Abstract
Surface water bodies are constantly exposed to pollutant inputs of different origin. Wastewater effluents discharge directly on the receiving natural streams, and are among the main entrance pathways for sulfonamides. Strong contrast between seasons, with the consequent fluctuations in the flow rates, and heavy contamination pressures from extensive urban, industrial, and agricultural activities are characteristics of water courses located in the Mediterranean area. The low base flows of Mediterranean rivers makes their hydrology cycle heavily dependent on wastewater inputs, and therefore removal efficiencies of wastewater treatment plants are key to the health of the aquatic ecosystem.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Environmental risk assessment
- Mediterranean region
- Removal efficiency
- Sulfonamide
- Surface waters
- Wastewater treatment plant
1 Introduction
As a consequence of the increasing human population density and more intensive animal farming techniques, fresh water systems have become highly susceptible to be at risk of potential contamination by different pharmaceutical products (PhPs) from both human and veterinary use. Awareness of the presence of PhPs in wastewaters and aquatic ecosystems is growing as investigations regarding new pollutants increase and analytical techniques for detecting these chemicals improve. At present, approximately 3,000 different pharmaceutical ingredients are used in the European Union (EU), including antibiotics, β-blockers, lipid regulators, antidepressants, etc. [1]. Estimations of the potential environmental impact of PhPs are usually based on the quantities produced and consumed, their potency and also on their tendency to bioaccumulate in the environment. The risk posed by antibiotics could be explained in terms of any of these premises. In first place, their role is superlative in modern agriculture and livestock, and this fact is reflected in their high consumption rates. Although information on their usage is not available to the general public either in the United States (US) or in the European Union (EU), estimations indicate sales over the 16,000 t in US in 2001, of which 9,300 t are used in animal-feeding operations [2]. According to the European Federation of Animal Health (FEDESA), the annual consumption of antibiotics in the EU in 1999 was in total 13,288 t with 29% for veterinary medicine, 6% as antibiotic feed additives, and 65% in human medicine. In addition, prescription drugs are generally sold in quantities one order of magnitude lower than nonprescription drugs [3]. Regarding their potency, these substances are designed to cause a biological effect in the target organism or patient at relatively low concentrations. Once discharged in the environment, they may have numerous unexpected effects on nontarget, or as yet unknown, receptors. It has been demonstrated in different studies that the environmental presence of antimicrobials leads to the development of antibiotic resistance in bacteria, threat that has been recognized by, among others, the World Health Organization (WHO) and is a well-documented fact nowadays. They can also be toxic to different nontarget organisms, including beneficial bacteria in both natural and urban environments; for instance, wastewater treatment processes may be disrupted [4, 5] or degrading microbiota from different ecosystems can be negatively affected [6]. Finally, antimicrobial resilience and persistence in the environment has been demonstrated [7, 8]. This is a direct consequence of their physicochemical properties such as polarity or liposolubility (they can go through biological membranes), which makes them very persistent compounds in order to stay active and therefore very prone to bioaccumulate
2 Environmental Presence of Sulfonamide Antibiotics: Sources and Occurrence
Sulfonamides (SAs) are one of the most widely used antibiotics in human and especially in animal husbandry and fish farming [9, 10]. They are usually applied in combination with diaminopyrimidines such as trimethoprim due to the enhancement of their activity [11]. In EU, SAs are the second most widely used veterinary antibiotics, representing 21% of the sales in the United Kingdom in 2000, and 11–23% in several other European countries. In US, SAs account for the 2.3% of the total amount of antibiotics used [a2]. SAs are widely used because they are inexpensive, effective against a broad spectrum of common bacterial infections, and have high effectiveness in growth promotion in veterinary applications, although this last use has been banned in the EU since 2006 for all antibiotics [12]. The increase in the number of confined animal-feeding operations (CAFOs), which often lack proper waste management practices, has led to a higher use of these antibiotics and, therefore, to a greater occurrence of these substances in the environment. Following treatment, livestock will excrete 50–90% of the administered dose, the parent drug making up for 9–30%. These amounts of the unchanged substance vary depending on the form of the drug and the animal age and species [13, 14]. Animal excreta are considered one of the major sources of environmental contamination by SAs; residues of these antimicrobials have been detected in manure from medicated animals, which is frequently applied as nutrient amendment in agriculture as it is regarded as a very valuable fertilizer containing essential nutrients for plant growth such as nitrogen, phosphorous, organic carbon or potassium [15–19]. The extensive use of manure in crop fields is among the major routes by which veterinary antibiotics enter the environment [19–21] and, eventually, the different water systems. The consequent diffuse pollution is difficult to prevent and deal with due to the large areas of application. Once on the topsoil and due to their weak sorption to soil tendency and high solubility, the excreted residues of SAs become very mobile and may reach surface waters during runoff episodes and even percolate and contaminate the aquifers [15, 22]. This possibility has already been proved in several publications, showing the presence of SAs at different concentrations in groundwater from various sites close to animal farming facilities [23–30]. On the other hand, although veterinary antibiotics such as SAs only reach wastewater treatment plants (WWTPs) to a limited extent, they have been frequently detected in influent and most importantly, in effluent wastewaters [31–34] due to their generally low biodegradation and elimination efficiency during sewage treatment. As these effluents commonly discharge into natural water courses, in the last decade a growing awareness in the scientific field has been manifested regarding the danger posed by the WWTPs inputs to river ecosystems. River basins and catchment areas can therefore be considered highly vulnerable systems regarding SAs contamination. It should also be considered the frequent application of biosolids from WWTPs as organic amendments in agriculture, opening a different entrance pathway into the environment for these substances [35]. Other secondary input pathways are waste effluents of the manufacturing processes or hospitals, the disposal of unused or expired drug products (solid waste or “flushing”), accidental spills during manufacturing or distribution and leakage from septic systems and agricultural waste-storage facilities [36–38]. Antibiotics that reach landfill sites as solid waste are subjected to biologic degradation processes, but some may persist and leach into surrounding groundwater or reach river courses after flood episodes [39–41]. Another critical scenario is that of aquaculture and antibiotics direct addition to receiving waters, formulated as feed additives, with 70–80% of the administered amount entering the environment [42].
2.1 Presence of Sulfonamides in Wastewater Treatment Plants
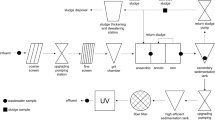
Given the relevance of WWTPs discharges as indirect entrance pathway for SAs and many other pollutants onto surface waters, a first step to evaluate the health of a river ecosystem would be to determine the loads of pollutants in these WWTPs effluents. Degradation and vulnerability of river systems are directly dependent on the removal efficiencies (RE%) of the WWTPs regarding these contaminants; however, data on the RE% of these compounds during wastewater treatment is still scarce. In general, Spanish WWTPs apply primary and secondary biologic treatments, the latter usually based on conventional activated sludge (CAS). Tertiary treatments such as ozonation, which have demonstrated to be highly efficient in the removal of different PhPs including SAs, are seldom applied [43–45]. Table 1 summarizes some of the RE% values found recently in the literature. Recently, frequencies of detection and RE%s were reported for the seven main WWTPs located along the Ebro River Basin [31]. SAs of human application such as sulfamethoxazole (SMX), sulfapyridine (SPY) and sulfadiazine (SDZ, also used in veterinary therapies) were the most frequently detected (>85%) and at the highest concentrations (650 ng L−1 for SMX and 227 ng L−1 for SPY) in both influent and effluent samples. RE% values obtained were hard to interpret, as SAs were not regularly present in all the WWTPs, and values ranged from negative removals to 100% elimination. SDZ was in average the SA eliminated most efficiently in these seven WWTPs, whereas SPY showed intermediate to high RE% values. SMX showed both RE% higher than 50% but also negative values in many WWTPs. These higher concentrations detected in the effluents are usually attributed to the presence of SA conjugates and metabolites, which usually are not comprised within the scope of the different studies; these conjugates can be transformed back during treatment into the original compound, as demonstrated recently [46] and could therefore explain higher concentrations of SAs in effluents than in influent waters [47, 48]. Alternative secondary treatments, such as membrane bioreactors (MBRs), have been investigated in recent years to obtain an improvement in the RE% values. However, this treatment technology has proved not to be especially good, in particular for SMX and SPY, the two most relevant SAs in terms of frequencies of detection and concentration. Recent works demonstrated that although elimination rates for SMX were higher in the MBRs than in CAS, removal was only partial as nearly half of the SMX input could still be detected in their respective effluents [49–52]. On the contrary, MBRs worked more efficiently than CAS for other SAs, such as SDZ, which was completely removed after MBR treatment, whereas it was removed only 49% during the CAS treatment. Regarding acetylated metabolites, N4-acetylsulfamethazine (AcSMZ) was 100% removed after MBR treatment, and only in a 54% after the CAS treatment [51]. Tertiary treatments such as ozonation and nanofiltration have demonstrated high efficiencies in SAs removal [44, 53–56], but still its application in WWTPs is scarce and the fate of the transformation products generated unknown [57].
2.2 Presence of Sulfonamides in Surface Waters
The first reported case of surface water contamination by SAs was in England in 1982, when Watts et al. detected at least one compound from SAs family in river water at concentrations of 1 μg L−1 [58]. Nowadays, in Europe the EU Water Framework Directive (WFD) specifies the need to monitor PPs (SAs among them) in surface waters as an informative step to protect and improve the quality of the European water resources [59]. Given that SAs have been frequently detected in WWTP effluents, several studies have aimed to highlight the state and vulnerability of the receiving freshwaters downstream of urban areas and WWTP facilities, focusing especially in the presence in these water matrices of SAs of human consumption, which are the most commonly detected in the wastewater effluents. The low natural biodegradation of SAs [60], and low tendency to adsorb to solid matrices (from the river bed) [61, 62] together with the SAs inputs, both agricultural and urban, that the river may receive all along the basin would lead to a marked concentration gradient from the source to the mouth of the water course. When interpreting the obtained data, seasonal changes should also be taken into account. Generally, the highest concentrations of human SAs (SMX, SPY) are expected during the dry seasons, as the dilution exerted by the receiving streams is lower. For instance, Kim and Carlson [63] detected SMX at a maximum average concentration of 230 ng L−1 during the winter and of 320 ng L−1 during the summer, in the dry season, in Cache La Poudre River, in northern Colorado. During the rainy season, whereas concentrations of SAs from human use would be more diluted, runoff from irrigated rural areas may increase the concentrations in freshwater of veterinary SAs, denoting its runoff origin from crop lands after heavy rain periods. For instance, in the study by Kim et al., runoff from irrigated rural areas increased the concentrations in freshwater of sulfamerazine (SMR) and sulfadimethoxine (SDM), veterinary SAs (40 and −60 ng L−1), respectively. Cold conditions can also contribute to higher concentrations due to reduced biodegradation of these contaminants in water. Other studies on the distribution of SAs in surface waters yielded similar outcomes, with higher levels of human SAs (SMX,SPY) detected during the dry periods and higher levels of veterinary SAs, such as SDM, sulfamethazine (SMZ), or SDZ during high flow conditions [37, 64, 65]. In some occasions, the release of untreated wastewaters due to strong rainfall events can also contribute to higher concentrations of human SAs than expected [66]. In Europe, the impact of urban inputs was also demonstrated during two sampling campaigns carried out along the Ebro River Basin (Spain) in 2007–2008 [31]. Samples corresponding to tributaries of the main water course presented the highest total concentration of SAs due to their lower flows and dilution exerted on the effluents loads. In 2008, strong rainfall and subsequent runoff events from agricultural land accounted for the highest total SAs concentrations detected in two sampling points in the Ebro River located upstream of two WWTPs (without urban influence). SMX was again the SA most frequently detected in the different surface water samples investigated, being present in the 100% of the samples during the dryer period (2007), with an average concentration of 89.8 ng L−1, and in the 69% of the samples during a higher waterfall period (2008), with an average concentration of 25.5 ng L−1. SPY was also detected in the 100% of the samples during the dry period, at an average concentration of 11 ng L−1, and in the 62% of the samples during the rainy season, with a lower average concentration of 2.7 ng L−1. Another 16 SAs and one acetylated metabolite were detected at concentrations ranging from 0.1 to 127 ng L−1. SMX was also present in freshwater from the Douro River in Portugal, with a maximum concentration of 53.3 ng L−1 and an occurrence of 33% [67]. SMX was detected in the Seine River in all the samples investigated over a period of 6 months in 2006, with average concentrations between 37 and 140 ng L−1 [66]. In this study, the concentration of SMX seemed to increase after heavy rain episodes, which was attributed to the release of untreated wastewaters and not to surface runoff in agriculture areas, as SMX is mainly used in human medicine. Lower concentrations of SMX were detected by the same author in the Oise River, Marne River, and again Seine River (12–26 ng L−1) [68]. SMX was detected also different sampling sites along the Elbe River in Germany and the Czech Republic during 1999 and 2000 at concentrations in the range of 30–70 ng L−1 [69]. The presence of SAs not only in river water samples but also in their sediments [7, 63, 65, 70–72], despite their low distribution coefficients (K d), highlights the river systems vulnerability against these antimicrobials. Furthermore, the presence of SAs metabolites such as their acetylated or glucuronidated moieties has been already demonstrated and the neglection of these compounds would mean to underestimate the real SAs concentration in the water matrix under study, and also the potential adverse effects derivated from the ecosystems exposure to these substances. For instance, the acetylated form of SMX has been detected in natural streams at higher frequencies and concentrations than its parent molecule [73]. A recent study has also demonstrated that N4-acetyl-SPY is more toxic than its parent compound, SPY, to aquatic bacteria [74].
3 Sulfonamide Presence in the Mediterranean Region: The Case of the Llobregat River Basin
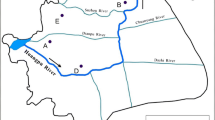
The semiarid conditions present in the Mediterranean region aggravate the adverse ecological effects derived from the presence of SAs and other PPs in natural water courses [75]. The hydrology of streams and rivers of these regions are characterized by high seasonal variability with periods of low or intermittent flow disrupted by acute floods [76, 77]. The increasing population density has resulted in not only a higher water demand for irrigation or human consumption, but also in the intensification of wastewater inputs on the receiving streams, which usually present low natural base flows due to the aforementioned long draught periods. These inputs are among the major stressors of receiving streams and rivers, as they contain an excess of nutrients together with a wide range of emerging contaminants. The Llobregat River is an illustrative example of the hydrological pattern of Mediterranean rivers, with low winter and summer discharges and periodic floods in spring and autumn. It is located in the northeast of Catalonia (Spain) and flows into the Mediterranean Sea south of the city of Barcelona. Along its 156 km, it covers a catchment area of about 4,957 km2, which is densely populated (3,089,465 inhabitants, data from 1999), especially in its middle and lower sections. Together with its two main tributaries, the Cardener River and the Anoia River, the Llobregat is subjected to heavy anthropogenic pressure, receiving extensive industrial and urban discharges from more than 50 WWTPs (137 Hm3 year−1; 92% from WWTPs) [78]. These inputs are only partially diluted by its natural flow (0.68–6.5 m3 s−1 basal flow). Furthermore, 30% of the annual discharge of the river (693 Hm3) is used for drinking water supply, including the city of Barcelona. The average monthly flow registered in 2000–2008 period showed peaks of 100 m3 s−1 together with minimum values of 1 m3 s−1 (www.gencat.cat/aca). The Llobregat has therefore been chosen in several studies as the typical case study of the problematic of a Mediterranean overexploited river. Recently, within the framework of the European project MODELKEY, several works have been devoted to study the presence of emerging contaminants [79–81]. During three sampling campaigns carried out along the Llobregat River and one of its main tributaries, the Anoia River, different types of emerging contaminants, including PhPs and SAs, were monitored [82]. Samples were taken in June and November of 2005 and May of 2006, covering spring and autumn periods (maximum flow periods). In the case of SAs, the highest concentrations were detected in the low course of the river and near its mouth (Fig. 1). Due to the cumulative effect along the basin mentioned in Sect. 2.2, SAs followed a pollution gradient and these high concentrations are due to both frequent WWTP discharges and accumulation. SMX was present at a maximum concentration of 4,297 ng L−1, followed by SMZ at 2,482 ng L−1, and its acetylated metabolite that was present at a concentration of 695 ng L−1. Furthermore, estimated values for SPY, sulfamethoxypyridazine (SMP), SDZ, and SPY were out of the analytical calibration range (>5,000 ng L−1) in the sampling location. These values are over two orders of magnitude above the values obtained in continental rivers (Fig. 2, Table 2) and, as can be observed, correspond to SAs of both veterinary and human use. In a recent work, SAs have been detected in effluents of four different WWTPs along this basin, but their concentrations were never higher than 300 ng L−1 [74]. In the Anoia River, despite its lower flow and dilution factor exerted on the incoming pollutants, concentrations were markedly lower, results that can be explained in terms of the lower number of discharging WWTPs to this tributary in comparison with the Llobregat. Urban inputs play a major role in both the hydrology and the presence of pollutants in this basin, as demonstrated with SAs.
4 Ecotoxicological Effects of Sulfonamides in the Aquatic Environment
There is a substantial lack of ecotoxicological data regarding adverse effects of SAs and their metabolites, which is probably one of the main reasons for the absence of European regulation on maximum levels of this family of antibiotics in any environmental compartment. Nowadays, none of the PhPs detected in surface water are considered in any of the Drinking Water Directives worldwide [1]. Recently, different PhPs such as carbamazepine or diclofenac were considered to be included in the list of priority substances of the new European Directive 2008/105/EC on environmental quality standards, although they were finally withdrawn. Whereas SAs are probably not pharmacologically active in humans at the concentrations detected so far (usually at the ng L−1 level), they might be potential micropollutants to key living organisms in aquatic ecosystems (e.g., fish, aquatic invertebrates and unicellular algae). These different taxonomic groups, belonging to different trophic levels, may be exposed and negatively affected to different extents. For example, severe toxic effects in primary producers may imply loss of the whole food-chain structure, as they represent a significant portion of the total biomass of the ecosystem and are important as a source of carbon for the rest of the aquatic biosphere. Despite the lack of toxicity data available in the literature, it has been demonstrated that generally microalgae are more sensitive than crustaceans and fish to antibacterial agents (e.g., triclosan and ciprofloxacin). However, SAs have proved to hardly pose any toxicity against green algae [83, 84]; estimated inhibitory concentration (IC) values were much higher than those expected in surface waters and SAs have been considered unlikely to be toxic to algae at environmental concentrations. SMX, as one of the most consumed SAs in human medicine and most frequently detected in natural waters, has been the target of different toxicity evaluations. Median effective concentrations (EC50) range from 80 mg L−1 against green algae [85] to values of 0.52 mg L−1 for algae and 0.21 mg L−1 for crustaceans [85], indicating that the risk posed by this substance should not be excluded in real environmental conditions. It has been demonstrated that aquatics plants [86], crustaceans and fish are also vulnerable to SAs; SMX also showed toxicity against rainbow trout (Oncorhynchus mykiss), but at concentrations so high that were not representative of the real situation in freshwaters [87]. Bioaccumulation of SMZ in sturgeon (Acipenser schrenkii) was also demonstrated, but considered of little environmental concern regarding presence in tissues consumed by humans or to biomagnification in fish consumed by fish predators [88]. On the other hand, toxicity and bioaccumulation in marine environment were observed in brine shrimp exposed to SDM, with the potential implications for the rest of the food chain in the marine community [89].
SAs are usually not detected as isolated drugs in the aquatic environment but together with other SAs, and synergistic effects could be expected when residues of different SAs are detected in the same study site [84, 90]. Belonging to the same family of compounds implies similar molecular structure and modes of action, so “concentration addition” is likely. Furthermore, it is necessary to take into account that the degradation products and metabolites of SAs may also be involved in the final toxic effects on the algae, making the interpretation of the toxic data more complex. Recently, EC50 values for Vibrio fischerii were calculated for SPY and its acetylated metabolite; concentrations of 27.4 mg L−1 and of 8.2 mg L−1 for SPY and the metabolite, respectively, after 15 min exposure were reported [46, 74]. According to the EU legislation (Directive 447 93/67/EEC) that categorizes the toxicity to aquatic organism depending on the EC50, SPY would be classified as harmful, and its metabolite as toxic. To the author’s knowledge, the only reference regarding harmful effects of acetylated SAs is that by Eguchi et al. [90], in which the metabolites of SDM, SMX, and SDZ showed much weaker growth inhibitory effects than the corresponding parent SA against microalgae, usually the more sensitive taxa. The simultaneous presence of the corresponding acetylated metabolites enhanced the inhibitory effect of the three SAs, and also the addition of the diaminopirimidine trimethoprim.
4.1 Ecotoxicity of Sulfonamide Intermediate Products
Whether or not SAs are biodegraded in the aquatic environment would settle the very first step for a complete environmental risk assessment (ERA). At the same time, the toxicity of the intermediate by-products of both biotic and abiotic degradation should be taken into account when evaluating the derived ecological risk. SAs undergo photocatalytic degradation [91, 92] and, if the photodegradation products generated are biodegradable, they can be removed during wastewater treatment using biological methods. If these products are persistent or not readily biodegradable, risks of ecotoxicity should be considered. Both inhibitory and stimulatory effects could be expected, as demonstrated by Baran et al. for sulfathiazole (STZ), SMX, SDZ, and sulfachloropyridazine (SCM) against green algae growth [93]. Photoenhanced toxicity under natural sunlight has already been demonstrated for three SAs (SMX, STZ, and SMZ) against crustacean Daphnia magna, suggesting that the photodegradation of the parent compound leads to the formation of more toxic by-products [94]. Also, the by-products of SMX after ozonation treatment were toxic against D. magna and P. subcapicata [95]. In both cases, the assayed concentrations of SAs that were acutely toxic to D. magna were much higher than levels detected in the environment and the ecological risks associated were considered to be limited.
4.2 Bacterial Resistance
So far, environmental research on antibiotics in general has focused mainly on the bacterial resistance acquired against antimicrobials in the different environmental compartments. Nowadays, the widespread presence of resistant bacterial strains has been demonstrated in several scientific works. In river ecosystems, the frequent presence of SMX has led to the detection of SMX-resistant bacteria belonging to Aeromonas spp., typical waterborne bacteria [96]. The Acinetobacter genera were also affected by the presence of this SA [97], and a correlation was established between SMX environmental concentration and occurrence of SMX-resistant bacteria. SAs-resistant genes have been found not only in surface water but also in river sediments [98]. The concentration of these genes was up to 1,200 times higher in sediments, indicating that they can be considered as important antibiotic resistance genes (ARGs) reservoirs. SAs may have qualitative and quantitative effects upon the resident microbial community found in sediment, which can in turn affect the degradation of organic matter. WWTP effluents have been considered as ARG sources in different works too [4, 99, 100].
4.3 Environmental Risk Assessment for Sulfonamides in Surface Waters
As mentioned above, little information is available regarding the ecological effects of SAs and other PhPs, due mainly to the fact that such investigations are not legally required as part of the licensing procedures for human medicaments. The risk assessment guidelines set up by the European Medicines Agency (EMEA) for the marketing authorization of new medicinal products have been used in a few occasions to prioritize the risk from drugs that are already in use and to assess the potential impact of drugs yet to be released [31, 48, 101–106]. Although they are designed as part of the process for registering new drugs, they are used nowadays as the only restrictive measure established so far to evaluate environmental risk from drugs that are already being consumed and that are being excreted in aquatic or terrestrial environments. The ERA protocol is a two-phase tiered process that begins with an approximate calculation of the predicted environmental concentration (PEC) of the drug in water. These guidelines recommend that any drug exceeding 10 ng L−1 in surface water should progress to Phase II, where standard acute toxicity tests will be carried out in order to estimate predicted no-effect concentration (PNEC) or nonobserved effect concentration (NOEC) [107]. Finally, the ratio of the PEC to PNEC, known as the hazard quotient (HQ), indicates whether a potential environmental impact is implicit and further testing might be needed (HQ > 1). It is also recommended that when the total concentration of metabolites is a 10% greater than the concentration of the corresponding parent drug, the metabolites are also to be further investigated (phase II tier B) in order to determine their ecotoxicological effects. The EMEA Committee for Medicinal Products for Veterinary Use also established similar guidelines to assess the potential for veterinary medicines to affect nontarget species in the environment, including both aquatic and terrestrial species [108]. When PNEC values are not available, an alternative PNEC can be derived by dividing EC50 or median lethal concentration (LC50) values (acute toxicity data) by an uncertainty factor of up to 1,000 [109], and so converting acute to chronic toxicity values, since data on chronic toxicity for SAs is lacking. Likewise, measured environmental concentrations (MECs) are used in the calculation instead of PECs. In order to set up a worst case scenario, maximum MECs and the lowest EC50 or LC50 values are used. In all cases, the MECs should be higher than the boundary value of 10 ng L−1 established by EMEA in Tier 1. Table 3 summarizes the HQ values reported to date in the literature. As can be observed, HQs > 1 were detected only for SMX and only for blue green algae. The highest risk corresponded to the exposure to concentrations detected in the Llobregat River, highlighting once more the vulnerability of water courses located in the Mediterranean climate region.
Abbreviations
- AcSMZ:
-
N4-acetylsulfamethazine
- ARGs:
-
Antibiotic resistance genes
- CAFO:
-
Confined animal-feeding operation
- CAS:
-
Conventional activated sludge
- EC50 :
-
Median effective concentration
- EMEA:
-
European medicine agency
- ERA:
-
Environmental risk assessment
- EU:
-
European Union
- FEDESA:
-
European federation of animal health
- HQ:
-
Hazard quotient
- LC50 :
-
Median letal concentration
- MBR:
-
Membrane bioreactor
- ME:
-
Measured environmental concentration
- NOEC:
-
Non observed effect concentration
- PEC:
-
Predicted environmental concentration
- PhP:
-
Pharmaceuticals
- PNEC:
-
Predicted no-effect concentration
- RE%:
-
Removal efficiency
- SA:
-
Sulfonamide
- SDM:
-
Sulfadimethoxine
- SDZ:
-
Sulfadiazine
- SMP:
-
Sulfamethoxypyridazine
- SMR:
-
Sulfamerazine
- SMX:
-
Sulfamethoxazole
- SMZ:
-
Sulfamethazine
- SPY:
-
Sulfapyridine
- STZ:
-
Sulfathiazole
- US:
-
United States
- WFD:
-
Water frame directive
- WHO:
-
World Health Organization
- WWTP:
-
Wastewater treatment plant
References
Ternes T, Joss A (2006) Human pharmaceuticals, hormones and fragrances. The challenge of micropollutants in urban water management. IWA Publishing, London
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (vas) in the environment. Chemosphere 65(5):725–759
Kümmerer K (2004) Pharmaceuticals in the environment – sources, fate, effects and risks. Springer, Berlin, pp 27–44
Costanzo SD, Murby J, Bates J (2005) Ecosystem response to antibiotics entering the aquatic environment. Mar Pollut Bull 51(1–4):218–223
Amin MM, Zilles JL, Greiner J, Charbonneau S, Raskin L, Morgenroth E (2006) Influence of the antibiotic erythromycin on anaerobic treatment of a pharmaceutical wastewater. Environ Sci Technol 40(12):3971–3977
Jones OAH, Voulvoulis N, Lester JN (2002) Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res 36:5013–5022
Löffler D, Römbke J, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water-sediment systems. Environ Sci Technol 39(14):5209–5218
Zuccato E, Calamari D, Natangelo M, Fanelli R (2000) Presence of therapeutic drugs in the environment. Lancet 355(9217):1789–1790
Chafer-Pericas C, Maquieira T, Puchades R, Company B, Miralles J, Moreno A (2010) Multiresidue determination of antibiotics in aquaculture fish samples by HPLC-MS/MS. Aquacult Res 41(9):e217–e225
Hamscher G, Priess B, Nau H (2006) A survey of the occurrence of various sulfonamides and tetracyclines in water and sediment samples originating from aquaculture systems in northern Germany in summer 2005. Untersuchung von teichwässern und -sedimenten in Niedersächsischen aquakulturen im sommer 2005 auf sulfonamide und tetracycline 57(4):97–101
Pérez-Trallero E, Iglesias L (2003) Tetracyclines, sulfonamides and metronidazole. Tetraciclinas, sulfamidas y metronidazol. Enferm Infec Micr Cl 21(9):520–529+533
UE (2003) Regulation 1831/2003/ec on additives for use in animal nutrition
Parfitt KE (1999) Martindale–the complete drug reference, 32nd edn. Pharmaceutical Press, London
Halling-Sorensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lutzhoft HC, Jorgensen SE (1998) Occurrence, fate and effects of pharmaceutical substances in the environment – a review. Chemosphere 36(2):357–393
Kwon JW (2011) Mobility of veterinary drugs in soil with application of manure compost. Bull Environ Contam Toxicol 87(1):40–44
Motoyama M, Nakagawa S, Tanoue R, Sato Y, Nomiyama K, Shinohara R (2011) Residues of pharmaceutical products in recycled organic manure produced from sewage sludge and solid waste from livestock and relationship to their fermentation level. Chemosphere 84(4):432–438
Pan X, Qiang Z, Ben W, Chen M (2011) Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in shandong province, china. Chemosphere 84(5):695–700
Hamscher G, Pawelzick HT, Hoper H, Nau H (2005) Different behavior of tetracyclines and sulfonamides in sandy soils after repeated fertilization with liquid manure. Environ Toxicol Chem 24(4):861–868
Boxall ABA, Kolpin DW, Halling-Sorensen B, Tolls J (2003) Are veterinary medicines causing environmental risks? Environ Sci Technol 37(15):286A–294A
Schauss K, Focks A, Heuer H, Kotzerke A, Schmitt H, Thiele-Bruhn S, Smalla K, Wilke BM, Matthies M, Amelung W, Klasmeier J, Schloter M (2009) Analysis, fate and effects of the antibiotic sulfadiazine in soil ecosystems. Trends Anal Chem 28(5):612–618
Kotzerke A, Sharma S, Schauss K, Heuer H, Thiele-Bruhn S, Smalla K, Wilke BM, Schloter M (2008) Alterations in soil microbial activity and n-transformation processes due to sulfadiazine loads in pig-manure. Environ Pollut 153(2):315–322
Diaz-Cruz MS, Garcia-Galan MJ, Barcelo D (2008) Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J Chromatogr A 1193(1–2):50–59
Batt AL, Snow DD, Aga DS (2006) Occurrence of sulfonamide antimicrobials in private water wells in washington county, Idaho, USA. Chemosphere 64(11):1963–1971
Lindsey ME, Meyer M, Thurman EM (2001) Analysis of trace levels of sulfonamide and tetracycline antimicrobials, in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal Chem 73(19):4640–4646
Sacher F, Lang FT, Brauch HJ, Blankenhorn I (2001) Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-wurttemberg, Germany. J Chromatogr A 938(1–2):199–210
Blackwell PA, Lutzhoft HCH, Ma HP, Halling-Sorensen B, Boxall ABA, Kay P (2004) Fast and robust simultaneous determination of three veterinary antibiotics in groundwater and surface water using a tandem solid-phase extraction with high-performance liquid chromatography-UV detection. J Chromatogr A 1045(1–2):111–117
Diaz-Cruz MS, Barcelo D (2006) Determination of antimicrobial residues and metabolites in the aquatic environment by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 386(4):973–985
Karthikeyan KG, Meyer MT (2006) Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci Total Environ 361(1–3):196–207
Batt AL, Aga DS (2005) Simultaneous analysis of multiple classes of antibiotics by ion trap LC/MS/MS for assessing surface water and groundwater contamination. Anal Chem 77(9):2940–2947
Watanabe N, Bergamaschi BA, Loftin KA, Meyer MT, Harter T (2010) Use and environmental occurrence of antibiotics in freestall dairy farms with manured forage fields. Environ Sci Technol 44(17):6591–6600
García-Galán MJ, Díaz-Cruz MS, Barceló D (2011) Occurrence of sulfonamide residues along the ebro river basin. Removal in wastewater treatment plants and environmental impact assessment. Environ Int 37(2):462–473
Gobel A, McArdell CS, Joss A, Siegrist H, Giger W (2007) Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci Total Environ 372(2–3):361–371
Gros M, Petrović M, Barceló D (2007) Wastewater treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro river basin (Northeast Spain). Environ Toxicol Chem 26(8):1553–1562
Ye S, Yao Z, Na G, Wang J, Ma D (2007) Rapid simultaneous determination of 14 sulfonamides in wastewater by liquid chromatography tandem mass spectrometry. J Sep Sci 30(15):2360–2369
Topp E, Monteiro SC, Beck A, Coelho BB, Boxall ABA, Duenk PW, Kleywegt S, Lapen DR, Payne M, Sabourin L, Li H, Metcalfe CD (2008) Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci Total Environ 396(1):52–59
Miao XS, Bishay F, Chen M, Metcalfe CD (2004) Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol 38(13):3533–3541
Lin AYC, Tsai YT (2009) Occurrence of pharmaceuticals in Taiwan's surface waters: impact of waste streams from hospitals and pharmaceutical production facilities. Sci Total Environ 407(12):3793–3802
Chang XS, Meyer MT, Liu XY, Zhao Q, Chen H, Chen JA, Qiu ZQ, Yang L, Cao J, Shu WQ (2010) Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of three gorge reservoir in China. Environ Pollut 158(5):1444–1450
Ahel M, Mikac N, Cosovic B, Prohic E, Soukup V (1998) The impact of contamination from a municipal solid waste landfill (Zagreb, Croatia) on underlying soil. Water Sci Technol 37(8):203–210
Schwarzbauer J, Heim S, Brinker S, Littke R (2002) Occurrence and alteration of organic contaminants in seepage and leakage water from a waste deposit landfill. Water Res 36(9):2275–2287
Bound JP, Voulvoulis N (2005) Household disposal of pharmaceuticals as a pathway for aquatic contamination in the united kingdom. Environ Health Perspect 113(12):1705–1711
Samuelsen OB, Lunestad BT (1996) Bath treatment, an alternative method for the administration of the quinolones flumequine and oxolinic acid to halibut Hippoglossus hippoglossus, and in vitro antibacterial activity of the drugs against some Vibrio sp. Dis Aquat Organ 27(1):13–18
Le-Minh N, Khan SJ, Drewes JE, Stuetz RM (2010) Fate of antibiotics during municipal water recycling treatment processes. Water Res 44(15):4295–4323
Garoma T, Umamaheshwar SK, Mumper A (2010) Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 79(8):814–820
Lin AY-C, Lin C-F, Chiou J-M, Hong PKA (2009) O3 and O3/H2O2 treatment of sulfonamide and macrolide antibiotics in wastewater. J Hazard Mater 171(1–3):452–458
García Galán MJ, Frömel T, Müller J,Peschka M, Knepper T, Díaz Cruz S, Barceló D (2011) Biodegradation studies of N4-acetylsulfapyridine and N4-acetylsulfamethazine in environmental water applying mass spectrometry techniques. Anal Bioanal Chem 402(9):2885–2896
Gobel A, McArdell CS, Suter MJF, Giger W (2004) Trace determination of macrolide and sulfonamide antimicrobials, a human sulfonamide metabolite, and trimethoprim in wastewater using liquid chromatography coupled to electrospray tandem mass spectrometry. Anal Chem 76(16):4756–4764
Gros M, Petrovic M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36(1):15–26
Tambosi JL, de Sena RF, Favier M, Gebhardt W, Jose HJ, Schroder HF, Moreira R (2010) Removal of pharmaceutical compounds in membrane bioreactors (MBR) applying submerged membranes. Desalination 261(1–2):148–156
Tambosi JL, de Sena RF, Gebhardt W, Moreira R, Jose HJ, Schroder HF (2009) Physicochemical and advanced oxidation processes – a comparison of elimination results of antibiotic compounds following an MBR treatment. Ozone Sci Eng 31(6):428–435
García Galán MJ, Díaz-Cruz, M.S., Barceló, D. (2011) Removal of sulfonamide antibiotics upon conventional activated sludge and advance membrane bioreactors treatment. J Hazard Mat (accepted)
Radjenovic J, Petrovic M, Barceló D (2007) Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal Bioanal Chem 387(4):1365–1377
Dodd MC, Huang CH (2004) Transformation of the antibacterial agent sulfamethoxazole in reactions with chlorine: kinetics, mechanisms, and pathways. Environ Sci Technol 38(21):5607–5615
Huber MM, Canonica S, Park GY, Von Gunten U (2003) Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ Sci Technol 37(5):1016–1024
Nakada N, Shinohara H, Murata A, Kiri K, Managaki S, Sato N, Takada H (2007) Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res 41(19):4373–4382
Kosutic K, Dolar D, Asperger D, Kunst B (2007) Removal of antibiotics from a model wastewater by RO/NF membranes. Sep Purif Technol 53(3):244–249
Zwiener C (2007) Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal Bioanal Chem 387(4):1159–1162
Watts CD, Crathorne B, Fielding M, Killops SD (1982) Nonvolatile organic compounds in treated waters. Environ Health Perspect 46:87–89
Feitosa-Felizzola J, Chiron S (2009) Occurrence and distribution of selected antibiotics in a small mediterranean stream (Arc river, Southern France). J Hydrol 364(1–2):50–57
Garcia-Galan MJ, Diaz-Cruz MS, Barcelo D (2008) Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trends Anal Chem 27(11):1008–1022
Thiele-Bruhn S, Aust MO (2004) Effects of pig slurry on the sorption of sulfonamide antibiotics in soil. Arch Environ Contam Toxicol 47(1):31–39
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US Streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36(6):1202–1211
Kim SC, Carlson K (2007) Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ Sci Technol 41(1):50–57
Kolpin DW, Skopec M, Meyer MT, Furlong ET, Zaugg SD (2004) Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci Total Environ 328(1–3):119–130
Zheng S, Qiu X, Chen B, Yu X, Liu Z, Zhong G, Li H, Chen M, Sun G, Huang H, Yu W, Freestone D (2011) Antibiotics pollution in Jiulong river estuary: source, distribution and bacterial resistance. Chemosphere 84(11):1677–1685
Tamtam F, Mercier F, Le Bot B, Eurin J, Tuc Dinh Q, Clement M, Chevreuil M (2008) Occurrence and fate of antibiotics in the Seine river in various hydrological conditions. Sci Total Environ 393(1):84–95
Madureira TV, Barreiro JC, Rocha MJ, Rocha E, Cass QB, Tiritan ME (2010) Spatiotemporal distribution of pharmaceuticals in the Douro river estuary (Portugal). Sci Total Environ 408(22):5513–5520
Tamtam F, Mercier F, Eurin J, Chevreuil M, Le Bot B (2009) Ultra performance liquid chromatography tandem mass spectrometry performance evaluation for analysis of antibiotics in natural waters. Anal Bioanal Chem 393(6–7):1709–1718
Wiegel S, Aulinger A, Brockmeyer R, Harms H, Löffler J, Reincke H, Schmidt R, Stachel B, von Tümpling W, Wanke A (2004) Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 57(2):107–126
Tamtam F, Le Bot B, Dinh T, Mompelat S, Eurin J, Chevreuil M, Bonté P, Mouchel JM, Ayrault S (2011) A 50-year record of quinolone and sulfonamide antimicrobial agents in Seine river sediments. J Soils Sediments 11:852–859
Cai-Ming T, Qiu-Xin H, Yi-Yi Y, Xian-Zhi P (2009) Multiresidue determination of sulfonamides, macrolides, trimethoprim, and chloramphenicol in sewage sludge and sediment using ultrasonic extraction coupled with solid phase extraction and liquid chromatography-tandem mass spectrometry. Fenxi Huaxue/Chinese J Anal Chem 37(8):1119–1124
Yang JF, Ying GG, Zhao JL, Tao R, Su HC, Chen F (2010) Simultaneous determination of four classes of antibiotics in sediments of the Pearl river using RRLC-MS/MS. Sci Total Environ 408(16):3424–3432
Ashton D, Hilton M, Thomas KV (2004) Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci Total Environ 333(1–3):167–184
García Galán MJ, Díaz-Cruz MS, Barceló D (2011) Removal of selected sulfonamides and its metabolites during conventional activated sludge treatment. Evaluation of the potential environmental impact of wastewater effluents on the receiving ecosystems. Water Res (submitted)
Petrovic M, Postigo C, Lopez de Alda M, Ginebreda A, Gros M, Radjenovic J, Barcelo D (2010) Occurrence and fate of pharmaceuticals and illicit drugs under water scarcity. In: Sabater S, Barcelo D (eds) Water scarcity in the Mediterranean: perspectives under global change, vol 8, Handbook of environmental chemistry. Springer, Berlin, pp 197–228
Gasith A, Resh VH (1999) Streams in mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annu Rev Eco Sys 30:51–81
Marti E, Aumatell J, Godé L, Poch M, Sabater F (2004) Nutrient retention efficiency in streams receiving inputs from wastewater treatment plants. J Environ Qual 33(1):285–293
Muñoz I, Lopez-Doval JC, Ricart M, Villagrasa M, Brix R, Geiszinger A, Ginebreda A, Guasch H, Lopez De Alda MJ, Romani AM, Sabater S, Barcelo D (2009) Bridging levels of pharmaceuticals in river water with biological community structure in the Llobregat river basin (northeast Spain). Environ Toxicol Chem 28(12):2706–2714
Köck-Schulmeyer M, Ginebreda A, Postigo C, López-Serna R, Pérez S, Brix R, Llorca M, Alda MLD, Petrovic M, Munné A, Tirapu L, Barceló D (2011) Wastewater reuse in mediterranean semi-arid areas: the impact of discharges of tertiary treated sewage on the load of polar micro pollutants in the Llobregat river (NE Spain). Chemosphere 82(5):670–678
Kuster M, López de Alda MJ, Hernando MD, Petrovic M, Martín-Alonso J, Barceló D (2008) Analysis and occurrence of pharmaceuticals, estrogens, progestogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). J Hydrol 358(1–2):112–123
López-Roldán R, de Alda ML, Gros M, Petrovic M, Martín-Alonso J, Barceló D (2010) Advanced monitoring of pharmaceuticals and estrogens in the Llobregat river basin (Spain) by liquid chromatography-triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography-time of flight-mass spectrometry. Chemosphere 80(11):1337–1344
Garcia-Galan MJ, Villagrasa M, Diaz-Cruz MS, Barcelo D (2010) LC-QqLIT MS analysis of nine sulfonamides and one of their acetylated metabolites in the Llobregat river basin. Quantitative determination and qualitative evaluation by ida experiments. Anal Bioanal Chem 397(3):1325–1334
Pro J, Ortiz JA, Boleas S, Fernández C, Carbonell G, Tarazona JV (2003) Effect assessment of antimicrobial pharmaceuticals on the aquatic plant lemna minor. Bull Environ Contam Toxicol 70(2):290–295
Yang LH, Ying GG, Su HC, Stauber JL, Adams MS, Binet MT (2008) Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ Toxicol Chem 27(5):1201–1208
Isidori M, Lavorgna M, Nardelli A, Pascarella L, Parrella A (2005) Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci Total Environ 346(1–3):87–98
Brain RA, Ramirez AJ, Fulton BA, Chambliss CK, Brooks BW (2008) Herbicidal effects of sulfamethoxazole in lemna gibba: using p-aminobenzoic acid as a biomarker of effect. Environ Sci Technol 42(23):8965–8970
Laville N, Ait-Aissa S, Gomez E, Casellas C, Porcher JM (2004) Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 196(1–2):41–55
Hou XL, Shen JZ, Zhang SX, Jiang HY, Coats JR (2003) Bioconcentration and elimination of sulfamethazine and its main metabolite in sturgeon (Acipenser schrenkii). J Agric Food Chem 51(26):7725–7729
Migliore L, Brambilla G, Grassitellis A, Dojmi di Delupis G (1993) Toxicity and bioaccumulation of sulphadimethoxine in artemia (crustacea, anostraca). Int J Salt Lake Res 2(2):141–152
Eguchi K, Nagase H, Ozawa M, Endoh YS, Goto K, Hirata K, Miyamoto K, Yoshimura H (2004) Evaluation of antimicrobial agents for veterinary use in the ecotoxicity test using microalgae. Chemosphere 57(11):1733–1738
Andreozzi R, Raffaele M, Nicklas P (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50(10):1319–1330
Boreen AL, Arnold WA, McNeill K (2004) Photochemical fate of sulfa drugs in the aquatic environment: sulfa drugs containing five-membered heterocyclic groups. Environ Sci Technol 38(14):3933–3940
Baran W, Sochacka J, Wardas W (2006) Toxicity and biodegradability of sulfonamides and products of their photocatalytic degradation in aqueous solutions. Chemosphere 65(8):1295–1299
Jung JYKY, Kim JK, Jung D-H, Choi K (2008) Environmental levels of ultraviolet light potentiate the toxicity of sulfonamide antibiotics in Daphnia magna. Ecotoxicology 17:37–45
MdM G-R, Mezcua M, Agüera A, Fernández-Alba AR, Gonzalo S, Rodríguez A, Rosal R (2011) Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution. J Hazard Mater 192(1):18–25
Hoa PTP, Managaki S, Nakada N, Takada H, Shimizu A, Anh DH, Viet PH, Suzuki S (2011) Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci Total Environ 409(15):2894–2901
Goñi-Urriza M, Pineau L, Capdepuy M, Roques C, Caumette P, Quentin C (2000) Antimicrobial resistance of mesophilic Aeromonas spp. Isolated from two european rivers. J Antimicrob Chemother 46(2):297–301
Luo Y, Mao D, Rysz M, Zhou Q, Zhang H, Xu L, Alvarez PJJ (2010) Trends in antibiotic resistance genes occurrence in the Haihe river, China. Environ Sci Technol 44(19):7220–7225
Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A (2010) Tracking antibiotic resistance genes in the south platte river basin using molecular signatures of urban, agricultural, and pristine sources. Environ Sci Technol 44(19):7397–7404
Storteboom H, Arabi M, Davis JG, Crimi B, Pruden A (2010) Identification of antibiotic-resistance-gene molecular signatures suitable as tracers of pristine river, urban, and agricultural sources. Environ Sci Technol 44(6):1947–1953
Ferrari B, Mons R, Vollat B, Fraysse B, Paxeaus N, Lo Giudice R, Pollio A, Garric J (2004) Environmental risk assessment of six human pharmaceuticals: are the current environmental risk assessment procedures sufficient for the protection of the aquatic environment? Environ Toxicol Chem 23(5):1344–1354
Ginebreda A, Muñoz I, de Alda ML, Brix R, López-Doval J, Barceló D (2010) Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the llobregat river (NE Spain). Environ Int 36(2):153–162
Grung M, Källqvist T, Sakshaug S, Skurtveit S, Thomas KV (2008) Environmental assessment of Norwegian priority pharmaceuticals based on the EMEA guideline. Ecotoxicol Environ Saf 71(2):328–340
Huschek G, Hansen PD, Maurer HH, Krengel D, Kayser A (2004) Environmental risk assesssment of medicinal products for human use according to European Commission recommendations. Environ Toxicol 19(3):226–240
Park S, Choi K (2008) Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 17(6):526–538
Santos JL, Aparicio I, Alonso E (2007) Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ Int 33(4):596–601
EMEA (CHMP) (2006) Guideline on the environmental risk assessment of medicinal products for human use
EMEA (CVMP) (2004) Guideline on environmental impact assessment for veterinary medicinal products phase II
Sanderson H, Johnson DJ, Wilson CJ, Brain RA, Solomon KR (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144(3):383–395
Acknowledgments
This work has been funded by the Spanish Ministry of Science and Innovation through the projects CEMAGUA (CGL2007-64551/HID) and SCARCE (Consolider Ingenio 2010 CSD2009-00065). MJ García acknowledges AGAUR (Generalitat de Catalunya, Spain) for economic support through an FI pre-doctoral grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
García-Galán, M.J., Díaz-Cruz, M.S., Barceló, D. (2012). Occurrence and Fate of Sulfonamide Antibiotics in Surface Waters: Climatic Effects on Their Presence in the Mediterranean Region and Aquatic Ecosystem Vulnerability. In: Sabater, S., Ginebreda, A., Barceló, D. (eds) The Llobregat. The Handbook of Environmental Chemistry, vol 21. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2011_140
Download citation
DOI: https://doi.org/10.1007/698_2011_140
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30938-0
Online ISBN: 978-3-642-30939-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)