Abstract
A novel derivatizing agent, 5-chloro-2,2,3,3,4,4,5,5-octafluoropentyl chloroformate (ClOFPCF), was synthesized and tested as a reagent for direct water derivatization of highly polar and hydrophilic analytes. Its analytical performance satisfactorily compared to a perfluorinated chloroformate previously described, namely 2,2,3,3,4,4,5,5-octafluoropentyl chloroformate (OFPCF). The chemical properties (reactivity, selectivity, derivatization products, and their chromatographic and spectral features) for ClOFPCF were investigated using a set of 39 highly polar standard analytes, including, among others, hydroxylamine, malic and succinic acids, resorcinol, hydroxybenzaldehyde, and dihydroxybenzoic acid. Upon derivatization, the analytes were extracted from the aqueous solvent and analyzed by gas chromatography (GC)-mass spectrometry (MS) in the electron-capture negative ionization (ECNI) mode. Positive chemical ionization (PCI)-MS was used for confirming the molecular ions, which were virtually absent in the ECNI mass spectra. ClOFPCF showed good reaction efficiency, good chromatographic and spectroscopic properties (better than with OFPCF), good linearity in calibration curves, and low detection limits (0.3–1 µg/L). A unique feature of the derivatizations with ClOFPCF, and, in general, highly fluorinated chloroformates, is their effectiveness in reacting with carboxylic, hydroxylic, and aminic groups at once, forming multiply-substituted non-polar derivatives that can be easily extracted from the aqueous phase and determined by GC-ECNI-MS. The entire procedure from raw aqueous sample to ready-to-inject hexane solution of the derivatives requires less than 10 min. Another benefit of this procedure is that it produced stable derivatives, with optimal volatility for GC separation, and high electron affinity, which allows their detection as negative ions at trace level. In addition, their mass spectra exhibits chlorine isotopic patterns that clearly indicate how many polar hydrogens of the analyte undergo derivatization. Finally, derivatization with ClOFPCF was used successfully to identify 13 unknown highly polar disinfection byproducts (DBPs) in ozonated fulvic and humic acid aqueous solutions and in real ozonated drinking water.

The derivatization of hydroxylamine with 5-chloro-2,2,3,3,4,4,5,5-octafluoropentyl chloroformate yields optimal gas chromatographic separation despite a 27-fold molecular weight increment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drinking water disinfection byproducts (DBPs) are mainly formed by reaction of a disinfectant (such as chlorine, ozone, chloramine, or chlorine dioxide) with natural organic matter (NOM) present in most water sources [1, 2]. While the use of disinfectants to kill bacteria and maintain the safety of drinking water saved many human lives in the last century, at the same time, some DBPs have been shown to cause cancer in laboratory animals [3]. Consequently, a few DBPs, including trihalomethanes (THMs), haloacetic acids, and bromate, have been regulated in the United States, European Union, and other countries, but a clear chemical and toxicological characterization of most DBPs has still to be accomplished. Currently, more than 50% of the total organic halogen (TOX) formed during the chlorination of drinking water and more than 50% of the assimilable organic carbon (AOC) formed during ozonation of drinking water has not been accounted for in identified DBPs [3]. Indeed, exhaustive DBP identification is very complex because of the variety of the disinfection methods and the extensive variability of NOM.
The use of ozone to disinfect drinking water results in a decreased concentration of regulated DBPs, such as THMs. In addition, ozone is a more effective biocide than chlorine, particularly for chlorine-resistant microbes and spores, such as Cryptosporidium oocysts. All disinfection strategies that make use of ozone produce (1) decreased formation of halogenated DBPs, (2) change of DBP speciation, and (3) appearance of few DBPs of potential toxicological concern, many of which are not formed by any other disinfection processes [1, 3]. It is believed that most DBPs formed by ozone are highly hydrophilic and non-toxic, but experimental results are lacking, since most of these compounds cannot be detected by common analytical techniques. DBPs of this sort are typically polyacids, hydroxyacids, ketoacids, glycoxals, hydroxylamines, aminoacids, aminoalcohols, and glycols [4–8].
The characterization of ozone DBPs in aqueous matrices is challenging, due to the lack of direct analytical methods, especially for small and highly polar DBPs [9]. The difficulties in determining such hydrophilic compounds are encountered in two fundamental steps: (a) their extraction from the aqueous matrix and (b) their chromatographic separation and detection. Most derivatization procedures require anhydrous conditions otherwise the reagents are instantly hydrolyzed. Therefore, an initial extraction step, followed by solvent evaporation is commonly performed before the derivatization. On the other hand, derivatization of highly polar substances directly in water became possible using hydrophobic chloroformates [10, 11], such as n-hexyl chloroformate (HCF) [12–15]. For hydrophobic chloroformates, the hydrolysis kinetics is generally slower than the derivatization kinetics because of their poor solubility in water. The derivatization reaction is believed to take place at the organic-water interface or through a phase-transfer mechanism, provided that adequate phase mixing is assured [14]. Despite the high derivatization efficiency of HCF with carboxylic, hydroxylic and aminic compounds, polysubstituted derivatives obtained from tri-, tetra-, and penta-functional analytes (e.g., dihydroxybenzoic acid, tartaric acid, citric acid) are not sufficiently volatile to efficiently elute from a GC column. To overcome this drawback and improve the sensitivity, highly fluorinated alkyl- and aryl chloroformates were synthesized [16–19] for the derivatization of a large variety of highly polar compounds, which were subsequently detected by electron-capture negative ionization (ECNI)-MS. Hušek, Šimek, and coworkers obtained successful derivatization of aminoacids using two fluorinated alkyl chloroformates (namely, 2,2,3,3,3-pentafluoropropyl- and 2,2,3,3,4,4,4-heptafluorobutyl chloroformate) [20–23].
The present study introduces a novel derivatizing agent, 5-chloro-2,2,3,3,4,4,5,5-octafluoropentyl chloroformate (ClOFPCF), which was purposely synthesized for the detection of highly polar DBPs, and compares its analytical performances with a perfluorinated chloroformate previously described, namely 2,2,3,3,4,4,5,5-octafluoropentyl chloroformate (OFPCF) [19].
Experimental
Chemicals and standard solutions
The following acids were purchased from Sigma-Aldrich (St. Louis, MO, USA): butyric, malic, malonic, methylmalonic, pyruvic, succinic, tartronic, citraconic, mesaconic, itaconic, trans-trans-muconic, 2- and 4-hydroxybenzoic, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- and 3,5-dihydroxybenzoic, and 4-hydroxyphenylacetic acid. 2,2,3,3,4,4,5,5-Octafluoropentan-1-ol, hydroxylamine, 3-aminophenol, 2-, 3-, and 4-hydroxybenzaldehyde, 2-, 3-, and 4-hydroxybenzyl alcohol, 2,3-, 2,5-, and, 3,5-dihydroxybenzyl alcohol, 2- and 5-methylresorcinol, o-, m- and p-cresol and pyridine were supplied by Sigma-Aldrich. Tartronic acid, 3-hydroxybenzoic acid and 3-aminopropanol were from Merck; 3-aminobenzoic acid was from Carlo Erba (Milan, Italy); 3,4-dihydroxybenzaldehyde was from Fluka (Buchs SG, Switzerland); 5-H-octafluoropentanoic acid and perfluoroheptane were from Apollo Scientific Ltd. (Bredbury, U.K.). Suwannee River fulvic and humic acids were supplied from the International Humic Substances Society, St. Paul, MN.
Separate stock solutions were prepared by dissolving the standards in ultrapure water. All standard solutions were stored at 4 °C until use.
Synthesis of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentyl chloroformate
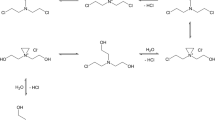
OFPCF was synthesized from 2,2,3,3,4,4,5,5-octafluoropentan-1-ol (commercially available) as previously described [18]. ClOFPCF was synthesized from 5-chloro-2,2,3,3,4,4,5,5-octafluoropentan-1-ol (not commercially available), which in turn was synthesized from 5-H-octafluoropentanoic acid. The overall synthetic protocol for the alcohol is summarized in Scheme 1, and the specific details follow.
Synthesis of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentanoic acid
Forty grams of 5-H-octafluoropentanoic acid was dissolved in 160 g of perfluoroheptane and placed in a photochemical reactor, which included a 150 W high pressure Hg lamp. To eliminate dissolved oxygen the solution was maintained under a low nitrogen stream for about 30 min. Once the Hg lamp was turned on, 6 L/h of chlorine gas was bubbled into the solution for about 45 min, while maintaining the reaction temperature between 35 and 40 °C. At the end of the reaction time, the lamp was kept on until the HCl formed and the excess of chlorine were eliminated by nitrogen sparging. Then, the solution was transferred into a 250-ml round-bottom flask and the perfluoroheptane solvent was distilled (b.p. = 79 °C), leaving a liquid residue of 35.2 g of 5-chloro-octafluoropentanoic acid (77%).
NMR analysis confirmed the structure of 5-chloro-octafluoropentanoic acid.
Chemical shifts 19F (ppm; ref. CFCl3), −69.0 (t, 2F(a), J F–F = 15 Hz); −120.5 (2F(b)); −122.6 (2F(c)); −119.8 (2F(d)).
Synthesis of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentanoate ethyl
Thirty grams of thionyl chloride was placed in a round-bottom flask with 500 mg of pyridine and the solution was stirred for 10 min at room temperature. In a dropping funnel 33.5 mg of 5-chloro-octafluoropentanoic acid was placed and cooled at −5 °C. Then the round-bottom flask was placed in an oil bath at 75 °C. While maintaining the solution under continuous magnetic stirring, the 5-chloro-octafluoropentanoic acid was slowly added dropwise into the flask. The flask was kept in the oil bath for 2 h. The excess thionyl chloride was eliminated by distillation (T head = 79 °C). The reaction was maintained at 40 °C under stirring, while 7.0 g of absolute ethanol was added dropwise. Subsequently, the temperature of the reaction batch was increased to 83 °C to eliminate the HCl formed under a stream of nitrogen. The excess ethanol was eliminated by distillation at 130 °C, leaving a residue of 25 g of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentanoate ethyl (yield, 75%), whose structure was confirmed by NMR analysis.
Chemical shifts 19F (ppm; ref. CFCl3), −69.0 (t, 2F(a), J F–F = 15 Hz); −120.4 (2F(b)); −122.6 (2F(c)); −119.4 (2F(d)).
Chemical shifts 1H (ppm; ref. TMS), 4.4 (q, 2H); 1.3 (t, 3H).
Synthesis of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentan-1-ol
Twenty-one grams of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentanoate ethyl was dissolved in absolute ethanol (6.2 g) and placed in a round-bottom flask. In a dropping funnel, NaBH4 (2.0 g) was dissolved in absolute ethanol (56 g). The round-bottom flask was submerged in an ice bath and cooled to 5 °C while keeping the solution under continuous stirring. The NaBH4 solution in the dropping funnel was added slowly to the reaction, while maintaining the temperature below 10 °C. Once the flask reached room temperature, 60 mL of distilled water was added and the resulting solution was acidified by slowly adding HCl 37% (5.2 g) under stirring for about 30 min. The solution was transferred to a separatory funnel together with 85 mL of distilled water and the two layers were allowed to separate. The reaction product was purified by distillation (bp = 66 °C at 42 mbar). 5-chloro-2,2,3,3,4,4,5,5-octafluoropentan-1-ol (10.4 g) was obtained. The structure was confirmed by NMR analysis:
Chemical shifts 19F (ppm; ref. CFCl3), −69.0 (t, 2F(a), J F–F = 15 Hz); −121.0 (2F(b)); −124.0 (2F(c)); −123.5 (2F(d)).
Chemical shifts 1H (ppm; ref. TMS), 3.9 (t, 2H, J H–F = 14 Hz).
Synthesis of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentylchloroformate
One gram of bis(trichloromethyl)carbonate was dissolved in acetone (5 mL) and cooled at −15 °C in a 25-mL septum-sealed vial. One hundred sixty microliters of pyridine was added by a syringe perforating the Teflon septum, and the solution was stirred for 1 h. Then, 313 µL of 5-chloro-2,2,3,3,4,4,5,5-octafluoropentan-1-ol dissolved in acetone (8 mL) was slowly added via a syringe (four aliquots of 2 mL each, at 20 min intervals), while maintaining the solution at −15 °C and eliminating equal volumes of gas (phosgene) from the vial before each addition (the same syringe was used). The gas eliminated from the reaction vial was neutralized in a NaOH solution. The reaction was kept at −15 °C for 2 h after the last addition and then allowed to reach room temperature.
The reaction yield was ∼95%. Although the crude chloroformate solution in acetone still contained small amounts of pyridinium chloride, hydrochloric acid, and phosgene, the presence of these contaminants proved to increase the stability of the chloroformate to at least one month (at −20 °C). Therefore, no further purification of the derivatizing agent solution was undertaken. As for other highly fluorinated chloroformates [18, 19], this raw solution was directly used in the derivatization reactions described below.
Sample preparation
Drinking water samples were collected at the Gwinnet County (GA, USA) ozonation plant. Three different samples were analyzed: (a) raw (untreated) water (b) ozonated water (intermediate ozonation step, before post chlorination) and (c) final, finished water (after secondary disinfection by chlorine). One liter of each water sample was lyophilized to dryness, the dry powder scraped from the lyophilization flask, and a small amount of methanol added afterwards to each flask to remove the remaining material. The solutions were placed in separate vials and blown down to dryness. From the solid residues scraped from the lyophilization flasks, aliquots of about 10 mg were weighted and dissolved in 10 mL of ultrapure water for the subsequent derivatization and analysis.
Ozonation of fulvic and humic acids
Stock solutions of humic and fulvic acids were prepared by dissolving 5 mg of solid material in about 50 mL of water, then carefully adding NaOH 0.1 N until pH 7.5–8.0 was reached. The final solution volume was adjusted to 100 mL, in order to obtain 50 mg/L solutions. Working solutions at 10 mg/L concentration were obtained by water dilution from the latter.
The working solutions of humic and fulvic acid were directly ozonated using an ozone generator which was connected to either an O2 or an air cylinder coupled with a pressure reducing valve. Oxygen or air were flowed through a quartz tube, constantly irradiated by a Pen-Ray® mercury discharge lamp (UVP Pen-Ray Lamps, Upland, CA) emitting at 185 nm radiation wavelength. A scrubber was used to bubble ozone into a calibrated vial. The best ozone yields were obtained using O2 as the feed gas and setting the oxygen flow in the 20–50 ml/min range. These conditions produced 3–4 × 10−5 mol of ozone/h of treatment, as measured by reaction with indigo dye.
The final ozonated solutions were subjected to immediate derivatizaton (2 mL aliquots) following the same procedure described below.
Derivatization procedure
Working solution mixtures at various concentration levels were prepared from stock standard solutions immediately before use by dilution with ultrapure water. These aqueous solutions (2 mL for all experiments in the present work) were basified with 200 µL of 1 M NaOH. Then, 150 µL of chloroformate solution was added (2 µmol of chloroformate in acetone), while keeping the reaction tube under ultrasonic mixing at optimized power and distance from the emitting tip (sonicator described below). Immediately, 5 µL of a saturated (400 mg/L) dicyclohexylcarbodiimide (DCC) solution in pyridine was added. The reaction was allowed to proceed for 3 min under sonication. The reaction products were extracted in n-hexane (600 µL) over 1 min. The organic layer was separated and analyzed by GC-MS. All the derivatization products proved stable for at least 24 h.
Warnings
All syntheses and derivatizations make use of highly reactive and toxic reagents, including phosgene, thionyl chloride, and perfluoroalkyl chloroformates. All of these chemicals have to be manipulated under a fume hood, using gloves and safety glasses. Crude chloroformate solution in acetone used in the derivatization still contains small amounts of HCl and phosgene that are converted into inert NaCl and NaCO3 upon NaOH addition. Ears should be protected with appropriate earplugs during the sonication step.
Instrumentation and analysis
A Branson Sonifier II W-450 (Danbury, CT) sonicator, with variable emission power, was used to enable the derivatizations under ultrasonic mixing. An ultrasonic bath was built for executing the derivatizations under optimized conditions [19], but similar results could be obtained using an ordinary water bath sonicator.
A benchtop PerkinElmer TurboMass (Norwalk, CT) spectrometer equipped with an AutoSystem XL gas chromatograph was utilized for most analyses. The quadrupole mass analyzer had an upper limit of m/z 1,200. A chemical ionization source was used to acquire both positive chemical ionization (PCI) and ECNI mass spectra. Isobutane was employed as the reagent gas for both positive and negative ion-mode experiments, at a pressure of 50 Pa. The ion source was maintained at the lowest temperature (140 °C) compatible with prevention of analyte condensation.
A DB-5MS (5% diphenyl dimethyl siloxane) capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness, Agilent, Folson, CA) was utilized. The samples were injected by an AutoSystem XL autosampler in the splitless mode at a temperature of 300 °C. The carrier gas (helium) was maintained constant at 1 mL/min. The oven temperature was programmed as follows: isothermal at 35 °C for 2 min, from 35 °C to 300 at 15 °C/min, isothermal at 300 for 5 min. The transfer line was maintained at 200 °C.
A Finnigan-MAT 95 (Bremen, Germany) mass spectrometer interfaced to a Varian 3900 gas chromatograph (Paolo Alto, California) was alternatively used for qualitative analyses. The GC splitless injector was set at 300 °C. A DB-5 (5% diphenyl dimethyl siloxane) capillary column (30 m, 0.25 mm i.d., 0.25 µm film thickness, J&W, Folson, California) was utilized. The carrier gas (helium) was maintained at constant pressure (13 psi) and the temperature program was the same as for the PerkinElmer instrument. The transfer line was maintained at 240 °C. The magnetic mass analyzer was continuously scanned over the mass range of interest, typical ranges were m/z 250–920 or 300–1,300, scanned at a rate of 1 s/decade. Isobutane was used as the moderating gas for ECNI, at a pressure of 50 Pa. The electron energy was set to 200 eV and the electron current to 0.2 mA. The ion source temperature was 200 °C for PCI and 150 °C for ECNI.
Analytical performance
Standard solutions of 37 DBP candidates were derivatized and analyzed, in order to determine the derivatives chromatographic retention times, representative fragment ions and the stability of their relative abundances.
Selectivity
Three ultrapure water samples (blanks) were derivatized and analyzed under the same conditions adopted for real and spiked samples. The occurrence of possible interferences from derivatization byproducts was tested by monitoring the selected ion chromatograms, characteristic for each investigated compound, at the retention time interval expected for their elution.
Linearity
Linearity was tested for 13 DBP candidates according to Table 2. At least five concentration levels (three replicates) for each analyte were analyzed to establish the calibration curves. Their linearity was tested using the least squares regression method and squared correlation coefficients (R 2). Calibration curves were also calculated in bi-logarithmic plots, in order to have homogeneous distribution of data points along the graph.
Limits
The limits of detection (LOD) were determined using the most abundant ion, where the response yielded a signal-to-noise ratio (S/N) greater than 3, after concentration of the n-hexane extracts by approximately fivefold. For each analyte, the noise was measured from −0.05 min before the peak onset until the beginning of the GC peak. Limits of quantitation (LOQs) were obtained from the calibration curves (with no concentration of the n-hexane extracts), as the lowest concentration yielding a linear response and a S/N equal to 10 or higher.
Precision
Intra-assay precision (%) was estimated by analyzing, in three consecutive days, nine replicates of two standard solution mixtures, at concentrations of 30 and 300 µg/L, respectively, for each analyte. Percentages refer to the ratio between the standard deviation and mean values.
Results and discussion
Derivatization products
Similar to other fluoroalkylchloroformates, ClOFPCF typically reacts with the polar groups of hydrophilic molecules in aqueous media by condensation and HCl elimination, as depicted in Scheme 2. Carboxylic acids are converted into the corresponding esters by eliminating CO2 and HCl. Hydroxylic and aminic groups are converted into carbonates and carbamates, respectively [10, 19].
Whenever the analyte carries two or more mobile hydrogens, the chloroformate reacts with each one to give a polysubstituted product. Each derivatization with ClOFPCF increases the analyte molecular weight by 248 or 292 Da, depending on whether CO2 elimination takes place. The derivatization products are hydrophobic and can be easily extracted into an organic solvent such as n-hexane.
Mass spectra
A significant advantage offered by the use of highly fluorinated derivatizing agents is that high electron affinity is conferred to the derivatization products, which is exploited in their detection by ECNI-MS. The introduction of a chlorine atom in place of a hydrogen further enhances the electron-capture cross-section of ClOFPCF derivatives with respect to OFPCF. Consequently, considerable sensitivity is expected in the target analysis of ClOFPCF derivatives, using negative ion detection mode.
However, ECNI yields rather unstable odd-electron molecular ion species. In addition, most ClOFPCF derivatives contain one or more weak carbonate and carbamate bonds. Both conditions tend to promote extensive fragmentation of the molecular ion, which is frequently undetectable in ECNI mass spectra of chloroformate derivatives. In contrast, PCI provides less sensitivity than ECNI, but PCI mass spectra of ClOFPCF derivatives often exhibit an abundant protonated molecular ion [M+H]+ and less extensive fragmentation. The combination of ECNI and PCI furnishes complete structural information for the ClOFPCF derivatization products.
For all the compounds examined in this study, both PCI and ECNI mass spectra were recorded, in order to clarify the main fragmentation mechanisms, particularly those typical of ClOFPCF derivatives. An example is provided in Fig. 1, which shows the PCI and ECNI mass spectra of the 2,4-dihydroxybenzoic acid ClOFPCF derivative. The PCI mass spectrum (A) exhibits an abundant protonated molecular ion [MH]+at m/z 987, as expected. The occurrence of significant fragment ions in the PCI mass spectrum is justified by the presence of two weak carbonate groups in the derivative's structure, resulting in an easy dissociation, even in the mild conditions provided by PCI. Carbonates typically fragment by cleavage on either side of the carbonyl group, with concurrent hydrogen rearrangement to maintain the electron parity.
Unlike PCI, the ECNI mass spectrum of the 2,4-dihydroxybenzoic acid derivative (B) does not exhibit a molecular ion. In its place, an abundant fragment ion at m/z 906 is observed. This originates by a sequence of hydrochloric acid and carbon dioxide losses, which represents a common dissociation pathway found in ECNI mass spectra of ClOFPCF derivatives. The most abundant fragment ions, at m/z 693 and 384, arise from the molecular ion by two consecutive cleavages of the carbonate groups. For all fragment ions, the isotopic pattern, characteristic of the presence of chlorine atoms, clearly indicate the number of substituents present in the structure.
It is interesting to compare the spectrum depicted in Fig. 1b with the one shown in Fig. 2, reporting the ECNI mass spectrum of the same compound (2,4-dihydroxybenzoic acid), upon derivatization by OFPCF. The two spectra were obtained on the same day, under the same experimental conditions. As with ClOFPCF, the OFPCF derivatives do not show a molecular ion and the main fragments at m/z 625 and 350 arise from carbonate cleavage mechanisms identical to ones active for ClOFPCF derivatives (Fig. 2). However, the fragment ion at high m/z is missing [M–HCl–CO2]− and no isotopic pattern distribution is present to support mass spectral interpretation. In practice, it is impossible to determine how many mobile hydrogens of the original molecule underwent derivatization using OFPCF. This is an advantage of ClOFPCF derivatives: they allow easy determination of how many mobile hydrogens underwent derivatization because of the chlorine isotopic pattern and the presence of fragment ions containing all the groups formed during the derivatization process.
As a general rule, ClOFPCF ester derivatives formed from carboxylic acids are rather stable and do not extensively fragment under PCI and ECNI conditions. The few fragment ions that can be observed arise from the loss of the ester group [M–265]− or the alkyloxycarbonyl substituent. On the other hand, carbonates and carbamates give extensive fragmentation in ECNI, especially when there are two or more of these groups in the derivative's structure. Fragmentation usually occurs by radical loss of the chloro-octafluoropentyloxycarbonyl or by neutral loss of HCl and/or CO2. Carbonates can alternatively eliminate the entire carbonate group ([M–309]− or release the terminal oxygen atom [M–293]–. The subsequent fragmentation steps frequently occur by elimination of a neutral molecule and hydrogen rearrangement (e.g. [M–293–310]–).
Chromatographic separation
In the study of highly polar DBPs originating from the reaction of ozone with NOM (i.e., humic and fulvic acids), it is suggested that polysubstituted oxidized aromatic and olefinic compounds are predominantly formed [4, 5]. Therefore, we selected a wide range of candidate ozone DBPs with multiple polar substituents in an aromatic or olefinic structure. These included poly-hydroxybenzoic acids, poly-dihydroxymethylbenzenes, hydroxybenzaldehydes, and a variety of polycarboxylic acids with saturated and unsaturated structures. Table 1 reports all candidate analytes tested, together with their molecular weight before and after ClOFPCF derivatization, their retention times and characteristic ions used for their determination by selective ion monitoring (SIM).
It is worth noting that Table 1 includes several isomeric compounds, with different chemical, physical and possibly toxicological properties. For example, six isomers of dihydroxybenzoic acid exists that are not easily discriminated by liquid chromatographic (LC) and LC/MS methods. Figure 3 reports the gas-chromatographic profile of an isomeric dihydroxybenzoic acid mixture, after derivatization with ClOFPCF. Although neither the chromatographic column nor the experimental conditions were intentionally optimized to improve separation (i.e., standard conditions were adopted), five sharp and symmetric peaks are neatly separated, with only 2,3- and 2,6-dihydroxybenzoic acids showing coelution.
Another important feature of the ClOFPCF derivatives is that their retention times appear to be more evenly distributed within a wider range, with respect to OFPCF derivatives [19], allowing easy allocation of the analytes in different SIM windows (Table 1). In particular, the groups of substances with a different number of mobile hydrogens undergoing derivatization are clearly separated from one another, whereas OFPCF derivatives with one, two or three substituents exhibit more extensive GC peak overlap.
Analytical performance
The analytical procedure described in the present study is aimed for qualitative screening purposes, particularly for the detection of ozone water DBPs, not previously recognized. When we tested spiked tap water and spiked ultrapure water in parallel experiments, identical results were obtained, within the experimental repeatability. The ECNI mass spectra for some of the candidate analytes (e.g., cresols) exhibited only one or two characteristic ions (Table 1), whereas the spectra for other substances (e.g., dihydroxybenzoic acids) included several significant ions. In the first case, the number of identification points is not sufficient for a conclusive identification, but it is still useful for screening purposes.
We also evaluated a series of pertinent performance parameters, including selectivity, linearity, repeatability, detection limits, and quantitation limits, for a collection of 13 analytes, representative of the various chemical classes under study (three dihydroxybenzaldehyde isomers, six saturated and unsaturated dicarboxylic acids, three hydroxybenzoic acid isomers, and 5-methylresorcinol). The experimental results are reported in Table 2.
Ion chromatograms obtained from the extracts of derivatized pure and tap water exhibited no interfering peaks (i.e., peaks with a S/N > 2) at the retention times where the analyte derivatives are expected to elute. This indicates that the ECNI-MS method is selective and free from positive interference from derivatization byproducts, at least in the retention time windows of interest.
Calibration curves for the selected analytes were built by plotting peak areas, averaged from three replicate values, against seven concentration levels (10, 30, 50, 100, 300, 1000, 3,000 μg/L). For all the analytes reported in Table 2, the curves were linear for the five central concentration levels, while the points at lower and higher concentration occasionally showed some deviation from linearity. All gradient values obtained from bi-logarithmic calibration plots were close to unity, as expected. Also, the R 2 values were close to unity for most analytes, with partial deviation for malic, malonic, and methylmalonic acids.
Given the simplicity of the matrix under study and the practical application undertaken, it was decided not to use the common mathematical algorithms to calculate LOD and LOQ values, but rather we preferred to obtain real experimental values, i.e., the lowest concentration that could be detected (LOD) and measured with reasonable accuracy and precision (LOQ). Since the derivatization procedure is followed by extraction into n-hexane (0.6 mL), these extracts could be further concentrated before GC-MS analysis whenever ultimate sensitivity was required. However, solvent evaporation to dryness was problematic because an oily residue was produced possibly due to the formation of bis(5-chloro-2,2,3,3,4,4,5,5,-octafluoropentyl)-carbonate as a reaction byproduct. Because it is problematic to determine the final sample volume following concentration (due to these oily residues), extracts were not further concentrated when determining calibration curves and LOQs. However, target analyte screening could take advantage of the increased sensitivity provided by a five- or sixfold concentration step, yielding a final sample volume of about 100 μl. Upon such a concentration, the LODs listed in Table 2 were obtained, ranging from 0.3 to 1.0 μg/L. These concentrations were positively tested and yielded S/N ratios exceeding 3.
Application to the detection of ozonation byproducts
In order to verify the effectiveness of this derivatization procedure in the application of interest, aqueous solutions of Suwannee River fulvic acid (SRFA) and humic acid (SRHA) were subjected to oxidative treatment with ozone for variable time intervals. The resulting solutions were subsequently derivatized with ClOFPCF, and the final n-hexane extracts were analyzed by GC-MS under both full-scan and SIM conditions, in order to identify any possible SRFA and SRHA ozonation products. Blank samples of untreated SRFA and SRHA in purified water were derivatized and analyzed to rule out components already present in SRFA and SRHA and possible reaction byproducts.
In practice, the concurrent and competing processes of DBP intermediate generation and subsequent decomposition by further oxidation make the resulting data quite complex. Several compounds were proven to be present in SRFA or SRHA solutions prior to ozonation but their concentration kept increasing as the treatment with ozone proceeded. In such cases, the substances were classified as ozonation byproducts even if they were already present at time zero of the process. An example of this is reported in Fig. 4, which shows the progressive increase of the itaconic acid signal as the ozonation of the SRHA solution progressed. Other ozonation byproducts were more easily identified when a chromatographic peak appeared only after the ozone generator was turned on. This was the case with tartronic acid, which was formed at low levels in the ozone treatment of SRFA solutions (Fig. 5).
A third interesting type of situation is represented by 2,6-dihydroxybenzoic acid (Fig. 6), which was found at relatively high concentrations in the SRFA starting solutions. Its chromatographic peak increased by a factor of 1.5 after a 10-min treatment with ozone, but rapidly declined in the subsequent time interval samplings. Although 2,6-dihydroxybenzoic acid generation from SRFA oxidation is highly plausible, it could barely be proven on statistical basis, since the kinetics for its decomposition favorably competes with that of its formation. We classified such cases as suspect, whereas we classified as negative the situations when a substance present in the initial SRFA or SRHA solutions showed a constant decline upon ozone treatment.
From a large series of comparative analyses, we identified six SRFA ozonation byproducts, namely 4-hydroxybenzoic acid, methyl-catechol (two isomers), 2-hydroxybenzaldehyde, tartronic acid and malonic acid, and suspect identification for 2,6-dihydroxybenzoic acid and itaconic acid. From SRHA, six byproducts were identified, including malonic acid, tartronic acid, malic acid, itaconic acid, maleic acid, and fumaric acid.
The derivatization procedure was also tested in the analysis of real ozonated drinking water samples from a Gwinnett County ozonation plant in metropolitan Atlanta (GA, USA). A large number of carboxylic and hydroxycarboxylic acids, mono- and di-hydroxybenzaldehydes, and mono- and dihydroxybenzoic acids were detected in the lyophilized raw water sample, as expected. However, from the comparative analysis of raw, ozonated and finished water samples, it was clear that most of the substances found in the raw water samples declined or disappeared upon ozone treatment. These preliminary results confirm the effectiveness of ozone for oxidizing and removing many organic pollutants and natural substances present in raw water. But, these results add little information on the possible generation of ozone-specific DBPs. Only two substances were detected in ozonated and finished water samples that were not present or were present in low abundance in the raw source water: maleic acid and itaconic acid. Systematic work on a variety of ozone water treatment plants needs to be done in the future to possibly detect a large set of ozone DBPs.
Conclusions
Derivatization with the novel 2-chloro-2,2,3,3,4,4,5,5-octafluoropentyl chloroformate proved to be an optimal tool for detecting small, highly polar and hydrophilic analytes in water samples for several reasons: (1) it is performed directly in the aqueous matrix, without preliminary extraction; (2) it benefits from an extremely wide applicability, since it is active on carboxylic, hydroxylic, aminic, and several other functional groups at the same time, releasing multiply-substituted derivatives; (3) it is rapid and quantitative; (4) it allows highly sensitive determination of the derivatives; (5) it is perfectly suited for target analysis, since it produces unique and stable derivatives, with optimal volatility for GC separation and high electron affinity. Moreover, (6) the presence of the chlorine isotopic pattern in the derivatives mass spectrum clearly indicates how many polar hydrogens of the analyte underwent derivatization. The last two aspects, together with a more moderate fragmentation in the mass spectra, represent a clear improvement of the novel derivatizing agent with respect to the previous highly fluorinated chloroformates.
On the other hand, it is difficult to identify unknown substances a priori within a complex mixture using ClOFPCF or other chloroformate derivatizing agents because the formation of weak carbonate and carbamate bonds promotes their extensive fragmentation both in EI and ECNI-MS, with frequent lack of the molecular ion information. Moreover, ECNI fragmentation provides limited insight into the analyte's original structure and is more often unpredictable than in EI. Therefore, it is concluded that a broadscreen search for ozone DBPs is difficult using only the present analytical procedure without target analysis. However, this analytical procedure may represent an extremely valuable tool to confirm or discount the presence of target analytes predicted to form by the reaction of disinfectants and NOM or anthropogenic materials. The process of predicting candidate DBPs structures can be assisted through a survey of products generated from the reaction of ozone with humic and fulvic acids, as we demonstrated here. This approach is likely to uncover a wide range of unknown hydrophilic DBPs that cannot be identified with existing procedures.
Highly fluorinated alkyl chloroformates are gaining increased popularity for the derivatization of a large variety of highly polar molecules in various matrices and applications, including clinical, biological, toxicological and environmental. The progressive commercial availability of these chloroformates will provide further impetus for their integration into routine analytical procedures.
References
Richardson SD (1998) In: Meyers RA (ed) Encyclopedia environmental analysis & remediation, vol 3. Wiley, New York, pp 1398–1421
Weinberg HS (1999) Anal Chem 71:801A–808A
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Mutat Res 636:178–242
Glaze WH, Weinberg HS (1993) Identification and occurrence of ozonation by-products in drinking water. American Water Works Association Research Foundation (AWWARF) and American Water Works Association (AWWA), Denver, CO
Krasner SW, Weinberg HS, Richardson SD, Pastor RC, Chinn R, Sclimenti MJ, Onstad GD, Thruston AD Jr (2006) Environ Sci Technol 40:7175–7185
Richardson SD, Thruston AD Jr, Caughran TV, Chen PH, Collette TW, Floyd TL, Schenck KM, Lykins BW Jr, Sun G-R, Majetich G (1999) Environ Sci Technol 33:3368–3377
Richardson SD, Thruston AD Jr, Krasner SW, Weinberg HS, Miltner RJ, Schenck KM, Narotsky MG, McKague AB, Simmons JE (2008) J Toxicol Environ Health A 71:1165–1186
Richardson SD (2009) Anal Chem 81:4645–4677
Zwiener C, Frimmel FH (2004) Anal Bioanal Chem 378:862–874
Hušek P (1998) J Chromatogr B 717:57–91
Hušek P, Šimek P (2006) Curr Pharm Anal 2:23–43
Minero C, Vincenti M, Lago S, Pelizzetti E (1994) Fresenius' J Anal Chem 350:403–409
Vincenti M, Minero C, Lago S, Rovida C (1995) J High Res Chromatogr 18:359–362
Angelino S, Maurino V, Minero C, Pelizzetti E, Vincenti M (1998) J Chromatogr A 793:307–316
Hall BJ, Parikh AR, Brodbelt JS (1999) J Forensic Sci 44:527–534
Simpson JT, Torok DS, Markey SP (1995) J Am Soc Mass Spectrom 6:525–528
Maurino V, Minero C, Pelizzetti E, Angelino S, Vincenti M (1999) J Am Soc Mass Spectrom 10:1328–1336
Vincenti M, Ghiglione N, Valsania MC, Davit P, Richardson SD (2004) Helv Chim Acta 87:370–375
Vincenti M, Biazzi S, Ghiglione N, Valsania MC, Richardson SD (2005) J Am Soc Mass Spectrom 16:803–813
Zahradnìčková H, Hušek P, Šimek P, Hartvich P, Maršálek B, Holoubek I (2007) Anal Bioanal Chem 388:1815–1822
Hušek P, Šimek P, Hartvich P, Zahradnìčková H (2008) J Chromatogr A 1186:391–400
Šimek P, Hušek P, Zahradnìčková H (2008) Anal Chem 80:5776–5782
Zahradnìčková H, Hartvich P, Šimek P, Hušek P (2008) Amino Acids 35:445–450
Acknowledgments
Financial support from the U.S. Environmental Protection Agency (U.S.-EPA; Cooperative Agreement No. R-82795101-1), from M.I.U.R. and Regione Piemonte is gratefully acknowledged. This paper has been reviewed in accordance with the U.S. EPA’s peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U.S. EPA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vincenti, M., Fasano, F., Valsania, M.C. et al. Application of the novel 5-chloro-2,2,3,3,4,4,5,5-octafluoro-1-pentyl chloroformate derivatizing agent for the direct determination of highly polar water disinfection byproducts. Anal Bioanal Chem 397, 43–54 (2010). https://doi.org/10.1007/s00216-010-3477-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3477-2