Abstract
Although aging is inexorable, aging well is not. From the perspective of research in rats and complementary models, reproductive experience has significant effects; indeed, benefits, which include better-than-average cognitive skills, a slowing of the slope of decline, and a healthier brain and/or nervous system well later into life. Work from our lab and others has suggested that the events of pregnancy and parturition, collectively referred to as reproductive experience—an amalgam of hormone exposure, sensory stimulation, and offspring behavioral experience and interaction—may summate to flatten the degree of decline normally associated with aging. Mimicking the effects of an enriched environment, reproductive experience has been shown to: enhance/protect cognition and decrease anxiety well out to two-plus years; result in fewer hippocampal deposits of the Alzheimer’s disease herald, amyloid precursor protein (APP); and, in general, lead to a healthier biology. Based on a suite of recent work in organisms as diverse as nematodes, flies, and mammals, the ubiquitous hormone insulin and its large family of related substances and receptors may play a major role in mediating some of the effects of RE on the parameters of aging studied thus far. We will discuss the current set of data that suggest mechanisms for successful biological and neurobiological aging, and the implications for understanding aging and senescence in their broadest terms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Amyloid precursor protein (APP)
- Enriched environment
- Hippocampus
- Lactation
- Learning and memory
- Medial preoptic area
- Oxytocin
- Parity effects
- Pregnancy
- Reproductive experience
- Senescence
- Steroid hormones
1 Introduction
Life leads inexorably to death. The path, however, may be smooth and clear of obstacles, or it can be strewn with impediments. The form that aging takes is subject to regulation and mediation through genetics, experience, diet, and a host of interwoven factors. Given the inevitability of the body’s descent into decrepitude, the focus is on extending the quality and/or quantity of life: modifying aging and the preserving of vitality for as long as possible. Of the many physiological factors that may control aging are those that, ironically, produce and protect life, namely, reproductive experience. This section will address the facets of aging that may be affected by the experiences associated with reproduction. Understanding these natural conditions and their effect on the aging process may elucidate principles that can be exploited to aid in successful aging.
2 Aging, in General, and Brain Aging and Consequences for Behavior and Physiological Function
Scientists studying a wide array of species, from worms to humans, have examined the factors that drive aging in an effort to better understand the process. Whether the motive is to discover the “fountain of youth”, or simply to identify why/how we age, recent research provides fascinating details of this process. Along with bodily changes that occur in humans with aging (i.e., metabolism slowing, decrease in heart and cardiovascular function, weakening of immune function, etc.) there are a host of cognitive changes occurring as well. Further, with Alzheimer’s disease and dementia rates on the rise, cognitive decline may be the greatest fear associated with aging. Descartes’ famous maxim, “I think, therefore, I am” is an appropriate descriptor for most people. That is, once one’s mind departs—they are no longer “themselves”. This section of the chapter will highlight recent work done in the field of the aging brain while emphasizing that aging is not a “fixed” process but one of plasticity and malleability.
Throughout the following chapter, signaling pathways and gene expression will be discussed as they are key players influencing the rate at which organisms age. Changes to intracellular organelles and the currency of neural activity, glucose utilization, form the very foundation for quality of aging. The “elderly” cell or neuron presents a complex and fascinating object for study. Among the factors that have attracted attention are those organelles that, for the entire life of the cell, fuel its activity, the mitochondrion. That, these change with age has attracted attention as primary sites of the initiation of aging in the neuron. Reduced expression of genes regulating mitochondrial function during aging is highly conserved across species (reviewed in Bishop et al. 2010). Specifically, brain and muscle are highly susceptible to faulty mitochondrial function (Bishop et al. 2010). Mitochondrial function begins to decline, and “longevity pathways” are likely activated as a compensatory mechanism to increase stress resistance (Bishop et al. 2010). As we discuss in more detail, stress has profound effects on the brain and cognition, but can exert its effects through multiple pathways. Mitochondrial integrity is an important bulwark against the many insults that can hasten the aging process. For the neuron, mitochondrial health and activity are crucial for defending against neurodegeneration. Factors that promote mitochondrial health would, therefore, be expected to facilitate longevity. (Interestingly, our laboratory has preliminary data showing enhanced mitochondrial activity—demonstrated by quantifying Mitotracker Green© activity in medial preoptic and hippocampal tissues—in lactating females compared to virgins [Morgan, Kinsley et al. unpublished observations]).
The insulin/IGF-1 pathway has been implicated in many aspects of aging. It functions as a nutrient sensor and has been demonstrated to regulate the DAF-2 gene, which in turn regulates reproductive development, resistance to oxidative stress, and autophagy among other things (reviewed in Kenyon 2010; see below). In the mammalian brain, decreased signaling of the insulin/IGF-1 pathway promotes a decrease in the brain pathology of Alzheimer’s disease, however, increased signaling may be neuroprotective (reviewed in Kenyon 2010). In mammals, insulin and IGF-1 have been shown to support learning and memory and promote neuronal survival through inhibition of apoptosis (Van der Heide et al. 2006). Further, as most pathways involved in aging, the insulin/IGF-1 pathway is plastic. For example, it has been shown that pregnancy-associated plasma protein (PAPPA) raises IGF-1 levels, thus activating a downstream signaling pathway shown to positively affect lifespan (Kenyon 2010). We explore more deeply the nervous system connections later-on in this chapter, but it should be kept in mind that a lifetime of battering via stress, nutrient challenges, injury, etc., takes its toll on the nervous system. Even normal activities, such as breathing, carry a price.
The phenomenon of oxidative stress refers to various pathologic changes observed in living organisms in response to excessive levels of cytotoxic oxidants and free radicals in the environment. Aging increases reactive oxygen species (ROS) such as free radicals that lead to cell and tissue damage. So what drives this change? An upregulation of oxidative stress-response genes has been identified in worms, flies, mice, rats, chimpanzees, and humans (reviewed in Bishop et al. 2010; Yankner et al. 2008). These data suggest that oxidative damage is conserved across species and is a mechanism of age related functional decline (Muller et al. 2007), and that such damage may be a fixed aspect of aging; other data indicate aging to be plastic, indeed. It may be surprising to some that changes in their daily routine may affect gene expression, as Fischer et al. (2007) demonstrated in the mouse. Here, the authors employed a mouse model capable of inducing expression of the p25 gene, which elicits such events as neurodegeneration, hippocampal synaptic degradation, and memory loss. They reported that environmental enrichment promoted the recovery of lost memories in these animals in addition to synaptic plasticity and induction of histone acetylation marks. Further, administration of a pharmacological histone deacetylase inhibitor evoked the same effect as environmental enrichment, including neuronal plasticity and recovery of memory (Fischer et al. 2007; reviewed in Bishop et al. 2010). Again, aging may not be a fixed process but one of many levels of malleability. As we will see later, the events associated with reproductive experience may be akin to a significant form of environmental enrichment.
Intra-cellular events, including intra-neuronal, regulate tissue aging responses. Autophagy, the cell’s ability to cannibalize its own internal organelles when an inadequate supply of nutrients exists, like the body raiding its fat stores in times of famine, is crucial to regulating the aging process (Bishop et al. 2010). In worms (Melendez et al. 2003), flies (Simonsen et al. 2008), and mice (Hara et al. 2006), increased autophagy extends the lifespan. The enhanced autophagy effect may be related to its reduction of insulin-like signaling (Melendez et al. 2003), as well as to the prevention/removal of the buildup of protein aggregates like those observed in Huntington’s or Alzheimer’s disease (Bishop et al. 2010). A key regulator in autophagy is the signaling pathway of the kinase, target of rapamycin (TOR; Bishop et al. 2010). The TOR kinase is a major amino acid and nutrient sensor that controls cell growth and blocks autophagy when food is abundant. It activates an array of anabolic processes including protein synthesis, transcription, and ribosome biogenesis in response to nutrient presence (Bishop et al. 2010). Dysfunction or misregulation of this pathway may lead to altered cell volumes, which in turn may lead to developmental errors and subsequent pathological conditions. In contrast, when the TOR signal is inhibited, it increases the lifespan of many species, from yeast to mice (for full review, see Kenyon 2010). Further, administration of the TOR inhibitor, rapamycin, extends the lifespan in mice even when given in late life (Harrison 2009). As nutrition may be a driving factor in this age related pathway, caloric restriction may play a role in aging as well. It is interesting to note that caloric restriction and enhanced metabolic demands are a normal feature of the state of the pregnant-lactating female.
Caloric restriction, not to be confused with malnutrition, increases the lifespan of many species, including primates (Bishop et al. 2010; Colman et al. 2009). A reduced intake of calories improves verbal memory in humans (Witte et al. 2009), reduces amyloid-β deposition, and improves learning and memory in transgenic mouse models of Alzheimer’s disease (Halagappa et al. 2007). This effect is believed to be mediated by gene expression changes—specifically in sirtuins. Sirtuins are NAD+-dependant protein deacetylases whose expression is upregulated in animals that are calorie restricted (for a complete review, see Kenyon 2010). But their effects are subtle and possibly context-dependent. Sirtuins can increase or decrease lifespan, may be neuroprotective or harmful to neurons, under different conditions (for full review, see Bishop et al. 2010; Kenyon 2010). Although little work has examined AMP kinase in this paradigm, it has been identified as a nutrient and energy sensor similar to TOR kinase. Overexpression of AMP kinase signals have also been shown to lengthen the lifespan of worms (Apfeld et al. 2004) and is thought to extend the lifespan in response to dietary restriction. Additionally, the anti-diabetic drug, metformin, which activates AMP kinase, can extend life in mice (Anisimov et al. 2008). The plasticity of aging again comes to the fore, extending the gene-by-environment interaction to the latter stages of life.
To what extent might a plastic brain, sensitive to reproductive hormones and experiences, be a model for aging-related modifications? Until recently, the dogma was that at birth, we possess a finite number of brain cells which, only through cell death and damage, changes. This notion has been dismissed, because many data have shown that the adult brain produces new neurons in two areas of the brain, well into senescence. The subventricular zone of the lateral ventricle where the newly born cells migrate to the olfactory bulbs, and subgranular zone of the dentate gyrus of the hippocampus have been repeatedly shown to be rife with new neurons. Though most work has been done in rats, adult neurogenesis has been observed in humans as well (Kemperman and Gage 1998). Age is the number one negative regulator of adult hippocampal neurogenesis (Kemperman 2006), with numbers dropping significantly as age increases. In rats, however, the rate at which the new neurons are born is affected by such factors as stress (negatively; Gould et al. 1998), enriched environment (positively; Nilsson et al. 1999; Kemperman et al. 1997), hormones (like estrogen and prolactin: positively; Tanapat et al. 1999; Shingo et al. 2003; Kemperman et al. 1998, respectively), to name a few. Furthermore, in some paradigms, disruption of neurogenesis leads to behavioral deficits if neurogenesis is interrupted (Enwere et al. 2004; Mak and Weiss 2010).
Neurogenesis occurs throughout the adult life of primates, but it is susceptible to stress effects, as well, as alluded to above (Gould et al. 1998). High levels of glucocorticoids, which can bind to receptors in the hippocampus, significantly inhibit neurogenesis in adult trees shrews (Gould et al. 1997), marmoset monkeys (Gould et al. 1998), and rats (Cameron and Gould 1994; Gould et al. 1992). The effect is reversed when an antidepressant is administered to chronically stressed rats (Dagyte et al. 2010). In contrast to stressed animals, animals living in an enriched environment display heavier brain weights (Cummins et al. 1973, 1977); more dendritic branching (Volkmar and Greenough, 1972); a significantly greater number of glial cells (Altman and Das 1965). Enriched animals also perform better on tasks of learning and memory (Cummins et al. 1973), and show enhanced neurogenesis in their hippocampal dentate gyri (Nilsson et al. 1999; Kemperman et al. 1997; Kemperman, et al. 1998).
Some earlier work suggests parallels between parity and brain enrichment. Diamond and colleagues reported and discussed structural plasticity in the reproductive brain five decades earlier. For example, the width of the cortex in late-pregnant rats that were housed in impoverished conditions was equivalent to those of non-pregnant rats that were exposed to enriched conditions (Diamond et al. 1971; Diamond, Krech and Rosenzweig 1964). The “pregnant” cortex was stimulated in a manner akin to an environmentally “enriched” cortex. Other support for these older data indicating cortical plasticity in the maternal brain has also been reported (Stern 1996; Xerri et al. 1994) in the somatosensory cortex. Data have indicated that this important cortical region (which receives many stimuli from the rooting and suckling pups) undergoes neuronal reorganization and is significantly larger in lactating compared to virgin and non-lactating postpartum rats. According to Pascual-Leone et al. (2005), such changes in cortical “maps” denote plasticity of the type that fits the definition of, as they state, a “dynamic shift in the strength of preexisting connections across distributed neural networks”. Further, such change clearly occurs “…in response to changes in afferent input or efferent demand” resulting from both pregnancy and offspring. That it supports reproduction fits both with Pascual-Leone’s “evolution’s invention” for the “value-added” superimposition of flexible learning mechanisms (onto genomic substrates), as well as with the requirement of marked neural responses to parity and enhancements in brain and behavior. Thus, the maternal brain is, by definition, a plastic organ, perhaps with effects that linger well into senescence.
Moreover, these enriching effects were observed in aged animals exposed to an enriched environment: they did not display the same rate of decline in neurogenesis compared to age matched, non-enriched controls (Kempermann and Gage 1998; Kempermann et al. 2002). These data are interesting as we learn that the brain (and the aging brain) has the potential for remarkable plasticity and change due to simple, natural changes in the environment. For example, exercise increases the endurance of cells and tissues in the brains of Alzheimer’s patients, thereby enhancing the brain environment for further neurogenesis, memory improvement, and brain plasticity (Radak et al. 2010). Running has also been shown to increase adult hippocampal neurogenesis, learning, and long-term potentiation in the mouse, as well (Van Praag et al. 1999). It has been suggested that insulin-like growth factor-1(IGF-1) may be mediating the effect of physical activity on adult neurogenesis (Carro et al. 2001). Here, aged animals administered IGF-1 that mimicked the exogenous levels of younger animals showed a restoration of levels to those seen in younger adults (Lichtenwalner et al. 2001). These data demonstrate that plasticity is not based on external influences alone. Intrinsic factors play a role in rates of neurogenesis in adult and aging brains as well.
Enwere et al. (2004) have reported reduced epidermal growth factor receptor signaling in aged mice accompanied by diminished olfactory neurogenesis, which may be responsible for the mouse’s impairment in fine olfactory discrimination. It has been shown that adult-born neurons are physiologically functional in the olfactory bulb (Carlen et al. 2002; Carleton et al. 2003). Hence, their role in late-in-life olfactory function. Estrogen and prolactin have been shown to enhance neurogenesis in adult animals. Ovariectomized females show a decrease in neurogenesis, and animals that receive replacement estradiol show an enhancement (Tanapat et al. 1999). When cell death was examined in these animals, estrogen replacement not only stimulated cell proliferation but increased the survivability of the neurons as well, which suggest that estrogen may be neuroprotective for neurons (Suzuki et al. 2001; Wise et al. 2001). These data have many implications for hormone replacement therapy and the aging brain, as well as to suggest that natural reproductive experience, of the sort discussed here, may itself be neuroprotective. The fluctuations in the above mentioned hormones and changes in environment mimic another time of life, namely, that of pregnancy, birth, lactation, and maternal behavior. Could the changes we see in maternal female brains contribute to current research about the aging brain?
Shingo et al. (2003) reported that pregnancy and postpartum were accompanied by nearly double the number of new olfactory bulb interneurons. When they examined the factors that may contribute to this increase in neurogenesis, more closely; they found that prolactin, a chief pregnancy and lacational hormone, played a major role. Is the effect limited to the female? Could such a “female oriented hormone” such as prolactin play a role in neurogenesis in males? Few studies have examined the male paternal brain, but Mak and Weiss (2004) recently examined paternal recognition of young and the associated changes in the brain. They found that newly born olfactory interneurons in males were preferentially activated by their offspring’s odors. The interruption of prolactin inhibited the production of new neurons, thus interfering with offspring recognition. Further, the recognition behavior was restored with the restoration of neurogenesis (Mak and Weiss 2010). So, could reproductive experience in this gender promote an “anti-aging” effect? Although there is a lack of thorough research pertaining to life expectancy and reproductive experience in males, one paper suggests that it may have a negative effect in males versus females. Rehm et al. (1984) revealed that Han:WIST female breeder rats live longer than virgins but that virgin males lived longer than their male breeder counterparts—an interesting trade-off, to be sure (sex versus longevity). These data would suggest that reproductive experience reduces lifespan in these animals, though it is important to keep in mind that this particular strain of rats does not often remain to rear the offspring, thus depriving themselves of the potential enriching benefits that may arise from the pup stimulation and care that we describe here. Interestingly, there are some data to suggest that “fatherhood” in primates results in similar brain changes as seen with animals living in an enriched environment (Kozorovitskiy et al. 2006). Specifically, primate fathers had a higher density of dendritic spines and vasopressin V1a receptors in the prefrontal cortex. Whereas we do not know if such changes would lead to a longer lifespan in males, parallel changes have been observed in the brains of maternal animals that appear to “age” better than their virgin counterparts. Thus, reproductive experience may improve the quality and not necessarily the quantity of lifespan. Further, paternal male mice have similar levels of circulating oxytocin compared to maternal mice, thereby making their brains “more maternal” when compared to non-parental males. As discussed above, the hormonal make-up of maternal animals is in part what contributes to their longevity or more successful aging. To the extent that males benefit from reproductive experience, those effects may be related more to care of offspring as opposed to reproductive experience, per se. In any event, the complexities of such endocrinological compromises certainly represent a fertile area for research. If such changes are occurring in maternal and paternal brains to promote and support cell birth and survival, would we expect to see positive persistent “anti-aging” effects in the maternal brain? As we will see in the next section, there are some interesting connections to suggest “yes”.
3 Evidence for Reproductive Experiential Mediation of Nervous System Development
As we saw earlier in this chapter, and in the book overall, the complexities involved in the phenomenon of aging of the nervous system are astronomically complex, and the likely interactions seemingly infinite. There are, however, many strong and long-lasting modifications that occur in the female rat during and following pregnancy, changes that are directed at successful reproduction, and some of which that relate in interesting ways to the aging brain and nervous system. It stands to reason, therefore, that significant events in the life of the animal possess substantial neural consequences. Note that early development and sexual differentiation, as well as puberty, mark the animal for the remainder of its life. No one questions that these events are life-defining. Here, we argue, too, that reproduction may take its place alongside the aforementioned epochs in the animal’s life.
For example, there are permanent modifications in the female’s behavior toward her and other young, a facilitation of maternal behavior, which suggests a likely permanent alteration of cognition associated with reproduction and hence, underlying neural structures. Earlier work by Moltz et al. (1969) had shown that females who experience reproduction and subsequent young retain the memory for pups, and act maternal toward them, many months after their initial exposure. Bridges (1975) demonstrated the lengthy effects that both pregnancy and the experiences of parturition had on the maternal memory for pup responsiveness. A single early reproductive event and associated maternal experience is sufficient to mark the female for many months, the equivalent of decades in the human, in her maternal responsiveness.
Other evidence shows a form of cumulative change to the brain with repeated reproductive experiences. Svare and Gandelman (1976) reported that, in Rockland-Swiss albino mice, postpartum aggression continued to increase in intensity, through parity experiences five or six. The animals displayed more aggression with more pregnancies and lactations, as if the combination of pregnancy, hormone exposure, and pup sensory stimulation built a foundation upon which substrate, subsequent pregnancies acted, like stories in a high-rise building. Parity-induced changes in the endogenous opioid system likewise show a step-wise pattern of effects, with primiparous females being significantly different than virgins, and multiparous being significantly different than primiparous in their sensitivities to both endogenous and exogenous opiates (Kinsley et al. 1999; Mann and Bridges 1992; and in humans, Agaram et al. 2009). Their underlying opioid systems are similarly modified (Bridges and Hammer 1992). Further, Felicio and colleagues have shown a similar effect (parity-induced) on dopamine activity (Bridges et al. 1993; Felicio et al. 1996; Bridges and Grimm 1982). Together, these data suggest a blanket effect of parity on numerous neurochemical systems, a plasticity likely directed at ensuring successful reproduction.
These long term effects on the brain and learning-and-memory and emotional/affective consequences indicate a powerful effect of the most basic and natural of life’s experiences: reproduction. Further, it begs the question: why should such manifest effects occur? We will explore these questions below.
Such neural effects suggest a significant maternal memory for young—for the many cues associated with pups (sights, smells, sounds, gustatory inputs, tactile stimulation, suckling stimulation, etc.)—that is resistant to the degradation of age or at least to interference. These data are suggestive of significant alterations of the substrate for learning, perhaps hippocampus, cortex, etc.
Recent data from our laboratory examined significant decreases in the number of degenerating neurons (as measured by silver-stained neurons) in the dorsal raphe, and frontal, parietal, and cingulate cortices (Love et al. 2005). The possible effects in the cortex are of interest because of recent reports which demonstrate that transient spatial memory information from the hippocampus is transferred to permanent storage in prefrontal and cingulate cortex (Maviel et al. 2004). Changes there include synaptogenesis and reorganization of the laminar layers, reminiscent of the cortical changes in somatosensory cortex in lactating females reported by Xerri et al. (1994). The data on the maternal brain, therefore, suggest an early, natural hormonal (estrogen, progesterone, oxytocin, etc.) mechanism that may eventually protect the aged, parous brain.
The same hormones shown to stimulate maternal behavior may also affect the foundation for such learning as presented above. For instance, in the hormonal profile characteristic of pregnancy, the powerful steroids progesterone (P) and estradiol (E2) regulate the transition from non- to high responsiveness to neonates (Bridges 1984, 1990, 2009) through neuron-hormone interactions and alterations of neuronal activity. The temporal patterning of exposure to E2 and P during pregnancy—in particular, the progressive alteration of the ratio of the two hormones over the 3 weeks of gestation, especially the rat’s third trimester where E2 is significantly elevated—are required for the eventual production and display of maternal behavior, and the requisite brain changes. Other substances, e.g., prolactin, placental lactogens, oxytocin, endogenous opioids, etc. also fluctuate temporally and are vital to both pregnancy and parturition, as well as to the onset of maternal behavior (see, e.g., Bridges et al. 1996, 1997; Kinsley 1994).
The striking influence exerted by natural levels of steroid and protein hormones extends into many areas. For example, the hormones of pregnancy exert significant changes in behavior and physiological regulation in the female, with the immediate onset of maternal behavior—characteristic of the postpartum, maternal female—being the most striking. These rapid and intense behavioral effects are preceded by significant actions of the hormones on the female’s neural substrate. Several studies have reported that P and E2 modify the structure of the neuron in the adult female brain, for example, increasing the concentration of apical dendritic spines in hippocampal neurons (McEwen and Woolley 1994; Woolley et al. 1990; Woolley and McEwen 1992, 1993; Yankova et al. 2001). These effects occur with relatively short exposure to the hormones, primarily E2, during the female’s estrous cycle; if the level or pattern of E2 and P associated with the estrous cycle is prolonged or increased, as occurs during pregnancy, there may be even greater and/or long lasting effects on the morphology of the neuron.
A study using Golgi-Cox silver-staining revealed an example of neural plasticity due to pregnancy and its hormonal exposure (Keyser et al. 2001). Neurons in the medial preoptic area (mPOA), a region that strongly regulates maternal behavior (Numan and Insel 2003), were examined in a group of females from different hormonal groups (ovariectomized [OVX], diestrus, sequential progesterone and estradiol exposed [P and E2, respectively], late-pregnant, and lactating [d5] rats). There was no difference between OVX and diestrous females, and both had smaller somal areas compared to P and E2-treated and late-pregnant females. The area of the soma returned to diestrus/ovx levels in lactating females, suggesting a return to baseline that follows the female’s pregnancy hormonal state. Further, there were similar hormone-induced effects on a number of dendritic branches and cumulative dendritic length in pregnant and hormone-treated groups compared to the OVX, diestrus, and lactating females. The increase in somal area denotes increased cellular activity (Miller and Erskine 1995), whereas the stimulatory effects on additional neuronal variables represent modifications in information processing capacity. Pregnancy and its attendant hormonal exposure, may stimulate neurons in the mPOA and, possibly other regions, which then contribute to the display of maternal behavior and its supporting activities.
For example, whereas the mPOA regulates pup-directed maternal behavior, ancillary sites, are also liable to undergo changes in their own right. Data show that the effects appear to extend to other behaviors and additional brain regions on which the female relies for carrying-out her maternal duties. Reproductive experience and exposure to offspring significantly modify the brain and behavior of the female, particularly those required for effective care and rearing of offspring. For example, age-matched female rats with multiple reproductive experiences (so-called multiparous females) exhibit reductions in opiate sensitivity relative to virgin or primiparous (one parous experience) females (Kinsley and Bridges 1988; Mann and Bridges 1992). Further, parous females performed significantly better in tests of spatial ability, compared to age-matched nulliparous (no reproductive experience) females (Kinsley et al. 1999). Together, the data suggest hormone-neuron interactions that have primarily maternal behavioral implications.
The potentiating experience of pregnancy, lactation, and pup exposure appear to alter female brain and behavior. In fact, it has been argued that these plastic changes are the hallmark of the female brain as it prepares to care for an expensive metabolic and genetic investment for a significant portion of its lifetime (Kinsley and Lambert 2006, 2008; Kinsley et al. 2008; Lambert et al. 2005; Love et al. 2005; Wartella et al. 2003). Our work here examines the extent to which the effects that are coming to light regarding RE are lengthy and widespread ( Lambert and Kinsley 2009). For example, Gatewood et al. (2005) investigated the ramifications of reproductive experience for lifetime effects. They examined the spatial learning and memory of nulliparous, primparous, and multiparous rats out to 2 years post-reproduction. Their data suggest that the effects of RE are long-lived, persistent, and may contribute to late-in-life hippocampal integrity and function.
In the Gatewood et al. work, reproductive experience in females appears to regulate spatial learning and memory throughout life. In this study, the animals were behaviorally tested at six, twelve, eighteen, and twenty-four months of age on a spatial learning task, which required them to remember the location of a baited food well in the dry land maze (DLM; a Morris water maze analog; Kesner and Dakis 1995). Beginning at 12 months of age, and continuing until 24 months, the animals underwent additional testing (on a so-called reversal task), which required that they “un-learn” the original contingency, and learn a new location for the food reward, thereby assessing the flexibility of their learning.
At each age, and for both main and reversal tasks, the multiparous females remembered the baited location significantly faster than both the nulliparous and primiparous females; the primiparous females learned the mazes significantly faster than the nulliparous females at 12, 18, and 24 months (in the main task) and at 12 and 24 months (in the reversal task). These animals appeared to retain the enhanced learning and memory abilities that they developed around the experiences of reproduction. They function more efficiently well out to the latter stage of their lives. We will discuss other aspects of this persistent effect of reproductive experience.
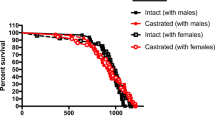
With the behavioral data in hand, the authors sought to examine likely neural regulators—or, at least, associates—of the enhanced memory effect. Thus, when the brains of these animals were examined, the parous animals also had a neural substrate that appeared healthier, as well, at least with regard to a single measure: neural health. The parous rats generally had fewer hippocampal deposits of the deleterious substance, amyloid precursor protein (APP). Compared to nulliparous females, the parous animals’ APP levels were negatively correlated with their performance in both the main and reversal tasks (i.e., more APP equaled poorer performance in the maze). This was a surprising find. In essence, all that these subjects had done to them was mating and pup exposure. Thus, the events surrounding reproductive experience (e.g., mating stimuli, elevations in pregnancy hormones such as estrogen, progesterone, oxytocin, etc., cues and/or stimulation from the young), may summate to produce a female brain that is both flexible and, perhaps, healthier in the aged female rat. The notion of pups as an enriched environment (see Kinsley et al. 1999; Vallée et al. 2001) is suggestive, and there is evidence that enriching environments have positive effects on Alzheimer’s disease pathology (Bennett et al. 2003). Early life experiences (including reproductive?) may help to forestall some negative components of the aging process.
In a preliminary test of the neuroprotective effects of reproductive experience (Kinsley and Brown, unpublished), we examined transgenic mice that over-express the APP protein (Jackson Lab’s B6.Cg-Tg[PDGFB-APP]5Lms/J). We mated half of these mice and allowed them to remain with their offspring through weaning. Then, ten days following weaning, the animals were trained to find a food reward in the DLM. These are preliminary data with a small N (3 three animals per group only.) Nonetheless, the data are provocative: the primiparous B6.Cg-Tg[PDGFB-APP]5Lms/J females significantly out-performed the un-mated females in a probe task which required the mice to spend time near the previously baited food well. Of special note is the fact that the behavioral improvements occurred at a time (~170d) when the age-progression of the APP effects begin to exert themselves in this animal model of age-related neurodegeneration. Therefore, a simple manipulation of these B6.Cg-Tg[PDGFB-APP]5Lms/J mice’s parity status appear capable of marked positive effects on an otherwise baleful protein product associated with senescence and deterioration of cognitive ability. Follow-up experiments are ongoing.
Other recent work has examined some of the gene expression patterns of mothers versus non-mothers (Contino et al. 2007; Kinsley et al. 2008). As we have discussed, life for the single, non-reproductive animal is harsh enough; with a full litter of demanding and “costly” offspring, however, the parous female faces the even more daunting task of keeping herself and her young alive. It follows, therefore, that mechanisms, expressed transiently or otherwise, might have evolved to assist in the dual survival of mother and young. Reproductive experience significantly enhances spatial learning and memory in rats (Kinsley et al. 1999). Others (Tomizawa et al. 2003) have demonstrated the role of oxytocin and other mechanisms in such improvements, coupled to significant modifications of hippocampal CA1 dendritic spine density (Kinsley et al. 2006). How general are the effects of parity on learning and memory, related tasks, and the various neural substrates that either support it directly or rely on it for survival? In a DNA microarray study (Kinsley et al. 2008), we timed-mated females, producing parous, lactating and non-parous groups. At their respective and specific stages of reproduction, we killed the females and isolated the CA1 region from each brain. Using a modified pipette tip punch, we excised bilateral tissue (15–20 mg of tissue/brain). The results showed a significant set of differences between the lactating and NULL females, especially and interestingly, for the set of genes associated with the insulin family. In particular, we found that, overall, there were 91 genes expressed in lactating but not nulliparous females, and 49 genes expressed in the former but not the latter females (Kinsley et al. 2008). Focusing on genes with a two-fold difference or greater (in parentheses), where lactating females >nulliparous females, the following gene expression differences were observed for:
-
Insulin-like growth factor (10.6)
-
Sensory neuron synuclein (8.6)
-
Synapotojanin (6.8)
-
Proenkephalin (4.7)
-
Calmodulin-dependent protein kinase (CaM kinase-GR) (4.6)
-
Insulin-like growth factor binding protein (4.5)
-
Insulin growth factor-binding protein (4.0)
-
SNAP-25a (3.2)
-
Huntington’s disease mRNA (2.8)
-
Potassium channel mRNA (2.7)
-
Glutamate receptor (2.5)
-
Interleukin-1 b-converting enzyme-related protease (2.2)
Where nulliparous females >lactating females, the following pattern was observed:
-
5-Hydroxytryptamine receptor (5HT5b) (11.2)
-
Olfactory inositol 1,4,5-triphosphate receptor (3.4)
-
Na–Ca Exchanger isoform NACA-1 (2.8)
-
RET Ligand-2 (RETL2) (2.6)
-
Neural receptor protein-tyrosine kinase (trkB) (2.4)
-
Glycine receptor a (2.3)
-
P2x (ATP) receptor (2.1)
These data (Kinsley et al. 2008) suggest some intriguing effects of reproductive experience. Among the genes showing expression pattern differences are those considered to be neuroplastic, in particular the insulin-family genes. The latter are interesting, too, in light of recent work by Toth et al. (2007) in which insulin growth factor (Igf) gene expression differences were implicated in the development of eusociality in insects. The authors here showed that in social wasps, females that engaged in brood care either through active reproduction or via foster care demonstrated an up-regulation of the same family of genes observed in mammalian mothers similarly engaged. It is intriguing to think that the act of parental care spreads across the phylogenetic scale a shared set of genetic changes uniting diverse species in the two core principles that have governed life on this planet since its inception: ingestion and reproduction. And it can be argued that feeding merely supports reproduction. Other effects on shared genes can be found in the next section. That feeding and aging well are related, has been shown in the earlier part of this chapter (and following) related to food restriction and insulin gene signaling in diverse species.
In other work, we time-mated females, producing parous (including MULT) and non-parous groups (Contino et al. 2007). At their respective and specific stages of reproduction, we killed the females and isolated the CA1 region from each brain. Using a modified pipette tip punch, we excised bilateral tissue (15–20 mg of tissue/brain). We then flash-froze the samples in –75°C acetone and stored them at –80°C until we determined relative levels of target mRNA expression via Quantitative, Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) analysis of the following neuroplasticity-related genes: brain derived neurotrophic factor (BDNF); cAMP-response-element binding (CREB) protein; neurotrophic tyrosine kinase receptor, type 2 (NTrk2); spinophilin; and syntaxin. To date, the data indicate general increases in gene expression levels for the aforementioned genes. Interestingly, recent work from our laboratory has also shown a different parity effect: lactating females display a significant reduction in pre-frontal cortical APP compared to nulliparous females. It is worthwhile noting that for all the genes examined thus far, APP is the only one that showed a decrease compared to nulliparous females.
The data thus far indicate that, in general, reproductive experience (viz., females that are pregnant, or primiparous/multiparous), modifies the expression of these genes. For each gene, there were significant differences or parallel but non-significant trends among nulliparous, pregnant, and parous females. We are currently evaluating the differences between up-/down-regulation of these genes and their downstream products. In total, the data suggest that the parity-related changes in activity of these specific genes may be at least in part responsible for the observed augmentation in spatial memory (and, possibly other functions) in response to pregnancy and presence of young. Such alterations identify a robust and far-ranging modification of basic neuronal activity in service to the mother and her offspring. Further addressing these intriguing neuroplastic and neurodegeneration-affecting genes is likely to elucidate ways to positively modify the aging process. The insulin gene family is a fascinating thread running through the metabolic changes, of aging, and reproductive plasticity effects, we and others have seen. Together with the diversity of species that show parallel influences, these relationships mark the gene as a major instigator in the path that aging may take in an individual. In fact, the unanimity of aging-related effects across species suggests common mechanisms and present a fascinating basis for future study. In the next section, we examine some of these cross-species relationships.
4 Mechanisms and Likely Connections to Disparate Species and Systems
Facing one’s own mortality may be unique to the human condition, but aging and death are not. In the continuing struggle against time, we have slowly begun to unlock the molecular mysteries of the aging phenomenon by studying the processes in other species. Among the goals in comparing humans to our distant (and not so distant; see Fig. 1) relatives is to find a mechanism—lifestyle, hormone treatment, or drug—that can not only to extend our existence, but to improve the time we do have. Among other benefits, improving aging could help prevent a wide range of age-related diseases and disorders, potentially including cancer and neurodegenerative conditions. Research on a wide array of species, including yeasts, nematodes, flies, rodents, monkeys, and humans has demonstrated conclusively that it is possible to increase lifespan (e.g., Fontana et al. 2010). Pro-aging benefits have been achieved in several different ways and, as we discuss here, it appears that reproductive experience could be playing a role as well. To understand the ways in which aging, longevity, and reproductive experience are interrelated, it is important to have a general understanding of the underlying genetics pathways.
Phylogenetic relationship among organisms in which aging and longevity have been studied (bold). Described here are studies involving mammals (humans, monkeys, mice), fruit flies (D. melanogaster), nematodes (C. elegans), and yeasts (S. cerevisiae). Despite the vast evolutionary distances between these species (approximately 1 billion years between yeasts and humans; Lucking et al. 2009), many of the same genetic pathways involved in regulation of aging are conserved. Tree modified from the Tree of Life Web Project
As we allude to above, perhaps the most thoroughly studied mechanism to increase longevity is caloric/dietary restriction (DR). Dietary restriction can increase the lifespan of the yeast Sacchromyces cerevisiae three-fold, has similar effects in the worm Caenorhabditis elegans and fruit fly Drosophila melanogaster, and can increase the lifespan of Mus musculus by up to 50% (Fontana et al. 2010). Lifespan-promoting benefits are primarily a result of decreases in gene expression in insulin or insulin-like pathways. Regulating insulin pathways result in decrease in age-related decline in function (e.g., errors in cell division) and disease (e.g., diabetes and cardiovascular disease; Fontana and Klein 2007; Anderson et al. 1999). Somewhat surprisingly, single-celled yeasts show these same age-related declines as found in rodents and humans, and improvements to longevity can be modulated through caloric restriction (Fabrizio et al. 2001).
In the presence of dietary restriction, yeasts are able to survive through modification of insulin pathways (Maroso 2005). Rather than dying off, S. cerevisiae actually experiences an extension lifespan. Studies have found that lifespan increases in yeast are possible through deletion of the serine-threonine kinase Sch9 (Fabrizio et al. 2001). Originally thought to be closely related to the human AKT—which has functions in insulin signaling, proliferation, and apoptosis—Sch9 is now considered to be homologous to the ribosomal protein kinase S6 K, another factor in the insulin signaling/aging pathway of a wide range of organisms (Kaeberlein et al. 2005). Under normal conditions, yeasts sense amino acids, activating the target of rapamycin kinase (TOR), whose functional properties include cell growth, proliferation, and cellular motility (Hay and Sonnenburg 2004; Beevers et al 2006), all of which drive the yeast to obtain the amino acids. TOR signaling then stimulates Sch9, eventually leading to cell growth, proliferation, etc. Disruption of this pathway, however, either by deletion of SCH9 (Fabrizio et al. 2001, 2003) or TOR (Kaeberlein et al. 2005; Pan and Shadel 2009) leads to a slowing of normal TOR function, but increases life expectancy by sending the yeasts into a state of reduced development and replication (Fig. 2). Further investigation of downstream targets of Sch9 has revealed how Sch9 mutants are able live up to three times as long as wild-type yeasts. Ge and collegues (2010) revealed that turning off Sch9 may trigger a longevity circuit that includes the regulation of rRNA processing and of a glucose response element. Evolutionarily, modification of the TOR-Sch9 pathway helps yeasts deal with stressful conditions, in particular, decreased amounts of glucose, their primary food source. At times of nutrient restriction, S. cerevisiae decreases cellular proliferation cycles, which in turn extends the time before replication-related dysfunction sets in (leading to aging and death). As an added bonus, modulation of the Sch9 and rRNA pathways confers additional stress-related buffers, including heat-shock resistance (Fabrizio et al. 2001, 2003; Ge et al. 2010). Given the multitude of advantages conferred to yeasts in this scenario, it is no surprise that pathways responding to food intake would be under significant evolutionary pressure and thus, be conserved by so-called “higher” organisms.
A simplified model for the regulation of aging genes shared by yeasts, worms, flies, and mammals (including humans). In yeasts (Sacchromyces cerevisiae, far left), food intake triggers the TOR pathway, which triggers Sch9 and normal cellular activity (respiration, division, etc.). Inhibition of this pathway, either through dietary restriction or inactivation of TOR or Sch9 results in a decrease in normal cellular activity and an increase in longevity. The TOR/Sch9 pathway is conserved in other organisms as well. Inactivation of the pathway again inhibits aging genes through an as-yet unknown mechanism. Additionally, aging may be regulated through the activity of forkhead box transcription factors in Caenorhabditis elegans, Drosophilia melanogaster, and Mus musculus (DAF-16, FOXO, and FOXO1, respectively). In all three species, disruption of the insulin pathway allows functioning of the forhead box transcription factors leading to longer lifespan. Additionally, it is possible to increase functioning of DAF-16, FOXO and FOXO1 through the activity of sirtuin proteins. These proteins are notably found in the plant compound resveratrol, which has received much attention lately. (Modified from Russel and Kahn 2007; Fontana et al. 2010)
The TOR—Sch9 pathway is conserved as a mechanism in Caenorhabditis elegans, Drosophila melanogaster, and Mus musculus (in these organisms, Sch9 is S6 K; Kapahi et al. 2004; Doonan et al. 2008; Selman et al. 2009). The major difference between yeasts and these groups is that the food-response system is indirect in worms, flies, and mice, going through insulin/insulin-like growth factors (Ins/IGF-1-like; Henderson and Johnson 2001; Wijchers et al. 2006; Johnson 2008). Normal functioning of the Ins/IGF-1-F pathway results in cell functioning and replication, which increases the amount of cellular waste products (i.e., oxidants) and opportunities for cellular dysfunctions that lead to aging.
Dietary restriction (DR) reduces the amount of insulin needed to process nutrients, and therefore, lead to down-regulation of the Ins/IGF-1-like signally pathway. In C. elegans, disrupting insulin signaling leads to up-regulation of a forkhead FoxO transcription factor, DAF-16. The activity of FOXO proteins is similar in worms, fruit flies, and mammals—they lead to a variety of activities that promote longer lifespan including fat storage (Feige et al. 2008); modulation of the cellular stress response (Brunet et al. 2004); autophagy (Klionsky and Emr 2000); removal of free radicals (Doonan et al. 2008); and protecting against neurodegeneration (Liu et al. 2004; Mojsilovic-Petrovic et al. 2009). The effects of FOXO are hardly modest—mutations in the Ins/IGF-1-like pathway that lead to FOXO expression can increase the lifespan of worms by 10 times and over-expression of the gene in D. melanogaster can yield a 52% increase (Hwangbo et al. 2004). In addition to decreasing the Ins/IGF-1-like signaling, dietary restriction can enhance the function of sirtuin deacetylase proteins (SIR/SIRT), which appear to help regulate FOXO in worms, flies, and mammals (Tissenbaum and Guarente 2001; Brunet et al. 2004; Frescas et al. 2005; Yang et al. 2005). Alteration to the amounts SIR/SIRT present in animals can lead to significant results. Loss of SIRT has been shown to increase aging in mice (Mostoslavsky et al. 2006) and increases in SIRT may increase lifespan in humans (Rose et al. 2003). Interestingly, the plant compound resveratrol—found in grapes, red wine, and peanuts—may target sirtuin proteins. Resveratrol has become a popular target in the popular media (“Drink wine to lose weight and live longer!”) due largely to studies implicating its role in increasing the lifespan of S. cerevisiae, C. elegans, and D. melanogaster, fish, and even mice (Howitz et al. 2003; Wood et al. 2004; Viswanathan et al. 2005; Baur et al. 2006; Valenzano et al. 2006; Gruber et al. 2007). In addition to the genes and proteins discussed here, there are many more that are being discovered as players in the complex regulation of aging through the general insulin pathway (for an in-depth review, see Greer and Brunet, 2010). Although DR can have profound effects in increasing life-expectancy, some reviewers feel that there are too many complications with humans—decrease in immune function, individuals not wanting to fast—for it to be feasible as a life-extending technique in humans (Phelan and Rose 2005). Fortunately, the genetic underpinnings of aging may help clarify the role of endocrine hormones and reproductive experience in the aging process.
The discovery that hormones critical to sexual reproduction are associated with the regulation of insulin and aging mechanism is striking. Specifically, modification of pregnenolone (C. elegans; Broue et al. 2007) and ecdysone pathways (D. melanogaster; Simon et al. 2003) have both been shown to increase life expectancy in their respective species. Even more interestingly, the changes in steroid hormones are not thought to happen independently from the insulin pathway. Rather, studies suggest that several hormone pathways could be operating downstream of insulin receptors themselves. Importantly, these findings do not propose new functions for already important hormones, but the use of hormones rather than proteins makes possible interactions between multiple cell and tissue types; neurons, fat tissues, and other targets such as heart or liver tissue can all work with one another to regulate growth, cellular proliferation, and reproduction. Understanding the actions of hormones is crucial to understanding the positive effects of reproduction experience on aging.
The regulation of the insulin pathway by hormones mean that aging and anti-aging mechanisms are also affected by hormones in worms, flies, and mammals (Fig. 3). Surprisingly, these hormones are generated not only by the neuroendocrine and reproductive system, but also in fatty tissues. Caenorhabditis elegans utilizes cholesterol from the intestines to produce pregnenolone, a mammalian steroid precursor, which then interacts with DAF16 to trigger anti-aging genes/stress responses as discussed above (i.e., entering at the dauer hibernation stage; Hsin and Kenyon 1999). This interaction may be modulated by another factor in the intestines before sending signals—perhaps KLOTHO, a protein generated in the brain that has been shown to affect longevity in mice by blocking function of insulin receptors (Kuro-o et al. 1997, 2009; Kuroshu et al. 2005)—to target tissues, but it does seem clear that pregnenolone is interacting with both insulin-like factors in neurons and target tissues. In rats, insulin from the pancreas attaches to receptors on fat cells and target tissues to inhibit FOXO1, and thus, anti-aging genes. If, however, insulin signaling is blocked or reduced by either SIRT2 (in adipocytes) or KLOTHO, FOXO1 and anti-aging genes are activated allowing mice to live longer (Coschigano et al. 2003; Flurkey et al. 2001).
A prospective model for the effect of hormones (gray background)—including those related to pregnancy (oval background)—on longevity. KLOTHO, generated in the brain, appears to block insulin receptors in mice and may be involved in signaling in C. elegans. In C. elegans, pregnenalone triggers DAF-16 activity which in turn promotes longevity. In flies, however, it is necessary to down-regulate the ovarian hormone ecdysone in order to see increases in longevity. The story is even more complex in mammals, as the reproductive hormones leptin and estrogen appear both to block FOXO1 signaling and to promote aging through another mediator. It appears that, in general, decreasing reproduction leads to an increase in aging. Pregnancy hormones do, however, appear to have regulatory and beneficial effects on the course of aging in a variety of organisms. Future focused research will enable a better understanding of these intriguing relationships
A link between fatty tissues and reproductive experience may be found in a study revealing that progesterone increases the number of insulin receptors (InR) in adipocytes, allowing the pregnant mother to store fat even though glucose is being preferentially shuttled to the fetus (Flint et al. 1979). This increase in InR would logically lead to decreased functioning of FOXO proteins and thus promote aging. More recent studies, however, have shown that a peptide hormone, leptin, is produced in adipocytes and usually correlates positively with overall body fat mass. Leptin function remains high, however, when insulin receptors are down regulated in fat-specific insulin-receptor (FIRKO) mice (Ahima and Flier 2000). FIRKO mice remain thin even when placed on a high-fat diet and outlive their littermates, who suffer from obesity-related diseases when fed the same diets, by 18% (Bluher et al. 2002, 2003). These data indicate that leptin may play a role in the anti-aging process by promoting insulin sensitivity and keeping animals thin (Russel and Kahn 2007). Lastly, Messines and collegues (2001) found that levels of leptin could be raised in non-pregnant women by adding a progesterone supplement. Thus, we might conclude that pregnancy-related progesterone not only induced adipocytes to create insulin receptors, but also to increase levels of leptin, potentially attenuating some of the negative side-effects of pregnancy on glucose insensitivity.
As previously noted, changes to the insulin pathway help yeasts and worms go into states of hibernation/developmental stasis in times of famine, a condition adaptive enough to have been preserved in metazoans such that mammals can now reap benefits from decreasing our caloric intake (conversely, increases in human obesity rates have recently lead to a decline in life expectancy (Olshansky et al. 2005). Whereas caloric restriction can increase life span, such decreases are also associated with negative side effects that could be considered, including decreased immune function (Fontana and Klein 2007) and infertility (Holliday 2005). The decrease in reproduction is a curious one; evolutionary theory would predict that organisms should optimize reproductive success by living longer. Although this can happen in certain species (Weladji et al. 2006), it is not the case for most organisms.
Several studies have looked for link between reproductive experience and longevity. Although one might suspect that living longer would allow for greater reproductive success, it appears that there is instead a trade-off between being long-lived and having reproductive success. The argument is essentially that there are costs associated with maintaining the mother and her offspring that a non-mother does not incur. In C. elegans, reproductive success does not increase with longevity during dietary restriction. Rather, reproduction is maximized when lifespan decreases to normal levels (Fontana et al. 2010). The same relationship is found in cheetahs (Pettorelli and Durant 2007) and humans. A study in 153 countries worldwide found that in every region there was a negative correlation between age and reproduction (Thomas et al. 2000). Indeed, pregnancy itself can have negative medical side effects such as gestational diabetes, (a disease associated with the insulin pathway; e.g., Kumangai et al. 2003) and work has suggested that knocking out reproduction entirely can have positive effects in mice (Conover and Bale 2007). What is likely happening, then, at least in the case of DR, is that disruption of the insulin pathway allows the organism to live longer and create better reproductive opportunities (i.e., in ideal environmental conditions rather than low-food conditions) rather than more opportunities. Aging better, it seems, is not just about the number of offspring produced.
There are a host of behavioral and biological modifications present in reproductive females that can lead to healthy aging, particularly enhancements in heart and brain function. Alterations to the insulin receptors have been shown to improve heart performance in fruit flies (Wessells et al. 2004). Estradiol, an ovarian sex steroid, helps mice survive oxidative stress (Behl et al. 1995), and pregnenolone, a steroid precursor hormone in humans (currently a popular, if unproven, anti-aging supplement), has been shown to improve neuronal survival (Gursoy et al. 2001) and modulate neurotransmitter systems by promoting microtubule assembly (Murakami et al. 2000). These protective features no doubt let the organism live a more productive (and perhaps happier) life, and may be related to the improvements we describe above.
Recent evidence from mice and rat approaches the question of aging from the opposite direction—discovering improvements in behavior and aging well and then attempting to discern the underlying hormonal basis for those changes. During pregnancy and into lactation, mother rats show superior performance at learning, memory, and physical challenges when compared to non-mothers (Kinsley et al. 1999) and mother rats show decreased stress and anxiety (Wartella et al. 2003). Our laboratory is even finding evidence that mother rats are better at “thinking” about the future (prospective memory) than virgins (Franssen, Rafferty et al. unpublished). What seems clear is that during pregnancy, steroid hormones—including pregnenolone progenitors—are reworking the mammalian brain. Oxytocin alters astrocyte morphology (Modney and Hatton 1994; Hatton and Zhao Yang 2002), neurogenesis increases (Furuta and Bridges 2005), and even short-term changes in estradiol and progesterone changes during pregnancy appear to be able to affect neuroplasticity in the brain (Kinsley et al. 2006; Kinsley 2008). Moreover, these changes are long-lasting. As we discuss above, mothers remain better at learning and memory, well beyond their reproductive years (Gatewood et al. 2005; Love et al. 2005; Kinsley et al. 2008), and are less likely to suffer from dementia (Victoria and Kinsley, unpublished).
5 Summary, Conclusions, and Future Directions
By studying the interactions among genes, proteins, and hormones, we can discover ways in which we can both increase life expectancy and improve our lifestyles in old age. Reproductive experience has been investigated regarding its relationship to life expectancy and in other sets of behavioral benefits. The next step is to incorporate the studies. It is important to discover what, exactly, is the molecular impact of pregnancy hormones and motherhood that lead to long-term memory, anxiety, and stress coping advantages over non-mothers. The work is being done. Progesterone receptors have been mapped in the hippocampus, the brain’s major memory center (e.g., Guerra-Araiza et al. 2001; Auger and De Vries 2002), and neuroprotective capabilities such as increased myelination, and stimulation of mitochondria, are being elucidated (Koenig et al. 1995; Azcoitia et al. 2003). Perhaps most encouragingly, there is evidence that pregnenolone may act as a neurosteroid that can reverse age-related deficits in mammals (including non-mothers), suggesting a possible future treatment for neural diseases like dementia (Schumacher et al. 2003). Although these studies were not necessarily focused on aging, neuroprotection, and enhancement of brain activity are definitely important components of aging well. Understanding the genetic, hormonal, and reproductive data at our disposal, coupled to cross-species comparative approaches, we may soon be able to identify specific lifestyle choices (eat less and reproduce!) and/or drug options to improve the quality of life as life itself wanes.
References
Agaram R, Douglas MJ, McTaggart RA, Gunka V (2009) Inadequate pain relief with labor epidurals: a multivariate analysis of associated factors. Int J Obstet Anesth 18:10–14
Ahima RS, Flier JS (2000) Adipose tissue as an endocrine organ. Trends in Endocrinol Metlab 11:327–332
Altman J, Das GD (1965) Post-natal origin of microneurons in the rat brain. Nature 207:953–956
Anisimov VN et al (2008) Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 7:2769–2773
Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R (2004) The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev 18:3004–3009
Auger CJ, De Vries GJ (2002) Distribution and steroid responsiveness of progestin receptor immunoreactivity within vasopressin-immunoreactive cells in the bed nucleus of the stria terminalis and the centromedial amygdala of male and female rat brain. J Neuroendocrinol 14:161–167
Azcoitia I, DonCarlos LL, Garcia-Segura LM (2003) Are gonadal steroid hormones involved in disorders of brain aging? Aging Cell 2:31–37
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Beevers C, Li F, Liu L, Huang S (2006) Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer 119:757–764
Behl C, Widmann M, Trapp T, Holsboer F (1995) 17-β Estradiol Protects Neurons from Oxidative Stress-Induced Cell Death in Vitro. Biochem Biophys Res Comm 216:473–482
Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes De Leon CF, Arnold SE, Barnes LL, Bienias JL (2003) Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60:1909–1915
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Bluher M, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR (2002) Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3:25–38
Bluher M, Khan BP, Kahn CR (2003) Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299:572–574
Bridges RS (1975) Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav 14:245–249
Bridges RS, Grimm CT (1982) Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science 218:166–168
Bridges RS (1984) A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 114:930–940
Bridges RS (1990) Endocrine regulation of parental behavior in rodents. In: Krasnegor NA, Bridges RS (eds) Mammalian Parenting: Biochemical, Neurobiological and Behavioral Determinants. Oxford University Press, New York, pp 93–117
Bridges RS, Hammer Jr RP (1992) Parity-associated alterations in medial preoptic opiate receptors in female rats. Brain Res 578:269–274
Bridges RS, Felicio LF, Pellerin LJ, Steuer AM, Mann PE (1993) Prior parity reduces post-coital diurnal and nocturnal prolactin surges in rats. Life Sci 53:439–445
Bridges RS, Robertson MC, Shiu RPC, Friesen HG, Stuer AM, Mann PE (1996) Endocrine communication between conceptus and mother: a role for placental lactogens in the induction of maternal behavior. Neuroendocrinology 64:57–64
Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE (1997) Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen. Endocrinol 138:756–763
Bridges RS (2009) The Neurobiology of the Parental Brain. Academic Press, New York
Broue F, Liere P, Kenyon C, Baulieu EE (2007) A steroid hormone that extends the lifespan of Caenorhabditis elegans. Aging Cell 6:87–94
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868
Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng H, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 303:2011–2015
Cameron HA, Gould E (1994) Adult neurogenesis is regulated by adrenal steroids in the rat dentate gyrus. Neuroscience 61:203–209
Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Freisen J (2002) Functional integration of adult-born neurons. Curr Biol 12:606–608
Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM (2003) Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 6:507–518
Carro E, Nunez A, Busiguina S, Torres-Aleman I (2001) Circulating insulin-like growth factorI mediates effects of exercise on the brain. J Neurosci 20:2926–2933
Colman RJ et al (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204
Conover CA, Bale LK (2007) Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell 6:727–729
Contino R, Friedenberg J, Christon L, Norkunas T, Worthington D, Jablow L, Drew M, Victoria L, Chipko C, Sirkin M, Ferguson T, Jones C, Bardi M, Lambert KG and Kinsley CH (2007). The expression of the maternal Brain: Specific genes involved in the neuroplasticity of motherhood. Paper presented at the Society for Neuroscience annual meeting, San Diego, CA, November
Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ (2003) Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144:3799–3810
Cummins RA, Walsh RN, Budtz-Olsen OE, Konstantinos, Horsfall CR (1973) Environmentally induced changes in the brains of elderly rats. Nature 243:516–518
Cummins RA, Livesey PJ, Evans JG (1977) A developmental theory of environmental enrichment. Science 197:692–694
Dagyte G, Trentani A, Postema F, Luiten PG, Den Boaer JA, Gabriel C, Mocaer E, Meerlo P, Van de Zee EA (2010) The novel antidepressant agomelatine normalizes hippocampal neuronal activity and promotes neurogenesis in chronically stressed rats. CNS Neurosci Ther 16:195–207
Diamond MC, Krech D, Rosenzweig MR (1964) The effects of an enriched environment on the histology of the rat cerebral cortex. J Comp Neurol 123:111–120
Diamond MC, Johnson RE, Ingham C (1971) Brain plasticity induced by environment and pregnancy. Int J Neurosci 2:171–178
Doonan R, Mcelwee JJ, Matthijssens F, Walker GA, Houthood K, Back P, Matscheski A, Vanfleteren JR, Gems D (2008) Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22:3226–3241
Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta O, Weiss S (2004) Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24:8354–8365
Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD (2001) Regulation of longevity and stress resistance by Sch9 in yeast. Science 292:288–290
Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD (2003) SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics 163:35–46
Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki PJ, Auwerx J (2008) Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Met 8:347–358
Felicio LF, Florio JC, Sider LH, Cruz-Casallas PE, Bridges RS (1996) Reproductive experience increases striatal and hypothalamic dopamine levels in pregnant rats. Brain Res Bull 40:253–256
Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodeling. Nature 447:178–182
Flint DJ, Sinnett-Smith PA, Clegg RA, Vernon RG (1979) Role of insulin receptors in the changing metabolism of adipose tissue during pregnancy and lactation in the rat. Biochem J 182:421–427
Flurkey K, Papaconstantinou J, Miller RA, Harrison DE (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci 98:6736–6741
Fontana L, Klein S (2007) Aging, adiposity and calorie restriction. JAMA 297:986–994
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span from yeasts to humans. Science 328:321–326
Frescas D, Valenti L, Accili D (2005) Nuclear trapping of the forkhead transcription factor FoxO1 via sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 280:20589–20595
Furuta M, Bridges RS (2005) Gestation-induced cell proliferation in the rat brain. Brain Res Dev Brain Res 156:61–66
Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyers EAA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH (2005) Motherhood mitigates aging-related decrements in learning and memory. Brain Res Bull 59:267–283
Ge H, Wei M, Fabrizio P, Hu J, Cheng C, Longo VD, Li LM (2010) Comparative analyses of time-course gene expression profiles of the long-lived sch9Δ mutant. Nucl Acids Res 38:143–158
Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS (1992) Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 12:3642–3650
Gould E, McEwan BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is reguilated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498
Gould E, Tanapat P, Hastings NB, Shors TJ (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci 95:3168–3171
Greer EL, Brunet A (2010) Signaling networks in aging. J Cell Sci 121:407–412
Gruber J, Tang SY, Halliwell B (2007) Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann NY Acad Sci 1100:530–542
Guerra-Araiza C, Reyna-Neyra A, Salazar AM, Cerbon MA, Morimoto S, Camacho-Arroyo I (2001) Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull 54:13–17
Gursoy E, Cardounel A, Kalimi M (2001) Pregnenolone protects mouse hippocampal (HT-22) cells against glutamate and amyloid beta protein toxicity. Neurochem Res 26:15–21
Halagappa VK et al (2007) Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 26:212–220
Hara T et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889
Harrison DE et al (2009) Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature 460:392–395
Hatton GI, Zhao Yang Q (2002) Peripartum interneuronal coupling in the supraoptic nucleus. Brain Res 932:120–123
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945
Henderson ST, Johnson TE (2001) DAF-16 integrates developmental and environmental inputs to mediat aging in the nematode Caenorhabditis elegans. Curr Biol 11:1975–1980
Holliday R (2005) Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays 10:125–127
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Hsin H, Kenyon C (1999) Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399:362–366
Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M (2004) Drosophila dFOXO controls lifespan and regulates insulin signaling in brain and fat body. Nature 492:562–566
Johnson TE (2008) Caenorhabditis elegans. (2007). The premier model for the study of aging. Exp Gerontol 43:1–4
Kaeberlein M, Powers III RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK (2005) Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310:1193–1196
Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S (2004) Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14:885
Kempermann G (2006) Adult neurogenesis. Oxford, Oxford Press
Kempermann G, Gage F (1998) Closer to neurogenesis in adult humans. Nat Med 4:555–557
Kempermann G, Kuhn G, Gage F (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495
Kempermann G, Brandon EP, Gage F (1998) Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol 8:939–942
Kempermann G, Gast D, Gage F (2002) Neuroplasticity in old age:sustained five-fold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52:135–143
Kenyon CJ (2010) The genetics of ageing. Nature 464:504–512
Kesner R, Dakis M (1995) Phencyclidine injections into the dorsal hippocampus disrupt long-but not short-term memory within a spatial learning task. Psychopharmacology 120:203–208
Keyser L, Stafisso-Sandoz G, Gerecke K, Jasnow A, Nightingale L, Lambert KG, Gatewood J, Kinsley CH (2001) Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull 55:737–745
Kinsley CH, Bridges RS (1988) Parity associated reductions in behavioral sensitivity to opiates. Biol Reprod 39:270–278
Kinsley CH (1994) Developmental psychobiological influences on rodent parental behavior. Neurosci Biobehav Rev 18:269–280
Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG (1999) Motherhood improves learning and memory: Neural activity in rats is enhanced by pregnancy and the demands of rearing offspring. Nature 402:137–138
Kinsley CH, Lambert KG, The Maternal Brain (2006) Pregnancy and motherhood change the structure of the female mammal’s brain, making mothers attentive to their young and better at caring for them. Scientific American January 2006
Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Keyser-Marcus L, Hearon C, Wightman N, Morgan MD, Kozub FJ, Lambert KG (2006) Motherhood and pregnancy hormones modify concentrations of hippocampal neuronal dendritic spines. Horm Behav 49:131–142
Kinsley CH (2008) The neuroplastic maternal brain. Horm Behav 54:1–4
Kinsley CH, Lambert KG (2008) Reproduction-induced neuroplasticity: natural behavioral and neuronal alterations associated with the production and care of offspring. J Neuroendocrinology 20:515–525
Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G (2008) Motherhood Induces and Maintains Behavioral and Neural Plasticity Across the Lifespan in the Rat. Arch of Sex Behav 37:43–56
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE (1995) Progesterone synthesis and myelin formation by Schwann cells. Science 268:1500–1503
Kozorovitskiy Y, Hughes M, Lee K, Gould E (2006) Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci 9:1094–1095
Kumangai S, Holmang A, Bjorntorp P (1993) The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Phys Scand 149:91–97
Kuro-o M (2009) Klotho and aging. Biochem Biophys Acta 1790:1049–1058
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kuroshu H, Yamamoto M, Clark JD, Pastor JV, Nandi A et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Lambert KG, Kinsley CH (2009) The neuroeconomics of motherhood: the costs and benefits of maternal investment. In Bridges RS (ed.), The Neurobiology of the Parental Brain. 481–492, Academic Press
Lambert KG, Berry AE, Griffin G, Amory-Meyer EA, Madonia-Lomas LF, Love G, Kinsley CH (2005) Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav 85:799–806
Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR (2001) Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age related decline in hippocampal neurogenesis. Neuroscience 107:603–613
Liu Y, Neve RL, Taylor P, Driscoll M, Clardy J, Merry D, Kalb RG (2004) FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci 29:8236–8247
Love G, Torrey N, McNamara I, Morgan M, Banks M, Wightman N, Glasper ER, DeVries AC, Kinsley CH, Lambert KG (2005) Maternal experience produces long lasting behavioral modifications in the rat. Behav Neurosci 119:1084–1096
Lucking R, Huhndorf S, Pfister D, Plata ER, Lumbsch H (2009) Fungi evolved right on track. Mycologia 101:810–822
Mak HY, Ruvkun G (2004) Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development 131:1777–1786
Mak GK, Weiss S (2010) Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci 13:753–758
Mann PE, Bridges RS (1992) Neural and endocrine sensitivities decline as a function of multiparity in the rat. Brain Res 580:241–248
Maroso EJ (2005) Overview of caloric restriction and ageing. Mech Ageing Dev 126:913–922
Maviel T, Durkin TP, Menzaghi F, Bontempi B (2004) Sites of cortical reorganization critical for remote spatial memory. Science 305:96–99
McEwen BS, Woolley CS (1994) Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol 29:431–436
Melendez A et al (2003) Autophagy genes are essential to dauer development and life-span extension in C elegans. Science 301:1387–1391
Messines IE, Papageorgiou I, Milingo S, Asprodini E, Kollios G, Seferiadis K (2001) Oestradiol plus progesterone treatment increases serum leptin concentrations in normal women. Hum Repro 16:1827–1832
Miller S, Erskine MS (1995) Ultrastructural effects of estradiol and 5-alpha-androstane on neurons within the ventromedial nucleus of the hypothalamus. Neuroendocrinology 61:669–679
Modney BK, Hatton GI (1994) Maternal behaviors: evidence that they feed back to alter brain morphology and function. Acta Paediatr 83:29–32
Mojsilovic-Petrovic J, Nedelsky N, Boccitto M, Mano I, Georgiades SN, Zhou W, Liu Y, Neve RL, Taylor PJ, Driscoll M, Clardy J, Merry D, Kalb RG (2009) FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neuro 29:8236–8247
Moltz H, Levin R, Leon M (1969) Differential effects of progesterone on the maternal behavior of primiparous and multiparous rats. J Comp Physiol Psychol 67:36–40
Mostoslavsky R, Chua KF, Lombard DB, Pang WW et al. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503
Murakami K, Fellous A, Baulieu E, Robel P (2000) Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly PNAS 97:3579–3584
Nilsson M, Perfilieva E, Johansson U, Orwar O, Erikccon PS (1999) Enriched environment increases neurogenesis in the adult rat and improves spatial memory. J Neurobiol 39:569–578
Numan M, Insel TR (2003) The neurobiology of parental behavior. Springer-Verlag, New York
Olshansky JS, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS (2005) A potential deline in life expectancy in the United States in the twenty-first century. N Engl J Med 352:1138–1145
Pan Y, Shadel GS (2009) Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging 1:131–145
Pascual-Leone A, Amedi A, Fregni F, Merabet LB (2005) The plastic human brain cortex. Ann Rev Neuroscience 28:377–401
Pettorelli N, Durant SM (2007) Longevity in cheetahs: the key to success? Oikos 116:1879–1886
Phelan JP, Rose MR (2005) Why dietary restriction substantially increases longevity in animal models but won’t work in humans. Age Res Rev 4:339–350
Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, Boldogh I (2010) Exercise plays a preventative role against Alzheimer’s disease. J Alzheimers Dis 20:777–783
Rehm S, Deerberg F, Rapp KG (1984) A comparison of life-span and spontaneous tumor incidence of male and female Han: WIST Virginia and retired breeders. Lab Anim Sci 34:458–464
Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, Bonafe M, Franceschi C, Tan Q, Boiko S, Yashin, AI, De Benedictis G (2003) Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol 38:1065–1070
Russel SJ, Kahn CR (2007) Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8:681–691
Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJM, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y (2003) Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 71:3–29
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ (2009) Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 326:140–144
Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S (2003) Pregnancy stimulated neurogenesis in the adult female forebrain is mediated by prolactin. Science 299:117–120
Simon AF, Shih C, Mack A, Benzer S (2003) Steroid control of longevity in Drosophila melanogaster. Science 299:1407–1410
Simonsen A et al (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistence in adult Drosophila. Autophagy 4:176–184
Stern JM (1996) Somatosensation and maternal care in Norway Rats. In: Rosenblatt JS, Snowden CT (eds) Parental care: Evolution, mechanisms, and adaptive significance. Academic Press, New York, NY
Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM (2001) Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol 6:1064–1075
Svare B, Gandelman R (1976) A longitudinal analysis of maternal aggression in Rockland-Swiss albino mice. Dev Psychobiol 9:437–446
Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19:5792–5801
Thomas F, Teriokhin AT, Budilova EV, Brown SP, Renaud F, Guegan JF (2000) Human longevity at the cost of reproductive success: evidence from global data. J Evo Biol 13:409–414
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–230
Tomizawa K, Iga N, Lu Y-F, Moriwaki A, Matsushita M, Li S-T, Miyamato O, Itano T, Matsui H (2003) Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6:384–390
Toth AL, Varala K, Newman TC, Miguez FE, Hutchison SK, Willoughby DA, Simons JF, Egholm M, Hunt JH, Hudson ME, Robinson GE (2007) Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318:441–444
Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A (2006) Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 16:296–300
Vallée M, Mayo W, Le Moal M (2001) Role of pregnenolone, hydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Brain Res Rev 37:301–312
Van der Heide LP, Ramakers GM, Smidt MP (2006) Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol 79:205–221
Van Praag H, Christie B, Sejnowski TJ, Gage F (1999) Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci 96:13427–13431
Viswanathan M, Kim SK, Berdichevsky A, Guarente L (2005) A Role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell 9:605–615
Volkmar FR, Greenough WT (1972) Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science 176:1145–1147
Wartella J, Amory E, Madonia-Lomas L, Macbeth AH, McNamara I, Stevens L, Lambert KG, Kinsley CH (2003) Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav 79:373–381
Weladji RB, Gaillard JM, Yoccoz NG, Holand O, Mysterud A, Loison A, Nieminen M, Stenseth NC (2006) Good reindeer mothers live longer and become better in raising offspring. Proc R Soc 273:15911239–124
Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R (2004) Insulin regulation of heart function in aging fruit flies. Nat Gen 21:127–1281
Wijchers PJ, Burbach JP, Smidt MP (2006) In control of biology: of mice, men and Foxes. Biochem J 397:233–246
Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M, Rosewell KL (2001) Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Brain Res Rev 37:313–319
Witte AV, Fobker M, Gellner R, Knecht S, Floel A (2009) Caloric restriction improves memory in elderly human. Proc Natl Acad Sci 106:1255–1260
Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D (2004) Sirtuin activators mimic caloric restriction and delay aging in metazoans. Nature 430:686–689
Woolley CS, McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult. J Neurosci 12:2549–2554
Woolley CS, McEwen BS (1993) Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306
Woolley C.S, Gould E, Frankfurt M, McEwen B.S (1990) Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 pyramidal neurons. J Neurosci 10:4035–3225
Xerri C, Stern JM, Merzenich MM (1994) Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci 14:1710–1721
Yang Y, Hou H, Haller EM, Nicosia SV, Bai W (2005) Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 24:1021–1032
Yankner BA, Lu T, Loerch P (2008) The aging brain. Annu Rev Pathol 3:41–66
Yankova M, Hart SA, Woolley CS (2001) Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci 98:3525
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kinsley, C.H., Franssen, R.A., Meyer, E.A. (2011). Reproductive Experience may Positively Adjust the Trajectory of Senescence. In: Pardon, MC., Bondi, M. (eds) Behavioral Neurobiology of Aging. Current Topics in Behavioral Neurosciences, vol 10. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2011_123
Download citation
DOI: https://doi.org/10.1007/7854_2011_123
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23874-1
Online ISBN: 978-3-642-23875-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)