Abstract
Maintaining integrity of the skin and its appendages still preserves its top-ranking in priorities of survival for the modern human as it probably once did for the ancient individual, −not only- because it is the primary barrier to external assaults, but also because of social and psychological impact of healthy skin during their life-span. Healing wounds in order to shield off the internal organs from infections and damage, restoring its ability to adapt to various environmental stimuli, and slowing-down and reversing aging of the skin in the quest for an everlasting youth can be named as a few of the main drivers behind the multi-million investments dedicated to the advancement of our understanding of skin’s physiology. Over the years, these tremendous efforts culminated in the breakthrough discovery of skin stem cells the regenerative capacity of which accounted for the resilience of the skin through their unique capacity as a special cell type that can both self-renew and differentiate into various lineages. In this review, first we summarize the current knowledge on this amazing organ both at a structural and functional level. Next, we provide a comprehensive -in depth- discussion on epidermal as well as dermal stem cells in terms of the key regulatory pathways as well as the main genetic factors that have been implicated in the orchestration of the skin stem cell biology in regards to the shifts between quiescence and entry into distinct differentiation programs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With roughly 1.85 m2 surface area and accounting for about 15% of the total body weight skin is -not only- the largest organ for most mammals, but also an essential barrier that protects the organisms from external insults such as pathogens, toxic chemicals, UV from the sun, and mechanical injury (Park 2015). In addition, skin also executes vital functions such as regulation of body temperature, prevention of excessive water loss, removal of waste metabolites through sweat, and production of pigments against the sunlight (Kolarsick et al. 2011). Furthermore, skin serves as a major site for the metabolic and secretory processes that yield an array of biomolecules, including lipids, proteins, glycans, and hormones. For example, it is one of the major endocrine sites where peripheral Vitamin D synthesis takes place (Gaur et al. 2017).
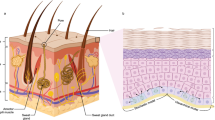
Anatomically human skin is composed of three main layers, including the outer layer of epidermis, the inner layer of dermis underlying the epidermis, and the inner-most layer of subcutaneous fat (also known as hypodermis or panniculus) (Arron 2016). Together with its appendages such as hair, nail, and mucous membranes of sudoriferous (sweat) and sebaceous (oil-secreting) glands, it forms the continuous integumentary system (McGrath et al. 2004). Moreover, several accessory structures such as specialized nerve receptors for regulation of responses to external as well as internal stimuli (such as touch, heat, pain, and pressure) aid the skin in execution of its vital functions (Garland 2012). The types of the cells in each layer and their thickness at a given anatomical location varies to a great extent. For example, while epidermis is the thinnest in the eyelids (0.1 mm) it reaches its thickest value in the palms and soles of the feet (1.5 mm). On the other hand, dermis is the thickest in the back (approximately 30–40 times thicker) than the overlaying epidermis in the same location (Kolarsick et al. 2011).
In this chapter, we aim first to introduce our reader to this organ that is recognized for its remarkable ability in tissue regeneration both in normal and repair homeostasis. Next, we continue our discussion by dissecting out the biology of skin stem cells which sets the basis of skin’s resilience. In a thorough summary, we report the findings of several elegant studies which unveiled distinct types of skin stem cells, their cell intrinsic as well as extrinsic signalling pathways, their complex interaction with local immune cells all of which play essential roles in proper operation of this “fountain of youth” in times of need. Finally, the contribution of cases to the oncogenic transformation in the skin when these signalling pathways lose their harmony and go astray is iterated.
1.1 The Epidermis
The layer of epidermis is renewed in a natural process known as cell (skin) turnover continually throughout life albeit with slowing kinetics with aging (Fuchs 2009; Li and Clevers 2010). In this natural cycle of turnover cells generated at the basal layer of the epidermis continually change form, which is known as differentiation in stem cell biology, until they reach the skin surface where they become shed having undergone apoptosis (Arron 2016).
Epidermis takes its embryonic roots from the ectoderm where in the course of the embryonic development mesenchymal cells populate the skin as they transmit instructive signals for the stratification of the epidermis and the positioning of down-growths that mark the initiation of formation of hair follicle (HF).
Epidermis is composed of several layers so-called strata, each with a unique composition of a number of main cell types, including keratinocytes at various stages of differentiation, dendritic cells, melanocytes, Merkel’s cells, and Langerhans’ cells. Keratinocytes constitute 95% of the cells in the epidermis (Arda et al. 2014). Numerous catabolic enzymes including lipases, phosphatases, esterases, nucleotides, and proteases remodel the extracellular space (Gaur et al. 2017).
In a way the tissue architecture of the epidermal layer can be envisioned as a continuous array of a binary unit that is compiled in countless numbers in an ordered and repeated fashion (Rasouli et al. 2018). The interfollicular epidermis (IFE), which is a stratified epithelium and hence forms the protective barrier against the outside environment and pilosebaceous unit (PSU) (Forni et al. 2012), which harbours some of the skin appendages such as the secretory glands and the hair follicle, constitute the two main components of binary unit that are associated with one another in this continuous array (Schepeler et al. 2014; Ceafalan et al. 2012).
Being the most abundant cell type of the epidermis, the main function of keratinocytes is to produce keratin, a protein that accounts for maintaining flexibility the skin and its ability to resist mechanical as well as hydraulic stress (Kolarsick et al. 2011). They can be readily distinguished from the “clear” dendritic cells by their relatively larger stainable amount of cytoplasm and their intercellular bridges (Gaur et al. 2017). In fact, the concerted morphological as well as spatial changes in keratinocyte population of each layer determines the distinct transitions from one stratum to the next (Bikle 2012). For example, the three bottom layers, including the basal cell layer of Stratum Germinativum bordering the Basal Membrane, the squamous cell layer of Stratum Spinosum, and the granular cell layer of Stratum Granulosum constitute the zone of the epidermis with living and nucleated cells all three of which are also collectively termed as Stratum Malpighii. While Stratum Lucidum and Stratum Corneum constitute the zone of the epidermis with dead and cornified or horny enucleated cells (corneocytes) (Kolarsick et al. 2011).
Hence, going outward from the Basement Membrane the dividing populations of the basal cells undergo proliferation cycles followed by the commitment to a terminal differentiation program that allows generation of the auxiliary structures of the integumentary systems such as the nails and the sweat glands (Leung et al. 2014). Considering the loss of thousands of cells upon each touch, these tightly controlled sprouts of self-renewal provided by the epidermal stem cells -not only- replenish the protective shell of the outer epidermal layer, but also ensure maintenance of a constant cell number constructed with relevant cell-to-cell and cell-to-basement membrane adhesions (Yang et al. 2019). The farther away from the Basement Membrane the more differentiated and the less viable the cells become, because in the absence of a capillary network carrying nutrients, the cells of the upper epidermal layers undergo a morphologically and biochemically distinct apoptotic program that eliminates cells without causing injury (Sigismund et al. 2012). In a sense the epidermal differentiation program is proposed as a type of apoptotic program overseeing proper conversion of keratinocytes into corneocytes (Gaur et al. 2017). In other words, during their migration the terminally differentiating cells alter their morphology on their way to death transcending multiple stages epidermal differentiation under the control of tightly regulated transcriptional programs (Lippens et al. 2005). Although these transcriptional read-out remains mostly the same at the early stages throughout the spinous and granular layers, ultimately they change to result in the dead flattened cells of the squamous layer and become removed from the skin surface very much like the process found in the gut (Deo and Deshmukh 2018; Fuchs and Nowak 2008). For example, advancing towards a more differentiated phenotype expression of Keratin 5 and 14 (KRT5/K5 and KRT14) is turned off, and expression of KRT1 and KRT10 become transcriptionally upregulated (Torma 2011; Alam et al. 2011). This change in transcription profile sets a pivotal switch in the keratin production because it establishes an intermediate filament protein network interlinked with desmosomes that serves as resilient structural scaffold fortifying cell-to-cell junctions and resisting against mechanical stresses (Gaur et al. 2017).
Almost all the nutrients feeding the epidermal layer is provided by the capillaries of the deeper dermis and are taken up by single layer of dividing cells of the Stratum Germinativum (Losquadro 2017). This strata hosts column-shaped keratinocytes attached to basement membrane as well as one another through desmosomal junctions at their short and long axes, respectively (Suzuki et al. 2000). Contrary to the predictions for this mitotically active compartment not all epidermal stem cells divide under normal conditions, they rather progress slowly through their long cell cycle (Alcolea and Jones 2014). However, stimuli such as wounding can increase the number dividing stem cells by inducing withdrawal of these non-dividing clones from quiescence. The migration of a basal cell from the basal layer reaching the surface of the skin is estimated as 28 days (Kolarsick et al. 2011).
A variety of cell types found in Stratum Spinosum present with highly different morphology, cellular structure, and properties (Freeman and Sonthalia 2019). In an interwoven architecture they provide support to the overall skin through the desmosomal plaques where -in fact- the keratin filaments are anchored. The spinose forms of these desmosomal plaques that mark the periphery of these cells gives this layer its name (Delva et al. 2009).
In the next upper layer of Stratum Granulosum further keratin production and the concomitant cell death takes place in its flattened cells with cytoplasmic keratohyaline granules (Bragulla and Homberger 2009). Owing to its elevated levels of lysosomal enzymes compared to those found in the lower to layers of Germinativum and Spinosum, Stratum Granulosum is known as the keratogenous layer where abrupt terminal differentiation converts these granular cells to the horny cells of the cornified layer (Kolarsick et al. 2011).
In the next layer up towards the surface of the skin the corneocytes of the thick layer of Stratum Lucindum serves as the barrier against UV damage from the sun or water loss (Yousef et al. 2019). Corneocytes are dense in protein, but not in lipid content while their extracellular millieu is a continuous lipid matrix (Haftek 2015). Desquamation, a term derived from the Latin verb “desquamare” (meaning scraping the scales of a fish) is namely peeling of the skin and describes the process for the shedding of the outermost layer of the skin tissue which involves physical and biochemical properties of the corneocytes at the cellular level (Murphrey and Zito 2019). For example, compact and tightly attached arrangement of the cells to one another in the lower levels becomes more scattered as they proceed to the surface of the skin through increased degradation of desmosomes at the intercellular attachments (Kolarsick et al. 2011). During this process keratinocytes move outward toward the surface of the skin, while millions of dead cells full with keratin are disposed daily from the outermost layer of Stratum Corneum, depositing the soft keratin that gives the skin its elasticity and protection to the underlying dermal and hypodermal tissues (Agarwal and Krishnamurthy 2019b). Overall, in every 35–45 days a new epidermal layer becomes created through the stunningly concordant orchestration of mitotic activity coupled to differentiation and followed by cell death, allowing the epidermis to sustain a dynamic tissue homeostasis in terms of the cell number and to be a selective barrier that keeps damaging microorganisms out and important body fluids in (Kolarsick et al. 2011).
Similar to the case of keratinocytes, melanocytes produce a complex polymer derived from the amino acid tyrosine called melanin -a dark pigment that -not only- protects core epidermal cells from UV damage, but also determines the color of both skin and hair- in membrane-bound organelles of melanosomes in a series of hormone-induced, receptor-mediated biochemical reaction cascades (Agarwal and Krishnamurthy 2019b). In healthy skin melanocytes become shed via skin turnover. However, interruptions to the normal skin turnover results in long term retention of melanin causing hyperpigmentation and formation of freckles and dark spots. Together with carotene (yellow to orange pigment) melanin gives skin it color (Chadwick et al. 2012).
Melanocytes are derived from the neural crest and are located at the basal layer (Cichorek et al. 2013). As they progress toward the surface of the skin they come in contact with the keratinocytes to which they transfer their melanin content without forming cellular junctions (Feller et al. 2014). Abundance of this skin-and-hair-coloring pigment is directly proportional to the abundance of melanosomes and their release into the keratinocytes that -overall- is associated with how much a human population is exposed to sun historically in a given geographical region (Schalka et al. 2014). Thereby, increased sun exposure is counteracted through increased melanogenesis in conjunction with increased surfacing of the melanin through its transfer to the keratinocytes (i.e., tanning of the skin), resulting in improved UV-absorbance for the ultimate goal of preserving genetic information from the radiation damage. Increase in sun exposure results in increases in melanin production changing the skin color temporarily (suntan). Similarly, on a permanent basis darker skin produces more melanin. The differences in skin colors amongst individuals is reflected rather through the differences in kind and amount of melanin, not the number of melanocytes (Del Bino et al. 2018). For example, while oriental skin color is a result of carotene in the stratum corneum, albinism is a skin color defect, in which skin does not produce melanin (Fajuyigbe and Young 2016). For example, individuals of African descent have larger melanosomes than Caucasians, who has membrane-bound melanosomes in smaller size and with distinct morphological differences compared to the spherical melanosomes of red-haired individuals (Del Bino et al. 2018). Conversely, loss of melanosomes in conjunction with loss of melanocytes results in graying of hair (Del Bino et al. 2018).
Merkel’s cells are type I mechanoreceptors that are found densely in high tactile sensitivity regions such as the finger tips, oral cavity, lips, and outer root sheath of hair follicles. They account for high touch sensitivity in these anatomical locations (Haeberle and Lumpkin 2008). Upon changes in their interaction within their assemblies of so-called “touch domes” they secrete neurotransmitters creating an action potential in the neighboring Sensory Neurons to relay the touch reception to the brain.
Langerhans cells that mediate various T Cell responses are present in all layers of the epidermis are densely present in the Stratum Spinosum and mainly function in the protection of the body by preventing pathogens from entering the body (Upadhyay et al. 2013). They are a type of dendritic cells (antigen-presenting immune cells) of the skin originating from bone marrow. Constituting 2–8% of the total epidermal cell population Langerhans are distributed in constant numbers in various squamous epithelia of the body, including epidermis, oral cavity, esophagus, vagina, lymphoid organs, and in normal dermis (Westerterp et al. 2005). Due to their key roles mediating T Cell responses, the hydrolytic enzymes of their phagolyosomes process the antigens found in the contents of their specialized organelles called Birbeck granules and ultimately contribute to T cell activation (Suhail et al. 2019).
The basement membrane residents, basal keratinocytes and the dermal fibroblasts, form the backbone of the Dermal-Epidermal junction at the interface between the epidermis and dermis and produce key extracellular matrix (ECM) components such as Collagen type IV, anchoring fibrils, and dermal microfibrils (Breitkreutz et al. 2013). Together with this dense meshwork of extracellular molecules the zone of Dermal-Epidermal junction provide support for the epidermis. In addition to housing the mitotically active basal cells it is also in charge of guiding cell polarity, direction of cell growth, organization of cytoskeleton in basal cells, providing growth stimulatory signals, and serving as a semipermeable barrier that controls trafficking of fluids and exchange of cells (Agarwal and Krishnamurthy 2019a; Yousef and Sharma 2018). For example, Laminin 5, which is an abundantly found factor in the architecture of ECM, utilizes α3β1-integrin for its assembly. As the cells of the basal layer leave the mitotically active compartment they exit the cell division cycle, commit to a terminal differentiation program, they switch off integrin and laminin expression (Kolarsick et al. 2011).
1.1.1 Skin Appendages
As mentioned above a group of auxiliary structures that grow down in the direction from the epidermis toward dermis become embedded in this zone, assist skin’s function in touch, temperature sensation, removal of toxins, perspiration, and thermoregulation (Brohem et al. 2011). These auxiliary structures that are derived from the ectoderm are collectively known as skin appendages (or adnexa), including eccrine and apocrine glands, ducts, pilosebaceous unit (PSU) that is comprised of hair, hair follicle, arrector pili muscle, and sebaceous gland. During wound healing these adnexal structures can be regenerated via migration of the keratinocytes from adnexal epithelium to the surface of the epidermis in a process called reepithelialization that takes place rather more rapidly in areas with higher number of pilosebaceous units (like the face and scalp) than those that have less (like the back) following an injury (Yousef and Badri 2019). Sudoriferous (from the latin word sudor for sweat) glands, Sebaceous glands, Ceruminous glands (present in the external auditory canal), and Mammary glands (present in the breast epithelium) are the four main types of glands of the integumentary system (Murphrey and Vaidya 2019).
1.1.1.1 Eccrine Sweat Glands
Eccrine sweat glands are formed by the downward growth of a group of epithelial cells from the epidermis towards the dermis in three compartments (Diao et al. 2019). Out of ~ 3,000,000 present in total overall the skin, highest number of eccrine sweat glands are found in anatomical areas such as palms, soles of the feet, forehead, and armpits while their number is the fewest on the back (Rittie et al. 2013). Each eccrine unit, transcends from a pore on the surface of the skin all the way to the depth of dermis. The overall function of the gland in heat control and electrolyte homeostasis is executed through the concerted operation of various cell types present in each compartment of the gland (Hodge and Brodell 2019; Lu and Fuchs 2014). In response to thermal stimuli a hypotonic solution is generated by the sodium-absorbing action of the cells in the straight dermal portion and the lowest coiled secretory duct where primarily glycogen-rich inner epithelial cells produce the sweat (Lu et al. 2012). Dark mucoidal cells and myoepithelial cells of the lowest coiled secretory duct compartment contribute to the formation of the sweat which then is transferred to the skin surface through the upper part of the straight dermal duct and the spiral intra-epidermal duct to be excreted from the skin pores (Flament et al. 2015).
1.1.1.2 Apocrine Sweat Glands
Apocrine glands are secretory glands of thick fluidic mixtures with characteristic odors distinct from those produced by bacteria that decompose skin secretions (Patel et al. 2019). Secretory fluids of the apocrine gland are more viscous than those secreted by the eccrine glands that are smaller in size are located closer to skin surface than the apocrine glands (Lu and Fuchs 2014). These highly viscous secretions by the apocrine glands contain pheromones, substances that mediate communication with other members of the species olfactory stimuli (sensing the environment through smell-detection of airborne substances) (Doty 2014). They regulate scent release as opposed to eccrine glands that control thermal adaptation. With distinct anatomical and physiological differences in comparison to the eccrine and apoeccrine sweat glands, intraepithelial ducts of the apocrine glands do not open directly to the skin surface, but open into the pilosebaceous duct (Briggman et al. 1981). Their secretory coiled base is entirely based in the subcutaneous fat and comprises of soly secretory cells without ductal cells as it is the case for the eccrinal coiled secretory base (George et al. 2004). Although exact composition of their secretion remains largely unknown due to difficulties in obtaining pure samples, it is a viscous fluid that is rich protein content (Witwer et al. 2013). Since apocrine gland activity is induced right before onset of puberty it is thought to be under a hormonal regulation. Specialized apocrine glands such as ceruminous and mammary glands have specific secreted cargo (Ohki and Kikuchi 2019). Ceruminous glands located in the external ear canal lining secrete the sticky cerumen (earwax) that repels foreign material. In the case of mammary glands the secreted fluid is the milk (Shokry and Filho 2017).
1.1.1.3 Apoeccrine Sweat Glands
Being derived from eccrine-like precursors during puberty Apoeccrine Sweat Glands (AEGs) open directly onto surface of the skin like the eccrine glands. However, the secretory rate of AEGs is 10 times higher than that of eccrine glands while their total number may vary in each individual (Cui and Schlessinger 2015).
1.1.1.4 Sebaceous Glands
Sebaceous glands are present in groups of two or more per hair follicle (Martel and Badri 2019). Major role of this gland is to keep hair soft and pliable by flushing the hair shaft constantly with its secretion called sebum, which is the term given to the lipid droplets available in ample amounts within the cytoplasm of the sebaceous cells, but in reality it is a cocktail of fats, waxes, and hydrocarbons (Martel and Badri 2019). Morphologically the gland has a lobular shape which is surrounded by the sebaceous gland connective tissue sheath on the periphery where a collagenous layer often contains blood vessels, nerve cells, and those from an immunogenic origin such as the Mast cells (Hoover and Krishnamurthy 2019). These cells are located in the upper segment of the hair follicle in a lobular arrangement. The lipid-packed cells are derived from an underlying germinative basal layer. Resting on this connective tissue zone there is a pool of proliferative sebocytes that forms the innermost cell layer of the gland (Blanpain and Fuchs 2006). Very much like the case seen in epidermal self-renewal, the basal sebocytes enter a maturation process whereby they become committed to a differentiation program as they migrate to the necrotic mid-zone of the gland that is aligned with the sebaceous duct concordant with their increased accumulation of lipid droplets (Niemann 2009; Zouboulis 2009). Hence, sebocytes at various points in their differentiation program can be visualized with distinct staining patterns (i.e., nuclear, membranous, and cytoplasmic) in immunohistochemical analyses (Xu et al. 2002). At the central zone of the gland lobule fully differentiated sebocytes undergo holocrine secretion releasing their sebum contents. Sebum then becomes transferred to the infundibular segment of the hair follicle inside the Follicular Canal via the Sebaceous Duct to be emitted on the hair shaft and skin surface together with the necrotic cellular debris (Schneider and Zouboulis 2018). Sebaceous glands are most densely populated in the face and scalp and are present in other anatomical parts of human body to lesser extent, however, they are absent in the palms, soles and dorsal sides of the feet (Taylor and Machado-Moreira 2013). Since lipids are poor conductors of heat, sebaceous glands help prevent water and heat loss (Pappas 2009). Throughout lifetime with the elevation in the plasma levels of sex hormones sebaceous glands become activated during puberty. The over-secretory activity of the glands may result in excessive sebum production, clogging of the gland and hair follicle, leading to lesions of “acne”, a common disorder seen in teenagers. From an evolutionary perspective sebaceous glands are proposed to be important for extra lubrication to facilitate birth during the passage through the birth canal (Kolarsick et al. 2011).
1.1.1.5 Nails
Fingernails are another important appendage of the integumentary system providing protection, improved sensation, and ability to grasp small sized objects (Shirato et al. 2017). The nail bed underneath the nail plate is part of the nail-matrix that has blood vessels, nerves, melanocytes, and keratinocytes (Brahs and Bolla 2019). The nail plate itself is formed from the keratin-producing keratinocytes in distinct matrices of the nail bed (Rice et al. 2010). The ventral (where the nail emerges), dorsal, and the intermediate/deep nail layers are produced by the nail bed, proximal matrix, and the intermediate matrix, respectively (Baswan et al. 2017). Fingernails (0.1 mm per day) grow 2–3 times faster than the toenails (Yaemsiri et al. 2010). Due to their slow growth rate toenails can provide information about toxic exposure of an individual such as the Mees lines -a form of horizontal hypopigmentation across the nail plate- are characteristic of arsenic poisoning (Kolarsick et al. 2011).
1.1.1.6 Hair Follicles
Although biological function of hair such as protection from external agents, providing insulation, and spreading glandular secretion products evenly were of higher degree of significance for the caveman, social and psychological role of hair has taken precedence over its former biological roles for the individuals of the modern society. To battle either hair loss (alopecia) or presence of excessive hair in undesired areas (hirsutism and hypertrichosis) or decolouring of hair sets the drive for a giant pharma-cosmetics-academic ecosystem that works with the goal of developing improved hair products annually (Sachdeva 2010). Hence, today, much of what we have learned about hair growth is the fruition of this momentum that propels a multibillion industry willing to prevent the emotional distress associated with having these conditions. Moreover, several investigators studying different biological processes picked the hair follicle as a model system resulting in an explosion of our knowledge on this particular skin appendage that regenerates in cycles (Paus and Cotsarelis 1999; Chuong et al. 2012).
The decisions towards establishing the number and distribution of hair follicles is made during the fetal stages and these decisions are not amended after birth (Lothian 2000). For example, density of the hair follicles in a given area of the skin are determined by early-expression gene products that are involved in the morphogenesis of the follicles. Although the size and shape of a hair follicle may vary depending on the location, its structure will be the same as others (Balana et al. 2015; Nowak et al. 2008). During embryonic development mesenchymal cells in the fetal dermis become congregated below the basal layer of the epidermis, an event that stimulates the basophilic cells of the epidermis resident to the basal cell layer to grow at a downward angle into the dermis (Schlessinger and Sonthalia 2019). In the second-half of the 8-staged-morphological development program the hair follicle continues to grow up until a bulb forms around those mesenchymal stem cells from which dermal papilla is derived (Rompolas and Greco 2014). Differentiation at the lower portion of the follicle gives rise to structures such as hair cone, the hair shaft, the cuticle, and the two inner-root sheaths, while the differentiation ongoing in the upper segments of the follicle gives rise to hair canal that spans from upper dermis throughout to the surface of the epidermis (Kobielak et al. 2003). Further structures such as the sebaceous gland and a groups of smooth muscle cells, called the Arrector Pili Muscle (APM), that attach the follicle to the external root sheath, and hair bulge forms from two distinct buds (Martel and Badri 2019). The hair bulge becomes located where the APMs are attached to the hair follicle while the opposite end of the APM is embedded in the papillary dermis. As it is discussed in detail in the next section, the bulge is thought to be a reservoir of stem cells that are in charge of regenerating follicles. Opposite to the side of the sebaceous gland a third bud emerges to give rise to the apocrine gland.
In a sense, HF is the compartment of the PSU where the sebaceous gland, the apocrine gland, and the AMP are housed (Mistriotis and Andreadis 2013). HF is further conceptualized as a two-compartment system where it has an upper part that includes the infundibulum and isthmus, whereas the lower part includes the hair bulb, hair bulge, the matrix, and the dermal papilla (DP) (Mistriotis and Andreadis 2013; Hsu et al. 2014). In collaboration with IFE, the main compartment of the epidermis, where progressive differentiation of keratinocytes after leaving the basal compartment form the barrier against the outside environment, PSU enables waterproofing of the skin through the secretion of sebum by the sebaceous gland (Schepeler et al. 2014; Fuchs et al. 2003).
The infundibulum compartment of the HF in a given PSU neighbours IFE and -hence- opens to the skin surface on one end and isthmus on the other (Schepeler et al. 2014). Isthmus is the mid-segment of the HF spanning from the infundibulum above and the top of the hair bulge below (Schepeler et al. 2014). The upper portion of the isthmus is defined as the junctional zone (JZ) which covers the region between the duct of the sebaceous gland and APM attachment (Schepeler et al. 2014).
Depending on the source, the lower compartment of the HF of a given PSU can be conceptualized differently. For example, while the hair bulge can be considered to reside in the isthmus according to one source (Mistriotis and Andreadis 2013), it is considered as a distinct segment beneath the isthmus as the upper segment of the permanent PSU which is proposed to consist of hair bulge and hair germ (Schepeler et al. 2014). Irrespective of the lack of consensus on its location, hair bulge is recognized as the reservoir for the hair follicular stem cell (HFSC) that is capable of regenerating the HF in cases of normalcy and damage. Committed HFSCs migrate from the bulge region toward the hair bulb where they proliferate and differentiate to generate the hair shaft and the rest of the epithelial cells of the HF such as the inner and outer sheaths composed of keratinocytes (Myung and Ito 2012; Woo and Oro 2011).
While the, inferior segment experiences cycles of involution and regeneration throughout life, same is not true for the infundibular and isthmus layers. Both the hair shaft and the inner & outer sheaths are derived from the proliferating cells of the hair bulb and these cells are called matrix cells (Martel and Badri 2019). Similar to the case with epidermal and sebaceous gland epithelial homeostasis, matrix cells of the follicle move upward as the hair grows, becoming more compressed as they enter the rigid inner root sheath which sheds when the growing hair (also in an upward direction) reaches the isthmus. Hence, it is not surprising that the number of cells entering the sheath determine the size of the hair while the dimensions and the curvature of the inner root sheath determine the shape of the hair (Alibardi 2004; Thibaut et al. 2005). Meanwhile, the color of the hair is determined by the number and shape of a melanosomes stretch lined up in the hair shaft after being synthesized by the melanocytes which transfer them to keratinocytes inside the bulb matrix (Slominski et al. 2005).
In contrast to the case of continually regenerating epidermis, hair grows in cycles stemming from each hair follicle that operates independent of others in humans. Each hair cycle comprises of three distinct phases called Anagen, Catagen, and Telogen phases. While these cycles could be out of synchrony for the human HF units, in mice the first two cycles take place in synchrony (Hsu et al. 2014). Anagen phase is known as the active growth phase during which hair growth is approximately at 0.33 mm and generally lasts about three to 5 years on the scalp. With age anagen phase lasts progressively shorter and it is profoundly shortened in individuals who suffer from alopecia (Qi and Garza 2014). During Catagen phase involution takes place whereby apoptosis prevails in many cells of the outer root sheath and this phase lasts about 2 weeks (Botchkareva et al. 2006). During the resting phase of Telogen, hairs of the scalp become pushed out by the growing hair shaft that are in anagen phase for about 3–5 months, while hairs in the other parts of the body present with shorter anagen, but longer telogen phases resulting in their shorter length, but longer retention on the skin (Pierard-Franchimont and Pierard 2013).
Interactions between the epithelial and mesenchymal cells determine the development of hair follicle (Sennett and Rendl 2012). As it will be discussed in detail in the “Stem Cells of the Skin” section, genes that play key roles in hair development are also important for the cycling of the hair follicle (Paus and Cotsarelis 1999). Insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 7 (FGF-7) are the two key molecules that regulate the development as well as cycling of hair follicles. In mice both are secreted by the dermal papilla and stimulate their receptors embedded in the membranes of overlying matrix cells (Seo et al. 2016). Estrogens, thyroid hormones, glucocorticoids, retinoids, prolactin, and growth factor are a few examples of hormonal factors that impact hair growth. Androgens such as testosterone and its active metabolite dihydrotestosterone have potent effects on hair growth through their receptor-mediated action exerted on dermal papilla cells by increasing hair follicle size, like seen in the case of beard area during puberty. Intriguingly, this promoting effect can become suppressive for the follicles in the scalp resulting in androgen alopecia later on in life (Zhang et al. 2018; Chen and Zouboulis 2009).
With the exception of congenital hair disorders that may be consequences of genetic mutations in keratins or other structural proteins, pathologies such as alopecia, hair loss, and undesired hair growth result from deviations from hair follicle cycling and, therefore, can be reversed (Zernov et al. 2016). A number of factors impact hair cycle, for example, telogen phase can be prolonged during pregnancy, while number of scalp hairs in anagen phase can be increased (Chueh et al. 2013). Upon equilibration of estrogen levels following childbirth telogen hairs become lost and anagen hairs simultaneously are converted to telogen hair which eventually becomes lost in 3–5 months. Another striking example of hair cycle disorder is seen in cases of telogen effluvium whereby synchronous termination of anagen or telogen results in massive hair loss in scalp, face, and other body parts (Ting and Barankin 2006). Severe trauma, childbirth, surgery, weight loss, severe stress, drug side effect, endocrine disorders, anemia, and malnutrition are found in association with telogen effluvium (Guo and Katta 2017).
Strikingly, hair follicle is the only organ that epitomizes its pre-natal development in each hair follicle cycle as it regenerates during postnatal stages of life. Several gene products including growth factors and their receptors, growth factor antagonists, transcription factors, adhesion molecules, and intracellular signal transduction components regulate both hair follicle development and hair follicle cycling (Lee and Tumbar 2012). Among these gene products many were historically discovered in Drosophila Melanogaster and hence are named after the phenotypes stemming from their specific mutant versions. For example, Decapentaplegic (Dpp/bone morphogenetic protein (BMP)), Engrailed (en), Homeobox (hox), hedgehog/patched (hh/ptc), notch, wingless/armadillo (wg/wnt/catenin) genes are known for their critical roles both for hair follicle and vertebrate development (O’Connor et al. 2006; Mizutani and Bier 2008).
1.2 Dermis
The dermis, also known as the “true skin”, is the layer of skin that lies between the epidermis and subcutaneous tissues of hypodermis as a thick layer of fibrous, filamentous, amorphous, and elastic tissue containing predominantly collagen (protein that gives skin its strength), reticular fibers (protein fibers that provide support) and elastin (protein that accounts for the skin’s elasticity) (Brown and Krishnamurthy 2018). In the dermal layer, those bio compounds that help maintain skin hydration and firmness (such as hyaluronic acid, collagen, and elastin) are manufactured by fibroblasts of various lineages (Ganceviciene et al. 2012).
While the epidermis serves as a protective barrier and hosts cell turnover, the main function of the dermis is to maintain skin’s firmness and elasticity. Dermis is about 2 mm accounting for 90% of skin’s thickness (Yousef et al. 2019). Collagen that maintain skin firmness, elastin that provides elasticity, and hyaluronic acid that maintains hydration make up approximately 70% of the dermis. The dermis plays a greater role on skin firmness and elasticity than the epidermis in that upon its damage skin becomes more prone to wrinkles and sagging that are harder to reverse (Yousef et al. 2019; Zhang and Duan 2018). Factors such as aging, inflammation, and UV exposure cause skin deterioration which is aggravated with slowed down metabolism, cell turnover, and fibroblastic cell division, and hence the amount of collagen (Phillip et al. 2015).
The high abundance of these elaborate filamentous dermal protein networks in the dermal layer accounts for the tensile strength, pliability, and elasticity of the skin. Moreover, due to housing receptors of sensory stimuli such as heat and touch, it regulates body temperature, protects the body from injury, and binds water (Phillip et al. 2015; Wang et al. 2015). Upon various stimuli dermal tissue allows an array of cell types, including cells of the nervous system and vascular epidermally-derived appendages, fibroblasts, macrophages, mast cells, lymphocytes, plasma cells, and leukocytes enter the dermis (Wang et al. 2015). The sustained interaction of the dermis with the epidermis promotes maintenance for the properties of both tissues. For example, collaboration of both is seen both during the morphogenesis of the dermal-epidermal junction and epidermal appendages during the development and during wound healing to accomplish proper repairing and remodelling (Pastar et al. 2014). Although dermis is not known to undergo waves of differentiation conspicuously like in the case of epidermis, distinct connective tissue compartments can be predicted depending on depth across the dermal cross-section. Likewise, depending -not only- on depth, but also on turnover and remodelling processes that may be governed by external stimuli in normal as well as diseased states of the skin, abundance of ECM components such as collagen and elastic connective tissue also vary (Bonnans et al. 2014).
In terms of embryonic origin, dermal layer is heterogeneous in nature in that various types of residential cells are derived from different embryonic fate. For example, while the constituents of the dermis originate from mesoderm, others such as nerve cells, melanocytes are descendants of the neural crest. Up to E6 (embryonic week 6) dermis is full with precursors of the fibroblasts which are dendritic shaped cells containing acid-mucopolysaccharides. By E12 fibroblasts commence synthesizing reticulum fibers as well as collagen and elastic fibers. Later on (by E24) both fats cells of an adipose layer and those of the vasculature emerge underneath the dermal layer (Domowicz et al. 2008; Agarwal and Krishnamurthy 2019b). Strikingly, only a few of the many fibroblasts present in infant dermis persist throughout adulthood where small collagen bundles are typical. However, it is noteworthy that infant dermis that comprises of small collagen bundles converts to an architecture that contains thicker collagen bundles (Lakos et al. 2004).
As the principal component of the dermis collagen is highly enriched in amino acids such as glycine, hydroxyproline, and hydroxylysine, and encoded by 15 distinct genetic variants that become translated into the members of a fibrous family of proteins in human skin (Shoulders and Raines 2009). For example, Type I collagen is the sub-type intrinsic to the dermis and while Types IV is found in the basement membrane zone, Type VII -produced by the keratinocytes- is important for the infrastructure of the anchoring fibrils. As a stress-resistant protein, collagen is a key structural component that is widespread throughout the body being present in tendons, ligaments, bones, and the dermis, while the elastic fibers of the dermal layer contribute marginally to the stress-resistance property of the skin in the face of mechanical injury (Shoulders and Raines 2009). The members of the fibrillar collagens found in the skin is predominating group of proteins in terms of abundance throughout the body. In line with the tissue texture pertained to the layers of the dermis, while loosely positioned collagen fibrils are typical of the papillary and adventitial dermis, heftier collagen bundles are more of a characteristic of the reticular layer of the dermis (Prost-Squarcioni et al. 2008).
In contrast to the structural and biochemical properties of the collagen fibers, elastin fibers have a binary structure where there is a protein filament and the amorphous protein component of elastin (van Eldijk et al. 2012). Elastin fibers are anchored into the glycosaminoglycan-rich ECM of the dermis via the fibroblasts. Parallel to the case of collagen fiber network, finer elastin fibers are found in the papillary dermis, while more coarse versions are found in the reticular layer. Although hyaluronic acid is a minor component of the normal dermis, becomes the highly accumulating mucopolysaccharide of the pathological states (Ushiki 2002; Tracy et al. 2016).
The layer of dermis can be envisioned in two such intermingled sub-layers that they are often hard to tell apart. One that consists of the loose connective tissue is called the Papillary Layer while the one that has the denser connective tissue is called the Reticular Layer (Brown and Krishnamurthy 2019a).
Papillary layer owes its name to the finger-like projections of papillae and in certain regions it entails a network of fine capillaries that nourish the epidermis, while other regions contain the so-called Meissner’s corpuscles (Tactile Corpuscles), which are a type of nerve ending mediating sensitivity to light touch (Piccinin and Schwartz 2019). The intricate network of capillaries serve the crucial functions of carrying nutrients to and removing waste metabolites from the local cells as well as maintain optimal body temperature by increasing or decreasing blood flow through pertinent contraction and relaxation cycles. Interestingly, the papillary layer of the dermis in the fingertips determines the pattern of the fingertips (Joyner and Casey 2015).
On the other hand, as the deepest layer of the dermis the reticular layer is composed of an elaborate meshwork of elastin and collagen fibers (which makes up about 70% of the extracellular matrix) (Frantz et al. 2010). The reticular layer collagen is produced by the resident fibroblasts. The strength and elasticity of the skin is attributed to its reversible property of viscoelasticity which provides resuming back to the resting state following a stretching up to a physiological limit upon elevation of the mechanical stress (Tepole et al. 2012). In other words, viscoelasticity of the skin gives its resilience to insults by external forces due to the tightly-woven elastin and collagen meshwork. While sliding and re-arrangement of these collagen fibers underlies the ability to persist a physical load by guarding tissue-integrity through allowing skin deformation whilst preventing damage, elastic fibers provide the ability to bounce/relapse back to the resting state once that physical load is removed (Ehrlich and Hunt 2012). However, when this property falters the architecture of the skin in terms of structural properties of collagen and elastin networks are subject to change like seen in cases of cancer, aging, toxic UV, and sunlight exposure (Marionnet et al. 2014).
In that regard, the collagen network exist in a rather dynamic than static state where its degradation due to catalytic activities such as spare collagenases is counteracted by its constant assembly following its synthesis and processing by the fibroblasts where a pro-collagen polypeptide chain becomes integrated and then secreted to be used in the construction of collagen fibrils (Abou Neel et al. 2016).
A set of sensory receptors called Pacinian corpuscles that are involved in reception of deep pressure are also found within the reticular layer which cushions the deep projections of skin appendages such as sweat glands, lymph vessels, smooth muscle, and hair follicles (Slominski et al. 2012).
1.3 Other Cell Types of the Dermal Architecture
Nerve cells, an intricate network of blood vessels, hair follicles, sebaceous, and sweat glands constitute the skin appendages that are embedded in the dermal layer. In addition, dermal adipose cells, mast cells, and infiltrating leukocytes also reside in the dermis. In this section we will brief our reader about these minority cell types of the dermal layer (Randall et al. 2018).
1.3.1 Vasculature
Most of the skin vasculature is embedded in the reticular dermis, however, according to the recent reports a branching and intricate network of capillaries is placed right above the bulge where they modulate hair growth by Hair Follicle Stem cells (HFSCs) via secretion of angiogenesis-derived factors. Major function of the skin vasculature is to carry nutrients, hormones, and immune cells (Hsu et al. 2014). Two types of intercommunicating plexuses encompass the dermal vasculature architecture. The first is known as the subpapillary (or superficial) plexus, that comprises of postcapillary venules, and it is located at the papillary-reticular junction of the dermis (Imanishi et al. 2008). The second is the lower plexus which is found at the dermal-subcutaneous interface. The capillaries, end arterioles, and venules of the subpapillary plexus supply to the papillae of the dermis. Meanwhile, the deeper plexus, −which is supplied by the larger blood vessels and more complex in structure than the subpapillary plexus-, supply to the adnexal structures. Being regulated by the preoptic-anterior hypothalamus blood flow, the skin is modulated in response to thermal stress in humans. (Ye and De 2017). Being regulated by the preoptic-anterior hypothalamus blood flow, the skin is modulated in response to thermal stress in humans. In order to cope with increased heat, vasodilation, increased skin blood flow, and sweating are important responses to disseminate heat (Greaney et al. 2016). Conversely, in response to cold, vasoconstriction in the skin helps preventing heat loss and hypothermia. Disturbance to the skin blood flow can significantly debilitate maintenance of normal body temperature as seen in the case of patients with type II Diabetes who may experience heat stroke and heat exhaustion upon elevation in external temperature and menopausal women who experience hot flashes induced by hormonal imbalance (Hifumi et al. 2018).
1.3.2 Muscles
As mentioned earlier the APM that are attached to the hair follicles below the sebaceous glands make up one of the muscle groups of the dermis (Fujiwara et al. 2011). Being situated in the connective tissue of the upper dermis APM fibers exist at such an angle to the hair follicle that upon contraction hair follicle becomes pulled into a vertical position resulting in a type of skin deformation known as “gooseflesh” or “goosebumps” (Brown and Krishnamurthy 2019b). Another group of smooth muscle bundle is found surrounding the veins and arteries of the skin. The specialized smooth muscles of glomus is in between the arterioles and venules. Striated (voluntary muscle groups) resident to the skin of the neck and face are known as muscle of expression (Haddad et al. 2001). Likewise, subpapillary muscles of the aponeurotic system (a network of aponeuroses connecting muscles and fascia) mediate movement of body parts.
1.3.3 Nerves
Together with the arterioles and venules the highly abundant nerve bundles make up the neurovascular bundles of the dermis. Among the sensory organs skin is the largest due to its dense innervation by innumerable primary sensory neuron fibers (Andreone et al. 2015). The cell bodies of this heterogeneous population of neurons including nociceptors, mechanoreceptors, and proprioceptors, are located in trigeminal and dorsal root ganglia (Hsu et al. 2014). For example, Meissner corpuscles -densely found in the ventral sides of the hands and feet and fingertips- are resident to the dermal papillae and convey the signals induced by touch to the central nervous system. Hence, sensory nerves are in close contact with the cells of the epidermis and the hair follicle with the nerve endings anchoring at the different layers of the epidermis (Hsu et al. 2014; Andreone et al. 2015). A region just above the hair bulge is surrounded by the mechanoreceptive nerve endings. Being located in the deeper portion of the dermis in the weight-bearing surfaces of the body Vater-Pacini corpuscles are large nerve-endings and mediate sense of pressure (Bell et al. 1994). The unmyelinated nerve fibers found around the hair follicles and papillary dermis transmit sensations such as pain, temperature, and itching (Park and Kim 2013). The postganglionic adrenergic fibers of the autonomic nervous system regulate the vasoconstriction. The latter also controls the secretions of the apocrine gland and the contractions of the AP muscles of the hair follicles. Secretions of the eccrine sweat glands are regulated by the cholinergic fibers. Signals emanating from the skin can affect the sensory innervation and dendritic arborisation (McCorry 2007; Gordan et al. 2015). Conversely, signals from peripheral nerves may influence hair follicles, in return. For example, neuropeptides such as substance P and calcitonin gene-related peptide (CGRP; a pro-inflammatory neurogenic bio compound), can induce hair follicle regression (Hsu et al. 2014).
1.3.4 Mast Cells
Mast cells are a sub-type of immune cells that originate in the bone marrow in a progenitor form of myeloid lineage and they localize widespread in the peripheral tissues bordering external environment, including the mucous-producing tissues of the gut and lungs as well as the skin and blood vessels (Krystel-Whittemore et al. 2016). Given that the skin is one such interface, it houses a large mast cell population (more densely found in papillary dermis than in hypodermis) as the first responders to the presence of parasitic invaders as well as allergens (da Silva et al. 2014). While Type I (connective tissue) mast cells are inherent to the dermis and submucosa, Type II (or mucosal Mast cells) are found in the mucosa of the respiratory tract and the bowel. Mast cell maturation takes place in response to the c-kit ligand, stem cell factor, and other stimuli released by their microenvironment (Krystel-Whittemore et al. 2016). Numerous peripheral large and long vili and round, oval or angular membrane-bound cytoplasmic granules encapsulating chemokines such as histamine and heparin, certain cytokines, serine proteinases, leukotrienes, and prostanoids are hallmarks of their morphology (Kunder et al. 2011). Anchoring of a variety of stimuli such as superoxides, complement proteins, neuropeptides, and lipoproteins to the immunoglobulin E (IgE) and consequential binding of IgE to its receptors embedded on mast cell surface triggers a process called “degranulation of mast cells” whereby -within seconds- inflammatory content of cytoplasmic granules are delivered to the microenvironment. In other words, crosslinking of hundreds of thousands of FcϵRI glycoprotein membrane receptors to their ligand IgE as a consequence of engaging stimulatory signals is the initiating event of Mast Cell activation (Shakoory et al. 2004). While these cells participate in regulation of vascular homeostasis, angiogenesis, venom detoxification as well as innate and adaptive immune responses in normal physiology, they have emerged as players with either promoting or suppressive roles in pathologies of allergy, asthma, atherosclerosis, several types of cancers, and gastrointestinal disorders when deregulation of their accumulation, proliferation, clearance or migration prevails (Chen et al. 2018).
1.3.5 The Hypodermis (Subcutaneous Fat)
Hypodermis is a cushioning layer present underneath the dermis. It insulates the body against physical trauma, heat, and cold, while serving as an energy storage area. The fat is exclusively deposited in adipocytes, held together by fibrous tissue (Driskell et al. 2014; Labusca and Zugun-Eloae 2018). This layer of the skin begins to develop toward the end of the fifth month with the appearance of the fat cells in the subcutaneous tissue. The fat cells are the most predominant cell type found in this layer while mesenchymal stem cells are also present in the hypodermis layer. Adipocytes are separated by large blood and lymph vessels (Labusca and Zugun-Eloae 2018).
In fact, recent findings rapidly established the notion that subcutaneous fat is a major endocrine organ that secretes an array of stimulatory factors, also termed as adipokines, that exert influential roles on lipid metabolism, energy balance, insulin sensitivity all of which are important in angiogenesis, immunomodulation, and inflammatory response. In other words, adipokines collectively constitute the secretory repertoire of the adipose tissue and they participate in maintenance of organ homeostasis at the autocrine, paracrine, and/or endocrine level by mediating communication between multiple cell types (Gaur et al. 2017; Al-Suhaimi and Shehzad 2013; Stern et al. 2016). For example, conversion of androstenedione into estrone by aromatase, production of leptin by lipocytes to control sateity takes place in this layer. Another example is seen in tissue repair processes of the skin. During epidermal and dermal repair adipokine repertoire participates in coordinating both proliferation and migration of keratinocytes and fibroblasts (Schmidt and Horsley 2013).
The fact that the mesenchymal stem cell content per gram of tissue is 500 times higher in subcutaneous adipose tissue (hypodermis) than bone marrow was probably one of the most exciting findings for the history of regenerative medicine, an exploding field which was summarized comprehensively in the previous issue of this journal by Cankirili et al. (2019). For the most part it is thought that the therapeutic effects of the dermal layer (discussed further in the following section) and subcutaneous fat compartment are executed by the mesenchymal stem cell residents of these layers through their healing capacity on sites of injury and inflammation through actions of their endogenous secretory repertoire including pro-regenerative, anti-fibrotic, anti-apoptotic, and growth factors required for the tissue repair processes (Gaur et al. 2017). Mesenchymal stem cells derived from the adipose tissue is proposed to be the “endogenous factories” that supply trophic factors capable of supporting all layers of the skin “in sickness (repair) and in health” (Gaur et al. 2017). For that matter we believe there will be benefit to introduce the common properties of mesenchymal stem cells before plunging into the amazing depths of skin stem cell biology.
2 Mesenchymal Stem Cells of the Skin
Mesenchymal Stem cells have the capacity to renovate the mesodermal tissues such as the connective, cartilage, and bone tissues by replenishing their cellular context. In addition to subcutaneous fat (hypodermis) they can easily be isolated from blood, fat, bone marrow, and foreskin in adequate quantities and this property is only one of their attractive features for the purposes of regenerative medicine (Augustine 2018). Second feature involves their ability to retain their stemness in tissue culture conditions over relatively high number of passages (Shim et al. 2013). Thirdly, under appropriately provided ex vivo conditions they can take the fate of various cell types, including myocytes, adipocytes, osteoblasts, neuronal sub-types, and chondrocytes (Jumabay and Bostrom 2015). Finally, the immunomodulatory properties of mesenchymal stem cells (especially those that are immunosuppressive in nature) allow them to execute an ideal management of tissue repair processes. Hence, in recent years numerous methodologies have emerged in the clinic, whereby mesenchymal stem cells were manipulated in order to accelerate the wound healing processes induced in response to a wide variety of injuries such as severe burns, myocardiac infarction, neurodegenerative damage disorders, hepatic injury, muscle degenerative disorders, bone injury, and chondrocyte erosion (Zachar et al. 2016).
2.1 Stem Cells and Their Niches in the Skin
Skin is home to a diversified community of stem cells and other cell types where each community is located in a friendly neighbourhood of various niches each which are resided by other cell types assisting stem cells at different stages of their lifetime from self-renewal to terminal differentiation (Lutolf and Blau 2009). In this section, we brief our reader with the current knowledge on different types of stem cells and their niches at both cellular and molecular scope. Although initial thought was that cells forming the niche come from a lineage distinct from the stem cells they regulate, recent reports underscore the co-presence of stem cells with their differentiated progeny, suggesting that cues from the descendant cells are as much important as those coming from the rest of cells forming the niche in governing the biology of their stem cell parents (Hsu et al. 2014).
Considering the constant regeneration capacity that has come forth repeatedly for several of the skin compartments described in the previous sections, it is not too surprising that replenishment of each compartment is provided by a unique set of stem cells that concertedly exit quiescence and execute a program of differentiation until the desired tissues are formed, a phenomenon underlying the maintenance of skin in normal homeostasis and wound healing (Gaur et al. 2017). Since the first exploitation of skin stem cells (keratinocytes) in treatment of burn patients (two children whose body surface was burnt by more than 90%), there has been prodigious amount of information accumulating about skin stem cell biology (Wabik and Jones 2015). The first hints about the presence of skin stem cells was coined by their ability to retain diploidy even after hundreds of clonal passages without any requirement for immortalization procedures when grown in the presence of feeder fibroblast layer to make tissue based on the findings obtained in Howard Green’s laboratory at MIT (Adam et al. 2018). Following this landmark work introducing us to the immense clinical potential of these skin stem cells, which are contemporarily known as epidermal (Fuchs et al. 2003) stem cells (also known as the epithelial stem cells of the skin), numerous studies continued the discovery of different types of stem cells resident to differential niches found in the skin. For example, over the years increasing evidence have pointed to the presence of a diversified presence of adult stem cells, including mesenchymal, hematopoietic, and neural stem cells residing in the skin (Shi et al. 2006).
In line with the current definition of stem cells, skin stem cells and/or progenitor cells can -not only- renew themselves (self-renewal) and commit to specific differentiation programs to generate various lineages of the skin (multipotency), but also produce cell types of other tissue types when provided proper ex vivo conditions (plasticity) (Shi et al. 2006). Furthermore, cellular quiescence, which is relatively unrelated to the these three traits, is also recognized as a stem cell property and methods involving uptake and retention of labelled nucleotide analogues are used for the identification of long-lived quiescent stem cell populations (Lang et al. 2013).
According to the results of the engraftment studies where labelled epidermal cultures are allowed to reconstitute epidermal tissue in vivo, about 10–12% of the basal cells were capable of generating a single column of differentiating cells (Potten and Booth 2002). Another method for labeling stem cells involves pulse-labeling of newly synthesized DNA in all dividing cells of a tissue with radiolabeled nucleotide analogs (such as bromodeoxyuridine (BrdUrd) or tritiated (Hrckulak et al. 2016) thymidine) and follows up the rarely dividing cells that retain the label in the tissue (Podgorny et al. 2018). Shedding light to the slow cycling nature of tissue stem cells, this pulse-chase method lead to the development of a model for the skin epithelial maintenance where periodic division of slow cycling so-called Label Retaining Cells LRCs (putative stem cells) in the basal layer generates a pool of cells termed as transiently amplifying cells (TACs). TACs that populate most of the basal layer typically divide two or three times before their commitment to a differentiation into mature skin cells as they migrate upward (Fuchs 2009; Li et al. 2017).
Later on studies done in mice, rats, and human pointed out that bulge region of the HF is one of the important niches where majority of LRS find -in fact- sanctuary. Historically, bulge region of the hair was characterized as a thickening area in the upper portion of the follicle where slow-growing LRCs are found (Lang et al. 2013). It is proposed that the reason for the hair bulge to be a niche of preference for most clonogenic cells and LRCs of high label retaining capacity is -most likely- because in mammals the hair bulge is fortressed amongst the upper column of cells and heavily keratinized hair shaft above and the supportive, innervated, and nourishing vasculature of the dermal pocket below (Fuchs et al. 2003).
In normal skin homeostasis, stem cells from various niches of the skin such as hair follicle (HF), interfollicular epidermis (IFE), and sebaceous glands play key roles in maintaining healthy epidermal and dermal layers (Gonzales and Fuchs 2017). Both intrinsic signalling pathways at the genetic and epigenetic levels and extrinsic crosstalk between the stem cells and resident cells of their niche are mediated via the secreted cytokines, chemokines, and growth factors, accounting for the overall regenerative capacity of the skin (Psarras et al. 2019; Kizil et al. 2015). In this section we summarize the most prominent long-term stem cells and progenitors found in the skin epithelium as well as current understanding of how stem cells and progenitor cells interact with each other to mobilize the tightly regulated sequence of events that result in adequate amount of tissues and stem cell pool.
The unipotent populations of epidermal stem cells that occupy the niche of the basal layer differentiate into keratinocytes to regenerate the epidermis in normal and injured adult skin. More specifically, these unipotent stem cells are proposed to originate from the multipotent progenitors of the bulge region of the hair follicle (Shi et al. 2006). In case of injury a subset of these follicle-derived multipotent stem cells can migrate out of the hair follicles to the wound site and participate in the repair of the damaged epithelium, while their contribution to the maintenance of the epidermis in normal homeostasis is limited. As it is discussed in detail below, the follicle-derived stem cells can give rise to the tissues of outer root sheath, inner root sheath, hair shaft, and sebaceous gland. Notably, being positive for the neural stem cell marker of Nestin, follicular stem cells have a capacity to differentiate into neurons, glia, keratinocytes, smooth muscle cells, melanocytes, and even blood vessels under appropriate conditions (Shi et al. 2006; Chen et al. 2009).
As mentioned earlier, IFE units comprise of differentiated layers of the stratified epithelium constituting the main component of the epidermis and the foundation of the protective barrier. The layers of epithelia are fueled by the epidermal stem cells found in the basal layer (Schepeler et al. 2014). As the basal cells depart from the Basement Membrane and commit to the terminal differentiation program an extensive transcriptional and post-translational remodeling take place involving modification of intracellular proteins, intercellular junctions, and nuclear fragmentation whereby dense cytoskeletal architecture of keratinocytes forms the highly crosslinked 10-nm intermediate filaments (IFs) that are key to confront external insults (Schepeler et al. 2014; Hsu et al. 2014). The undifferentiated proliferative progenitors expressing Keratin 5 (K5) and 14 (K14) participate in self-renewal as well as give rise to epidermal epithelium. Hierarchically they are proceeded with an increasing degree of differentiation by the nonproliferative, but transcriptionally active spinous and granular layers, that express K1, K10, and involucrin, and eventually by the to-be-shed cells of the dead stratum corneum (Hsu et al. 2014; Alam et al. 2011; Srivastava et al. 2018).
Results from mouse studies utilising various Cre-lineage tracer methodologies engineered under the control of Keratin promoters specific to basal layer support two models: According to the first, “Hierarchical model” a slow-cycling stem cell nested in the conceptual proliferative basal unit of each IFE gives rise to the short-lived transiently amplifying cells (TACs) which then exits the proliferative layer after a certain number of cell divisions to replenish the differentiating cells of the upper layers in a columnar fashion (Hsu et al. 2014; Zhang and Hsu 2017; Rangel-Huerta and Maldonado 2017). According to the second, “stochastic model” basal IFE layer comprises of a single type of proliferative progenitor, descendants of which randomly decide either retain their progenitor identity or to differentiate in which case both daughter cells have equal chances of remaining as stem cells or committing to a differentiation program (Hsu et al. 2014). Hence, it is tempting to speculate that the former model prevails under homeostatic conditions, whereby the stem cell pool is maintained through asymmetric division, where the parent stem cell divides into two cells one retaining stemness (particularly self-renewal) and the other assuming a more differentiated phenotype (differentiation). This way hierarchical model assumes that the body reserves a powerful reservoir of cells that can be readily engaged when tissue repair becomes needed while supplying a differentiated progeny for normal tissue maintenance (Bryder et al. 2006). On the other hand, in the case of engaging in a repair activity stochastic model could predominate. For the two stem cells generated in the symmetric division from each parent stem cell, it could completely depend on the signaling conjecture of the microenvironment whether these two daughter cells will continue to be stem cells or commit to a differentiation program to replenish the damaged tissue (Mistriotis and Andreadis 2013). Therefore, like other adult SCs, skin stem cells typically remain quiescent until they are coaxed to proliferate and/or differentiate in vivo, while in vitro they display a noteworthy proliferative as well as differentiation potential (Mistriotis and Andreadis 2013; Horsley et al. 2008).
Mitogens such as insulin-like growth factor (IGFs), fibroblast growth factor 7 (FGF-7), FGF-10, and epidermal growth factor receptor (EGFR) ligands are produced by dermal fibroblasts that facilitate potent pathways for epidermal proliferation (Seeger and Paller 2015). Upregulation of transforming growth factor alpha (TGF a), which is a positive regulator of EGFR signalling, or abrogation of Mig6 or LRIG1 (in humans), which is an inhibitory to EGFR-dependent signalling, stimulates epidermal proliferation (Hsu et al. 2014). Basal epidermal cells are anchored to the major basement membrane component laminin-5 through their receptors such as integrin alpha3beta1 and alpha6beta4 that signals through GTPase RAC1. For example, in humans higher beta1 integrin expression is indicative of greater stem cell potential (Hsu et al. 2014; Hamill et al. 2009; DiPersio et al. 2000).
Furthermore, certain factors of epigenetic modifications are also implicated in epidermal proliferation and differentiation homeostasis. For example, histone H3 Lys (H3K27) methyltransferases EZH1 and EZH2, histone H3K27 demethylase JMJD3 associated activities are essential for epidermal differentiation, respectively through modulating transcription of alpha 6 and beta1 integrin genes. Intriguingly, epidermal proliferation is proposed also to be under a temporal regulation exerted by the core clock factors of the circadian rhythm machine (Mistriotis and Andreadis 2013; Chen et al. 2012).
Epidermal stratification requires delamination of basal cells, whereby they lose their attachment to the basement membrane. Their journey moving upward is presumed to begin with an asymmetrical cell division which generates a committed suprabasal cell and a proliferative basal cell as a result of cytokinesis perpendicular to the basement membrane. The first commitment step in differentiating to spinous cells is dependent on Notch signaling which execute important roles in developmental processes (Hsu et al. 2014; Berika et al. 2014). Binding to its ligands triggers cleavage of Notch receptor proteins by gamma-secretase and the cytosolic domain then translocates to the nucleus to alleviate the transcriptional repression exerted by RBP-J -thereby- enabling the induction of Hes/Hey-dependent transcriptome. For example, expression of Notch ligand DELTA by the basal cells and consequent induction of Notch signaling as a result of DELTA binding to the receptors allows commitment to spinous cell differentiation by promoting detachment from the basement membrane and mediating downstream events of asymmetric cell division to balance epidermal cell proliferation and differentiation (Hsu et al. 2014; Bazzoni and Bentivegna 2019).
2.1.1 Hair Follicle Stem Cells
The dynamism of the HFs is unanimously attributed to a diversified and rich pool of stem cells known as Hair Follicle Stem Cells (HFSCs) that are continuously self-renewing, differentiating, and regulating hair growth. HFSCs emanate from distinct developmental origins and localizing to distinct anatomical locations within the hair follicle (Soteriou et al. 2016). Because HFSCs are easily accessible they have been extensively studied in vitro demonstrating a highly proliferative and multipotent characteristic which -in vivo- is believed to be the major contributory factor to skin homeostasis (Mistriotis and Andreadis 2013). Observations made in the in vitro studies of HFSCs, have advanced to enabling engineering of various tissues for organ replacement. Furthermore, combined with the tools of genetic engineering HFSCs offer encouraging venues for the treatment of genetic diseases of skin or hair disorders (Ormandy et al. 2011).
Several anatomic locations within the HF -by itself- are home to distinct type of stem cell populations, including HFSCs and MSCs (Mistriotis and Andreadis 2013; Lang et al. 2013). Both HFSCs in charge of regenerating hair and MSCs in charge of regenerating UV-absorbing melanocytes co-reside in the hair bulge and hair germ (Hsu et al. 2014).
In regards to the exact identity of the stem cell population harboured in the bulge region, current model accepts that the bulge niche includes both proliferative (CD34+ and LGR5+) and quiescent (label retaining; CD34+ but LGR5-) stem cells (Mistriotis and Andreadis 2013). Interestingly, LGR5+ cells that are fully capable of regenerating the HF, do not coincide with the label retaining cell populations of the bulge.
Intriguingly, cells from the Isthmus/Infundibulum region display multipotent properties due to the observation that they can -not only- differentiate into the epithelial cells of the HF, but also those of sebaceous gland and the epidermis (Mistriotis and Andreadis 2013). These cells isolated from the region between the sebaceous gland and the bulge lack bulge stem cell (Bu-SC) markers (KRT15- and CD34-), but they are highly proliferative in vivo, remain clonogenic in vitro, and give rise to new HFs upon transplantation or epidermis upon injury (Mistriotis and Andreadis 2013).
With a quiescent subpopulation residing in the bulge Bu-Scs and another with higher propensity to proliferate residing in the hair germ just neighboring the bulge, HFSCs are generally considered in two subpopulations (Hsu et al. 2014). While Bu-SCs ultimately proceed to generate the outer root sheath (ORS) cells, those derived from the hair germ differentiate to form the TACs of the matrix that give rise to the inner root sheath (IRS) (a channel that surrounds the hair shaft during anagen) cells. In catagen, the next generation of HFSCs become deposited in the newly formed bulge and hair germ that are derived from the upper ORS and middle ORS cells, respectively, for the consecutive hair cycle. In a series of elegant experiments Hsu and co-workers demonstrated that during anagen although some of the Bu-SCs that exit the bulge, they retain their stemness including remaining quiescent. This fraction of BuSCs constitute the reservoir of stem cells for the next cycle, some differentiate, yet still return to the bulge having lost their stemness despite expressing stem cell markers (Mistriotis and Andreadis 2013). In case of injury, but not in normal skin homeostasis, Bu-SCs can migrate to the wound site and differentiate into keratinocytes (Mistriotis and Andreadis 2013). Further studies demonstrated the robust multipotent capacity of Bu-SCs both in vivo where they took part in angiogenesis and in vitro where they were able to differentiate into keratinocytes as well as cells of neuronal (such as neurons, glial cells, and melanocytes) and mesenchymal origin (Mistriotis and Andreadis 2013).
Although pool of HFSCs remain mostly in quiescence throughout the hair cycle, their proliferation and differentiation become triggered due to the action of several factors secreted by the stem cell progeny and dermal cells in anagen (Hsu et al. 2014). One such factor is the bone morphogenetic proteins (BMPs) that play important roles in bone and cartilage formation. Strikingly, recent studies uncovered that BMPs contribute to the maintenance of quiescence of HFSCs while they execute additional roles that are essential, in embryogenesis, organ homeostasis and other developmental processes. For example, BMP4 secreted by the dermal fibroblasts, BMP2 expressed by the subcutaneous fat, BMP6 secreted by the K6+ cells of the inner bulge layer, as well as the quiescence factor FGF-18, all function in maintenance of quiescence of both Bu-SCs and hair germ SCs during telogen (Hsu et al. 2014). In support of these findings, conditional ablation of the bone morphogenetic protein receptor 1α (Bmpr1α) gene promotes beta-catenin stabilization, expansion of HFSCs that fail to enter terminal differentiation, and reduction in slow-cycling cell population (Kobielak et al. 2007). Conversely, overexpression of Bmpr1α promotes precocious commitment of HF-SCs to differentiation (Mistriotis and Andreadis 2013; Sotiropoulou and Blanpain 2012). In agreement with the inhibitory role of BMP in hair cycling, expression of BMP antagonists -such as NOGGIN, FGF-7, FGF-10, TGF-β2 (hair germ activating factors) in dermal papillae concordant with fall in BMP4 and BMP2 levels in dermal fibroblasts and adipocytes, respectively, allows the HFSC pool to promote hair growth (Sotiropoulou and Blanpain 2012).
In addition to its widely renowned pro-oncogenic effects and those in development, accumulation of the WNT effector, nuclear beta-catenin and activation of its target genes following complex formation with TCF/LEF transcription factor, is a major propeller in hair germ and dermal papillae stimulation (Hrckulak et al. 2016; Li et al. 2018). Indeed, phenotype of mouse models where WNT inhibitor DKK1 is overexpressed under the control of Krt14 is hairless revealing the potent inhibitory role of DKK1 in HF development confirming the essential role of WNT-dependent signalling in hair growth (Dela Cruz et al. 2012). Conversely, overexpression of a constitutively active truncated beta-catenin under the control of an epidermal promoter permanently promotes formation of new HFs as well as tumors in adult mice, while transient activation of beta-catenin results in increased hair formation (Hsu et al. 2014; Enshell-Seijffers et al. 2010).
An activated state of WNT signalling within hair germ as well as dermal papillae is crucial for the normal transition of hair follicles from telogen to the next anagen. This fact is underscored by the findings that abrogation of beta-catenin nuclear accumulation either in hair germ or dermal papillae results in a telogen arrest of the hair cycle or delayed regeneration of the hair follicles, respectively (Hsu et al. 2014). Although precise compartments of the HF supplying WNT stimulatory signals are to be determined, hair germ itself and dermal fibroblasts are strong candidate sources (Hsu et al. 2014). Reduction of matrix cell proliferation and subsequent precocious entry into catagen in response to loss of beta-catenin nuclear accumulation in DP points to the importance of WNT signalling in maintenance of the mesenchymal and epithelial interactions between the DP and bulge cells (Sotiropoulou and Blanpain 2012; Lowry et al. 2005). Moreover, appearance of nuclear beta-catenin and other WNT effectors in hair germ during the initial stages of anagen preceding that by the dermal papillae is required for normal entry into anagen, because targeted ablation of WNT ligand secretion in the HF epithelium in telogen results in suppression of WNT signalling in DP during anagen and a subsequent potent arrest of hair cycle (Sotiropoulou and Blanpain 2012; Myung et al. 2013).
Parallel to the HF-activating output of WNT signalling, activated TGFβ family member TGF-β2 also contributes to regenerative cues in the HF (Vaidya and Kale 2015). For example, prevention of TGF-β2-dependent signalling within HFSCs through SMAD2/3 resulted in delayed coat recovery in conditional TGF-β reporter II knock-out mouse model (Oshimori and Fuchs 2012b). Furthermore, elevating TGF-β2 levels exogenously was stimulatory to HFSC proliferation both in in vivo and in vitro conditions by antagonizing BMP-mediated maintenance of quiescence through transcriptional upregulation of TGF-β2-target gene Tmeff1 (tomoregulin) (Vaidya and Kale 2015) (Sakaki-Yumoto et al. 2013). Hence, together with other BMP-inhibitory factors secreted by DP during late telogen, TGF-β2 overrides quiescence signals to drive follicular regeneration into the next anagen.
Dermal papilla underlying the hair germ produces several factors such as FGF-7, FGF-10, TGF-β2, BMP2 inhibitor NOGGIN during early-to-late telogen to stimulate hair follicle activation for the consecutive hair cycle (Plikus 2012). Meanwhile, expression levels of pro-quiescence genes BMP4 in dermal fibroblasts and BMP2 in mature adipocytes become decreased to perpetuate HFSC activation. Finally, platelet-derived-growth-factor alpha (PDGF-alpha) produced by the adipocyte precursor cells turn PDGF signalling in dermal papillae on (Andrae et al. 2008; Gonzales and Fuchs 2017).
Most likely, this fine balance within the bulge niche and the interaction between the bulge and DP rests on an elaborate stimulatory and inhibitory signalling network in a manner that couples HFSC quiescence/activation to the hair cycling. Therefore, the signalling events discussed above will –most likely- be followed by the discovery of additional ones (Oshimori and Fuchs 2012a).
Although murine models have been instrumental in advancing our knowledge on molecular details of HF biology, findings from these invaluable studies should be evaluated with caution in the sense that there are several differences between the murine HF biology and that of human. For example, in contrast to humans who have only two types of hair (vellus and heavily pigmented hairs), mice have several distinct types of hair, including pelage, vibrissae, cilia, hairs on the tail, ear, genital, nipples, perianal area, and around the feet) (Mistriotis and Andreadis 2013). Apart from type, human HFs cycle independently after birth, while those of mouse (e.g., pelage hair) cycle in synchrony. Finally, there are profound differences between biological markers used in characterization of hair follicular stem cell populations of human and mouse all of which are described in detail in a comprehensive review (Mistriotis and Andreadis 2013).
Several challenges exist in path of stem cell marker discovery. First of all, these markers are rare and often not linked to stem cell function (Lang et al. 2013). Second, these putative markers can be transiently expressed by a small stem cell population or can be co-expressed by another non-dividing cell resident to the niche raising reliability issues (Carulli et al. 2014). Hence, a true stem cell marker must be expressed by such candidate stem cell populations in the niche that that population must meet all of the criteria for stemness including self-renewal, multipotency, and plasticity. Moreover, these hallmarks of stem cells must be cross-confirmed by the use of other stem cell characterization/identification methods, including measurement of quiescence and behavior of the cells in culture (the ability to behave as stem cells in in vivo lineage tracing experiments) upon transplantation (Lang et al. 2013; Cai et al. 2004). All of these methods have pros and cons in terms of their accuracy in diagnosing stemness (Zhu et al. 2017). For a detailed description, we recommend two comprehensive reviews written by Mistriotis and Andreadis (2013) and Lang et al. (2013). Nonetheless, novel stem cell markers are in need in the isolation of these populations to aid therapeutic means of regenerative medicine. In this direction, engineering reliable in vitro models that can mimic the in vivo HF microenvironment sets the foci of several research groups (Mistriotis and Andreadis 2013).
In contrast to the case of hematopoietic stem cells, where both parent stem cell and every member of its progeny across the differentiation hierarchy can be identified, such specific markers for the precise identification of epidermal stem cells are still lacking impeding the development of reliable isolation protocols (Firth and Yuan 2012). Members of the integrin transmembrane receptor family that mediate the attachment of the basal layer to the substratum of the epidermis are proposed as candidate stem cell markers (Barczyk et al. 2010; Alvares et al. 2008). If epidermal stem cells residing either in the hair follicle bulge or inter-follicular epidermis require strong adherence to the basement membrane to maintain both their stemness and their location in the niche, molecules of anchorage to the cell-substratum attachment can be putative markers for stem cells (Chermnykh et al. 2018). Transferrin receptor is another surface marker, the expression of which differs between stem cells and their proliferating progeny, the former presenting with reduced expression of the transferrin receptor (Brekelmans et al. 1994).
So far several stem cell markers were suggested for the murine HFs, however, human HFs remain relatively underexplored. One reason is the difficulty in isolation of human cells due to the challenges in identification of human bulge region anatomically in contrast to the case of murine bulge (Joulai Veijouye et al. 2017). According to the results of a recent screen carried out by Klöpper and co-workers CD200, KRT15, and KRT19 were proposed as putative bulge stem cells markers, although their location is not restricted to the bulge, but extends to a wider area of isthmus as well (Mistriotis and Andreadis 2013; Tiede et al. 2007). In contrast to mouse, human bulge cells are CD34, NES, or LHX2 negative (Kloepper et al. 2008). In this study although CD200 positive cells were isolated from a population of LRCs using laser capture microdissection and they showed an increased clonogenic potential in vitro, their multipotency was not examined (Mistriotis and Andreadis 2013; Ghadially 2012). On the other hand, KRT15high/CD200+/CD34-/CD271- bulge-derived cells had an increased clonogenic potential as compared to KRT15low/CD200+/CD34-/CD271- cells (Mistriotis and Andreadis 2013; Sari et al. 2010). More recently, it was reported by the Andreadis group that human DP/DS cells displayed a cell surface profile characteristic of MSCs being positive for CD90, CD44, CD49b, CD105, and CD73. These cells were clonally multipotent as individual clones could be induced to differentiate into fat, bone, cartilage, and smooth muscle with high efficiency (Mistriotis and Andreadis 2013).
Given the differential slower activation kinetics of Bu-SC compared to those of the hair germ which gives rise to the matrix TACs upon anagen initiation, it is conceivable that the HFSC subpopulations are stimulated by distinct set of signals (Panteleyev 2018). For example, production of the potent mutagenic signalling factor Sonic hedgehog (SHH) by the newly formed matrix TACs is shown to trigger and maintain the Bu-SC activation (Guo et al. 2018). In addition to the bulge activation, SHH-dependent signalling enhances the expression of anti-quiescence genes such as noggin and FGF-7 in dermal papillae to sustain a highly proliferative state in the matrix and lower ORS (Che et al. 2012). Therefore, once proliferating TACs start sending mutagenic factors -not only- the Bu-SCs are stimulated, but also they mediate a cross-stimulatory signalling between the hair germ and dermal papillae parallel to the progression throughout the anagen (Ren et al. 2017). Impact of core circadian clock genes on HFSC biology will need to be addressed in further studies.
HFSCs also appear in control of the formation and attachment of the APM. Bu-SCs express nephronectin, an ECM protein from the same family of proteins including EGFL6 (Hsu et al. 2014; Linton et al. 2007). Both nephronectin, which is enriched in the basement membrane of the bulge, and EGFL6 are ligands for α8β1 integrin and nephronectin recruits α8β1+ dermal cells. Upon nephronectin-integrin engagement dermal cells up-regulate the expression of smooth muscle actin, an APM marker (Linton et al. 2007). Remarkably, in nephronectin null mice, fewer APMs are formed and their anchorage becomes shifted to the EGFL6-expressing cells above the bulge, suggesting that EGFL6 may compensate for the loss of nephronectin-dependent anchorage (Hsu et al. 2014; Tsepkolenko et al. 2019). Overall these findings suggest that different hair follicle compartments recruit and assemble different hair follicle–associated structures, including peripheral nerves, blood vessels, and APMs via involvement of different ECM proteins (Tsepkolenko et al. 2019; Shimoda et al. 2014).
Sebaceous gland is also thought to be derived from the Bu-SCs that have migrated out of the bulge. Another view advocates differentiation of a unipotent stem cell population located above the bulge and that express BLIMP1 to replenish the sebocyte pool (Mistriotis and Andreadis 2013; Blanpain and Fuchs 2009).
Another niche that is proposed to contain HFSCs is the Dermal Papilla (DP) and Dermal Sheath (DS) compartments which are derived from the mesoderm, unlike the hair bulge that is derived from the ectoderm (Mistriotis and Andreadis 2013; Morgan 2014). Both DP and DS cells crosstalk to the bulge in regulation of the hair cycling. Both DP and DS stem cells display a profound differentiation potential that is demonstrated in ability to form hematopoietic cell subtypes upon engraftment in lethally irradiated mice (Morgan 2014). Moreover, there is evidence to DP and DS stem cells being the precursor’s dermal stem cells that function in dermal maintenance in normalcy and tissue repair. Strikingly, both rat and human-derived DP and DS stem cells reveal broad plasticity to generate myogenic, osteogenic, chondrogenic, and adipogenic lineages which is comparable to that seen with mesenchymal stem cells of bone marrow under appropriately provided culture conditions (Mistriotis and Andreadis 2013; Fitzsimmons et al. 2018). Taken together with the ability of single human DP and DS cell to differentiate into mesenchymal lineages these HFSC subpopulations are recognized for their multipotent trait.
2.1.2 Melanocyte Stem Cells (MSCs)
Proliferation of the Melanocyte stem cells (MSC), which co-reside together with HFSCs in the bulge and hair germ, seems to be closely coupled to that of the latter in the sense that MSCs also initiate generating differentiating progenitor melanocytes at the beginning of the anagen (Mull et al. 2015). Therefore, it is not too surprising that the synchronization of MSC activation and differentiation with that of HFSCs is achieved by the signals originating from HFSCs and dermal papilla. Factors such as the KIT ligand (produced by the dermal papilla) and endothelins (produced by the matrix) conduct differentiation of melanocytes in the hair bulb during anagen. Endothelin-1 is a downstream target of WNT signalling in HFs of early anagen, therefore, elevation of WNT signaling or conditional deletions of Nftb results in expansion of melanocytes which can be rescued by the injection of endohelin receptor B antagonists (Hsu et al. 2014; Chang et al. 2013). As the new hair cycle initiates, MSCs are co-activated along with the HFSCs to produce proliferative committed progenitor melanocytes (Hsu et al. 2014). Later in mature HFs these melanocytes are found in the inner core of the matrix where they transfer the melanin synthesized to the differentiating HFSCs (Hsu et al. 2014; Gola et al. 2012). During the following phase of catagen melanocytes also degenerate together with the rest of the matrix cells. Several findings support the notion that activation of the WNT signaling and beta-catenin-associated activity in MSCs promotes proliferation and differentiation of MSCs (Lang et al. 2013). Strikingly, stabilization of the beta-catenin in the HFSCs induced an extrinsic effect on the MSCs in the sense that the melanocyte pool was expanded, the bulge region was enlarged in a endothelin-dependent manner (Lang et al. 2013). Several Wnt inhibitors are present in the MSC niche, including DKK3, Sfrp1, and Dab2 and in MSCs themselves, including DKK4, Sfrp1, and Wif1 (Lang et al. 2013; Svensson et al. 2008). Hence, these findings suggest presence of a seesaw-like mechanism whereby suppression of WNT signalling promotes stemness of MSCs while its activation induces differentiation of MSCs into melanocytes (Lang et al. 2013; Gola et al. 2012).
In addition to WNT ligands TGF-betas are also presumed to be produced by HFSCs which -in return- might be responding and coordinating signals released by MSCs (Hsu et al. 2014; Svensson et al. 2008; Bogaerts et al. 2014). TGF-beta-dependent signalling is implicated in maintaining MSC quiescence and their undifferentiated state (Lang et al. 2013). TGF-beta signalling is normally involved in cellular growth and survival processes, however, in the HF biology TGF-beta proteins become up-regulated (indicated by the presence of the nuclear phospho-Smad2) as the HF regresses during catagen and promote degeneration of the epithelial-derived components of the HF (Lang et al. 2013; Bogaerts et al. 2014). Both during normal hair growth and following UV exposure, changes in TGF-beta expression are reported. Transcription factors such as MITF and PAX3, melanin-associated enzymes such as tyrosinase decrease upon activation of TGF-beta-dependent signalling (Lang et al. 2013).
Disruption of Notch signaling also hampers MSCs from self-renewal (Lang et al. 2013). The four known Notch receptors can be activated by ligands (Jagged 1 and 2, Delta-like 1,3, 4) which stimulates the cleavage of the receptor by gamma secretase to release Notch intracellular domain (NID) (Rutz et al. 2005). The release of NID from the membrane and its subsequent translocation to the nucleus results in activation of CBF1-dependent transcription (RBP-J kappa in mice). Blockade of Notch signalling (through use of gamma secretase inhibitor (GSI)) results in permanent hair greying due to complete depletion of both melanocytes and MSC pool, in contrast to the reversible de-coloration seen with the blockade of KIT receptors (Lang et al. 2013; Rutz et al. 2005).
HFSCs regulate themselves and the MSCs by their increased expression of ECM proteins such as the hemi-desmosomal transmembrane collagen (Collagen XVII also known as COL171A, BP180 or BPAG2) which mediates anchorage to the basement membrane (Lang et al. 2013). Therefore, loss of this protein results in graying and loss of hair as well as loss of HF integrity, suggesting this anchorage protein provides docking both to the HFSC and MSC population (Walko et al. 2015).
Coupling of hair cycle events to those of the HFSC life cycle from self-renewal throughout expansion and differentiation demands intricate crosstalk amongst the HFSC subpopulations (Mulloy et al. 2003). In this respect it is quite remarkable that during the generation of a pigmented hair shaft Bu-SC subpopulations that come from diverse developmental origins such as follicular epithelium ectoderm, melanocyte stem cell-neural crest, DP/DS-mesoderm all crosstalk to one another in a concerted fashion (Mulloy et al. 2003; Sakaki-Yumoto et al. 2013).
The hair bulge is also home to the MSCs that are TACs activated during anagen to produce melanocytes to maintain hair pigmentation during each hair cycle (Mistriotis and Andreadis 2013). Due to the relative ease to harvest MSCs from skin and their close synchrony with the expansion and differentiation of HFSCs during the hair cycle have promoted them as a popular model to studies of stem cell biology (Hsu et al. 2014; Tobin 2009). Furthermore, quiescence or growth of melanocytes can be controlled through depilation while any dysfunction in this population can be readily traced through loss in pigmentation, for example, BcL2 loss-associated apoptosis of MSCs results in greying of the hair (Lang et al. 2013; Jo et al. 2018). Having their embryonic roots in the highly plastic tissue of neural crest, being very multipotent and prolific, and capable of migrating to new locations are inherent qualities of MSCs that allow them to be an excellent stem cell source for the application of regenerative medicine (Achilleos and Trainor 2012). Moreover, because of their relatively longer lifespan compared to that of keratinocytes and their ability to work as a single-cell unit the growth and differentiation capacities of which can be controlled by surrounding cells enables manipulation of MSCs in in vivo and ex vivo in stem cell based therapies (Lang et al. 2013; Zakrzewski et al. 2019). As it will be discussed in detail in “Stem Cells and Cancer” section the very same properties could account for the aggressiveness and highly malignant nature of the tumors derived from melanocytes. Melanomas the incidence of which is rising steadily in contrast to the case of other types of cancers tend to metastasize very early raising fatality (Lang et al. 2013; Schatton and Frank 2008).
Due to the pairing of proliferation and apoptosis of the melanocytes to the hair growth cycle in mice, most of the studies on MSCs tend to be based on this model system (Preston et al. 2018). Using a transgenic mouse model where beta-galactosidase as a reporter under the control of the dopachrome tautomerase promoter (gene encoding an enzyme involved in melanin synthesis, it was understood that bulge region (also defined as the lower permanent portion (LLP)) is the niche for the MSCs (Hsu et al. 2014). The bulge microenvironment provides direct contact with the adjacent cells, scaffold proteins of the ECM, and secreted signaling factors to regulate MCSs (Hsu et al. 2014; Gentile and Garcovich 2019).
Nevertheless, our understanding of MSC biology is beginning to take in terms of how melanocyte stem cells function within their niche, how their quiescence and proliferation is controlled by external signals, and how plastic this population is (Lang et al. 2013). MSCs, that locate to the bulge of the hair follicle are impacted by other cell types such as HFSCs, extracellular matrix proteins, and a number of secreted factors that either promote or suppress multipotency or self renewal (Hsu et al. 2014). Deregulation of these regulatory signals lead to inability of the stem cell to maintain their stem cell pools or provide pigmented progeny and could potentially contribute to transformation processes in the context of acquiring oncogenic mutations (Lang et al. 2013; Aponte and Caicedo 2017).
2.1.3 Dermal Stem Cells
Dermal Stem Cells (DSCs) are another stem cell population the presence of which has gained evidence parallel to those obtained for epidermal stem cells (Blanpain and Fuchs 2006; Martin et al. 2016). In recent years, DSCs has entered the clinic as an accessible and abundant stem cell source for cell-based therapies (Vapniarsky et al. 2015). For that matter their isolation, purity, safety, viability, characterization, and in vitro propagation have been main focus of research in several laboratories around the world. DSCs display plasticity in the sense that they can be coaxed to generate mesenchymal, ectodermal as well as endodermal cell lineages under properly provided ex vivo conditions (Vapniarsky et al. 2015; Ojeh et al. 2015). Various lineages of dermal fibroblasts as the most predominant cell type of the dermis has been studied extensively (Driskell and Watt 2015). Particularly they have been considered as the source that is mobilized to wound site to accomplish dermal regeneration. However, recent evidence obtained in the past decade-and-a-half points out that dermal fibroblast/myoblast pool might be derived from other sources (Vapniarsky et al. 2015). Among candidate sources there are bone marrow-derived, tissue-derived mesenchymal stem cells as well as a source that ensues “epithelial-mesenchymal-transition” a process that involves de-differentiation of epithelial cells (Vapniarsky et al. 2015; Stone et al. 2016).
In this respect, hair follicle DP and DS were the first niches proposed for the DSCs that can give rise to cells of the hair-supporting papilla in the lower region of the follicle and migrate out into the dermis of the adjoining skin (Agabalyan et al. 2017). For example, hair follicle dermal sheath cells of the (Martin et al. 2016; Balañá et al. 2015). Conversely, transitions of dermal sheath cells into dermal papillae cells have been reported (Lachgar et al. 1996; Darby et al. 2014). For example, dermal stem cells derived from the follicle are transplanted onto wounds (Cha and Falanga 2007), they contribute to the new dermal tissues in a manner reminiscent of the wound-healing fibroblasts. In this regard, DP and DS-associated cells can differentiate into several mesenchymal, neuronal, and glial lineages (Vapniarsky et al. 2015).
Although, hair follicle is taken as an epidermal stem cell source, −especially the bulge region- emerge as a key source for the regeneration of both epidermal and dermal cell populations (Cheng et al. 2018). It was demonstrated that the hair bulge contains stem cells of neural origin such as the human epidermal neural crest stem cells (EPI-NCSCs), most likely by the epidermal ORS being in strong association with DS of the hair bulge (Vapniarsky et al. 2015; Hu et al. 2006). These cells display multipotency covering major neural derivatives such as the bone, cartilage, neurons, Schwann cells, myofibroblasts, and melanocytes (Vapniarsky et al. 2015).
As it will be discussed in detail in the next section cells isolated from foreskin, reveal that hair follicle is not the only source for the DSCs. The perivascular niche consists of stem cells in the adipose tissue, placenta, skeletal muscle, pancreas, and others (Corselli et al. 2010; da Silva Meirelles et al. 2008). Like the DSCs of the perivascular niche, those recently identified in the stroma of the sweat glands can also differentiate into adipogenic, chondrogenic, and osteogenic lineages (Vapniarsky et al. 2015).
2.1.4 Stem Cells of the Foreskin
Circumcision is described as the removal of foreskin tissue from the tip of the penis. It is done due to religious, cultural or medical reasons. Removal of foreskin tissue is surgically performed by professionals under sterile conditions. Circumcision should be carried out during the neonatal stage due to the higher risk of complications that may arise during later stages of development like puberty (Schoen et al. 2000). Basically, towards the end of the first trimester, a twist of skin evolves at the tip of the penis. Skin distally expands folding to turn into the foreskin. In the presence of certain androgens, the cells keratinize, spread, and migrate to the prepuce. This process is not completed at birth, but rather continues progressing throughout the childhood (Taylor et al. 1996).
As mentioned in the previous sections mesenchymal stem cells are important for gene therapy and regenerative medicine. Recent studies show that human adult stem cells offer to be a therapeutic alternative to embryonic stem cells. Adult stem cells can be isolated from different sources such as bone marrow (Tasli et al. 2014), adipose (Kalinina et al. 2011), placenta (Li et al. 2012), and dental tissues (Tasli and Sahin 2014). It was shown that skin is also a source of MSCs (Blanpain and Fuchs 2006). Like in the case of fibroblasts derived from dermal skin which are multipotent, express mesenchymal stem cell markers, and display immunosuppressive properties in common with those derived from the bone marrow. Fibroblastic tissue derived from the human foreskin tissue is one of the most important sources of mesenchymal stem cells. Moreover, it was reported that fibroblast-like cells derived from tissue support pluripotency and self-renewal of human embryonic stem cells (hESCs) (Unger et al. 2009; Mamidi et al. 2011). Human foreskin tissue is preferable because it is accessible, cheap, and can be acquired through ways that do not entail ethical issues (Ullah et al. 2015).
There are different types of studies performed using foreskin cells. In 2015, Somuncu et al. showed that foreskin cells have the capability to turn into adipogenic, chondrogenic, osteogenic cells as well as neurogenic and epithelial cells in vitro (Ullah et al. 2015). Normally, mesenchymal stem cells do not express hematopoietic stem cell markers. However, it was also reported that foreskin cells express mesenchymal and hematopoietic stem cell markers (Somuncu et al. 2015).
In another study, foreskin cells have been used as feeder cell lines for the human Embryonic Stem (ES) cells. It was shown that ES cells preserved their stem cell properties such as proliferation capacity, immortality, and pluripotency up to 70 passages. The main role of foreskin feeder cells is their potency to be cultured for 42 passages that allows suitable diagnosis for unknown agents and some genetic modifications (Hovatta et al. 2003; Mamidi et al. 2011).
There are studies investigating the migration potential of human foreskin cells. As known, fibronectin is described as a glycoprotein and found in an insoluble form on the surfaces of fibroblasts. It was shown that the presence of fibronectin averted the migration capacity of human foreskin cells through the matrix. These studies are given advises about cell-matrix and cell-cell interactions of human foreskin cells (Schor et al. 1981; Hovatta et al. 2003).
As emphasized, human foreskin cells is a promising tool for cell therapy and tissue engineering applications. They have the ability to differentiate into neurogenic, osteogenic, chondrogenic, and adipogenic cells. Due to their neurogenic differentiation capacity, human new-born foreskin cells can be used in neurodegenerative diseases such as Parkinson’s or Alzheimer’s disease (Bredesen et al. 2006). Moreover, they express hematopoietic stem cell markers making them appropriate candidates for cancer types such as multiple myeloma and leukaemia. Moreover, human foreskin tissue is abundant and can be easily obtained from circumcision treatments. Mostly, circumcised tissues are thrown away but these tissues can be used for stem cell isolation and further experiments (Somuncu et al. 2015).
3 Immunomodulatory Properties of Skin Stem Cells
Hair follicle displays unique immunological profile that accounts for its immune privilege described by the virtual absence of MHC Class I expression and relatively low numbers of immune cells in the hair follicle. Due to this immune privilege property, transplantation of follicle dermal-sheath cells from an individual to another does not involve tissue rejection processes (which are typically encountered in allograft procedures) while permitting new hair growth. Thus, being universal donors skin-derived stem cells became an attractive therapeutic source in cell-based therapies.
Highly proliferative Mesenchymal Stem Cells (MSCs) can be harvested from almost every tissue type in human body such as adipose (Zuk et al. 2001), cartilage (Conrad 1979), liver (Campagnoli et al. 2001), amniotic fluid (Scherjon et al. 2003), tooth germ (Taşlı and Şahin 2014), hair follicle (Rahmani et al. 2014), foreskin (Somuncu et al. 2015), and so on (Benvenuto et al. 2007; Gucciardo et al. 2009; Phinney and Prockop 2007). Also according to the data obtained from immunology-related studies, these MSCs can be used in treatment of immune disorders. For example, systematic infusion of MSCs proved them as useful tools in management of Graft Versus Host Disease (GVHD) due to their immunosuppressive properties (Le Blanc et al. 2003; Prasad et al. 2011). Modulation, activation, and proliferation ability of MSCs may help tissue renewal that would also be applicable for curing of variety immune diseases including GVHD.
It is not surprising that skin is home to a diversified pool of immune cells due to its essential function of forming the barrier to external assaults. Therefore, there is a dynamic crosstalk between the epithelial skin stem cells and resident immune cells whereby function and action of both cell types are coupled to each other to sustain or resume tissue homeostasis during normalcy or tissue repair. While dendritic epidermal γδ T cells (DETCs) and Langerhans cells reside in the epidermal layer, dendritic cells, mast cells, macrophages, γδ T cells, and αβ T cells are typically found in the dermal layer. Upon injury or infection, repair processes are initiated by the inflammatory cues, which are collectively known as “damage associated molecular patterns (DAMPS) or “pathogen associated molecular patterns (PAMPS)” disseminating from either dying cells or pathogens, respectively, to alert the immune system. In case of muscle repair, muscle stem cells (also known as satellite cells) rely on signals emanating from macrophages and Tregs to initiate the tissue repair processes such as proliferation of myoblasts (Naik et al. 2018). Release of distinct factors by the inflammation-activated macrophages (M1) provides suppression of NOTCH function to allow muscle stem cells to exit quiescence. During later stages of the repair, differentiation of the myoblast pool into myocytes ensues when regenerative M2 macrophages take over. For proper completion of the repair of the injured muscle tissue damage-induced inflammatory response must be subdued by the Tregs, expansion of which takes place at the time when macrophages phenotype switches from M1 and M2 and secrete stimulatory factors for regeneration. In contrast, during recovery from hair depilation release of CCL2 by the HFSCs guide the congregation of macrophages in the follicular niche possibly through augmented WNT signalling the loss of which delays hair cycling (Naik et al. 2018).
DETCs in injured skin produce FGF-7, FGF-10 and IGF-1, which are significant for endurance, proliferation, and relocation of epidermal cells. It has been shown that in mice secretion of fibroblast growth factor (FGF-9) by the dermal γδ T cells stimulates WNT expression and signalling in the dermal fibroblasts of the wound site promoting hair follicle neogenesis (Gay et al. 2013). In a positive feedback mechanism FGF9 produced by the wound fibroblasts fuels further WNT stimulation to augment regenerative processes of hair follicle neogenesis in the injured epidermis. However, human dermal layer lacks such an abundant dermal γδ T cell population which accounts for the failure to restore hair growth following damage in humans (Gay et al. 2013). Similar or varying mechanisms of immune cell participation in wound healing are reported in skin and other tissue types. For example, in an organ culture model activated human T cells likewise upregulate IGF-1 contributing to wound healing. Strikingly, same study reports that while both αβ T cells and γδ T cells demonstrate secretion of IGF1 when they are isolated from acute wounds, same is not true for these epidermal immune cell pools, which remain unresponsive to stimulation, when they are isolated from chronic wounds (Toulon et al. 2009).
Throughout the hair cycle the distribution and utility of immune cells change proposing that morphogenetic changes in the hair follicles may orchestrate concordant immune cell alignment in the skin. One such example is seen in the association of the pre-Langerhans Cells (pre-LCs, precursors of epidermal dendritic cells, see above) with the HFs before they appear in the IFE both in humans and rodents (Nagao et al. 2012). In this elegant study authors report that upon external stress, entry of the pre-LCs was permitted to the desired regions of HF via CCR2 and CCR6 chemokine receptor-dependent recruitment-permissive, but CCR8 chemokine receptor-dependent recruitment-nonpermissive mechanisms. Further evidence unveils the stunningly well-orchestrated biology of LC-recruitment to the HFs by demonstrating that ligands for the recruiter receptors of CCR2 and CCR6 are expressed by the distinct regions of the HF. While chemokine ligand CCL20 (activates CCR6 receptor) is secreted by the keratinocytes in the infundibulum region and chemokine ligand CCL2 (activates its CCR2 receptor) by the keratinocytes in the isthmus region, secretion of CCL8 is executed by the keratinocytes in the bulge region that probably accounts for evasion of pre-LC and LC repopulation in the bulge region. On the contrary, absence of immunogenic rather predominance of immunosuppressive signalling in the bulge region, which is recognized as the “stem cell sanctuary” and the matrix, reconciles with the “immune privilege’ property of the HFs. Nonetheless, restrictions to the “immune privilege” properties of the HF conceivably exist as suggested by the fact that besiege of both hair follicular and epidermal tissues by the immune cells can be encountered in allo-transplantation procedures (Paus et al. 2003).
Understanding impact of wounding on both skin stem cells and resident immune cells can aid treatment of autoimmune diseases like alopecia areata, pathology of which involves sustained activity of inflammatory immune cells aiming the hair bulb and the matrix, avoiding only Bu-SCs, and results in hair loss (Ito et al. 2005). In the cases of disorders such as discoid lupus erythematosus and lichen planopilaris, Bu-SCs are demolished and, therefore, ensuing hair loss is permanent (Harries et al. 2013; Al-Refu and Goodfield 2009). Therefore, precise identification of the molecules in charge of the interaction between the epithelial stem cells and immune cells will undoubtedly result in fruition of novel treatments that tap into the capacity of the skin in fine-tuning the adequate stimulation of immune response upon damages and pathogens without allowing over-stimulus of inflammatory responses to prevent autoimmune disorders (Patzelt et al. 2008).
Finally, lessons learned from the p120-catenin conditional knock-out mice underscore the intimate relation between the epidermal cells and resident immune cells (Perez-Moreno et al. 2006). As a crucial component of the adherens junctions (AJs), that serve as the intercellular glue-molecules in all epithelial tissues, p120 is a sister molecule of β-Catenin in the family of Armedillo repeat catenins. Both p120 and β-Catenin docks and stabilizes to the core E-Cadherin transmembrane protein at distinct sites of the cytoplasmic regions to bridge the dynamics of the intra-cellular actin skeleton to the extracellular domain of E-Cadherin that functions as the intercellular physical connector. While β-Catenin turns on Lef1/Tcf-dependent gene expression in response to WNT stimuli, p120 turns on Kaiso-dependent transcription. At the neonatal stage, despite of having decreased levels of intercellular AJ components, the epidermis of the p120-Catenin null mice displays no overt failure of the epidermal barrier function. However, as these animals age, they develop epidermal hyperplasia, chronic inflammation, hair degeneration, and profoundly decreased abdominal fat. All of these pathologies are linked to aberrant activation of proinflammatory signals downstream to NFκB-dependent transcriptional cascade. These findings propose that in the context of combating infectious assaults pathogen-induced proinflammatory signalling cascades that coordinate immune responses in the skin, possess a potential to participate in neoplastic progression in the absence of pathogens (Perez-Moreno et al. 2006).
3.1 T Lymphocyte-Mediated Suppression
T cells play the most important role in the memory creation of the adaptive immunity reaction which is generated as a specialized immune response to specific pathogens (Zhang et al. 2012). T cell activation can be hampered from direct cell to cell interaction with MSCs. These interactions between MSCs and cytotoxic (CD8+) or regulatory (CD4+) T cells, which control the secretion of related cytokines for signaling and inhibition of T cell activation, are necessary for effective immune suppression (Nauta and Fibbe 2007; Uccelli et al. 2008). Also, it has been shown that MSCs can express the Fas ligand (FasL), also known as a binding partner for the death receptor, that prevents T cell migration (Zhang et al. 2012), by prompting initiated T cell death via direct cell-to-cell interaction (Akiyama et al. 2012). Researchers demonstrated the inhibitory effect of MSCs on T cell proliferation, however, these suppressive effects of MSCs haven’t been fully understood either in cases of autologous or allogeneic cell transfer (Krampera et al. 2003; Le Blanc et al. 2003). Cytokine secretion from MSCs and immune cells increase the levels of interferon γ (IFNγ) and interleukin (IL)-17, which trigger T helper cells (TH0) to produce IL-4 that is in charge of inducing differentiation of regulatory T cells (TH2) cells (Aggarwal and Pittenger 2005; Sun et al. 2009). The (observation) conclusion that MSCs can suppress immunity might, at first, appear as an unwanted effect, however, it becomes a desired consequence in cases of organ transplantation of this type of stem cells, especially in cases of Solid Organ Transplantation (SOT). While there isn’t any research showing the effect of MSCs against Solid Organ Allograft (SOA) refusal, a plentiful and increasing amount of facts obtained from in vitro and in vivo studies claim that this approach might be a promising in management of SOA (Bartholomew et al. 2002; Zhou et al. 2006; Crop et al. 2009; Renner et al. 2009). While results from the human clinical studies are not satisfactory yet, preclinical studies demonstrated that MSCs could become usable therapeutic agents for OR applications. Furthermore, not just that they can modulate the host immune reaction in a way that may enhance the tolerance of the transplanted organ, but also their ability to trigger tissue regeneration and induce unique gene expression profile may assist the processes involved in reduction of the inflammatory reaction to the allograft. Further studies must be designed and carefully carried out to explain the MSC-dependent immune suppression. Nonetheless, the less toxicity and possible long term suppression of immunity by MSCs make them a potentially outstanding therapeutic tool compared to the traditional T cell suppressive agents (Corry et al. 1999; Kawai et al. 2014).
3.2 B Lymphocyte-Mediated Suppression
While T cells are the main player of an immune reaction, B cells have important roles in antibody secretion to modulate immune response and also they closely work together with T cells. While many studies have shown that MSCs can inhibit B cell proliferation, differentiation, and cytokine secretion (Augello et al. 2005; Gerdoni et al. 2007; Rasmusson et al. 2007; Asari et al. 2009), it has also been shown that MSCs can increase B cell proliferation and cytokine secretion from B cells in in vitro and in vivo studies (Rasmusson et al. 2007; Traggiai et al. 2008). Even though, the exact mechanism-of-action how MSC-mediated B cell suppression operates remains unknown, researchers claim that this suppression can take place as a result of differentiated B cell or the direct effect of the local stimulating signals. Co-culture studies displayed that triggering MSC-mediated immune response suppression needs some inducing cues from B cells, suggesting that in order for MSCs to suppress an immune reaction they need to be activated by relevant stimuli derived from B cells in the first place. This back-and-forth crosstalk between MSCs and B cells ultimately result in the inhibition of B cell proliferation, pointing to a feedback loop inhibition type mechanism in control of B Cell proliferation. An alternate mechanism for the inhibition of B cell proliferation involves the effect of MSCs on the activity of T cells through which they might inhibit B cells indirectly (Gerdoni et al. 2007). One other important point about these experiments is that majority them were done in in vitro conditions not in vivo or ex vivo.
3.3 Dendritic Cell-Mediated Suppression
Additionally, MSCs are effective against monocytes, monocyte-derived dendritic cells, macrophages, natural killer cells, and neutrophils. Few studies demonstrated that MSCs are able to suppress the generation dendritic cells from monocytes by blocking the antigen presentation, in vitro (Gerdoni et al. 2007; Ramasamy et al. 2007). Also, it has been claimed that dendritic cells are key factors of immune response and tolerance, depending on the activation and maturation stage and the cytokine milieu at sites of inflammation (Rutella et al. 2006). Suggesting that, inhibition of differentiation, maturation, and action of dendritic cells might occur by suppressing the CD14+ monocyte differentiation into mature dendritic cells and through promoting cytokine secretion (Beyth et al. 2005; Jiang et al. 2005).
3.4 Natural Killer Cell (NKs)-Mediated Suppression
NK cells play important roles in the intrinsic pathway, mainly during anti-tumor and anti-viral infections. As a result of their highly cytotoxic function, and they have the ability to secrete large amount of pro-inflammatory cytokines, including TNFα and IFNγ (Malhotra and Shanker 2011). Moreover, MSCs inhibit IL-2 and IL-15 mediated NK cell proliferation, IFNγ secretion, and cytotoxicity of both latent and stimulated NK cells (Rasmusson et al. 2003; Meisel et al. 2004; Aggarwal and Pittenger 2005).
Immunosuppression ability of MSCs are making them outstanding candidates for immunosuppressive agents for the prevention and treatment of various inflammatory and autoimmune diseases (Uccelli et al. 2008; Rasmusson et al. 2007). Starting from the 2000, unique MSC sources have been obtained from several dental tissues, which exhibit remarkable tissue regenerative and immunosuppressive properties (Yamaza et al. 2010). Other than having spectacular self-renewal property and multipotency, hFSSCs have powerful immunosuppressive functions comparable to other stem cell types, making them promising cell sources for MSC mediated transplantation treatment.
Foreskin is a waste tissue that’s why they might be more beneficial than other cell sources as obtainability of MSCs. Additionally FSSCs have a high growth and clonogenic capacity (Najar et al. 2016). Several immunomodulatory cytokines and factors including IL-6, IL-12 and TNF-α secreted from FSSCs, potentially involved in FSK-MSC immunomodulation were identified. Hence, this immunomodulation function of FSSCs might be make these cells hopeful tolerogenic agent for developing stem cell-based immunotherapy.
4 Stem Cells in Skin Cancer
Skin cancer is a common form of cancer diagnosed in men and women. During the last decade, there has been a rise in the prevalence of skin cancer globally (Bergers et al. 2016). Skin phototype, hair color, multiple nevi, family history, and the degree of exposure to ultraviolet (UV) radiation are cited amongst the etiologic factors that can cause skin cancer (Hernando et al. 2016; Pfeifer 2015; Raimondi et al. 2008). There are three major types of skin cancer: (i) malignant melanoma (ii) basal cell carcinomas (BCCs) and (iii) squamous cell carcinomas (SCCs) (Simões et al. 2015). Melanoma is one of the most aggressive, complex, and heterogeneous cancer type with 132,000 new cases diagnosed worldwide each year (Lohcharoenkal et al. 2018; WHO 2017).
Human malignant melanoma is an extremely aggressive type of skin cancer which is characterized by its astonishing heterogeneity, tendency for spreading throughout the body, and developing resistance to cytotoxic mediators.
Even though standard care such as chemotherapy and immunotherapy have been assessed in clinical studies, most of these therapeutic agents fall short in providing an effective treatment for those with progressive disease. Lack of effective treatment in melanoma patients is ascribed primarily to a high degree of tumor heterogeneity that results in countless number of genetically distinct subpopulations. Therefore, in a given tumor mass, some of these subpopulations belong to cancer stem cells (CSCs), while the rest are composed of non-CSCs which constitute majority of the tumor (Alamodi et al. 2016). The consensus on the commonly found features of a CSCs include: (i) their potential to initiate tumor growth, ability for (ii) self-renewal and (iii) differentiate into tumor cells from different epithelial origin that make up the bulk of the tumor (Aponte and Caicedo 2017).
The CSC model includes various subpopulations of malignant cells and for long time it has been suggested that the carcinogenic component of primary melanoma is not homogeneous, as one would expect in a stochastic model of clonal carcinogenesis (Zabala et al. 2016). Fairly, melanoma display ‘polyclonism’ (Laga and Murphy 2010), in the sense that structurally, cytologically, and immune-histochemically distinct populations often co-exist inside of a single tumor nodule. In consistence with this model of melanoma, a hierarchical order of cell types, that is a consequence of tumor differentiation, is documented in vivo. Each of these cell types has distinct capacity for self-renewal that can propel tumorigenesis through the engagement of signature signalling pathways (Maniotis et al. 1999).
Several malignant features of melanomas such as intratumoral heterogeneity, tumor progression, and drug resistance are determined by their stem cells, also known as melanoma stem cells (MSCs) (Nguyen et al. 2015). MCSs are a subpopulation of melanoma tumors and their molecular characteristics may either account for increased progression, drug resistance, and recurrence of melanomas or are a consequence of these malignant events (Alamodi et al. 2016). Distinct populations of MSCs are described by their signature proteomic as well as genomic context that includes several driver-mutations promoting tumor growth. There are certain features of melanoma stem cells that offer an excellent disease model to study (El-Khattouti et al. 2014, 2015; Leikam et al. 2015; Li et al. 2015). First, MSCs may be isolated from the skin through moderately non-invasive methods as they are mainly present in the interior of a particular anatomic niche inside of the skin, thus allowing their easy isolation. For example, MSCs are located within the bulge area of the hair follicle in the murine skin (El-Khattouti et al. 2015). Due to the developmental origins of MSCs, they are multipotent and they have the innate property to migrate to different sites (Stecca and Santini 2015).
Both BCCs and SCCs are thought to originate from a stem cell ancestry although this idea needs further proof. The rationale for this claim bases on the fact that the continuously renewing tissue of epidermis is a niche for all keratinocytes that undergo a well-defined differentiation program upon leaving the stem cell compartment and terminate this process by the generation of dead horn squames (Leikam et al. 2015). In relevance with this concept, should a damage take place in the cells of a tissue-type other than the stem cell compartment, these cells will most likely be cleared due to the differentiation process. On the other hand, in case of an insult to the pool of epidermal stem cells, several genetic changes that can potentially prevent cell cycle control and stimulate neoplastic growth, may accumulate due to the long-lived nature of these cells that are much less frequently divided (Li et al. 2015). Alternatively, non-stem cells may prevail and -thereby- contribute to transformation through acquiring genetic changes, all of which may hinder their clearance. Support for this hypothesis comes from the observation for the heterogeneity found in human BCCs. It was demonstrated previously that inactivation of p53 through several mutations -mostly those caused by UV exposure- is an early event in the development of skin carcinoma (Brash et al. 2010; Shannan et al. 2016). For example, nuclear accumulation of mutant p53 protein was detected in clones of keratinocytes with normal morphology as well as potential stem cell populations isolated from skin samples of individuals who were chronically exposed to sun. Since mutant p53 is devoid of function, normally executed by the wild type protein, UV-induced apoptosis would be abrogated (Pacifico et al. 2017). Moreover, there is a striking heterogeneity in the mutations characterized for p53 in human BCCs based on several reports (Benjamin and Ananthaswamy 2016). Studies, where clonality of tumors was delineated by microdissection-based approaches, indicated that cellular composition of these tumors are shaped by a dominating clone with a high degree of genomic instability. Nonetheless, a second or -even- a third genomic variation in p53 was detected in subclones found within the same tumor area (Pontén et al. 1997).
Overall, subpopulations found in melanoma lesions display a CSC-like phenotype including tumorigenesis, self-renewal, and differentiation characteristics in that these putative melanoma CSCs have enhanced ability of forming and maintaining melanoma tumors with a propensity to resist treatment.
5 Conclusion
Due to their exceptional regenerative properties stem cells of the skin will continue being an attractive research area-of-interest for numerous research studies in future. There is no doubt understanding the precise nature of the communication of the distinct stem cell populations with the niche components, their progeny, and with one another will hold promise for the improvement of clinical methods of regenerative medicine. Improvement of the current clinical practice will be -further- enabled by the accurate identification of skin stem cell subtypes that is dependent on the characterization of subtype-specific markers. With increased sensitivity of isolation methods, advanced understanding of the biology for a specific sub-type of skin stem cells, their therapeutic manipulation could offer more effective treatment modalities for a wide variety of degenerative disorders of the skin.
Abbreviations
- AEG:
-
Apoeccrine Sweat Glands
- APM:
-
Arrector Pili Muscle
- BCCs:
-
Basal Cell Carcinomas
- BMP:
-
Bone Morphogenetic Protein
- BMPs:
-
Bone Morphogenetic Proteins
- Brdurd:
-
Bromodeoxyuridine
- Bu-SC:
-
Bulge Lack Bulge Stem Cell
- CGRP:
-
Calcitonin Gene-Related Peptide
- CSCs:
-
Cancer Stem Cells
- DETCs:
-
Dendritic Epidermal Γδ T Cells
- DP:
-
Dermal Papilla
- DP:
-
Dermal Papilla
- DS:
-
Dermal Sheath
- DSCs:
-
Dermal Stem Cells
- E6:
-
Embryonic Week 6
- EGFR:
-
Epidermal Growth Factor Receptor
- FGF-7:
-
Fibroblast Growth Factor 7
- GSI:
-
Gamma Secretase İnhibitor
- GVHD:
-
Graft Versus Host Disease
- HF:
-
Hair Follicle
- HFSC:
-
Hair Follicular Stem Cell
- Hh/Ptc:
-
Hedgehog/Patched
- H3K27:
-
Histone H3 Lys
- Hox:
-
Homeobox
- Hescs:
-
Human Embryonic Stem Cells
- EPI-Ncscs:
-
Human Epidermal Neural Crest Stem Cells
- IRS:
-
Inner Root Sheath
- IGF-1:
-
Insulin-Like Growth Factor 1
- IFE:
-
Interfollicular Epidermis
- IFs:
-
Intermediate Filaments
- K14:
-
Keratin 14
- K5:
-
Keratin 5
- LRCs:
-
Label Retaining Cells
- LLP:
-
Lower Permanent Portion
- M-SCs:
-
Melanocyte Stem Cells
- MSCs:
-
Mesenchymal Stem Cells
- NKC:
-
Natural Killer Cell
- NID:
-
Notch İntracellular Domain
- ORS:
-
Outer Root Sheath
- PSU:
-
Pilosebaceous Unit
- PDGF-Alpha:
-
Platelet-Derived-Growth-Factor Alpha
- SOA:
-
Solid Organ Allograft
- SOT:
-
Solid Organ Transplantation
- SCCs:
-
Squamous Cell Carcinomas
- TGF Alpha:
-
Transforming Growth Factor Alpha
- TACS:
-
Transiently Amplifying Cells
- Wg/Wnt/Catenin:
-
Wingless/Armadillo
References
Abou Neel EA, Aljabo A, Strange A, Ibrahim S, Coathup M, Young AM, Bozec L, Mudera V (2016) Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine 11:4743–4763. https://doi.org/10.2147/IJN.S107624
Achilleos A, Trainor PA (2012) Neural crest stem cells: discovery, properties and potential for therapy. Cell Res 22(2):288–304. https://doi.org/10.1038/cr.2012.11
Adam RC, Yang H, Ge Y, Lien W-H, Wang P, Zhao Y, Polak L, Levorse J, Baksh SC, Zheng D (2018) Temporal layering of signaling effectors drives chromatin remodeling during hair follicle stem cell lineage progression. Cell Stem Cell 22(3):398–413. e397
Agabalyan NA, Rosin NL, Rahmani W, Biernaskie J (2017) Hair follicle dermal stem cells and skin-derived precursor cells: exciting tools for endogenous and exogenous therapies. Exp Dermatol 26(6):505–509
Agarwal S, Krishnamurthy K (2019a) Histology, skin. In: StatPearls [Internet]. StatPearls Publishing
Agarwal S, Krishnamurthy K (2019b) Histology, skin. In: StatPearls. Treasure Island (FL)
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822
Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S (2012) Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10(5):544–555
Alam H, Sehgal L, Kundu ST, Dalal SN, Vaidya MM (2011) Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22(21):4068–4078. https://doi.org/10.1091/mbc.E10-08-0703
Alamodi AA, Eshaq AM, Hassan S-Y, Al Hmada Y, El Jamal SM, Fothan AM, Arain OM, Hassan S-L, Haikel Y, Megahed M (2016) Cancer stem cell as therapeutic target for melanoma treatment. Histol Histopathol 31(12):1291–1301
Alcolea MP, Jones PH (2014) Lineage analysis of epidermal stem cells. Cold Spring Harb Perspect Med 4(1):a015206. https://doi.org/10.1101/cshperspect.a015206
Alibardi L (2004) Comparative aspects of the inner root sheath in adult and developing hairs of mammals in relation to the evolution of hairs. J Anat 205(3):179–200. https://doi.org/10.1111/j.0021-8782.2004.00324.x
Al-Refu K, Goodfield M (2009) Hair follicle stem cells in the pathogenesis of the scarring process in cutaneous lupus erythematosus. Autoimmun Rev 8(6):474–477
Al-Suhaimi EA, Shehzad A (2013) Leptin, resistin and visfatin: the missing link between endocrine metabolic disorders and immunity. Eur J Med Res 18:12. https://doi.org/10.1186/2047-783X-18-12
Alvares SM, Dunn CA, Brown TA, Wayner EE, Carter WG (2008) The role of membrane microdomains in transmembrane signaling through the epithelial glycoprotein Gp140/CDCP1. Biochim Biophys Acta 1780(3):486–496. https://doi.org/10.1016/j.bbagen.2008.01.010
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22(10):1276–1312. https://doi.org/10.1101/gad.1653708
Andreone BJ, Lacoste B, Gu C (2015) Neuronal and vascular interactions. Annu Rev Neurosci 38:25–46. https://doi.org/10.1146/annurev-neuro-071714-033835
Aponte PM, Caicedo A (2017) Stemness in Cancer: stem cells, Cancer stem cells, and their microenvironment. Stem Cells Int 2017:5619472. https://doi.org/10.1155/2017/5619472
Arda O, Goksugur N, Tuzun Y (2014) Basic histological structure and functions of facial skin. Clin Dermatol 32(1):3–13. https://doi.org/10.1016/j.clindermatol.2013.05.021
Arron S (2016) Anatomy of the skin and pathophysiology of radiation dermatitis. In: Skin care in radiation oncology. Springer, Cham, pp 9–14
Asari S, Itakura S, Ferreri K, Liu C-P, Kuroda Y, Kandeel F, Mullen Y (2009) Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol 37(5):604–615
Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G (2005) Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35(5):1482–1490
Augustine R (2018) Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks. Prog Biomater 7(2):77–92. https://doi.org/10.1007/s40204-018-0087-0
Balana ME, Charreau HE, Leiros GJ (2015) Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J Stem Cells 7(4):711–727. https://doi.org/10.4252/wjsc.v7.i4.711
Balañá ME, Charreau HE, Leirós GJ (2015) Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J Stem Cells 7(4):711
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 339(1):269–280. https://doi.org/10.1007/s00441-009-0834-6
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30(1):42–48
Baswan S, Kasting GB, Li SK, Wickett R, Adams B, Eurich S, Schamper R (2017) Understanding the formidable nail barrier: a review of the nail microstructure, composition and diseases. Mycoses 60(5):284–295. https://doi.org/10.1111/myc.12592
Bazzoni R, Bentivegna A (2019) Role of notch Signaling pathway in Glioblastoma pathogenesis. Cancers 11(3). https://doi.org/10.3390/cancers11030292
Bell J, Bolanowski S, Holmes MH (1994) The structure and function of Pacinian corpuscles: a review. Prog Neurobiol 42(1):79–128
Benjamin CL, Ananthaswamy HN (2016) Etiology of nonmelanocytic skin cancer. In: Skin cancer prevention. CRC Press, pp 39–66. https://doi.org/10.3109/9781420021097. ISBN: 9780429163425
Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A (2007) Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 25(7):1753–1760
Bergers LI, Reijnders CM, van den Broek LJ, Spiekstra SW, de Gruijl TD, Weijers EM, Gibbs S (2016) Immune-competent human skin disease models. Drug Discov Today 21(9):1479–1488
Berika M, Elgayyar ME, El-Hashash AH (2014) Asymmetric cell division of stem cells in the lung and other systems. Front Cell Dev Biol 2:33. https://doi.org/10.3389/fcell.2014.00033
Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J (2005) Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105(5):2214–2219
Bikle DD (2012) Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord 13(1):3–19. https://doi.org/10.1007/s11154-011-9194-0
Blanpain C, Fuchs E (2006) Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22:339–373. https://doi.org/10.1146/annurev.cellbio.22.010305.104357
Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10(3):207–217. https://doi.org/10.1038/nrm2636
Bogaerts E, Heindryckx F, Vandewynckel YP, Van Grunsven LA, Van Vlierberghe H (2014) The roles of transforming growth factor-beta, Wnt, notch and hypoxia on liver progenitor cells in primary liver tumours (review). Int J Oncol 44(4):1015–1022. https://doi.org/10.3892/ijo.2014.2286
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801. https://doi.org/10.1038/nrm3904
Botchkareva NV, Ahluwalia G, Shander D (2006) Apoptosis in the hair follicle. J Invest Dermatol 126(2):258–264. https://doi.org/10.1038/sj.jid.5700007
Bragulla HH, Homberger DG (2009) Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat 214(4):516–559. https://doi.org/10.1111/j.1469-7580.2009.01066.x
Brahs AB, Bolla SR (2019) Histology, nail. In: StatPearls. Treasure Island (FL)
Brash DE, Heffernan TP, Nghiem P (2010) Carcinogenesis: UV radiation. In: Textbook of aging skin. Springer, Berlin, pp 567–578
Bredesen DE, Rao RV, Mehlen P (2006) Cell death in the nervous system. Nature 443(7113):796
Breitkreutz D, Koxholt I, Thiemann K, Nischt R (2013) Skin basement membrane: the foundation of epidermal integrity – BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res Int 2013:179784. https://doi.org/10.1155/2013/179784
Brekelmans P, van Soest P, Voerman J, Platenburg PP, Leenen PJ, van Ewijk W (1994) Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol 159(2):331–339. https://doi.org/10.1006/cimm.1994.1319
Briggman JV, Bank HL, Bigelow JB, Graves JS, Spicer SS (1981) Structure of the tight junctions of the human eccrine sweat gland. Am J Anat 162(4):357–368. https://doi.org/10.1002/aja.1001620406
Brohem CA, Cardeal LB, Tiago M, Soengas MS, Barros SB, Maria-Engler SS (2011) Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res 24(1):35–50. https://doi.org/10.1111/j.1755-148X.2010.00786.x
Brown TM, Krishnamurthy K (2018) Histology, dermis. In: StatPearls [Internet]. StatPearls Publishing
Brown TM, Krishnamurthy K (2019a) Histology, dermis. In: StatPearls. Treasure Island (FL)
Brown TM, Krishnamurthy K (2019b) Histology, hair and follicle. In: StatPearls. Treasure Island (FL)
Bryder D, Rossi DJ, Weissman IL (2006) Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169(2):338–346. https://doi.org/10.2353/ajpath.2006.060312
Cai J, Weiss ML, Rao MS (2004) In search of “stemness”. Exp Hematol 32(7):585–598. https://doi.org/10.1016/j.exphem.2004.03.013
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98(8):2396–2402
Çankirili NK, Altundag O, Çelebi-Saltik B (2019) Skin stem cells, their niche and tissue engineering approach for skin regeneration. Adv Exp Med Biol. https://doi.org/10.1007/5584_2019_380
Carulli AJ, Samuelson LC, Schnell S (2014) Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr Biol 6(3):243–257. https://doi.org/10.1039/c3ib40163d
Ceafalan L, Gherghiceanu M, Popescu LM, Simionescu O (2012) Telocytes in human skin--are they involved in skin regeneration? J Cell Mol Med 16(7):1405–1420. https://doi.org/10.1111/j.1582-4934.2012.01580.x
Cha J, Falanga V (2007) Stem cells in cutaneous wound healing. Clin Dermatol 25(1):73–78
Chadwick S, Heath R, Shah M (2012) Abnormal pigmentation within cutaneous scars: a complication of wound healing. Indian J Plast Surg 45(2):403–411. https://doi.org/10.4103/0970-0358.101328
Chang CY, Pasolli HA, Giannopoulou EG, Guasch G, Gronostajski RM, Elemento O, Fuchs E (2013) NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature 495(7439):98–102. https://doi.org/10.1038/nature11847
Che L, Yuan YH, Jia J, Ren J (2012) Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chin J Cancer Res = Chung-kuo yen cheng yen chiu 24(4):323–331. https://doi.org/10.3978/j.issn.1000-9604.2012.10.10
Chen WC, Zouboulis CC (2009) Hormones and the pilosebaceous unit. Derm Endocrinol 1(2):81–86. https://doi.org/10.4161/derm.1.2.8354
Chen M, Przyborowski M, Berthiaume F (2009) Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng 37(4–5):399–421
Chen S, Ma J, Wu F, Xiong LJ, Ma H, Xu W, Lv R, Li X, Villen J, Gygi SP, Liu XS, Shi Y (2012) The histone H3 Lys 27 demethylase JMJD3 regulates gene expression by impacting transcriptional elongation. Genes Dev 26(12):1364–1375. https://doi.org/10.1101/gad.186056.111
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204–7218. https://doi.org/10.18632/oncotarget.23208
Cheng C-C, Tsutsui K, Taguchi T, Sanzen N, Nakagawa A, Kakiguchi K, Yonemura S, Tanegashima C, Keeley SD, Kiyonari H (2018) Hair follicle epidermal stem cells define a niche for tactile sensation. elife 7:e38883
Chermnykh E, Kalabusheva E, Vorotelyak E (2018) Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci 19(4). https://doi.org/10.3390/ijms19041003
Chueh SC, Lin SJ, Chen CC, Lei M, Wang LM, Widelitz R, Hughes MW, Jiang TX, Chuong CM (2013) Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther 13(3):377–391. https://doi.org/10.1517/14712598.2013.739601
Chuong CM, Randall VA, Widelitz RB, Wu P, Jiang TX (2012) Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology 27(2):61–72. https://doi.org/10.1152/physiol.00028.2011
Cichorek M, Wachulska M, Stasiewicz A, Tyminska A (2013) Skin melanocytes: biology and development. Postepy Dermatol Alergol 30(1):30–41. https://doi.org/10.5114/pdia.2013.33376
Conrad G (1979) Cartilage cell differentiation. Clin Orthop Relat Res 139:185–205
Corry RJ, Chakrabarti PK, Shapiro R, Rao AS, Dvorchik I, Jordan ML, Scantlebury VP, Vivas CA, Fung JJ, Starzl TE (1999) Simultaneous administration of adjuvant donor bone marrow in pancreas transplant recipients. Ann Surg 230(3):372
Corselli M, Chen C-W, Crisan M, Lazzari L, Péault B (2010) Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol 30(6):1104–1109
Crop MJ, Baan CC, Korevaar SS, IJzermans JN, Alwayn IP, Weimar W, Hoogduijn MJ (2009) Donor-derived mesenchymal stem cells suppress alloreactivity of kidney transplant patients. Transplantation 87(6):896–906
Cui CY, Schlessinger D (2015) Eccrine sweat gland development and sweat secretion. Exp Dermatol 24(9):644–650. https://doi.org/10.1111/exd.12773
da Silva Meirelles L, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26(9):2287–2299
da Silva EZM, Jamur MC, Oliver C (2014) Mast cell function: a new vision of an old cell. J Histochem Cytochem 62(10):698–738
Darby IA, Laverdet B, Bonté F, Desmoulière A (2014) Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7:301
Del Bino S, Duval C, Bernerd F (2018) Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci 19(9). https://doi.org/10.3390/ijms19092668
Dela Cruz F, Terry M, Matushansky I (2012) A transgenic, mesodermal specific, Dkk1 mouse model recapitulates a spectrum of human congenital limb reduction defects. Differ Res Biol Divers 83(4):220–230. https://doi.org/10.1016/j.diff.2012.01.001
Delva E, Tucker DK, Kowalczyk AP (2009) The desmosome. Cold Spring Harb Perspect Biol 1(2):a002543. https://doi.org/10.1101/cshperspect.a002543
Deo PN, Deshmukh R (2018) Pathophysiology of keratinization. J Oral Maxillofac Pathol 22(1):86–91. https://doi.org/10.4103/jomfp.JOMFP_195_16
Diao J, Liu J, Wang S, Chang M, Wang X, Guo B, Yu Q, Yan F, Su Y, Wang Y (2019) Sweat gland organoids contribute to cutaneous wound healing and sweat gland regeneration. Cell Death Dis 10(3):238. https://doi.org/10.1038/s41419-019-1485-5
Di Persio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, Hynes RO (2000) alpha3beta1 and alpha6beta4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci 113(Pt 17):3051–3062
Domowicz MS, Sanders TA, Ragsdale CW, Schwartz NB (2008) Aggrecan is expressed by embryonic brain glia and regulates astrocyte development. Dev Biol 315(1):114–124. https://doi.org/10.1016/j.ydbio.2007.12.014
Doty RL (2014) Human pheromones: do they exist? In: Mucignat-Caretta C (ed) Neurobiology of chemical communication. Frontiers in neuroscience. CRC Press, Boca Raton
Driskell RR, Watt FM (2015) Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 25(2):92–99
Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V (2014) Defining dermal adipose tissue. Exp Dermatol 23(9):629–631. https://doi.org/10.1111/exd.12450
Ehrlich HP, Hunt TK (2012) Collagen organization critical role in wound contraction. Adv Wound Care 1(1):3–9. https://doi.org/10.1089/wound.2011.0311
El-Khattouti A, Selimovic D, Haïkel Y, Megahed M, Gomez CR, Hassan M (2014) Identification and analysis of CD133+ melanoma stem-like cells conferring resistance to taxol: an insight into the mechanisms of their resistance and response. Cancer Lett 343(1):123–133
El-Khattouti A, Sheehan NT, Monico J, Drummond HA, Haikel Y, Brodell RT, Megahed M, Hassan M (2015) CD133+ melanoma subpopulation acquired resistance to caffeic acid phenethyl ester-induced apoptosis is attributed to the elevated expression of ABCB5: significance for melanoma treatment. Cancer Lett 357(1):83–104
Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA (2010) Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell 18(4):633–642. https://doi.org/10.1016/j.devcel.2010.01.016
Fajuyigbe D, Young AR (2016) The impact of skin colour on human photobiological responses. Pigment Cell Melanoma Res 29(6):607–618. https://doi.org/10.1111/pcmr.12511
Feller L, Masilana A, Khammissa RA, Altini M, Jadwat Y, Lemmer J (2014) Melanin: the biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med 10:8. https://doi.org/10.1186/1746-160X-10-8
Firth AL, Yuan JX (2012) Identification of functional progenitor cells in the pulmonary vasculature. Pulm Circ 2(1):84–100. https://doi.org/10.4103/2045-8932.94841
Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA (2018) Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int 2018:8031718. https://doi.org/10.1155/2018/8031718
Flament F, Francois G, Qiu H, Ye C, Hanaya T, Batisse D, Cointereau-Chardon S, Seixas MD, Dal Belo SE, Bazin R (2015) Facial skin pores: a multiethnic study. Clin Cosmet Investig Dermatol 8:85–93. https://doi.org/10.2147/CCID.S74401
Forni MF, Trombetta-Lima M, Sogayar MC (2012) Stem cells in embryonic skin development. Biol Res 45(3):215–222. https://doi.org/10.4067/s0716-97602012000300003
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123(Pt 24):4195–4200. https://doi.org/10.1242/jcs.023820
Freeman SC, Sonthalia S (2019) Histology, keratohyalin granules. In: StatPearls. Treasure Island (FL)
Fuchs E (2009) The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137(5):811–819. https://doi.org/10.1016/j.cell.2009.05.002
Fuchs E, Nowak JA (2008) Building epithelial tissues from skin stem cells. Cold Spring Harb Symp Quant Biol 73:333–350. https://doi.org/10.1101/sqb.2008.73.032
Fuchs PA, Glowatzki E, Moser T (2003) The afferent synapse of cochlear hair cells. Curr Opin Neurobiol 13(4):452–458
Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM (2011) The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144(4):577–589. https://doi.org/10.1016/j.cell.2011.01.014
Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC (2012) Skin anti-aging strategies. Derm Endocrinol 4(3):308–319. https://doi.org/10.4161/derm.22804
Garland EL (2012) Pain processing in the human nervous system: a selective review of nociceptive and biobehavioral pathways. Prim Care 39(3):561–571
Gaur M, Dobke M, Lunyak V (2017) Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int J Mol Sci 18(1):208
Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD (2013) Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 19(7):916
Gentile P, Garcovich S (2019) Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth-factor, and Mesenchymal stem cell Signaling impact analysis on cell growth and hair follicle development. Cell 8(5). https://doi.org/10.3390/cells8050466
George JM, Smita M, Kadalmani B, Girija R, Oommen OV, Akbarsha MA (2004) Secretory and basal cells of the epithelium of the tubular glands in the male Mullerian gland of the caecilian Uraeotyphlus narayani (Amphibia: Gymnophiona). J Morphol 262(3):760–769. https://doi.org/10.1002/jmor.10276
Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R (2007) Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol 61(3):219–227
Ghadially R (2012) 25 years of epidermal stem cell research. J Invest Dermatol 132(3 Pt 2):797–810. https://doi.org/10.1038/jid.2011.434
Gola M, Czajkowski R, Bajek A, Dura A, Drewa T (2012) Melanocyte stem cells: biology and current aspects. Med Sci Monit 18(10):RA155–RA159. https://doi.org/10.12659/msm.883475
Gonzales KAU, Fuchs E (2017) Skin and its regenerative powers: an Alliance between stem cells and their niche. Dev Cell 43(4):387–401. https://doi.org/10.1016/j.devcel.2017.10.001
Gordan R, Gwathmey JK, Xie LH (2015) Autonomic and endocrine control of cardiovascular function. World J Cardiol 7(4):204–214. https://doi.org/10.4330/wjc.v7.i4.204
Greaney JL, Kenney WL, Alexander LM (2016) Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci Basic Clin 196:81–90. https://doi.org/10.1016/j.autneu.2015.11.002
Gucciardo L, Lories R, Ochsenbein-Kölble N, Zwijsen A, Deprest J (2009) Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG Int J Obstet Gynaecol 116(2):166–172
Guo EL, Katta R (2017) Diet and hair loss: effects of nutrient deficiency and supplement use. Derm Pract Concep 7(1):1–10. https://doi.org/10.5826/dpc.0701a01
Guo S, Liao H, Liu J, Liu J, Tang F, He Z, Li Y, Yang Q (2018) Resveratrol activated sonic hedgehog Signaling to enhance viability of NIH3T3 cells in vitro via regulation of Sirt1. Cell Physiol Biochem 50(4):1346–1360. https://doi.org/10.1159/000494593
Haddad A, Laicine EM, Tripathi BJ, Tripathi RC (2001) An extensive system of extravascular smooth muscle cells exists in the choroid of the rabbit eye. Exp Eye Res 73(3):345–353. https://doi.org/10.1006/exer.2001.1042
Haeberle H, Lumpkin EA (2008) Merkel Cells in Somatosensation. Chemosens Percept 1(2):110–118. https://doi.org/10.1007/s12078-008-9012-6
Haftek M (2015) Epidermal barrier disorders and corneodesmosome defects. Cell Tissue Res 360(3):483–490. https://doi.org/10.1007/s00441-014-2019-1
Hamill KJ, Hopkinson SB, DeBiase P, Jones JC (2009) BPAG1e maintains keratinocyte polarity through beta4 integrin-mediated modulation of Rac1 and cofilin activities. Mol Biol Cell 20(12):2954–2962. https://doi.org/10.1091/mbc.E09-01-0051
Harries MJ, Meyer K, Chaudhry I, E Kloepper J, Poblet E, Griffiths CE, Paus R (2013) Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol 231(2):236–247
Hernando B, Ibarrola-Villava M, Fernandez LP, Peña-Chilet M, Llorca-Cardeñosa M, Oltra SS, Alonso S, Boyano MD, Martinez-Cadenas C, Ribas G (2016) Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol Sex Differ 7(1):17
Hifumi T, Kondo Y, Shimizu K, Miyake Y (2018) Heat stroke. J Intensive Care 6:30. https://doi.org/10.1186/s40560-018-0298-4
Hodge BD, Brodell RT (2019) Anatomy, skin sweat glands. In: StatPearls. Treasure Island (FL)
Hoover E, Krishnamurthy K (2019) Physiology, sebaceous glands. In: StatPearls. Treasure Island (FL)
Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E (2008) NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132(2):299–310. https://doi.org/10.1016/j.cell.2007.11.047
Hovatta O, Mikkola M, Gertow K, Strömberg AM, Inzunza J, Hreinsson J, Rozell B, Blennow E, Andäng M, Ährlund-Richter L (2003) A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod 18(7):1404–1409
Hrckulak D, Kolar M, Strnad H, Korinek V (2016) TCF/LEF transcription factors: an update from the internet resources. Cancers 8(7). https://doi.org/10.3390/cancers8070070
Hsu Y-C, Li L, Fuchs E (2014) Emerging interactions between skin stem cells and their niches. Nat Med 20(8):847
Hu YF, Zhang ZJ, Sieber-Blum M (2006) An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells 24(12):2692–2702
Imanishi N, Kishi K, Chang H, Nakajima H, Aiso S (2008) Three-dimensional venous anatomy of the dermis observed using stereography. J Anat 212(5):669–673. https://doi.org/10.1111/j.1469-7580.2008.00890.x
Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11(12):1351
Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, Mao N (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105(10):4120–4126
Jo SK, Lee JY, Lee Y, Kim CD, Lee JH, Lee YH (2018) Three streams for the mechanism of hair graying. Ann Dermatol 30(4):397–401. https://doi.org/10.5021/ad.2018.30.4.397
Joulai Veijouye S, Yari A, Heidari F, Sajedi N, Ghoroghi Moghani F, Nobakht M (2017) Bulge region as a putative hair follicle stem cells niche: a brief review. Iran J Public Health 46(9):1167–1175
Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95(2):549–601. https://doi.org/10.1152/physrev.00035.2013
Jumabay M, Bostrom KI (2015) Dedifferentiated fat cells: a cell source for regenerative medicine. World J Stem Cells 7(10):1202–1214. https://doi.org/10.4252/wjsc.v7.i10.1202
Kalinina NI, Sysoeva VY, Rubina KA, Parfenova YV, Tkachuk VA (2011) Mesenchymal stem cells in tissue growth and repair. Acta Nat 3(4):30–37
Kawai T, Sachs D, Sprangers B, Spitzer T, Saidman S, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao B (2014) Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant 14(7):1599–1611
Kizil C, Kyritsis N, Brand M (2015) Effects of inflammation on stem cells: together they strive? EMBO Rep 16(4):416–426. https://doi.org/10.15252/embr.201439702
Kloepper JE, Tiede S, Brinckmann J, Reinhardt DP, Meyer W, Faessler R, Paus R (2008) Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol 17(7):592–609. https://doi.org/10.1111/j.1600-0625.2008.00720.x
Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E (2003) Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol 163(3):609–623. https://doi.org/10.1083/jcb.200309042
Kolarsick PA, Kolarsick MA, Goodwin C (2011) Anatomy and physiology of the skin. J Derm Nurses Assoc 3(4):203–213
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F (2003) Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101(9):3722–3729
Krystel-Whittemore M, Dileepan KN, Wood JG (2016) Mast cell: a multi-functional master cell. Front Immunol 6:620
Kunder CA, St John AL, Abraham SN (2011) Mast cell modulation of the vascular and lymphatic endothelium. Blood 118(20):5383–5393. https://doi.org/10.1182/blood-2011-07-358432
Labusca L, Zugun-Eloae F (2018) The unexplored role of intra-articular adipose tissue in the homeostasis and pathology of articular joints. Front Vet Sci 5:35. https://doi.org/10.3389/fvets.2018.00035
Lachgar S, Moukadiri H, Jonca F, Charveron M, Bouhaddioui N, Gall Y, Bonafe JL, Plouët J (1996) Vascular endothelial growth factor is an autocrine growth factor for hair dermal papilla cells. J Investig Dermatol 106(1):17–23
Laga AC, Murphy GF (2010) Cellular heterogeneity in vertical growth phase melanoma. Arch Pathol Lab Med 134(12):1750–1757
Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, Wang XJ, DiPietro LA, Varga J (2004) Targeted disruption of TGF-beta/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol 165(1):203–217. https://doi.org/10.1016/s0002-9440(10)63289-0
Lang D, Mascarenhas JB, Shea CR (2013) Melanocytes, melanocyte stem cells, and melanoma stem cells. Clin Dermatol 31(2):166–178. https://doi.org/10.1016/j.clindermatol.2012.08.014
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O (2003) HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31(10):890–896
Lee J, Tumbar T (2012) Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol 23(8):906–916. https://doi.org/10.1016/j.semcdb.2012.08.003
Leikam C, Hufnagel A, Otto C, Murphy D, Mühling B, Kneitz S, Nanda I, Schmid M, Wagner T, Haferkamp S (2015) In vitro evidence for senescent multinucleated melanocytes as a source for tumor-initiating cells. Cell Death Dis 6(4):e1711
Leung Y, Kandyba E, Chen YB, Ruffins S, Chuong CM, Kobielak K (2014) Bifunctional ectodermal stem cells around the nail display dual fate homeostasis and adaptive wounding response toward nail regeneration. Proc Natl Acad Sci U S A 111(42):15114–15119. https://doi.org/10.1073/pnas.1318848111
Li L, Clevers H (2010) Coexistence of quiescent and active adult stem cells in mammals. Science 327(5965):542–545
Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y (2012) Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 19(9):1505–1513. https://doi.org/10.1038/cdd.2012.26
Li S, Yue D, ChEN X, Wang L, Li J, Ping Y, Gao Q, Wang D, Zhang T, Li F (2015) Epigenetic regulation of CD271, a potential cancer stem cell marker associated with chemoresistance and metastatic capacity. Oncol Rep 33(1):425–432
Li Y, Watanabe K, Fujioka M, Ogawa K (2017) Characterization of slow-cycling cells in the mouse cochlear lateral wall. PLoS One 12(6):e0179293. https://doi.org/10.1371/journal.pone.0179293
Li Z, Xu Z, Duan C, Liu W, Sun J, Han B (2018) Role of TCF/LEF transcription factors in bone development and Osteogenesis. Int J Med Sci 15(12):1415–1422. https://doi.org/10.7150/ijms.26741
Linton JM, Martin GR, Reichardt LF (2007) The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development 134(13):2501–2509. https://doi.org/10.1242/dev.005033
Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W (2005) Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ 12(Suppl 2):1497–1508. https://doi.org/10.1038/sj.cdd.4401722
Lohcharoenkal W, Mahapatra KD, Pasquali L, Crudden C, Kular L, Ulum YZA, Zhang L, Landén NX, Girnita L, Jagodic M (2018) Genome-wide screen for MicroRNAs reveals a role for miR-203 in melanoma metastasis. J Investig Dermatol 138(4):882–892
Losquadro WD (2017) Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plast Surg Clin North Am 25(3):283–289. https://doi.org/10.1016/j.fsc.2017.03.001
Lothian JA (2000) The birth plan revisited. J Perinat Educ 9(2):viii–vixi. https://doi.org/10.1624/105812400X87581
Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E (2005) Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev 19(13):1596–1611. https://doi.org/10.1101/gad.1324905
Lu C, Fuchs E (2014) Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med 4(2). https://doi.org/10.1101/cshperspect.a015222
Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150(1):136–150. https://doi.org/10.1016/j.cell.2012.04.045
Lutolf MP, Blau HM (2009) Artificial stem cell niches. Adv Mater 21(32–33):3255–3268. https://doi.org/10.1002/adma.200802582
Malhotra A, Shanker A (2011) NK cells: immune cross-talk and therapeutic implications. Immunotherapy 3(10):1143–1166
Mamidi MK, Pal R, Mori NAB, Arumugam G, Thrichelvam ST, Noor PJ, Abdullah HMF, Gupta PK, Das AK, Zakaria Z (2011) Co-culture of mesenchymal-like stromal cells derived from human foreskin permits long term propagation and differentiation of human embryonic stem cells. J Cell Biochem 112(5):1353–1363
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155(3):739–752
Marionnet C, Tricaud C, Bernerd F (2014) Exposure to non-extreme solar UV daylight: spectral characterization, effects on skin and photoprotection. Int J Mol Sci 16(1):68–90. https://doi.org/10.3390/ijms16010068
Martel JL, Badri T (2019) Anatomy, hair follicle. In: StatPearls. Treasure Island (FL)
Martin MT, Vulin A, Hendry JH (2016) Human epidermal stem cells: role in adverse skin reactions and carcinogenesis from radiation. Mutat Res Rev Mutat Res 770:349–368
McCorry LK (2007) Physiology of the autonomic nervous system. Am J Pharm Educ 71(4):78. https://doi.org/10.5688/aj710478
McGrath J, Eady R, Pope F (2004) Anatomy and organization of human skin. In: Rook’s textbook of dermatology. https://doi.org/10.1002/9781444317633.ch3
Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2, 3-dioxygenase–mediated tryptophan degradation. Blood 103(12):4619–4621
Mistriotis P, Andreadis ST (2013) Hair follicle: a novel source of multipotent stem cells for tissue engineering and regenerative medicine. Tissue Eng Part B Rev 19(4):265–278. https://doi.org/10.1089/ten.TEB.2012.0422
Mizutani CM, Bier E (2008) EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat Rev Genet 9(9):663–677. https://doi.org/10.1038/nrg2417
Morgan BA (2014) The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med 4(7):a015180. https://doi.org/10.1101/cshperspect.a015180
Mull AN, Zolekar A, Wang YC (2015) Understanding melanocyte stem cells for disease Modeling and regenerative medicine applications. Int J Mol Sci 16(12):30458–30469. https://doi.org/10.3390/ijms161226207
Mulloy JC, Cammenga J, Berguido FJ, Wu K, Zhou P, Comenzo RL, Jhanwar S, Moore MA, Nimer SD (2003) Maintaining the self-renewal and differentiation potential of human CD34+ hematopoietic cells using a single genetic element. Blood 102(13):4369–4376. https://doi.org/10.1182/blood-2003-05-1762
Murphrey MB, Vaidya T (2019) Histology, apocrine gland. In: StatPearls. Treasure Island (FL)
Murphrey MB, Zito PM (2019) Histology, stratum corneum. In: StatPearls. Treasure Island (FL)
Myung P, Ito M (2012) Dissecting the bulge in hair regeneration. J Clin Invest 122(2):448–454. https://doi.org/10.1172/JCI57414
Myung PS, Takeo M, Ito M, Atit RP (2013) Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol 133(1):31–41. https://doi.org/10.1038/jid.2012.230
Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K (2012) Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13(8):744
Naik S, Larsen SB, Cowley CJ, Fuchs E (2018) Two to tango: dialog between immunity and stem cells in health and disease. Cell 175(4):908–920
Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L (2016) Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy 18(2):160–171
Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110(10):3499–3506
Nguyen N, Couts KL, Luo Y, Fujita M (2015) Understanding melanoma stem cells. Melanoma Manag 2(2):179–188
Niemann C (2009) Differentiation of the sebaceous gland. Derm Endocrinol 1(2):64–67. https://doi.org/10.4161/derm.1.2.8486
Nowak JA, Polak L, Pasolli HA, Fuchs E (2008) Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3(1):33–43. https://doi.org/10.1016/j.stem.2008.05.009
O’Connor MB, Umulis D, Othmer HG, Blair SS (2006) Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133(2):183–193. https://doi.org/10.1242/dev.02214
Ohki M, Kikuchi S (2019) Apocrine adenoma of the external Auditory Canal with Pseudoepitheliomatous hyperplasia. Case Rep Otolaryngol 2019:7395856. https://doi.org/10.1155/2019/7395856
Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O (2015) Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci 16(10):25476–25501
Ormandy EH, Dale J, Griffin G (2011) Genetic engineering of animals: ethical issues, including welfare concerns. Can Vet J = La revue veterinaire canadienne 52(5):544–550
Oshimori N, Fuchs E (2012a) The harmonies played by TGF-beta in stem cell biology. Cell Stem Cell 11(6):751–764. https://doi.org/10.1016/j.stem.2012.11.001
Oshimori N, Fuchs E (2012b) Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10(1):63–75
Pacifico A, Leone G, Ananthaswamy HN (2017) Molecular basis of skin carcinogenesis. In: The molecular basis of human cancer. Springer, New York, pp 497–503
Panteleyev AA (2018) Functional anatomy of the hair follicle: the secondary hair germ. Exp Dermatol 27(7):701–720. https://doi.org/10.1111/exd.13666
Pappas A (2009) Epidermal surface lipids. Derm Endocrinol 1(2):72–76. https://doi.org/10.4161/derm.1.2.7811
Park K (2015) Role of micronutrients in skin health and function. Biomol Ther 23(3):207
Park B, Kim SJ (2013) Cooling the skin: understanding a specific cutaneous Thermosensation. J Lifestyle Med 3(2):91–97
Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M (2014) Epithelialization in wound healing: a comprehensive review. Adv Wound Care 3(7):445–464. https://doi.org/10.1089/wound.2013.0473
Patel BC, Treister AD, McCausland C, Lio PA (2019) Anatomy, skin, sudoriferous gland. In: StatPearls. Treasure Island (FL)
Patzelt A, Knorr F, Blume-Peytavi U, Sterry W, Lademann J (2008) Hair follicles, their disorders and their opportunities. Drug Discov Today Dis Mech 5(2):e173–e181
Paus R, Cotsarelis G (1999) The biology of hair follicles. N Engl J Med 341(7):491–497
Paus R, Ito N, Takigawa M, Ito T (2003) The hair follicle and immune privilege. In: Journal of investigative dermatology symposium proceedings, vol 2. Elsevier, pp 188–194
Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E (2006) p120-catenin mediates inflammatory responses in the skin. Cell 124(3):631–644
Pfeifer GP (2015) How the environment shapes cancer genomes. Curr Opin Oncol 27(1):71
Phillip JM, Aifuwa I, Walston J, Wirtz D (2015) The mechanobiology of aging. Annu Rev Biomed Eng 17:113–141. https://doi.org/10.1146/annurev-bioeng-071114-040829
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25(11):2896–2902
Piccinin MA, Schwartz J (2019) Histology, meissner corpuscle. In: StatPearls. Treasure Island (FL)
Pierard-Franchimont C, Pierard GE (2013) Alterations in hair follicle dynamics in women. Biomed Res Int 2013:957432. https://doi.org/10.1155/2013/957432
Plikus MV (2012) New activators and inhibitors in the hair cycle clock: targeting stem cells’ state of competence. J Invest Dermatol 132(5):1321–1324. https://doi.org/10.1038/jid.2012.38
Podgorny O, Peunova N, Park JH, Enikolopov G (2018) Triple S-phase Labeling of dividing stem cells. Stem Cell Rep 10(2):615–626. https://doi.org/10.1016/j.stemcr.2017.12.020
Pontén F, Berg C, Ahmadian A, Ren Z-P, Nistér M, Lundeberg J, Uhlén M, Pontén J (1997) Molecular pathology in basal cell cancer with p53 as a genetic marker. Oncogene 15(9):1059
Potten CS, Booth C (2002) Keratinocyte stem cells: a commentary. J Invest Dermatol 119(4):888–899. https://doi.org/10.1046/j.1523-1747.2002.00020.x
Prasad VK, Lucas KG, Kleiner GI, Talano JAM, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J (2011) Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 17(4):534–541
Preston S, Aras S, Zaidi MR (2018) Spatiotemporal labeling of melanocytes in mice. Int J Mol Sci 19(5). https://doi.org/10.3390/ijms19051469
Prost-Squarcioni C, Fraitag S, Heller M, Boehm N (2008) [Functional histology of dermis]. Annales de dermatologie et de venereologie 135(1 Pt 2):1S5–20. https://doi.org/10.1016/S0151-9638(08)70206-0
Psarras S, Beis D, Nikouli S, Tsikitis M, Capetanaki Y (2019) Three in a box: understanding Cardiomyocyte, fibroblast, and innate immune cell interactions to orchestrate cardiac repair processes. Front Cardiovasc Med 6:32. https://doi.org/10.3389/fcvm.2019.00032
Qi J, Garza LA (2014) An overview of alopecias. Cold Spring Harb Perspect Med 4(3). https://doi.org/10.1101/cshperspect.a013615
Rahmani W, Abbasi S, Hagner A, Raharjo E, Kumar R, Hotta A, Magness S, Metzger D, Biernaskie J (2014) Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev Cell 31(5):543–558
Raimondi S, Sera F, Gandini S, Iodice S, Caini S, Maisonneuve P, Fargnoli MC (2008) MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer 122(12):2753–2760
Ramasamy R, Fazekasova H, Lam EW-F, Soeiro I, Lombardi G, Dazzi F (2007) Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 83(1):71–76
Randall MJ, Jungel A, Rimann M, Wuertz-Kozak K (2018) Advances in the biofabrication of 3D skin in vitro: healthy and pathological models. Front Bioeng Biotechnol 6:154. https://doi.org/10.3389/fbioe.2018.00154
Rangel-Huerta E, Maldonado E (2017) Transit-amplifying cells in the fast lane from stem cells towards differentiation. Stem Cells Int 2017:7602951. https://doi.org/10.1155/2017/7602951
Rasmusson I, Ringdén O, Sundberg B, Le Blanc K (2003) Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76(8):1208–1213
Rasmusson I, Le Blanc K, Sundberg B, Ringden O (2007) Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol 65(4):336–343
Rasouli M, Chen Y, Basu A, Kukreja SL, Thakor NV (2018) An extreme learning machine-based Neuromorphic tactile sensing system for texture recognition. IEEE Trans Biomed Circuits Syst 12(2):313–325. https://doi.org/10.1109/tbcas.2018.2805721
Ren N, Atyah M, Chen WY, Zhou CH (2017) The various aspects of genetic and epigenetic toxicology: testing methods and clinical applications. J Transl Med 15(1):110. https://doi.org/10.1186/s12967-017-1218-4
Renner P, Eggenhofer E, Rosenauer A, Popp F, Steinmann J, Slowik P, Geissler E, Piso P, Schlitt H, Dahlke M (2009) Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. In: Transplantation proceedings, vol 6. Elsevier, pp 2607–2611
Rice RH, Xia Y, Alvarado RJ, Phinney BS (2010) Proteomic analysis of human nail plate. J Proteome Res 9(12):6752–6758. https://doi.org/10.1021/pr1009349
Rittie L, Sachs DL, Orringer JS, Voorhees JJ, Fisher GJ (2013) Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am J Pathol 182(1):163–171. https://doi.org/10.1016/j.ajpath.2012.09.019
Rompolas P, Greco V (2014) Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol 25–26:34–42. https://doi.org/10.1016/j.semcdb.2013.12.005
Rutella S, Danese S, Leone G (2006) Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108(5):1435–1440
Rutz S, Mordmuller B, Sakano S, Scheffold A (2005) Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol 35(8):2443–2451. https://doi.org/10.1002/eji.200526294
Sachdeva S (2010) Hirsutism: evaluation and treatment. Indian J Dermatol 55(1):3–7. https://doi.org/10.4103/0019-5154.60342
Sakaki-Yumoto M, Katsuno Y, Derynck R (2013) TGF-beta family signaling in stem cells. Biochim Biophys Acta 1830(2):2280–2296. https://doi.org/10.1016/j.bbagen.2012.08.008
Sari T, Yuksel MK, Topcuoglu P, Tol M, Ayyildiz E, Ozcan M, Ilhan O (2010) The effect of CD34 count and clonogenic potential of hematopoietic stem cells on engraftment. Transfus Apher Sci 43(3):315–320. https://doi.org/10.1016/j.transci.2010.09.020
Schalka S, Steiner D, Ravelli FN, Steiner T, Terena AC, Marcon CR, Ayres EL, Addor FA, Miot HA, Ponzio H, Duarte I, Neffa J, Cunha JA, Boza JC, Samorano Lde P, Correa Mde P, Maia M, Nasser N, Leite OM, Lopes OS, Oliveira PD, Meyer RL, Cestari T, Reis VM, Rego VR, Brazilian Society of D (2014) Brazilian consensus on photoprotection. An Bras Dermatol 89(6 Suppl 1):1–74. https://doi.org/10.1590/abd1806-4841.20143971
Schatton T, Frank MH (2008) Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 21(1):39–55. https://doi.org/10.1111/j.1755-148X.2007.00427.x
Schepeler T, Page ME, Jensen KB (2014) Heterogeneity and plasticity of epidermal stem cells. Development 141(13):2559–2567
Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102(4):1548–1549
Schlessinger DI, Sonthalia S (2019) Embryology, epidermis. In: StatPearls. Treasure Island (FL)
Schmidt BA, Horsley V (2013) Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 140(7):1517–1527. https://doi.org/10.1242/dev.087593
Schneider MR, Zouboulis CC (2018) Primary sebocytes and sebaceous gland cell lines for studying sebaceous lipogenesis and sebaceous gland diseases. Exp Dermatol 27(5):484–488. https://doi.org/10.1111/exd.13513
Schoen EJ, Colby CJ, Ray GT (2000) Newborn circumcision decreases incidence and costs of urinary tract infections during the first year of life. Pediatrics 105(4):789–793
Schor SL, Schor AM, Bazill GW (1981) The effects of fibronectin on the migration of human foreskin fibroblasts and Syrian hamster melanoma cells into three-dimensional gels of native collagen fibres. J Cell Sci 48(1):301–314
Seeger MA, Paller AS (2015) The roles of growth factors in keratinocyte migration. Adv Wound Care 4(4):213–224. https://doi.org/10.1089/wound.2014.0540
Sennett R, Rendl M (2012) Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol 23(8):917–927. https://doi.org/10.1016/j.semcdb.2012.08.011
Seo HS, Lee DJ, Chung JH, Lee CH, Kim HR, Kim JE, Kim BJ, Jung MH, Ha KT, Jeong HS (2016) Hominis placenta facilitates hair re-growth by upregulating cellular proliferation and expression of fibroblast growth factor-7. BMC Complement Altern Med 16:187. https://doi.org/10.1186/s12906-016-1180-3
Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G (2004) The role of human mast cell-derived cytokines in eosinophil biology. J Interf Cytokine Res 24(5):271–281. https://doi.org/10.1089/107999004323065057
Shannan B, Perego M, Somasundaram R, Herlyn M (2016) Heterogeneity in melanoma. In: Melanoma. Springer, pp 1–15
Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T (2006) Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res 133(2):185–192
Shim JH, Lee TR, Shin DW (2013) Novel in vitro culture condition improves the stemness of human dermal stem/progenitor cells. Mol Cell 36(6):556–563. https://doi.org/10.1007/s10059-013-0260-1
Shimoda M, Principe S, Jackson HW, Luga V, Fang H, Molyneux SD, Shao YW, Aiken A, Waterhouse PD, Karamboulas C, Hess FM, Ohtsuka T, Okada Y, Ailles L, Ludwig A, Wrana JL, Kislinger T, Khokha R (2014) Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat Cell Biol 16(9):889–901. https://doi.org/10.1038/ncb3021
Shirato R, Abe A, Tsuchiya H, Honda M (2017) Effect of fingernail length on the hand dexterity. J Phys Ther Sci 29(11):1914–1919. https://doi.org/10.1589/jpts.29.1914
Shokry E, Filho NRA (2017) Insights into cerumen and application in diagnostics: past, present and future prospective. Biochem Med 27(3):030503. https://doi.org/10.11613/BM.2017.030503
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78:929–958. https://doi.org/10.1146/annurev.biochem.77.032207.120833
Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP (2012) Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev 92(1):273–366. https://doi.org/10.1152/physrev.00005.2011
Simões M, Sousa J, Pais A (2015) Skin cancer and new treatment perspectives: a review. Cancer Lett 357(1):8–42
Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ (2005) Hair follicle pigmentation. J Invest Dermatol 124(1):13–21. https://doi.org/10.1111/j.0022-202X.2004.23528.x
Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD (2012) Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 212:v, vii, 1–115
Somuncu ÖS, Taşlı PN, Şişli HB, Somuncu S, Şahin F (2015) Characterization and differentiation of stem cells isolated from human newborn foreskin tissue. Appl Biochem Biotechnol 177(5):1040–1054
Soteriou D, Kostic L, Sedov E, Yosefzon Y, Steller H, Fuchs Y (2016) Isolating hair follicle stem cells and epidermal keratinocytes from dorsal mouse skin. J Vis Exp 110. https://doi.org/10.3791/53931
Sotiropoulou PA, Blanpain C (2012) Development and homeostasis of the skin epidermis. Cold Spring Harb Perspect Biol 4(7):a008383
Srivastava SS, Alam H, Patil SJ, Shrinivasan R, Raikundalia S, Chaudhari PR, Vaidya MM (2018) Keratin 5/14mediated cell differentiation and transformation are regulated by TAp63 and Notch1 in oral squamous cell carcinomaderived cells. Oncol Rep 39(5):2393–2401. https://doi.org/10.3892/or.2018.6298
Stecca B, Santini R (2015) Insight into melanoma stem cells: the role of the hedgehog signaling in regulating self-renewal and tumorigenicity. Innov Strateg Tissue Eng 2:107
Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, Leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23(5):770–784. https://doi.org/10.1016/j.cmet.2016.04.011
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M (2016) Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365(3):495–506
Suhail S, Sardashti N, Jaiswal D, Rudraiah S, Misra M, Kumbar SG (2019) Engineered skin tissue equivalents for product evaluation and therapeutic applications. Biotechnol J 14:1900022
Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S (2009) Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27(6):1421–1432
Suzuki K, Tanaka T, Enoki M, Nishida T (2000) Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Invest Ophthalmol Vis Sci 41(9):2495–2500
Svensson A, Norrby M, Libelius R, Tagerud S (2008) Secreted frizzled related protein 1 (Sfrp1) and Wnt signaling in innervated and denervated skeletal muscle. J Mol Histol 39(3):329–337. https://doi.org/10.1007/s10735-008-9169-y
Tasli PN, Sahin F (2014) Effect of lactoferrin on odontogenic differentiation of stem cells derived from human 3rd molar tooth germ. Appl Biochem Biotechnol 174(6):2257–2266. https://doi.org/10.1007/s12010-014-1204-8
Taşlı PN, Şahin F (2014) Effect of lactoferrin on odontogenic differentiation of stem cells derived from human 3rd molar tooth germ. Appl Biochem Biotechnol 174(6):2257–2266
Tasli PN, Aydin S, Yalvac ME, Sahin F (2014) Bmp 2 and bmp 7 induce odonto- and osteogenesis of human tooth germ stem cells. Appl Biochem Biotechnol 172(6):3016–3025. https://doi.org/10.1007/s12010-013-0706-0
Taylor NA, Machado-Moreira CA (2013) Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extreme Physiol Med 2(1):4. https://doi.org/10.1186/2046-7648-2-4
Taylor JR, Lockwood AP, Taylor AJ (1996) The prepuce: specialized mucosa of the penis and its loss to circumcision. Br J Urol 77(2):291–295. https://doi.org/10.1046/j.1464-410x.1996.85023.x
Tepole AB, Gosain AK, Kuhl E (2012) Stretching skin: the physiological limit and beyond. Int J Non-Linear Mech 47(8):938–949. https://doi.org/10.1016/j.ijnonlinmec.2011.07.006
Thibaut S, Gaillard O, Bouhanna P, Cannell DW, Bernard BA (2005) Human hair shape is programmed from the bulb. Br J Dermatol 152(4):632–638. https://doi.org/10.1111/j.1365-2133.2005.06521.x
Tiede S, Kloepper JE, Bodò E, Tiwari S, Kruse C, Paus R (2007) Hair follicle stem cells: walking the maze. Eur J Cell Biol 86(7):355–376
Ting PT, Barankin B (2006) Patches of hair loss on the scalp. Can Fam Physician 52(957):960
Tobin DJ (2009) Aging of the hair follicle pigmentation system. Int J Trichology 1(2):83–93. https://doi.org/10.4103/0974-7753.58550
Torma H (2011) Regulation of keratin expression by retinoids. Derm Endocrinol 3(3):136–140. https://doi.org/10.4161/derm.3.3.15026
Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL (2009) A role for human skin–resident T cells in wound healing. J Exp Med 206(4):743–750
Tracy LE, Minasian RA, Caterson EJ (2016) Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care 5(3):119–136. https://doi.org/10.1089/wound.2014.0561
Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A (2008) Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells 26(2):562–569
Tsepkolenko A, Tsepkolenko V, Dash S, Mishra A, Bader A, Melerzanov A, Giri S (2019) The regenerative potential of skin and the immune system. Clin Cosmet Investig Dermatol 12:519
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8(9):726
Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells – current trends and future prospective. Biosci Rep 35(2). https://doi.org/10.1042/BSR20150025
Unger C, Gao S, Cohen M, Jaconi M, Bergstrom R, Holm F, Galan A, Sanchez E, Irion O, Dubuisson JB (2009) Immortalized human skin fibroblast feeder cells support growth and maintenance of both human embryonic and induced pluripotent stem cells. Hum Reprod 24(10):2567–2581
Upadhyay J, Upadhyay RB, Agrawal P, Jaitley S, Shekhar R (2013) Langerhans cells and their role in oral mucosal diseases. N Am J Med Sci 5(9):505–514. https://doi.org/10.4103/1947-2714.118923
Ushiki T (2002) Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol 65(2):109–126
Vaidya A, Kale VP (2015) TGF-beta signaling and its role in the regulation of hematopoietic stem cells. Syst Synth Biol 9(1–2):1–10. https://doi.org/10.1007/s11693-015-9161-2
van Eldijk MB, McGann CL, Kiick KL, van Hest JC (2012) Elastomeric polypeptides. Top Curr Chem 310:71–116. https://doi.org/10.1007/128_2011_205
Vapniarsky N, Arzi B, Hu JC, Nolta JA, Athanasiou KA (2015) Concise review: human dermis as an autologous source of stem cells for tissue engineering and regenerative medicine. Stem Cells Transl Med 4(10):1187–1198
Wabik A, Jones PH (2015) Switching roles: the functional plasticity of adult tissue stem cells. EMBO J 34(9):1164–1179. https://doi.org/10.15252/embj.201490386
Walko G, Castanon MJ, Wiche G (2015) Molecular architecture and function of the hemidesmosome. Cell Tissue Res 360(3):529–544. https://doi.org/10.1007/s00441-015-2216-6
Wang Y, Xu R, He W, Yao Z, Li H, Zhou J, Tan J, Yang S, Zhan R, Luo G, Wu J (2015) Three-dimensional histological structures of the human dermis. Tissue Eng Part C Methods 21(9):932–944. https://doi.org/10.1089/ten.TEC.2014.0578
Westerterp M, Busch OR, Bergman JJ, Ten Kate FJ, van Lanschot JJ (2005) A “crackleware” oesophagus. J Clin Pathol 58(12):1325–1327. https://doi.org/10.1136/jcp.2005.026807
WHO (2017) World Health Organization Skin Cancer. https://www.who.int/uv/faq/skincancer/en/index1.html
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-‘t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2. https://doi.org/10.3402/jev.v2i0.20360
Woo WM, Oro AE (2011) SnapShot: hair follicle stem cells. Cell 146(2):334–334.e332. https://doi.org/10.1016/j.cell.2011.07.001
Xu X, Genega EM, Nasuti JF (2002) Differential immunocytochemical staining patterns of uroplakin observed on neoplastic and nonneoplastic tissue fragments obtained from upper urinary tract brush specimens. Acta Cytol 46(4):684–689. https://doi.org/10.1159/000326976
Yaemsiri S, Hou N, Slining MM, He K (2010) Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol 24(4):420–423. https://doi.org/10.1111/j.1468-3083.2009.03426.x
Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S (2010) Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1(1):5
Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K (2019) Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther 10(1):229. https://doi.org/10.1186/s13287-019-1312-z
Ye H, De S (2017) Thermal injury of skin and subcutaneous tissues: a review of experimental approaches and numerical models. Burns 43(5):909–932. https://doi.org/10.1016/j.burns.2016.11.014
Yousef H, Badri T (2019) Histology, skin appendages. In: StatPearls. Treasure Island (FL)
Yousef H, Sharma S (2018) Anatomy, skin (Integument), epidermis. StatPearls Treasure Island (FL); StatPearls Publishing LLC: St Petersburg, FA, USA
Yousef H, Alhajj M, Sharma S (2019) anatomy, skin (Integument), Epidermis. In: StatPearls. Treasure Island (FL)
Zabala M, Lobo N, Qian D, van Weele L, Heiser D, Clarke M, Liu H, Lathia J (2016) Overview: cancer stem cell self-renewal. In: Cancer stem cells. Academic, pp 25–58
Zachar L, Bacenkova D, Rosocha J (2016) Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res 9:231–240. https://doi.org/10.2147/JIR.S121994
Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z (2019) Stem cells: past, present, and future. Stem Cell Res Ther 10(1):68. https://doi.org/10.1186/s13287-019-1165-5
Zernov NV, Skoblov MY, Marakhonov AV, Shimomura Y, Vasilyeva TA, Konovalov FA, Abrukova AV, Zinchenko RA (2016) Autosomal recessive Hypotrichosis with woolly hair caused by a mutation in the keratin 25 gene expressed in hair follicles. J Invest Dermatol 136(6):1097–1105. https://doi.org/10.1016/j.jid.2016.01.037
Zhang S, Duan E (2018) Fighting against skin aging: the way from bench to bedside. Cell Transplant 27(5):729–738. https://doi.org/10.1177/0963689717725755
Zhang B, Hsu YC (2017) Emerging roles of transit-amplifying cells in tissue regeneration and cancer. Wiley Interdiscip Rev Dev Biol 6(5). https://doi.org/10.1002/wdev.282
Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M (2012) The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481(7380):157
Zhang Y, Xu J, Jing J, Wu X, Lv Z (2018) Serum levels of androgen-associated hormones are correlated with curative effect in androgenic alopecia in Young men. Med Sci Monit 24:7770–7777. https://doi.org/10.12659/MSM.913116
Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, Huso DL, Semenza GL (2006) Spontaneous transformation of cultured mouse bone marrow–derived stromal cells. Cancer Res 66(22):10849–10854
Zhu S, Qing T, Zheng Y, Jin L, Shi L (2017) Advances in single-cell RNA sequencing and its applications in cancer research. Oncotarget 8(32):53763–53779. https://doi.org/10.18632/oncotarget.17893
Zouboulis CC (2009) Sebaceous gland receptors. Derm Endocrinol 1(2):77–80. https://doi.org/10.4161/derm.1.2.7804
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deniz, A.A.H., Abdik, E.A., Abdik, H., Aydın, S., Şahin, F., Taşlı, P.N. (2019). Zooming in across the Skin: A Macro-to-Molecular Panorama. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 8. Advances in Experimental Medicine and Biology(), vol 1247. Springer, Cham. https://doi.org/10.1007/5584_2019_442
Download citation
DOI: https://doi.org/10.1007/5584_2019_442
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45892-8
Online ISBN: 978-3-030-45893-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)