Abstract
A large amount of residues from active pharmaceutical ingredients (API) that are currently in use are known to reach aquatic ecosystems and have potentially adverse effects on living organisms. Prioritization methods are useful tools for both regulation and surveillance purposes in the environmental policy of APIs. Their use has largely increased over the last decade, and the different existing methodologies can lead to large discrepancies between the highlighted substances. This chapter aims at discussing studies conducted in the context of hospitals. Perhaps more important than the results themselves, the methodologies with the set of selected criteria are discussed, as well as their advantages and associated uncertainties. A case study of API prioritization applied to a Swiss university hospital is presented with two different approaches: a ranking-based OPBT approach (Occurrence, Persistence, Bioaccumulation, and Toxicity) and an environmental risk assessment (ERA), with the calculation of risk quotient (RQ). The ERA results combined with those of other studies dealing with ERA-based API prioritization in hospitals highlighted several compounds presenting high risks for the aquatic ecosystems (RQ > 1): antibiotics (ciprofloxacin, amoxicillin, piperacillin, azithromycin), anti-inflammatory drugs (diclofenac, mesalazine), as well as the hormone estradiol and the antidiabetic metformin. Nevertheless, only the antibiotic ciprofloxacin was commonly determined as problematic. Finally, the most critical issues for API prioritization in hospitals were identified from the literature overview and the results of the presented case study: handling of the consumption data, involvement of expert judgment, uncertainties linked with the predicted environmental concentration (PEC) calculation, and quality of the hazard evaluation. Although prioritization procedures applied to hospitals can be burdensome to apply in practice and many associated uncertainties remain, they represent essential tools to establish lists of priority molecules to follow via monitoring programs and allow their theoretical risk assessment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Environmental risk assessment
- Hospital effluents
- Pharmaceuticals
- Predicted environmental concentrations
- Prioritization
1 Introduction
A large amount of the many active pharmaceutical ingredients (APIs) currently in use reaches aquatic ecosystems and generates potentially adverse effects for living organisms [1,2,3]. Once in the environment, API residues can indeed cause some adverse effects to wildlife, such as the feminization of male fish from synthetic hormones [3, 4] or the impairment of organs by the nonsteroidal anti-inflammatory drug diclofenac in trout [5].
Sources of APIs in surface water are diverse: they may come from human and animal use, waste disposal, and/or manufacturing [2, 6]. Generally, urban wastewater treatment plants (WWTPs) are the main contributors of API residues into aquatic ecosystems through human consumption in households [7, 8]. Urban wastewater was shown to have contained pharmaceutical residues for decades [9]. Recently, however, increasing attention was paid to hospitals and healthcare facilities as a source of environmental pollution through their effluents [10,11,12]. They differentiate themselves from domestic sources by the nature of the administered molecules [10, 13]. Hospitals represent only a small proportion of the urban API load source found at the watershed outlet on average: <10% [2], <15% [11], and 20–25% [12]. Nevertheless, this fraction can vary from 3 to 74% according to the compound type and the hospital bed/inhabitant ratio of the watershed [14]. An in-depth analysis is developed in the chapter by Chonova et al., in this book.
All APIs currently in use, which vary between 3,000 and 5,000 [15,16,17], cannot be measured in monitoring campaigns or be assessed for environmental risk. Prioritization methods are thus necessary because they allow the establishment of priority lists of molecules to be monitored, based on a set of selected criteria. Prioritization was thus identified by a panel of 40 international experts as the second most important question in the area of ecotoxicology and environmental risks of pharmaceuticals [15]. The use of prioritization methodologies for APIs increased in the past 15 years, but some methodological discrepancies are largely observed between studies due to different objectives. Indeed, they were implemented with different emphases: spatial variability [18, 19]; some specific types of drugs, such as veterinary drugs [20, 21] or anticancer drugs [18, 22]; and hospital effluents [23, 48].
This chapter aims at presenting, first, an overview of the different prioritization methodologies currently used, both in a broader context and for hospital wastewater. Second, these approaches are illustrated with a case study from a major hospital in Switzerland. The priority list obtained is detailed and compared with other studies. Finally, the most important parameters when applying API prioritization in hospitals are identified and discussed.
2 API Prioritization Approaches
2.1 Method Overview
In general, API prioritization methods are based on consumption data and on a simplified risk assessment for the environment and/or human health [11, 24, 25]. The parameters considered are environmental persistence, the bioaccumulation potential, and the effects [26,27,28,29]. The elaboration of a priority list of pharmaceuticals thus strongly depends on the quantity and the quality of the available data for these three parameters [13, 30]. Some studies also consider the mode of action [31] or the analytical feasibility in the procedure [7]. Therefore, the relevance of the chosen criteria is of great importance and may lead to important methodological discrepancies from one study to another and can also induce many uncertainties in the results [30, 32].
One commonly used method for prioritizing is the persistence, bioaccumulation, and toxicity (PBT) approach proposed in Europe in the framework of the registration, evaluation, authorization, and restriction of chemicals (REACH). Specific studies dealing with pharmaceuticals were also applied in this approach, which consists of calculating a ranking of concern according to the PBT properties of the substances [28, 33, 34]. For example, the national approach developed in Sweden established a ranking from 1 to 3 for each parameter, with a maximum global rank of 9.Footnote 1 Unfortunately, the experimental data are still scarce, inducing a lack of realistic information regarding the PBT properties and the behavior of pharmaceuticals in the environment [34].
This leads researchers to use computational tools such as Quantitative Structure Activity Relationship (QSAR) models to predict the missing values [35]. However, computed values cannot replace experimental ones [27, 36, 37]. Nevertheless, the use of two different QSAR models for the prioritization of more than 1,200 APIs was shown to be in agreement with each other’s models (86%) [34]. More importantly, several priority compounds that were highlighted, such as clotrimazole, sertraline, loratadine, or miconazole, were in line with previous studies and have already been detected in the environment [28, 36].
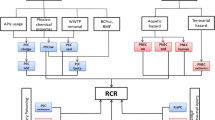
Another method for prioritizing pharmaceuticals is proposed by the European Medicines Agency in the preapproval phase for the authorization of new medicinal products for human use [25]. The EMEA guidelines require an environmental risk assessment for the new compounds introduced into the European market since 2006 [25]. It is worth stressing that the environmental risks for APIs registered before that date are therefore not properly assessed or are not assessed at all [34]. This ERA consists of a tier-based environmental risk assessment procedure for APIs, which comprises two phases: the estimation of exposure (Phase I) and the environmental fate and effects analysis (Phase II). This procedure has been adopted by several authors and is adapted according to the study’s specific needs [13, 26, 29].
Phase I comprises a PBT approach and the calculation of the predicted environmental concentrations (PEC) in receiving surface water (PECSW), which are calculated as follows:
with DOSEai being the maximum daily dose consumed per inhabitant, F pen as the fraction of the market penetration, WWinhab as the amount of wastewater per inhabitant per day, and DIL as a dilution factor, which represents the dilution of wastewater in surface water. Thus, PECsw does not consider degradation or retention in WWTP, nor patient metabolism. Moreover, the default values of 0.01 for F pen, 200 L inh−1 d−1 for WWinhab, and 10 for DIL are proposed in the EMEA guidelines.
F pen may also be calculated according to the consumption data and defined daily doseFootnote 2 (DDD) values proposed by the World Health Organization (WHO). Thus, by using PBT properties derived from QSAR modeling and PECsw generated with a number of default values, the procedures following the guidelines are likely unrealistic. Nevertheless, if the results of Phase I show that the active ingredient in question has a bioaccumulation tendency (Log K ow > 4.5) or exhibits a PEC above 10 ng/L, then Phase II is needed.
Phase II addresses the calculation of the environmental risk quotient (RQ) as the ratio between exposure (PEC) and effects (PNEC):
PNEC is defined as a predicted non-effect concentration and is calculated by applying an assessment factor (AF) to the non-observed effect concentration (NOEC), which is calculated based on ecotoxicological tests performed on several species. AF can vary between 10 and 1,000 according to the number of tested species [38].
In 2008, Besse and Garric [26] reviewed studies from eight different countries that prioritized and identified the most problematic pharmaceutical compounds for the environment in Europe and the USA between 2000 and 2008. They noted that despite similar methods used to determine the exposure, i.e., the PEC calculation, there were some important discrepancies in the methodologies used to assess PNEC values, making a proper comparison of the results difficult. For example, PNEC values can be associated with either acute or chronic toxicity tests, and it has been shown that the acute risks linked with API were rather negligible, whereas chronic risks could not be ruled out because of the scarcity of the ecotoxicological data [36]. Thus, ERA studies based on acute toxicity tests do not reflect the risks of long-term exposure to subacute levels.
More recently, Mansour et al. [39] identified 33 studies in a broader context and discussed the different criteria used: sale values, exposure data (measured environmental concentrations, MEC, or PEC values), toxicity data, pharmacological data, physicochemical properties, wastewater treatment plant removal efficiencies, and other criteria. They pointed out that almost all of the prioritization studies were performed in North America, Europe, and China, and that priority lists in other regions of the world may be different due to other types of pharmaceuticals consumed, other wastewater treatment systems, and/or other climatic conditions. They applied prioritization approaches to the most commonly consumed APIs in Lebanon, and these international concerns will probably follow an increasing trend.
In Europe, prioritization studies are used to develop monitoring strategies from a regulatory perspective [40], and several pharmaceuticals were recently proposed to be placed on a watch list with ten high-priority compounds [41]. The highlighted APIs were the NSAIDFootnote 3 diclofenac, the hormones estrone (E1), 17-β-estradiol (E2), and 17-α-ethinyl estradiol (EE2), as well as the macrolide antibiotics erythromycin, azithromycin, and clarithromycin. Indeed, the clearly established deleterious effect of diclofenac on trout kidneys [5] and the endocrine-disrupting problems observed in fish [3, 4] were already mentioned. Due to their antimicrobial properties and their role in the propagation of resistance, antibiotics are considered one of the most hazardous pharmaceutical classes for the aquatic environment [42]. Although diclofenac and hormones are not likely to be found in high concentrations in hospital wastewater, antibiotics residues have proven to be a major driver in the propagation of resistance in the environment [43, 44].
2.2 Prioritization Studies Applied to Hospital Wastewater
Prioritization methods can also be applied to hospital wastewater but need to be adjusted. The PBT approach is readily transposable to the consumed APIs in hospitals, but when performing an environmental risk assessment, some slightly different parameters are often considered while calculating predicted concentrations or risk quotients.
Predicted concentrations in hospital wastewater (PECHWW) are obtained by dividing the excreted mass – i.e., the consumed mass (M) multiplied by the excretion factor (F excr) – by the volume of wastewater (V) [13, 29, 36, 38]:
Often, the volume of consumed water is used instead of the volume of wastewater, which is not easy to assess. Excretion factors are considered the sum of excretion in urine and feces as unchanged drugs but do not take into account metabolites. Some authors assume that the glucuronide conjugates are cleaved in the environment and should therefore be taken into account in the calculation [45]. However, glucuronide bonds are known to be unstable, and their behavior in aquatic environment is unknown. The glucuronide conjugates of some compounds were already detected in the surface water [46].
The predicted concentrations in receiving surface water (PECSW) are calculated by considering the removal efficiencies in WWTP (R) and the dilution (DIL) that hospital effluents undergo in the watershed [13, 29, 36]:
The dilution factor (DIL) is usually fixed at 10 for pharmaceuticals in WWTPs, according to the European guidelines [25]. However, this factor accounts for the dilution of municipal wastewater into the receiving aquatic ecosystem but not for hospital effluents, which are first diluted in the urban sewer network. Kümmerer [42] suggests that the dilution of hospital effluents in municipal wastewater is more important than the dilution of the latter into rivers or lakes and proposes a dilution factor of 100, which is close to reality when calculated [36].
It is not realistic to calculate the risk for hospital effluents, because the exposure of living organisms is null in hospital sewers; thus the calculation of hazard quotient (HQ) has been suggested [47]. HQ is thus calculated for hospital wastewater (HQHWW) and risk quotient for surface water (RQSW) while considering the dilution in the aquatic environment. Generally, a RQSW ≥ 1 means that the considered API poses a high risk for the aquatic ecosystems, with 0.1 ≤ RQSW < 1 denoting medium risk and RQSW < 0.1 denoting low risk [48]. HQHWW ≥ 1 would only mean that the considered API contributes significantly to the environmental hazard of the hospital effluents.
To our knowledge, only a few studies applied prioritization methods to the hospital-based consumption of APIs (Table 1). The prioritization is always realized with a subset of substances, varying from 15 to 250 according to the limiting factors defined through expert judgment. Expert judgment is indeed very often applied for the inclusion/exclusion of APIs, either before or after the prioritization, and chosen criteria are very diverse: previously highlighted compounds, reported measured environmental concentrations (MEC), or a focus on drugs with bioaccumulation potential [27] or anticancer drugs [22]. Other criteria considered for the prioritization are human metabolism through the excreted fraction (F excr), the environmental behavior according to drug properties (pK a , K oc, K ow, etc.), the removal efficiencies in the wastewater treatment plants, as well as the potential effects on living organisms through PNEC values.
Hereafter, we will present the results of our prioritization study, which were generated in a major hospital in Switzerland and were previously published [23]. The results will be compared to those of other studies, and the discrepancies observed in terms of the methodologies and criteria used will be discussed.
3 Geneva University Hospitals: A Swiss Case Study
3.1 Setting and Consumption Data Collection
The Geneva University Hospitals (HUG) are some of the most important hospitals of Switzerland. They comprise eight different hospitals (general, pediatric, psychiatric, maternity, etc.) and approximately 40 other healthcare facilities, providing both primary and tertiary care. In 2012, 8,443.2 full-time equivalent collaborators and a total of 671,709 days of hospitalization were registered for 1,908 beds, 48,112 inpatients, and over 860,000 outpatient consultations. The average daily water consumption was approximately 760 m3.
The aggregated data for drugs dispensed in both the inpatient and outpatient settings in 2012 were first obtained from the hospital pharmacy database using the “Business Object®” software. These data correspond to the drugs ordered by the different medical units to the pharmacy to treat their patients – as well as the returns (stock and delivery errors, discharged or deceased patients, etc.). The data give an approximation of the yearly inpatient consumption of APIs by transforming the overall unit doses (UD) in grams of active ingredients while considering their dosages [27]. Moreover, the pharmacy data are delivery data, which can differ from real consumption in the service due to lack of patient compliance, outside consumption, or other reasons [27]. All confidential health information was removed to create anonymous analytic datasets in conformity with Swiss data protection regulations.
According to the consumption data, 4,301 kg of APIs were delivered in 2012. Given the hypothesis that 100% of the administered drugs is consumed in the hospitals, this results in a ratio of 90 g/patient. However, while taking into account outpatient consultations, a much more realistic ratio of 4.8 g/patient is obtained. Thus, it is important to consider outpatient consumption, which can represent an important fraction of the consumption depending on the nature of the compounds. Indeed, outpatient treatments have increased significantly with, in some cases, only 20% of the drugs prescribed to outpatients excreted on-site [49]. Weissbrodt et al. [50] showed that 50% of iodinated X-ray contrast media and 70% of antineoplastic agents prescribed in the studied hospital were excreted at home. Concerning systemic antiviral drugs, they are specifically prescribed and delivered in the HUG as in a city pharmacy, but they are likely to be excreted at home by outpatients [11, 51].
In general, antibiotic drugs are the most commonly consumed class of drugs in hospitals [10, 11], but in our case, analgesics were more important (31.3%) than antibiotics (11.4%), followed by antiviral (6.4%) and anti-inflammatory (4.9%) drugs. While taking the excretion rate into account, antiviral and antibiotic drugs are excreted at a higher proportion compared to another Swiss hospital, whereas a lower fraction of iodinated X-ray contrast media and laxatives is excreted [36, 51]. This is probably due to the size difference between the two hospitals (338 vs. 1,908 beds) and the difference in prescriptions and activities between a cantonal hospital and a university hospital.
3.2 Prioritization
3.2.1 PBT Approach
The prioritization procedure applied to active pharmaceutical ingredients (APIs) consumed in the Geneva University Hospitals was adapted from previous studies [26, 27, 29]. Among the approximately 1,000 APIs delivered by the hospital pharmacy in 2012, only 150 APIs with more than 10,000 unit doses (UD) were first retained. After the conversion from UD to grams of API, only 84 APIs, for which more than 1 kg were sold in 2012, were kept. The objective was to obtain a list of priority compounds to monitor; thus, the less consumed APIs were thought to be undetectable in hospital wastewater. Nevertheless, antineoplastic and immunomodulant drugs (Code L, according to the Anatomical Therapeutic Classification, ATC) with more than 10,000 UD were added to these 84 APIs due to their inherent toxicity, resulting in a total of approximately 100 APIs for the prioritization. Each API has been given 4 rankings, from 1 to 5, based on 4 criteria: Occurrence (O), Persistence (P), Bioaccumulation (B), and Environmental Toxicity (T). A final ranking was then obtained by the addition of the ranks of the four criteria, which are weighted according to the data quality. Indeed, to take into account the data quality, the ranks of the different criteria were multiplied by a quality factor: this factor is equal to 1 if no data were available, 2 if the PNEC or the Log K ow were modeled with a QSAR approach, and 3 if the experimental values were available.
Among most of the priority compounds highlighted were NSAIDs (ibuprofen, diclofenac, and mefenamic acid), antiviral drugs (ritonavir, raltegravir), the antidepressant sertraline, anesthetics and analgesics (lidocaine, gabapentin, propofol), as well as antibiotics (sulfamethoxazole, trimethoprim, ciprofloxacin, and metronidazole), drugs for the cardiovascular system (metoprolol, oxazepam), and antineoplastic drugs (paclitaxel). Ritonavir was previously identified as a problematic hospital compound [36], and sertraline shows adverse effects in aquatic organisms [3] and is considered a priority compound by several authors [26, 28]. Note that 55% of the compounds were present in the top 20 list when taking into account data quality. This means that weighting according to the data quality changes the order of importance for about half of the compounds. More details can be found in Daouk et al. [23].
3.2.2 Environmental Risk Assessment
Among the 20 APIs with the highest PECHWW, 8 antibiotics and 5 antiviral drugs were identified. Note that PECHWW were calculated assuming that 100% of drug consumption occurs on site, and thus they are certainly over-evaluated due to the fractions excreted by outpatients [11]. Moreover, the volume of wastewater (V) was assumed to be equal to the known volume of consumed water (760 m3). In our case, the predicted loads of the most frequently consumed APIs (paracetamol, ibuprofen, and the antibiotics metronidazole, ciprofloxacin, and sulfamethoxazole) were in agreement with the measured loads, but over- and underestimations are observed for other APIs (Fig. 1).
In general, overestimations of PEC are commonly observed when compared to measured concentrations (MEC) [11, 28]. They are mostly due to uncertainties linked with wastewater volume measurements and excretion factors [52]. Other parameters can also influence the observed overestimations, such as the retention or degradation processes. Indeed, although the excreted fractions of hydroxyl metabolites were considered to predict concentrations and loads, the latter were overestimated compared to the measured fractions. One possible explanation would be a rapid transformation in wastewater due to a chemical instability or biodegradation tendency. This was probably also the case for the antibiotic piperacillin, which was rarely detected and only in trace concentrations [53], although it was identified as a problematic compound in another study [54]. Unfortunately, no information was found in the literature.
Underestimations were also observed for the analgesics morphine and codeine, as well as for the antiepileptic carbamazepine and gadolinium (Gd). Some possible explanations, such as private consumption outside the hospital and excretion within the hospital, are depicted in more detail in Daouk et al. [53]. Nevertheless, overestimation is more frequent than underestimation [52].
In our case, a dilution factor of 296 was used for the dilution of hospital wastewater in the urban network (obtained by dividing the volume of hospital water consumption by the volume of urban wastewater for the year 2012), and a factor of 10 was applied to the second dilution in receiving water (DIL = 2,960). Thus, the PECSW are only representative of the hospital contribution and do not take into account domestic consumption. PECSW highlighted the high probability of finding the antibiotic drugs piperacillin (69 ng/L) and amoxicillin (33 ng/L) and the antidiabetic metformin (32 ng/L) in the freshwater environment. In our case, PECSW were lower than MEC in the grab surface water samples of the downstream river [55]. This is easily explained by the fact that the predicted values only consider hospital consumption (and not domestic consumption) and were thus only representative of the API hospital fraction.
The hazard quotient calculated for hospital wastewater (HQHWW) varies widely (from 10−3 to 103), and for the 71 calculated HQHWW, 32 were above 1 (45%). The ten most hazardous compounds were ciprofloxacin, amoxicillin, trimethoprim, 5-fluorouracil, ibuprofen, lidocaine, sulfamethoxazole, paracetamol, ritonavir, and lopinavir (Table 2). These results are consistent with previous studies [47, 56]. Although the prescribed drugs can differ between hospitals, heavily consumed APIs, such as the latter, are likely to participate in generating environmental hazards. HQHWW can thus help hospital managers and local authorities to identify priority compounds and develop strategies to reduce their input into aquatic ecosystems.
The environmental risk quotient calculated for surface water (RQSW) revealed that a priori only the hospital fraction of ciprofloxacin was likely to pose a high risk to aquatic ecosystems (RQSW > 1). This was further confirmed with measurements [53] and confirms previous results obtained in another Swiss hospital [57]. The antibiotics amoxicillin, trimethoprim, sulfamethoxazole, the cytostatic fluorouracil, and the antiviral ritonavir were shown to pose a medium risk (RQSW > 0.1).
The 20 highest priority compounds were consistent with other studies (see Table 2). Sulfamethoxazole, ciprofloxacin, and ibuprofen were indeed identified as high-priority pharmaceuticals for the water cycle by de Voogt et al. [58]. Ritonavir was identified as a risky hospital compound by Escher et al. [36], and lidocaine, amoxicillin, ciprofloxacin, and sulfamethoxazole were selected as typical hospital compounds for monitoring by Helwig et al. [12] and are likely to pose problems when reaching the aquatic ecosystem [47, 59, 60].
Trimethoprim is generally administered in combination with sulfamethoxazole, and it has also been identified as problematic by Valcarcel et al. [59]. Fluorouracil (5-FU) and capecitabine were predicted as having low concentrations in European surface water [61], but they were not considered together by the latter authors. Capecitabine is a prodrug that is enzymatically transformed into 5-FU in the body and thus should be considered together with 5-FU. Although capecitabine was not among the priority compounds according to the OPBT approach because it is not excreted in high amounts, it contributes to the environmental risk to aquatic species (RQSW = 0.2).
3.3 Sensitivity Analysis
A sensitivity analysis was performed for 34 APIs to assess the variability of the predicted risk quotients associated with the different parameters taken into account for prediction: consumption, excretions factors (F excr), removal efficiencies in WWTP, hospital water consumption, and PNEC values [23]. In general, the excretion rates (F excr) and the ecotoxicological data (PNEC values) are likely to influence most of the final RQ values, while API consumption (M) and removal efficiencies (R) have moderate consequences, and the water consumption pattern has a small impact. Indeed, RQ values varied up to one order of magnitude according to changes in the excretion rates and up to three orders of magnitude with the uncertainties associated with the PNEC values [23]. In our case, the excretion rates of cytostatic and antiviral drugs were highly uncertain, as well as the PNEC values of cytostatics and antibiotics. The influence of the monthly variability of API consumption on RQ values is mass dependent: highly consumed APIs such as anti-inflammatory (ibuprofen) or analgesic drugs (paracetamol) exhibited much lower variations than the least commonly consumed cytostatic drugs (methotrexate, epirubicin). In the end, according to the worst-case scenario (maximum values for M and F excr and minimum values for V, R, and PNEC), 5 compounds exhibited high risk and 4 moderate risk compared to 1 and 5, respectively, according to the mean scenario.
3.4 Discussion
3.4.1 Methodologies Comparison
The comparison of the highest priority compounds resulting from the different approaches applied to the API consumption of the Geneva University Hospitals – the ERA and the PBT with and without weighting – highlighted that 8 drugs were revealed by the 3 methodologies (40%) and 12 by at least 2 different methodologies (60%). The ERA highlighted more antibiotics (4 in the top 5), whereas PBT ranked more NSAIDs (3 in the top 5). This difference can be explained by the fact that the ERA does not take into account the bioaccumulation potential (Log K ow). Furthermore, PNEC values were not available for 27 molecules, and, thus, the latter were not taken into account with the ERA. According to our point of view, both approaches are complementary, and combined evaluations should therefore be considered. One possibility of doing so includes adding the ranks of both OPBT and ERA approaches. In our study, the NSAID ibuprofen becomes the highest priority compound, and diclofenac, mefenamic acid, the antidepressant sertraline, and the antibiotic sulfamethoxazole are in the top 5 [23]. Antiviral drugs (ritonavir and raltegravir), analgesics (lidocaine and propofol), and antibiotics (trimethoprim, amoxicillin, ciprofloxacin, and metronidazole) were also highlighted in the top 20 with the rank combination of both methods. Nine compounds (ibuprofen, paracetamol, diclofenac, ciprofloxacin, sulfamethoxazole, trimethoprim, metronidazole, metoprolol, and carbamazepine) were previously highlighted as priority compounds in at least two different studies as reported by Al Aukidy et al. [48].
It is worth stressing that these prioritizations have some drawbacks: they deal only with the most consumed drugs (>1 kg/year); many PNEC and Log K ow values are obtained by QSAR models, and the excretion factors were fixed to mean values. Nevertheless, and despite being a theoretical approach, the highlighted priority compounds – NSAIDs, antiviral drugs, the antidepressant sertraline, the sedative propofol, and/or the antibiotics sulfamethoxazole, trimethoprim, ciprofloxacin, and amoxicillin – are consistent with results of previous studies [11, 14, 26, 36].
3.4.2 Comparison with Other Studies
Hereafter, the prioritized compounds according to ERA are compared to the results of other prioritization studies dealing with ERA in hospitals (Table 2). To summarize, we obtained consumption data from the central pharmacy of the Geneva University Hospitals, and 32 APIs showed a hazard quotient (HQHWW) above 1 [23]. However, by taking into account dilution in the surface water, only the antibiotic ciprofloxacin had a risk quotient above 1. Nevertheless, when considering both urban and hospital consumptions, 7 APIs out of the 15 measured had RQ above 1: ciprofloxacin, ibuprofen, piperacillin, mefenamic acid, diclofenac, gabapentin, and sulfamethoxazole [53].
Helwig et al. [54] obtained consumption data from both Scottish hospitals and community pharmacies. While considering both urban and hospital consumptions, 9 antibiotics had RQs >1: amoxicillin, piperacillin, flucloxacillin, penicillin V, tazobactam, erythromycin, ketoconazole, ciprofloxacin, and oxytetracycline (Table 2). This is not really surprising as low PNEC values were determined for antibacterial drugs during the last decade [54]. Furthermore, they observed that half of the API with risk quotients above 1 had high hospital contributions.
Guo et al. [40] analyzed 146 APIs that are used in the community or in hospital settings in the UK (England, Scotland, and Wales). The risk scores were calculated as the ratio between exposure in the different environmental compartments (PEC) and the hazard toward living organisms from different trophic levels (PNEC). Forty international experts were also solicited to identify compounds with low use and of potential high concern. Expert judgment was used to exclude 12 substances with high usage but falling outside the scope of the project, such as calcium carbonate or ferrous sulfate (see Table 1). They identified 13 compounds with risk quotients above 1 for the aquatic ecosystem. The highlighted compounds were antibiotics (amoxicillin, clarithromycin, ciprofloxacin, azithromycin), anti-inflammatory drugs (diclofenac, mesalazine), an antidiabetic (metformin), an antidepressant (amitriptyline), atorvastatin and its metabolites, omeprazole, and the hormone estradiol (Table 2).
Although this comparison should be considered with precaution because of the differences in the parameters used, it allows the complementary identification of priority APIs: antibiotics (ciprofloxacin, amoxicillin, piperacillin, azithromycin), anti-inflammatory drugs (diclofenac, mesalazine), hormone estradiol, and the antidiabetic metformin. However, ciprofloxacin is the only compound identified as highly problematic in the three studies.
4 Conclusions and Perspectives
Though differences are commonly observed between countries and hospitals, we were able to identify five key issues for the prioritization of pharmaceuticals in hospitals based on a literature review and our Geneva case study:
-
(1)
Access to consumption data. Although the availability and quality of consumption data improved in the last few years, many uncertainties remain due to the tediousness of data handling and conversion [54]. Note that hospitals are not likely giving their consumption data, the availability of which is often limited by their costs. Moreover, many different drug prescription systems exist, and thus the list of prescribed drugs may differ in each hospital. Indeed, a commission of experts often evaluates the list of medicines and chooses the allowed candidates, at least in Switzerland.
-
(2)
Quality and handling of the consumption data. Tedious and time-consuming manipulation is required to extract usable data for API prioritization and/or environmental risk assessment. Indeed, pharmaceutical dataset are not readily suitable for environmental assessment needs, and the transformation into grams of active ingredient per time unit is not easily performed.
-
(3)
Expert judgment. Criteria used by experts for the inclusion-exclusion of compounds, either before or after the prioritization, are highly variable (previously highlighted compounds, reported measured concentrations (MEC), etc.) and induce some large discrepancies in established lists of priority compounds.
-
(4)
Uncertainties associated with the PEC model. The PEC model, when applied to both hospital wastewater and surface water, can be of help during the selection process for monitoring campaigns and allow the calculation of risk quotients, but they include strong limitations or associated uncertainties:
-
a.
100% consumption hypothesis – the assumption of the complete consumption of the delivery data is a source of uncertainty. Moreover, the seasonal variation of the consumption is difficult to take into account and can, for some compounds such as antibiotics, strongly influence the resulting concentrations [62].
-
b.
Wastewater volume – as we discussed, water consumption is often considered equal to the volume of wastewater, which is not always the case. Moreover, when the volume of wastewater is measured, high uncertainties are associated with the measurement techniques [52, 53].
-
c.
Excretion factors – human metabolism is highly variable, and the elimination of APIs by the human body is not reproducible from one patient to another. Therefore, when considered, the excretion factor values are average values with high intrinsic uncertainties. Excretion factors were indeed identified as major sources of uncertainties by Verlicchi and Zambello [52], as well as by the sensitivity analysis of the presented case study.
-
d.
Local dilution factors – from both hospital effluents to urban wastewater and from urban wastewater to surface water, as well as the associated uncertainties for ERA, the dilution rates used are often not properly calculated for local hydrological conditions.
-
e.
Degradation – the often considered conservative mass transfer of substances during their transport through the urban wastewater network and surface water and the huge variability linked with the few available WWTP removal data are both sources of uncertainties.
-
a.
-
(5)
Quality of the hazard/risk evaluation. Although the availability and quality of ecotoxicological data improved in the last few years, many shortcomings remain, leading to major uncertainties in the PNEC calculation. Indeed, PNEC values are likely to be highly variable due to the way in which they are calculated, and the uncertainties associated to the calculated risk quotients are highly dependent to these PNEC values. This was stressed by the sensitivity analysis of the presented case study.
Therefore, prioritization procedures applied to hospitals can be burdensome to apply in practice, and many uncertainties are linked with the different issues detailed above. Nevertheless, prioritization approaches represent essential procedures when dealing with the very high number of API currently in use. They indeed allow for the theoretical identification of the degree of environmental threat for each pharmaceutical product, as well as establishing lists of priority molecules to follow for monitoring programs. Prioritization methods thus represent helpful tools for creating the environmental policy of APIs, for both regulation and surveillance purposes.
There will certainly be an increasing use of prioritization methods for APIs consumed in hospitals in the future. Nevertheless, ecopharmacovigilance is a relatively new domain of investigation, and methodological adaptations to the new challenges for the water quality monitoring are somehow necessary. Environmental risk assessment studies of API residues must indeed consider the risks of long-term exposure to subacute levels, as well as the risks of mixtures of pollutants in the aquatic ecosystems. Moreover, the emphasis is widely placed on parent compounds and the risks toward aquatic organisms, whereas metabolites and soil or sediment compartments are scarcely considered. The recent development of pharmaceutical drugs based on biotechnologies (monoclonal antibodies and vaccines) will certainly induce some methodological adjustments to take them into account. Thus, along with the development of green pharmacy, an update of the European guidelines proposed by the EMEA will be necessary in the future.
Notes
- 1.
- 2.
DDD “is the assumed average maintenance dose per day for a drug used for its main indication in adults. DDDs only give a rough estimate of consumption and not an exact picture of actual use” (Source: www.whocc.no).
- 3.
Nonsteroidal anti-inflammatory drug.
References
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(Suppl 6):907–938
Kümmerer K (2010) Pharmaceuticals in the environment. Annu Rev Environ Resour 35:57–75
Santos LHMLM, Araújo AN, Fachini A, et al (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175:45–95
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159
Hoeger B, Kollner B, Dietrich DR, et al (2005) Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat Toxicol (Amsterdam, Netherlands) 75:53–64
Daughton CG (2003) Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ Health Perspect 111:775–785
Götz C, Stamm C, Fenner K, et al (2010) Targeting aquatic microcontaminants for monitoring: exposure categorization and application to the Swiss situation. Environ Sci Pollut Res 17:341–354
Michael I, Rizzo L, Mcardell CS, et al (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995
Richardson ML, Bowron JM (1985) The fate of pharmaceutical chemicals in the aquatic environment. J Pharm Pharmacol 37:1–12
Kümmerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources – a review. Chemosphere 45:957–969
Le Corre KS, Ort C, Kateley D, et al (2012) Consumption-based approach for assessing the contribution of hospitals towards the load of pharmaceutical residues in municipal wastewater. Environ Int 45:99–111
Helwig K, Hunter C, Maclachlan J, et al (2013) Micropollutant point sources in the built environment: identification and monitoring of priority pharmaceutical substances in hospital effluents. J Environ Anal Toxicol 3:177
Mullot J-U (2009) Modélisation des flux de médicaments dans les effluents hospitaliers, vol 11. Faculté de Pharmacie de Chatenay-Malabry, Paris-Sud, p 334. http://www.lspe.u-psud.fr/These%20Ju%20Mullot.pdf. Accessed Feb 2017
Santos LHMLM, Gros M, Rodriguez-Mozaz S, et al (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461–462:302–316
Boxall AB, Rudd MA, Brooks BW, et al (2012) Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect 120:1221–1229
Hughes SR, Kay P, Brown LE (2013) Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol 47:661–677
Donnachie RL, Johnson AC, Sumpter JP (2016) A rational approach to selecting and ranking some pharmaceuticals of concern for the aquatic environment and their relative importance compared with other chemicals. Environ Toxicol Chem/SETAC 35:1021–1027
Oldenkamp R, Huijbregts MJ, Hollander A, et al (2013) Spatially explicit prioritization of human antibiotics and antineoplastics in Europe. Environ Int 51:13–26
Oldenkamp R, Huijbregts MA, Ragas AM (2016) The influence of uncertainty and location-specific conditions on the environmental prioritisation of human pharmaceuticals in Europe. Environ Int 91:301–311
Kim Y, Jung J, Kim M, et al (2008) Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environ Toxicol Pharmacol 26:167–176
Wang N, Guo X, Shan Z, et al (2014) Prioritization of veterinary medicines in China’s environment. Hum Ecol Risk Assess Int J 20:1313–1328
Booker V, Halsall C, Llewellyn N, et al (2014) Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci Total Environ 473–474:159–170
Daouk S, Chevre N, Vernaz N, et al (2015) Prioritization methodology for the monitoring of active pharmaceutical ingredients in hospital effluents. J Environ Manag 160:324–332
De Jongh CM, Kooij PJF, De Voogt P, et al (2012) Screening and human health risk assessment of pharmaceuticals and their transformation products in Dutch surface waters and drinking water. Sci Total Environ 427–428:70–77
EMEA (2006) Guideline on the environmental risk assessment of medicinal products for human use. European Medicines Agency, London, p. 12
Besse J-P, Garric J (2008) Human pharmaceuticals in surface waters: implementation of a prioritization methodology and application to the French situation. Toxicol Lett 176:104–123
Jean J, Perrodin Y, Pivot C, et al (2012) Identification and prioritization of bioaccumulable pharmaceutical substances discharged in hospital effluents. J Environ Manag 103:113–121
Ortiz De Garcia S, Pinto GP, Garcia-Encina PA, et al (2013) Ranking of concern, based on environmental indexes, for pharmaceutical and personal care products: an application to the Spanish case. J Environ Manag 129:384–397
Perazzolo C, Morasch B, Kohn T, et al (2010) Occurrence and fate of micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part I: Priority list for environmental risk assessment of pharmaceuticals. Environ Toxicol Chem/SETAC 29:1649–1657
Coutu S, Rossi L, Barry DA, et al (2012) Methodology to account for uncertainties and tradeoffs in pharmaceutical environmental hazard assessment. J Environ Manag 98:183–190
Christen V, Hickmann S, Rechenberg B, et al (2010) Highly active human pharmaceuticals in aquatic systems: a concept for their identification based on their mode of action. Aquat Toxicol 96:167–181
Morais SA, Delerue-Matos C, Gabarrell X (2014) An uncertainty and sensitivity analysis applied to the prioritisation of pharmaceuticals as surface water contaminants from wastewater treatment plant direct emissions. Sci Total Environ 490:342–350
Wennmalm Å, Gunnarsson B (2009) Pharmaceutical management through environmental product labeling in Sweden. Environ Int 35:775–777
Sangion A, Gramatica P (2016) PBT assessment and prioritization of contaminants of emerging concern: pharmaceuticals. Environ Res 147:297–306
Pavan M, Worth AP (2008) Publicly-accessible QSAR software tools developed by the Joint Research Centre. SAR QSAR Environ Res 19:785–799
Escher BI, Baumgartner R, Koller M, et al (2011) Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res 45:75–92
Orias F, Perrodin Y (2013) Characterisation of the ecotoxicity of hospital effluents: a review. Sci Total Environ 454–455:250–276
European Commission E (2003) Technical guidance document on risk assessment. TGD part II. European Chemical Bureau, Institute for Health and Consumer Protection, Ispra
Mansour F, Al-Hindi M, Saad W, et al (2016) Environmental risk analysis and prioritization of pharmaceuticals in a developing world context. Sci Total Environ 557–558:31–43
Guo J, Sinclair CJ, Selby K, et al (2016) Toxicological and ecotoxicological risk-based prioritization of pharmaceuticals in the natural environment. Environ Toxicol Chem/SETAC 35:1550–1559
Carvalho RN, Ceriani L, Ippolito A et al (2015) Development of the first watch list under the environmental quality standards directive. In: Sustainability EEJRCIfEa. Publications Office of the European Union, Luxembourg, p 166
Kümmerer K (2009) Antibiotics in the aquatic environment – a review – part I. Chemosphere 75:417–434
Czekalski N, Berthold T, Caucci S, et al (2012) Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland. Front Microbiol 3:106
Laffite A, Kilunga PI, Kayembe JM et al (2016) Hospital effluents are one of several sources of metal, antibiotic resistance genes, and bacterial markers disseminated in Sub-Saharan urban rivers. Front Microbiol 7:1128. doi:10.3389/fmicb.2016.01128. Accessed Feb 2017
Besse J-P, Kausch-Barreto C, Garric J (2008) Exposure assessment of pharmaceuticals and their metabolites in the aquatic environment: application to the French situation and preliminary prioritization. Hum Ecol Risk Assess Int J 14:665–695
Bonvin F, Chèvre N, Rutler R, et al (2012) Pharmaceuticals and their human metabolites in Lake Geneva: occurrence, fate and ecotoxicological relevance. Arch Sci 65:143–155
Orias F, Perrodin Y (2014) Pharmaceuticals in hospital wastewater: their ecotoxicity and contribution to the environmental hazard of the effluent. Chemosphere 115:31–39
Al Aukidy M, Verlicchi P, Voulvoulis N (2014) A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci Total Environ 493:54–64
Besse JP, Latour JF, Garric J (2012) Anticancer drugs in surface waters: what can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ Int 39:73–86
Weissbrodt D, Kovalova L, Ort C, et al (2009) Mass flows of X-ray contrast media and cytostatics in hospital wastewater. Environ Sci Technol 43:4810–4817
Mcardell C, Kovalova L, Siegrist H, et al (2011) Input and elimination of pharmaceuticals and disinfectants from hospital wastewater. Eawag, Düebendorf, p. 95
Verlicchi P, Zambello E (2016) Predicted and measured concentrations of pharmaceuticals in hospital effluents. Examination of the strengths and weaknesses of the two approaches through the analysis of a case study. Sci Total Environ 565:82–94
Daouk S, Chevre N, Vernaz N, et al (2016) Dynamics of active pharmaceutical ingredients loads in a Swiss university hospital wastewaters and prediction of the related environmental risk for the aquatic ecosystems. Sci Total Environ 547:244–253
Helwig K, Hunter C, Mcnaughtan M, et al (2016) Ranking prescribed pharmaceuticals in terms of environmental risk: inclusion of hospital data and the importance of regular review. Environ Toxicol Chem/SETAC 35:1043–1050
Ortelli D, Edder P, Rapin F et al (2011) Métaux et micropolluants organiques dans les rivières et les eaux du Léman. Rapp Comm Int Prot Eaux Léman contre Pollut (CIPEL) Campagne 2010:65–86
Verlicchi P, Al Aukidy M, Galletti A, et al (2012) Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ 430:109–118
Chèvre N, Coutu S, Margot J, et al (2013) Substance flow analysis as a tool for mitigating the impact of pharmaceuticals on the aquatic system. Water Res 47:2995–3005
De Voogt P, Sacher F, Janex-Habibi M, et al (2008) Development of an international priority list of pharmaceuticals relevant for the water cycle. Water Sci Technol 59:39–46
Valcarcel Y, Gonzalez Alonso S, Rodriguez-Gil JL, et al (2011) Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 84:1336–1348
Verlicchi P, Al Aukidy M, Zambello E (2012) Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment – a review. Sci Total Environ 429:123–155
Johnson AC, Oldenkamp R, Dumont E, et al (2013) Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of Europe. Environ Toxicol Chem 32:1954–1961
Coutu S, Wyrsch V, Wynn HK, et al (2013) Temporal dynamics of antibiotics in wastewater treatment plant influent. Sci Total Environ 458–460:20–26
Acknowledgments
We would like to thank R. Aebersold, P. Bonnabry, P. Dayer, A. Perrier, and A. Samson from the Geneva University Hospitals (HUG), as well as M. Enggist and F. Pasquini from the Water Ecology Service of the Geneva State (SECOE) for their support in this project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Daouk, S., Chèvre, N., Vernaz, N., Daali, Y., Fleury-Souverain, S. (2017). Prioritization of Active Pharmaceutical Ingredients in Hospital Wastewater. In: Verlicchi, P. (eds) Hospital Wastewaters. The Handbook of Environmental Chemistry, vol 60. Springer, Cham. https://doi.org/10.1007/698_2017_14
Download citation
DOI: https://doi.org/10.1007/698_2017_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62177-7
Online ISBN: 978-3-319-62178-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)