Abstract
One hundred sixty-nine groundwater samples were collected, chemically analyzed, and classified into shallow, intermediate, and deep zones to evaluate the vertical and lateral change in groundwater quality in the central part of the middle Nile Delta. To estimate the groundwater suitability for drinking, parameter’s concentrations were evaluated according to WHO drinking water guidelines to delineate the samples of desirable and undesirable range in every zone. According to the computed WQI, most part of the shallow groundwater is unsuitable for drinking [unfit (8 wells, 14.55%), very poor (3 wells, 5.45%), and poor drinking quality (26 wells, 47.3%)]. Intermediate groundwater zone is mostly suitable [excellent (4 wells, 8.9%) and good (24 wells, 53.3%)]. The deep groundwater quality is classified into unfit (3 wells, 4%), very poor (5 wells, 7%), poor water (27 wells, 40%), good quality (30 wells, 45%), and excellent (2 wells, 3%).
Groundwater suitability was also evaluated using TDS, Na%, SAR, RSC, Cl, KI, PI, MH, CAI, and CR. Irrigation water quality index (IQW) was also used as an integrated method. The studied groundwater is mostly of medium suitability where a number of samples which fall within this class are 36 (65.5%), 29 (64%), and 34 (51%) for the shallow, intermediate, and deep groundwater. Water samples have good irrigation quality which increases downward where 15 (27.2%), 13 (29%), and 32 (48%) samples are recorded in this class, respectively. Samples belonging to the poor quality class are mostly located in the northern part, and its sample numbers are 4 (7.3%), 3 (7%), and 1 (1%), respectively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Drinking water quality index
- Groundwater pollution
- Irrigation water quality index
- Nile Delta
- Water quality assessment
1 Introduction
Resources of freshwater distribution are uneven all around the world, and the freshwater accessibility is gradually becoming rare and attributable to population growth and varies human activities. The lack of fresh surface water leads to misuse of groundwater to meet the demand required by different areas. Groundwater quality is similarly essential as its amount is attributable to the appropriateness of water for different targets. Spatial differences in groundwater quality in certain areas are a component of physical, chemical, and biological factors that are significantly affected by aquifer geology and human activities [1, 2]. Groundwater became the main water supply for residential, industrial, and irrigation divisions of numerous nations. Spatial change in the quality of groundwater because of geologic setup and anthropogenic elements warrants the assessment of the groundwater quality for any use including that for human utilization. Evaluation of the water quality for drinking purposes includes the determination of the chemical composition of groundwater and the remedial measures for the restoration of the water quality in case of its deterioration demand and the estimation of probable sources of the groundwater pollution [3]. About 33% of the world’s population use groundwater for drinking [4, 5].
Low quality of water unfavorably influences human health and plant development. In developing nations like Egypt, about 60% of all infections are specifically occurring due to the poor quality of the drinking water [6]. The spatial distribution of water quality and significant components should be compared with the geology and area using land use/land cover spread maps in GIS environment [7, 8]. Therefore the chemical processes of water and the methods of their acquisition could be clearly understood [9]. Egyptian population is basically situated in the narrow valley and delta of the Nile. This high population density is accompanied with a continuous growing size of the population, wastewater, development of industrialization, living standards, the expanded utilization of chemicals in agriculture, the lack of real control on the transfer of unsafe waste materials, and in addition the absence of ecological open mindfulness. This study aims to evaluate the quality characteristics of the groundwater in the central Nile Delta part wherein the people of the terrain are mostly dependent on the groundwater for their needs and to assess the groundwater suitability for drinking and irrigation purposes. Many researchers have investigated the water quality in the Nile Delta, among them Salem [10], Elewa [11], Gemail et al. [12], Morsy and El-Fakharany [13], Salema et al. [14], Ghoraba et al. [15], Khalil et al. [16], El Bedawy [17], Bennett et al. [18], Elkafoury et al. [19], Fattah and Ragab [20], El-Kowrany et al. [21], Negm and Armanuos [22], Negm and Eltarabily [23], Salem et al. [24], Sharaky et al. [25], and Salem and Osman [26].
No encountered research work on the central Nile Delta used the integrated method of water quality indices to evaluate the groundwater for irrigation and drinking purposes as will be shown in this study.
2 Study Area
The Nile Delta, one of the biggest depocenters in the Mediterranean, is currently mainly a man-altered beachfront plain where the Nile sediments have stopped accumulating in the Mediterranean and locally are retreating. The Nile Delta area is around 22,000 km2 and records for 66% of Egypt’s agrarian surface. Its smooth coastline is 225 km long and lies 160 km north of Cairo (Fig. 1). Land elevation diminishes gradually northward, from 18 m masl (meter above ocean level) close to Cairo to less than 1.0 m close to the coast [27]. The Nile water is flowing from the Aswan High Dam to the delta. Thirty-three percent of this water volume is lost by evapotranspiration and penetration to groundwater aquifers, and the other two third flows slowly through the intense system of irrigation channels and drains. Agricultural activities are overwhelming in the Nile Delta because of the soil salinity [27, 28] and a watering system framework setup. The seawater intrusion might strongly influence the quality of the groundwater in the area. The rise in the sea level as well as the changes of Nile River flow leads to salinity increase [28, 29] and water logging in the northern part of the Nile Delta [30]. Furthermore, the present and future anthropogenic activities, particularly intensive unplanned groundwater pumping, are bringing about the disintegration of the accessible groundwater resources.
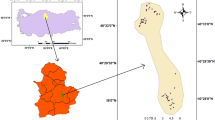
Location map of the study area in the central part of the middle Nile Delta. A–A′ is the location of the cross section shown in Fig. 2
The study area occupied the central part of Nile Delta (Fig. 1) and is located between the Damietta branch (in the east) and Rosetta branch (in the west). It lies between latitudes 30° 06′ and 31° 10′ north. The study area extends from the northern part of Monufia Governorate into the southern part of Kafr El Sheikh Governorate and including the whole area of Gharbia Governorate.
3 Hydrogeological Setting
Several studies dealing with the geology, hydrogeology, and hydrogeochemistry of the Nile Delta quaternary aquifer were done. The significant part of the annual recharge of the Nile Delta aquifer is gotten from the immediate seepage from the watering system trenches and from the infiltration through soils due to irrigation activities. The yearly general groundwater recharge to the aquifer is evaluated at 6.70 km3/year [24, 31,32,33,34].
The Nile Delta quaternary aquifer is of semi-confined type [29, 35]. It occupies the entire Nile Delta. This aquifer changes in thickness from 200 m in the south where Cairo is located to 1,000 m in the northern direction [36] (Fig. 2). The depth to water in this aquifer ranges between 1 and 2 m in the north, 3–4 m in the middle (study region), and 5 m in the south [29]. Distinctive evaluated groundwater depth has been accounted for by RIGW [37] and Morsy [38].
Hydrogeological cross section from south to north in the Nile Delta showing the groundwater regime [11]
Bilqas formation is a thin clay layer that covers the top of the quaternary aquifer of the Nile Delta [24, 29, 39, 40]. This clay layer is responsible for the semi-confining characters of the aquifer. Bilqas formation’s thickness differs from 5 to 20 m in the southern and the central part of the delta and achieves 50 m in the northern part [24, 29, 41]. The lithological and thickness characteristics of the Bilqas formation greatly affect the level of interaction between the surface water and the groundwater [42]. Mit Ghamr formation is the main aquifer in the Nile Delta which is formed by quaternary sediments [24, 29] (Fig. 2). The variable hydraulic parameters and water salinity of the aquifer are related to the various deltaic depositional conditions [39]. These sediments show various aggradations and degradation cycles that were typically produced by changes in sea level [41]. These quaternary sediments made the Nile Delta aquifer as a large water reservoir that is recharged by the Nile water flowing from south to north through an intensive network watering system [43]. The quaternary Nile Delta aquifer is not hydraulically connected with the underlying tertiary formations where the latter rocks act as an aquiclude [42].
4 Methodology
The performed working methodology of this work is shown in Fig. 3. One hundred sixty-six groundwater samples were gathered during field inventory in 2014–2015. Samples were collected from the drinking and irrigation water wells with depths ranging from around 15 to 120 m. Samples were gathered in newly washed plastic bottles. The performed field measurements and laboratory major ions analysis were discussed in Salem et al. [24] and Salem and El-horiny [44], where electrical conductance (EC, mS/cm), pH (hydrogen ion activities), temperature (°C), and TDS (total dissolved solids, mg/l) were measured in situ. Hach’s portable EC/TDS meter and portable Consort pH meter (model P 314) were utilized [45]. Chemical analysis of the water samples was carried out by the central laboratory of the ministry of environmental affairs in Tanta City and included the determination of the major ions (i.e., K, Na, Ca, Mg, Cl, HCO3, CO3, and SO4) and trace elements (Fe, Mn, Cu, and Zn). The chemical analysis was finished within few days of water sample gathering. The laboratory analyses of the major ions were done utilizing the standard analytical techniques depicted by Hach [45]. Precipitation with barium chloride method was used for sulfate measuring by spectrophotometer. Chloride, bicarbonate, calcium, and magnesium were measured utilizing a digital titrator. Titration method using a standard solution of mercuric nitrate and diphenyl carbazone reagent powder was used for chloride ion measurements. Measurements of bicarbonates were done by titration using standard sulfuric acid solution. Concentrations of calcium and magnesium were measured through TH test (total hardness) and calcium hardness test. Atomic absorption was utilized for measuring Fe, Mn, Cu, and Zn. To have a definite depiction of the groundwater quality of the study zone, the gathered samples are classified into shallow groundwater (Fig. 4a), intermediated groundwater (Fig. 4b), and profound groundwater tests (Fig. 4c). The statistical assessment of the water chemical analysis for drinking and irrigation objects is listed in Tables 1 and 2.
4.1 Estimation of Drinking Water Quality Index (WQI)
Chemically based drinking water guidelines according to WHO [46, 47] including the desirable limits of the measured parameters are listed in Table 3. Depending on the relative importance of each parameter for drinking water quality, parameter’s weight is assigned. TDS, EC, NO3, and Pb each has a maximum weight of 5. SO4, TH, and Mn assigned a weight of 4. 3 is used as the weight of pH, Cl, and Na. K, Mg, Ca, HCO3, Cu, Fe, and Zn weight is 2 [48]. The calculating method of WQI is as follows:
As stated by Armanuos et al. [49], “Qi is the ith quality rating and is given by Eq. (2), Wi is the ith relative weight of the parameter I and is calculated by Eq. (3), Ci is the ith concentration of parameter and Si is the ith drinking water guideline according to WHO guidelines in mg/l, wi is the weight of ith parameter, and n is the number of chemical parameters.”
4.2 Estimation of Irrigation Water Quality (IWQ) Index
Five parameter groups are utilized to compute the IWQ. The used parameters were classified into groups according to the rules introduced by Ayers and Westcost [50] and Simsek and Gunduz [51] (Tables 4 and 5). The IWQ index calculating method in the current study was done according to what was stated in Simsek and Gunduz [51] and Spandana et al. [52] as follows:
where i is an incremental index and G is the contribution of each one of the five hazard groups that are essential to assess the quality of water resource for irrigation. Salinity hazard as EC value represents the first category and is determined as:
where w is the group weighting, and r is the parameter rating value. EC–SAR combination is the second category and represents the infiltration and permeability hazard that is computed as:
where w and r are the group weight and the parameter rating value, respectively. The third group is the specific ion toxicity of SAR, chloride, and boron ions in the water and is calculated as a weighted average of the three ions:
where j is an incremental index, w is the weighting of the third group, and r is the rating value of each parameter. The fourth group is the trace element toxicity that is calculated as a weighted average of all the ions available for analysis:
where k is an incremental index, N is the total number of trace element available for the analysis, w is the weight value of this group, and r is the rating value of each parameter. The fifth and the final category is the miscellaneous effects to sensitive crops that are represented by nitrate–nitrogen and bicarbonate ions and the pH of the water and is formulated as a weighted average:
where m is an incremental index, w is the weight value of this group, and r is the rating value of each parameter.
5 Results and Discussion
5.1 Evaluation of Groundwater Quality for Drinking
Drinking water characteristics are soft, low TDS, and has no dissolved toxic components. WHO [47] drinking water guidelines are taken as the basis for the groundwater quality assessment. The statistical analysis of the measured chemical parameters which are used to assess the studied groundwater drinking quality is listed in Table 1.
5.1.1 Shallow Groundwater
The shallow groundwater pH values range from 6.8 to 8.31 with an average value of 7.74. One hundred percent of the samples of the shallow zone are within the desirable limits. TDS vary in the range from 210 to 8,820 mg/l, and the average value is 1,165.7 mg/l. Based on WHO [47] for water suitability according to TDS values, 65.5% of the wells (36 well) are unsuitable water for drinking, and 34.5% of wells are suitable [53]. Spatially the samples have unsuitable TDS values, occupying most of the study area except some areas to the east and the west (Fig. 5a). TH values range from 136.55 to 1,946.4 mg/l with 342.1 mg/l average value. 67.3% (37 wells) of the groundwater samples have soft characters; 32.7% (18 wells) are of hard to very hard category which is beyond the suggested safe limit [54]. The latter unsuitable groundwater samples occupy the northern and southeastern parts of the area (Fig. 5b). NO3 concentration varies from 0.022 to 42 mg/l with an average value of 3.05 mg/l. One hundred percent of the shallow groundwater samples have NO3 concentrations under the permissible limit (Table 1) [55]. Higher NO3 values are recognized in the southern and northwestern parts of the area.
Na concentrations range from 24.2 to 2,160 mg/l with an average of 244.4 mg/l. Based on WHO [46] guidelines, 11 samples (20%) are above the limit and spatially located in the northern parts (Fig. 6a). One hundred percent of the shallow groundwater samples have K concentration below the desirable limit and ranges from 0.060 to 11.40 mg/l with an average value of 1.70 mg/l (Table 1). Ca concentrations range from 27 to 620 mg/l with an average of 78 mg/l. About 5.5% (three wells) of the shallow samples have Ca concentration which exceeded the desirable limit and is located in the northern part (Fig. 6b). Mg concentrations range from 10.50 to 206.56 mg/l, and its average value is 33 mg/l. Most of the samples (98.2%, 54 wells) have Mg concentrations within the desirable range (Fig.6c). The concentration of HCO3 ranges from 137.20 to 2,562.81 mg/l with an average value of 378 mg/l. Thirty-nine wells (about 70.9%) of the total wells have HCO3 concentrations lower than the desirable limit and occupy most of the study area. 29.1% of wells are above HCO3 limit and mostly located in the northern parts of the area (Fig. 6d). It was found that concentrations of SO4 ions range from 0.20 to 246.20 mg/l with an average of 43.30 mg/l. One hundred percent of the samples are within the desirable limit (Table 1). Chloride concentrations vary from 50 to 3,166 mg/l with an average of 362.80 mg/l. Forty-three wells (78.2%) are within the desirable range and located in the southern and central parts. The samples of the northern part of the area (12 wells, 21.8%) are above the permissible limit (Fig. 6e). The concentration of Fe ranges from 0 to 3.42 mg/l with an average of 0.96 mg/l. Twenty-one wells (about 38.2%) are within the desirable range and represented at different localities in the study area. Thirty-four wells (61.8%) are above the permissible limit and cover the most part of the study area (Fig. 7a). Mn concentrations vary from 0.042 to 2.76 mg/l with an average of 0.677 mg/l. Twenty-five wells (45.5%) of the shallow groundwater samples have unsuitable Mn concentrations and occupy the most part of the study area. Thirty wells (54.5%) of the samples are within the desirable range and occupy the area located to the northeastern and southwestern parts (Fig. 7b). A higher concentration of Mn is toxic and is usually bad in terms of taste, odor, and discoloration of food. It was found that the amount of Cu ions ranges from 0.002 to 1.15 mg/l with an average of 0.91 mg/l. One hundred percent of wells are within the desirable limit with concentration increase toward NE parts as a general trend (Table 1). The concentration of Zn varies from 0.006 to 2.63 mg/l with an average of 0.314 mg/l. Most of the wells (41 wells, 74.5%) are within desirable limit. Fourteen wells (25.5%) exceeded the permissible limit and are located to the northern and eastern directions (Fig. 7c).
5.1.2 Intermediate Zone
The pH values of the intermediate groundwater range from 7.34 to 8.40 with an average value of 7.75. One hundred percent of the wells in the study area are within the desirable pH limits. TDS of the intermediate groundwater vary in the range from 173 to 6,430 mg/l with an average value of 804.43 mg/l. According to WHO [46], 58% (26 wells) of the wells are unsuitable water for drinking, and 42% (19 wells) are of suitable water for drinking. The wells above the desirable limit are located at the northern and the southern parts of the study area (Fig. 8). TH as CaCO3 in the intermediate groundwater ranges from 2.4 to 141.5 mg/l with an average value of 23.43 mg/l (Table 1). All the groundwater wells fall in the soft category (100%), and the concentration increases toward the northern direction as a general trend. NO3 concentration ranges from 0.0124 to 42 mg/l with an average value of 3.9 mg/l. All the samples of this zone have NO3 concentrations under the permissible limit with an increasing trend toward the northwestern direction (Table 1).
Na concentration ranges from 15.20 to 1,249.5 mg/l with an average of 135.40 mg/l and increases toward northern parts as a general trend (Fig. 9a). Five wells (11.11%) are above the desirable limit and are located in northern parts. K concentration ranges from 1.1 to 3.20 mg/l with an average value of 0.78 mg/l, all samples are under the desirable limit (Table 1), and concentrations increase toward the northern direction. Ca concentrations range from 8.4 to 750 mg/l with an average of 85.40 mg/l. About 95.6% (43 wells) of the samples are within the permissible limit and show an increase toward the northern direction (Fig. 9b). The concentrations of Mg range from 4 to 141.4 mg/l with an average value of 39.9 mg/l. Most of the Mg concentrations (44 wells, 97.8%) are within the desirable limit and increase toward the northern parts as a general trend (Fig. 9c). The concentration of HCO3 in the intermediate groundwater ranges from 80 to 542.7 mg/l with an average value of 288.40 mg/l. Thirty-one samples (about 68.9%) of the total wells are under the desirable limit and occupy most of the study area, while the wells above the permissible limit are located in the northern parts and some localities at central and southern parts (Fig. 9d). It was found that 100% of wells are within the desirable limit of SO4 concentrations and range from 0.48 to 189 mg/l with an average of 60.10 mg/l (Table 1). The concentrations of Cl ion vary from 16 to 3,610 mg/l with an average of 256.40 mg/l. Thirty-nine wells (86.7%) are within the desirable limit and increase toward the northern parts as a general trend (Fig. 9e).
The concentration of Fe ranges from 0 to 2.81 mg/l with an average of 0.693 mg/l. Sixteen samples (about 35.6%) are within the desirable range, and concentrations increase toward the northwest and northeast. The wells above the desirable limit (29 wells, 64.4%) are located in the northern, western, and southwestern parts of the study area (Fig. 10a). The concentration of Mn varies from 0.068 to 1.95 mg/l with an average of 0.65 mg/l. Most of the wells (24 wells, 53.3%) are within the desirable range, and the unsuitable samples (21 wells, 46.7%) are located at the southern and northern parts (Fig. 10b). It was found that concentrations of Cu ion range from 0 to 1.28 mg/l with an average of 0.246 mg/l. One hundred percent of wells are within the desirable limit (Table 1) with concentrations increasing toward the northern parts as a general trend. The concentration of Zn varies from 0.006 to 1.54 mg/l with an average of 0.361 mg/l. Most of the wells (34 wells, 75.6%) are within the permissible limit, and 11 wells (24.4%) located mostly in the central and southeastern parts exceed the permissible limit (Fig. 10c).
5.1.3 Deep Zone
pH values of the deep groundwater range from 7.30 to 8.45 with an average value of 7.65. One hundred percent of the deep groundwater samples are within the desirable limits with higher values in some locations in the northern and southern direction. The TDS values vary in the range from 180 to 1,940 mg/l with an average value of 635.11 mg/l. Thirty-nine wells (58.2%) are unsuitable, and 28 wells (41.8%) are of suitable water for drinking. The suitable water samples are located in the eastern and western directions (Fig. 11a). TH in this zone ranges from 91.96 to 1,026 mg/l with an average value of 306.24 mg/l. Forty-one wells (61.2%) of the groundwater wells fall in the soft category; 26 wells (38.8%) are located in the central part of the area and fall in hard to very hard category (Fig. 11b). NO3 concentration ranges from 0.006 to 30.4 mg/l with an average value of 2.7 mg/l. All the samples of this zone have NO3 concentrations under the permissible limit with an increasing trend toward the south and southeast (Table 1).
Na concentrations show a range from 17.20 to 427.7 mg/l with an average of 98.41 mg/l. Most of the deep groundwater has Na concentrations within the desirable range except the five wells (7.5%) which are mostly located in the northeastern part (Fig. 12a).All the deep samples have K concentration under the desirable limit and range from 0.15 to 2 mg/l with an average value of 0.66 mg/l (Table 1). The concentration of Ca ranges from 30.70 to 230 mg/l with an average of 75 mg/l. Sixty-six wells (98.51%) of the groundwater samples are within the desirable range except one sample located in the southern part (Fig. 12b). The concentrations of Mg range from 9.72 to 110 mg/l with an average value of 28.92 mg/l. All of the Mg concentrations (100%) are within the desirable limit of 125 mg/l (Table 1). The concentration of HCO3 in this zone ranges from 87.92 to 904.3 mg/l with an average value of 303.7 mg/l. Seventeen wells (25.4%) are above the desirable limit. These samples are recognized in different localities within the study area (Fig. 12c). It was found that the amount of SO4 ion ranges from 0.31 to 252.90 mg/l with an average of 17 mg/l and about 65 wells (97%) are within the desirable limit of 250 mg/l (Fig. 12d). The concentrations of Cl varies from 17 to 748.30 mg/l with an average 160.30 mg/l. Fifty-nine wells (88.1%) are within the desirable range, and eight wells (11.9%) are above the limit and located mostly at the northern parts (Fig. 12e).
The concentrations of Fe range from 0 to 1.76 mg/l with an average of 0.38 mg/l. Forty-two samples (62.7%) are under the desirable limit (0.3 mg/l), and 25 wells (37.3%) are unsuitable (Fig. 13a). The unsuitable Fe-related groundwater samples are represented mostly in the northwestern direction and some localities in the southeastern and southwestern directions. The concentrations of Mn have ranged from 0.036 to 2.89 mg/l with an average of 0.524 mg/l. 49.3% of the samples are within the desirable range and 50.7% are above the limit. The unsuitable groundwater extended along the southeastern–northwestern direction (Fig. 13b). All wells are within the desirable limit of Cu concentration with a range from 0.003 to 1.95 mg/l and an average value equal to 0.62 mg/l (Table 1). The concentration of Zn varies from 0 to 1.37 mg/l with an average of 0.283 mg/l. Most of the wells (53 well, 79.1%) are within the desirable range (0.5 mg/l), and 14 wells (20.9%) exceed the limit (Fig. 13c). The unsuitable wells are located in the southwestern corner of the area.
5.1.4 Drinking Water Quality Index (WQI)
Drinking water quality index of the shallow, intermediate, and deep groundwater zones (Table 6, Fig. 14a–c, respectively) and spatial distribution have different patterns. Most of the area in the shallow zone (Fig. 14a) is characterized by poor drinking quality (26 wells, 47.3%) with patches of good quality (18 wells, 32.7%). The northern and southern parts of the area show very poor (3 wells, 5.45%) to unfit (8 wells, 14.55%) groundwater quality. The groundwater of the intermediate zone has excellent (4 wells, 8.9%) to good (24 wells, 53.3%) water quality in the central part of the area. On the other hand, the samples from the southern part have poor quality (13 wells, 29%), and in the northern part, it has very poor (2 wells, 4.4%) to unfit (2 wells, 4.4%) characters (Fig. 14b). In the deep zone (Fig. 14c), the poor water quality class (27 wells representing 40%) covers most of the study area. Its water quality changed into very poor (5 wells, 7%) to unfit (3 wells, 4%) in the southern direction and excellent (2 wells, 3%) to good quality (30 wells, 45%) to the eastern and western directions.
The correlation matrix (Table 7) shows the relationships between WQI and different water quality parameters. As the correlation coefficient increases, the elements’ bad effects on the drinking water quality increase. In the shallow groundwater, there is a strong positive relationship between water quality deterioration (higher WQI) and TDS, EC, TH, Ca, Na, Mg, K, Cl, HCO3, and Zn. It means all these parameters increase with increasing TDS which are the mean factor for quality deterioration. Mn is moderately affecting the groundwater quality, but SO4, NO3, Fe, and Cu show weak to a very weak relationship with WQI. This means the shallow groundwater is not affected by seawater intrusion because SO4 does not increase linearly with TDS and NO3, Fe, and Cu concentrations are not related to salinity increase, but it could be related to groundwater–sediment interaction.
Similar to the shallow water, the intermediate zone WQI shows a strong to an intermediate positive relationship with seawater intrusion-related parameters which are TDS, EC, Na, Mg, Cl, as well as SO4. In contrast, the freshwater-related water quality parameters (Ca, K, HCO3, and NO3) show a weak relationship with WQI which could be related to a higher freshwater fraction. As the deep samples generally have lower salinity, therefore WQI has intermediate positive relationships (>0.5) with seawater-related parameters and weak relationship with the freshwater-related parameters. Iron and Mn show an increasing effect on water quality deterioration in the intermediate zone for iron and deep zone for manganese. In contrast, Cu is highly affecting the water quality in the shallow zones and nearly with no effect on the intermediate and deep zones.
5.2 Evaluation of Groundwater Quality for Irrigation
In the Nile Delta, groundwater is the second water resource for irrigation. Therefore, TDS, Na%, SAR, RSC, Cl, KI, PI, TH, MH, CAI, and CR (Table 2) and irrigation water quality index (IQW) were computed to survey the suitability of this groundwater for irrigation purposes. Sodium is a vital element since it indicates the soluble alkali/sodium effect to soils. Since sodium decreases soil penetrability which badly affects cultivation process, SAR (sodium adsorption ratio) was calculated using the following equation:
Sodium percent is a factor likewise calculated to assess the appropriateness of water for irrigation purposes [56, 57]. This factor is computed by the accompanying equation:
The relative wealth of sodium regarding alkaline earths and boron and the amount of HCO3 and CO3 in abundance of soluble earths additionally impact the appropriateness of water for irrigation (RSC) [58]. RSC (residual sodium carbonate) is computed as follows:
The soil penetrability is influenced by water use for a long time. Na, Mg, Ca, and HCO3 component in the soil impact it. Permeability index (PI) was developed by Doneen [59] to evaluate the appropriateness of water for irrigation where
Measured Na versus Mg and Ca was used by Kelly [60] and Paliwal [61] as Kelley index. This factor is computed as shown in this equation:
Magnesium ratio (MH) was suggested by Szabolcs and Darab [62] to assess the irrigation water by the given formula:
CAI (chloro-alkaline index) is an important factor and characterized as the ion-exchange characters between the groundwater and aquifer sediments [63]. CAI is computed according to the following equation:
CR (corrosivity ratio) is essential to assess the ability to transfer water in metallic pipes. Water with CR < 1 is safe, while >1 shows corrosive characters and thus not to be transferred in metal pipes. The CR is computed utilizing this equation:
5.2.1 Shallow Zone
The classification of the shallow groundwater suitability for irrigation using the abovementioned parameters is listed in Table 2, and the spatial distribution is shown in Figs. 15 and 16. According to Jain et al. [64], most parts of the study area (50 wells, 90.9%) have desirable TDS range less than 3,000 mg/l except the northern parts which have unfit TDS values (five samples) (Fig. 15a). Na% in the shallow groundwater zone ranged between 19.4 and 80.1%, with an average of 44.7% (Table 1). It is observed that most of the shallow groundwater samples located in the southeastern and western parts fall into the category of good (58.2%, 32 sample). Groundwater samples located in the northern part of the study area have permissible (21.8%, 12 samples) and doubtful (20%, 11 samples) ranges (Fig. 15b). The calculated value of SAR ranges from 0.83 to 21.4 and has been classified mostly as excellent (45 samples, 81.8%) and good (7 samples, 12.7%) for irrigation. Fair category for groundwater (3 samples, 5.5%) is represented in the northeastern part of the study area (Fig. 15c). RSC reveal that most of the samples fall within good category (48 sample, 87.3%) suitable for irrigation. Two samples (3.6%) and five samples (9.1%) restricted to the northeastern part fall within the category of doubtful and unsuitable, respectively (Fig. 15d). Permeability index reveals that most of the shallow samples fall within class II category (42 sample, 76.4%) except the northeastern part where 13 wells (23.6%) are classified as class I (Fig. 15e). Based on CAI, 30 wells (54.5%) of groundwater samples have cation–anion exchange reaction characters and occupy various locations in the study area. Samples belong to the base-exchange reaction category are represented by 25 wells (45.5%) and have no definite spatial distribution pattern (Fig. 15f). According to chloride concentrations, 34 wells (61.8%) fall within fresh category suitable for irrigation, 9 wells (16.4%) are considered fresh–brackish, 6 wells (10.9%) are brackish, and 6 wells (10.9%) are located in the northern part and fall within brackish–salt range (Fig. 16a). Magnesium hazard (MH) shows that 46 wells (83.6%) occupy most of the study area and are under the suitable category. Nine wells (16.4%) are unsuitable and located as spots in the southeastern, northeastern, and northwestern parts (Fig. 16b). According to KI, most of the shallow samples fall within suitable category (41 samples, 74.5%) which occupy most of the study area. Fourteen wells (25.5%) are classified as unsuitable and located in the northern parts (Fig. 16c). The calculated CR values reveal that most of the shallow samples fall within corrosive category (38 wells, 69.1%). Noncorrosive category (17 wells, 30.9%) is represented mostly in the western part of the area (Fig. 16d).
5.2.2 Intermediate Zone
The classification of the intermediate groundwater suitability for irrigation is shown in Table 2, and the spatial distribution is presented in Figs. 17 and 18. Forty-three wells (95.6%) and two samples (4.4%) have suitable (<3,000 mg/l) and unfit TDS values for irrigation, respectively. The unfit groundwater is represented in the northern part (Fig. 17a). Calculated Na% reveals that most of the samples fall into the category of excellent (28 wells, 62.2%) and good water (3 wells, 6.7%). Permissible (12 wells, 26.7%) and doubtful (2 wells, 4.4%) categories are represented mostly in the northern parts (Fig. 17b). Intermediate groundwater is classified as suitable for irrigation ranging from excellent to good according to SAR-calculated values (Fig. 17c). Most of the groundwater samples have RSC values falling within good category (42 wells, 93.3%) and occupying most of the study area. Doubtful (2 wells, 4.5%) and unsuitable (1 well, 2.2%) categories are represented as scattered few locations (Fig. 17d). Class II is the predominant PI category (38 wells, 84.4%) and represented in all parts of the study area while 7 wells (15.6%) are related to class I and represented as three scattered small locations (Fig. 17e). Base-exchange reactions are the common CAI category (25 wells, 55.4%) and occupying an area extending from the southwestern to the northeastern and northern directions (Fig. 17f). Cation–anion exchange reactions (20 wells, 44.6%) mostly characterize the water of the southeastern and northwestern parts of the area. Chloride concentrations reveal that most of the groundwater samples fall within fresh category (30 wells, 66.7%) and occupy the central parts, two samples (4.4%) are very fresh, and ten wells (22.3%) fell within the fresh–brackish category and are located in the southern and the northern parts. Brackish (1 well, 2.2%) and brackish–salt (2 wells, 4.4%) groundwater are located in the northern parts as shown in Fig. 18a. According to the calculated magnesium hazard (MH), 29 wells (64.4%) are under suitable category and occupy most of the study area, and 16 wells (35.6%) fell within the unsuitable category and are represented mostly in the southwestern part of the area (Fig. 18b). Most of the samples of the intermediate zone fall within the suitable KI category (35 wells, 77.8%), while ten wells (22.2%) are unsuitable and located in the northern parts (Fig. 18c). As shown in Fig. 18d, the calculated CR values reveal that most of the intermediate samples fall within corrosive category (34 wells, 75.6%). Eleven samples (24.4%) are of noncorrosive properties and located in the central part of the area and other few locations.
5.2.3 Deep Zone
Parameters used for examining of the deep groundwater suitability for irrigation were listed in Table 2 and spatially distributed in Figs. 19 and 20. Based on TDS values, all samples are suitable for irrigation (TDS < 3,000 mg/l) (Fig. 19a). In respect to Na%, 44 wells (65.7%) fall within the category of good and occupy most of the area, and 15 wells (22.4%) are of permissible values and located in the southeastern and the northeastern directions. Few samples fall in excellent category (2 wells, 2.9%), three wells (4.5%) are in doubtful category, and three wells (4.5%) are unsuitable (Fig. 19b). The calculated values of SAR reveal that most of the samples fall within excellent category (65 wells, 97%), and the rest of the samples (2 wells, 3%) fall into the good category (Fig. 19c). Calculated RSC reveal that 63 wells (94%) fall into good category and 4 wells (6%) located in the northeastern part fall within categories of doubtful and unsuitable (Fig. 19d). Class II is the predominant permeability index category (58 wells, 86.6%), while 9 wells (13.4%) located in the eastern part fall within class I (Fig. 19e). The calculated CAI has no definite trend where 32 wells (47.8%) are under cation–anion exchange reaction category, and 35 wells (52.2%) fall within base-exchange reaction category (Fig. 19f). Chloride concentrations show that 4 wells (6%) and 46 wells (56.7%), respectively, fall within very fresh and fresh category and occupy most of the study area. Seventeen wells (25.4%) have fresh–brackish characters, and eight wells (11.9%) are brackish and located mostly in the northeastern direction (Fig. 20a). Calculated magnesium hazard (MH) values reveal that 60 wells (89.55%) are under suitable category and occupy most of the study area, and 7 wells (10.45%) are unsuitable and mostly located in the northwestern direction (Fig. 20b). KI reveal that most of the deep samples fall within suitable category (56 wells, 83.6%), and 11 wells (16.4%) are unsuitable and characterize small areas in the south and northeastern parts (Fig. 20c). The calculated CR has no definite trend, and most of the samples fall within corrosive category (45 wells, 67.2%), while 22 samples (32.8%) are characterized by noncorrosive properties (Fig. 20d).
The binary relationships between salinity hazards and SAR (Fig. 21) and total ion concentrations and Na% (Fig. 22) reveal that most of the shallow, intermediate, and deep groundwater samples fall within ranges from good to permissible. Some samples are of the unsuitable category. It is obvious that the quality of groundwater for irrigation in the southern and middle parts is good to permissible, but the groundwater in the northern parts is of doubtful to unsuitable characters.
5.2.4 Irrigation Water Quality (IQW) Index
Irrigation water quality index of the shallow, intermediate, and deep groundwater zones (Table 8, Fig. 23a–c, respectively) has different patterns. IQW of the shallow zone (Fig. 23a) shows that 4 wells (7.3%) fell within poor category and are located in the northeastern and northwestern parts, and 15 wells (27.2%) are of good water quality. The medium water quality (36 wells, 65.5%) is the predominant category and occupies most of the study area. The intermediate groundwater (Fig. 23b) is predominantly of good (13 wells, 29%) to medium (29 wells, 64%) water quality and changes to low quality to the northern part (3 wells, 7%). The deep zone (Fig. 23c), in general, looks similar to the intermediate zone with medium (34 wells, 51%) to good (32 wells, 48%) water quality changed to low quality (1 wells, 1%) in the northern part.
The correlation matrix (Table 7) shows the relationships between IQW and different water quality parameters. In the shallow groundwater, EC, SAR, Cl, and HCO3 are the most effective parameters in IQW where the correlation coefficients range from 0.65 to 0.91. Weak correlation values between IWQ and NO3, pH, Fe, Mn, Zn, and Cu are noticed. As the soil hydrogeochemical processes decrease in the intermediate and deep groundwater, the effect of the major parameters like EC, SAR, and Cl on the IQW decreases downward. In contrast to the shallow groundwater, correlation coefficient values of EC, Cl, and HCO3 with IQW decrease downward reaching weak class in the deep groundwater. On the other hand, the effect of the minor elements on the irrigation water quality index increases downward reaching 0.3, 0.45, 0.35, 0.56, and 0.59 for NO3, Fe, Mn, Zn, and Cu, respectively. This could be related to the predominance of the water–rock interaction in the intermediate and deep groundwater zones.
6 Conclusions
Based on WHO [46, 47] guidelines for drinking water, Na, K, Mg, Ca, SO4, Cl, Cu, and NO3 concentrations are lower than the desirable limits in around 80% or more of the collected samples. Mostly, the samples have undesirable concentrations of these ions which are located in the northern part of the study area. Around 68–75% of the samples have HCO3 desirable concentration. TDS, iron, and manganese are the most effective individual factors for quality evaluation of this groundwater. 65%, 42%, and 58% of the samples have TDS higher than 500 mg/l for the three zones, respectively. Regarding iron concentrations, 61%, 64%, and 37% of the wells have undesirable concentrations. Around half of the collected water samples have undesirable manganese concentrations. Ninety-eight percent of the intermediate groundwater samples have desirable TH concentrations, while around 67% and 61% are recorded for the shallow and deep zones, respectively.
According to WQI, most part of the area in the shallow zone is characterized by poor quality (26 wells, 47.3%), and 18 wells (32.7%) fall in good categories. The northern and southern parts of the area show very poor (3 wells, 5.45%) to unfit (8 wells, 14.55%) groundwater. The intermediate zone has excellent (4 wells, 8.9%) to good (24 wells, 53.3%) water quality in the central part of the area. While in the southern part, it has poor quality (13 wells, 29%), and in the northern part, it has very poor (2 wells, 4.4%) to unfit (2 wells, 4.4%) characters. In the deep zone, the poor (27 wells, 40%) water quality class covers most of the study area. The latter class changed into very poor (5 wells, 7%) to unfit (3 wells, 4%) in the southern direction and excellent (2 wells, 3%) to good quality (30 wells, 45%) to the eastern and western directions.
Ten individual parameters were utilized to assess the quality of studied groundwater and its reasonableness for irrigation. These parameters are TDS, SAR, Na%, Cl, RSC, PI, KI, CAI, C.R, and MH. Groundwater wells were ordered concurring TDS as around 90, 99, and 100% of wells for shallow, intermediate, and deep zone separately have TDS under 3,000 mg/l. Subsequently groundwater is reasonable for irrigation. Concerning Na%, the vast majority of the groundwater samples fall into the classification of good to permissible. The computed estimation of SAR varies from 0.83 to 21.37 for the shallow zone, 0.57 to 12.1 for the intermediate zone, and 0.7 to 17.38 for the deep zone and has been classified as about 82% for shallow, 92% for intermediate, and 100% for deep zones of groundwater wells are suitable for irrigation. Most samples have good RSC category, class II (76–86%, PI), fresh and fresh–brackish characters (Cl), and suitable KI and MH. Samples that have base-exchange and cation-exchange characters are mostly present with equal percentages in the three zones. A considerable number of samples are of corrosive properties, 69%, 75%, and 67%, respectively, for the shallow, deep, and intermediate zones.
Few samples located in the northern part of the area have IWQ values less than 22 and are considered to be of poor irrigation quality. Groundwater of such regions could decrease soil quality and led to yield misfortune. As a general guideline, water pumping from such regions must be stopped. It was estimated that the quality of the groundwater in the studied aquifer is fairly accepted and in the most parts of the study area is reasonable for irrigation. Such spatial distribution of the factors controlling the studied groundwater quality for drinking and irrigation in the three zones is a reasonable tool to be used in future domestic and rural administration plans and in deciding the most appropriate site for drilling wells and for evaluating the general groundwater suitability.
7 Recommendations
The Nile Delta groundwater aquifer is influenced by many environmental factors like human activity on the surface, sea water intrusion, and the type of aquifer sediments. Therefore, the decision-maker must take into consideration the lateral and vertical changes in groundwater quality when digging wells for different uses in order to achieve the highest utilization of wells. Microbial contamination should also be studied in spatial form to have a complete image about the Nile Delta aquifer. The researchers should not study this aquifer as a single homogenous body, but they should consider the vertical and horizontal change of groundwater chemistry. To complete this topic, the authors will conduct a hydrogeochemical study in the three dimensions of the aquifer.
References
Singh CK, Shashtri S, Mukherjee S, Kumari R, Avatar R, Singh A, Singh RP (2011) Application of GWQI to assess effect of land use change on groundwater quality in lower shiwaliks of Punjab: remote sensing and GIS based approach. Water Resour Manag 25:1881–1898. https://doi.org/10.1007/s11269-011-9779-0
Subramaniam T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use Chithar River basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Shabbir R, Ahmad SS (2015) Use of geographic information system and water quality index to assess groundwater quality in Rawalpindi and Islamabad. Arab J Sci Eng 40:2033. https://doi.org/10.1007/s13369-015-1697-7
Toumi N, Hussein BHM, Rafrafi S, El Kassas N (2015) Groundwater quality and hydrochemical properties of Al-Ula region, Saudi Arabia. Environ Monit Assess 187:84–100. https://doi.org/10.1007/s10661-014-4241-4
UNEP (United Nations Environment Program) (1999) Global environment outlook 2000. Earthscan, London
Vincy V, Brilliant R, Pradeepkumar AP (2015) Hydrochemical characterization and quality assessment of groundwater for drinking and irrigation purposes: a case study of Meenachil River basin. Western Ghats, Kerala, India. Environ Monit Assess 187:4217. https://doi.org/10.1007/s10661-014-4217-4
Bruce BW, Becker MF, Pope LM, Gurdak JJ (2003) Ground-water quality beneath irrigated agriculture in the central high plains aquifer, 1999–2000. U.S. Geological Survey Water-Resources Investigations Report 03–4219, U.S. Geological Survey, Reston, VA, p 39
Zhang W, Kinniburgh D, Gabos S (2013) Assessment of groundwater quality in Alberta, Canada Using GIS Mapping. Third international conference on medical, biological and pharmaceutical sciences (ICMBPS’2013), Bali, pp199–203
Arumaikkani GS, Chelliah S, Gopalan M (2017) Mapping the spatial distributions of water quality and their interpolation with land use/land cover using GIS and remote sensing in Noyyal River basin, Tamil Nadu, India. J Geosci Environ Protection 5:211–220
Salem ZE (2009) Natural and human impacts on the groundwater under an Egyptian village, central Nile Delta e a case study of Mehallet Menouf. In: 13th International Water Technology Conference (IWTC), 12–15 March 2009, Hurghada, 3, pp 1397–1414
Elewa HH (2010) Potentialities of water resources pollution of the Nile River Delta, Egypt. Open Hydrol J 4:1–13
Gemail K, El-Shishtawy AM, El-Alfy M, Ghoneim MF, El-Bary MHA (2011) Assessment of aquifer vulnerability to industrial waste water using resistivity measurements. A case study, along El-Gharbyia main drain, Nile Delta, Egypt. J Appl Geophys 75:140–150
Morsy W, El-Fakharany Z (2012) Predicting the impact of surface wastewater on groundwater quality in quesna industrial area. J Am Sci 8:772–781
Salema MG, El-Awady MH, Amine E (2012) Enhanced removal of dissolved iron and manganese from nonconventional water resources in Delta District, Egypt. Energy Procedia 18:983–993
Ghoraba SM, Zyedan BA, Rashwan IMH (2013) Solute transport modeling of the groundwater for quaternary aquifer quality management in Middle Delta, Egypt. Alex Eng J 52:197–207
Khalil MA, Salem ZE, Gheda SF, El-Sheekh MM (2013) Quality assessment of drinking water in Tanta City, Egypt. J Environ Sci Eng B 2:257–275
El Bedawy R (2014) Water resources management: alarming crisis for Egypt. J Manag Sustain 4:108–124
Bennett PC, El Shishtawy AM, JM S, Atwia MG (2014) Source and migration of dissolved manganese in the central Nile Delta aquifer, Egypt. J Afr Earth Sci 96:8–20
Elkafoury A, Dawoud W, Negm A, Bady M, Aly MH (2014) Integrated framework for evaluating the impact of urban transportation gaseous emissions on groundwater quality. Int Water Technol J 4:114–124
Fattah MK, Ragab EG (2014) Assessment of groundwater vulnerability to pollution in the southern part of Nile Delta, Egypt. Standard Sci Res Essays 2:725–738
El-Kowrany SI, El-Zamarany EA, El-Nouby KA, El-Mehy DA, Ali EA, Othman AA, Salah W, El-Ebiary AA (2016) Water pollution in the middle Nile Delta, Egypt: an environmental study. J Adv Res 7:781–794
Negm AM, Armanuos AM (2016) GIS-based spatial distribution of groundwater quality in the western Nile Delta, Egypt. In: Negm AM (ed) The Nile Delta. The handbook of environmental chemistry. Springer, Cham. https://doi.org/10.1007/698_2016_66
Negm AM, Eltarabily MGA (2016) Modeling of fertilizer transport through soil, case study: Nile Delta. In: Negm AM (ed) The Nile Delta. The handbook of environmental chemistry. Springer, Cham. https://doi.org/10.1007/698_2016_66
Salem ZE, Al Temamy AM, Salah MK, Kassab M (2016) Origin and characteristics of brackish groundwater in Abu Madi coastal area, Northern Nile Delta, Egypt. Estuar Coast Shelf Sci 178:21–35
Sharaky AM, El Hasanein AS, Atta SA, Khallaf KM (2016) Nile and groundwater interaction in the western Nile Delta, Egypt. In: Negm AM (ed) The Nile Delta. The handbook of environmental chemistry. Springer, Cham. https://doi.org/10.1007/698_2016_66
Salem ZE, Osman OM (2017) Use of major ions to evaluate the hydrogeochemistry of groundwater influenced by reclamation and seawater intrusion, West Nile Delta, Egypt. Environ Sci Pollut Res 24:3675–3704
Negm AM, Saavedra O, El-Adawy A (2016) Nile Delta biography: challenges and opportunities. In: Negm A (ed) The Nile Delta. The handbook of environmental chemistry, vol 55. Springer, Cham
Dawoud M (2004) Design of national groundwater quality monitoring network in Egypt. J Environ Monit Assess 96:99–118
Mabrouk MB, Jonoski A, Solomatine D, Uhlenbrook S (2013) A review of seawater intrusion in the Nile Delta groundwater system – the basis for assessing impacts due to climate changes and water resources development. Hydrol Earth Syst Sci Discuss 10:10873–10911
Barrocu G, Dahab K (2010) Changing climate and saltwater intrusion in the Nile Delta, Egypt. In: Taniguchi M, Holman IP (eds) Groundwater response to changing climate. CRC Press, Boca Raton, pp 11–25. https://doi.org/10.1201/b10530-3
Kashef AI (1983) Salt water intrusion in the Nile Delta. Ground Water 21:160–167
Mikhailova M (2001) Hydrological regime of the Nile Delta and dynamics of its coastline. Water Resour 28:477–490
Sefelnasr A, Sherif MM (2014) Impacts of seawater rise on seawater intrusion in the Nile Delta aquifer, Egypt. Groundwater 52:264–276
Sherif MM, Sefelnasr A, Javadi A (2012) Incorporating the concept of equivalent freshwater head in successive horizontal simulations of seawater intrusion in the Nile Delta aquifer. Egypt. J Hydrol 464:465
Ball J (1939) Contribution to the Geography of Egypt, Survey, Cairo, pp 23–84
RIGW (1992) Hydrogeological map of Nile Delta, scale 1:500,000, 1st edn. Nile Delta
RIGW (2002) Nile Delta groundwater modeling report. Research Institute for Groundwater, Kanater El- Khairia, Egypt
Morsy S (2009) Environmental management to groundwater resources for Nile Delta region. PhD thesis, Faculty of Engineering, Cairo University, Egypt
Farid M (1980) Nile Delta groundwater study. MSc thesis, Cairo University, Egypt
Wilson J, Townley R, SaDa Costa A (1979) Mathematical development and verification of a finite element aquifer flow model AQUIFEM-1. Technology adaptation program, Report No. 79–2, Cambridge, MA
Diab S, Dahab K, El Fakharany M (1997) Impacts of the paleohydrological conditions on the groundwater quality in the northern part of Nile Delta, the geological society of Egypt. J Geol 4112B:779–795
Saleh F (1980) Some hydrological and hydrochemical studies on the Nile Delta. MSc. thesis, Faculty of Science, Ain Shams University, Egypt
Abdel Maged H (1994) Water logging phenomena in the north of the delta region. MSc thesis, Faculty of Science, Cairo University, Egypt
Salem ZE, El-horiny MM (2014) Hydrogeochemical evaluation of calcareous eolianite aquifer with saline soil in a semiarid area. Environ Sci Pollut Res 21:8294–8314
HACH (1990) Chemical procedures explained. Hach Technical Center for Applied Analytical Chemistry, Colorado
WHO (World Health Organization) (1996) Guidelines for drinking-water quality, 2nd edn
WHO (World Health Organization) (2011) Guidelines for drinking-water quality, 4th edn
Abbasi T, Abbasi A (2012) Water quality indices. Elsevier, Amsterdam, pp 19–28
Armanuos AM, Negm A, Valeriano OCS (2015) Groundwater quality investigation using water quality index and ArcGIS: case study: western Nile Delta aquifer, Egypt. Proceedings of the 18th water technology conference (IWTC18), Sharm ElSheikh, 12–14 March
Ayers S, Westcost W (1985) Water quality for agriculture, FAO irrigation and drainage paper. Food Agriculture Organization, Rome, p 29
Simsek C, Gunduz O (2007) IWQ index: a GIS-integrated technique to assess irrigation water quality. Environ Monit Assess 128:277–300
Spandana MP, Suresh KR, Prathima B (2013) Developing an irrigation water quality index for Vrishabavathi command area. Int J Eng Res Technol 2:821–830
Srinivas Y, Oliver DH, Raj AS, Chandrasekar N (2013) Evaluation of groundwater quality in and around Nagercoil town, Tamilnadu, India: an integrated geochemical and GIS approach. Appl Water Sci 3:631. https://doi.org/10.1007/s13201-013-0109-y
Ramesh K, Seetha K (2013) Hydrochemical analysis of surface water and groundwater in tannery belt in and around Ranipet, Vellore district, Tamil Nadu, India. Int J Res Chem Environ 3:36–47
Brindha K, Neena Vaman KV, Srinivasan K, Sathis Babu M, Elango L (2014) Identification of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, southern India. Appl Water Sci 4:159. https://doi.org/10.1007/s13201-013-0138-6
Tiwari N, Manzoor A (1988) River pollution in Kathmandu valley (Nepal), suitability of river water for irrigation. Indian J Environ Protection 8:269–274
Wilcox V (1948) The quality of water for irrigation use. US Department of Agriculture, Technical Bulletin, Washington
Richards A (1954) Diagnosis and improvement of saline and alkali soils. USDA Hand Book
Doneen D (1954) Salination of soil by salts in the irrigation water. Am Geophys Union Trans 35:943–950
Kelly P (1940) Permissible composition and concentration of irrigation waters. Proc Am Soc Civ Eng 66:607–613
Paliwal V (1967) Effect of gypsum application on the quality of irrigation waters. Madras Agric J 59:646–647
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content on soil. In: Szabolics I (ed) Proceedings of the 8th international congress soil science, sodics soils. Res Inst Soil Sci, Agric Chem, Hungarian Acad Sci, ISSS Trans II, pp 802–812
Schoeller H (1967) Geochemistry of ground water. An international guide for research and practice, vol 15. UNESCO, Paris, pp 1–18
Jain K, Kumar P, Sharma K (2003) Ground water qualities of ghataprabha command area Karnataka. Indian J Environ Ecoplann 7:251–262
Acknowledgments
The authors are grateful to Tanta University for the financial support offered by the project number “TU-01-12-03” during the course of this research work. The authors thank the editor Prof. Dr. Abdelazim Negm for his constructive remarks.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Salem, Z.E., Elsaiedy, G., ElNahrawy, A. (2017). Assessment of the Groundwater Quality for Drinking and Irrigation Purposes in the Central Nile Delta Region, Egypt. In: Negm, A. (eds) Groundwater in the Nile Delta . The Handbook of Environmental Chemistry, vol 73. Springer, Cham. https://doi.org/10.1007/698_2017_137
Download citation
DOI: https://doi.org/10.1007/698_2017_137
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-94282-7
Online ISBN: 978-3-319-94283-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)