bellingham MC (2011) A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17:4–31
Beneyto M, Meador-Woodruff JH (2008) Lamina-specific abnormalities of NMDA receptor associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 33(9):2175–2186
Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI (2008) N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475
Berk M, Dean O, Cotton SM, Gama CS, Kapczinski F, Fernandes BS, Kohlmann K, Jeavons S, Hewitt K, Allwang C, Cobb H, Bush AI, Schapkaitz I, Dodd S, Malhi GS (2011a) The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: an open label trial. J Affect Disord 135:389–394
Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M et al (2011b) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35(3):804–817
Berk M, Turner A, Malhi GS, Ng CH, Cotton SM, Dodd S, Samuni Y, Tanious M, McAulay C, Dowling N, Sarris J, Owen L, Waterdrinker A, Smith D, Dean OM (2019) A randomized controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo. BMC Med 17:18
Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC (2002) Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry 7(8):845–850
Brietzke E, Kapczinski F (2008) TNF-alpha as a molecular target in bipolar disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 32:1355–1361
Brown ES, Bobadilla L, Rush AJ (2001) Ketoconazole in bipolar patients with depressive symptoms: a case series and literature review. Bipolar Disord 3:23–29
Burket JA, Deutsch SI (2019) Metabotropic functions of the NMDA receptor and an evolving rationale for exploring NR2A-selective positive allosteric modulators for the treatment of autism spectrum disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 90:142–160
Burt T, Sachs GS, Demopulos C (1999) Donepezil in treatment-resistant bipolar disorder. Biol Psychiatry 45:959–964
Calabrese JR, Guelfi JD, Perdrizet-Chevallier C, Agomelatine Bipolar Study Group (2007) Agomelatine adjunctive therapy for acute bipolar depression: preliminary open data. Bipolar Disord 9:628–635
Cannon DM, Carson RE, Nugent AC, Eckelman WC, Kiesewetter DO, Williams J, Rollis D, Drevets M, Gandhi S, Solorio G, Drevets WC (2006) Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. Arch Gen Psychiatry 63:741–747
Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, Cleare AJ, Pariante CM (2013) Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord 148(1):136–140
Claustrat B, Brun J, Chazot G (2005) The basic physiology and pathophysiology of melatonin. Sleep Med Rev 9:11–24
Coyle JT, Duman RS (2003) Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38:157–160
Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163
Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM (2011) Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes. Psychiatr Genet 21(1):1–4
Davis KL, Berger PA, Hollister LE, Defraites E (1978) Physostigmine in mania. Arch Gen Psychiatry 35:119–122
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, Alvarado-Alanis P, Ramírez-Bermúdez J, Graff-Guerrero A (2013) Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiat 70(10):1057–1066
de Sousa RT, van de Bilt MT, Diniz BS, Ladeira RB, Portela LV, Souza DO, Forlenza OV, Gattaz WF, Machado-Vieira R (2011) Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4-week study. Neurosci Lett 494(1):54–56
Descamps-Latscha B, Drüeke T, Witko-Sarsat V (2001) Dialysis-induced oxidative stress: biological aspects, clinical consequences, and therapy. Semin Dial 14(3):193–199
Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R (2014) Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 28:536–544
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802
Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC et al (2010) Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol 13(10):1369–1381
Duman RS, Malberg J, Nakagawa S, D’as C (2000) Neuronal plasticity and survival in mood disorders. Biol Psychiatry 48(8):732–739
Eden Evins A, Demopulos C, Nierenberg A, Culhane MA, Eisner L, Sachs G (2006) A double-blind, placebo-controlled trial of adjunctive donepezil in treatment-resistant mania. Bipolar Disord 8:75–80
Fitzpatrick FA (2004) Cyclooxygenase enzymes: regulation and function. Curr Pharm Des 10(6):577–588
Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K et al (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31(5):326–332
Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S (2001) Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology 158:100–106
Furey ML, Drevets WC (2006) Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63:1121–1129
Gallagher P, Malik N, Newham J, Young AH, Ferrier IN, Mackin P (2008) Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev 1:CD005168
Gartside SE, Leitch MM, Young AH (2003) Altered glucocorticoid rhythm attenuates the ability of a chronic SSRI to elevate forebrain 5-HT: implications for the treatment of depression. Neuropsychopharmacology 28(9):1572–1578
Geddes JR, Miklowitz DJ (2013) Treatment of bipolar disorder. Lancet 381(9878):1672–1682
Gibbons AS, Scarr E, McLean C, Sundram S, Dean B (2009) Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord 116:184–191
Giridharan VV, Sayana P, Pinjari OF, Ahmad N, da Rosa MI, Quevedo J, Barichello T (2019) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25(1):94–113
Gitlin MJ, Swendsen J, Heller TL, Hammen C (1995) Relapse and impairment in bipolar disorder. Am J Psychiatry 152(11):1635–1640
Guardiola-Lemaitre B, De Bodinat C, Delagrange P, Millan MJ, Munoz C, Mocaer E (2014) Agomelatine: mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol 171:3604–3619
Guloksuz S, Altinbas K, Aktas Cetin E, Kenis G, Bilgic Gazioglu S, Deniz G, Oral ET, van Os J (2012) Evidence for an association between tumor necrosis factor-alpha levels and lithium response. J Affect Disord 143(1–3):148–152
Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J (2020) Modulation of the inflammatory response benefits treatment-resistant bipolar depression: a randomized clinical trial. J Affect Disord 261:145–152
Hallahan B (n.d.) Scopolamine in bipolar depression (SCOPE-BD). https://clinicaltrials.gov/ct2/show/NCT04211961
Hendler NH (1978) Spironolactone prophylaxis in manic-depressive disease. J Nerv Ment Dis 166(7):517–520
Horrobin DF, Lieb J (1981) A biochemical basis for the actions of lithium on behaviour and on immunity: relapsing and remitting disorders of inflammation and immunity such as multiple sclerosis or recurrent herpes as manic-depression of the immune system. Med Hypotheses 7(7):891–905
Hu Y, Yu X, Yang F, Si T, Wang W, Tan Y, Zhou D, Wang H, Chen D (2010) The level of serum brain-derived neurotrophic factor is associated with the therapeutic efficacy of modified electroconvulsive therapy in Chinese patients with depression. J ECT 26(2):121–125
Husain MI, Chaudhry IB, Hamirani MM, Minhas FA, Kazmi A, Hodsoll J, Haddad PM, Deakin JF, Husain N, Young AH (2016) Minocycline and celecoxib as adjunctive treatments for bipolar depression: a study protocol for a multicenter factorial design randomized controlled trial. Neuropsychiatr Dis Treat 13:1–8
Husain MI, Strawbridge R, Stokes PR, Young AH (2017) Anti-inflammatory treatments for mood disorders: systematic review and meta-analysis. J Psychopharmacol 31:1137–1148
Husain MI, Chaudhry IB, Khoso AB, Husain MO, Hodsoll J, Ansari MA, Naqvi HA, Minhas FA, Carvalho AF, Meyer JH, Deakin B, Mulsant BH, Husain N, Young AH (2020) Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomized controlled trial. Lancet Psychiatry 7(6):515–527
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34(2):145–169
Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic-adrenergic hypothesis of mania and depression. Lancet 2:632–635
Jun C, Choi Y, Lim SM, Bae S, Hong YS, Kim JE, Lyoo IK (2014) Disturbance of the glutamatergic system in mood disorders. Exp Neurobiol 23:28–35
Juruena MF (2014) Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav 38:148–159
Juruena MF, Gama CS, Berk M, Belmonte-de-Abreu PS (2009) Improved residual symptoms in bipolar affective disorder with adjunctive spironolactone (mineralocorticoid receptor antagonist): case series. J Psychopharmacol 23(8):985–987
Juruena MF, Pariante CM, Papadopoulos AS, Poon L, Lightman S, Cleare AJ (2009a) Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br J Psychiatry 194:342–349
Juruena MF, Ottoni G, Machado-Vieira R, Carneiro R, Weingarthner N, Fleig SS, Broilo L, Busnello ED (2009b) Bipolar I and II disorder residual symptoms: Oxcarbazepine and carbamazepine as add-on treatment to lithium in a double-blind, randomized trial. Prog Neuro-Psychopharmacol Biol Psychiatry 33:94–99
Kargar M, Yousefi A, Mojtahedzadeh M, Akhondzadeh S, Artounian V, Abdollahi A, Ahmadvand A, Ghaeli P (2014) Effects of celecoxib on inflammatory markers in bipolar patients undergoing electroconvulsive therapy: a placebo-controlled, double-blind, randomised study. Swiss Med Wkly 144:w13880
Kato T (2007) Mitochondrial dysfunction as themolecular basis of bipolar disorder: therapeutic implications. CNS Drugs 21(1):1–11
Keck PE Jr, Hsu HA, Papadakis K, Russo J Jr (2009) Memantine efficacy and safety in patients with acute mania associated with bipolar I disorder: a pilot evaluation. Clin Neuropharmacol 32:199–204
Kew JN, Kemp JA (2005) Ionotropic andmetabotropic glutamate receptor structure and pharmacology. Psychopharmacology 179(1):4–29
Khairova R, Pawar R, Salvadoran G, Juruena MF, De Sousa RT, Soeiro-de-Souza MG, Salvador M, Zarate CA, Gattaz WF, Machado-Vieira R (2012) Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep 5:680–682
Laeng P, Pitts RL, Lemire AL, Drabik CE, Weiner A, Tang H, Thyagarajan R, Mallon BS, Altar CA (2004) The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem 91:238–251
Lanfumey L, Mongeau R, Hamon M (2013) Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther 138:176–184
Leach MJ, Baxter MG, Critchley MA (1991) Neurochemical and behavioral aspects of lamotrigine. Epilepsia 32(2):4–8
Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R, Kupfer DJ (2012) Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord 141(1):1–10
Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang YS, Wang LJ, Lee IH, Wang TY, Yeh TL, Yang YK, Hong JS, Lu RB (2014) The effects of add-on low-dose memantine on cytokine levels in bipolar II depression: a 12-week double-blind, randomized controlled trial. J Clin Psychopharmacol 34:337–343
Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. Prog Neurobiol 69:341–374
Li X, Ketter TA, Frye MA (2002) Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J Affect Disord 69(1–3):1–14
López-Muñoz F, Shen WW, D’Ocon P, Romero A, Álamo C (2018) A history of the pharmacological treatment of bipolar disorder. Int J Mol Sci 19(7):E2143
Lund C, Tomlinson M, De Silva M, Fekadu A, Shidhaye R, Jordans M, Petersen I, Bhana A, Kigozi F, Prince M, Thornicroft G, Hanlon C, Kakuma R, McDaid D, Saxena S, Chisholm D, Raja S, Kippen-Wood S, Honikman S, Fairall L, Patel V (2012) Prime. A programme to reduce the treatment gap for mental disorders in five low- and middle-income countries. PLoS Med 9(12):e1001359
Machado-Vieira R, Ibrahim L, Henter ID, Zarate CA Jr (2012) Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav 100(4):678–687
Maes M (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35(3):664–675
Magalhaes PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M (2011) N-acetylcysteine for major depressive episodes in bipolar disorder. Braz J Psychiatry 33:374–378
Mahableshwarkar AR, Calabrese JR, Macek TA, Budur K, Adefuye A, Dong X, Hanson E, Sachs GS (2017) Efficacy and safety of sublingual ramelteon as an adjunctive therapy in the maintenance treatment of bipolar I disorder in adults: a phase 3, randomized controlled trial. J Affect Disord 221:275–282
Malhi GS, Bassett D, Boyce P, Bryant R, Fitzgerald PB, Fritz K, Hopwood M, Lyndon B, Mulder R, Murray G, Porter R, Singh AB (2015) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 49(12):1087–1206
McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH (2007) Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res 1127(1):108–118
McElroy SL, Winstanley EL, Martens B, Patel NC, Mori N, Moeller D, McCoy J, Keck PE Jr (2011) A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol 26:48–53
McLoughlin GA, Ma D, Tsang TM, Jones DN, Cilia J, Hill MD, Robbins MJ, Benzel IM, Maycox PR, Holmes E, Bahn S (2009) Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res 8(4):1943–1952
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698(1–3):6–18
Modabbernia A, Taslimi S, Brietzke E, Ashrafi M (2013) Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry 74(1):15–25
Mousavi SY, Khezri R, Karkhaneh-Yousefi MA, Mohammadinejad P, Gholamian F, Mohammadi MR, Zeinoddini A, Akhondzadeh S (2017) Randomized double-blind placebo-controlled trial on effectiveness and safety of celecoxib adjunctive therapy in adolescents with acute bipolar mania. J Child Adolesc Psychopharmacol 27:494–500
Moylan S, Maes M, Wray N, Berk M (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606
Murck H, Schüssler P, Steiger A (2012) Renin-angiotensin-aldosterone system: the forgotten stress hormone system: relationship to depression and sleep. Pharmacopsychiatry 45(3):83–95
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547
Norris ER, Karen B, Correll JR, Zemanek KJ, Lerman J, Primelo RA, Kaufmann MW (2013) A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord 144:141–147
O’Brien PM, Craven D, Selby C, Symonds EM (1979) Treatment of premenstrual syndrome by spironolactone. Br J Obstet Gynaecol 86(2):142–147
Paraskevas GP, Triantafyllou NI, Kapaki E, Limpitaki G, Petropoulou O, Vassilopoulos D (2006) Add-on lamotrigine treatment and plasma glutamate levels in epilepsy: relation to treatment response. Epilepsy Res 70(2–3):184–189
Park LT, Lener MS, Hopkins M, Iadorola N, Machado-Vieira R, Ballard E, Nugent A, Zarate CA Jr (2017) A double-blind, placebo-controlled, pilot study of riluzole monotherapy for acute bipolar depression. J Clin Psychopharmacol 37:355–358
Pereira C, Chavarria V, Vian J, Ashton MM, Berk M, Marx W, Dean OM (2018) Mitochondrial agents for bipolar disorder. Int J Neuropsychopharmacol 21:550–569
Rantamaki T, Knuuttila JEA, Hokkanen ME, Castrén E (2006) The effects of acute and long-term lithium treatments on TrkB neurotrophin receptor activation in the mouse hippocampus and anterior cingulate cortex. Neuropharmacology 50:421–427
Rao JS, Rapoport SI (2009) Mood-stabilizers target the brain arachidonic acid cascade. Curr Mol Pharmacol 2:207–214
Rao JS, Lee HJ, Rapoport SI, Bazinet RP (2008) Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry 13(6):585–596
Rao JS, Harry GJ, Rapoport SI, Kim HW (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15(4):384–392
Rapaport MH, Manji HK (2001) The effects of lithium on ex vivo cytokine production. Biol Psychiatry 50(3):217–224
Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. Behav Brain Res 140:1–47
Roitman S, Green T, Osher Y, Karni N, Levine J (2007) Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord 9:754–758
Rosa AR, Frey BN, Andreazza AC, Cereser KM, Cunha AB, Quevedo J, Santin A, Gottfriend C, Gonçalves CA, Vieta E, Kapczinski F (2006) Increased serum glial cell line-derived neurotrophic factor immunocontent during manic and depressive episodes in individuals with bipolar disorder. Neurosci Lett 407(2):146–150
Rosa AR, Singh N, Whitaker E, de Brito M, Lewis AM, Vieta E, Churchill GC, Geddes JR, Goodwin GM (2014) Altered plasma glutathione levels in bipolar disorder indicates higher oxidative stress; a possible risk factor for illness onset despite normal brain-derived neurotrophic factor (BDNF) levels. Psychol Med 44:2409–2418
Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasaio A, Nawa H, Aloyz EP, Castren E (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant induced behavioral effects. J Neurosci 23:349–357
Sarris J, Mischoulon D, Schweitzer I (2012) Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry 73:81–86
Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, Drevets D, Yates W, Gleason O, Drevets WC, Preskorn SH (2018) Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2x2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 8:27
Scaini G, Fries GR, Valvassori SS, Zeni CP, Zunta-Soares G, Berk M et al (2017) Perturbations in the apoptotic pathway and mitochondrial network dynamics in peripheral blood mononuclear cells from bipolar disorder patients. Transl Psychiatry 7(5):e1111
Scheefhals N, MacGillavry HD (2018) Functional organization of postsynaptic glutamate receptors. Mol Cell Neurosci 91:82–94
Shao L, Young LT, Wang JF (2005) Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry 58:879–884
Sitges M, Chiu LM, Guarneros A, Nekrassov V (2007) Effects of carbamazepine, phenytoin, lamotrigine, oxcarbazepine, topiramate and vinpocetine on Na+ channel-mediated release of [3H]glutamate in hippocampal nerve endings. Neuropharmacology 52:598–605
Soczynska JK, Kennedy SH, Alsuwaidan M, Mansur RB, Li M, McAndrews MP, Brietzke E, Woldeyohannes HO, Taylor VH, McIntyre RS (2017) A pilot, open-label, 8-week study evaluating the efficacy, safety and tolerability of adjunctive minocycline for the treatment of bipolar I/II depression. Bipolar Disord 19:198–213
Sourial-Bassillious N, Rydelius PA, Aperia A, Aizman O (2009) Glutamate-mediated calcium signaling: a potential target for lithium action. Neuroscience 161(4):1126–1134
Tatay-Manteiga A, Balanzá-Martínez V, Bristot G, Tabarés-Seisdedos R, Kapczinski F, Cauli O (2017) Clinical staging and serum cytokines in bipolar patients during euthymia. Prog Neuropsychopharmacol Biol Psychiatry 77:194–201
Toniolo RA, Silva M, Fernandes FBF, Amaral J, Dias RDS, Lafer B (2018) A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression. J Neural Transm 125:247–257
Wang M, Hammarbäck S, Lindhe BA, Bäckström T (1995) Treatment of premenstrual syndrome by spironolactone: a double-blind, placebo-controlled study. Acta Obstet Gynecol Scand 74(10):803–808
Wang JF, Shao L, Sun X, Young LT (2009) Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Hron 11(5):523–529
Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH (2004) Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry 184:496–502
Watson S, Gallagher P, Porter RJ, Smith MS, Herron LJ, Bulmer S, North-East Mood Disorders Clinical Research Group, Young AH, Ferrier IN (2012) A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biol Psychiatry 72:943–949
Wernze H (2000) Surprising effect of spironolactone on eating behavior and mood in bulimia nervosa. Psychopharmakotherapie 7:33–39
Yatham LN, Vieta E, Goodwin GM, Bourin M, de Bodinat C, Laredo J, Calabrese J, Agomelatine Study Group (2016) Agomelatine or placebo as adjunctive therapy to a mood stabilizer in bipolar I depression: randomized double-blind placebo-controlled trial. Br J Psychiatry 208:78–86
Yoon SJ, Lyoo IK, Haws C, Kim TS, Cohen BM, Renshaw PF (2009) Decreased glutamate/glutamine levels may mediate cytidine’s efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology 34:1810–1818
Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN (2004) Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology 29:1538–1545
Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK (2005) An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57(4):430–432
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71(11):939–946
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Juruena, M.F., Jelen, L.A., Young, A.H., Cleare, A.J. (2020). New Pharmacological Interventions in Bipolar Disorder. In: Young, A.H., Juruena, M.F. (eds) Bipolar Disorder: From Neuroscience to Treatment. Current Topics in Behavioral Neurosciences, vol 48. Springer, Cham. https://doi.org/10.1007/7854_2020_181
Download citation
DOI: https://doi.org/10.1007/7854_2020_181
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-72142-8
Online ISBN: 978-3-030-72143-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)