Abstract
The single-tablet regimen of sofosbuvir (SOF), an HCV nucleotide analog NS5B polymerase inhibitor, and velpatasvir (VEL), a second-generation HCV NS5A inhibitor, provides a highly efficacious, safe, and simple treatment regimen for patients with genotype 1–6 HCV infection. The clinical development program for SOF/VEL focused on generating safety and efficacy data across a broad range of patient populations to support a single treatment duration for all patients and therapeutic options for patients with compensated and decompensated liver disease. Three Phase 2 studies defined the optimal dose of VEL as 100 mg for a fixed-dose combination tablet with 400 mg of SOF and demonstrated that the treatment duration of 12 weeks provided high SVR rates across all genotypes irrespective of cirrhosis status, prior treatment history, or the presence of baseline resistance-associated substitutions (RASs). The Phase 3 studies enrolled and treated over 1,000 genotype 1–6 HCV-infected patients with 12 weeks of SOF/VEL. In patients with compensated cirrhosis, the overall SVR rate was 98%, and with SOF/VEL + RBV in patients with decompensated cirrhosis, the SVR rate was 94%. With minimal drug-drug interactions and no need for on-treatment safety monitoring, SOF/VEL for 12 weeks provides an important treatment option for patients of all genotypes and is ideally suited to address the global epidemic of chronic HCV infection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hepatitis C virus infection is a global health challenge with approximately 80 million persons infected worldwide [1]. Even with interferon-based therapy targeting the host immune system, treatment response rates varied based on genotype, likely due to the substantial genetic variability across genotypes. Early direct-acting antivirals (DAAs) were designed for maximal efficacy against genotype 1 reflecting its predominance in North America and Europe, and, importantly, the first in vitro HCV replicons were limited to genotype 1 only. At Gilead, the ultimate goal for hepatitis C treatment was to develop an all-oral, pangenotypic regimen that could be safely and simply administered across a broad population. Based on the success of tenofovir disoproxil fumarate-containing single tablet regimens for HIV treatment in both in the developed and developing world, there was a keen recognition of the need for this simplicity to have the maximal impact globally on chronic HCV infection. With this goal in mind, a pangenotypic NS5A inhibitor was developed to coformulate with sofosbuvir (SOF), a pangenotypic nucleotide analog nonstructural protein (NS) 5B polymerase inhibitor.
2 Phase 1 Studies
Sofosbuvir had been well characterized from a clinical pharmacology perspective at the time velpatasvir (VEL) was developed. Thus, the Phase 1 program focused on studies with VEL alone initially and, then, in combination with SOF to further define drug interactions. The plasma half-life for VEL of approximately 15 h supported once daily dosing. Velpatasvir is absorbed relatively rapidly, with a median time to Cmax (Tmax) of 3 h (Gilead). Velpatasvir is highly protein bound (>99.5%) and is minimally metabolized with biliary excretion of unchanged VEL as the major route of elimination accounting for 77% of recovered drug in a clinical absorption, distribution, metabolism, and excretion study (Gilead). Studies conducted in patients with renal insufficiency or hepatic impairment demonstrated that no dose adjustment is needed for VEL in patients with end-stage renal disease or those with severe hepatic insufficiency (Gilead). Furthermore, population pharmacokinetic analysis in HCV-infected patients indicated that race, gender, age, and BMI have no clinically relevant effect on the exposure of VEL (or SOF or its major metabolite, GS-331007). Velpatasvir exposure increases approximately 30% when coadministered with a meal, supporting the dosing recommendation of SOF/VEL to be administered without regard to food.
In vitro, VEL was determined to be a substrate and an inhibitor of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), a substrate of CYP2B6, CYP2C8, and CYP3A4 with slow turnover and an inhibitor of OATP1B1, OATP1B3, and OATP2B1. A large number of drug interaction studies were conducted with SOF/VEL to assess for the potential of clinically meaningful drug interactions. Overall, SOF/VEL has a clinical pharmacology and drug interaction profile that make it well suited for a diverse patient population. Potent inducers of P-gp and/or moderate or potent inducers of CYP2B6, CYP2C8, or CYP3A4 (e.g., rifampin, St. John’s wort, carbamazepine) will reduce plasma concentrations of SOF and/or VEL and should be avoided. Immunosuppressants such as cyclosporine and tacrolimus can be safely coadministered as can opiate substitution therapy and oral contraceptives. Efavirenz and tipranavir are the two antiretroviral agents that should be avoided with SOF/VEL coadministration, and statin exposure can increase with SOF/VEL coadministration – the risk of rhabdomyolysis may be increased for these patients, and for rosuvastatin, a dose no higher than 10 mg daily should be used. Absorption of VEL is pH-dependent, and therefore acid-reducing agents can lower exposure. This effect can be minimized with specific dosing instructions: antacid dosing should be separated by at least 4 h from SOF/VEL; H2-receptor antagonists should be given simultaneously or 12 h apart from SOF/VEL at a dose no higher than famotidine 40 mg twice daily or equivalent; the effect of proton-pump inhibitors up to a dose of omeprazole 20 mg daily or its equivalent can be largely mitigated through coadministration of SOF/VEL with food.
3 Phase 1b Study
Once preliminary safety and pharmacokinetic data were obtained from single and multiple doses of VEL ranging from 50 to 450 mg in healthy subjects, a Phase 1b study, GS-US-281-0102, was undertaken to assess the antiviral activity and safety and pharmacokinetic profiles of VEL administration for 3 days at doses of 5–150 mg in genotype 1–4 HCV-infected patients [2]. A total of 11 dosing cohorts were enrolled across 10 sites in the United States and Puerto Rico: five cohorts of patients with genotype 1a HCV infection (5, 25, 50, 100, and 150 mg VEL); one cohort each of patients with genotype 1b, 2, or 4 HCV infection (150 mg VEL); and three cohorts of patients with genotype 3 HCV infection (25, 50, and 150 mg VEL). Within each cohort, patients were randomized in a 4:1 ratio to VEL or placebo except for the cohort of patients with genotype 4 HCV infection, all of whom received VEL. Patients were excluded from participation if they had cirrhosis or prior exposure to an HCV NS5A inhibitor.
Of the 87 patients treated, 84 completed 3 days of dosing and 2 weeks follow-up (Day 17). One patient discontinued on Day 1 due to an adverse event of nausea, one withdrew consent on Day 4 after completing dosing, and one was lost to follow-up after the Day 7 visit. A total of 61 patients completed 48 weeks of long-term follow-up. Most patients were male (78%), nearly one-third (31%) were black or African-American, and baseline viral load was similar across dosing groups and genotypes with a mean HCV RNA of 6.43 log10 IU/mL. Between Day 1 and Day 17, 21/87 patients (24%) reported at least one adverse event: 18/70 (26%) of the VEL-treated patients and 3/17 (18%) of the placebo-treated patients. All adverse events were mild or moderate in severity with headache being the most frequently reported adverse event (6/87 patients, 7%). No deaths or serious adverse events were reported from Day 1 through week 48 of follow-up. There was no trend in adverse events relative to the dose of VEL and no clinically relevant changes in laboratory values, vital signs, physical examination findings, or ECGs. The pharmacokinetics of VEL were similar to those observed in healthy volunteers and confirmed that VEL is suitable for once-daily dosing in patients with HCV infection.
Administration of three daily doses of VEL resulted in rapid reductions in HCV RNA such that the median maximal decline in HCV RNA across all genotypes at all VEL doses evaluated was >3 log10 IU/mL (Fig. 1). Among patients with genotype 1a HCV infection, the median maximum HCV RNA decline was >3.6 log10 IU/mL at all doses from 5 to 150 mg. Patients with genotype 1b and 2 HCV infection who received VEL 150 mg for 3 days had median (Q1, Q3) maximal viral load reductions of 4.3 (4.2, 4.4) and 4.4 (4.1, 4.8) log10 IU/mL, respectively. The median (Q1, Q3) maximal viral load reductions in patients with genotype 3 HCV infection were 3.2 (1.0, 4.0) log10 IU/mL, 3.1 (1.9, 3.3) log10 IU/mL, and 3.1 (2.9, 3.8) log10 IU/mL for the 25, 50, and 150 mg VEL doses, respectively. The two patients with genotype 4 HCV infection had maximal viral load reductions of 3.9 and 3.0 log10 IU/mL. Patients receiving the 5 mg VEL dose experienced more rapid viral rebound after treatment than patients receiving higher VEL doses although all patients had HCV RNA return to baseline levels during the follow-up period. Analysis of NS5A sequences was also undertaken. At baseline, 22/70 patients (31%) had pretreatment NS5A resistance-associated substitutions (RASs) detected using a cutoff of 1%. Patients with genotype 1 or 3 HCV infection without pretreatment RASs had greater declines in HCV RNA compared to patients with pretreatment RASs. This difference was most notable at the 25 and 50 mg doses of VEL in genotype 3 HCV-infected patients, whereas at the 150 mg dose level, the difference was not observed. Among the patients with 48 weeks of follow-up, RASs that were present at baseline generally persisted through the follow-up period, whereas those that had emerged during treatment tended to decline over time.
Viral load reductions over time following three doses of velpatasvir in (a) genotype 1 HCV-infected patients and (b) genotype 2, 3, or 4 HCV-infected patients (Reproduced from [2])
Based on the totality of safety, pharmacokinetic, and antiviral activity, 25 and 100 mg doses of VEL were selected to move forward in combination with SOF for Phase 2 trials in HCV-infected patients.
4 Phase 2 Studies
The potent antiviral activity of VEL across genotypes 1–4 and the previously demonstrated efficacy of SOF as well as the combination of ledipasvir (LDV) and SOF as an approved single-tablet regimen for genotype 1, 4, 5, or 6 HCV infection suggested that the combination of SOF/VEL would be highly efficacious as a therapeutic regimen. Thus, the Phase 2 program was designed to address three fundamental questions. The first was regarding dose selection for VEL (25 mg versus 100 mg); the second was regarding duration of treatment (8 weeks versus 12 weeks); the third was regarding the contribution of ribavirin to safety and efficacy. Recognizing that SOF/VEL had the potential with its pangenotypic activity to be a cornerstone of an HCV elimination strategy globally which would include resource-limited settings, the goal was to determine the optimal dose and duration to provide maximal efficacy and safety across a broad patient population irrespective of genotype, prior treatment history, or fibrosis status to advance into Phase 3 clinical trials and, ultimately, to patients where genotyping would no longer be a necessary component of the HCV treatment algorithm. Safety, efficacy, and pharmacokinetic data were generated from three Phase 2 studies described separately below.
4.1 Study GS-US-342-0102
Study GS-US-342-0102 enrolled treatment-naïve genotype 1–6 HCV-infected patients without cirrhosis [3]. The study was conducted at 48 sites in the United States from August 2013 through August 2014 in two parts. In Part A, genotype 1–6 HCV-infected patients were randomized to receive SOF 400 mg with velpatasvir, 25 or 100 mg, for 12 weeks (groups 1–6). In Part B which was initiated following a review of the safety and efficacy of patients enrolled in Part A, genotype 1 or 2 HCV-infected patients were randomized to receive SOF 400 mg with velpatasvir 25 or 100 mg, with or without weight-based RBV (1,000–1,200 mg daily) for 8 weeks (groups 7–14). Patients were required to have cirrhosis excluded by either liver biopsy within 2 years of screening, a FibroTest score of 0.48 or less and an aspartate aminotransferase-platelet ratio index of 1 or less during screening, or a Fibroscan score of 12.5 kPa or less within 6 months of baseline. Additional exclusion criteria included HIV or HBV coinfection, hepatic decompensation, prior treatment for HCV, and select laboratory abnormalities. The primary endpoint was sustained virologic response 12 weeks after treatment completion (SVR12).
A total of 377 patients were randomized and treated. Table 1 shows demographic, disease, and baseline characteristics by dose and duration. In general, patients were representative of a treatment-naïve population in the United States. Within the different dosing groups, demographic factors were balanced across the different genotypes. All but three patients completed study treatment. One genotype 3 HCV-infected patient receiving SOF + VEL 25 mg for 12 weeks discontinued at week 8 due to virologic failure; one genotype 1 HCV-infected patient receiving SOF + VEL 25 mg for 8 weeks discontinued at Day 6 due to adverse events of abdominal pain, palpitations, and dizziness; and one genotype 1 HCV-infected patient receiving SOF + VEL 100 mg for 8 weeks discontinued due to noncompliance with study drugs.
Overall, among the 377 patients randomized and treated, 337 (89%) achieved SVR12 (Table 2). In part A, assessing 12 weeks of SOF + VEL treatment, the SVR12 rate was 96% (26/27) in those receiving SOF + VEL 25 mg (group 1) and 100% (28/28) in those receiving SOF + VEL 100 mg (group 2). Among patients with genotype 3 HCV infection, the SVR12 rate was 93% (25/27) in those receiving SOF + VEL 25 mg (group 3) as well as SOF + VEL 100 mg (group 4). The two patients who did not achieve SVR12 in group 3 experienced virologic failure – one subject had a 5 log10 IU/mL HCV RNA reduction after 4 weeks of treatment but failed to fully suppress by week 8 and thus met a virologic stopping criterion; the other patient relapsed at posttreatment week 4. In group 4, one patient experienced virologic relapse, and one patient had evidence for reinfection with genotype 2b that was not detectable with deep sequencing prior to treatment. The SVR12 rate in patients with genotype 2, 4, 5, or 6 HCV infection receiving SOF + VEL 25 mg (group 5) or SOF + VEL 100 mg was 96% (22/23) and 95% (21/22), respectively. There were no virologic failures in either of these groups; one patient committed suicide prior to posttreatment week 12, and the other patient was lost to follow-up after completing treatment. The high SVR rate and low rate of virologic failure in treatment-naïve, genotype 1–6 HCV-infected patients without cirrhosis treated for 12 weeks with SOF + VEL 25 mg or 100 mg supported assessing a shorter treatment duration.
In order to examine both the 25 and 100 mg doses of VEL and the impact of RBV on an 8-week treatment duration, part B was limited to genotype 1 or 2 patients only. This facilitated enrollment, as well, given the genotype distribution within the United States. The shortened treatment duration of 8 weeks for genotype 3 HCV-infected treatment-naïve patients was assessed in a Phase 2 study conducted in New Zealand and is discussed below. Rates of SVR12 among genotype 1 HCV-infected patients were 87% (26/30) for those receiving VEL 25 mg, 83% (25/30) for those receiving VEL plus RBV, 90% (26/29) for those receiving VEL 100 mg, and 81% (25/31) for those receiving VEL plus RBV. Other than one patient in the SOF + VEL 25 mg group who discontinued treatment on Day 6 and one patient in the SOF + VEL 100 mg group who was lost to follow-up, virologic relapse occurred in patients not achieving SVR12. Among genotype 2 HCV-infected patients, SVR12 rates were 77% (20/26) with VEL 25 mg, 88% (22/25) with VEL 25 mg plus RBV, 88% (23/26) with both VEL 100 mg and VEL 100 mg plus RBV. One patient in the VEL 25 mg plus RBV group did not complete posttreatment assessments, and all other non-SVR12 patients experienced virologic relapse.
Deep sequencing of the HCV NS5A and NS5B genes was performed from pretreatment samples from all patients and from posttreatment samples from all patients with virologic failure. Of the 377 patients enrolled, 375 and 372 had sequencing data for HCV NS5A and NS5B, respectively. The prevalence of pretreatment NS5A RASs detected with a 15% cutoff was 34% (128/375) and 18% (25/142), 23% (7/31), 48% (58/122), and 24% (13/54) in patients with genotype 1a, 1b, 2, and 3 HCV infections, respectively. In contrast, the rates of pretreatment HCV NS5B RASs were much lower with only 5% (17/372) of patients overall having these RASs at baseline. Overall, rates of SVR12 were similar among patients with pretreatment NS5A RASs (90%) as compared to those without pretreatment NS5A RASs (92%). The impact of pretreatment NS5A RASs did not substantially differ based on treatment duration and/or genotype.
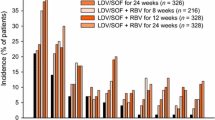
Overall, treatment with SOF + VEL with or without RBV was well tolerated, with only one patient (<1%) discontinuing treatment due to an adverse event. This patient, a 19-year-old white woman with genotype 1 HCV infection, was receiving SOF + VEL 25 mg and experienced mild abdominal pain, mild palpitations, and moderate dizziness on treatment Day 6. The investigator assessed these events as related to study drug, and treatment was discontinued on the following day. All of these events resolved by Day 2 of follow-up. Across all treatment groups, there were low rates of serious adverse events (2%), none of which were assessed by the investigator as related to study drugs, and one death occurred in the study: a 36-year-old man with genotype 2 HCV infection and preexisting psychiatric disease committed suicide after completing 12 weeks of treatment with SOF + VEL 25 mg (group 5). Patients administered with RBV-containing regimens had a higher incidence of RBV-associated toxicities such as fatigue, insomnia, and rash and laboratory abnormalities consistent with hemolysis such as decreased hemoglobin and elevated bilirubin levels. No difference in the type or incidence of adverse events between treatment regimens with respect to dose of VEL or treatment duration was observed. Fatigue and headache were the only adverse events occurring in >10% of patients in the SOF + VEL 100 mg 12-week treatment groups.
Sofosbuvir with VEL 25 or 100 mg for 12 weeks was well tolerated and resulted in high SVR12 rates in noncirrhotic patients infected with genotypes 1–6. With 8 weeks of treatment, higher relapse rates were observed among the genotype 1 or genotype 2 HCV-infected patients at both the 25 and 100 mg dose of VEL, and the addition of RBV did not impact SVR12 rates. These data supported the further development of a fixed-dose combination tablet of SOF/VEL at the 12-week treatment duration.
4.2 Study GS-US-337-0122
The impact of shortening SOF + VEL treatment duration from 12 to 8 weeks in genotype 1 or 2 HCV-infected patients was assessed in GS-US-342-0102, conducted in the United States where genotype 3 HCV infection represents approximately 6% of total HCV-infected patients. In contrast, genotype 3 HCV-infected patients make up over 30% of total HCV infections in New Zealand. Study GS-US-337-0122 (ELECTRON-2) was an ongoing Phase 2 clinical trial at two sites in New Zealand. This trial was amended to assess the safety and efficacy of SOF + VEL 25 or 100 mg with or without RBV for 8 weeks in treatment-naïve genotype 3 HCV-infected patients without cirrhosis [4].
A total of 104 patients were randomized to one of the four treatment groups. Demographic and baseline characteristics are provided in Table 3. The patient population was similar to that enrolled in GS-US-342-0102 with the exception of a higher percentage of native Hawaiian/Pacific Islander patients enrolled in this trial and higher percentage of black patients enrolled in GS-US-342-0102. All but two patients completed treatment. One patient withdrew consent for participation in the study, and one patient discontinued treatment due to a flare of preexisting eczema.
Virologic outcomes following 8 weeks of SOF + VEL at both dose levels and with or without RBV are presented in Table 4. All patients who received SOF + VEL 25 mg for 8 weeks achieved SVR12. The SVR12 rate was 88% in patients who received SOF + VEL 25 mg + RBV for 8 weeks with two patients experiencing virologic relapse and one patient discontinuing treatment prior to virologic suppression. In the SOF + VEL 100 mg groups, SVR rates were 96% and 100%, without and with RBV, respectively. There were no virologic failures in either treatment group; one patient withdrew consent from the trial.
The combination of SOF + VEL 25 or 100 mg with or without RBV for 8 weeks was well tolerated. One patient discontinued SOF + VEL 25 mg + RBV treatment due to an exacerbation of facial eczema and eye inflammation. A second patient in the SOF + VEL 100 mg group discontinued RBV only due to dyspepsia and lethargy. Only one serious ADVERSE EVENT occurred in the trial (convulsion) which was assessed by the investigator as unrelated to study drug. No difference in the type or incidence of ADVERSE EVENTs between treatment regimens with respect to dose of VEL or treatment duration was observed.
This Phase 2 study suggested that high SVR12 rates could be achieved in genotype 3 HCV-infected patients without cirrhosis treated for 8 weeks with SOF + VEL. The higher dose of VEL 100 mg was associated with a slightly higher SVR rate – no virologic failures were observed in the SOF + VEL 100 mg treatment groups. The addition of RBV increased hematologic toxicity but did not improve efficacy.
4.3 Study GS-US-342-0109
Study GS-US-342-0109 was conducted in parallel with Study GS-US-342-0102 and enrolled treatment-experienced genotype 1 or 3 HCV-infected patients with or without cirrhosis [5]. The study was conducted at 58 sites in the United States, Australia, and New Zealand from June 2013 through August 2014. Three cohorts of patients were enrolled: treatment-experienced genotype 3 HCV-infected patients without cirrhosis, treatment-experienced genotype 3 HCV-infected patients with cirrhosis, and treatment-experienced genotype 1 HCV-infected patients with or without cirrhosis. For genotype 3 HCV-infected patients, treatment-experienced was defined as having failed prior therapy with an interferon-based regimen, whereas for genotype 1 HCV-infected patients, prior treatment experience was limited to patients who had failed an NS3/4A protease inhibitor in combination with peginterferon and RBV. Within these three cohorts, patients were randomized to one of four treatment groups to receive SOF 400 mg with VEL, 25 or 100 mg, with or without weight-based RBV (1,000–1,200 mg daily) for 12 weeks. Inclusion and exclusion criteria were otherwise similar to GS-US-342-0102, the Phase 2 trial of SOF + VEL in treatment-naïve genotype 1–6 HCV-infected patients. The primary endpoint was SVR12.
A total of 321 patients were randomized and treated. Table 5 shows demographic, disease, and baseline characteristics by dose and duration. In general, patients were representative of a treatment-experienced population. As compared to treatment-naïve patients without cirrhosis enrolled in GS-US-342-0102, these patients were slightly older, and there was a higher percentage of males, non-IL28B CC genotype, higher viral load, and abnormal ALT levels at baseline. Within the different dosing groups, demographic factors were balanced across genotype 1 and genotype 3 patients. Approximately 1/3 of the genotype1 HCV-infected patients had cirrhosis. All but two patients completed study treatment. One genotype 3 HCV-infected patient without cirrhosis receiving SOF + VEL 25 mg + RBV for 12 weeks discontinued treatment due to elevated gamma glutamyltransferase (GGT) and ALT, and one genotype 3 HCV-infected patient with cirrhosis receiving SOF + VEL 25 mg + RBV for 12 weeks discontinued due to noncompliance with study drugs and subsequently withdrew consent.
Table 6 shows SVR12 rates in all treatment groups. Among the treatment-experienced patients with genotype 3 HCV infection without cirrhosis who received SOF plus VEL 25 mg without or with RBV, the SVR12 rates were 85% and 96%, respectively. All treatment-experienced patients with genotype 3 HCV infection without cirrhosis who received SOF plus VEL 100 mg without or with RBV achieved SVR12. Among the treatment-experienced patients with genotype 3 HCV infection and cirrhosis who received SOF plus VEL 25 mg without or with RBV, the SVR12 rates were 58% and 84%, respectively. The SVR12 rates in treatment-experienced patients with genotype 3 HCV infection and cirrhosis who received SOF plus VEL 100 mg without or with RBV were 88% and 96%, respectively. Among patients with genotype 1 HCV infection who had not achieved SVR after previous treatment with a protease inhibitor regimen, SVR 12 rates were 100% and 97% in those treated with SOF plus VEL 25 mg without and with RBV, respectively, and 100% and 96% in those treated with SOF plus VEL 100 mg without and with RBV, respectively. In contrast to the efficacy data generated in treatment-naïve patients in Study GS-US-342-0102 which didn’t differentiate between the two doses of VEL, in the current study including genotype 3 treatment-experienced patients, the VEL 100 mg dose demonstrated higher SVR rates as compared to those observed with SOF + VEL 25 mg.
Deep sequencing of the HCV NS5A and NS5B genes was successfully performed from pretreatment samples for 321 and 318 patients, respectively, and from posttreatment samples from all patients with virologic failure. The prevalence of pretreatment NS5A RASs detected with a 15% cutoff was 17% (53/321) overall: 17% (36/210) in patients with genotype 3 HCV infection and 15% (17/111) in patients with genotype 1 HCV infection. Among patients with genotype 3 HCV infection without cirrhosis, SVR12 rates were similar in patients with and without NS5A RASs. Only 1 of the 11 genotype 3 HCV-infected patients with cirrhosis who relapsed following treatment with SOF plus VEL 25 mg had pretreatment NS5A RASs. The SVR12 rate among genotype 1 HCV-infected patients with RASs was 96% (16/17). Overall, these data suggested that NS5A RASs did not influence treatment outcome even with the lower dose of VEL. The prevalence of HCV NS5B RASs overall was lower with only 4% (11/318) of patients having NS5B RASs at baseline. All but one of these patients achieved SVR12.
Treatment with SOF + VEL with or without RBV was well tolerated, with only one patient (<1%) discontinuing treatment due to an adverse event. This patient, a 58-year-old white woman without cirrhosis and genotype 3 HCV infection, was receiving SOF + VEL 25 mg plus RBV and experienced an elevated ALT and GGT levels on treatment Day 80. The investigator assessed these events as related to a study drug, and treatment was discontinued on the following day; she achieved SVR12. This patient’s GGT level returned to pretreatment levels by posttreatment Day 11 and ALT level normalized by posttreatment Day 33. Total bilirubin levels remained normal throughout. Across all treatment groups, there was a low rate of serious adverse events (2%), and none was assessed by the investigator as related to study drugs. Patients administered with RBV-containing regimens had a higher incidence of RBV-associated toxicities such as fatigue, insomnia, and rash and laboratory abnormalities consistent with hemolysis such as decreased hemoglobin and elevated bilirubin levels. No difference in the type or incidence of adverse events between treatment regimens with respect to dose of VEL or treatment duration was observed. Adverse events were similar to those observed in Study GS-US-342-0102 and did not differ based on cirrhosis status.
Among treatment-experienced genotype 1 or 3 HCV-infected patients with or without cirrhosis, SOF with VEL 100 mg for 12 weeks resulted in consistently high SVR12 rates. With the lower dose of VEL, higher relapse rates were observed among the genotype 3 HCV-infected patients with or without cirrhosis; the addition of RBV improved SVR12 rates to some extent in this situation. Given the goal of the SOF/VEL program to have a single-tablet regimen supporting a single treatment duration for patients irrespective of genotype, prior treatment, or cirrhosis status, these data, in combination with those from GS-US-342-0102, supported the further development of a fixed-dose combination tablet of SOF 400 mg/VEL 100 mg for a 12-week treatment duration.
As described above, these Phase 2 studies assessed the combination of SOF and VEL coadministered as separate agents. It is worth noting that while these trials were ongoing, significant formulation efforts were underway to develop a fixed-dose combination (FDC). Since the dose for Phase 3 was unknown at the time, both the 25 and 100 mg doses of VEL were coformulated with SOF 400 mg. As the SOF/VEL 400/100 mg FDC was selected to move ahead into Phase 3, this formulation was assessed in a bioavailability study comparing the pharmacokinetics of the two drugs coadministered as separate agents as compared to administered as an FDC. The exposure to SOF, SOF metabolites, and VEL was similar across both formulations thus enabling transition to the single-tablet regimen for the registrational Phase 3 trials. This Phase 1 study, 342-0104, also assessed the impact of high-fat or medium-fat meal on the pharmacokinetics of the SOF/VEL FDC and demonstrated that food modestly increased VEL exposure to a degree that would not be anticipated to impact efficacy or safety based on the clinical data. These results enabled coadministration of SOF/VEL without regard to food in the Phase 3 studies.
5 Phase 3 Studies
The dose of VEL (100 mg) in combination with SOF 400 mg and the duration of therapy (12 weeks) were established based on the safety and efficacy results in genotype 1–6 HCV-infected patients enrolled in the three Phase 2 studies. The SOF/VEL Phase 3 studies were designed to evaluate the efficacy and safety of treatment with SOF/VEL in a diverse subject population with respect to HCV genotypes and subtypes, demographic characteristics, and geographical regions. Three multicenter studies evaluated SOF/VEL in subjects without cirrhosis or with compensated cirrhosis, and one multicenter study evaluated regimens of SOF/VEL in subjects with decompensated cirrhosis. At this time (first half of 2014), SOF had been approved, in combination with pegylated interferon for genotype 1 and 4 HCV-infected patients and in combination with RBV for 12 or 24 weeks in genotype 2 or 3 HCV-infected patients, respectively. This treatment landscape informed the study design for each trial, as outlined below. The goal was to demonstrate that SOF/VEL could be a highly effective, single-tablet 12-week treatment regimen for all HCV-infected patients with compensated liver disease, irrespective of HCV genotype or subtype, stage of fibrosis, prior interferon-based treatment, or pretreatment viral resistance. In addition, a Phase 3 study in HCV-infected patients with decompensated cirrhosis was also conducted – a treatment population without any approved treatment options at that time.
5.1 ASTRAL-1
ASTRAL-1 was a Phase 3, double-blind, placebo-controlled study involving untreated and previously treated patients with chronic HCV genotype 1, 2, 4, 5, or 6 infection, including those with compensated cirrhosis [6]. A separate trial with an active comparator group was deemed necessary for patients with genotype 3 HCV infection in light of the special clinical challenges presented in this population, particularly those with cirrhosis and/or prior treatment failure. In ASTRAL-1, patients with HCV genotype 1, 2, 4, or 6 were randomly assigned in a 5:1 ratio to receive SOF/VEL (400 mg/100 mg) in a once-daily, fixed-dose combination tablet or matching placebo for 12 weeks at 81 sites in the United States, Canada, Europe, and Hong Kong from July 18, 2014, through December 19, 2014. Because of the low prevalence of genotype 5 in the study regions, patients with genotype 5 did not undergo randomization but were assigned to the SOF/VEL group. Patients in the placebo group were eligible for deferred treatment with 12 weeks of SOF/VEL. The primary endpoint was SVR12. The protocol allowed enrollment of patients with compensated cirrhosis as well as those who had previously been treated for HCV with a regimen not containing an HCV NS5B inhibitor or NS5A inhibitor. No upper limits were placed on age or body mass index.
Of the 740 patients treated, 35 patients with genotype 5 HCV infection were enrolled directly into the SOF/VEL group, 624 were randomized to receive SOF/VEL, and 116 patients were randomized to receive matching placebo. Demographic and baseline characteristics were generally balanced across these groups (Table 7). In the SOF/VEL group, 34% of the patients had HCV genotype 1a, 19% genotype 1b, 17% genotype 2, 19% genotype 4, 6% genotype 5, and 7% genotype 6. Most patients were white (79%) and male (60%). Nineteen percent of the patients had cirrhosis, 69% had a non-CC IL28B genotype (which has been associated with a reduced response to interferon-based HCV treatment), and 32% had received previous unsuccessful treatment for HCV. Of the 201 patients in the SOF/VEL group who had received previous treatment, 28% had received a regimen of peginterferon, RBV, and a protease inhibitor, and 61% had received peginterferon and RBV; 48% of these patients had persistently detectable HCV RNA while receiving previous treatment, and 51% had a virologic relapse or breakthrough. A total of 51% of patients were enrolled in Europe, 46% in North America (Canada and the United States), and 3% in Hong Kong.
Overall, the rate of SVR12 among patients who received 12 weeks of SOF/VEL was 99% (95% confidence interval [CI], 98 to >99), which was significantly superior to the prespecified performance goal of 85% (P < 0.001) (Fig. 2). None of the 116 patients in the placebo group had an SVR. Rates of SVR were similar regardless of the HCV genotype: 98% (95% CI, 95 to >99) in patients with genotype 1a infection, 99% (95% CI, 95–100) with genotype 1b, 100% (95% CI, 97–100) with genotype 2, 100% (95% CI, 97–100) with genotype 4, 97% (95% CI, 85 to >99) with genotype 5, and 100% (95% CI, 91–100) with genotype 6. Of the 121 patients with any genotype who had cirrhosis, 120 (99% [95% CI, 95 to >99]) had a SVR. Of the 624 patients who received at least one dose of SOF/VEL, 2 (<1%) had virologic failure: a 56-year-old white man without cirrhosis who had received no previous treatment for genotype 1a HCV infection and a 58-year-old black man with cirrhosis who had persistently detectable HCV RNA during previous peginterferon – RBV treatment for genotype 1b HCV infection. The two men had undetectable serum HCV RNA at week 4 of treatment, and both had a virologic relapse by posttreatment week 4. Four other patients in the SOF/VEL group did not achieve an SVR. Two of the four were lost to follow-up (one did not return after completing 45 days of treatment; the other completed treatment and had undetectable serum HCV RNA at posttreatment week 4 but did not return for the posttreatment week 12 visit), one discontinued treatment because of an adverse event, and one died during follow-up. Rates of SVR in all patient subgroups, including those with cirrhosis (99%) and prior treatment experience (>99%), were high.
SVR12 rates in ASTRAL-1 overall and by genotype (Reproduced from [6]). Error bars represent 95% confidence intervals
At baseline, NS5A resistance-associated variants were detected in 257 of 616 patients (42%) for whom sequencing data were available. Of these 257 patients, 255 (99%) had an SVR. The two patients who had virologic failure did not have NS5A-resistant variants at baseline but did so at the time of relapse. The patient with HCV genotype 1a infection who had a relapse had the Y93N variant detected in more than 99% of the viral population. The second patient (with HCV genotype 1b who had a relapse) had the Q30R (in 98.7%) and L31M (in >99%) at baseline and Q30R (in >99%), L31M (in >99%), and Y93H (in 99%) at the time of relapse. The Q30R variant confers an increase by a factor of 2.2 in the 50% effective concentration (EC50) of VEL in the HCV genotype 1a replicon. Arginine (R) variants at position 30 of the NS5A protein were present at baseline in 62 patients in the entire study population: 5 patients with genotype 1, 5 with genotype 2, 50 with genotype 4, and 2 with genotype 5. Of these 62 patients, 60 (97%) had an SVR. Variants associated with resistance to NS5B nucleoside inhibitors were detected at baseline in 54 of the 601 patients (9%) for whom sequencing data were available. No S282 variants were detected. All 54 patients had an SVR.
Twelve weeks of SOF/VEL treatment was well tolerated with the type, frequency, and severity of nonserious adverse events generally similar in both groups (Table 8). Of the 624 patients in the SOF/VEL group, 1 (<1%) discontinued treatment prematurely because of an adverse event. This patient, a 52-year-old white woman with genotype 1a HCV infection without cirrhosis, discontinued treatment because of an anxiety attack on the 13th day of treatment. Of the 116 patients in the placebo group, 2 (2%) discontinued treatment because of an elevated aminotransferase level, a prespecified criterion for discontinuation. A total of 15 patients (2%) in the SOF/VEL group had 19 serious adverse events. No single serious adverse event occurred in more than one patient. There was one death in the SOF/VEL group. This patient, a 55-year-old white man with HCV genotype 5a without cirrhosis who had a history of dyslipidemia for which he was taking ezetimibe–simvastatin, died during sleep 8 days after the completion of treatment. The cause of death was not determined. The patient was not taking amiodarone. None of the patients in the placebo group had a serious adverse event. There was no significant difference in the rates of any adverse event in the SOF/VEL group and the placebo group (78% and 77%, respectively). The rates of individual adverse events did not differ significantly between the two groups. The most common adverse events were headache, fatigue, nasopharyngitis, and nausea. Hematologic abnormalities were infrequent in the SOF/VEL group, affecting 1% of patients or less. No patients in the placebo group had hematologic abnormalities. No patient in either study group had a Grade 3 or 4 elevation in creatinine (>3.0 mg/dL) or total bilirubin (>2.5 mg/dL).
5.2 ASTRAL-2
After the protocol for ASTRAL-1 was finalized and trial activity had begun, the US Food and Drug Administration requested a separate study be conducted with an active comparator for patients with HCV genotype 2. ASTRAL-2 was a Phase 3, open-label, active comparator trial involving untreated and previously treated patients with chronic HCV genotype 2 infection, including those with compensated cirrhosis [7]. Patients with HCV genotype 2 were randomly assigned in a 1:1 ratio to receive SOF/VEL (400 mg/100 mg) in a once-daily, fixed-dose combination tablet or SOF + RBV for 12 weeks, the standard of care at the time the study was conducted. The primary endpoint was SVR12. The protocol allowed enrollment of patients with compensated cirrhosis as well as those who had previously been treated for HCV with an interferon-based regimen. Inclusion and exclusion criteria were similar to those in ASTRAL-1 including no upper limits on age or body mass index. Due to the need for RBV coadministration, a creatinine clearance of greater than 50 mL/min at screening was required.
A total of 266 patients were randomized and treated at 51 sites in the United States from October 15, 2014, through December 18, 2014. Randomization was stratified by cirrhosis status and prior treatment history. The demographic and baseline characteristics of patients were generally balanced across treatment groups (Table 9). Most of the patients were white men and had non-CC IL28B genotype. A total of 14% of patients had cirrhosis, and 14–15% had received unsuccessful treatment for HCV. All but two patients (<1%), one in each treatment group, completed treatment. One patient in the SOF/VEL group discontinued treatment on Day 1 due to adverse events of difficulty concentrating, headache, and anxiety. One patient in the SOF + RBV group completed the week 10 visit and was subsequently lost to follow-up.
The rate of SVR12 was 99% (95% confidence interval [CI], 96–100) among those who had received SOF/VEL for 12 weeks, as compared with 94% (95% CI, 88–97) among those who had received SOF + RBV for 12 weeks (Fig. 3). The study met its primary statistical endpoint in that those treated with SOF/VEL had an SVR12 rate that was significantly superior to that among patients who had received the standard treatment of SOF + RBV for 12 weeks, with a strata-adjusted absolute difference of 5.2 percentage points (95% CI, 0.2–10.3, P = 0.02 with the Cochran–Mantel–Haenszel test stratified according to cirrhosis status and previous treatment). There were no virologic failures among patients receiving SOF/VEL. One 57-year-old black man discontinued study treatment on Day 1 after receiving one dose of the study drug because of adverse events. Of the 132 patients who received SOF + RBV, 6 (5%) had a virologic relapse, and 2 other patients were lost to follow-up. Deep sequencing indicated that approximately 60% of the 134 patients in the SOF/VEL group had NS5A RASs and 10% had NS5B RASs at baseline. The most prevalent NS5A variant observed at baseline was L31M in 52% of the patients. Despite the presence of pretreatment NS5A and NS5B RASs, no patient receiving SOF/VEL had virologic failure.
Overall, treatment with SOF/VEL or SOF + RBV for 12 weeks was generally safe and well tolerated (Table 10). A smaller percentage of subjects in the SOF/VEL 12-week group experienced any adverse event (69%, 92 of 134) compared with the SOF + RBV 12-week group (77%, 101 of 132), including treatment-related adverse events (SOF/VEL, 34%; SOF + RBV, 57%) and adverse events leading to modification or interruption of any study drug (SOF/VEL, 0; SOF + RBV, 10%). The most common adverse events were reported by a smaller percentage of subjects in the SOF/VEL 12-week group compared with the SOF + RBV 12-week group, including fatigue (15% vs 36%), headache (18% vs 22%), nausea (10% vs 14%), and insomnia (5% vs 14%). Most adverse events were Grade 1 (mild) or Grade 2 (moderate) in severity. Grade 3 (severe) adverse events were rare (SOF/VEL, 2%; SOF + RBV, 2%). No Grade 4 (life-threatening) adverse events were reported. Serious adverse events were also rare (2%, four of 266 subjects [2 in each treatment group]). No serious adverse event was reported in >1 subject. All serious adverse events were considered by the investigators to be not related to study drug. Two nontreatment-emergent deaths were reported during the study (metastatic lung cancer and cardiac arrest after treatment completion). Only one subject permanently discontinued any study drug (SOF/VEL) due to adverse events. Hematologic laboratory abnormalities consistent with RBV-induced hemolysis were observed in the SOF + RBV arm but not in patients treated with SOF/VEL.
5.3 ASTRAL-3
Before the availability of direct acting antiviral agents, HCV genotypes 2 and 3 were grouped together in treatment guidelines as “easy-to-cure” genotypes. However, in the era of direct acting antivirals, HCV genotype 3 has been associated with fewer available treatment options and lower rates of treatment response. Furthermore, some studies have suggested HCV genotype 3 is associated with more rapid disease progression and a higher rate of complications such as hepatocellular carcinoma. A simple, RBV-free regimen that would be highly effective in patients irrespective of genotypes would be highly desirable. The ASTRAL-3 study was a Phase 3, open-label, active comparator study involving untreated and previously treated patients with chronic HCV genotype 3 infection, including those with compensated cirrhosis [7]. Patients with HCV genotype 3 were randomly assigned in a 1:1 ratio to receive SOF/VEL (400 mg/100 mg) in a once-daily, fixed-dose combination tablet or SOF + RBV for 24 weeks, the standard of care at the time the study was conducted. The primary endpoint was SVR12. The protocol allowed enrollment of patients with compensated cirrhosis as well as those who had previously been treated for HCV with an interferon-based regimen. Inclusion and exclusion criteria were similar to those in ASTRAL-1 and ASTRAL-2 including no upper limits on age or body mass index. Due to the need for RBV coadministration, a creatinine clearance of greater than 50 ml/min was required at screening.
A total of 552 patients were randomized and treated at 76 sites in the United States, Canada, Europe, Australia, and New Zealand from July 30, 2014, through December 17, 2014. Randomization was stratified by cirrhosis status and prior treatment history. The demographic and baseline characteristics of patients were generally balanced across treatment groups (Table 11). Most of the patients were white men and had non-CC IL28B genotype. Nearly a third of patients had cirrhosis, and just over one quarter had undergone unsuccessful treatment.
The rate of SVR12 was 95% (95% CI, 92–98) among those who had received SOF/VEL for 12 weeks, as compared with 80% (95% CI, 75–85) among those who had received 24 weeks of SOF + RBV (Fig. 4). The SVR12 rate with 12 weeks of SOF/VEL was significantly superior to that with 24 weeks of SOF + RBV. The strata-adjusted absolute difference was 14.8 percentage points (95% CI, 9.6–20.0; P < 0.001 with the Cochran–Mantel–Haenszel test stratified according to cirrhosis status and previous treatment). Among the 277 patients who received SOF/VEL, 11 (4%) had virologic failure after the end of treatment, and 2 patients were lost to follow-up. Among the 275 patients who received SOF + RBV, 38 (14%) had a relapse after treatment, 1 had virologic failure during treatment, 6 were lost to follow-up, 4 discontinued treatment because of adverse events, 2 withdrew consent, 2 died, and 1 discontinued treatment before achieving undetectable HCV RNA. Among patients who received SOF/VEL, the SVR rate was 91% among those with cirrhosis, as compared with 97% among those without cirrhosis. Among patients who received SOF + RBV, the rates of SVR among patients with and those without cirrhosis were 66% and 87%, respectively. A similar pattern of response was seen according to whether patients had received previous treatment. Among patients in the SOF/VEL group, the rate of SVR was 90% among those who had received previous HCV treatment, as compared with 97% among those who had received no previous treatment. The corresponding rates among patients in the SOF + RBV group were 63 and 86%. The rate of SVR among patients who had received previous treatment and who had evidence of cirrhosis was 89% in the SOF/VEL group as compared with 58% in the SOF + RBV group. Sustained virologic response did not appear to be correlated with the IL28B genotype or early viral kinetics.
Of the 274 patients in the SOF/VEL group who had available data on virologic outcome with deep sequencing data, 43 (16%) had detectable NS5A RASs (A30K, L31M, and Y93H) at baseline. Of these patients, 38 (88%) had an SVR. Of the 25 patients with the Y93H variant at baseline, 21 (84%) had an SVR. Of the 231 patients without NS5A RASs at baseline, 225 (97%) had an SVR. All ten patients with baseline NS5B resistance-associated variants (N142T, L159F, E237G, L320I, and V321A/I) had an SVR.
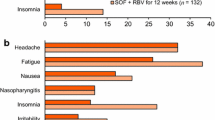
Treatment with SOF/VEL for 12 weeks was well tolerated in this study and compared favorably with SOF + RBV for 24 weeks (Table 12). Overall, adverse events and Grade 3 and 4 adverse events occurred less frequently in patients in the SOF/VEL 12-week group than in the SOF + RBV 24-week group. For the majority of adverse events that occurred in ≥10% of patients in either treatment group, there was a lower incidence of adverse events in SOF/VEL-treated patients than in SOF + RBV-treated patients. Adverse events associated with the hematological, constitutional, dermatologic, and neuropsychiatric toxicities of RBV were, as expected, markedly less common in the SOF/VEL 12-week group than in the SOF + RBV 24-week group: anemia (0.4% vs 9%), fatigue (26% vs 38%), arthralgia (4% vs 8%), pruritus (3% vs 13%), dry skin (0.7% vs 9%), insomnia (11% vs 27%), irritability (8% vs 15%), and anxiety (3% vs 8%). There were no discontinuations due to adverse events in the SOF/VEL 12-week group compared with nine discontinuations due to adverse events in the SOF + RBV 24-week group, suggesting that the more favorable safety and tolerability profile of the SOF/VEL 12-week group resulted in a higher rate of treatment completion. There were no Grade 4 adverse events or treatment-related serious adverse events in the SOF/VEL 12-week group. Three deaths were reported in the study: one due to gunshot wounds, one due to natural causes, and one due to an unknown cause; all three patients were in the SOF + RBV 24-week group. There were no clinically meaningful Grade 3 or 4 laboratory abnormalities in the SOF/VEL 12-week group. Consistent with the expected toxicity profile of RBV, decreases in hemoglobin and lymphocytes and increases in reticulocytes, platelets, and total bilirubin were observed in the SOF + RBV 24-week group.
5.4 ASTRAL-4
Prior to SOF-based therapies, the only treatment option for HCV-infected patients who had progressed to decompensated cirrhosis was liver transplantation; interferon-based therapies were associated with poor response rates and unacceptable toxicities including death. The compassionate use program with SOF + RBV demonstrated proof-of-concept that patients with advanced liver disease could be safely and effectively treated. However, treatment durations of 24–48 weeks were required, and efficacy was not optimized. Ledipasvir/sofosbuvir plus RBV for 12 weeks subsequently showed high SVR rates and excellent tolerability in both pretransplant and posttransplant genotype 1 or 4 HCV-infected patients. With these data and the Phase 1 study demonstrating that velpatasvir pharmacokinetics were not substantially altered in severe hepatic impairment, ASTRAL-4 was undertaken to assess the possibility for a pangenotypic treatment option for HCV-infected patients with decompensated cirrhosis. The study set out to address both the impact of treatment duration and the need for RBV in HCV-infected patients with Child-Pugh-Turcotte (CPT) B cirrhosis. The ASTRAL-4 study was a Phase 3, open-label study involving untreated and previously treated patients with chronic HCV genotype 1–6 infection and CPT B cirrhosis [8]. Patients were randomly assigned in a 1:1:1 ratio to receive SOF/VEL once-daily for 12 weeks, SOF/VEL plus weight-based RBV for 12 weeks, or SOF/VEL for 24 weeks. The primary endpoint was SVR12.
A total of 267 patients were randomized and treated at 47 sites in the United States from August 17, 2014, through December 19, 2014. Randomization was stratified by genotype. The demographic and baseline characteristics of patients were generally balanced across treatment groups (Table 13). Overall, 60% of patients had HCV genotype 1a, 18% genotype 1b, 4% genotype 2, 15% genotype 3, 3% genotype 4, and less than 1% genotype 6; no patients had genotype 5. A total of 6% of patients were black, and 55% had received prior treatment for HCV infection. The median baseline CPT score was 8 (range, 5–10), the median baseline MELD score was 10 (range, 6–24), and the median creatinine clearance was 84.7 mL/min (range, 15–198). The majority of patients (95%) had a baseline MELD score of 15 or less. All the patients had CPT class B cirrhosis at screening, but 27 patients (10%) had CPT class A or CPT class C cirrhosis at treatment baseline, which reflects the dynamic changes in CPT scoring in this population.
Rates of SVR were 83% (95% confidence interval [CI], 74–90) in patients who received SOF/VEL for 12 weeks, 94% (95% CI, 87–98) among those who received SOF/VEL + RBV, and 86% (95% CI, 77–92) among those who received SOF/VEL for 24 weeks (Fig. 5). Post hoc analyses did not detect any significant differences in SVR rates among the three treatment groups. Among patients with HCV genotype 1, the SVR rate was 88% for those who received SOF/VEL for 12 weeks, 96% for those who received SOF/VEL + RBV, and 92% for those who received SOF/VEL for 24 weeks. Among the smaller population of patients with HCV genotype 3, the SVR rate among patients who received SOF/VEL + RBV was 85%, as compared with 50% for the two groups that received SOF/VEL alone. All the patients with HCV genotype 2, 4, or 6 had an SVR except for one patient with HCV genotype 2 who was assigned to receive SOF/VEL for 24 weeks; this patient died of liver failure after completing 28 days of treatment. A total of 22 patients had virologic failure: 11 of 90 patients (12%) who received SOF/VEL for 12 weeks, 3 of 87 patients (3%) who received SOF/VEL + RBV, and 8 of 90 patients (9%) who received SOF/VEL for 24 weeks. Of the 22 patients who had virologic failure, 20 had a relapse, and 2 (both with HCV genotype 3) had virologic breakthrough. One of the patients with virologic breakthrough, a 56-year-old white man who was assigned to receive SOF/VEL + RBV, had undetectable plasma levels of study drugs at the time of virologic failure, which suggests nonadherence. The other patient with virologic breakthrough was a 52-year-old white man with HCV genotype 3a who was assigned to receive SOF/VEL for 24 weeks. This patient had an HCV RNA level of less than 15 IU/mL from week 4 through week 10 with low levels of HCV RNA (26–80 IU/mL) at week 12 and week 16; the patient’s participation in the study was terminated early at week 16 because he met the stopping criteria for virologic failure. There was no evidence to suggest nonadherence. Also counted among the patients with treatment failure were four who were lost to follow-up and seven who died before the primary endpoint.
Of the 255 patients for whom pretreatment NS5A sequencing data were available, 72 (28%) had pretreatment NS5A RASs. Of these 72 patients, 64 (89%) had an SVR, as compared with 169 of 183 patients (92%) who did not have pretreatment NS5A RASs. Among patients with HCV genotype 1 receiving SOF/VEL + RBV, the SVR rate in those with NS5A RASs was 100%, and the rate without such variants was 98%. Among patients with HCV genotype 1 in the SOF/VEL groups who had pretreatment RASs, the SVR rate was 80% among those who received 12 weeks of treatment and 90% among those who received 24 weeks of treatment; among those who did not have RASs, the rates were 96% and 98%, respectively. An analysis of the effect of resistance on treatment outcome in patients with HCV genotype 3 was limited by the small number (six patients) with RASs in our study. The majority of patients who had virologic failure had NS5A RASs at the time of failure; NS5B RASs were less common and typically observed at low levels. Of the 251 patients for whom pretreatment NS5B deep-sequencing data were available, 8 had pretreatment RASs (at positions N142T, L159F, E237G, and M289I). All eight patients had an SVR.
In this population of patients with decompensated liver disease, understanding whether achieving SVR is associated with improved outcomes is important [9]. Among patients who achieved SVR24 and had CPT and MELD scores available, 54% had an improvement in the CPT score over baseline, 36% had no change in the CPT score, and 10% had a worsening in the CPT score (Table 14). Of the 223 patients with a baseline MELD score of less than 15 for whom MELD data were available at posttreatment week 24, a total of 49% had an improved MELD score, 25% had no change in the MELD score, and 26% had a worsening in the MELD score. Among patients with a baseline MELD score above 15 who achieved SVR24, 72% had an improved MELD score, 4% had no change, and 24% had a worsened MELD score at posttreatment week 24.
A total of 9 patients discontinued study treatment prematurely because of an adverse event: 1 of 90 patients (1%) who received SOF/VEL for 12 weeks, 4 of 87 patients (5%) who received SOF/VEL + RBV, and 4 of 90 patients (4%) who received SOF/VEL for 24 weeks (Table 15). No adverse event that led to discontinuation of a study drug was reported in more than one patient. Serious adverse events occurred in 19% of patients who received SOF/VEL for 12 weeks, 16% of those who received SOF/VEL + RBV, and 18% of those who received SOF/VEL for 24 weeks. The most common serious adverse events were hepatic encephalopathy and sepsis (with each event occurring in five patients across groups). The most common adverse events in all groups were fatigue (29%), nausea (23%), and headache (22%), although anemia, diarrhea, and insomnia were also common among the patients who received SOF/VEL + RBV. Overall, 81% of patients in the groups who received SOF/VEL alone had at least one adverse event, as compared with 91% of patients receiving SOF/VEL + RBV. Nine deaths occurred during the study. Two patients died after discontinuing study treatment but within 30 days after the end of treatment, and seven patients died more than 30 days after the end of treatment. Most of the deaths were due to complications of end-stage liver disease (i.e., liver failure, sepsis, or multiorgan failure). The nine deaths were evenly divided among the three treatment groups; none were considered to be related to therapy by the investigator. Reductions in hemoglobin, lymphocytes, and platelets were common in all three groups. In the group that received SOF/VEL + RBV, decreases in hemoglobin to less than 10.0 g/dL occurred in 23% of patients and decreases to less than 8.5 g/dL in 7% of patients. In the groups that received SOF/VEL, the rates of decrease in hemoglobin were 8% and 1%, respectively, among those who received 12 weeks and 9% and 1% among those who received 24 weeks. Anemia or reductions in hemoglobin were successfully managed in the majority of patients with a modification of or interruption in the RBV dose, although one patient was treated with erythropoietin. Two patients who received SOF/VEL for 12 weeks required the infusion of packed red cells for the treatment of gastrointestinal bleeding. Hyperbilirubinemia that was consistent with hemolysis was primarily observed in patients receiving SOF/VEL + RBV.
5.5 Summary of Phase 3 Data Supporting Initial Registration of SOF/VEL
A total of 1,035 patients were treated with 12 weeks of SOF/VEL across the three Phase 3 studies in genotype 1–6 patients with compensated liver disease. The overall SVR rate was 98% (1,015/1,035) with a 1% rate of virologic failure. These data conclusively demonstrated that SOF/VEL for 12 weeks provides a simple, highly effective, and safe single-tablet regimen for HCV-infected patients irrespective of genotype or demographic or baseline disease characteristics. This simplicity can enable expansion of treatment to nearly all HCV-infected patients and allow extension of the provider network beyond specialist physicians. Removing the need to genotype reduces overall cost of care, as does the absence of a need for on-treatment laboratory monitoring. Among patients with decompensated cirrhosis, SOF/VEL + RBV for 12 weeks resulted in a 94% SVR rate and was associated with an improvement in CPT and/or MELD scores in the majority of patients enrolled in ASTRAL-4. These data from the four registrational Phase 3 studies supported the approval of SOF/VEL (Epclusa®) in the United States on June 28, 2016, as the first interferon-free pangenotypic regimen and the only pangenotypic regimen indicated for both patients with compensated and decompensated liver disease. Since then, Epclusa has been approved in the European Union and in many other regions worldwide.
5.6 ASTRAL-5
HIV-infected patients coinfected with chronic HCV have more rapid progression of liver disease. In the interferon era, response rates to treatment were low, and tolerability was poor such that the decision to treat these patients was challenging and the management complex, involving a coordinated effort across hepatology and infectious disease experts. In light of these differences in efficacy and safety among the HIV/HCV coinfected population, these patients were considered a “special population,” and dedicated studies were required in some regions to gain approval for treatment of these patients. The ASTRAL-5 study was a Phase 3 open-label, single-arm trial of 12 weeks of SOF/VEL in HIV/HCV coinfected patients with genotype 1–6 HCV infection [10]. Patients with compensated cirrhosis and/or prior treatment failure were permitted, and most HIV antiretroviral regimens were allowed, as well as enrollment of subjects not on current treatment for HIV. The primary endpoint of the trial was SVR12.
Of the 106 patients enrolled at 17 sites in the United States, 91 (86%) were men, 48 (45%) were black, and 19 (18%) had cirrhosis. SVR12 was achieved by 101 (95%; 95% CI 89–99) of 106 patients; 74 (95%, 87–99) of 78 with genotype 1, all 11 (100%, 72–100) with genotype 2, 11 (92%, 62–100) of 12 with genotype 3, and all 5 (100%, 48–100) with genotype 4. All 19 patients with cirrhosis had SVR12. Two patients relapsed, two were lost to follow-up, and one withdrew consent. Two discontinued treatment due to adverse events and two had serious adverse events. The most common adverse events were fatigue (25%), headache (13%), arthralgia (8%), and upper respiratory tract infection (8%). These data were consistent with the Phase 3 studies in HCV monoinfected patients and demonstrated that SOF/VEL for 12 weeks was safe and provided high rates of SVR12 in patients with HCV and HIV coinfection. This study supported the supplemental indication in the United States for SOF/VEL for the treatment of coinfected patients, granted on August 2, 2017 [11].
6 Conclusion
The single-tablet regimen of SOF/VEL combines a pangenotypic nucleotide HCV NS5B polymerase inhibitor (SOF) with a pangenotypic HCV NS5A inhibitor (VEL). Large Phase 3 studies in diverse patient populations inclusive of individuals with multiple traditionally negative predictive factors for treatment response (e.g., cirrhosis, prior treatment failure) demonstrated high SVR rates as well as a favorable safety profile. These data support the use of SOF/VEL for 12 weeks in genotype 1–6 HCV-infected patients without cirrhosis or with compensated cirrhosis and SOF/VEL + RBV for 12 weeks in genotype 1–6 HCV-infected patients with decompensated cirrhosis. With SOF/VEL as a treatment option, the only decision a healthcare provider needs to make is regarding the addition of RBV for those patients with decompensated cirrhosis. The simplicity of the regimen (one pill once daily for a single duration for HCV-infected patients with compensated liver disease), limited drug interactions, and a favorable safety profile make it ideally suited to address the global health challenge of chronic HCV and fulfill the World Health Organization’s target of HCV elimination by 2030.
References
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H (2014) Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 61:S45–S57
Lawitz E, Freilich B, Link J et al (2015) A phase 1, randomized, dose-ranging study of GS-5816, a once-daily NS5A inhibitor, in patients with genotype 1-4 hepatitis C virus. J Viral Hepat 22:1011–1019
Everson GT, Towner WJ, Davis MN et al (2015) Sofosbuvir with velpatasvir in treatment-naïve noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection: a randomized trial. Ann Intern Med 163:818–826
Gane EJ, Hyland RH, An D, McNally J, Brainard DM, Symonds WT, McHutchison JG, Stedman DA (2014) Once daily sofosbuvir with GS-5816 for 8 weeks with or without ribavirin in patients with HCV genotype 3 without cirrhosis result in high rates of SVR12: the ELECTRON2 study. Hepatology 60(4 (suppl)):236A
Pianko S, Flamm SL, Shiffman ML et al (2015) Sofosbuvir plus velpatasvir combination therapy for treatment-experienced patients with genotype 1 or 3 hepatitis C virus infection: a randomized trial. Ann Intern Med 163:809–817
Feld JJ, Jacobson IM, Hezode C et al (2015) Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 373:2599–2607
Foster GR, Afdhal N, Roberts SK et al (2015) Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 373:2608–2617
Curry MP, O’Leary JG, Bzowej N et al (2015) Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 373:2618–2628
O’Leary J. EASL, 2016, #SAT-169
Wyles D, Brau N, Kottilil S et al (2017) Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label phase 3 study. Clin Infect Dis 65:6–12
Gilead Sciences Inc. (2018) EPCLUSA® (sofosbuvir and velpatasvir) tablets, for oral use. US Prescribing Information, Foster City
Acknowledgment
The authors would like to thank the patients and their families as well as study site staff who participated in the clinical trials of Epclusa.
Compliance with Ethical Standards
Conflict of Interest Diana M. Brainard and John G. McHutchison are employees of Gilead Sciences, Inc.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. The authors would like to thank the patients and their families as well as study site staff who participated in the clinical trials of Epclusa.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Brainard, D.M., McHutchison, J.G. (2019). The Clinical Development of Sofosbuvir/Velpatasvir (SOF/VEL, Epclusa®). In: Sofia, M. (eds) HCV: The Journey from Discovery to a Cure. Topics in Medicinal Chemistry, vol 32. Springer, Cham. https://doi.org/10.1007/7355_2018_43
Download citation
DOI: https://doi.org/10.1007/7355_2018_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28399-5
Online ISBN: 978-3-030-28400-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)