Abstract
Drinking water disinfection by-products (DBPs) are an unintended consequence of using chemical disinfectants to kill harmful pathogens in water. DBPs are formed by the reaction of disinfectants with naturally occurring organic matter, bromide, and iodide, as well as from anthropogenic pollutants. Potential health risks of DBPs from drinking water include bladder cancer, early-term miscarriage, and birth defects. Risks from swimming pool DBP exposures include asthma and other respiratory effects. Several DBPs, such as trihalomethanes (THMs), haloacetic acids (HAAs), bromide, and chlorite, are regulated in the U.S. and in other countries, but other “emerging” DBPs, such as iodo-acids, halonitromethanes, haloamides, halofuranones, and nitrosamines, are not widely regulated. DBPs have been reported for the four major disinfectants: chlorine, chloramines, ozone, and chlorine dioxide (and their combinations), as well as for newer disinfectants, such as UV treatment with post-chlorination. Each disinfectant can produce its own suite of by-products. Several classes of emerging DBPs are increased in formation with the use of alternative disinfectants (e.g., chloramines), including nitrogen-containing DBPs (“N-DBPs”), which are generally more genotoxic and cytotoxic than those without nitrogen. Humans are exposed to DBPs not only through ingestion (the common route studied), but also through other routes, including bathing, showering, and swimming. Inhalation and dermal exposures are now being recognized as important contributors to the overall human health risk of DBPs. Analytical methods continue to be developed to measure known DBPs, and research continues to uncover new highly polar and high-molecular-weight DBPs that are part of the missing fraction of DBPs not yet accounted for. New studies are now combining toxicology and chemistry to better understand the health risks of DBPs and uncover which are responsible for the human health effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chloramination
- Chlorination
- Chlorine dioxide
- DBPs
- Disinfection by-products
- Drinking water
- Occurrence
- Ozonation
- Swimming pools
- Toxicity
1 Introduction
The disinfection of drinking water has been rightly hailed as a public health triumph of the twentieth century. Before its widespread use, millions of people died from waterborne diseases. Now, people in developed nations receive quality drinking water every day from their public water systems. However, chemical disinfection has also produced an unintended health hazard: the potential for cancer and reproductive and developmental effects (including early-term miscarriages and birth defects) that are associated with chemical disinfection by-products (DBPs) [1–6]. Research is being conducted worldwide to solve these important human health issues.
Chemical disinfectants, such as chlorine, ozone, chloramines, and chlorine dioxide, are used to kill harmful pathogens in drinking water, and produce safe, potable water. However, these disinfectants are also powerful oxidants, and can chemically react with the naturally occurring organic matter (NOM), mostly present from decaying leaves and other plant matter, and also with bromide and iodide salts naturally present in some source waters (rivers, lakes, and groundwaters). NOM, which is mostly comprised of humic and fulvic acids, serves as the primary precursor to DBP formation. Anthropogenic contaminants can also react with disinfectants to form contaminant DBPs. These contaminants mostly enter drinking water sources from treated wastewater, but can also enter from other sources, such as agricultural run-off. Contaminant DBPs have been reported from pharmaceuticals, antibacterial agents, estrogens, pesticides, textile dyes, bisphenol A, alkylphenol surfactants, UV filters (used in sunscreens), and diesel fuel [1]. Many times, the contaminants react directly with the disinfectants, but sometimes, it is an environmental degradation product of these initial contaminants that react to form DBPs.
Chlorine, ozone, chlorine dioxide, and chloramines are the most common chemical disinfectants in use today, and each produces its own suite of DBPs in drinking water. Two nonchemical means of disinfecting drinking water – UV light and reverse osmosis (RO) membranes – are also gaining in popularity for disinfecting water, and these technologies may hold promise in reducing levels of DBPs formed in drinking water. However, these other nonchemical disinfectants may not be without drawbacks. For example, there is some early evidence that medium-pressure UV can react with NOM to form higher levels of some DBPs after post-treatment with chlorine [7]. And, the use of RO has issues with disposal of the resulting brines left over from treatment. RO is increasingly being used at desalination plants that convert seawater into potable drinking water. Iodine point-of-use treatments were also recently investigated for formation of iodo-DBPs [8]. These point-of-use treatments are used by the military in remote locations (iodine tincture), by campers and hikers (iodine tablets), and for rural consumers in developing countries (e.g., the new Lifestraw, a reusable device that uses an iodinated anion exchange resin material with activated carbon post-treatment).
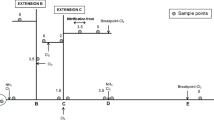
Over the last 30 years, significant research efforts have been directed toward increasing our understanding of DBP formation, occurrence, and health effects. More than 600 DBPs have now been reported in the scientific literature [9, 10]. Examples of DBP chemical classes are shown in Table 1. However, only less than 100 have been addressed either in quantitative occurrence or toxicity studies. The DBPs that have been quantified in drinking water range from parts-per-trillion (ng/L) to parts-per-billion (μg/L) levels. However, more than 50% of the halogenated DBP material (containing chlorine, bromine, or iodine) formed during the chlorination of drinking water (Fig. 1), and more than 50% of the DBPs formed during ozonation of drinking water are still not accounted for [11, 13], and nothing is known about the potential toxicity of many of the DBPs present in drinking water. Much of the previous health effects research has focused on cancer, genotoxicity, mutagenicity, or cytotoxicity. There are concerns that the types of cancer observed in animal studies (primarily liver cancer) for the regulated DBPs do not correlate with the types observed in human epidemiology studies (primarily bladder cancer). It is possible that emerging, unregulated DBPs may be responsible. It is also possible that ingestion (the primary route included in animal studies) is not the only important route of exposure.
This chapter will provide an overview of regulated and emerging, unregulated DBPs, including discussion of their occurrence and formation from different disinfectants and their toxicity. Discussions will include classical DBPs formed by reactions of disinfectants with NOM and contaminant DBPs formed by reaction of disinfectants with anthropogenic contaminants. Analytical methods used in the discovery of new DBPs and for the measurement of known DBPs will also be discussed, as well as new research investigating other routes of exposure beyond ingestion.
2 Regulated DBPs
Chloroform and other trihalomethanes (THMs) were the first DBPs identified in chlorinated drinking water in 1974 [14, 15]. Soon after their discovery, the THMs were found to cause cancer in laboratory animals [16]. As a result, they became regulated in the U.S. in 1979 [17], and later in several other countries. A few additional DBPs are now regulated in the U.S., including five haloacetic acids (HAAs), chlorite, and bromate (Fig. 2). The regulated THMs are sometimes referred to as THM4, regulated HAAs as HAA5, and the nine commonly occurring chloro-bromo-HAAs as HAA9. THMs and HAAs are formed primarily by chlorine and chloramines; chlorite is a DBP from chlorine dioxide, and bromate is mostly from ozonation. Table 2 lists the DBPs currently regulated in the U.S. and Europe, as well the current World Health Organization (WHO) guidelines.
Of the four major disinfectants used today, chlorine generally produces the highest levels of THMs and HAAs. Because drinking water-treatment plants can have difficulty in meeting the regulatory limits, many plants have changed their disinfection practices. Often, the primary disinfectant is changed from chlorine to “alternative” disinfectants, including ozone, chlorine dioxide, chloramines, or UV. In some cases, chlorine is used as a secondary disinfectant following primary treatment with an alternative disinfectant, particularly for ozone, chlorine dioxide, and UV to maintain a disinfectant residual in the water distribution system. However, new issues and problems can result with changes in disinfection practices. For example, the use of ozone can significantly reduce or eliminate the formation of THMs and HAAs, but it can result in the formation of bromate, especially when elevated levels of bromide salts are present in the source waters. Bromide (and iodide) salts can be present in source waters (e.g., rivers) near coastal areas, due to salt water intrusion into the water supplies, and also in inland locations, due to “fossilized seawater,” where salts from ancient seas impact surface water or groundwater. Bromate is a concern because it causes cancer in laboratory animals [18]. Several other DBPs, including nitrosamines, iodo-acids, iodo-THMs, and bromonitromethanes, can also be increased in formation with the use of alternative disinfectants. They will be discussed in detail in later sections on emerging DBPs. Differences in source water conditions, including concentrations of bromide or iodide salts, concentrations of NOM, and pH, can have a dramatic effect on the formation of various DBPs (chlorine-, bromine-, or iodine-containing) and the levels formed.
3 Emerging DBPs
3.1 Overview
Emerging DBPs beyond those that are currently regulated are becoming important. In general, brominated DBPs are now being recognized as toxicologically important because there is indication that brominated DBPs may be more carcinogenic than their chlorinated analogs, and new studies are indicating that iodinated compounds may be more toxic than their brominated analogs [19–21]. Brominated and iodinated DBPs form due to the reaction of the disinfectant (such as chlorine) with natural bromide or iodide present in source waters. Coastal cities, whose groundwaters and surface waters can be impacted by salt water intrusion, and some inland locations, whose surface waters can be impacted by natural salt deposits from ancient seas or oil-field brines, are examples of locations that can have high bromide and iodide levels. A significant proportion of the U.S. population and several other countries now live in coastal regions that are impacted by bromide and iodide; therefore, exposures to brominated and iodinated DBPs can be important. Early evidence in epidemiologic studies also gives indication that brominated DBPs may be associated with the new reproductive and developmental effects [3, 4], as well as cancer effects.
Specific DBPs that are of current interest include iodo-acids, bromonitromethanes, iodo-THMs, halooamides, halofuranones, halopyrroles, haloquinones, haloaldehydes, halonitriles, and nitrosamines. Many of these were predicted to be carcinogens [22] and were the subject of a nationwide occurrence study in the U.S., which reported the most extensive quantitative occurrence of priority, unregulated DBPs [11, 13]. In addition, many of these are nitrogen-containing DBPs (the so-called “N-DBPs”), which have recently been shown to be more genotoxic and cytotoxic than those without nitrogen [19]. N-DBPs can be increased in formation through the use of chloramination, and new research also indicates that algae and amino acids can serve as precursors in their formation [23–25].
3.2 Iodo-Acids and Iodo-THMs
Iodo-acids are a new and potentially toxicologically significant class of DBP identified as part of the U.S. Nationwide Occurrence Study [11, 12, 20] and quantified in a recent 23-city occurrence study in the U.S. [21]. Five iodo-acids have been identified in finished drinking water: iodoacetic acid, bromoiodoacetic acid, (Z)-3-bromo-3-iodopropenoic acid, (E)-3-bromo-3-iodopropenoic acid, and (E)-2-iodo-3-methylbutenedioic acid [13]. Iodo-acids, including diiodoacetic acid, were also recently found in waters treated with iodine [8]. They were initially discovered in chloraminated drinking water, and have been found up to 1.7 μg/L individually [21].
Iodoacetic acid is the most genotoxic DBP studied to-date in mammalian cells, approximately 2x more genotoxic than bromoacetic acid [20], which is regulated in drinking water, but rarely detected. The rank order for genotoxicity monohaloacetic acids follows: iodo- > bromo- ≫ chloroacetic acid [20]. New research is revealing a potential mechanism for this rank order. Monohalogenated acids have been found to inhibit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity in a concentration-dependent manner with the same rank order, and the rate of inhibition and toxic potency were highly correlated with their alkylating potential and their propensity of the halogen leaving group. Other iodo-acids have also been recently shown to be genotoxic, and iodo-acids are also highly cytotoxic in mammalian cells [21]. The rank order for genotoxicity is iodoacetic acid ≫ diiodoacetic acid > bromoiodoacetic acid > (E)-2-iodo-3-methylbutenedioic acid > (E)-3-bromo-3-iodopropenoic acid > (E)-3-bromo-2-iodopropenoic acid. Iodoacetic acid is also teratogenic, producing developmental effects (neural tube closures) in mouse embryos, at levels (nM) similar to levels that induce DNA damage in mammalian cells [26, 27].
Iodo-THMs have been known as DBPs since the mid-1970s [28] and dichloroiodomethane was even referred to as the “5th trihalomethane” (after the original four regulated THMs) [29]. They have since been measured in drinking waters treated with chlorination or chloramination [11, 21, 30, 31], with highest levels observed in chloraminated water (up to 15 μg/L individually). In chloraminated drinking water, iodo-THMs can be formed at levels comparable to the regulated THMs (THM4). In the U.S. Nationwide Occurrence Study, one location showed iodo-THMs at 81% of the THM4 levels in a chloraminated drinking water [11]. Iodo-THMs identified and measured include dichloroiodomethane, bromochloroiodomethane, dibromoiodomethane, chlorodiiodomethane, bromodiiodomethane, and iodoform. Point-of-use treatment with iodine was also recently shown to produce iodo-THMs, with highest levels observed in iodine tincture treatment [8].
Until recently, their major concern had to do with taste and odor problems in drinking water (due to a low threshold concentration of medicinal tastes and odors in drinking water – as low as 0.02–5 μg/L) [30]. It was not until 2008 that they were investigated for genotoxicity and cytotoxicity [21]. One iodo-THM (chlorodiiodomethane) is highly genotoxic in mammalian cells, and all six iodo-THMs are cytotoxic [21]. With the exception of iodoform, the iodo-THMs are less cytotoxic than the iodo-acids.
Iodo-DBPs are of concern not only for their potential health risks, but also because research indicates that they are formed at increased levels (along with iodo-THMs) in waters treated with chloramines. Chloramination has become a popular alternative to chlorination for water-treatment systems that have difficulty meeting the regulations with chlorine, and also for treatment plants with long distribution systems because chloramines can provide a more stable residual than chlorine. Chloramines are generated from the reaction of chlorine with ammonia, and it appears that the length of free chlorine contact time (before ammonia addition to form chloramines) is an important factor in the formation of iodo-DBPs [21].
Scheme 1 illustrates the reactions involved in the formation of iodo-DBPs from chloramination vs. chlorination and helps to explain their increased formation with chloramination. Analogous to the formation of brominated DBPs from naturally occurring bromide, iodo-DBPs can be formed by the reaction of disinfectants with naturally occurring iodide and NOM. With chlorine, reactions to form iodate are much faster than reactions to form other iodo-DBPs, but the corresponding reactions with monochloramine (to form iodite and iodate) are much slower, such that iodo-DBPs increase in formation [32, 33]. Because of chlorine’s competing reaction to form iodate as a sink for the natural iodide, it is likely that treatment with significant free chlorine contact time before the addition of ammonia will not produce substantial levels of iodo-acids or iodo-THMs [21, 32, 33]. New research has also revealed that anthropogenic contaminants (i.e., compounds used for medical imaging) can also be a source of iodine in the formation of iodo-DBPs [34]. This new work will be discussed in detail later in the section on Contaminant DBPs.
3.3 Halonitromethanes
Just as there are nine possible chloro-bromo haloacetic acids (HAA9) that can form in drinking water, nine halonitromethanes can be formed. Chloropicrin (trichloronitromethane) has been the most commonly measured example in this class, but has not been a concern for toxicity in drinking water. Bromonitromethanes, however, have shown significant toxicity [35] and have been found in drinking water, particularly that treated with preozonation [11, 12, 35–37]. Bromonitromethanes are more cytotoxic and genotoxic than most DBPs currently regulated in drinking water [35]. Dibromonitromethane is more than an order of magnitude more genotoxic to mammalian cells than MX (3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone, a carcinogenic DBP), and is more genotoxic than all of the regulated DBPs, except for monobromoacetic acid. Other brominated forms are also potent in this assay. Halonitromethanes are also mutagenic in the Salmonella bacterial cell assay [38], with mutagenic potencies greater than that of the regulated THMs [39]. The halonitromethanes were also at least 10x more cytotoxic than the THMs, and the greater cytotoxic and mutagenic activities of the halonitromethanes was indicated to be likely due to the greater intrinsic reactivity conferred by the nitro group [39].
Bromonitromethanes are substantially increased in formation with the use of pre-ozonation before chlorine or chloramine treatment, and concentrations up to 3 μg/L individually have been reported [11, 12]. Laboratory-scale formation studies indicate that nitrite may play a role in the formation of the nitro group in these DBPs [40]. Tribromonitromethane (bromopicrin) and other trihalonitromethanes (which include bromodichloro- and chlorodibromonitromethane) require particular analytical conditions for their analysis. These compounds are thermally unstable and decompose under commonly used injection port temperatures during gas chromatography (GC) or GC/mass spectrometry (MS) analysis [41].
3.4 Nitrosamines
Nitrosamines have been of significant interest since they were discovered to be DBPs in 2002 [42, 43]. Their structures are shown in Fig. 3. N-Nitrosodimethylamine (NDMA) is a probable human carcinogen, and there are toxicological concerns regarding other nitrosamines. NDMA was initially discovered in chlorinated drinking waters from Ontario, Canada [44], and has since been found in other locations [42, 43, 45]. The detection of NDMA in drinking water is largely due to improved analytical techniques that have allowed its determination at low ng/L concentrations. NDMA is generally found at highest levels in chloraminated drinking water, where the nitrogen in monochloramine (NH2Cl) is incorporated into the structure of the NDMA by-product [42]. Chlorination can also form NDMA to some extent when nitrogen precursors are present (e.g., natural ammonia in the source water or nitrogen-containing coagulants or ion-exchange resins used in the water-treatment process) [46–48].
NDMA is regulated in California at 10 ng/L [49] and Ontario, Canada at 9 ng/L [50]. A Canadian national drinking water guideline is also under development [51], and the U.S. Environmental Protection Agency (EPA) has recently announced that they intend to regulate a group of nitrosamines in the U.S. NDMA was included in the U.S. EPA’s second Unregulated Contaminant Monitoring Rule (UCMR-2), along with five other nitrosamines (N-nitrosodiethylamine, N-nitrosodibutylamine, N-nitrosopropylamine, N-nitrosomethylethylamine, and N-nitrosopyrrolidine), and national occurrence data are currently available [52]. This new national data reveals a maximum level of 530 ng/L for NDMA in chloraminated drinking water, which surpasses the previous highest level (180 ng/L) observed in chloraminated drinking water from Canada [53]. In addition, NDMA and four other nitrosamines are also on the U.S. EPA’s final Contaminant Candidate List (CCL-3), a priority list of contaminants for potential future regulation in drinking water [54].
An EPA method was created for measuring NDMA and six additional nitrosamines in drinking water (EPA Method 521) [55]. This method uses GC/chemical ionization (CI)-MS/MS and enables the measurement of NDMA and six other nitrosamines (N-nitrosomethylethylamine, N-nitrosodiethylamine, N-nitroso-di-n-propylamine, N-nitroso-di-n-butylamine, N-nitrosopyrrolidine, and N-nitrosopiperidine) in drinking water at detection limits ranging from 1.2 to 2.1 ng/L. A liquid chromatography (LC)/MS/MS method [56] can also be used to measure nine nitrosamines, including N-nitrosodiphenylamine, which is thermally unstable and cannot be measured using the EPA Method.
NDMA (and other nitrosamines) can dramatically increase in concentration in distribution systems (relative to finished water at the drinking water-treatment plant). For example, an initial level of 67 ng/L in drinking water-treatment plant effluent was shown to increase to 180 ng/L in the distribution system [53]. As a result, measurements taken at water-treatment plants may substantially underestimate the public’s exposure to this carcinogen.
While generally attributed to the use of chloramines or chlorine, NDMA was recently identified in ozonated drinking water from Germany [57]. An anthropogenic contaminant containing a dimethylamine group was discovered to be the precursor in its formation (discussed in more detail in the Contaminant DBP section).
3.5 Haloamides
Haloamides are formed primarily by chlorine or chloramine, and they were quantified for the first time in the Nationwide Occurrence Study. They have been measured in finished drinking waters from several U.S. states, up to 9.4 μg/L, individually [11, 13]. There is some indication that haloamides may be increased with chloramination. Because nitriles can hydrolyze to form amides [58, 59], it is possible that some of their formation is due to hydrolysis of the corresponding halonitriles, which are commonly found as DBPs. The first iodo-amide – bromoiodoacetamide – was recently identified in chloraminated drinking waters from several cities in the U.S. that had high bromide levels in their source waters [60]. This iodo-amide is highly cytotoxic and genotoxic in mammalian cells, as are other haloamides. As a class, haloamides are the most cytotoxic of all DBP classes measured to-date, and they are the second-most genotoxic DBP class, very close behind the halonitriles [19].
3.6 Halonitriles
Although they are not regulated in the U.S., haloacetonitriles (HANs) have been measured in several occurrence studies. Dichloro-, bromochloro-, dibromo-, and trichloroacetonitrile (HAN4) are the most commonly measured HAN species and have been included in a survey of 35 U.S. water utilities [61], a survey of 53 Canadian water utilities [62], and the US EPA’s Information Collection Rule (ICR) effort [63]. In the ICR, HANs were found up to 41 μg/L and were generally present at 12% of the levels of the four regulated THMs. HANs are formed by treatment with chlorine, chloramine, chlorine dioxide, or ozone disinfection; plants using chloramines (with or without chlorine) had the highest levels in their finished drinking water. Several other HANs, including a number of brominated species, were also measured in the U.S. Nationwide Occurrence Study. Total HAN levels reached a maximum of 14 μg/L, and were approximately 10% of the levels of the four regulated THMs combined, although a maximum of 25% was observed. When higher bromide levels were present in the source waters, more brominated HAN species were formed. This shift in speciation was observed in another study of high-bromide waters in Israel, which also provided evidence that chlorine dioxide disinfection can form HANs (dibromoacetonitrile) [31]. Two other halonitriles, cyanogen chloride (CNCl) and cyanogen bromide (CNBr), can be formed by chlorine or chloramines, but are generally found with chloramination [61]. CNCl was measured at several chloramination plants as part of the ICR effort, with levels ranging from submicrogram per liter to 21 μg/L. CNBr can also be formed with ozonation when source waters contain natural bromide [64]. Other halonitriles, including three- and four-carbon halonitriles, have also been identified, but have not been quantified in drinking water.
As a class, halonitriles are the most genotoxic of the DBPs studied in mammalian cells [19], and they are third in cytotoxicity, similar to other N-DBPs, haloamides, and halonitromethanes.
3.7 Halofuranones
Before it was discovered to be a drinking water DBP, MX (3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone) was originally identified in pulp mill effluent; subsequently, it was found in chlorinated drinking water from a number of samples taken around the world. MX has both an open and closed form that is dependent on pH; the ring-opened, oxo-butenoic acid form is present at the pH of drinking water (ZMX, Fig. 4). Other analogs of MX were also later identified in chlorinated drinking water, including its geometric isomer (EMX) [65], oxidized and reduced forms of MX (ox-MX and red-MX), as well as brominated analogs (the so-called BMXs) [66]. Structures of several of these analogs are shown in Fig. 4.
Bacterial mutagenicity tests were the original cause of concern for MX, as MX was found to be a potent mutagen in the Salmonella Ames assay, and MX can account for as much as 20–50% of the total mutagenicity in chlorinated drinking water [67]. At the time it was identified, MX was the most mutagenic DBP ever identified in drinking water, and in 1997, it was found to be a carcinogen in rats [68]. However, the genotoxic effects in mammalian cells are relatively moderate, such that several other classes of DBPs (including iodo-acids, halonitromethanes, haloamides, and halonitriles) show greater genotoxicity [19]. The concentration of MX required to produce a genotoxic effect in vivo is usually very high, around 100 mg/kg mouse oral administration [69]. Mutagenicity studies with transgenic medaka fish showed that MX did not induce mutations in the liver (for 96 h exposures) [70].
In the few occurrence studies that had been previously carried out, measured concentrations of MX were generally 60 ng/L or lower. In 2002, Wright et al. reported levels as high as 80 ng/L of MX found in drinking waters from Massachusetts [71], and in the U.S. Nationwide Occurrence Study, which specifically focused on waters high in natural organic matter and/or bromide, much higher levels were found (frequently >100 ng/L and as high as 850 ng/L) in finished drinking waters across the U.S. [11, 12]. Halogenated furanones were highest at a plant that disinfected with chlorine–chloramines (2.38 μg/L in plant effluent drinking water) and at a plant that disinfected with (1.02 μg/L in the distribution system). In drinking water plant effluents, a maximum level of 0.31 μg/L was observed for MX; maximum levels of brominated MX analogs included measurements of 0.72 and 0.81 μg/L for BEMX-1 and BEMX-2, respectively.
It is also interesting to note that the halogenated furanones were often stable in the distribution system and in simulated distribution system tests. Previous controlled laboratory studies had suggested that halogenated furanones, particularly MX, may not be stable in distribution systems. In at least five instances, MX levels actually increased in concentration from the plant effluent to the distribution system point sampled [11, 12]. Occasionally, MX levels decreased in the distribution system, but in these instances, it was still generally present at detectable levels.
3.8 Haloaldehydes
Haloaldehydes are formed primarily with chlorine or chloramine disinfection, but they are increased in formation with preozonation. In the Nationwide Occurrence Study, haloaldehydes were the third largest DBP class by weight (behind THMs and HAAs) of all the DBPs studied. Dichloroacetaldehyde was the most abundant of these haloaldehydes, with a maximum concentration of 16 μg/L. Before this study, chloral hydrate (trichloroacetaldehyde) was the only commonly measured haloaldehyde, and it was included in the ICR. Chloral hydrate and monochloroacetaldehyde are mutagenic in vitro [1], and tribromoacetaldehyde and chloral hydrate were recently found to be genotoxic in human cells [72].
New work on the entire class of haloaldehydes indicates that many are highly cytotoxic and genotoxic in mammalian cells [19].
3.9 Halopyrroles
In 2003, a new halogenated pyrrole – 2,3,5-tribromopyrrole (structure in Table 1) – was identified in drinking water [31]. This represents the first time that a halogenated pyrrole has been observed as a drinking water DBP for any disinfectant. This halopyrrole was found in finished drinking water from a full-scale drinking water-treatment plant in Israel that used pre-chlorination (at an initial reservoir) followed by primary treatment with combined chlorine dioxide–chlorine or combined chlorine dioxide–chloramine to treat a high bromide source water (approximately 2 ppm). This identification resulted from the first study of chlorine dioxide DBPs formed under high bromide/iodide conditions. Bromide levels in U.S. source waters generally range up to a maximum of approximately 0.5 ppm, and so to-date, this tribromopyrrole has not been identified in drinking waters from the U.S.
Mammalian cell toxicity testing revealed tribromopyrrole to be 8x more cytotoxic than dibromoacetic acid (a regulated DBP) and to have about the same genotoxic potency as MX. When the formation of tribromopyrrole was investigated using isolated humic and fulvic acid fractions collected from the source waters (as NOM precursors), tribromopyrrole was found to be formed primarily from humic acid, whereas the THMs, HAAs, and aldehydes were mostly formed from fulvic acid. It is interesting to note that a soil humic model proposed by Schulten and Schnitzer that was based on 13C NMR, pyrolysis, and oxidative degradation data, includes a pyrrole group in its structure [73]. In addition, the elementary analysis (C, H, N, X) for these natural humic and fulvic acids showed a greater contribution from N in the humic acid as compared to that in the fulvic acid. In none of the samplings from this research was tribromopyrrole found in pre-chlorinated waters (with chlorine treatment only). Thus, the combination of chlorine dioxide and chlorine (or chloramines) may be necessary for its formation. It is also possible that chloramination alone may also be important for its formation.
3.10 Haloquinones
In 2010, the first haloquinone DBP was reported in drinking water – 2,6-dichloro-1,4-benzoquinone – using SPE and LC/MS/MS [74]. Quantitative structure-toxicity relationship (QSTR) analysis had predicted that haloquinones are highly toxic and may be formed during drinking water treatment. The chronic lowest observed adverse effect levels (LOAELs) of haloquinones are predicted to be in the low μg/kg body weight per day range, which is 1,000× lower than most regulated DBPs, except bromate. This new DBP was found in drinking water treated with chlorine and chloramines, as well as chloramines and UV irradiation, at levels ranging from 5.3 to 54.6 ng/L. It has a predicted LOAEL of 49 μg/kg body weight per day.
Following this initial discovery, three additional haloquinones were identified in drinking water using LC/ESI-MS/MS: 2,6-dichloro-3-methyl-1,4-benzoquinone, 2,3,6-trichloro-1,4-benzoquinone, and 2,6-dibromo-1,4-benzoquinone [75]. Following their discovery in chlorinated drinking water, they were quantified, along with 2,6-dichloro-1,4-benzoquinone. Levels ranged from 0.5 to 165 ng/L. An unusual feature about these compounds is that, using negative ion-ESI, they form (M+H)- ions through a reduction step, rather than the classic (M–H)- ions that are typically observed. The authors used tandem-MS and accurate mass measurements to confirm the identity of these unusual ions. The structures of the haloquinone DBPs are shown in Fig. 5.
4 Other DBPs
4.1 Other Haloacids
There are four bromochloro-HAAs that are not currently regulated in the U.S., bromochloroacetic acid, bromodichloroacetic acid, chlorodibromoacetic acid, and tribromoacetic acid. Many laboratories routinely measure them as part of the nine total bromochloro-HAAs (HAA9). A recent study by Singer and colleagues makes the case that measuring all nine bromochloro-HAAs is important because measuring only the five regulated ones can significantly underestimate the total exposure, especially for water systems that contain appreciable levels of bromide in their source waters. The additional four unregulated HAAs are bromine-containing species that can be found at increased levels in drinking waters that have high bromide in their source waters, and their concentrations can be similar to the five regulated HAAs. Also, because bromine-containing DBPs are generally more toxic than chlorine-containing DBPs, knowing their concentrations can be important.
Other haloacids with longer carbon chains can also be formed in drinking water, mostly with chlorine and chloramine. One of these, 3,3-dichloropropenoic acid, was included as a priority DBP measured in the U.S. Nationwide Occurrence Study [11, 12]. It was found at a maximum of 4.7 μg/L and was present in nearly all of the water-treatment plants studied. The corresponding brominated acid, 3,3-dibromopropenoic acid, has also been identified as a DBP in drinking water, as well as several other three-, four-, and five-carbon acids and diacids [10].
Two of the more unusual bromoacids include the bromo-oxoacids 3,3-dibromo-4-oxopentanoic acid and 3-bromo-3-chloro-4-oxopentanoic acid. So far, there are no quantitative data on these other brominated acids, but preliminary toxicity data indicate that they may be toxicologically important.
4.2 Haloketones
Haloketones can be formed in waters treated with chlorine, chloramines, chlorine dioxide, as well as ozone–chlorine and ozone–chloramine combinations. Two haloketones, 1,1-dichloropropanone and 1,1,1-trichloropropanone, were measured in the ICR effort [63], where they ranged up to 10.0 and 17.0 μg/L, respectively. Other haloketones, including chloropropanone, 1,3-dichloropropanone, 1,1-dibromopropanone, 1,1,3-trichloropropanone, 1-bromo-1,1-dichloropropanone, 1,1,1-tribromopropanone, 1,1,3-tribromopropanone, 1,1,3,3-tetrachloropropanone, 1,1,1,3-tetrachloropropanone, and 1,1,3,3-tetrabromopropanone, were also measured in the U.S. Nationwide Occurrence Study [11, 12], and were found in drinking waters treated with a variety of disinfectants, though generally at sub-μg/L levels. To-date, they have not been investigated for toxicity, but some were predicted to cause cancer in the prioritization effort mentioned earlier [22].
4.3 Chlorate and Iodate
Chlorate is a DBP from chlorine dioxide and can also be present in chlorinated drinking water when hypochlorite bleach solutions are used for treatment (due to decomposition of the hypochlorite upon storage). In chlorine dioxide-treated drinking water, chlorate levels can approach 20% of the original chlorine dioxide dose. Chlorate concentrations in drinking water are typically much higher than other DBPs, including THMs. From the U.S. EPA ICR data, which represents the most extensive data for chlorate, the median level of chlorate was 120 μg/L at plants using chlorine dioxide for disinfection, but plants can sometimes exceed the health reference level of 210 μg/L. Recent measurements of chlorate included a study of full-scale treatment plants in Israel, in which chlorate was found up to 52 μg/L [31]; a full-scale treatment plant in Virginia, where chlorate was found at a median level of 14 μg/L [76]; and full-scale treatment plants in Quebec, where chlorate was present at a maximum of 190 μg/L [77].
Chlorate is mutagenic in Salmonella and induces chromosome aberrations and micronuclei in mammalian cells [78]. It has also been shown to induce thyroid tumors in laboratory animals [79]. The U.S. EPA has placed chlorate on the current CCL-3 [54], as well as its Unregulated Contaminant Monitoring Rule-3 (UCMR-3) [80] to collect further national data, and is currently considering it for regulation.
Iodate can be formed as a chlorination or ozonation DBP when elevated levels of natural iodide are present in the source waters [32, 33]. Unlike bromate, iodate is not a concern for toxicity because it is reduced to iodide in the body.
4.4 Aldehydes and Ketones
Several nonhalogenated aldehydes were measured in the U.S. ICR effort, including formaldehyde, acetaldehyde, glyoxal, and methyl glyoxal [63]. These aldehydes are DBPs produced primarily by ozone, although both chlorine and chlorine dioxide treatment can also form low parts per billion levels of formaldehyde. In the ICR, these aldehydes were detected at higher concentrations in water-treatment systems using ozone (up to 30.6 μg/L) than chlorine dioxide. Formaldehyde was detected at more than 50% of the treatment plants using chlorine dioxide at a mean of 5.3 μg/L and 90th percentile of 9.0 μg/L. Acetaldehyde, glyoxal, and methyl glyoxal were observed at maximum levels of 11, 16, and 6 μg/L, respectively, in ozonated drinking water, but were generally below the detection limit (5 μg/L) in chlorine dioxide-treated waters. Pentafluorobenzylhydroxylamine (PFBHA) derivatization is important for measuring these polar aldehydes and ketones by GC/MS because, without derivatization, they are almost impossible to extract from water. This derivatization process is discussed later in this chapter.
Additional aldehydes and ketones were also included in the U.S. Nationwide Occurrence Study: dimethylglyoxal (2,3-butanedione), cyanoformaldehyde, 2-butanone (methyl ethyl ketone), trans-2-hexanal, 5-keto-1-hexanal, and 6-hydroxy-2-hexanone [11, 13]. Dimethylglyoxal was the most consistently detected of these carbonyl compounds (up to 3.5 μg/L) and was found at higher levels in plants using ozone. Maximum levels of 0.3, 5.0, and 0.7 μg/L were observed for cyanoformaldehyde, 2-butanone, and trans-2-hexenal, respectively; 6-hydroxy-2-hexanone and 5-keto-1-hexanal were only detected in early stages of treatment, and not in finished waters.
4.5 Carboxylic Acids
Nonhalogenated carboxylic acids are also common DBPs from chlorine, chloramines, ozone, and chlorine dioxide [10]. In addition to halogenation reactions that can occur (primarily with chlorine and chloramine), oxidation reactions also occur, and can produce carboxylic acids. There is generally not a concern for toxicity for them, as many are naturally present in foods.
5 Discovery Research for New Highly Polar and High-Molecular-Weight DBPs
As mentioned earlier, more than 50% of the total organic halogen (TOX) formed in chlorinated drinking water remains unidentified, and much less is accounted for ozone, chloramine, and chlorine dioxide treated water. Because DBPs are typically present at nanogram per liter to microgram per liter levels, they are usually extracted into an organic solvent (with SPE or liquid–liquid extraction) and concentrated before measurement by GC or GC/MS. This means that most previous DBP research has focused on low molecular weight, volatile and semivolatile DBPs that are easy to extract from water. As a result, high-molecular-weight DBPs and highly polar DBPs are likely to be found in the “missing” DBP fraction. Ultrafiltration (UF) studies indicate that >50% of the TOX in chlorinated drinking water is >500 Da in molecular weight [81], and new research is revealing that highly polar DBPs are also part of this “missing” fraction. For example, new LC/MS/MS research using precursor scans of bromine (m/z 79 and 81) and iodine (m/z 127) has allowed the identification of new polar compounds, such as 1,1,2-tribromo-1,2,2-tricarboxylethane, 1-bromoamino-1,2-dibromo-1,2,2-tricarboxylethane, chloroiodoacetic acid, (E)- and (Z)-iodobutenedioic acid, 4-iodobenzoic acid, 3-iodophthalic acid, 2,4-diiodobenzoic acid, 5,6-diiodosalicylic acid, and 5,6-diiodo-3-ethylsalicylic acid [82, 83]. The iodo-acids were found at higher levels in chloraminated drinking water, consistent with previous results for other iodo-acids [21].
High-molecular-weight DBPs, which are not possible to measure using GC/MS, are also being investigated using ESI-MS/MS. Most of this work is very preliminary, due to the complexity of the mass spectra obtained. ESI-MS/MS has been used to generate chlorine and bromine fragment ions that can be used to select halogenated DBPs from the complex mixture of high-molecular-weight DBPs. In addition, radiolabeled chlorine (36Cl) has been used to further probe high-molecular-weight DBPs formed on chlorination of drinking water [84].
6 Contaminant DBPs
All of the DBPs previously discussed are “classical DBPs”, formed by the reaction of disinfectants with natural organic matter in source waters. However, source waters are also impacted by municipal and industrial emissions [85], and recent investigations have shown that some of these water contaminants can also react with disinfectants used in drinking water treatment to form their own by-products, some of which are toxic or estrogenic. Contaminant DBPs have been reported for several classes of drugs, pesticides, personal care products, estrogens, bisphenol A, alkylphenol surfactants, and algal toxins. Most of these contaminant DBPs were found in controlled laboratory studies and not in actual drinking water, but the potential is there for their formation in drinking water treatment. It is not surprising that DBPs can form from these contaminants because many of them have activated aromatic rings that can readily react with oxidants like chlorine, chloramines, ozone, and chlorine dioxide.
6.1 Pharmaceutical DBPs
Several classes of antibiotics, e.g., tetracyclines [86], fluoroquinolones [87, 88], and β-lactams [89] were observed to react with chemical oxidants such as chlorine dioxide (ClO2) and free chlorine. Oxidation with ClO2 yields hydroxylated and oxygenated products in the case of tetracyclines, and leads to dealkylation, hydroxylation, and intramolecular ring closure at the piperazine moiety of the fluoroquinolones [86, 88]. Reaction of these antibiotics with chlorine mostly generated chlorinated and OH-substituted by-products [86, 87]. Unlike fluroquinolones, whose quinolone ring is left mostly intact, disinfection with ClO2 may diminish the antibiotic capacity of tetracyclines because it leads to cleavage of the tetracyclines’ ring system [86, 88]. On the other hand, oxidation of β-lactam antibiotics such as penicillin, amoxicillin, and cefadroxil with ClO2 leads to the formation of hydroquinone and a wide range of substituted phenols [89].
Adachi and Oka investigated the formation of cyanide during the reaction of chlorine with 20 different pharmaceuticals containing nitrogen in their molecular structure [90]. High levels of cyanide were generated by chlorination of hexamine and losartan potassium aqueous solutions. Other precursors of cyanide included metronidazol, dacarbazine, and allopurinol.
The antibacterial agent sulfamethoxazole produced chlorinated and nonchlorinated DBPs when reacted with chlorine [91]. A ring-chlorinated product was formed via halogenation of the aniline moiety at sub-stoichiometric concentrations of chlorine. 3-Amino-5-methylisoxazole, sulfate, and N-chloro-p-benzoquinone imine were formed via rupture of the sulfamethoxazole sulfonamide moiety at stoichiometric excess of chlorine.
Carbadox, a veterinary antibacterial agent, also formed oxidation products when reacted with chlorine [92]. These products are believed to maintain the antibacterial activity because they kept the biologically active N-oxide group in their structure.
Chlorination of the antacid cimetidine leads to the formation of four major DBPs: cimetidine sulfoxide, 4-hydroxylmethyl-5-methyl-1H-imidazole, 4-chloro-5-methyl-1H-imidazole, and a product proposed to be either a β- or δ-sulfam. The formation of the last three products resulted from unexpected reactions and more substantial structural changes than those typically observed in chlorination [93].
The reaction of the lipid-regulator gemfibrozil with free chlorine yielded four chlorinated derivatives of this compound [94]. Chlorination of acetaminophen (paracetamol) generated 11 discernible DBPs, including the toxic compounds 1,4-benzoquinone and N-acetyl-p-benzoquinone imine and two ring chlorination products, chloro-4-acetamidophenol and dichloro-4-acetamidophenol [95].
Shen and Andrews investigated several pharmaceuticals containing dimethylamine or diethylamine in their structures as potential precursors of NDMA and N-nitrosodiethylamine during chloramination [96]. Eight out of 19 pharmaceuticals yielded molar conversions higher than 1%. Ranitidine, one of the most prescribed drugs in the world, showed the strongest potential to form NDMA, as previously reported [97]. NDMA-related compounds are also suggested to be generated when controlled drugs like amphetamine-type drugs react with chloramines [98]. Although the latter is still to be proven, two chlorinated ring products, 4-chloro-1,3-benzodioxole and 1-chloro-3,4-dihydroxybenzene were identified during the chlorination of amphetamine-type drugs [98].
Ozonation of the antiepileptic drug carbamazepine resulted in the formation of three main DBPs: 1-(2-benzaldehyde)-4-hydro-(1H,3H)-quinazoline-2-one, 1-(2-benzaldehyde)-(1H,3H)-quinazoline-2,4-dione, and 1-(2-benzoic acid)-(1H,3H)-quinazoline-2,4-dione [99]. Acridine, a compound with known carcinogenic properties, acridine-9-carbaldehyde, and 9-hydroxy-acridine were DBPs observed during the treatment of carbamazepine solutions with chlorine dioxide [100]. Acridine and 1-(2-benzaldehyde)-(1H,3H)-quinazoline-2,4-dione were also identified as major DBPs of ozonation and chlorination of oxcarbazepine, a keto analog of carbamazepine [101].
Major oxidation products of propanolol and metoprolol formed during ozonation in aqueous solution were investigated by Benner et al. [102, 103]. In the case of propanolol, the main ozonation product is a ring-opened compound with two aldehyde moieties, which results from ozone attack to the naphthalene ring [103]. Formation of aldehyde moieties was also one of the main oxidation routes during metoprolol ozonation, together with hydroxylation [102].
Recently, Duirk et al. [34] showed evidence that iodinated X-ray contrast media (ICM), such as iopamidol, constitute an iodine source to form iodo-THM DBPs, e.g., dichloroiodomethane, and iodo-acid DBPs, e.g., iodoacetic acid, in chlorinated and chloraminated drinking waters. However, the complete reaction pathway is not fully understood yet, and it is under further investigation. Chloraminated and chlorinated source waters with iopamidol were genotoxic and cytotoxic in mammalian cells. This is in agreement with the previously reported high genotoxicity and cytotoxicity of the iodo-acids and iodo-THMs [20, 21].
6.2 Estrogen DBPs
As reviewed by Pereira et al. [104], the reaction of estrogens, that is estrone, estradiol, and ethinylestradiol, with free chlorine occurs mainly via an electrophilic substitution at the ortho and para positions, which results eventually in cleavage of the aromatic structure. Several authors have reported that dichlorinated derivatives present less estrogenic activity than monochlorinated derivatives, and in most cases, estrogen DBPs are less potent in terms of estrogenicity than the parent compounds.
Molecular ozone not only reacts easily with double bonds, activated aromatic structures, or hetero-atoms, but it can also form highly reactive and nonselective free radicals, e.g., HO•. Therefore, and due to the latter reaction mechanism, some of the estrogens DBPs generated during the ozonation of estradiol water solutions are common to those formed during diverse photocatalytic processes (O3/UV, TiO2/UV, and photo-Fenton). In addition to forming hydroxylated derivatives from estrogens, ozone can also form dicarboxylic acids via the opening of an aromatic ring. This transformation route was also identified during the heterogeneous photocatalysis with TiO2 of estradiol [104].
6.3 Pesticide DBPs
Oxidation of triazine herbicides with chlorine and chlorine dioxide has been widely studied [105–108]. In the case of sulfur-containing triazines, oxidation occurs mainly via cleavage of the weakened R–S–CH3 bond rather than by addition of chlorine. Reactions of S-triazines with chlorine are faster than with chlorine dioxide, and form sulfoxide, sulfone, and a sulfone hydrolysis product. Chlorination with chlorine dioxide only produced sulfoxide [108]. Lopez et al. identified the formation of sulfonate esters during the chlorination of ametryn and terbutryn [106, 107]. Triazine DBPs identified by Brix et al. exhibited higher toxicities than the parent compounds [105]. Similar to triazines, clethodim, a cyclohexanedione herbicide, is oxidized by hypochlorite and chloramines to clethodim sulfoxide and then to sulfone [109].
Chlorpyrifos reacted with free chlorine to form chlorpyrifos oxon, which is more toxic than the parent compound. Both compounds further hydrolyze to a more stable product, 3,5,6-trichloro-2-pyridinol [110].
Chlorination products of glyphosate, one of the most widely used herbicide in the world, and glycine, one of the intermediates in glyphosate chlorination, were investigated by Mehrsheikh et al. [111]. Both compounds followed a similar degradation route, with the final glyphosate chlorination products identified as methanediol and other small molecules, such as phosphoric acid, nitrate, CO2, and N2.
Isoxaflutole is an isoxazole herbicide that, in the presence of hypochlorite, hydrolyzed to a stable and phytotoxic metabolite, diketonitrile. This intermediate further degraded to yield benzoic acid as the major end product, which is nonphytotoxic [112].
Chlorination of waters containing two phenylurea-type herbicides, isoproturon and diuron, results in the formation of THMs. The reaction of the phenylurea-type herbicide isoproturon with chlorine produced compounds that still contained the aromatic ring of the herbicide with the urea side-chain unmodified. The formation of chlorinated and brominated derivatives was related to the bromide concentration present in the water [113].
Zambodin et al. [114] studied the DBPs of the herbicide chlortoluron generated during chlorination. In this case, halogenation (chlorination) and hydroxylation reactions were the main transformation routes observed, taking place exclusively on the aromatic ring of the molecule. Xu et al. [115] reported the formation of six volatile DBPs, including chloroform, dichloroacetonitrile, 1,1-dichloropropanone, 1,1,1-trichloropropanone, dichloronitromethane, and trichloronitromethane.
Ozonation of organophosphorous pesticides led to the formation of oxon intermediates (diazooxon, methyl paraoxon, and paraoxon for diazinon, methyl parathion, and parathion, respectively) [116]. These compounds accumulated to a different extent as a function of the solution pH.
The fungicide tolylfluanide was recently shown to form a new microbial transformation product, N,N-dimethylsulfamide, which subsequently reacts with ozone to form the carcinogenic NDMA [57]. This was discovered after high ng/L levels of NDMA were observed in ozonated drinking water from Germany and came as a surprise because ozone does not form NDMA by reaction with natural organic matter. The chlorination products of N,N-dimethylsulfamide have not been investigated yet.
6.4 Personal Care Product DBPs
Parabens are widely used as preservatives in the cosmetic and pharmaceutical industries and also as food additives, due mainly to their bactericidal and fungicidal properties. Terasaki and Makino identified 14 monochloro- and dichloro-parabens formed by chlorination of parabens [117]. Ozonation of parabens in aqueous solutions produced paraben DBPs mainly through hydroxylation of their aromatic ring and/or their ester chain [118].
Chlorination and chloramination of a widely used antibacterial additive, triclosan, which is used in many household personal care products, results in the formation of chloroform, 5,6-dichloro-2-(2,4-dichlorophenoxy)phenol, 4,5-dichloro-2-(2,4-dichlorophenoxy)phenol, 4,5,6-trichloro-2-(2,4-dichlorophenoxy)phenol, 2,4-dichlorophenol, and 2,4,6-trichlorophenol [119]. The reaction of triclosan with monochloramine is slow, however, compared to chlorine [120]. The chlorophenoxyphenols are formed via bimolecular electrophilic substitution of triclosan.
Two UV filters (used to block UV-rays in sunscreens and other products), octyl-p-methoxycinnamate and octyl-dimethyl-p-aminobenzoate, reacted with chlorine, producing chlorine-substituted compounds as intermediates that finally cleaved to smaller ester products [121]. Some of the identified octyl-p-methoxycinnamate DBPs showed weak mutagenic properties. Chlorinated and brominated intermediates were formed during chlorination of 2-ethylhexyl-4-(dimethylamine)benzoate and 2-hydroxy-4-methoxybenzophenone, with trichloromethoxyphenol the most abundant DBP [122].
Chlorine DBPs of the polycyclic musks 6-acetyl-1,1,2,4,4,7-hexamethyltetraline (AHTN) and 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran (HHCB), which are widely used fragrances in cosmetics, daily care products, and cleaning products for household and industry, were investigated by Kuhlich et al. [123]. This study evidenced chlorination of HHCB as a potential new source of HHCB-lactone in the environment, other than biological transformation.
Terpenoid DBPs were investigated by Joll et al. [124] and Qi et al. [125]. The main ozonation product of 2-methylisoborneol was camphor, which was further oxidized to formaldehyde, acetaldehyde, propanal, buntanal, glyoxal, and methyl glyoxal [125]. Chlorination of β-carotene, retinol, β-ionone, and geranyl acetate resulted in the formation of THMs [124].
6.5 Alkylphenol Surfactant and Bisphenol A DBPs
Alkylphenol ethoxylate surfactants are widely used in laundry detergents. Chlorination of these compounds results in the formation of halogenated nonylphenolic DBPs, most of them brominated acidic compounds [126].
Bisphenol A, a compound highly used in the production of epoxy resins and polycarbonate plastics, forms monochloro-, dichloro, trichloro-, and tetrachloro derivatives when chlorinated [127]. Its reaction with ozone produces as major transformation products, catechol, orthoquinone, muconic acid derivatives of bisphenol A, benzoquinone, and 2-(4-hydroxyphenyl)-propan-2-ol [128].
6.6 Algal Toxin DBPs
Cyanobacterial toxins are toxins produced by certain species of blue-green algae that have become a major environmental and public health concern. The behavior of cyanotoxins during chlorination treatment has been recently reviewed by Merel et al. [129]. Chlorination DBPs have been reported only for the hepatotoxins microcystin-LR and cylindrospermopsin. Other cyanotoxins, such as nodularins, saxitoxins, and anatoxins, have yet to be investigated. Different isomers of six chlorination products of microcystin-LR have been characterized: dihydroxy-microcystin, monochloro-microcystin, monochloro-hydroxy-microcystin, monochloro-dihydroxy-microcystin, dichloro-dihydroxy-microcystin, and trichloro-hydroxy-microcystin. Only two chlorination DBPs have been reported so far for cylindrospermopsin: 5-chloro-cylindrospermopsin and cylindrospermopsic acid [129]. Chlorination of microcystin, cylindrospermopsin, and nodularins seems to reduce the mixture toxicity; however, this aspect has not been extensively studied [129].
7 Human Exposure
New research also indicates that exposures from other activities, including showering, bathing, and swimming in chlorinated swimming pools can increase exposures to certain DBPs [130–147]. DBPs are not only ingested by drinking the water, but some can also be inhaled or can penetrate the skin [130–132, 134, 135]. In particular, volatile DBPs that easily transfer from the water to the air (including THMs) can be inhaled during showering or visiting an indoor chlorinated swimming pool – either through active swimming or from sitting near the pool, breathing in the pool vapors. THMs, HAAs, and haloketones have been measured in human blood, urine, or exhaled breath after showering, bathing, or swimming [130, 132, 134, 135, 141, 142, 145, 148]. These exposure routes are now being recognized in human exposure and human epidemiologic studies.
Recent results indicate that these other exposure routes may increase the risk of bladder cancer [149]. There is also new evidence that genetic susceptibility may play a role in bladder cancer. A recent epidemiologic study conducted in Spain revealed that people who carry a particular glutathione S-transferase zeta-1 (GSTZ1) polymorphism and are missing one or both copies of glutathione S-transferase theta-1 (GSTT1) were particularly susceptible to bladder cancer when exposed to >49 μg/L THMs in drinking water [6]. Approximately 29% of the Spanish study population had this genetic susceptibility, and approximately 25% of the U.S. population would also have this genetic susceptibility.
Chlorinated swimming pool exposures have also been linked with respiratory effects, including asthma [140, 150–152]. Trichloramine, which is formed by the reaction of chlorine with urea (from sweat and urine), has been suspected in these cases of asthma. In addition to sweat and urine, pool waters also contain other DBP precursors, such as skin cells, hair, and lotions/sunscreens.
To-date, only two efforts to comprehensively identify DBPs in swimming pools have been reported. In the first, 19 DBPs were identified in outdoor swimming pools [136]. In the second, >100 DBPs were identified in indoor chlorinated and brominated pools, including many nitrogenous DBPs (haloamides, halonitriles, haloanilines, haloamines, haloanisoles, and halonitro-compounds), likely due to the nitrogen-containing precursors from swimmers (urine, sweat, etc.) [143]. Trichloramine and THMs were also measured in the pool air [143]. Nitrosamines have been measured in chlorinated pools and hot tubs, up to a maximum of 429 ng/L [137]. Levels observed were up to 500× greater than the level (0.7 ng/L) associated with a one in a million lifetime cancer risk. Volatile DBPs, such as trichloramine, dichloromethylamine, and dichloroacetonitrile, have also been measured in pool waters using membrane introduction mass spectrometry (MIMS) [133]. Brominated DBPs from sunscreens have been reported [136], as have DBPs from the reaction of chlorine with parabens used in lotions, cosmetics, and sunscreens [117].
The mutagenicity, genotoxicity, and cytotoxicity of swimming pool waters have recently been reported [143, 144, 146]). One study showed that pool waters were significantly more toxic than their tap water sources [146]. Because THM concentrations are similar between tap waters and pool waters, using THMs to monitor exposure in epidemiological studies may not be the best metric. Pools treated with a combination of UV light and chlorine disinfection indoors, or outdoor sunlight exposure exhibited lower cytotoxicity than their indoor counterparts disinfected with chlorine [146].
8 Combining Chemistry with Toxicology
More studies are combining DBP identification/measurement efforts with toxicology to understand their potential health effects. For example, a large integrated multidisciplinary study (called the Four Lab Study) was recently published [13, 153, 154]. This effort involved the collaboration of chemists, toxicologists, engineers, and risk assessors from the four National Research Laboratories of the U.S. EPA, as well as collaborators from academia and the water industry. For this study, a new procedure using reverse osmosis was developed for producing chlorinated drinking water concentrates for animal toxicology experiments. DBPs were then comprehensively identified (resulting in the identification of >100 DBPs), and 75 priority and regulated DBPs were quantified to assess what DBPs the animals were exposed to. An extensive battery of in vivo and in vitro toxicity assays were used, with an emphasis on reproductive and developmental effects. When the NOM was concentrated first and disinfected with chlorine afterward, DBPs (including volatiles and semivolatiles) were formed and maintained in a water matrix suitable for animal studies. DBPs were relatively stable over the course of the animal studies (125 days) with multiple chlorination events, and a significant proportion of the TOX was accounted for through a comprehensive identification approach. Many DBPs were reported for the first time, including previously undetected and unreported haloacids and haloamides. The new concentration procedure not only produced a concentrated drinking water suitable for animal experiments but also provided a greater TOC concentration factor (136x), enhancing the detection of trace DBPs that are often below detection using conventional approaches.
9 Analytical Methods for Identifying and Quantifying DBPs
Experiments to identify disinfection by-products (DBPs) have been carried out using two different procedures. In the first, natural waters (e.g., river, lake) are reacted with the disinfectant, either in a pilot plant, an actual treatment plant, or in a controlled laboratory study. In the second type of procedure, aquatic humic material is isolated and reacted with the disinfectant in purified water in a controlled laboratory study. This latter type of study is relevant because humic material is an important precursor of THMs and other DBPs. Aquatic humic material is present in nearly all natural waters, and isolated humic material reacts with disinfectants to produce most of the same DBPs found from natural waters. Because DBPs are typically formed at low levels (ng/L-μg/L), samples are usually concentrated to allow for DBP detection. Concentration methods that are commonly used include solid phase extraction (SPE), solid phase microextraction (SPME), liquid–liquid extraction, and XAD resin extraction (for larger quantities of water) [9].
9.1 GC/MS
GC/MS was the primary tool for identifying the first DBPs, and it remains an important tool for measuring and identifying new DBPs. Large mass spectral libraries (NIST and Wiley databases, which contain >200,000 spectra) enable rapid identifications. When DBPs are not present in these databases, high-resolution MS, chemical ionization-MS, and sometimes GC/infrared spectroscopy (IR) have been used with GC/MS to obtain structural information. Examples of the use of GC/MS for identifying new DBPs include the recent identification of iodo-acids. The iodo-acids were discovered in drinking water treated with chloramination through the use of full-scan GC/MS on the methylated extracts. Empirical formula information for both the molecular ions and the fragment ions was obtained by high-resolution electron ionization (EI)-MS, and the spectra were interpreted to yield tentative identifications of five new iodo-acids (iodoacetic acid, bromoiodoacetic acid, (E)-3-bromo-3-iodopropenoic acid, (Z)-3-bromo-3-iodopropenoic acid, and (E)-2-iodo-3-methylbutenedioic acid). Structural assignments were then confirmed by the match of mass spectra and GC retention times to authentic chemical standards, several of which had to be synthesized.
GC/MS(/MS) is also popular for quantifying DBPs. Selected ion monitoring (SIM) or multiple reaction monitoring (MRM) mode are used with GC/MS and GC/MS/MS, respectively, to maximize the sensitivity and provide low detection limits. Some EPA Methods utilize GC/MS, including EPA Method 524.2, which uses GC/EI-MS for THM analysis [155], and EPA Method 521, which uses for GC/CI-MS/MS for nitrosamine analysis [55]. In addition, many priority unregulated DBPs have been measured using GC/MS in a U.S. Nationwide Occurrence Study [11, 12].
9.2 GC/ECD
GC/electron capture detection (ECD) is also used to measure DBPs. In particular, EPA Method 552.2 and 552.3 are commonly used to measure haloacetic acids in drinking water [156, 157]. ECD is very sensitive toward halogenated compounds and allows low-level detection for HAAs (0.012–0.17 μg/L detection limits for EPA Method 552.3).
9.3 LC and UPLC/MS/MS
LC/MS/MS and ultraperformance liquid chromatography (UPLC)/MS/MS are increasingly being used to identify and quantify highly polar DBPs and probe high-molecular-weight DBPs [158]. For example, LC/MS/MS was used to discover the first haloquinone DBP found in drinking water: 2,6-dichloro-1,4-benzoquinone [74]. In addition, a new nitrosamine method was created using LC/MS/MS, and with this method, two new nitrosamine DBPs were found in drinking water – nitrosopiperidine and nitrosodiphenylamine [56]. LC/MS/MS was essential for detecting nitrosodiphenylamine, as it is thermally unstable and cannot be measured by GC/MS. Derivatizing agents, such as 2,4-dinitrophenylhydrazine (DNPH), have also been used with LC/MS to enable the detection of highly polar DBPs; these are discussed in the later section on derivatizing agents.
The presence of high-molecular-weight DBPs had been indicated in research using UF membranes and TOX analysis. This research revealed that >50% of the total halogenated material in chlorinated drinking water may be >1,000 Da in molecular size [81]. Subsequent LC/ESI-MS/MS studies have been used to probe its chemical composition [159]. 36Cl-labeled HOCl (aqueous chlorine) has also been used to react with NOM to enhance the detection of chlorine-containing DBPs in the high-molecular-weight fractions [84, 159]. Results revealed a highly dispersed molecular weight distribution, and an average molecular mass of 2,000 Da.
Precursor ion scans of chlorine (m/z 35), bromine (m/z 79 and 81), and iodine (m/z 127) have also been used to target chlorinated, brominated, and iodinated DBPs, respectively [82, 83, 160]. For example, precursor scans of m/z 127 were used with UPLC/ESI-MS/MS to provide a more comprehensive picture of polar iodinated DBPs formed in drinking water [83]. This recently allowed the detection of 17 iodo-DBPs, including a few that had not been previously reported.
9.4 IC/ICP-MS and IC/ESI-MS
A few DBPs, such as bromate, chlorate, iodate, and chlorite, are present as anions in drinking water. As a result, they are not volatile and cannot be analyzed by GC/MS. They are also difficult to separate by LC, but will separate nicely using ion chromatography (IC). At neutral pH, HAAs are also anions and can be separated using IC. A number of methods have been created for these DBPs using both IC/inductively coupled plasma (ICP)-MS and IC/ESI-MS. Pretreatment to remove interfering ions (e.g., sulfate and chloride), along with the use of a suppressor column prior to introduction into the MS interface, is beneficial for trace-level measurement.
Several EPA Methods have been created for measuring bromate, a carcinogenic DBP that is currently regulated in U.S. drinking waters at 10 μg/L. Two of these use mass spectrometry: EPA Method 321.8 and EPA Method 557. EPA Method 321.8 uses IC/ICP-MS and can achieve 0.3 μg/L detection limits [161], EPA Method 557 uses IC/ESI-MS/MS and can achieve 0.02 μg/L detection limits [162]. EPA Method 557 can also be used to measure the commonly occurring chloro-bromo-HAAs (HAA9) and dichloropropanoic acid, with detection limits ranging from 0.015 to 0.20 μg/L. Roehl et al. published a good review covering the use of IC/ESI-MS for analyzing HAAs and bromate [163], and Paull and Barron published a nice review of IC applications (including IC/ESI-MS and IC/ICP-MS) for measuring HAAs [164].
Shi and Adams recently created a rapid IC/ICP-MS method for simultaneously measuring iodoacetic acids, bromoacetic acids, iodate, and bromate in drinking water, groundwater, surface water, and swimming pool water [165]. Method detection limits were sub-μg/L for iodinated DBPs, and low-μg/L for brominated DBPs. However, mono-, di-, and tri-chlorinated species could not be detected because the sensitivity of ICP-MS for chlorine is poor.
9.5 IC/Conductivity
Bromate has also been measured using IC with conductivity detection. For example, EPA Method 302.0 uses two-dimensional IC with suppressed conductivity detection to measure bromate at 0.12 μg/L detection limits [166]. Bromate, chlorite, and chlorate can also be measured by an earlier EPA Method (Method 300.1), which uses IC with conductivity detection [167]. Method detection limits ranging from 0.45 to 1.28 μg/L can be achieved.
9.6 MIMS
MIMS is a technique that uses a semipermeable membrane for directly introducing analytes into the mass spectrometer. This allows analytes to be measured in real-time with little or no sample preparation. MIMS has been previously used to measure the stability of CNCl in chlorinated and chloraminated drinking water [168], to quantify CNCl and CNBr in drinking water [169], to measure chloramines and chlorobenzenes in water samples [170], and investigate the mechanism and kinetics of chloroform formation in drinking water [171]. More recently, it has been used to measure volatile DBPs in indoor swimming pools [138, 172].
9.7 FAIMS-MS
High-field asymmetric waveform ion mobility spectrometry (FAIMS)-MS offers an additional degree of separation of analytes, based on the differences in the ratio of ion mobility at high electric field vs. low field [173]. When used with ESI-MS, FAIMS can significantly reduce chemical backgrounds and enhance the detection of DBPs. Low ng/L detection limits can often be achieved without preconcentration, extraction, or derivatization. ESI-FAIMS-MS has been used to measure HAAs in drinking water and human urine [174, 175]; bromate, chlorate, and iodate in drinking water [176]; and nitrosamines in drinking water [177, 178]; and in wastewater-treatment plant effluents [179].
9.8 Derivatization Techniques
For some classes of compounds, derivatizations are performed to enable their detection (Table 3). For example, methylations enable the detection and measurement of carboxylic acids (including haloacids) by GC/MS. Derivatization with o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA) is popular for the GC/MS analysis of polar aldehydes and ketones that are difficult or impossible to extract from water without derivatization. And, N-methyl-bis-trifluoroacetamide (MBTFA) derivatization with GC/ion trap-MS/MS was recently shown to offer improved detection limits for measuring MX (3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone) in drinking water [180]. Silylating agents, such as bis(trimethylsilyl)trifluoroacetamide (BSTFA) and N-methyltrimethylsilyltrifluoroacetamide (MSTFA), are sometimes used to derivatize alcohols/phenols to enable their detection by GC/MS. For example, BSTFA derivatization has been used recently with GC/MS to identify several DBPs formed by the chlorination of parabens (para-hydroxybenzoate esters), which are water contaminants used as preservatives in a wide variety of personal care products (e.g., sunscreens, bath gels, shampoos, and toothpaste) [117].
Several newer derivatization techniques have also been developed for enabling the identification of new, highly polar DBPs with GC/MS or LC/MS(/MS). For example, chloroformate derivatizing agents have been developed for extracting highly polar DBPs with multiple hydroxyl, carboxyl, and amino substituents for analysis with GC/negative chemical ionization-MS [181, 182]. DNPH [183], O-(carboxymethyl hydroxylamine) (CMHA) [184], and 4-dimethylamino-6-(4-methoxy-1-naphthyl)-1,3,5-triazine-2-hydrazine (DMNTH) [185] have been used with LC/MS(/MS) to extract and identify highly polar carbonyl DBPs in drinking water.
9.9 Near Real-Time Methods
Researchers continue to pursue the development of new instruments to enable real-time measurements of DBPs in drinking water, which would be a tremendous benefit to drinking water utilities and to epidemiologists, who could obtain more accurate exposure information for their studies. A recent development includes a new instrument that can selectively measure THMs and HAAs in near real-time directly from drinking water distribution systems [186]. The instrument uses a capillary membrane sampler-flow injection analyzer and is based on the fluorescence of the reaction of nicotinamide in basic solution with THMs and HAAs. The analyzer alternates sampling between two sample loops connected to a capillary membrane sampler, which discriminates between the volatile THMs and the nonvolatile HAAs. Low μg/L detection limits are possible for the four regulated THMs and five regulated HAAs (chloro-, dichloro-, trichloro-, bromo-, and dibromoacetic acid), and results compare favorably to EPA Methods. This method provided automated online sampling with hourly sample analysis rates.
9.10 Total Organic Chlorine, Bromine, and Iodine
A few years ago, Minear’s group at the University of Illinois pioneered the development of a method to speciate TOX, such that total organic chlorine (TOCl), total organic bromine (TOBr), and total organic iodine (TOI) could be differentiated [187]. This method involves the sorption of analytes onto activated carbon, followed by removal of inorganic analytes, combustion of the activated carbon, bubbling the combustion gas into ultrapure water, and injection of this water onto an ion chromatograph for measurement of chloride, bromide, and iodide. The original TOX measurement served a useful purpose in providing an idea of the total halogenated material formed in chlorinated and other disinfected waters, so that it could be determined how much of the halogenated DBPs were being accounted for through quantification of targeted DBPs. This measurement has been widely used and has revealed that more than 50% of the halogenated DBPs in drinking water are still not accounted for. The development of the TOCl/TOBr/TOI method allowed an even finer distinction of these DBPs, and has become an important measurement because of increased toxicity among the brominated and iodinated DBPs. This method was recently used to measure the contribution of chlorinated, brominated, and iodinated DBPs to the mixture of halogenated DBPs formed in iodine point-of-use treatments [8]. This method was also recently used to follow the formation of TOCl and TOBr over time in a kinetic study of DBPs from chlorination [188].
10 Conclusions
Through more than 30 years of research, many DBPs have been identified, and we have a greater understanding of how they are formed, as well as ways to reduce or eliminate many of them. However, despite much research, more than 50% of the halogenated DBPs in chlorinated drinking water remains unaccounted for, and much less is accounted for with ozone, chloramine, and chlorine dioxide treatment. It is especially important to investigate DBPs formed by alternative disinfectants because more water-treatment plants in the U.S. are changing from chlorine to alternative disinfectants to meet requirements of the new regulations. Beyond the three most popular alternative disinfectants (chloramines, ozone, and chlorine dioxide), there is also a trend toward nonchemical disinfection, such as UV irradiation and membrane technology. UV irradiation is sometimes presented as a DBP-free disinfectant, but it has the potential to form hydroxyl radicals in water (as ozone does), which can produce oxygen-containing DBPs and has been shown to activate NOM to make it more reactive toward chlorine. The use of membranes in desalination plants can cause shifts to brominated DBPs when the disinfectant is added (due to the considerable amount of bromide that can traverse the membrane). It will be important to continue to investigate these new treatments to determine their relative safety compared to existing treatment technologies.
In addition, it is important to continue research on contaminant DBPs. With increased drought and increased populations in many parts of the world, our rivers contain increasingly higher concentrations of anthropogenic contaminants, which can also form hazardous DBPs. It is important to study their formation and devise wastewater and drinking water-treatment methods that will remove them.
Finally, it is paramount to determine which DBPs are responsible for human health effects observed and eliminate or minimize them in drinking water. As mentioned earlier, it is still not known which DBPs are responsible for the bladder cancer observed in human epidemiologic studies or which DBPs are responsible for the reproductive/developmental effects observed. Investigating new, emerging DBPs that show a toxic response is an important element in solving this important human health issue, as is investigating human health effects from routes of exposure beyond ingestion. In this regard, it will be important to consider inhalation and dermal exposure in future toxicity studies, so it can be determined which exposure route(s) are responsible for the adverse human health effects and also which DBPs are responsible.
References
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242
Villanueva CM, Cantor KP, Cordier S, Jaakkola JJK, King WD, Lynch CF, Porru S, Kogevinas M (2004) Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology 15(3):357–367
Waller K, Swan SH, DeLorenze G, Hopkins B (1998) Trihalomethanes in drinking water and spontaneous abortion. Epidemiology 9:134–140
Savitz DA, Singer PC, Hartmann KE, Herring AH, Weinberg HS, Makarushka C, Hoffman C, Chan R, Maclehose R (2005) Drinking water disinfection by-products and pregnancy outcome. AWWA Research Foundation, Denver, CO
Nieuwenhuijsen MJ, Toledano MB, Eaton NE, Fawell J, Elliott P (2000) Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: a review. Occup Environ Med 57(2):73–85
Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, Malats N, Chanock S, Yeager M, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castaño-Vinyals G, Samanic C, Rothman N, Kogevinas M (2010) Polymorphisms in GSTT1, GSTZ1, AND CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ Health Perspect 118(11):1545–1550
Liu W, Cheung LM, Yang X, Shang C (2006) THM, HAA and CNCl formation from UV irradiation and chlor(amination) of selected organic waters. Water Res 40:2033–2043
Smith EM, Plewa MJ, Lindell CL, Richardson SD (2010) Comparison of byproduct formation in waters treated with chlorine and iodine: relevance to point-of-use treatment. Environ Sci Technol 44:8446–8452
Richardson SD (1998) Drinking water disinfection by-products. In: Meyers RA (ed) The encyclopedia of environmental analysis and remediation, vol 3. Wiley, New York, pp 1398–1421
Richardson SD (2011) Disinfection by-products: formation and occurrence in drinking water. In: Nriagu JO (ed) Encyclopedia of environmental health, vol 2. Elsevier, Burlington, M. A., pp 110–136
Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad G, Thruston AD Jr (2006) The occurrence of a new generation of disinfection by-products. Environ Sci Technol 40:7175–7185
Weinberg HS, Krasner SW, Richardson SD, Thruston AD Jr (2002) The occurrence of disinfection by-products (DBPs) of health concern in drinking water: results of a nationwide DBP occurrence study. EPA/600/R02/068. Available at www.epa.gov/athens/publications/reports/EPA_600_R02_068.pdf
Richardson SD, Thruston AD Jr, Krasner SW, Weinberg HS, Miltner RJ, Schenck KM, Narotsky MG, McKague AB, Simmons JE (2008) Integrated disinfection by-products mixtures research: comprehensive characterization of water concentrates prepared from chlorinated and ozonated/postchlorinated drinking water. J Toxicol Environ Health A 71(17):1165–1186
Rook JJ (1974) Formation of haloforms during chlorination of natural waters. Water Treat Examination 23:234–243
Bellar TA, Lichtenberg JJ, Kroner RC (1974) Occurrence of organohalides in chlorinated drinking waters. J Am Water Works Assoc 66(12):703–706
National Cancer Institute (1976) Report on the carcinogenesis bioassay of chloroform (CAS no. 67-66-3), MD: National Cancer Institute. TR-000 NTIS Rpt No PB264018, Bethesda, MD
U.S. Environmental Protection Agency (1979) National interim primary drinking water regulations: control of trihalomethanes in drinking water: Final rules. Fed Regist 44:68624–68705
Kurokawa Y, Aoki S, Matsushima Y (1986) Dose–response studies on the carcinogenicity of potassium bromate in F344 rats after long-term oral administration. J Nat Cancer Inst 77(4):977–982
Plewa MJ, Wagner ED, Muellner MG, Hsu KM, Richardson SD (2008) Comparative mammalian cell toxicity of N-DBPs and C-DBPs. In: Karanfil T, Krasner SW, Westerhoff P, Xie Y (eds) Occurrence, formation, health effects and control of disinfection by-products in drinking water, vol 995. American Chemical Society, Washington DC, pp 36–50
Plewa MJ, Wagner ED, Richardson S, Thruston ADJ, Woo YT, McKague AB (2004) Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts. Environ Sci Technol 38:4713–4722
Richardson SD, Fasano F, Ellington JJ, Crumley FG, Buettner KM, Evans JJ, Blount BC, Silva LK, Waite TJ, Luther GW, McKague AB, Miltner RJ, Wagner ED, Plewa MJ (2008) Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ Sci Technol 42:8330–8338
Woo YT, Lai D, McLain JL, Manibusan MK, Dellarco V (2002) Use of mechanism-based structure-activity relationships analysis in carcinogenic potential ranking for drinking water disinfection by-products. Environ Health Perspect 110(suppl 1):75–87
Chu WH, Gao NY, Deng Y, Krasner SW (2010) Precursors of dichloroacetamide, an emerging nitrogenous DBP formed during chlorination or chloramination. Environ Sci Technol 44(10):3908–3912
Fang J, Yang X, Ma J, Shang C, Zhao Q (2010) Characterization of algal organic matter and formation of DBPs from chlor(am)ination. Water Res 44(20):5897–5906
Yang X, Fan C, Shang C, Zhao Q (2010) Nitrogenous disinfection byproducts formation and nitrogen origin exploration during chloramination of nitrogenous organic compounds. Water Res 44(9):2691–2702
Hunter ES III, Rogers EH, Schmid JE, Richard A (1996) Comparative effects of haloacetic acids in whole embryo culture. Teratology 54:57–64
Hunter ES III, Tugman JA (1995) Inhibitors of glycolytic metabolism affect neurulation-stage mouse conceptuses in vitro. Teratology 52:317–323
Glaze WH, Henderson JE, Smith G (1975) Analysis of new chlorinated organic compounds formed by chlorination of municipal wastewater. In: Jolley RJ (ed) Water chlorination: environmental impact and health effects. Ann Arbor Science, Ann Arbor, MI, pp 139–159
Thomas RF, Weisner MJ, Brass HJ (1980) The fifth trihalomethane: dichloroiodomethane. Its stability and occurrence in chlorinated drinking water. In: Jolley RL, Brungs WA, Cumming RB, Jacobs VA (eds) Water chlorination: environmental impact and health effects, vol 3. Ann Arbor Science, Ann Arbor, MI, pp 161–168
Cancho B, Ventura F, Galceran M, Diaz A, Ricart S (2000) Determination, synthesis and survey of iodinated trihalomethanes in water treatment processes. Water Res 34:3380–3390
Richardson SD, Thruston AD Jr, Rav-Acha C, Groisman L, Popilevsky I, Juraev O, Glezer V, McKague AB, Plewa MJ, Wagner ED (2003) Tribromopyrrole, brominated acids, and other disinfection byproducts produced by disinfection of drinking water rich in bromide. Environ Sci Technol 37(17):3782–3793
Bichsel Y, Von Gunten U (1999) Oxidation of iodide and hypoiodous acid in the disinfection of natural waters. Environ Sci Technol 33(22):4040–4045
Bichsel Y, Von Gunten U (2000) Formation of iodo-trihalomethanes during disinfection and oxidation of iodide-containing waters. Environ Sci Technol 34:2784–2791
Duirk SE, Lindell C, Cornelison CC, Kormos J, Ternes TA, Attene-Ramos M, Osiol J, Wagner ED, Plewa MJ, Richardson SD (2011) Formation of toxic iodinated disinfection by-products from compounds used in medical imaging. Environ Sci Technol 45:6845–6854
Plewa MJ, Wagner ED, Jazwierska P, Richardson SD, Chen PH, McKague AB (2004) Halonitromethane drinking water disinfection byproducts: chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ Sci Technol 38(1):62–68
Krasner SW, Chinn R, Hwang CJ, Barrett S (1991) Analytical methods for brominated organic disinfection by-products. In: Proceedings of the 1990 American Water Works Association water quality technology conference, American Water Works Association, Denver, CO
Richardson SD, Thruston AD Jr, Caughran TV, Chen PH, Collette TW, Floyd TL, Schenck KM, Lykins BW Jr, Sun G, Majetich G (1999) Identification of new drinking water disinfection byproducts formed in the presence of bromide. Environ Sci Technol 33:3378–3383
Kundu B, Richardson SD, Swartz PD, Matthews PP, Richard AM, DeMarini DM (2004) Mutagenicity in Salmonella of halonitromethanes: a recently recognized class of disinfection by-products in drinking water. Mutat Res 562(1–2):39–65
Kundu B, Richardson SD, Granville CA, Shaughnessy DT, Hanley NM, Swartz PD, Richard AM, DeMarini DM (2004) Comparative mutagenicity of halomethanes and halonitromethanes in Salmonella TA100: structure-activity analysis and mutation spectra. Mutat Res 554(1–2):335–350
Choi J, Richardson SD (2005) Formation of halonitromethanes in drinking water. In: Proceedings of the international workshop on optimizing the design and interpretation of epidemiologic studies to consider alternative disinfectants of drinking water, U.S. EPA, Raleigh, NC
Chen PH, Richardson SD, Krasner SW, Majetich G, Glish GL (2002) Hydrogen abstraction and decomposition of bromopicrin and other trihalogenated disinfection byproducts by GC/MS. Environ Sci Technol 36(15):3362–3371
Choi J, Valentine RL (2002) Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: a new disinfection by-product. Water Res 36(4):817–824
Mitch WA, Sedlak DL (2002) Formation of N-nitrosodimethylamine (NDMA) from dimethylamine during chlorination. Environ Sci Technol 36:588–595
Jobb DB, Hunsinger R, Meresz O, Taguchi VY (1992) A study of the occurrence and inhibition of formation of N-nitrosodimethylamine (NDMA) in the Ohsweken water supply. In: Proceedings of the fifth national conference on drinking water, Winnipeg, Manitoba, Canada
Boyd JM, Hrudey SE, Li XF, Richardson SD (2011) Solid-phase extraction and high-performance liquid chromatography mass spectrometry analysis of nitrosamines in treated drinking water and wastewater. Trends Anal Chem 30(9):1410–1421
Mitch WA, Sharp JO, Trussell RR, Valentine RL, Alvarez-Cohen L, Sedlak DL (2003) N-nitrosodimethylamine (NDMA) as a drinking water contaminant: a review. Environ Eng Sci 20:389–404
Wilczak A, Assadi-Rad A, Lai HH, Hoover LL, Smith JF, Berger R, Rodigari F, Beland JW, Lazzelle LJ, Kincannon EG, Baker H, Heaney CT (2003) Formation of NDMA in chloraminated water coagulated with DADMAC cationic polymer. J Am Water Works Assoc 95(9):94–106
Kemper JM, Wale SS, Mitch WA (2010) Quaternary amines as nitrosamine precursors: a role for consumer products? Environ Sci Technol 44:1224–1231
California Department of Publich Health. Drinking water notification levels and response levels: an overview. Available at: http://www.cdph.ca.gov/certlic/drinkingwater/Documents/Notificationlevels/notificationlevels.pdf
Ontario Regulation 169/03. Ontario drinking water quality standards. Safe drinking water act, 2002. Available at: www.e-laws.gov.on.ca/html/regs/english/elaws_regs_030169_e.htm
Health Canada. N-nitrosodimethylamine (NDMA) in drinking water. Document for public comment. Prepared by the Federal-Provincial-Territorial Committee on drinking water. Available at: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/consult/_2010/ndma/draft-ebauche-eng.pdf
U.S. Environmental Protection Agency. Occurrence data accesing unregulated contaminant monitoring data – UCMR 2. Available at: http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/data.cfm#ucmr2010
Charrois JW, Arend MW, Froese KL, Hrudey SE (2004) Detecting N-nitrosamines in drinking water at nanogram per liter levels using ammonia positive chemical ionization. Environ Sci Technol 38:4835–4841
U.S. Environmental Protection Agency. Contaminant Candidate List 3 – CCL. Available at http://water.epa.gov/scitech/drinkingwater/dws/ccl/ccl3.cfm
Munch JW, Bassett MV (2004) EPA Method 521. Determination of nitrosamines in drinking water by solid-phase extraction and capillary column gas chromatography with large volume injection and chemical ionization tandem mass spectrometry (MS/MS), U.S. EPA, Cincinnati, OH. Available at: www.epa.gov/nerlcwww/m_521.pdf
Zhao YY, Boyd J, Hrudey SE, Li XF (2006) Characterization of new nitrosamines in drinking water using liquid chromatography tandem mass spectrometry. Environ Sci Technol 40(24):7636–7641
Schmidt CK, Brauch HJ (2008) N, N-dimethosulfamide as precursor for N-nitrosodimethylamine (NDMA) formation upon ozonation and its fate during drinking water treatment. Environ Sci Technol 42:6340–6346
Glezer V, Harris B, Tal N, Iosefzon B, Lev O (1999) Hydrolysis of haloacetonitriles: linear free energy relationship kinetics and products. Water Res 33:1939–1948
Reckhow DA, MacNeill AL, Platt TL, MacNeill AL, McClellan JN (2001) Formation and degradation of dichloroacetonitrile in drinking waters. J Water Supp Res Technol Aqua 50(1):1–13
Plewa MJ, Muellner MG, Richardson SD, Fasano F, Buettner KM, Woo YT, McKague AB, Wagner ED (2008) Occurrence, synthesis, and mammalian cell cytotoxicity and genotoxicity of haloacetamides: an emerging class of nitrogenous drinking water disinfection byproducts. Environ Sci Technol 42:955–961
Krasner SW, McGuire MJ, Jacangelo JG, Patania NL, Reagan KM, Aieta EM (1989) The occurrence of disinfection by-products in United States drinking water. J Am Water Works Assoc 81:41–53
Williams DT, LeBel GL, Benoit FM (1997) Disinfection by‐products in Canadian drinking water. Chemosphere 34:299–316
McGuire MJ, McLain JL, Obolensky A (2002) Information collection rule data analysis. American Water Works Association Research Foundation, Denver, CO
Najm IN, Krasner SW (1995) Effects of bromide and NOM on by-product formation. J Am Water Works Assoc 87(1):106–115
Kronberg L, Vartiainen T (1988) Ames mutagenicity and concentration of the strong mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and of its geometric isomer E-2-chloro-3-(dichloromethyl)-4-oxo-butenoic acid in chlorine-treated tap waters. Mutat Res 206:177–182
Suzuki N, Nakanishi J (1995) Brominated analogues of MX (3-chloro-4-(dichloromethyl)-5-hydroxy-2 (5H)-furanone) in chlorinated drinking water. Chemosphere 30:1557–1564
Kronberg L, Holmbom B, Reunanen M, Tikkanen L (1988) Identification and quantification of the Ames mutagenic compound 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone and of its geometric isomer (E)-2-chloro-3-(dichloromethyl)-4-oxobutenoic acid in chlorine-treated humic water and drinking water extracts. Environ Sci Technol 22:1097–1103
Komulainen H, Kosma VM, Vaittinen SL, Vartiainen T, Kaliste-Korhonen E, Lotjonen S, Tuominen RK, Tuomisto J (1997) Carcinogenicity of the drinking water mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone in the rat. J Nat Cancer Inst 89:848–856
Sasaki YF, Nishidate E, Izumiyama F, Watanabe-Akanuma M, Kinae N, Matsusaka N, Tsuda S (1997) Detection of in vivo genotoxicity of 3-chloro-4-(dichloromethyl)-5-hydroxy-2[5H]-furanone (MX) by the alkaline single cell gel electrophoresis (Comet) assay in multiple mouse organs. Mutat Res 393:47–53
Geter DR, Winn RN, Fournie JW, Norris MB, DeAngelo AB, Hawkins WE (2004) MX [3-chloro-4-(dichloromethyl)-5-hydroxy-2[5H]-furanone], a drinking-water carcinogen, does not induce mutations in the liver of cll transgenic medaka (Oryzias latipes). J Toxicol Environ Health A 67(5):373–383
Wright JM, Schwartz J, Vartiainen T, Mäki-Paakkanen J, Altshul L, Harrington JJ, Dockery DW (2002) 3-Chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) and mutagenic activity in Massachusetts drinking water. Environ Health Perspect 110(2):157–164
Liviac D, Creus A, Marcos R (2010) DNA damage induction by two halogenated acetaldehydes, byproducts of water disinfection. Water Res 44:2638–2646
Schulten HR, Schnitzer MA (1993) State of the art structural concept for humic substances. Naturwissenschaften 80:29–30
Qin F, Zhao YY, Zhao Y, Boyd JM, Zhou W, Li XF (2010) A toxic disinfection by-product, 2,6-dichloro-1,4-benzoquinone, identified in drinking water. Angew Chem Int Ed 49(4):790–792
Zhao Y, Qin F, Boyd JM, Anichina J, Li XF (2010) Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal Chem 82:4599–4605
Hoehn RC, Ellenberger CS, Gallagher DL, Wiseman ETV Jr, Benninger RW, Rosenblatt A (2003) ClO2 and by-product persistence in a drinking water system. J Am Water Works Assoc 95(4):141–150
Baribeau H, Prevost M, Desjardins R, Lafrance P, Gates DJ (2002) Chlorite and chlorate ion variability in distribution systems. J Am Water Works Assoc 94(7):96–105
Kurokawa Y, Takayama S, Konishi Y (1986) Long-term in vivo carcinogenicity tests of potassium bromate, sodium hypochlorite, and sodium chlorite conducted in Japan. Environ Health Perspect 69:221–235
National Toxicology Program (2005) Toxicology and carcinogenesis studies of sodium chlorate (CAS no. 7775-09-9) in F344/N rats and B6C3F1 mice (drinking water studies). Natl Toxicol Program Tech Rep, Ser 517, pp 1–255
U.S. Environmental Protection Agency. Unregulated Contaminant Monitoring Rule 3 (UCMR 3). http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/ucmr3/index.cfm
Khiari D, Krasner SW, Hwang CJ, Chinn R, Barrett S (1997) In: Proceedings of the 1996 American Water Works Association water quality technology conference, American Water Works Association, Denver, CO
Zhang X, Talley JW, Boggess B, Ding G, Birdsell D (2008) Fast selective detection of polar brominated disinfection byproducts in drinking water using precursor ion scans. Environ Sci Technol 42(17):6598–6603
Ding G, Zhang X (2009) A picture of polar iodinated disinfection byproducts in drinking water by (UPLC/)ESI-tqMS. Environ Sci Technol 43(24):9287–9293
Zhang X, Minear RA (2006) Formation, adsorption and separation of high molecular weight disinfection byproducts resulting from chlorination of aquatic humic substances. Water Res 40(2):221–230
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36:1202–1211
Wang P, He Y-L, Huang C-H (2011) Reactions of tetracycline antibiotics with chlorine dioxide and free chlorine. Water Res 45:1838–1846
Dodd MC, Shah AD, Von Gunten U, Huang C-H (2005) Interactions of fluoroquinolone antibacterial agents with aqueous chlorine: reaction kinetics, mechanisms, and transformation pathways. Environ Sci Technol 39:7065–7076
Wang P, He Y-L, Huang C-H (2010) Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: reaction kinetics, product and pathway evaluation. Water Res 44:5989–5998
Navalon S, Alvaro M, Garcia H (2008) Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three β-lactam antibiotics with ClO2. Water Res 42(8–9):1935–1942
Adachi A, Okano A (2008) Generation of cyanide ion by the reaction of hexamine and losartan potassium with sodium hypochlorite. J Health Sci 54(5):581–583
Dodd MC, Huang C-H (2004) Transformation of the antibacterial agent sulfamethoxazole in reactions with chlorine: kinetics, mechanisms, and pathways. Environ Sci Technol 2004:5607–5615
Shah AD, Kim JH, Huang C-H (2006) Reaction kinetics and transformation of carbadox and structurally related compounds with aqueous chlorine. Environ Sci Technol 40:7228–7235
Buth JM, Arnold WA, McNeill K (2007) Unexpected products and reaction mechanisms of the aqueous chlorination of cimetidine. Environ Sci Technol 41(17):6228–6233
Krkosek WH, Koziar SA, White RL, Gagnon GA (2011) Identification of reaction products from reactions of free chlorine with the lipid-regulator gemfibrozil. Water Res 45:1414–1422
Bedner M, MacCrehan WA (2006) Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ Sci Technol 40:516–522
Shen R, Andrews SA (2011) Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res 45:944–952
Schmidt CK, Sacher F, Brauch HJ (2006) Strategies for minimizing formation of NDMA and other nitrosamines during disinfection of drinking water. In: Proceedings of the AWWA Water Quality Technology Conference, Denver, CO
Huerta-Fontela M, Galcerán MT, Ventura F (2011) Presence and removal of illicit drugs in conventional drinking water treatment plants. In: Castiglioni S, Zuccato E, Fanelli R (eds) Illicit drugs in the environment: occurrence, analysis, and fate using mass spectrometry. Wiley, Hoboken, New Jersey, pp 205–222
McDowell DC, Huber MM, Wagner M, Von Gunten U, Ternes TA (2005) Ozonation of carbamazepine in drinking water: Identification and kinetic study of major oxidation products. Environ Sci Technol 39(20):8014–8022
Kosjek T, Andersen HR, Kompare B, Ledin A, Heath E (2009) Fate of carbamazepine during water treatment. Environ Sci Technol 43(16):6256–6261
Li Z, Fenet H, Gomez E, Chiron S (2011) Transformation of the antiepileptic drug oxcarbazepine upon different water disinfection processes. Water Res 45:1587–1596
Benner J, Ternes TA (2009) Ozonation of metoprolol: Elucidation of oxidation pathways and major oxidation products. Environ Sci Technol 43(14):5472–5480
Benner J, Ternes TA (2009) Ozonation of propanolol: formation of oxidation products. Environ Sci Technol 43(13):5086–5093
Pereira RO, Postigo C, López de Alda M, Daniel LA, Barceló D (2011) Removal of estrogens through water disinfection processes and formation of by-products. Chemosphere 82:789–799
Brix R, Bahi N, López de Alda MJ, Farre M, Fernandez JM, Barceló (2009) Identification of disinfection by-products of selected triazines in drinking water by LC-Q-ToF-MS/MS and evaluation of their toxicity. J Mass Spectrom 44(3):330–337
Lopez A, Mascolo G, Tiravanti G, Passino R (1997) Degradation of herbicides (ametryn and isoproturon) during water disinfection by means of two oxidants (hypochlorite and chlorine dioxide). Water Sci Technol 35:129–136
Lopez A, Mascolo G, Tiravanti G, Passino R (1998) Formation of herbicide degradation by-products during groundwater disinfection: an LC-MS investigation. J Anal Chem 53:856–860
Mascolo G, Lopez A, Passino R, Ricco G, Tiravanti G (1994) Degradation of sulphur containing S-triazines during water chlorination. Water Res 28:2499–2506
Sandin-España P, Magrans JO, Garcia-Baudin JM (2005) Study of clethodim degradation and by-product formation in chlorinated water by HPLC. Chromatographia 62(3–4):133–137
Duirk SE, Collette TW (2006) Degradation of chlorpyrifos in aqueous chlorine solutions: pathways, kinetics and modeling. Environ Sci Technol 40:546–551
Mehrsheikh A, Bleeke M, Brosillon S, Laplanche A, Roche P (2006) Investigation of the mechanism of chlorination of glyphosate and glycine in water. Water Res 40(16):3003–3014
Lin CH, Lerch RN, Garrett HE, George MF (2003) Degradation of isoxaflutole (Balance) herbicide by hypochlorite in tap water. J Agric Food Chem 51:8011–8014
Mascolo G, Lopez A, James H, Fielding M (2001) By-products formation during degradation of isoproturon in aqueous solution. II: chlorination. Water Res 35(7):1705–1713
Zambodin CG, Losito II, Palmisano F (2000) Liquid chromatography/electrospray ionisation sequential mass spectrometric identification of the main chlortoluron by-products during water disinfection using chlorine. Rapid Commun Mass Spectrom 14:824–828
Xu B, Tian F-X, C-y Hu, Lin Y-L, S-j X, Rong R, Li D-P (2011) Chlorination of chlortoluron: kinetics, pathways and chloroform formation. Chemosphere 83:909–916
Wu J, Chongyu L, Sing Chan GY (2009) Organophosphorous pesticide ozonation and formation of oxon intermediates. Chemosphere 76:1308–1314
Terasaki M, Makino M (2008) Determination of chlorinated by-products of parabens in swimming pool water. Int J Environ Anal Chem 88(13):911–922
Tay KS, Rahman NA, Bin Abas MR (2010) Ozonation of parabens in aqueous solutions: kinetics and mechanisms of degradation. Chemosphere 81:1446–1453
Rule KL, Ebbett VR, Vikesland PJ (2005) Formation of chloroform and chlorinated organics by free-chlorine-mediated oxidation of triclosan. Environ Sci Technol 39:3176–3185
Greyshock AE, Vikesland PJ (2006) Triclosan reactivity in chloraminated waters. Environ Sci Technol 40:2615–2622
Nakajima M, Kawakami T, Niino T, Takahashi Y, Onodera S (2009) Aquatic fate of sunscreen agents octyl-4-methoxycinnamate and octyl-4-dimethylaminobenzoate in model swimming pools and the mutagenic assays of their chlorination byproducts. J Health Sci 55(3):363–372
Negreira N, Canosa P, Rodriguez I, Ramil M, Rubi E, Cela RJ (2008) Study of some UV filters stability in chlorinated water and identification of halogenated by-products by gas chromatography–mass spectrometry. J Chromatogr A 1178(1–2):206–214
Kuhlich P, Göstl R, Teichert P, Piechotta C, Nehls I (2011) Transformation of polycyclic musks AHTN and HHCB upon disinfection with hypochlorite: two new chlorinated disinfection by-products (CDBP) of AHTN and a possible source for HHCB-lactone. Anal Bioanal Chem 399:3579–3588
Joll CA, Alessandrino MJ, Heitz A (2010) Disinfection by-products from halogenation of aqueous solutions of terpenoids. Water Res 44(1):232–242
Qi F, Xu BB, Chen ZL, Ma J, Sun DZ, Zhang LQ (2009) Efficiency and products investigations on the ozonation of 2-methylisoborneol in drinking water. Water Environ Res 81:2411–2419
Petrovic A, Diaz A, Ventura F, Barceló D (2003) Occurrence and removal of estrogenic short-chain ethoxy nonylphenolic compounds and their halogenated derivatives during drinking water production. Environ Sci Technol 37:4442–4448
Hu JY, Aizawa T, Ookubo S (2002) Products of aqueous chlorination of bisphenol A and their estrogenic activity. Environ Sci Technol 36:1980–1987
Deborde M, Rabouan S, Mazellier P, Duguet J-P, Legube B (2008) Oxidation of bisphenol A by ozone in aqueous solutions. Water Res 42:4299–4308
Merel S, Clement M, Thomas O (2010) State of the art on cyanotoxins in water and their behaviour towards chlorine. Toxicon 55:677–691
Ashley DL, Blount BC, Singer PC, Depaz E, Wilkes C, Gordon S, Lyu C, Masters J (2005) Changes in blood trihalomethane concentrations resulting from differences in water quality and water use activities. Arch Environ Occup Health 60(1):7–15
Haddad S, Tardif GC, Tardif R (2006) Development of physiologically based toxicokinetic models for improving the human indoor exposure assessment to water contaminants: trichloroethylene and trihalomethanes. J Toxicol Environ Health A 69(23):2095–2136
Leavens TL, Blount BC, DeMarini DM, Madden MC, Valentine JL, Case MW, Silva LK, Warren SH, Hanley NM, Pegram RA (2007) Disposition of bromodichloromethane in humans following oral and dermal exposure. Toxicol Sci 99(2):432–445
Li J, Blatchley ER III (2007) Volatile disinfection byproduct formation resulting from chlorination of organic - Nitrogen precursors in swimming pools. Environ Sci Technol 41(19):6732–6739
Xu X, Weisel CP (2005) Dermal uptake of chloroform and haloketones during bathing. J Exp Anal Environ Epidemiol 15(4):289–296
Xu X, Weisel CP (2005) Human respiratory uptake of chloroform and haloketones during showering. J Exp Anal Environ Epidemiol 15(1):6–16
Zwiener C, Richardson SD, DeMarini DM, Grummt T, Glauner T, Frimmel FH (2007) Drowning in disinfection byproducts? Assessing swimming pool water. Environ Sci Technol 41:363–372
Walse SS, Mitch WA (2008) Nitrosamine carcinogens also swim in chlorinated pools. Environ Sci Technol 42:1032–1037
Weaver WA, Li J, Wen YL, Johnston J, Blatchley MR, Blatchley ER III (2009) Volatile disinfection by-product analysis from chlorinated indoor swimming pools. Water Res 43:3308–3318
Weisel CP, Richardson SD, Nemery B, Aggazzotti G, Baraldi E, Blatchley ER III, Blount BC, Carlsen KH, Eggleston PA, Frimmel FH, Goodman M, Gordon G, Grinshpun SA, Heederik D, Kogevinas M, LaKind JS, Nieuwenhuijsen MJ, Piper FC, Sattar SA (2009) Childhood asthma and environmental exposures at swimming pools: state of the science and research recommendations. Environ Health Perspect 117:500–507
LaKind JS, Richardson SD, Blount BC (2010) The good, the bad, and the volatile: can we have both healthy pools and healthy people? Environ Sci Technol 44(9):3205–3210
Font-Ribera L, Kogevinas M, Zock J, Gómez FP, Barreiro E, Nieuwenhuijsen MJ, Fernandez P, Lourencetti C, Perez-Olabarria M, Bustamante M, Marcos R, Grimalt JO, Villanueva CM (2010) Short-term changes in respiratory biomarkers after swimming in a chlorinated pool. Environ Health Perspect 118(11):1538–1544
Kogevinas M, Villanueva CM, Font-Ribera L, Liviac D, Bustamante M, Espinoza F, Nieuwenhuijsen MJ, Espinosa A, Fernandez P, DeMarini DM, Grimalt JO, Grummt T, Marcos R (2010) Genotoxic effects in swimmers exposed to disinfection by-products in indoor swimming pools. Environ Health Perspect 118(11):1531–1537
Richardson SD, DeMarini DM, Kogevinas M, Fernandez P, Marco E, Lourencetti C, Balleste C, Heederik D, Meliefste K, McKague AB, Marcos R, Font-Ribera L, Grimalt JO, Villanueva CM (2010) What's in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118(11):1523–1530
Liviac D, Wagner ED, Mitch WA, Altonji MJ, Plewa MJ (2010) Genotoxicity of water concentrates from recreational pools after various disinfection methods. Environ Sci Technol 44(9):3527–3532
Cardador MJ, Gallego M (2011) Haloacetic acids in swimming pools: swimmer and worker exposure. Environ Health Perspect 118:1545–1550
Plewa MJ, Wagner ED, Mitch WA (2011) Comparative mammalian cell cytotoxicity of water concentrates from disinfected recreational pools. Environ Sci Technol 45:4159–4165
Amer K, Karanfil T (2011) Formation of disinfection by-products in indoor swimming pool water. The contribution from filling water natural organic matter and swimmer body fluids. Water Res 45:926–932
Erdinger L, Kuhn KP, Kirsch F, Feldhues R, Frobel T, Nohynek B, Gabrio T (2004) Pathways of trihalomethane uptake in swimming pools. Int J Hygiene Environ Health 207(6):571–575
Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castaño-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M (2007) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165(2):148–156
Bernard A, Nickmilder M, Voisin C, Sardella A (2009) Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics 124(4):1110–1118
Goodman M, Hays S (2008) Asthma and swimming: a meta-analysis. J Asthma 45(8):639–647
Voisin C, Sardella A, Marcucci F, Bernard A (2010) Infant swimming in chlorinated pools and the risks of bronchiolitis, asthma and allergy. Eur Respiratory J 36(1):41–47
Simmons JE, Richardson SD, Teuschler LK, Miltner RJ, Speth TF, Schenck KM, Hunter ES III, Rice G (2008) Research issues underlying the four-lab study: integrated disinfection by-products mixtures research. J Toxicol Environ Health A 71:1125–1132
Pressman JG, Richardson SD, Speth TF, Miltner RJ, Narotsky MG, Hunter ES III, Rice GE, Teuschler LK, McDonald A, Parvez S, Krasner SW, Weinberg HS, McKague AB, Parrett CJ, Bodin N, Chinn R, Lee CFT, Simmons JE (2010) Concentration, chlorination, and chemical analysis of drinking water for disinfection byproduct mixtures health effects research: U.S. EPA’s four lab study. Environ Sci Technol 44:7184–7192
Munch JW (1995) EPA Method 524.2. Measurement of purgeable organic compounds in water by capillary column gas chromatography/mass spectrometry, rev 4.1, U.S. EPA, Cincinnati, OH. Available at: www.caslab.com/EPA-Methods/PDF/524_2.pdf
Munch DJ, Munch JW, Pawlecki AM (1995) EPA Method 552.2. Determination of haloacetic acids and dalapon in drinking water by liquid-liquid extraction, derivatization and gas chromatography with electron capture detection. U.S. EPA, Cincinnati, OH, Available at: http://www.caslab.com/EPA-Methods/PDF/552_2.pdf
Domino MM, Pepich BV, Munch DJ, Fair PS, Xie Y (2003) EPA Method 552.3. Determination of haloacetic acids and dalapon in drinking water by liquid-liquid microextraction, derivatization, and gas chromatography with electron capture detection. EPA 815-B-03-002. U.S. EPA, Cincinnati, OH. Available at: http://www.epa.gov/ogwdw/methods/pdfs/methods/met552_3.pdf
Zwiener C, Richardson SD (2005) Drinking water disinfection by-product analysis by LC/MS and LC/MS/MS. Trends Anal Chem 24:613–621
Zhang X, Minear RA (2002) Characterization of high molecular weight disinfection byproducts resulting from chlorination of aquatic humic substances. Environ Sci Technol 36(19):4033–4038
Zhang X, Minear RA, Barrett SE (2005) Characterization of high molecular weight disinfection byproducts from chlorination of humic substances with/without coagulation pretreatment using UF-SEC-ESI-MS/MS. Environ Sci Technol 39(4):963–972
Creed JT, Brockhoff CA, Martin TD (1997) EPA Method 321.8. Determination of bromate in drinking waters by ion chromatography inductively coupled plasma-mass spectrometry. U.S. EPA, Cincinnati, OH, Available at: http://www.epa.gov/microbes/m_321_8.pdf
Zaffiro AD, Zimmerman M, Pepich BV, Slingsby RW, Jack RF, Pohl CA, Munch DJ (2009) EPA Method 557. Determination of haloacetic acids, bromate, and dalapon in drinking water by ion chromatography electrospray ionization tandem mass spectrometry (IC-ESI-MS/MS). U.S. EPA, Cincinnati, OH, Available at: http://water.epa.gov/scitech/drinkingwater/labcert/upload/met557.pdf
Roehl R, Slingsby R, Avdalovic N, Jackson PE (2002) Applications of ion chromatography with electrospray mass spectrometric detection to the determination of environmental contaminants in water. J Chromatogr A 956(1–2):245–254
Paull B, Barron L (2004) Using ion chromatography to monitor haloacetic acids in drinking water: a review of current technologies. J Chromatogr A 1046(1–2):1–9
Shi HL, Adams C (2009) Rapid IC-ICP/MS method for simultaneous analysis of iodoacetic acids, bromoacetic acids, bromate, and other related halogenated compounds in water. Talanta 79:523–527
Wagner P, Pepich BV, Pohl C, Srinivasan K, De Borba B, Lin R, Munch DJ (2009) EPA Method 302.0. Determination of bromate in drinking water using two-dimensional ion chromatography with suppressed conductivity detection. U.S. EPA, Cincinnati, OH, Available at: http://water.epa.gov/scitech/drinkingwater/labcert/upload/met302_0.pdf
Pfaff JD, Hautman DP, Munch DJ (1997) EPA Method 300.1. Determination of inorganic anions in drinking water by ion chromatography. U.S. EPA, Cincinnati, OH, Available at: http://water.epa.gov/scitech/drinkingwater/labcert/upload/met300.pdf
Na C, Olson TM (2004) Stability of cyanogen chloride in the presence of free chlorine and monochloramine. Environ Sci Technol 38(22):6037–6043
Yang X, Shang C (2005) Quantification of aqueous cyanogen chloride and cyanogen bromide in environmental samples by MIMS. Water Res 39(9):1709–1718
Riter LS, Charles L, Turowski M, Cooks RG (2001) External interface for trap-and-release membrane introduction mass spectrometry applied to the detection of inorganic chloramines and chlorobenzenes in water. Rapid Commun Mass Spectrom 15:2290–2295
Rios RVRA, Da Rocha LL, Vieira TG, Lago RM, Augusti R (2000) On-line monitoring by membrane introduction mass spectrometry of chlorination of organics in water. Mechanistic and kinetic aspects of chloroform formation. J Mass Spectrom 35(5):618–624
Kristensen GH, Klausen MM, Hansen VA, Lauritsen FR (2010) On-line monitoring of the dynamics of trihalomethane concentrations in a warm public swimming pool using an unsupervised membrane inlet mass spectrometry system with off-site real-time surveillance. Rapid Commun Mass Spectrom 24(1):30–34
Guevremont R, Ding L, Ells B, Barnett DA, Purves RW (2001) Atmospheric pressure ion trapping in a tandem FAIMS-FAIMS coupled to a TOFMS: studies with electrospray generated gramicidin S ions. J Am Soc Mass Spectrom 12(12):1320–1330
Ells B, Barnett DA, Purves RW, Guevremont R (2000) Detection of nine chlorinated and brominated haloacetic acids at part-per-trillion levels using ESI-FAIMS-MS. Anal Chem 72(19):4555–4559
Gabryelski W, Wu F, Froese KL (2003) Comparison of high-field asymmetric waveform ion mobility spectrometry with GC methods in analysis of haloacetic acids in drinking water. Anal Chem 75(10):2478–2486
Barnett DA, Guevremont R, Purves RW (1999) Determination of parts-per-trillion levels of chlorate, bromate, and iodate by electrospray ionization/high-field asymmetric waveform ion mobility spectrometry/mass spectrometry. Appl Spectrosc 53(11):1367–1374
Planas C, Palacios O, Ventura F, Rivera J, Caixach J (2008) Analysis of nitrosamines in water by automated SPE and isotope dilution GC/HRMS - Occurrence in the different steps of a drinking water treatment plant, and in chlorinated samples from a reservoir and a sewage treatment plant effluent. Talanta 76:906–913
Yuan YZ, Liu X, Boyd JM, Qin F, Li J, Li XF (2009) Identification of N-nitrosamines in treated drinking water using nanoelectrospray ionization high-field asymmetric waveform ion mobility spectrometry with quadrupole time-of-flight mass spectrometry. J Chromatogr Sci 47(1):92–96
Zhao YY, Liu X, Boyd JM, Qin F, Li J, Li XF (2009) Identification of N-nitrosamines in treated drinking water using nanoelectrospray ionization high-field asymmetric waveform ion mobility spectrometry with quadrupole time-of-flight mass spectrometry. J Chromatogr Sci 47:92–96
Kubwabo C, Stewart B, Gauthier SA, Gauthier BR (2009) Improved derivatization technique for gas chromatography–mass spectrometry determination of 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone in drinking water. Anal Chim Acta 649(2):222–229
Vincenti M, Biazzi S, Ghiglione N, Valsania MC, Richardson SD (2005) Comparison of highly-fluorinated chloroformates as direct aqueous sample derivatizing agents for hydrophilic analytes and drinking-water disinfection by-products. J Am Soc Mass Spectrom 16(6):803–813
Vincenti M, Fasano F, Valsania MC, Guarda P, Richardson SD (2010) Application of the novel 5-chloro-2,2,3,3,4,4,5,5-octafluoro-1-pentyl chloroformate derivatizing agent for the direct determination of highly polar water disinfection byproducts. Anal Bioanal Chem 397(1):43–54
Richardson SD, Caughran TV, Poiger T, Guo Y, Gene Crumley F (2000) Application of DNPH derivatization with LC/MS to the identification of polar carbonyl disinfection by-products in drinking water. Ozone Sci Engin 22(6):653–675
Zwiener C, Glauner T, Frimmel FH (2003) Liquid chromatography/electrospray ionization tandem mass spectrometry and derivatization for the identification of polar carbonyl disinfection by-products. In: Ferrer I, Thurman EM (eds) Liquid chromatography/mass spectrometry, MS/MS and time-of-flight MS, ACS symposium series, vol 850. American Chemical Society, Washington DC, pp 356–375
Richardson SD, Karst U (2001) A new tailor-made derivatizing agent for identifying polar carbonyl DBPs in drinking water. In: Proceedings of the American Chemical Society conference. American Chemical Society, Washington DC
Emmert GL, Geme G, Brown MA, Simone PS Jr (2009) A single automated instrument for monitoring total trihalomethane and total haloacetic acid concentrations in near real-time. Anal Chim Acta 656(1–2):1–7
Echigo S, Zhang X, Minear RA, Plewa MJ (2000) Differentiation of total organic brominated and chlorinated compounds in total organic halide measurement: a new approach with an ion chromatographic technique. In: Barrett SE, Krasner SW, Amy GL (eds) Natural organic matter and disinfection by-products: characterization and control in drinking water. American Chemical Society, Washington DC, pp 330–342
Zhai H, Zhang X (2011) Formation and decomposition of new and unknown polar brominated disinfection byproducts during chlorination. Environ Sci Technol 45(6):2194–2201
Acknowledgments
This manuscript has been reviewed in accordance with the U.S. EPA’s peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U.S. EPA. Cristina Postigo acknowledges support from the EU through the FP7 program: Marie Curie International Outgoing Fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg 2011
About this chapter
Cite this chapter
Richardson, S.D., Postigo, C. (2011). Drinking Water Disinfection By-products. In: Barceló, D. (eds) Emerging Organic Contaminants and Human Health. The Handbook of Environmental Chemistry(), vol 20. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2011_125
Download citation
DOI: https://doi.org/10.1007/698_2011_125
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-28131-0
Online ISBN: 978-3-642-28132-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)