Abstract
Soil remediation by electro-Fenton (EF) process has been recently proposed in literature. Being applied for solution treatment, EF is mainly combined with soil washing (SW)/soil flushing (SF) separation techniques to remove the organic pollutants. The main criteria influencing the combined process have been identified as (1) operating parameters (electrode materials, current density, and catalyst (Fe2+) concentration), (2) the matrix composition (nature and dose of extracting agent, pH, complexity of SW/SF solutions), and (3) the environmental impact (acute ecotoxicity and biodegradability of effluent as well as impact on soil microbial activity). The influence of these parameters on the SW/EF and SF/EF integrated processes has been reviewed. Energy consumption calculations have been finally considered as it constitutes the main source of operating cost in EF process.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nowadays, soil pollution is a topic of the major importance not only because of the direct consequences of this pollution on ecosystems but also because it may lead to the pollution of supply water reservoirs and, consequently, prevent their use. This is especially important in regions that traditionally lack water and in areas where periodic droughts (now intensified with the climate change) make water a very valuable resource, which may even limit its economic and social subsistence. One of the types of pollution, which is gaining more and more attention in the scientific community because of its relevance, is the pollution with organic compounds, in particular with non-biodegradable anthropogenic organic species such as solvents, hydrocarbons, and pesticides. It is not a simple problem because these species can have very different characteristics in terms of hazardousness, biodegradability, solubility in water, and volatility, and, hence, there is not a unique efficient treatment that can be successfully applied for their depletion [1,2,3].
Instead, there are many types of competing technologies that can be applied to solve this important problem, and, nowadays, scientists are trying to shed light on the choice of the best for each type of pollutant and soil. Some of them, like soil washing (SW) of vapor extraction, transfer the pollutant from the soil to a different phase (liquid or gas), which is later treated ex situ in a more efficient way, removing rapidly the pollution from soil and avoiding its dispersion. They are very important, in fact, key technologies in the solution of the problem, because treatment of a large volume of soil affected by diffuse pollution is more difficult and, overall, more expensive than the treatment of a much lower volume of soil highly polluted with the same contaminant.
Regarding the transport of pollution from soil to a liquid, there are two main technologies: SW (ex situ) or soil flushing (SF) (in situ). The first needs the excavation of the soil and its transport to a washing unit, in which pollutants are removed in the best operation conditions by selecting the optimal washing fluid composition and volume, mixing rate, temperature, and contact time [2, 4, 5]. It may attain a very good removal of pollutants from the chemical point of view, but other soil characteristics like compaction are dramatically modified during this treatment, and special care should be taken after the treatment to try to come back to the pristine properties, once the soil is cleaned and placed again in the zone that it occupied before the pollution event. The composition of the SW fluid is rather important and in case of removal of low-solubility pollutants, the addition of extracting agents is key to extract them in efficient conditions [1, 2, 6]. Treatment of the SW wastes produced becomes a very important point to have an integrated solution to the problem, because it typically consists of highly loaded wastewater containing the soil pollutant, extracting agents, and many other species extracted from soil. Selective removal of pollutant in order to try to regenerate the SW fluid for reuse is the optimum solution looked for, because it may lead to a very efficient treatment technology from the viewpoint of sustainability and economy.
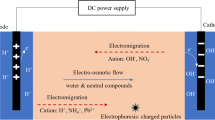
The other alternative consists of flushing a fluid throughout the soil to drag the pollutants contained and to collect this fluid into a special zone, where the flushing fluid is pumped to a subsequent liquid treatment [7,8,9]. This alternative modifies much less importantly soil characteristics, but it is more difficult to select the best extraction operation conditions because soil remains in its position during the treatment. In case of high permeability soil, the flushing fluid is pumped and collected directly without further requirements, using the gradient of hydrostatic pressure (pump and treat technology) as driving force for the transport of fluid. For low-permeability soils, this driving force is not efficient, and, here, the application of an electric field between pairs of anode-cathode may activate more complex transport processes such as electroosmosis, electromigration, and electrophoresis, commonly known as electrokinetic treatment. As in the SW technologies, these processes can be combined with an efficient composition of flushing fluid, which helps to drag efficiently pollutants that cannot be dragged directly by water. At this point, extracting agents may play a very important role as in the SW processes, although in SF, interactions are much more complex. These treatments also produce a polluted flushing fluid which should be treated once produced and the ideal final point of this treatment is to remove pollutants without affecting extracting agents and other possible additives in order to regenerate the flushing fluid and recycle it to the treatment.
There are many technologies that can be used to treat the SW and SF wastes. Initially, biological process should be the primary election because of their lower cost. However, it is important to remind that SW and SF are applied when in situ bioremediation technologies are not efficient and this means that pollutant should be hardly removed by microorganisms either in soil or in a liquid waste. In this context, advanced physicochemical technologies become the target for the treatment of these types of wastes. Among them, electrochemical advanced oxidation processes (EAOPs) are very promising [10], and one of them is going to be widely described in this chapter, i.e., the electro-Fenton (EF) process. In parallel, there have been many work carried out in the recent years in the development of other EAOPs such as anodic oxidation, photoelectrolysis, and sonoelectrolysis [11,12,13,14,15]. EF has the advantages (1) to generate in situ Fenton’s reagent leading to the formation of •OH, (2) to be less dependent on the mass transport of the pollutants thanks to homogeneous catalysis, (3) to avoid sludge formation and •OH wasting reactions thanks to controlled generation of H2O2 and Fe2+, and (4) to favor some selective oxidation as discussed later in this chapter.
EF treatment has been conventionally applied ex situ for SW/SF solutions [1, 2, 16] or a mixture of solutions with solid particles [17, 18], by generating hydroxyl radicals (•OH) through Fenton reaction in bulk solution [19] (Eq. 1):
A synthetic table (Table 1) summarizes the different research articles studying the combination between SW/SF and EF treatment for soil remediation.
All the SW/EF and SF/EF studies have been focused on hydrophobic organic contaminants (HOCs) such as petroleum hydrocarbons [9], polycyclic aromatic hydrocarbons (PAHs) including phenanthrene (PHE) and the 16 PAHs from US Environmental Protection Agency (USEPA) list [16, 18, 21, 22], pesticides [pentachlorophenol (PCP)] [23], explosives [trinitrotoluene (TNT)] [20], and dyes (Lissamine Green B) [18].
Three main criteria have been identified to be crucial in the cost-effectiveness of EF treatment of contaminated soil (Table 1): (1) the influence of operating parameters, (2) the matrix composition, and (3) the environmental impact. The significance of these parameters is discussed in the following sections.
2 Influence of Operating Parameters
In EF process, the main operating parameters playing a role at laboratory scale are (1) the nature of electrode materials, (2) the applied current density, and (3) the catalyst (ferrous iron) concentration, whose respective impacts on SW effluent degradation and mineralization efficiency are discussed in the three following subsections.
2.1 Influence of Electrode Materials
The electrode materials play a major role in EF process. According to the cathode materials employed, hydrogen peroxide (H2O2) can be electro-generated through the two-electron reduction of dissolved O2 (Eq. 2) along with simultaneous ferrous ion (Fe2+) regeneration through Fe3+ reduction (Eq. 3). Both reagents react to form hydroxyl radicals (•OH) in bulk solution through the Fenton reaction (Eq. 1).
Carbon-based materials are preferentially employed for their high hydrogen (H2) evolution overvoltage and their low catalytic activity for H2O2 decomposition. Carbon felt has especially shown good performance for its high specific surface area and its mesoporous structure, facilitating the O2 diffusion and its subsequent adsorption [24, 25]. This material was therefore used in EF treatment of SW solutions [16, 21, 22]. However, the use of porous carbon sponge cathode has shown to easily adsorb HOCs such as humic substances [26] – a fraction of soil organic matter – that are typically present in real SW solutions. Hydroxyl radicals produced homogeneously in the electrochemical cell could also oxidize these substances into more hydrophilic by-products leading to a rebound effect of the total organic carbon in bulk solution. To avoid this phenomena, non-porous cathode such as graphite or stainless steel could be used [18], though the H2O2 electro-generation at their surface is poor [27]. In that case, the amount of •OH generated through the Fenton reaction is limited.
Alternatively, adequate anode materials can be combined to such cathode materials. Two kinds of anode materials have been used in EAOPs: (1) active anodes such as platinum (Pt), carbon (e.g., graphite), and mixed metal oxides [e.g., dimensionally stable anode (DSA)] and (2) non-active anodes such as lead dioxide (PbO2), doped tin dioxide (e.g., F-SnO2 and Sb-SnO2), and boron-doped diamond (BDD). The first category is dedicated to materials that have a low O2 evolution overpotential, e.g., around 1.5 V vs. SHE with DSA, 1.6 V vs. SHE with Pt, and 1.7 V vs. SHE with graphite. In these conditions, •OH are chemisorbed at the anode surface, being barely available for pollutant oxidation. Contrastingly, the non-active anodes exhibit a high O2 evolution overpotential, e.g., 1.9 V vs. SHE with SnO2 and PbO2 and 2.3 V vs. SHE with BDD. As a consequence, •OH are generated in a large potential window and are physisorbed at the anode surface, resulting in the mineralization of the organic pollutants. Unlike •OH that are produced from the Fenton’s reaction in the bulk, these •OH are generated in a heterogeneous way on the anode surface. Therefore, their reaction is limited to the anode surface.
The influence of anode materials, i.e., Pt, DSA, and BDD, has been studied in the EF treatment of SW solutions containing PHE as representative pollutant and hydroxypropyl-beta-cyclodextrin (HPCD) as representative washing agent (Fig. 1). The kinetics rates of PHE and HPCD degradation are displayed in Fig. 1a.
Influence of anode materials during EF treatment of SW solution: (a) kinetics rate constant of pollutant (PHE) and extracting agent (HPCD) degradation and (b) mineralization. Operating conditions: current density, 6.7 mA cm−2; catalyst concentration, [Fe2+] = 0.2 mM; treatment time in mineralization graph (b), 4 h. (adapted with permission from [21, 22]) (Copyright 2014 Elsevier)
Interestingly, the pollutant is more quickly degraded with active anode such as Pt and DSA than with BDD anode. Inversely, the extracting agent is faster degraded with BDD than with Pt and DSA. This difference is attributed to the ways of oxidation of •OH from the bulk in the presence of cyclodextrin (Sect. 3.1) and the nature of electrode material as explained below. This trend further highlights the competitive oxidation between PHE and HPCD, which can be further underlined by the degradation kinetics ratio between the pollutant and the washing agent. It was noticed that the HPCD degradation rates were inversely correlated to the pollutant decay rates, i.e., when the kinetics rate of HPCD increased, the kinetics rate of PHE decreased inversely. Moreover, PHE was quicker degraded than HPCD whatever the anode employed, which is interesting if a recirculation loop is considered by reusing the solubilizing agent present in the partially oxidized SW solution as discussed in Sect. 3.1.
Looking at the comparison of mineralization power (Fig. 1b), the superiority of BDD is clear as compared to Pt and DSA. It was attributed to the high amount of heterogeneous •OH formed at BDD surface and their availability (physisorption) and the subsequent oxidation of organic compounds (Eqs. 4 and 5) [28]:
Thus, the involvement of two sources of •OH in the EF process using BDD anode implies higher degradation yield of extracting agent that predominate in washing solution as well as higher mineralization degree.
2.2 Influence of Current Density
The current density is another important parameter that plays a role on the electrochemical reaction rates and on the yield of electro-generated oxidants. Increasing the current density amplifies the in situ generation of Fenton reagent (H2O2 and Fe2+) at the cathode (Eqs. 2 and 3) and generation rate of heterogeneous hydroxyl radical (M(•OH)) at the anode. In this way, the current density is usually determined by normalizing the current intensity with the cathode surface area that is the working electrode in traditional EF process in which an active anode is employed as counter electrode. In the aim at comparing all the EF processes whatever the anode employed (active or non-active), the cathode area was considered in the current density values given in this chapter.
Figure 2a illustrates an increase of the kinetics rates of the washing agent when the current density increased from 3.3 to 6.7 mA cm−2. In this range of current density, the kinetics rates of the pollutant remain constant, the oxidation being mainly focused on the solubilizing agent. Besides, raising the current density until 13.3 mA cm−2 could not improve the degradation efficiency of both pollutant and extracting agent. This is due to the increase of reaction rate of parasitic reactions such as the H2O2 decomposition at the cathode (Eq. 6), at the anode (Eqs. 7 and 8), and in a lesser extent in bulk solution (Eq. 9) as well as hydrogen (H2) formation (Eq. 10):
Influence of current density during EF treatment of SW solution: (a) kinetics rate constant of pollutant (PHE) and extracting agent (HPCD) degradation and (b) mineralization. Operating conditions: catalyst concentration, [Fe2+] = 0.2 mM; anode material in kinetic constants graph (a), BDD; treatment time in mineralization graph (b), 4 h (adapted with permission from [21, 22]) (Copyright 2014 Elsevier)
These reactions are in competition with H2O2 electro-generation (Eq. 2) at the cathode.
In addition, the slight decrease of the degradation kinetics ratio between the pollutant and the washing agent at high current intensity indicates that current intensity may modify oxidation mechanisms in the electrochemical cell. For example, mediated oxidation is favored at high current intensity due to the generation of other strong oxidants such as persulfates, sulfate radicals, or ozone [10].
Considering the mineralization (Fig. 2b), the yields were increasing when the current density increased from 3.3 to 13.3 mA cm−2 with BDD anode material, while the yields remained quasi-constant with Pt and DSA anodes (considering standard deviations around ±1.4%). Still, BDD depicted much higher mineralization performance due to the paired electro-catalysis process.
2.3 Influence of Catalyst (Fe2+) Concentration
Ferrous ion acts as a catalyst in the EF process and is therefore added at a catalytic amount in the solution.
By varying the concentration of Fe2+ from 0.05 to 10 mM in a synthetic SW solution containing PHE and HPCD (Fig. 3a), the decay rate of the pollutant increased until a ferrous ion concentration of 0.2 mM. Increasing the catalyst concentration makes increase the amount of hydroxyl radicals formed through the Fenton reaction (Eq. 1).
Remarkably, higher Fe2+ concentration did not improve the kinetics rate of the pollutant degradation. It can be explained by the progressive inhibition of the oxidant generation, because of the greater extent of the waste reaction between Fe2+ and •OH (Eq. 11):
In these conditions, 0.2 mM was defined as the optimal Fe2+ concentration, which is in the range of concentration (0.1–0.2 mM) usually employed in EF processes at lab scale in batch experiments [18, 21, 22, 29].
The difference of the presence or absence of Fe2+ has been tested by Rosales et al. [18] in a soil slurry batch reactor. It is noticed that the dye decoloration rates was 1.35-fold higher with ferrous ion (2.3 h−1) than without addition of Fe2+ (1.7 h−1) by using graphite material as cathode and anode. It highlights the high oxidation efficiency of •OH formed by Fenton reaction (Eq. 1) as compared to the direct electro-oxidation treatment. In addition, the comparison between a BDD anode treatment in synthetic SW solution without the addition of Fe2+ − namely, anodic oxidation (AO) – and the mineralization efficiency of EF is displayed in Fig. 3b. By treating the same synthetic SW solution (PHE and HPCD), EF process gave 1.3 times higher efficiency as compared to AO process, and the mineralization yield was higher whatever the applied current density. This again emphasized the superiority of EF due to the double source of •OH production, by the additional presence of Fe2+ leading to •OH generation in the bulk.
More excitingly, the combination between SW/SF and EF treatment remains interesting since the presence of iron extracted from soil in SW/SF solution can be used as an iron source for the electrochemical treatment. This was evidenced by treating real SF solution [9] and real SW solution [16] by EF process without any addition of iron, since dissolved iron was present initially in the SW/SF solution at a concentration ranging from 0.02 to 0.06 mM. These amounts of concentration are sufficient to involve the Fenton reaction (Eq. 1). Thus, this parameter also strongly depends on the nature of the soil treated (particularly the concentration and availability of iron in the soil).
3 Effect of the Matrix
Apart from the EF parameters, the matrix composition has a great influence on the process efficiency, especially the washing agent, the pH of SW/SF solution, and the degree of complexity of the SW/SF solution (presence of soil organic matter, inorganic ions, etc.). The impacts of those parameters are discussed hereafter.
3.1 Influence of Nature of Extracting Agent and Possibility of Recovery
In SW- and SF-pollution transfer, technologies extracting agents are used to enhance the pollutant extraction by a two-step mechanism: (1) the desorption of the contaminant from the binding site in the solid matrix and (2) the elution from the solid phase into the extraction fluid [2, 6]. Several families of agents have been used in literature in SW/SF techniques such as surfactants, cyclodextrins, co-solvents, dissolved organic matter, deoxyribonucleic acid, chelating agents, fatty acid methyl esters, and vegetable oil [2]. In the case of surfactants, the pollutant extraction occurs when the agent is added in solution at concentrations higher than their critical micelle concentration (CMC) [30]. There are several criteria that prevail in the selection of these agents: low or even absence of CMC, low adsorption onto soil, and high pollutant extraction efficiency.
Nonionic surfactants correspond to these criteria, especially Tween 80 that possesses higher PAHs extraction capacity than Brij 35, Tergitol NP10, Tween 20, Tyloxapol, Igepal CA-720, and Triton X-100 [31, 32]. Tween 80 is therefore often selected as representative surfactant in literature, especially for combination with an electrochemical treatment [4, 15, 16, 21, 31, 33, 34]. Surfactants are amphiphilic molecules whose hydrophilic heads constitute a first barrier between •OH and the pollutant (HOC) (Fig. 4a). Before the oxidation of pollutant, the surfactant needs to be degraded first as it has been observed that the size of micelles decreases with treatment time [35]. In addition, the ratio between the pollutant and the surfactant is key in the size of these micelles and hence on the time course of a later treatment technology. The higher the dose of surfactant, the lower the size of the micelles and the higher is the resulting organic load in the SW fluid [35]. Therefore, the soil/liquid ratio determines not only the concentration of pollutant in the washing/flushing fluid but also the speciation that is particularly important in terms of the occurrence of micelles. Furthermore, steric hindrance of large micelles could prevent direct oxidation of micelles on the BDD anode surface [12], which could underscore the significant oxidation role of homogeneous •OH formed by Fenton reaction (Eq. 1) in bulk solution as well as other oxidant species leading to mediated oxidation of organic compounds in the bulk.
Schematic representation of two different ways of •OH oxidative degradation of hydrophobic organic pollutant in the presence of (a) surfactant (Tween 80) or (b) cyclodextrin (HPCD) in aqueous solution (adapted with permission from [21]) (Copyright 2014 Elsevier)
Alternatively, cyclodextrins have been used as washing agent since they do not have CMC and they do not form high viscosity emulsions [23]. These semi-natural molecules have a toroidal shape that allows trapping the pollutant inside their cavity (Fig. 4b). On the contrary to surfactant, in the case of HPCD, the HOC is trapped into the hydrophobic cavity, and the formation of a ternary complex between Fe2+, pollutant (HOC), and HPCD (Fe2+:HPCD:HOC) – evidenced by UV spectrophotometry measurements (formation constant of 56 mM−1; [21]) – allows the •OH to directly react with the pollutant (Eqs. 12 and 13) [21, 23]:
The binding between Fe2+ and the cyclodextrin depends on the functional group. In the case of HPCD, Fe2+ is likely coordinated with the hydroxyl group present on the rim of the molecule [36].
Thus, two different mechanisms have been highlighted according to the way to form cyclodextrin/HOC and surfactant/HOC complexes [21]. However, when considering a treatment of SW/SF solutions, the recycling abilities of the extracting agent are another important criterion to take into account aiming at reducing both the operating cost of reagents for the SW/SF step and energy requirements during the EF treatment of SW solution. Therefore, a synthetic solution containing Tween 80 (0.75 g L−1) or HPCD (10 g L−1) and PHE at the same initial concentration (17 mg L−1) has been treated by EF using a carbon felt cathode (150 cm2) and a Pt grid anode in a 400 mL undivided cell (Fig. 5) [21]. After 4 h of treatment, 95% of PHE was degraded with a pseudo-first order rate constant of 0.013 min−1, while 50% of Tween 80 was removed. In the case of cyclodextrin, the pollutant was completely removed after 4 h at a rate of 0.026 min−1 though HPCD was barely degraded at a 10% yield. The two times higher degradation rate of PHE in the presence of HPCD could be explained by the ternary complex as abovementioned. However, it is important to note that 13.3 times higher HPCD concentration was required to solubilize the same amount of PHE as compared to Tween 80. Therefore, after the removal of more than 90% of PHE, 1 g L−1 of HPCD was removed, while 0.375 g L−1 of Tween 80 was only degraded. Thus, considering the amount of extracting agent removed per quantity of pollutant degraded, Tween 80 has better recycling abilities compared to HPCD, because of the less solubilization power of the cyclodextrin.
Influence of nature of extracting agent [HPCD (10 g L−1) or Tween 80 (0.75 g L−1)] on pollutant [PHE (17 mg L−1)] degradation. Operating conditions: current density, 13.3 mA cm−2; catalyst concentration, [Fe2+] = 0.05 mM; anode material, Pt (Reprinted with permission from [21]) (Copyright 2014 Elsevier)
All these statements therefore emphasize the importance of two main criteria in the recycling abilities of extracting agent: (1) the shape of extracting agents and their functional groups, i.e., the toroidal shape of cyclodextrins allowing making selective the •OH degradation unlike the micelles shape, and (2) the concentration of the washing agent required to solubilize the pollutant, i.e., more than ten times with cyclodextrins as compared to surfactants. It is also important to mention that the oxidation by-products and the extracting agent would be in contact with the soil during the reuse of the agent, which means that the solution pH and the ecotoxicity of soil and solution are other parameters to monitor as discussed in Sects. 3.2 and 4, respectively.
3.2 Influence of pH
The pH of solution is determinant in processes involving Fenton reaction, due mainly to the pH dependency of iron ion species. At pH below 2, there is formation of peroxonium ion (H3O2 +) that is less reactive with Fe2+ which makes a decrease in the rate of Fenton’s reaction [19]. At pH higher than 4, the precipitation of ferric hydroxide (Fe(OH)3) occurs [29]. Thus, most of the EF studies are performed at an optimal pH of 3 [18, 20,21,22]. However, adjusting the pH requires acid reagents that increase the operating costs. That is why some efforts have been devoted to operate at circumneutral pH. Interestingly, in an experiment at an initial pH of 6 of PHE polluted-SW HPCD solution, the pollutant removal rate (0.026 min−1) was very similar to the one obtained at pH 3 (0.027 min−1) [21]. Additionally, when degrading by EF a PAHs contaminated SW-HPCD or Tween 80 solution with an initial pH of 8, the pH decreased quickly until a plateau around 3 after only 1 h of treatment (Fig. 6) [37]. In addition, the drop of pH occurs whatever the kind of anode material employed, e.g., active anode (Pt) [16] and non-active anode (BDD) [37]. This phenomenon is due to the formation of carboxylic acids that can be formed very quickly, especially from the opening of aromatic rings during the oxidative degradation of pollutants. The presence of carboxylic acids and aromatics molecules in organic matter – much more present in Tween 80 solutions (due to its higher extraction capacity) – can also contribute to the acidification of solutions.
Interestingly, recycling the partially treated SW solution for a second SW step did not affect the soil pH, as the pH value equaled the initial one (pH = 8) [16]. This is due to the strong buffering capacity of the soil with the presence of clay minerals and organic matter. Ionic exchange between the protons from SW solutions and the clay-humic complex saturated in Ca2+, K+, and Mg2+, and Na+ restores the alkaline soil pH.
3.3 Synthetic vs. Real Effluent
Synthetic effluents are usually preferred as a first experimental approach at laboratory scale. However, these treated solutions do not contain all the components that can be found in real SW/SF effluents such as inorganic ions (Ca2+, Na+, Mg2+, K+, etc.) and organic matter.
The potential presence of iron in soil can positively influence the electrochemical process efficiency as discussed in Sect. 2.3. During SW/SF extraction, iron can be solubilized and can then be involved in the Fenton reaction as demonstrated by our previous reports [9, 16]. In that case, the addition of ferrous iron – as traditionally performed in synthetic solutions – is useless.
The presence of organic matter is a parameter impacting the process efficiency by being easily adsorbed on porous carbon electrodes due to hydrophobic interactions [26] as abovementioned in Sect. 2.1. Dissolved organic matter (DOM) is also well known to decrease process efficiency (1) by decreasing the pollutant availability and (2) by increasing the competition with the pollutant since fulvic acids from DOM react very quickly with •OH [38, 39]. In addition, synthetic SW solutions are usually spiked with only one pollutant or several compounds from a contaminant family, whereas in real SW solutions, mixed pollutions are commonly found including numerous pollutants that are even not analyzed. This also makes rise the •OH consumption by wasting reactions.
To clarify the above statements, the EF treatments using BDD anode at a constant current density (6.7 mA cm−2) of synthetic and real SW solutions polluted by PAHs have been compared in Fig. 7 [16, 22, 37].
Influence of synthetic vs. real SW effluent using (a) HPCD or (b) Tween 80 as washing agent. Operating conditions: current density, 6.7 mA cm−2; anode material, BDD (adapted with permission from [22]) (Copyright 2014 Elsevier)
Interestingly, whatever the extracting agent employed (HPCD or Tween 80), the mineralization rates and yields are very similar for the treatment of synthetic and real SW solutions. This result is attributed to the negligible organic carbon fraction [4–5% of total organic carbon (TOC)] coming from the pollutants and organic matter as compared to the fraction from the washing agent itself (95–96% of TOC). It is important to keep in mind that the organic matter content as well as the level of organic pollution in soil could still have a role on the mineralization efficiency. In the presented data, an organic matter content of 4.7% was present in the studied soil with PAHs content of 1,000 mg kg−1 [16]. Higher concentration of pollution along with higher organic matter content would have implied lower mineralization efficiency as compared to studies in synthetic media.
4 Impacts on Ecotoxicity, Biodegradability, and Soil Respirometry
The environmental impact is a critical issue that needs to be assessed especially if successive washings are considered after EF treatment of partially oxidized SW solutions and/or if a pre−/post-biological treatment is performed.
Two kinds of bioassays have been mainly performed with SW solutions: (1) acute ecotoxicity tests of EF-treated SW solutions have been performed by monitoring the bioluminescence of Vibrio fischeri marine bacteria as representative eco-organism and (2) biodegradability tests represented by the BOD5/COD ratio, BOD5 being the biochemical oxygen demand after 5 days and COD being the chemical oxygen demand [21, 22, 37]. The influence of three parameters on ecotoxicity and biodegradability could be reviewed: (1) the nature of extracting agent (Fig. 8), (2) the nature of pollutant and matrix composition (Fig. 9), and (3) the anode material (Fig. 10).
Influence of (a, b) pollutants and (c, d) matrix composition on (a, c) Vibrio fischeri inhibition and (b, d) biodegradability (BOD5/COD) evolution during EF treatment of SW-Tween 80 solutions. Operating conditions: current density, 6.7 mA cm−2; anode material, BDD; [Tween 80]hydrocarbons = 11 g L−1; [Tween 80]PAHs = 7.5 g L−1; [Tween 80]synthetic matrix = 9 g L−1; [Tween 80]real matrix = 7.5 g L−1 (adapted with permission from [9]) (Copyright 2015 Elsevier)
Influence of anode materials on (a) Vibrio fischeri inhibition and (b) biodegradability (BOD5/COD) evolution during EF treatment of SW-HPCD solutions. Operating conditions: current density, 6.7 mA cm−2 (adapted with permission from [22]) (Copyright 2014 Elsevier)
Figure 8 compares the bioassays evolution during EF treatment of real SW solutions using HPCD or Tween 80 extracting agent in the same following conditions [37]: (1) both agents at the same initial concentration (7.5 ± 0.2 g L−1), considering that less than 2% of extracting agent adsorb onto the soil, (2) in the same operating conditions (BDD anode, 6.7 mA cm−2), and (3) from the same historically PAHs-contaminated soil. With both solubilizing agents, the ecotoxicity was high during the first hours of treatment. At this time, oxidation by-products are formed and can be more toxic than the initial molecule [21, 22, 40]. After 12 h of EF treatment, the toxicity of HPCD solutions starts decreasing until the end of treatment, due to the transformation of toxic intermediates to short-chain carboxylic. Contrastingly, experiments with Tween 80 do not show any drop of toxicity. It could be explained by the higher solubilization power of Tween 80 that extracted more toxic and recalcitrant pollutants [9] and/or by the lower ability of cyclodextrins to generate toxic intermediates [21]. Biodegradability assays corroborate these trends by highlighting a lag phase during the first 4 h of EF treatment whatever the agents employed, followed by a great increase of BOD5/COD ratio with HPCD solutions and slight rise with Tween 80 matrix. Considering that a threshold BOD5/COD ratio value of 33% is the acceptable level to consider a biological posttreatment [41], it could be considered after 8.5 and 20 h for HPCD solutions and Tween 80 solutions, respectively. Though the required treatment time was 2.3 times longer with Tween 80 solutions, the COD was 2.1-fold lower (2,900 mg-O2 L−1) compared to HPCD solution (6,200 mg-O2 L−1), meaning that a shorter biological treatment time would be then needed with Tween 80 effluent. It is further interesting to note that the initial biodegradability of SW solutions was very low (BOD5/COD <0.5%) whatever the extracting agent employed (Tween 80 or HPCD). However, the biodegradability enhancement factor (Eq. 14) reached more than 98% in all the cases after 8 h of treatment proving the high ability of EF process to increase the biodegradability of SW solutions.
Where R and R i are the BOD5/COD ratio and BOD5/COD initial ratio, respectively.
Figure 9a, b compare the EF experiments performed with Tween 80 present in two different kinds of matrix: (1) one is coming from a historically PAHs-contaminated soil [37] and (2) the second comes from a genuinely hydrocarbon-contaminated soil [9]. It is clearly shown that the influence of pollutants does not play a great role in EF treatment of SW solutions as similar trends in bioluminescence inhibition and biodegradability evolution are observed whatever the nature of pollutant. When considering the TOC ratio (%) between the TOC of pollutants and the TOC of surfactant, i.e., 4.8% in PAHs solutions and 3.2% in hydrocarbons solutions, it could be the reason why the contaminants have a negligible impact on the bioassay results. Similarly, the influence of the matrix composition (Fig. 9c, d) has a negligible impact on acute ecotoxicity when comparing synthetic SW solution (PHE, surfactant) with real SW solution (PAHs, surfactant, organic matter, and inorganic compounds). However, the biodegradability was lower with real effluent, with a BOD5/COD ratio of 33% reached after 12 and 20 h for EF treatment of synthetic and real solutions, respectively. The presence of organic matter and numerous pollutants induced the formation of less biodegradable compounds. Though it is noticeable that the initial biodegradability was very low, the biodegradability enhancement factors reached more than 97% after 8 h of EF treatment whatever the composition of the SW matrix.
Considering the influence of Pt, DSA, and BDD anode materials on bioassay results (Fig. 10), it is noticed that active anodes (Pt and DSA) had worse trend than non-active anode (BDD) when studying the EF treatment of synthetic SW-HPCD solutions [22]. The lag phase appearing at the beginning of all the treatments might be due to the production of hydroxylated degradation by-products such as, for example, hydroxylated PHEs, well known to be more toxic than the pristine compound [42].
The combination between EF process and a biological posttreatment has been proposed successfully for the mineralization of pharmaceuticals [43, 44] and pesticides [45]. Still, it has never been suggested for the treatment of SW/SF solutions. Recently, a combination between AO and an aerobic biological treatment was implemented to treat synthetic SW solution containing PHE and Tween 80 [15]. A synergistic effect was observed with a 3-h pretreatment by AO at 21 mA cm−2, leading to 80% overall COD removal after the biological treatment. The addition of Fe2+ and the use of a cathode allowing H2O2 generation should even increase the process efficiency in an EF setup, upon validation with supplementary experiments.
When considering a recirculation loop in SW/SF combined to EF treatment, the impact on the general soil microbial activity has to be considered since by-products are present in acidic SW solutions as abovementioned. It can be assessed by soil respirometry tests [16]. Interestingly, after a second SW cycle with EF-treated SW solution, the oxygen consumption rates were higher (0.81 μg-O2 (gh)−1 with Tween 80 and 0.34 μg-O2 (gh)−1 with HPCD) than a second fresh washing cycle (0.70 μg-O2 (gh)−1 with Tween 80 and 0.20 μg-O2 (gh)−1 with HPCD) (Fig. 11) [16]. It was also noticed that the oxygen consumption rates decreased when the number of successive washings increased, whatever the washing agent employed, even with only ultrapure water [16]. This could be assumed to be the result of the decrease in nutrient concentration, since nutrients are solubilized in each step of SW extraction [16]. It further highlighted that the oxidation of SW solutions did not affect the general soil microbial activity, which is corroborated by the quite similar oxygen consumption rates between the first SW cycle (0.93 μg-O2 (gh)−1 with Tween 80 and 0.37 μg-O2 (gh)−1 with HPCD) and the second cycle with treated SW solution. This trend would be explained by the hydrophilicity properties of oxidation by-products due to the formation of hydroxylated products (by •OH addition reactions), which makes the interactions negligible between the intermediates and soil particles.
Soil respirometry rates obtained after successive washings with different extracting agents (Tween 80 and HPCD) (adapted with permission from [16]) (Copyright 2016 Elsevier)
5 Energy Considerations and Concluding Remarks
Energy requirement represents the main part in operating cost of such electrochemical process. Therefore, authors try to reduce as much as possible the energy consumption in order to be competitive. The energy (E consumption) is usually calculated as follows (Eq. 15) [29]:
where E cell is the average cell voltage (V), I is the applied current intensity (A), t is the electrolysis time (h), and V s is the solution volume (L).
The energy requirements are compared according to the washing agent employed, the degree of complexity of the treated SW solution, and the mineralization time [partial mineralization or quasi-complete (>99%)] (Table 2) [9, 22, 37].
EF treatment of SW-HPCD solutions required between 1.4 and 2.8 times less energy than SW-Tween 80 solutions [37]. However, in such combined process, the solubilization efficiency of the extracting agent needs to be also taken into account in the calculations. Considering that ten more SW cycles are required with HPCD to extract the same PAHs concentration than with Tween 80, the energy required to treat the SW solutions would be ten times more, by assuming a linear relation between the initial organic load and the EF treatment time [37]. Another interesting feature would be to estimate the energy consumed per amount of pollutant degraded, so that the energy efficiency comparison could be more reliable. However, at the time to reach 33% of biodegradability or quasi end of mineralization, all the pollutants are already degraded. It means that global parameter such as COD or TOC of pollutant removed needs to be taken into account. The challenge will be then to estimate the TOC coming from the washing agent and its intermediates as well as the TOC coming from the pollutants and their oxidation by-products.
The SF/EF treatment of real Tween 80 solution required more energy (508 kWh m−3) than the EF treatment of SW-Tween 80 solutions (443 kWh m−3). Considering the pollutant removal efficiency, SW could extract around 41% of PAHs pollutant (1,090 mg kg−1 initially) after one cycle (24 h), while SF could extract only 1% of hydrocarbons (3,900–6,100 mg kg−1 initially) in 24 h. Further experiments would be required to compare the efficiency of SW with SF techniques in similar conditions as the energy calculation only takes into account the EF treatment and not the whole process.
Furthermore, achieving an EF treatment until quasi-complete mineralization with BDD anode material was less energy efficient per volume of treated effluent than reaching 33% of biodegradability whatever the washing agent employed and the degree of complexity of solution. Thus, the EF combination with a biological treatment has to be considered and experimented for the treatment of SW/SF solutions as only biodegradability assays have been performed for now. An optimal EF treatment time could be determined at a minimal energy consumed.
In addition, the energy required to completely degrade PHE from a synthetic HPCD solution was around 41 kWh m−3 with BDD anode [22]. Interestingly, it was around 60 times less than the energy consumed in another electrochemical setup developed to treat a synthetic SW-HPCD solution spiked with 35 mg L−1 of PHE [11]. The superiority of the EF process was imputed to the electrocatalytic formation of •OH radicals.
Though EF treatment of SW/SF solutions was efficient, the electric energy devoted to the pollutant degradation itself is low as compared to the energy devoted to the waste reactions and washing agent oxidation, which makes the energy strongly depend on the concentration of extracting agent used. Still, the possibility to implement an EF process allowing to reuse SW/SF solution and to recycle extracting agent is an interesting research area in order to improve the cost-effectiveness of the whole integrated process (SW/EF or SF/EF) and needs further development. In parallel, experiments could be performed to optimize EF treatment of soil slurry without addition of solubilizing agent or at concentration close to their CMC (ranging from 10 to 200 mg L−1) as proposed by Rosales et al. [18]. In such conditions, appropriate electrode materials would be required to avoid electrode fouling while keeping a high oxidant generation efficiency by minimizing the adverse effect on soil integrity due to strong oxidizing conditions. It could be an alternative to the in situ electrokinetic-Fenton proposed in literature. Finally, EF treatment can be a good alternative to replace or improve existing soil remediation technologies as it is clean (electron reagent), safe (mild conditions), easy to handle (simple equipment required), and versatile (adaptable to wide ranges of flow rates and organic load). The next step would be to scale up the suggested integrated processes by combining kinetics, hydrodynamics, and modeling studies to optimize the reactor design, the removal rates, and the energy efficiency. It will bring EF closer to industrial development.
References
Trellu C, Mousset E, Pechaud Y, Huguenot D, Van HED, Esposito G, Oturan MA (2016) Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review. J Hazard Mater 306:149–174
Mousset E, Oturan MA, Van Hullebusch ED, Guibaud G, Esposito G (2014) Soil washing/flushing treatments of organic pollutants enhanced by cyclodextrins and integrated treatments: state of the art. Crit Rev Environ Sci Technol 44:705–795
Rodrigo MA, Oturan N, Oturan MA (2014) Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev 114:8720–8745
Sáez C, López-Vizcaíno R, Canizares P, Rodrigo MA (2010) Conductive-diamond electrochemical oxidation of surfactant-aided soil-washing effluents. Ind Eng Chem Res 49:9631–9635
López-Vizcaíno R, Sáez C, Cañizares P, Rodrigo MA (2012) The use of a combined process of surfactant-aided soil washing and coagulation for PAH-contaminated soils treatment. Sep Purif Technol 88:46–51
Paria S (2008) Surfactant-enhanced remediation of organic contaminated soil and water. Adv Colloid Interface Sci 138:24–58
Risco C, Rubí-juárez H, Rodrigo S, López-vizcaíno R, Saez C, Cañizares P, Barrera-díaz C, Navarro V, Rodrigo MA (2016) Removal of oxyfluorfen from spiked soils using electrokinetic soil flushing with the surrounding arrangements of electrodes. Sci Total Environ 559:94–102
dos Santos EV, Souza F, Saez C, Canizares P, Lanza MRV, Martinez-huitle CA, Rodrigo MA (2016) Application of electrokinetic soil flushing to four herbicides: a comparison. Chemosphere 153:205–211
Huguenot D, Mousset E, van Hullebusch ED, Oturan MA (2015) Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons. J Environ Manage 153:40–47
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res Int 21:8336–8367
Gómez J, Alcántara MT, Pazos M, Sanromán MÁ (2010) Soil washing using cyclodextrins and their recovery by application of electrochemical technology. Chem Eng J 159:53–57
dos Santos EV, Sáez C, Martínez-Huitle CA, Cañizares P, Rodrigo MA (2015) The role of particle size on the conductive diamond electrochemical oxidation of soil-washing effluent polluted with atrazine. Electrochem Commun 55:26–29
dos Santos EV, Sáez C, Martínez-huitle CA, Cañizares P, Rodrigo MA (2016) Removal of oxyfluorfen from ex-situ soil washing fluids using electrolysis with diamond anodes. J Environ Manage 171:260–266
dos Santos EV, Sáez C, Cañizares P, Martínez-huitle CA, Rodrigo MA (2017) Treating soil-washing fluids polluted with oxyfluorfen by sono-electrolysis with diamond anodes. Ultrason Sonochem 34:115–122
Trellu C, Ganzenko O, Papirio S, Pechaud Y, Oturan N, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2016) Combination of anodic oxidation and biological treatment for the removal of phenanthrene and Tween 80 from soil washing solution. Chem Eng J 306:588–596
Mousset E, Huguenot D, Van Hullebusch ED, Oturan N, Guibaud G, Esposito G, Oturan MA (2016) Impact of electrochemical treatment of soil washing solution on PAH degradation efficiency and soil respirometry. Environ Pollut 211:354–362
Pazos M, Iglesias O, Gómez J, Rosales E, Sanromán MA (2013) Remediation of contaminated marine sediment using electrokinetic-Fenton technology. J Ind Eng Chem 19:932–937
Rosales E, Pazos M, Longo MA, Sanroman MA (2009) Influence of operational parameters on electro-Fenton degradation of organic pollutants from soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 44:1104–1110
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641
Murati M, Oturan N, van Hullebusch ED, Oturan MA (2009) Electro-Fenton treatment of TNT in aqueous media in presence of cyclodextrin. Application to ex-situ treatment of contaminated soil. J Adv Oxid Technol 12:29–36
Mousset E, Oturan N, van Hullebusch ED, Guibaud G, Esposito G, Oturan MA (2014) Influence of solubilizing agents (cyclodextrin or surfactant) on phenanthrene degradation by electro-Fenton process – study of soil washing recycling possibilities and environmental impact. Water Res 48:306–316
Mousset E, Oturan N, van Hullebusch ED, Guibaud G, Esposito G, Oturan MA (2014) Treatment of synthetic soil washing solutions containing phenanthrene and cyclodextrin by electro-oxidation. Influence of anode materials on toxicity removal and biodegradability enhancement. Appl Catal Environ 160–161:666–675
Hanna K, Chiron S, Oturan MA (2005) Coupling enhanced water solubilization with cyclodextrin to indirect electrochemical treatment for pentachlorophenol contaminated soil remediation. Water Res 39:2763–2773
Sirés I, Garrido JA, Rodríguez RM, Brillas E, Oturan N, Oturan MA (2007) Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene. Appl Catal Environ 72:382–394
Hu J, Sun J, Yan J, Lv K, Zhong C, Deng K, Li J (2013) A novel efficient electrode material: activated carbon fibers grafted by ordered mesoporous carbon. Electrochem Commun 28:67–70
Trellu C, Péchaud Y, Oturan N, Mousset E, Huguenot D, van Hullebusch ED, Esposito G, Oturan MA (2016) Comparative study on the removal of humic acids from drinking water by anodic oxidation and electro-Fenton processes: mineralization efficiency and modelling. Appl Catal Environ 194:32–41
Sopaj F (2013) Study of the influence of electrode material in the application of electrochemical advanced oxidation processes to removal of pharmaceutical pollutants from water. University of Paris-Est
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New York
Alcántara MT, Gómez J, Pazos M, Sanromán MA (2008) Combined treatment of PAHs contaminated soils using the sequence extraction with surfactant-electrochemical degradation. Chemosphere 70:1438–1444
Dhenain A, Mercier G, Blais J-F, Bergeron M (2006) PAH removal from black sludge from Aluminium industry by flotation using non-ionic surfactants. Environ Technol 27:1019–1030
Gómez J, Alcántara MT, Pazos M, Sanromán MA (2010) Remediation of polluted soil by a two-stage treatment system: desorption of phenanthrene in soil and electrochemical treatment to recover the extraction agent. J Hazard Mater 173:794–798
Mousset E, Oturan N, van Hullebusch ED, Guibaud G, Esposito G, Oturan MA (2013) A new micelle-based method to quantify the Tween 80® surfactant for soil remediation. Agron Sustain Dev 33:839–846
dos Santos EV, Saez C, Martinez-Huitle CA, Canizares P, Rodrigo MA (2015) Combined soil washing and CDEO for the removal of atrazine from soils. J Hazard Mater 300:129–134
Lindsey ME, Xu G, Lu J, Tarr MA (2003) Enhanced Fenton degradation of hydrophobic organics by simultaneous iron and pollutant complexation with cyclodextrins. Sci Total Environ 307:215–229
Mousset E (2013) Integrated processes for removal of persistent organic pollutants: soil washing and electrochemical advanced oxidation processes combined to a possible biological post-treatment. University of Paris-Est – University of Cassino and The Southern Lazio – UNESCO-IHE for Water Education
Westerhoff P, Aiken G, Amy G, Debroux J (1999) Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Res 33:2265–2276
Lindsey ME, Tarr MA (2000) Inhibited hydroxyl radical degradation of aromatic hydrocarbons in the presence of dissolved fulvic acid. Water Res 34:3–7
Dirany A, Sirés I, Oturan N, Ozcan A, Oturan MA (2012) Electrochemical treatment of the antibiotic sulfachloropyridazine: kinetics, reaction pathways, and toxicity evolution. Environ Sci Technol 46:4074–4082
Rodier J, Legube B, Merlet N (2009) Analyse de l’eau (water analysis), 9th edn. Dunod, Paris, (in French)
Fernandes D, Porte C (2013) Hydroxylated PAHs alter the synthesis of androgens and estrogens in subcellular fractions of carp gonads. Sci Total Environ 447:152–159
Mansour D, Fourcade F, Huguet S, Soutrel I, Bellakhal N, Dachraoui M, Hauchard D, Amrane A (2014) Improvement of the activated sludge treatment by its combination with electro Fenton for the mineralization of sulfamethazine. Int Biodeter Biodegr 88:29–36
Olvera-Vargas H, Oturan N, Buisson D, Oturan MA (2016) A coupled bio-EF process for mineralization of the pharmaceuticals furosemide and ranitidine: feasibility assessment. Chemosphere 155:606–613
Fontmorin J-M, Fourcade F, Geneste F, Floner D, Huguet S, Amrane A (2013) Combined process for 2,4-dichlorophenoxyacetic acid treatment – coupling of an electrochemical system with a biological treatment. Biochem Eng J 70:17–22
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mousset, E., Trellu, C., Oturan, N., Rodrigo, M.A., Oturan, M.A. (2017). Soil Remediation by Electro-Fenton Process. In: Zhou, M., Oturan, M., Sirés, I. (eds) Electro-Fenton Process. The Handbook of Environmental Chemistry, vol 61. Springer, Singapore. https://doi.org/10.1007/698_2017_38
Download citation
DOI: https://doi.org/10.1007/698_2017_38
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6405-0
Online ISBN: 978-981-10-6406-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)