Abstract
Electrokinetic remediation of soils contaminated with organic contaminants has gained interest due to the large number of contaminated sites with harmful organics. The organic contaminants in soils include pesticides, hydrocarbons, halogenated organics, and other hydrophobic compounds used in industry and agriculture. There is not still a reliable technology for the effective removal of organics from soils. In this context, the electrokinetic technology has attracted the interest of researchers, companies, and governments as a practical technology for the remediation of soils contaminated with organic compounds. The direct application of the electrokinetic technology to a contaminated soil results in limited remediation because the organic contaminants are hydrophobic and remain adsorbed to the soil particles and organic matter. In order to improve the remediation results, various alternatives have been proposed. These alternatives include the use of co-solvents or solubilizing agents (surfactants, cyclodextrins) to enhance the solubility of the organics in the interstitial fluid. Electrokinetic remediation can also be coupled with other remediation technologies such as phytoremediation, biodegradation, and chemical oxidation/reduction. Those coupled technologies aim to degrade the organics in the soil. The aim of this chapter is to review and summarize the remediation of soils contaminated with organics by electrokinetic soil flushing. This chapter includes the different approaches for the removal of organic contaminants in soil and assesses the advantages and drawbacks of the different electrokinetic-based remediation technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oil contamination
- Oil recovery
- Electrochemical transformation
- Diffuse double layer
- Electrokinetic remediation
1 Introduction
Human activities have led to contamination of air, waterbodies, soil, and groundwater. The contamination of soil and groundwater is mainly associated with mining activities, industry, the use of chemicals in agriculture and the lack of proper management of municipal, industrial, and hazardous waste. The contamination of soil has received less attention than the contamination of other media; however, it is a serious problem that affects ecosystems, public health, and the economic activities associated with the use of soil (agriculture, cattle rising, residential, recreational areas, etc.). The European Union was paying attention to the problem of soil contamination, and it has issued regulations to protect the soil and to restore the contaminated sites. The European regulation stressed the need to adopt measures to prevent, limit, and reduce the impact of the human activities in soil [1, 2]. Moreover, it is necessary to develop feasible technologies for the remediation of contaminated sites.
The remediation of contaminated sites requires the application of physical, chemical, and/or biological processes to separate, remove, degrade, or eliminate the contaminants. Since late 1980s and early 1990s of the twentieth century, various innovative soil remediation technologies were developed and tested [3]. Despite the research and development during about 30 years, there is not still a reliable technology for the remediation of contaminated sites. This is probably due to the complex geochemical interactions among soil components and contaminants. In this context, the electrochemical remediation of contaminated soils has been proposed as a new technology with the capacity of removing organic and inorganic contaminants, even in low permeability soils [4]. The studies at laboratory and field scale have proved that electrokinetics is a practical technology for the remediation of contaminated soils, sediments, and sludge, especially for the removal of inorganic contaminants, such as heavy metals, metalloids, and inorganic anions. The remediation of contaminated sites with organic pollutants is more complex due to the hydrophobicity of most of the common organics. However, the operation of electrokinetics in the appropriate conditions with the use of facilitating agents may result in an effective removal and degradation of organic contaminants. The objective of this chapter is to present the scientific and technical bases of electrokinetic remediation and to give an overview of the capacity of this technology for the remediation of organic contaminants.
2 Basis of Electrokinetic Remediation
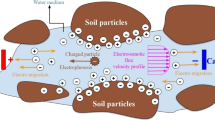
Electrokinetic remediation is an in situ technology specially designed and developed for the restoration of contaminated soils, sediments, and sludge. The electrokinetic process relies on the application of a low-intensity DC electric field directly to the soil to be remediated. The electric current induces the mobilization of the contaminants and their transportation toward the main electrodes, anode and cathode. The electrodes are commonly installed in situ in a well filled with a processing fluid, typically water with chemicals that favor the removal of contaminants. The processing fluid is pumped out of the well and treated to remove or eliminate the contaminants. The processing fluid is recycled back to the well. Figure 1 depicts the in situ application of electrokinetics in a contaminated site [4].
The electrokinetic treatment of a contaminated soil is basically a separation process. The electric field induces the mobilization and transportation of the contaminants by two main transport mechanisms: electromigration and electroosmosis. Electromigration is the transportation of ions toward the electrode of opposite charge. Cations, such as Cu2+, Pb2+, Na+, etc. will be transported toward the cathode (the negative electrode) and anions, such as CrO42−, F−, SO42−, etc. will be transported toward the anode (the positive electrode). Electroosmosis is the net flux of water through the soil induced by the electric field. The electroosmotic flow is the result of the interaction of ions in the interstitial fluid and the charged solid surface of the soil particles. These particles are usually negatively charged, and as a result, the electroosmotic flow goes from anode (−) to cathode (+). All the soluble contaminants in the interstitial fluid (water) can be removed from soil by electroosmosis, including ionic and non-ionic contaminants. The electrokinetic studies have proved that electromigration is the main transport mechanism for ionic contaminants (heavy metals, inorganic anions, etc.), whereas electroosmosis is more effective removing non-ionic contaminants (organic compounds) from the soil [4].
The application of the electric current to a soil specimen also induces chemical reactions upon the main electrodes but also in the mass of soil. These reactions include solubilization, precipitation, neutralization, and redox reaction. The main reaction is the electrolysis of water upon the electrodes: oxidation of water in the anode and reduction of water in the cathode (Eqs. 1 and 2). These reactions have an enormous influence in the solubilization and speciation of the contaminants because the hydronium ions generated at the anode and the hydroxyl ions generated in the cathode are transported through the soil modifying the soil pH. Any change in the pH of the soil and interstitial fluid affects the desorption of contaminants, the precipitation of metals, the ionization of organics, and in general, the speciation of the contaminants. The electrolysis of water is inevitable in a water–soil system, but the operation conditions can be adjusted to favor the pH conditions in the soil that enhance the mobilization of the target contaminants [4]. As an example, Ricart et al. [5] favored the acidification of soil specimen to enhance the mobilization of Mn from soil as Mn2+ and its transportation toward the cathode. On the contrary, Ottosen et al. [6] used ammonia to increase the pH of the soil specimen and mobilize the contaminant copper as [Cu(NH3)4]2−. These studies demonstrate that the understanding of the geochemistry of soils and contaminants is of utmost importance in electrokinetic remediation.
3 Removal of Organic Contaminants by Electrokinetics
Electrokinetic remediation was initially tested for the restoration of soils contaminated with heavy metals and other ionic inorganic contaminants; however, later studies have proved that the electrokinetic treatment can also be satisfactorily applied to soils contaminated with organic contaminants. The main transport mechanism for ionic contaminants is electromigration, especially at high contaminant concentrations. At low concentrations, electroosmosis may also play a significant role in the removal of ionic contaminants. In the case of organic contaminants, the main transport mechanism is electroosmosis because most of the organic contaminants are non-ionic, and they are not affected by the presence of an electric field. Some organic molecules are ionic or ionizable in the soil under the electrokinetic treatment conditions. These molecules can be transported through the soil porous matrix by electromigration. The relative contribution of electromigration and electroosmosis to the transportation of a specific compound depends on the chemical nature of the compound, its concentration in the soil, the characteristics of soil and water content. It has been proved that ion migration if about 10–300 times higher than electroosmosis. In the case of non-ionic organics, they can be removed by soil flushing in high permeability soil, but in low permeable soils, the hydraulic flow is negligible. It is in this case where electroosmosis plays a prominent role in the transportation of non-ionic contaminants. The operating condition of the electrokinetic treatment must be adjusted to favor and maintain a high electroosmotic flow in the soil. However, electroosmosis is very much affected by the physicochemical conditions of soil, ionic concentration in the interstitial fluid, and pH. The acidification of soil in the electrokinetic treatment operated at constant electric potential provokes the drop in the soil zeta potential and the decrease of electroosmotic flow.
The capacity of electrokinetic remediation to remove organic contaminants has been tested with a variety of organic compounds of environmental concern. These compounds include hydrophobic and toxic organics such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated organic compounds (PCBs), pesticides, herbicides, and energetic compounds. Considering that the most toxic, persistent, and dangerous organic contaminants are non-ionic or non-ionizable, electromigration does not play a role in the removal of these compounds, and only electroosmosis is able to remove non-ionic organic contaminants. However, the mass transport by electroosmosis implies the solubilization of organics in the interstitial fluid, water. The solubility in water of most of organic contaminants of concern is very low or even completely insoluble. The removal of organics by electroosmosis requires the addition of facilitating agents that enhance the solubility of the organic contaminants in water. This facilitating agents includes surfactants, co-solvents, cyclodextrins, and others. Other approaches to achieve an effective removal of elimination of organics imply the combination of electrokinetics with other remediation technologies such as chemical oxidation, chemical reduction, permeable reactive barriers, or electrolytic reactive barriers [4].
4 Electrokinetic Removal of Soluble Organics
The dye reactive black 5 is an organic compound commonly used in the textile industry and dyeing processes. This compound is toxic for living organisms, and it can be found in the discharge effluents of the textile industry. Once released to the environment, this compound can be adsorbed and retained in soils and sediments. The chemical structure of the reactive black 5 (Fig. 2) shows a complex molecule of an azo dye with four aromatic rings and four sulfonic groups. The complex structure of the azo dye explains why this molecule is difficult to degrade, as well as its negative effect in the environment that may last for long time. The sulfonic groups confers to the molecule the necessary polar characteristics to explain its solubility in water. Overall, reactive black is a toxic compound, difficult to biodegrade, and it is soluble in water, which increases its bioavailability and toxicity.
The removal of reactive black 5 from soils and sediments by electrokinetics is quite challenging because it is necessary to desorb the molecule from the solid matrix (soil or sediment) and then transport the molecule by electromigration or electroosmosis to be accumulated in the electrode chambers. The desorption of reactive black 5 from soil can be achieved using potassium sulfate in the processing fluid in anode and cathode. Potassium sulfate is transported into the soil specimen by electromigration and electroosmosis. The ionic exchange of K+ with reactive black molecules allows for desorption of the dye from the solid matrix. Once the molecule of reactive black 5 was in solution in the interstitial fluid, it can be transported by electroosmosis and electromigration toward the electrode chambers. The pH of the soil (and the interstitial fluid) is also important in the speciation of the molecule and its transportation by electrokinetics. As it is shown in Fig. 2, reactive black 5 has four sulfonic groups that confer to the molecule the characteristics of a weak acid. In neutral or acid environment, reactive black 5 remains as a neutral molecule, but in alkaline environment, the molecule is ionized forming an anion with four negative charges. The different speciation of reactive black 5 in acid or alkaline pH can be used to favor its transportation by electromigration or electroosmosis. When the pH is alkaline, the anion of reactive black 5 can be transported by electromigration toward the anode. In neutral acid environment, reactive black 5 remains as neutral molecule, and only electroosmosis could be effective in its removal from soil.
The pH of the soil specimen can be modified by the electrokinetic process. The electrolysis of water tends to acidify the pH close to the anode and alkalinize the soil close to cathode. If the reduction of water in the cathode is suppressed by the controlled addition of an acid, the soil is acidified by the hydronium ions electro-generated in the anode. Conversely, the addition of sodium hydroxide in the anode suppress the formation of H+ ions, and the hydroxyl ions generated in the cathode electro-migrates through the soil specimen increasing the pH. In the study for the electrokinetic removal of reactive black 5 [7] very different results were obtained depending on the soil pH. The direct application of the electric field with no pH control in the electrode chambers resulted in no significant removal of reactive black 5 from soil. The dye remained adsorbed to the soil. In a second experiment, the OH− ions in the cathode were neutralized by the controlled addition of sulfuric acid. The acid pH in the soil kept the reactive black 5 as a neutral molecule. No transportation or removal of reactive black 5 was observed in these conditions. The molecule is too big to be transported through the porous matrix of kaolinite by electroosmosis, and it probably remained adsorbed to the soil in acidic pH conditions. However, the electrokinetic treatment with the neutralization of the acid environment with the addition of NaOH in the anode resulted in a complete removal of reactive black 5 from soil. In these conditions, the soil pH was alkaline due to the electromigration of OH− generated in the cathode. The alkaline environment in the soil favored the desorption of reactive black 5 (in the presence of potassium sulfate). However, the most important effect of the alkaline pH is the ionization of the molecule of reactive black 5. At pH higher than 7, reactive black 5 forms an anion with four negative charges than can be electromigrated toward the cathode. Figure 3 shows the electromigration of reactive black 5 toward the anode. The transportation of the dye is evident in the picture sequence. After 5 days of treatment, all the dye was accumulated in the anode chamber. Then, reactive black 5 was degraded by anodic oxidation. This study combined electrokinetic transport and electrochemical oxidation to remove reactive black from soil and its degradation in the anodic solution. This is a good example of the strategic use of chemistry and electrokinetic transport to achieve a complete remediation of the contaminated soil and the degradation of the contaminant simultaneously in the same experimental setup.
5 Electrokinetics with Co-solvents
The main limitation of the electrokinetic remediation of soils with organic contaminants is the low solubility of the contaminants in water. Hydrocarbons, trichloroethylene, pesticides, and energetic compounds usually show very low solubility in water that makes difficult the removal of these compounds in contaminated soils. The transport mechanisms in electrokinetics are electromigration and electroosmosis, and both mechanisms require that the contaminants are in solution in the interstitial fluid. The fluid in the pores of soil is always water in in situ applications. As a result, the remediation of hydrophobic organics by electrokinetics needs the addition of facilitating agents that increase the solubility of the target contaminant in the interstitial fluid. One possibility to enhance the solubility of organics is the use of a co-solvent in the processing fluid.
The co-solvent for the enhanced electrokinetic remediation of organics in soil need to be carefully selected based on technical, environmental, and legal aspects. The co-solvent has to show an important solubility toward the target contaminant to assure fast and effective desorption and solubilization of the contaminants. Moreover, the co-solvent has to be miscible with water since water is always present in soil in in situ applications. The co-solvent must be safe for the environment, with minor environmental impact in the soil and groundwater, and it must be recovered from the soil after the remediation process. The use of co-solvents with water also provokes some technical limitations in the application of electrokinetics. The solubility of salts in the interstitial fluid decreases due to the presence of organic co-solvents, so the electric conductivity of the soil decreases too with the subsequent impact in the electrokinetic transport of the contaminants in soil. The co-solvent also affects the interaction between the soil surface and the interstitial fluid and may change the viscosity of the processing fluid. These two aspects have a major impact in the development of the electroosmotic flow, which is the main transport mechanism for organic contaminants. The use of a co-solvent may have two opposite effects: increases the contaminant solubility and decreases electroosmosis, and the combined result of the two effects may even be negative for the removal of the contaminants.
Various studies in the literature have tested the use of co-solvents for the removal of phenanthrene, an aromatic polycyclic hydrocarbon widely studied as a persistent and hydrophobic contaminant [8,9,10]. The electrokinetic treatment using water as a processing resulted in no removal of phenanthrene, independently of the pH of the soil. Various organic co-solvents were tested to enhance the solubility of phenanthrene in the processing fluid. The tested co-solvents include ethanol, n-butanol, n-butylamine, tetrahydrofuran, or acetone. The mobilization and removal of phenanthrene from soil were evident with the use of co-solvents, specially n-butylamine that resulted in the removal of 43% of phenanthrene in 127 days from a model soil specimen. The removal may be enhanced avoiding the acidification of soil due to the electrolysis of water in the anode, or enhancing the electroosmotic flow with the operation of higher voltage gradient (2 V/cm) or with a periodic voltage application (5 days on: 2 days off). The combined benefits of the enhanced electroosmosis and the contaminant solubilization with the co-solvent resulted in the effective removal of phenanthrene from soil [11].
6 Enhancing Solubility with Surfactants
The solubility of hydrophobic organics in soil remediation may be enhanced with the use of surfactants in the processing fluid in the electrode wells or chambers. The electrokinetic transport phenomena introduce the surfactants in the soil solubilizing the organic contaminants. Then, the solubilized organics can be transported out of the soil by electroosmosis. The combination of the electroosmotic flow and the solubilization of organics with surfactants is a practical approach for the electrokinetic removal of organic contaminants.
Surfactants are a group of chemical compounds with the capacity to modify the surface tension of water. The interest of using surfactants in soil remediation is their capacity to decrease the interfacial tension of water, increasing the solubility in water of hydrophobic organic contaminants. The molecules of surfactants include a hydrophilic group in one end of the molecule and a hydrophobic group in the opposite end. Surfactants are soluble in water due to the activity of the hydrophilic group. At the same time, the hydrophobic group assures the interaction with the organic contaminants in soil. Surfactants in water tend to form spherical structures called micelles. The micelles are formed with the surfactant molecules oriented with the hydrophilic group to the external part of the sphere and the hydrophobic group oriented to the inner part of the sphere. The inner space in the sphere is a hydrophobic environment very appropriate for the solubilization of the organic contaminants. Surfactants only form micelles when the concentration of surfactant in the interstitial fluid reach a specific critical micelle concentration (CMC). It means that the dose of surfactant to the soil needs to be adjusted to reach that specific CMC; lower concentrations are not appropriate for the effective solubilization of the organic contaminants [10].
Surfactant compounds can be classified into four groups considering the electric charge in the molecule. The four groups are neutral, cationic, anionic and zwitterionic. The latter are molecules that include positive and negative charges at the same time in the chemical structure of the molecule. In general, cationic surfactants are not effective in soil remediation application due to the electronegativity of the soil particles. The cationic surfactants tend to interact with soil particles, lowering the mass transportation and, therefore, their efficiency in contaminant solubilization. The most common surfactant in soil remediation are anionic and cationic molecules. Zwitterionic surfactants were also tested in soil remediation. The most important factor in the selection of a surfactant for an in situ application, apart from the contaminant solubilization capacity, is the toxicity for the soil microflora. This is the reason why the most interesting surfactants for soil remediation are natural compounds or biosurfactants. These compounds shows minor environmental impact, and they are easy to degrade after the remediation process [12].
Some examples of electrokinetic studies of contaminated soils with hydrophobic organics includes compounds such as phenanthrene, DDT, diesel hydrocarbons, dinitrotoluene, hexachlorobenzene, and others. These compounds were mobilized with the use of various surfactants: Sodium dodecyl sulfate (SDS), Brij 35, Tween 80, Igepal CA-720, Tergitol, and others. The removal efficiency of the target contaminants in electrokinetic applications with water as processing fluid was negligible. The contaminants remained adsorbed to the soil particles and were not mobilized in the testing conditions. However, the use of surfactants increases the removal efficiency over 80% at lab scale tests with various model and real soils [13, 14]. Phenanthrene was the target contaminant in a study by Reddy and Saichek [9]. Phenanthrene is a low solubility organic compound classified as “acute toxicity, category 4” in the globally harmonized system. In the unenhanced electrokinetic treatment using water as processing fluid, an important electroosmotic flow was registered. Despite the large electroosmotic flow, there was no removal of phenanthrene. Three surfactants were used to improve the solubility of phenanthrene in the interstitial fluid; Tween 80, Witconol, and Igepal CA-720. The addition of the surfactants in the interstitial fluid resulted in a significant decreasing of the electroosmotic flow. This decreasing was due to the increasing viscosity of the interstitial fluid, the different interaction with the soil particle surface and the lower electric conductivity. However, the removal of phenanthrene in the cathodic solution clearly increased despite the lower electroosmotic flow. This was due to the important solubilization of the phenanthrene in the interstitial fluid. Other variables that affect the solubilization and removal of phenanthrene are the soil pH, the ionic strength of the interstitial fluid, and the geochemical characteristics of soil. These variables mainly affect the development of the electroosmotic flow. The acidification of soil by the H+ ions electrogenerated at the anode tend to decrease and even suppress the electroosmotic flow. The acidification of soil is only important in soils with low buffering capacity. In any case, the use of a buffering solution in the anode chamber or the controlled addition of NaOH in the anode may help to avoid the acidification of soil [10, 11]. Overall, the effective removal of phenanthrene may be achieved by the combination of a surfactant and maintaining a high electroosmotic flow. Various strategies were tested to increase the electroosmotic flow: the operation at higher voltage gradients (2 V/cm) or use a periodic application of the voltage gradient, with 5 days on and 2 days off. The off time was used to let reactions to occur in the soil sample. The periodic voltage application resulted in about 90% of the phenanthrene removed on the cathode solution [11].
7 Selective Complexation with Cyclodextrins
Cyclodextrins are a family of compounds with a special structure in the form of a truncated cone. They are composed of glucose units, forming three different cyclodextrins namely α-cyclodextrin (6 units of glucose), β-cyclodextrin (7 units of glucose), and γ-cyclodextrin (8 units of glucose). These compounds are soluble in water due to the hydrophilic interaction of the –OH groups on both the ends of the cone. At the same time, the inner cavity of the cyclodextrin molecule shows a hydrophobic behavior that is very appropriate to allocate non-polar and hydrophobic organic contaminants. The size of the inner cavity of cyclodextrin depends on the number of glucose units that form the molecule. The inner cavity of the α-cyclodextrin is 0.45–0.53 nm, β-cyclodextrin is 0.60–0.65 nm, and γ-cyclodextrin is 0.75–0.85 nm. The cyclodextrins show a selective behavior based on the size of the organic molecule that can be allocated in the inner cavity. The cyclodextrin used in each application can be selected based on the target contaminant and some selective solubilization and removal can be observed based on the size of the contaminant molecules.
Various studies have used cyclodextrins in soil remediation applications for the removal of contaminants such as phenanthrene [15], dinitrotoluene [16], the herbicide atrazine [17], and other contaminants [18] in kaolinite-spiked specimens and real soil samples. The use of cyclodextrins resulted in better removal of the target contaminants compared with the unenhanced electrokinetic treatment. However, the removal results with cyclodextrins are usually lower than that with surfactants, or other remediation technologies (zero iron nanoparticles, in situ chemical oxidation, etc.). The activity of cyclodextrins can be enhanced with their combination with other removal enhancing options. As an example, the combined use of cyclodextrins and ultrasounds [19] and cyclodextrins and chemical oxidation with hydrogen peroxide [20] was tested with a significant improvement in the remediation results of hexachlorobenzene and phenanthrene in kaolinite model soils. An additional aspect to be considered in the use of cyclodextrins is the high cost of these compounds and the difficulty in their recovery from soil in in situ applications. Overall, the limited capacity for organics removal, compared to other remediating alternatives, and the high cost of the compound, result in not good perspectives in contaminated site applications.
8 Polycyclic Aromatic Hydrocarbons
Polycyclic aromatic hydrocarbons (PAH) have received a lot of attention in soil remediation studies. These compounds show a structure based on the condensation of aromatic rings. The most common PAHs studied were phenanthrene and anthracene. These compounds are insoluble and tend to absorb in the soil. Due to these characteristics, PAH are not be removed by electrokinetics in an enhanced test using water as processing fluid. The strategies to improve the electrokinetic removal include the use of facilitating agents that increase the solubility of PAHs in the processing fluid. The most common facilitating agents are surfactants, biosurfactants, co-solvents, and cyclodextrins. As a result, PAHs can be removed from soil based on the combined effect of the solubilization by the facilitating agents and the transportation toward the cathode by electroosmosis. However, the use of facilitating agents (surfactants, co-solvents, etc.) also affects the physicochemical properties of the processing fluid and its interaction with the soil particle surface. These changes in the chemistry of soil have an enormous effect in the development and maintenance of the electroosmotic flow, and hence the removal results will be affected. The pH in the electrode chambers and in the soil must be monitored to avoid the soil acidification that tend to suppress the electroosmotic flow. The periodic voltage application or the use of higher potential gradients may help in the development of the electroosmotic flow. Overall, the combined effect of the electroosmotic flow and surfactants as solubilizing agent results in very effective remediation of soils contaminated with PAH [21].
9 Chlorinated Aliphatic Hydrocarbons, Chlorophenols, and Chlorobenzenes
This group of organic contaminants is characterized for its toxicity toward aquatic organisms and soil microflora. The most common representative of this group is the trichloroethylene (TCE); other common soil contaminants of this group are chlorinated aliphatic hydrocarbons: pentachlorethylene (PCE), trichloroacetate (TCA), and trichlorethylene (TCE); chlorophenols: pentachlorophenol; and chlorobenzenes: PCB (polychloro biphenyls). TCE is found as a soil contaminant due to the lack of proper management of TCE wastes. This compound is relatively more soluble in water (1.280 g/L of TCE) than other components of this group. This means that contaminant TCE in soil can be mobilized to contaminate ground water and surrounding areas. The removal of chlorinated organics from soil is difficult due to the tendency to adsorb in the soil. However, some components of this group can be dissociated, so electromigration also plays a role in the transportation during the electrokinetic treatment. The removal of chlorinated organics from soil by electrokinetics requires the use of solubilizing agents due to the low solubility of these compounds in water. The solubilizing agents are surfactants and co-solvents. Yuan and Weng [22] reach a complete removal of ethylbenzene with the use of SDS (sodium dodecylsulfate) as a surfactant in the anodic solution. The authors claimed that the surfactant aided electrokinetic treatment was cost-effective, and it can be considered as a suitable method for large-scale applications.
10 Herbicides and Pesticides
The continuous use of pesticides and herbicides in agriculture resulted in the contamination of many agricultural fields. Common contaminants found in agricultural soils are the pesticides: DDT, aldrin, dieldrin, and endrin; and the herbicides: atrazine, molinate, and bentazone. These compounds tend to adsorb to the soil. Therefore, it is not possible to remove these contaminants from soil by an unenhanced electrokinetic treatment using water as processing fluid. It is not possible to remove many of those pesticides by electromigration because they form neutral species, and electroosmosis is ineffective due to the negligible solubility in water. The typical approach to remove these contaminants imply the use of surfactants to desorb the contaminants from soil and the subsequent removal by electroosmosis. Suanon et al. [23] reported the effective removal of organochlorine pesticides from a historically contaminated soil. These authors claimed to remove 50% of DDT and 77% of hexachlorobenzene in 15 days using the surfactant Triton X-100 in the anodic solution. The electrokinetic removal of the herbicides, molinate and bentazone, from soil, was studied by Ribeiro et al. [24]. These two herbicides were removed from soil by a combination of electromigration and electroosmosis. Molinate was concentrated in the cathodic solution. Both transport mechanisms, electromigration and electroosmosis, contributed to molinate transportation. Conversely, bentazone was accumulated in the anode and in the cathode depending on the electrokinetic conditions. At high electric density conditions, the transport of bentazone was faster than electroosmosis and bentazone was accumulated in the anode chamber. At low electric density conditions, the transport by electroosmosis was more effective and bentazone was accumulated in the cathode compartment. These results proved the importance of the geochemical interactions of soil-interstitial fluid-contaminant and their effect on the transportation of the contaminants out of the soil.
11 Nitroaromatic Compounds
Manufacture and use of ammunition are the causes of the contamination of soil with a specific group of contaminants called energy compounds. In this group, the most relevant contaminating substances are nitroaromatic compounds (TNT, DNT, and RDX). These compounds show a good affinity for organic matter and clay minerals. Therefore, they tend to remain adsorbed to the soil. Moreover, these compounds show non-polar molecules and low solubility in water. Removal of these compounds from soil by electrokinetics requires the use of solvents or surfactants to enhance the solubility in the interstitial fluid. Kessler et al. [25] showed that removal of DNT can be enhanced with cyclodextrins (CD) and cyclodextrin derivatives such as carboxymethyl-β-CD, amino-β-CD, and hydroxypropyl-β-CD. Further research is required in this field to find suitable and effective solubilizing agents for a complete removal of energetic compounds in contaminated soils.
12 Mixtures of Heavy Metals and Organic Pollutants
Contaminated sites often contain a mixture of contaminants including heavy metals and organics. The remediation of these sites by electrokinetics is challenging due to the different physicochemical characteristics of the contaminants and the different behavior under electrokinetic test conditions [26]. In general, heavy metals and other inorganic contaminants form ions that can be transported by electromigration. Conversely, organic contaminants are usually neutral species and show very low solubility in water, and their transportation is mainly by electroosmosis. The pH conditions in soil play a key role in the solubilization and removal of heavy metals. Cationic metals (Cd2+, Cu2+, Pb2+, etc.) can be solubilized at acidic pH favoring the advance of the acid front from the anode and neutralizing the alkaline environment of the cathode with the controlled addition of an acid. The penetration of OH− ions in the soil from the cathode may also be avoided with cationic exchange membranes. The use of complexing agents in the processing fluid is an alternative option to keep the metals in solution and avoid their precipitation in the alkaline environment close to the cathode. Anionic metals (CrO4−, etc.) can be mobilized at alkaline pH due to the reduced adsorption of the metal anions to the soil. On the other hand, organic contaminants require the addition of solubilizing agents to increase the solubility in water. The operating conditions of the electrokinetic treatment must be adjusted to favor the electroosmotic flow. The combined effect of the solubilizing agents and high electroosmotic flow results in the effective removal of the organic contaminants. However, the electroosmotic flow may be largely affected by the pH changes, especially the acidification in soil. When the pH of soil decreases and reaches acidic values, the electroosmotic flow sharply decreases and even reverses due to the change of the soil surface charge from negative to positive. As a result, the simultaneous removal of both heavy metals and organics is not always possible [27].
Various methods and processes have been developed for the sequential removal of organics and heavy metals from soils. Elektorowicz and Hakimipour [28, 29] developed the so-called SEKRIOP process that uses surfactants to dissolve hydrocarbons and EDTA to mobilize metals in the electrokinetic treatment of contaminated soils. This technology uses cationic exchange membranes in the cathode to retain free cationic metals transported by electromigration. The anionic complexes of heavy metals with EDTA are retained in anionic exchange membranes is the anode. This method resulted in very good removal ratios of heavy metals and hydrocarbons when used in model soils at lab scale. However, the SEKRIOP technology did not show such a good performance when processing actual soil specimens from contaminated sites with heavy metals and hydrocarbons. The research was then focused in improving the removal ratio, the treatment time, and the adequate management of the wastes from the electrode solutions [30].
13 Coupled Electrokinetic-Chemical Oxidation/Reduction
The coupled technology electrokinetic remediation with chemical oxidation/reduction is an interesting alternative to the treatment of contaminated soils with organic contaminants. The main limitation of organic contaminants is the low solubility in the processing fluid (water). That is the reason for the difficulty in removing organics by electrokinetics. The remediation by in situ chemical oxidation does not require the mobilization and transportation of the organic contaminants. In this technology, the contaminants are degraded in the soil by the action of chemical oxidants. The contaminants are transformed in smaller molecules, typically less toxic and harmful than the original contaminants. Eventually, the organic contaminants are completely oxidized to carbon dioxide and water. The limitation of chemical oxidation is the effective delivery of the oxidant in to the soil, especially in low permeability soils. It is exactly here where the combination of electrokinetics and chemical oxidation may result in a synergistic effect to achieve a fast remediation. In the coupled technology electrokinetic chemical oxidation, the electrokinetic transport phenomena are used to introduce into the soil the oxidants. Depending on the chemical nature of the oxidants, these reagents are dissolved in the anodic solution or the cathodic solution, and they are transported into the soil by electromigration or electroosmosis. As an example, permanganate and persulfate can be transported into the soil by electromigration from the cathodic solution, whereas hydrogen peroxide has to be added to the anodic solution and transported by electroosmosis into the soil because hydrogen peroxide is a neutral molecule. Other chemical reactants can be used for the degradation of the organic contaminants by a reductive chemical process to obtain less toxic organics. For example, the reductive dechlorination of organochloride pesticides or chlorinated solvents is based on the removal of chloride ions from the organic molecule, resulting in much less toxic products than can be degraded by monitored natural attenuation [31].
Yukselen-Aksoy and Reddy [32] tested the electrokinetic delivery of persulfate in a contaminated soil with PCB. Sodium persulfate is one of the common oxidizing agents used in environmental applications. The standard reduction potential of persulfate is 2.7 V, which assures the oxidation of organic contaminants. In this study, persulfate was added to the anodic solution, and it was transported into the soil by a combination of electromigration and electroosmosis. Persulfate needs to be activated to be able to degrade the organic contaminants. The activation in electrokinetic can be done by temperature or pH. Persulfate requires a minimum of 45 °C to be activated or a pH below 4. Those conditions can be achieved in electrokinetics adjusting the electric field intensity and favoring the advance of the acid from electrogenerated in the cathode. These authors reported that about 78% of PCB in the kaolinite soil specimen was degraded by persulfate activated by temperature and pH.
Oonnittan et al. [20, 33] tested the in situ chemical oxidation of hexachlorobenzene (HCB) contaminated soils. These authors used hydrogen peroxide as chemical reagent added to the anolyte solution. Hydrogen peroxide was transported into the soil by electroosmosis and attacked the organic contaminant in a Fenton-like process where the iron content in the soil was sufficient to activate the H2O2 for the generation of hydroxyl radicals (˙OH). The Fenton reagent can be deactivated at alkaline pH, so it is important to maintain the pH of the soil below 7 to assure an effective reaction of the hydrogen peroxide over the HCB. At alkaline pH, H2O2 decomposes in water and oxygen and does not form ˙OH radicals. In acid conditions, about 60% of HCB was eliminated from the soil in 10 days. The removal efficiency may be improved increasing the treatment time and controlling the soil pH in the optimum range for Fenton reaction (slightly acidic pH).
14 Nanoparticle Transport by Electrokinetics
The use of zero valent iron in environmental applications grew very fast due to its high capacity to reduce and degrade a variety of contaminants. One of the most interesting application of zero valent iron is the catalysis of reductive dechlorination of organic compounds such as trichloroethylene, pentachlorophenol, hexachlorobenzene, and others. The use of zero valent iron in the form of nanoparticles largely increases its activity and its application in environmental remediation. Reddy and Karri [34] tested the use of iron nanoparticles with electrokinetics to remove pentachlorophenol. In the coupled technology, the electrokinetic process is used as a driving force to introduce the nanoparticles in soil. The transportation of the nanoparticles is carried out by electroosmosis. The nanoparticles can be added to the anodic solution, but the oxidative environment in the anode may affect the stability of the nanoparticles. To avoid such effect, the addition of the nanoparticles is commonly done in the soil or in an additional chamber separated from the anode. In the study by Reddy and Karri [34], the advance of the nanoparticles through the soil specimen was followed by analyzing the increase in iron concentration. This method does not assure that the zero valent iron was transported as nanoparticles. The concentration of pentachlorophenol decreases at the end of the treatment by about 50% in the soil specimen. A complete removal of pentachlorophenol was found in the cathode side due to the combined effect of nanoparticles and reductive dechlorination. In order to improve the remediation results, it is necessary to favor the transportation of nanoparticles through the soil. The main limitation for an effective transportation of iron nanoparticles is their tendency to interact among them, to aggregate, and to settle very fast. Moreover, the high reactivity of iron nanoparticles results in their premature oxidation. Cameselle et al. [35] studied the zeta potential of iron nanoparticles and the influence of groundwater in the electrokinetic behavior. These authors have proposed the use of dispersants in the addition of iron nanoparticles to soil in order to avoid premature aggregation and settlement. Cameselle et al. [35] concluded that aluminum lactate showed good properties in the dispersion of iron nanoparticles in large-scale applications for the removal of organochloride contaminants. Other microscale and nanoscale particles, including bimetallic particles with copper and iron, or palladium and iron, were tested in the dechlorination of organic contaminants. Zheng et al. [36] proved the good activity of Cu/Fe nanoparticles in the complete removal of hexachlorobenzene, whereas the Pd/Fe nanoparticles showed only 60% removal in the study by Wan et al. [37].
15 Coupled Electrokinetic-Permeable Reactive Barriers
Permeable reactive barriers (PRB) have been satisfactorily used for the remediation of contaminant plumes in groundwater. PRB are a passive remediation system installed in the path of groundwater. Basically, a PRB is a trench in the path of groundwater filled with a reactive material. The contaminants in the groundwater are absorbed or react with the filling material of the PRB. The reactive filling material must be carefully selected to remove the target contaminants. In the case of organic contaminants, hydrocarbons and organochlorides, zero iron nanoparticles are preferred for their capacity to degrade organics and to decrease the toxicity of the contaminants by reductive dechlorination. Activated carbon can be used for the removal of organics and heavy metals. Precipitations reagents, calcium carbonate, can be used for the neutralization and precipitation of heavy metals in the groundwater. The PRB have to be designed to receive all the flow of groundwater, avoiding bypass. A PRB with a hydraulic conductivity much higher than the surrounding soil assures the flow of groundwater through the PRB. This technology is, in general, practical and affective in the removal of contaminants. Moreover, the operation costs and maintenance are minimal. A well-designed PRB may operate for a year with minor supervision.
A modification of the PRB is the electrokinetic barriers, which consist of a series of electrodes around a contaminated area or in front of the advance of contaminated groundwater. The electrodes are arranged in rows perpendicular to the direction of the groundwater flow. The depth of the electrodes must be at least coincident with the depth of the contaminated area. The electric current is established within the soil by the alternation of anodes and cathodes, which are connected to independent hydraulic circuits to adjust the most suitable conditions for the anolyte and the catholyte (pH, addition of solubilizing or complexing agents). Periodically, the contaminants in the electrolytes are removed by different techniques such as adsorption and ion exchange.
15.1 Electrokinetic Bio-barriers
Electrokinetic bio-barriers are basically electrokinetic barriers designed to contain pollutants and promote their biodegradation, both in soils and in groundwater. This technology consists of the installation of a row of anodes and cathodes perpendicular to the direction of the groundwater flow. A series of wells sandwiched between anodes and cathodes are also drilled and used to inject the nutrient solutions such as nitrogen, phosphorous, and substances capable of supplying oxygen to the medium. The chemical species that make up the nutrients are electrically charged and can therefore be dispersed through the soil homogeneously by electromigration. The organic pollutants present in the soil and transported by the groundwater are degraded at the level of the bio-barrier and downstream, thanks to the microbial activity favored by the supply of nutrients and oxygen.
15.2 Reactive Electrolytic Barriers
The electrolytic reactive barriers consist of two rows of electrodes (anodes and cathodes) very close to each other with a permeable filler material between both rows of electrodes. The barrier is installed in a trench perpendicular to the direction of the groundwater flow so that it intercepts the advance of contaminants carried by the groundwater (similar to reactive permeable barriers). A low electrical potential is applied to the electrodes that induces oxidation conditions at the anodes and reduction conditions at the cathodes. This system allows the transformation or degradation of pollutants by redox reactions into new products that are less toxic or dangerous for the environment. A wide range of redox contaminants such as arsenic, chlorinated hydrocarbons (TCE, TCA), and energy compounds (TNT and RDX), including mixtures of contaminants (difficult to treat with other technologies), can be treated with electrolytic barriers. This approach offers several advantages including: (1) the effective degradation of contaminants and reaction intermediates through oxidation and sequential reduction, (2) controlling the formation of contaminant precipitates through periodic inversion of the electrode potentials, (3), the contribution of chemical products is not necessary for the transformation; (4) simple operation, and (5) low operating cost. This technology has shown very good results in the treatment of soils and groundwater contaminated with chromium or TCE.
16 Bioelectroremediation
Electrokinetics can be combined with bioremediation to achieve a synergistic effect in the remediation of soil contaminated with organics. Bioremediation uses the capacity of the soil microflora to degrade the organic contaminants in situ. The main limitation of the biological degradation in the bioavailability of the contaminants. Electrokinetics may be used to mobilize and increase the availability of the contaminants. The electric field favors the desorption of the contaminants to be dissolved in the interstitial fluid, and transport the contaminants out of small pores where the microorganisms cannot enter. Moreover, electromigration and electroosmosis can be used for the supply of nutrients (ammonium, phosphate, etc.) and oxygen (e.g., oxygen in the form of H2O2) to the subsoil in in situ applications. An interesting effect of the coupled technology electrokinetic bioremediation is the transport of bacteria. The electric field may transport the bacteria, even in short distances, increasing the probability to access the contaminants, i.e., the electrokinetic transport of bacteria and contaminants increases the bioavailability of contaminants [38, 39]. Overall, the electric field is a simple and effective way to increase the bioremediation activity.
The electrokinetic biofence (EBF) technology developed by Lageman and Pool [40] is another way to combine electrokinetics and biodegradation. In the EBF, a series of alternating anodes and cathodes are installed perpendicular to the contaminated water flow. The electrode wells are filled with a nutrient solution (ammonium nitrate, potassium phosphate, etc.) that is dispersed in the subsoil by the electrokinetic transport. The increasing concentration of nutrients in the contaminated groundwater favors the biodegradation of the contaminants. This technology was applied for the remediation of a contaminated soil with organochlorine solvents. After 2 years of operation, the decreasing of the chlorine index (the amount of compounds with chlorine in the molecules) was observed. The operation requires low maintenance and supervision. The electricity for the electrokinetic process was provided by solar panels.
17 Electric Amendment of Phytoremediation
Phytoremediation is a benign and sustainable technology for the remediation of contaminated soils with heavy metals, inorganic contaminants, and biodegradable organic contaminants. Phytoremediation uses green plants to remove and/or degrade contaminants in the rhizosphere, the layer of soil occupied by the roots. The benefits of phytoremediation are the capability of removing organic and inorganic contaminants, minimum maintenance, and operational costs, which is visually pleasing and improves the quality of soil during the remediation, unlike other remediation technologies that seriously damage the quality of soil. The main limitations of the phytoremediation are the bioavailability of contaminants, the slow growth of the plants, the remediation limited to the layer of soil occupied by the roots, and the contaminant concentration has to be low or moderate, because plants will not survive in highly contaminated soils. The coupled technology electrokinetics phytoremediation was proposed to avoid in part the limitations of phytoremediation. The application of an electric field around a growing plant shows various positive effects in the plant and in the remediation process. The electric field mobilizes the nutrients that are transported toward the roots. Selected nutrient solutions can be added to the electrode wells and transported toward the roots. Similarly, the contaminants in soil can be mobilized by the electric field and transported toward the roots, where the contaminants are accumulated and degraded. The contaminants can be transported from soil zones out of the rhizosphere. Overall, the simultaneous application of electrokinetics to phytoremediation enhances the plant growing, increases de bioavailability of contaminants, and extends the remediation further than the rhizosphere.
The research results in electro-phytoremediation have proved that low or moderate electric gradients (below 2 DCV/cm) are beneficial for the plant and the remediation process. High-intensity electric field may provoke damage in soil microflora and plants. The damage is associated with pH changes due to the electrolysis of water upon the electrodes (acid pH on the anode side and alkaline pH on the cathode side). Rapid mobilization and transportation of contaminants toward the roots, reaching concentrations that may be toxic for the plant, is another limitation associated with high-intensity electric fields. These limitations may be avoided using alternate current instead of direct current. More research is still needed to determine the real benefits of electro-phytoremediation over the phytoremediation itself. The research must focus on the physiological changes induced by the electric current and how those changes in plant physiology may contribute to a better and faster soil remediation [41, 42].
18 Future Perspectives
The design of an electrokinetic application for the remediation of soils contaminated with organics must consider the scientific knowledge accumulated in the last three decades. In general, removal of organics requires the addition of facilitating agents (surfactants) to enhance the solubility of contaminants and, at the same time, keep a high electroosmotic flow. The combined effect of surfactants and electroosmotic flow results in the effective removal of organics. However, the surfactant-enhanced electrokinetics is costly, requires long treatment time, and generates wastes that require further treatment. As alternative, the combination of electrokinetics with chemical oxidation, bioremediation, or phytoremediation shows better perspectives. Chemical oxidation can be used in soils with toxic contaminants at high concentrations, whereas biological technologies can be used in low to moderate contaminated sites. Biological technologies are preferred because they do not damage the soil properties and do not require expensive chemicals, and the implementation and operational costs are relatively low.
References
S. Paleari, Is the European Union protecting soil? A critical analysis of community environmental policy and law. Land Use Policy 64, 163–173 (2017)
B. Vanheusden, Recent developments in European policy regarding brownfield remediation. Environ. Pract. 11(4), 256–262 (2009)
H.D. Sharma, K.R. Reddy, Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies (Wiley, Hoboken, 2004)
K.R. Reddy, C. Cameselle, Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater (Wiley, Hoboken, 2009)
M.T. Ricart, C. Cameselle, T. Lucas, J.M. Lema, Manganese removal from spiked kaolinitic soil and sludge by electromigration. Sep. Sci. Technol. 34(16), 3227–3241 (1999)
L.M. Ottosen, H.K. Hansen, G. Bech-Nielsen, A. Villumsen, Electrodialytic remediation of an arsenic and copper polluted soil-continuous addition of ammonia during the process. Environ. Technol. 21(12), 1421–1428 (2000)
M. Pazos, M.T. Ricart, M.A. Sanromán, C. Cameselle, Enhanced electrokinetic remediation of polluted kaolinite with an azo dye. Electrochim. Acta 52(10), 3393–3398 (2007)
A. Li, K.A. Cheung, K.R. Reddy, Cosolvent-enhanced electrokinetic remediation of soils contaminated with phenanthrene. J. Environ. Eng. 126(6), 527–533 (2000)
K.R. Reddy, R.E. Saichek, Effect of soil type on electrokinetic removal of phenanthrene using surfactants and cosolvents. J. Environ. Eng. 129(4), 336–346 (2003)
R.E. Saichek, K.R. Reddy, Electrokinetically enhanced remediation of hydrophobic organic compounds in soils: a review. Crit. Rev. Environ. Sci. Technol. 35(2), 115–192 (2005)
R.E. Saichek, K.R. Reddy, Effect of pH control at the anode for the electrokinetic removal of phenanthrene from kaolin soil. Chemosphere 51(4), 273–287 (2003)
S.N. Seyed Razavi, A. Khodadadi, H. Ganjidoust, Treatment of soil contaminated with crude-oil using biosurfactants. J. Environ. Stud. 37(60), 107–116 (2012)
H.I. Gomes, C. Dias-Ferreira, A.B. Ribeiro, Electrokinetic remediation of organochlorines in soil: enhancement techniques and integration with other remediation technologies. Chemosphere 87(10), 1077–1090 (2012)
A.T. Yeung, Y. Gu, A review on techniques to enhance electrochemical remediation of contaminated soils. J. Hazard. Mater. 195, 11–29 (2011)
K. Maturi, K.R. Reddy, Simultaneous removal of organic compounds and heavy metals from soils by electrokinetic remediation with a modified cyclodextrin. Chemosphere 63(6), 1022–1031 (2006)
A.P. Khodadoust, K.R. Reddy, O. Narla, Cyclodextrin-enhanced electrokinetic remediation of soils contaminated with 2,4-dinitrotoluene. J. Environ. Eng. 132(9), 1043–1050 (2006)
G. Wang, W. Xu, X. Wang, L. Huang, Glycine-β-cyclodextrin-enhanced electrokinetic removal of atrazine from contaminated soils. Environ. Eng. Sci. 29(6), 406–411 (2012)
K.R. Reddy, P.R. Ala, S. Sharma, S.N. Kumar, Enhanced electrokinetic remediation of contaminated manufactured gas plant soil. Eng. Geol. 85(1–2), 132–146 (2006)
T.D. Pham, R.A. Shrestha, M. Sillanpää, Removal of hexachlorobenzene and phenanthrene from clayey soil by surfactant- and ultrasound-assisted electrokinetics. J. Environ. Eng. 136(7), 739–742 (2010)
A. Oonnittan, R.A. Shrestha, M. Sillanpää, Effect of cyclodextrin on the remediation of hexachlorobenzene in soil by electrokinetic Fenton process. Sep. Purif. Technol. 64(3), 314–320 (2009)
M.O. Boulakradeche, D.E. Akretche, C. Cameselle, N. Hamidi, Enhanced electrokinetic remediation of hydrophobic organics contaminated soils by the combination of non-ionic and ionic surfactants. Electrochim. Acta 174, 1057–1066 (2015)
C. Yuan, C.H. Weng, Remediating ethylbenzene-contaminated clayey soil by a surfactant-aided electrokinetic (SAEK) process. Chemosphere 57(3), 225–232 (2004)
F. Suanon, L. Tang, H. Sheng, Y. Fu, L. Xiang, Z. Wang, et al., Organochlorine pesticides contaminated soil decontamination using TritonX-100-enhanced advanced oxidation under electrokinetic remediation. J. Hazard. Mater. 122, 388 (2020)
A.B. Ribeiro, E.P. Mateus, J.M. Rodríguez-Maroto, Removal of organic contaminants from soils by an electrokinetic process: the case of molinate and bentazone. Experimental and modeling. Sep. Purif. Technol. 79(2), 193–203 (2011)
D.A. Kessler, C.P. Marsh, S.W. Morefield, Electrokinetic Removal of Energetic Compounds (Wiley, Hoboken, 2009)
M.T. Ricart, M. Pazos, S. Gouveia, C. Cameselle, M.A. Sanroman, Removal of organic pollutants and heavy metals in soils by electrokinetic remediation. J. Environ. Sci. Health A 43(8), 871–875 (2008)
K.R. Reddy, C. Cameselle, P. Ala, Integrated electrokinetic-soil flushing to remove mixed organic and metal contaminants. J. Appl. Electrochem. 40(6), 1269–1279 (2010)
M. Elektorowicz, M. Hakimipour, Electrical field applied to the simultaneous removal of organic and inorganic contaminants from clayey soil, in 18th Eastern Research Symposium on Water Quality, October, Montreal, Canada: CAWQ (2001)
M. Elektorowicz, M. Hakimipour, Practical consideration for electrokinetic remediation of contaminated soil, in CSCE 8th Environmental and Sustainable Eng. Specialty Conference and Offshore Engineering, Moncton (2003), pp. 689–698
K.R. Reddy, K. Maturi, C. Cameselle, Sequential electrokinetic remediation of mixed contaminants in low permeability soils. J. Environ. Eng. 135(10), 989–998 (2009)
S. Grandel, A. Dahmke, Monitored natural attenuation of chlorinated solvents: assessment of potential and limitations. Biodegradation 15(6), 371–386 (2004)
Y. Yukselen-Aksoy, K.R. Reddy, Effect of soil composition on electrokinetically enhanced persulfate oxidation of polychlorobiphenyls. Electrochim. Acta 86(24), 164–169 (2012)
A. Oonnittan, R.A. Shrestha, M. Sillanpää, Remediation of hexachlorobenzene in soil by enhanced electrokinetic Fenton process. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 43(8), 894–900 (2008)
K.R. Reddy, M.R. Karri, Effect of electric potential on nanoiron particles delivery for pentachlorophenol remediation in low permeability soil. in Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering: The Academia and Practice of Geotechnical Engineering (2009)
C. Cameselle, K.R. Reddy, K. Darko-Kagya, A. Khodadoust, Effect of dispersant on transport of nanoscale iron particles in soils: zeta potential measurements and column experiments. J. Environ. Eng. 139(1), 23–33 (2013)
Z. Zheng, S. Yuan, Y. Liu, X. Lu, J. Wan, X. Wu, et al., Reductive dechlorination of hexachlorobenzene by Cu/Fe bimetal in the presence of nonionic surfactant. J. Hazard. Mater. 170(2–3), 895–901 (2009)
J. Wan, Z. Li, X. Lu, S. Yuan, Remediation of a hexachlorobenzene-contaminated soil by surfactant-enhanced electrokinetics coupled with microscale Pd/Fe PRB. J. Hazard. Mater. 184(1–3), 184–190 (2010)
S.T. Lohner, A. Tiehm, S.A. Jackman, P. Carter, Coupled electrokinetic-bioremediation: applied aspects, in Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater, ed. by K. R. Reddy, C. Cameselle, (Wiley, Hoboken, 2009), pp. 389–416
L.Y. Wick, Coupling electrokinetics to the bioremediation of organic contaminants: principles and fundamental interactions, in Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater, ed. by K. R. Reddy, C. Cameselle, (Wiley, Hoboken, 2009), pp. 369–387
R. Lageman, W. Pool, Electrokinetic biofences, in Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater, ed. by K. R. Reddy, C. Cameselle, (Wiley, Hoboken, 2009), pp. 357–366
C. Cameselle, S. Gouveia, Phytoremediation of mixed contaminated soil enhanced with electric current. J. Hazard. Mater. 361, 95–102 (2019)
C. Cameselle, R.A. Chirakkara, K.R. Reddy, Electrokinetic-enhanced phytoremediation of soils: status and opportunities. Chemosphere 93(4), 626–636 (2013)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cameselle, C., Gouveia, S., Cabo, A. (2021). Electrokinetic Soil Flushing. In: Rodrigo, M.A., Dos Santos, E.V. (eds) Electrochemically Assisted Remediation of Contaminated Soils. Environmental Pollution, vol 30. Springer, Cham. https://doi.org/10.1007/978-3-030-68140-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-68140-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68139-5
Online ISBN: 978-3-030-68140-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)