Abstract
Ultrasound (US) allows the non-invasive evaluation of morphological changes of kidney structure (by means of B-Mode) and patterns of renal and extrarenal vascularization (by means of color-Doppler and contrast-enhanced US). In hypertensive subjects it offers a relevant contribution to the diagnosis of early renal damage, acute or chronic nephropathies and nephrovascular disease. However, morphological changes are often detected late and non-specific and in recent years evidence has increased regarding the clinical relevance of renal resistive index (RRI) for the study of vascular and renal parenchymal renal abnormalities. RRI is measured by Doppler sonography in an intrarenal artery, as the difference between the peak systolic and end-diastolic blood velocities divided by the peak systolic velocity. At first RRI was proved to be a marker of renal disease onset and progression; later the influence of systemic vascular properties on RRI was shown and authors claimed its use as an independent predictor of cardiovascular risk rather than of renal damage. Indeed, renal vascular resistance is only one of several renal (vascular compliance, interstitial and venous pressure), and extrarenal (heart rate, pulse pressure) determinants that concur to determine RRI individual values but not the most important one. The clinical relevance of RRI measurement as a surrogate endpoint of specific renal damage or/and as surrogate endpoint of atherosclerotic diffuse vascular damage is still debated.To summarize, from the literature: (a) In hypertensives with normal renal function and no albuminuria, especially in younger people, RRI is an early marker of renal damage that is especially useful when hypertension and diabetes concur in the same subjects. In these subjects RRI could improve current clinical scores used to stratify early renal damage. In older subjects RRI increases in accordance with the increase in systemic vascular stiffness and, because of this close relationship, RRI is also a marker of systemic atherosclerotic burden and the role of renal determinants can weaken. The clinical relevance was not specifically investigated. (b) In transplant kidney and in chronic renal disease high (>0.80) RRI values can independently predict renal failure. The recent claim that systemic (pulse pressure) rather than renal hemodynamic determinants sustain this predictive role of RRI, does not significantly reduce this predictive role of RRI. (c) Doppler ultrasound allows diagnosis and grading of renal stenosis in both fibromuscolar dysplastic and atherosclerotic diseases. Moreover, by RRI assay Doppler ultrasound can indirectly measure the hemodynamic impact of renal artery stenosis on the homolateral kidney, by virtue of the stenosis-related decrease in pulse pressure. However, in elderly subjects with atherosclerotic renal artery stenosis coexisting renal diseases can independently increase RRI by the augmentation in renal vascular stiffness and tubulo-interstitial pressure and hidden changes due to renal artery stenosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The pathophysiological relationship between increased blood pressure and kidneys is complex. High blood pressure causes progressive renal damage but also vascular or parenchymal renal disease can sustain increase in blood pressure.
Ultrasound (US) has a key role in evaluating both morphological changes of kidney structure (by means of B-Mode) and patterns of renal and extrarenal vascularization (by means of color-Doppler and contrast-enhanced US), thus contributing to the diagnosis of early renal damage, acute or chronic nephropathies and nephrovascular disease in hypertensive patients. Maximum renal diameter is a morphological marker of CKD. It decreases contemporarily to Glomerular Filtration Rate (GFR) and a significant correlation of both renal diameter and cortical thickness with renal function has been demonstrated (Meola et al. 2016a). A direct correlation between the number of functional renal units, nephrons, renal mass, renal function and ultrasound-measured renal volume has also been reported. Kidney size was measured by US which compared with other radiologic methods gave results that came closest to the actual size of the kidney measured during surgery (Vegar Zubović et al. 2016). Ultrasound-measured volume of kidneys correlates well with the stage and the progression of CKD and can be used to evaluate CKD progression. However, ultrasound-detected morphological changes are late and not specific. In recent years increasing attention has been paid to the study of renal resistive index (RRI) obtained by Doppler arterial waveform analysis of intrarenal arteries as an independent marker of early renal damage when albuminuria and glomerular filtration rate are still normal, and as an independent predictor of renal failure progression in chronic renal disease (Radermacher et al. 2002; Ikee et al. 2005; Sugiura 2011; Bigè et al. 2012).
Infact, as well synthesized in the review by Viazzi et al. (Viazzi et al. 2014), not only does Doppler ultrasonography detect renal macroscopic vascular abnormalities that allows diagnosis and grading of renal artery stenosis, but it also identifies changes in blood flow at the microvascular level that reflect functional or structural changes within the kidneys. Specifically, acute functional changes in renovascular resistance physiologically induced by sympathetic activation or pharmacologically by ACE inhibitors (Bardelli et al. 1992; Jensen et al. 1994),acute increase in tubulo-interstitial pressure by hydronephrosis or acute kidney injury and chronic structural damage of arteriolar or tubule-interstitial rather than glomerular compartment do affect RRI.
Recent clinical and experimental evidence indicates that increased RRI in patients with primary hypertension with normal or reduced renal function may reflect and score changes in intrarenal perfusion because of arteriolar and/or tubule-interstitial renal damage that can occur independently of glomerular damage. Moreover,in hypertensive patients high RRI is also associated with worse systemic hemodynamics and atherosclerotic burden. Due to this relationship,RRI has been also proposed as a new independent marker and predictor of systemic cardiovascular risk in asymptomatic subjects. The clinical relevance and the possible therapeutic implications of this use need dedicated studies (Chirinos and Townsend 2014; O’Neill 2014). This review tries to give information on the knowledge of physiopathological renal and extra-renal determinants of RRI, necessary for the correct use of RRI ultrasound measurement in clinical practice when focused on the study of early and late renal damage in essential hypertension and in the diagnosis and grading of renal artery stenosis. Specifically, we want to show whether and when the measurement of RRI should be considered as a specific marker of renal damage to use together and in addition to Glomerular Filtration Rate (GFR) and microalbuminuria or as a parameter of systemic cardiovascular risk to use together and in addition to intima-media-thickness and other surrogate ultrasound endpoints for cardiovascular risk stratification of asymptomatic patients.
According to recent recommendations by the major societies for the study of hypertension (the American Heart Association, the American College of Cardiology and the Centers for Disease Control and Prevention, the American Society of Hypertension and the International Society of Hypertension, the Canadian Hypertension Educational Program, the European Society of Cardiology and the European Society of Hypertension, the National Institute for Health and Care Excellence, The French Society of Hypertension, the Taiwan and the Chinese Society of Hypertension) (James et al. 2014; Go et al. 2014; Weber et al. 2014; Dasgupta et al. 2014; Mancia et al. 2013; National Institute for Health and Care Excellence 2014; Blacher et al. 2014; Chiang et al. 2010; Liu 2010), the study of hypertensive patients by renal ultrasonography is mainly dedicated to patients with the clinical suspicion of secondary hypertension. This is because ultrasound can detect the presence of renal parenchymal disease, polycystic renal disease, and urinary tract obstruction. Specifically, when clinical characteristics point to renovascular hypertension, ultrasound screening is recommended to confirm or rule out the diagnosis of renal artery stenosis, grading the stenosis and investigating its hemodynamic impact on the homolateral kidney. At present, the ultrasound study of renal target-organ damage in essential hypertension has not been codified; and the determination of GFR and of albuminuria excretion rate are recommended.
This review wants to give strong support to the use of RRI for investigating early and late renal damage in hypertensive patients, as an independent predictor of renal failure and/or of cardiovascular risk. To have a high RRI selects subjects at increased risk of developing renal failure and of having cardiovascular events beyond the pattern of other current renal and ultrasound markers of risk.
2 Renal Resistive Index (RRI)
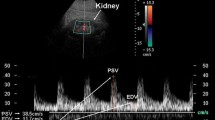
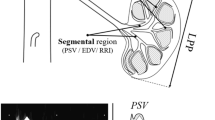
RRI, derived from the Doppler spectrum of intrarenal (segmental or interlobar) arteries, is obtained by the difference between maximum (peak systolic) and minimum (end-diastolic) flow velocity to maximum flow velocity (Fig. 1):
The morphology of Doppler spectrum of RRI is mainly determined by the velocity/time (V/t) curve that is assayed along the main renal artery, that can be defined as a “low resistance” curve. The systolic phase quickly increases to peak velocity and is followed by a progressive and gradual deceleration phase with a telediastolic velocity that does not decrease below 30–40 cm/sec. This Doppler spectrum is common and peculiar to all parenchymal flows, because a sufficient oxygen supply must be assured throughout the cardiac cycle (Meola et al. 2016b).
RRI was introduced in 1950 and initially proposed for the semi-quantitative assay of intra-renal vascular resistance by Pourcelot in (1974). He showed that the ratio was influenced by changes in vascular resistance distally to the point of RRI assay. The term RRI has been kept to the present time, even if the strict relationship between RRI and actual renal vascular resistance has become very weak (Chirinos and Townsend 2014; O’Neill 2014).
According to these findings RRI was initially used for the diagnosis and follow-up of acute and chronic renal disease (Radermacher et al. 2002; Viazzi et al. 2014) which are associated to dynamic and/or structural changes in intra-renal vessels. Later on RRI was proved to be a strong independent predictor of renal failure (Radermacher et al. 2002; Sugiura 2011). However, in the meantime growing evidence showed that RRI is the result of many intra and extra-renal determinants and that renal vascular resistance is only one of these, and not the most important (Boddi et al. 2015) (Fig. 2). Remarkably, in 1991 Gosling et al. (1991), and in 1999 Bude and Rubin (1999), clearly showed by in vitro experiments performed in simple artificial circuits, that RRI is dependent on both renal vascular compliance and resistance, becoming less dependent on resistance as compliance decreases. When compliance is zero, RRI is independent of changes in renal vascular resistance. Moreover, new experimental (Chirinos and Townsend 2014; Tublin et al. 2003) and clinical data (Chirinos and Townsend 2014; O’Neill 2014) were obtained showing that RRI was markedly affected by the changes in renal (renal interstitial and venous pressure) and systemic (pulse pressure) determinants of vascular compliance, and only scarcely by the chronic increase in renal vascular resistance. In chronic renal diseases and in transplant recipients, RRI mainly depends on systemic vascular compliance assayed as pulse pressure, rather than renal vascular properties (Chirinos and Townsend 2014; O’Neill 2014). In these patients the increase in RRI is strictly associated with the decrease in systemic vascular compliance assayed as pulse pressure that is negatively modulated by aging and traditional cardiovascular (CV) risk factors, among which hypertension plays a major role (Chirinos and Townsend 2014). According to this point of view, in hypertensive patients with and without renal function impairment, the increase in RRI predicts worse renal and general outcomes, as a marker of systemic atherosclerotic burden rather than of local renal damage. However, this statement is still a matter of debate and do not weaken the clinical relevance of RRI measurement for risk scoring.
Different renal and extrarenal systemic determinants concur to determine RRI. (1a) and (2a): renal and systemic determinants that decrease RRI. (b): glomerular resistance scarcely or not affect RRI. (1c) and (2c): renal and systemic determinants that increase RRI (Adapted by Boddi et al. 2015)
On the contrary full agreement was reached on the clinical use of RRI as a specific marker of renal damage, in subjects affected by those renal pathologies that can promote the progression of renal damage in hypertensive patients, i.e. hydronephrosis, renal vein thrombosis, increased abdominal pressure and acute kidney injury. In all these conditions the acute and marked increase in renal tubule-interstitial and venous pressure due to hydrostatic or inflammatory edema leads to the direct increase in RRI values (Boddi et al. 2015).
3 RRI Threshold in Clinical Practice
Aging is associated with a progressive quantitative decrease of renal microvascular bed and with an increased thickness of tunica media of renal arterioles (Fig. 2). This determines a decrease in the lumen/vessel wall ratio. Both these changes result in a progressive increase in RRI (Boddi et al. 1996) that can be amplified by the contemporaneous increase in systemic arterial stiffness. The steep age-dependent rise in RRI values is specific to the renal vasculature and is not seen in other vascular beds. The age-related hypertrophic remodelling of the vessel wall of renal microvessels can be further amplified by hypertension and/or diabetes (Pontremoli et al. 1999a; Maestripieri et al. 2012). In healthy adults most authors use >0.70 as the cut-off limit for pathological RRI and do not establish normal values according to age. However, in healthy subjects >70 year, RRI >0.70 can be measured in the absence of renal diseases, whereas in subjects aged >40 year can be the first marker of renal damage that anticipates GRF reduction and the occurrence of albuminuria (Boddi et al. 1996). Further an emerging clinical issue is the evaluation of the actual renal function in elderly normal patients or diabetics, since the estimated values of GFR by math formula suffer of limits in these subgroups. So that, in elderly subjects with normal renal function or diabetics, RRI assessment might be considered an other non-invasive way to reveal early renal damage.
In a recent large multicentric family-based population study, age was confirmed as a determinant of RRI. Ponte et al. (2014) also showed that the relationship of RRI with age is nonlinear and that RRI increases sharply after the age of 40.
In the same multicentric study female sex was associated with higher RRI values due to hormone differences and the fact that RRI has a genetic tract was reported (Ponte et al. 2014); the clinical relevance of these findings must be investigated by dedicated studies.
In newborns and in children under the age of four, RRI > 0.70 can be found because of renal anatomical structure in this period and is not associated with renal pathologies (Bude et al. 1992).
4 Systemic and Renal Determinants of RRI
In any arterial vascular tract, Doppler waveform is the integrated result of what happens before and downstream from the point where the flow is assayed.
4.1 Systemic and Extrarenal Determinants
4.1.1 Stiffness-Related Systemic Pulse Pressure
The ratio of systolic to diastolic blood pressure (see RRI equation) is an inverse function of pulse pressure. Thus, for any given intra-renal vascular resistance an increase in systemic systolic arterial pressure promotes a higher peak renal velocity and/or a decrease in diastolic arterial pressure. That results in a lower end-diastolic velocity. As a direct consequence, in vivo any increase in systemic arterial stiffness that causes increased pulse pressure is associated with high RRI values, both in physiological (aging) and pathological (hypertension) conditions (Fig. 2c). Changes in pulse pressure can also be tonic or phasic, as during an infusion of L-NG-monomethyl argirine (L-NMMA), an inhibitor of endothelial NOS. Neither RRI under baseline conditions nor RRI during L-NMMA infusion were related with renal vascular resistance or renal perfusion, assayed by para-aminohippuric acid and insulin clearance (Raff et al. 2010). On the contrary, RRI changed according to variations of central pulse pressure.
The relationship between RRI and pulse pressure has also been investigated in recipients of kidney transplants where systemic pulse pressure is recipient-specific, whereas the compliance of interlobular arteries is donor specific; in these kidneys RRI correlated with the age of the recipient but not of the donor, with recipient pulse pressure but not parameters of allograft function and with RRI of other (i.e. splenic) districts of the recipient (Naesens et al. 2013). As a whole the findings observed in transplant recipients strongly support that RRI primarily reflects the properties of the systemic vasculature that can hidden or weaken the effects of local renal damage on intrarenal vasculature.
4.1.2 Stenosis-Related Pulse Pressure
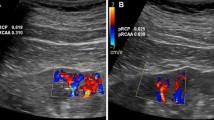
Severe (>80 %) renal artery thoracic or sovra-renal abdominal aorta or valvular aortic stenosis all decrease pulse pressure in vascular districts distal to stenosis, and decrease RRI values (<0.60) as a result of low peak systolic velocity (Fig. 2, 2a). The dampened flow is revealed by the peculiar Doppler wave pattern characterized by a “tardus”, slow, and “parvus”, small pulsus (Figs. 2 and 3). The finding of low RRI in the homolateral kidney and the lateralization of RRI (delta > 0.05) is indirect but reliable proof of severe renal artery stenosis (Fig. 4). In fact the gradual reduction of renal perfusion pressure up to 40 % does not substantially change renal blood flow and glomerular filtration rate, thanks to the self-regulating mechanisms of intrarenal circulation. In these conditions RRI is not affected. This mechanism becomes ineffective when morphological renal arterial stenosis is >75 %, renal perfusion pressure falls >40 % and renal systolic pressure is <70–80 mm Hg (Textor and Wilcox 2001; Jacobson 1988). This renal stenosis is defined hemodynamically significant, because it activates the renal renin angiotensin system (Meola et al. 2016b; Butterly and Schwab 2000) and demodulates Doppler waveform at intrarenal arteries. However, when distal renal vascular disease coexists due to chronic ischemic kidney, the hemodynamic effects of renal artery stenosis may be hidden. In these patients RRI is symmetrically high, not lateralized and the hemodynamic effect of arterial stenosis on renal parenchyma cannot be evaluated by Doppler ultrasound (Meola et al. 2016b; Boddi et al. 2015) (Fig. 4) (see also Ultrasound diagnostics of renal artery stenosis, page 14).
Schematic representation of possible RRI changes. From the left to the right:(a) low RRI values (0.50) because of low peak systolic velocity (PSV) with peculiar Doppler wave pattern of post-stenotic flow characterized by a “tardus”, slow, and “parvus”, little pulsus; (b) normal Doppler wave pattern and PSV/EDV at interlobar arteries; (c, d) high RRI (0.75–0.90) due to high peak systolic (PSV) and decreased end-diastolic velocity (EDV) (Adapted by Boddi et al. 2015)
Schematic representation of Doppler flow patterns assayed at and distal to a hemodynamically arterial renal stenosis; RRI is lateralized (delta > 0.05); when vascular or parenchimal nephropathies coexist, RRI values symmetrically increase and the hemodinamic effect of renal artery stenosis is hidden (Adapted from Boddi et al. 2015)
4.1.3 Heart Rate
Changes in heart rate can affect RRI independently from the other hemodynamic parameters because of changes in diastolic duration that modulate end-diastolic velocity. During bradicardia diastolic duration increases and high RRI is measured. On the contrary during tachicardia diastolic duration shortens and RRI decreases (Fig. 2, 2a and c).
4.2 Renal Determinants
4.2.1 Renal Interstitial and Venous Pressure
The renal capillary wedge pressure (interstitial tissue plus venous pressure) is a major renal determinant of RRI. In ex vivo rabbit kidney model elevations in ureteral pressure were significantly correlated with increased RRI values, mean renal vascular resistance (pressure/flow) and decreased mean conductance (flow/pressure) (Tublin et al. 1999). In humans in vivo the acute increase of renal interstitial pressure by hydronephrosis or of venous pressure by venous thrombosis, or of both by abdominal hypertension, results in a linearly related increase in RRI (Fig. 2, 1c). Also renal hematoma can acutely increase the pressure of interstitial compartment and elevate RRI (Platt et al. 1989).
Most importantly, acute kidney injury (AKI) is associated with an acute increase in interstitial pressure because of sustained vasoconstriction and ischemic and inflammatory damage of the tubulo-interstial compartment by sustained hypoperfusion. In all these clinical conditions the occurrence, severity and progression of renal damage can be well monitored by changes in RRI values (Platt et al. 1989; Schnell et al. 2012; Dewitte 2013; Le Dorze et al. 2012; Darmon et al. 2011). Recently, in critical patients admitted for medical, surgical or trauma disease, high RRI values at admission were significantly and independently associated with in-ICU mortality and persistent AKI at ICU discharge (Boddi et al. 2016).
4.2.2 Histological Renal Parameters – RRI and the Tubulo-Interstitial Compartment
Twenty years ago Platt et al. showed that RRI was significantly higher in nephropathies with tubulo-interstitial and/or vascular injury than in isolated glomerulopathies (Platt et al. 1990). Glomerular arterial resistance, that accounts for about 20 % of total renal vascular resistance, scarcely concurs to the determination of RRI; and nephropathies characterized by prevalent glomerular involvement are not associated with increased RRI. RRI is not a marker of renal function (Fig. 2).
The studies on the relationship between tubular, interstitial and arterial damage and RRI in renal disease and in kidney transplants show conflicting results: according to Ikee et al., only arteriolosclerosis out of all histological parameters independently correlated with RRI in chronic renal disease (Ikee et al. 2005), whereas in renal transplants investigated at 3, 12 and 24 months after transplantation RRI was not associated with any renal allograft histological features. On the contrary, other Authors reported that high RRI values were related to more severe tubulo-interstitial damage score, and an association between RRI values and the extension of interstitial fibrosis was shown, probably due to the rise in pressure exerted by interstitial fibrosis on adjacent vessels. Remarkably, interstitial fibrosis closely correlated to renal function and long-term prognosis and could underline the role of RRI as an independent marker of renal and clinical outcome in patients with CKD (Sugiura 2011; Bigè et al. 2012).
The possible use of RRI as a marker of tubulo-interstitial nephropathy is supported by the findings that the detection of high RRI values allowed the early identification of both normotensive and hypertensive patients with chronic tubulo-interstitial nephropathy diagnosed by 99 mTc DMSA scintigraphy and signs of tubular dysfunction, when renal function was still preserved (Boddi et al. 2006). Moveover, in hypertensive patients with normal creatinine clearance and no albuminuria, high RRI values were associated with low grade inflammation (Protein C reactive >2 mg/dl) and hyperuricemia (>6.5 mg/dl) (Berni et al. 2012, 2010). Both sustain a tubulo-interstitial nephropathy. In hypertensive patients, serum uric acid strongly correlated with RRI, independently of renal function or albuminuria, but the altered intrarenal hemodynamics did not explain the pathophysiology of hyperuricemic renal damage (Geraci et al. 2016).
A generalized consensus was reached that tubulo-interstitial and not glomerular nephropathies affect RRI and that RRI does not measure renal function.
4.2.3 Role of Arterial Vascular Resistance
Based on early experimental animal data (Bude and Rubin 1999; Tublin et al. 1999), RRI was long considered to directly mirror intrarenal resistance, thus allowing a non-invasive glimpse into intrarenal (patho) physiology (Norris and Barnes 1984). Under physiological conditions RRI assay could detect phasic increase in renal vascular resistance induced by sympathetic activation obtained by cold pressor test or handgrip; in the same subjects the increase of blood volume by acute hydration resulted in an RRI decrease (Boddi et al. 1996). Repeated daily sessions of music-guided slow-breathing increased parasympathetic modulation and decreased RRI early in the study.These changes were being followed by a positive modulation of baroflex sensitivity and decrease in blood pressure (Modesti et al. 2015). In patients with heart failure high RRI values were associated with increased intrarenal vascular resistance due to neurohormonal hyperactivity and independently predicted heart failure progression (Ciccone et al. 2014). In septic shock Doppler ultrasonography and RRI measurements may help determine in each patient the optimal mean aortic pressure for renal blood flow and may be a relevant end-point to titrate the haemodynamic treatment by fluid and norepinephrine administration (Deruddre et al. 2007).Catheter-based renal sympathetic denervation in patients with resistant hypertension reduced RRI probably through a decrease in intraparenchymal resistance, not mediated by reduction in systolic blood pressure (Mahfoud et al. 2012). As a whole these findings sustain that the RRI can detect phasic changes in renal vascular resistance.
On the contrary, RRI changes during dynamic vasodilation caused by nytroglicerin or (L-NMMA) infusion were poorly associated with the concurring direct measurement of renal resistance by scintigraphy, even if the changes in RRI and in renal vascular resistance moved in the same direction. Rather, RRI changes were directly related to changes in pulse pressure (Raff et al. 2010). Increased RRI has been shown to correlate with systemic arterial stiffness measured by ambulatory blood pressure derived by Ambulatory Arterial Stiffness Index (Ratto et al. 2006). Moreover, a close relationship between RRI and other markers of systemic atherosclerotic burden, as intima-media thickness and ankle brachial index, was shown in hypertensive patients with chronic renal disease, independently of renal damage (Pontremoli et al. 1999b).
For many years the role of high RRI values as an independent marker of renal outcome in patients with CKD was mainly due to the assumption that RRI increase was determined above all by the progressive “tonic” increase in vascular resistance because of: (a) decrease in arterial compliance due to renal arteriosclerosis; (b) elevation of extra-vascular renal pressure exerted by interstitial fibrosis in adjacent vessels; (c) vasoconstriction secondary to hypoxia and to loss of capillaries associated with renal fibrosis. All these are associated with decline in renal function (Boddi et al. 2015).
In recent years evidence has been gathered around RRI being an independent marker of renal and cardiovascular outcomes, because it measures systemic and not renal hemodynamic parameters, and reflects systemic vascular disease (Granata et al. 2014). We agree with O Neill’s title “Renal resistive index. A case of mistaken identity” (O’Neill 2014). However, there is no doubt that both phasic (sympathetic activation) and tonic (arteriolosclerotic ) changes in renal arterial resistance can modulate RRI.
4.2.4 RRI and Subclinical Renal Damage in Hypertension
In clinical practice albuminuria is measured to define subclinical renal damage in hypertensive patients, and the combination of eGFR and albuminuria is a useful predictor of CV disease (Viazzi et al. 2014). In recent years RRI was also validated as a clinical marker of subclinical renal damage as well as a prognostic predictor of renal and CV outcomes to use in addition to the above mentioned markers in order to improve their performance.
In untreated patients with primary hypertension and normal renal function, high RRI (>0.70) highlights subclinical signs of renal damage and shows a direct relationship with the amount of urine albumin excretion (Miyoshi et al. 2016). Further RRI was proved to be a useful index to predict increase in urinary albumin excretion in patients with essential hypertension (Viazzi et al. 2014). With the progression of hypertensive renal damage, high RRI values are often associated with a mild reduction in glomerular filtration rate and increased albuminuria or both (Doi et al. 2012). In hypertensive patients high (>0.70) RRI predicts renal dysfunction evaluated at 12 months by Cystatin C determination (Okura et al. 2010). Evaluation of both eGFR and RRI instead of albuminuria could be another investigative option to identify essential hypertensive subjects without clinical evidence of renal damage and cardiovascular disease, predisposed to worse renal and CV outcomes.
In hypertensive patients undergoing chronic antihypertensive therapy with no microalbuminuria and normal renal function, higher RRI values were found in those with hyperuricemia or low grade inflammation (PCR >2 mg/dl), both associated with tubulo-interstitial inflammation and endothelial dysfunction (Berni et al. 2012). Remarkably, in experimental studies it was found that hyperuricemia causes glomerular hypertension, vasoconstriction and ischemia, a potent stimulus for tubulo-interstitial inflammation and fibrosis (Berni et al. 2010; Sanchez-Losada et al. 2005).
Dynamic evaluation of RRI in normoalbuminuric patients with newly diagnosed hypertension showed that the decrease in RRI induced by nytroglicerine was lower in hypertensives than in controls despite similar baseline RRI (Bruno et al. 2011). Reduced renal vasodilation was independently related to the increase of systemic arterial stiffness and suggests a role of systemic hemodynamic load in determining early renal microvascular alteration in hypertension. RRI determination could help to understand the intricate link between hypertension and subclinical renal damage, till now mainly supported by the relationship between hypertension and microalbuminuria. The unifying mechanism that accounts for the different roles of RRI as a marker of subclinical renal damage and a prognostic predictor of renal and cardiovascular outcomes was suggested by Hashimoto et al. (Hashimoto and Hito 2011) who recorded aortic pressures, aortic and peripheral pulse wave velocities and RRI in 133 hypertensive patients: (a) RRI depends strongly on aortic pulse pressure and aortic stiffness; (b) RRI correlates inversely with the femoral reverse-flow and diastolic forward-flow indices; and (c) RRI predicts urinary albumin excretion together with the aortic pulse pressure. In these hypertensive patients the altered renal hemodynamics due to increased central pulse pressure and aortic stiffness contributed to the development of renal microvascular damage marked by high RRI. Every 0.1 increase in renal RRI was associated with a 5.4-fold increase in the adjusted relative risk of albuminuria (Hashimoto and Hito 2011). According to these findings atherosclerosis increases systemic arterial stiffness, predisposes renal circulation to a greater hemodynamic load (pulse pressure) and results in higher renal microvascular resistance. Increased systemic arterial stiffness underlines the strict relationship between RRI and atherosclerotic damage such as left ventricular hypertrophy, carotid intima media thickness and ankle brachial index (Pontremoli et al. 1999a; Calabia et al. 2014; Geraci et al. 2015). On the other hand high RRI might contribute to systemic arterial stiffening by renal dysfunction and activate a self-perpetuating process. Moreover, RRI proved to be an independent predictor of worse renal and CV outcomes in 426 patients with primary hypertension and no previous CV disease followed for a mean of 3.1 years (Doi et al. 2013). We can conclude that in hypertensive patients with normal renal function RRI is an early clinical marker of subclinical renal damage, that can anticipate the occurrence of microalbuminuria, but also signals systemic atherosclerotic burden. For both reasons high RRI is a good predictor of worse renal and cardiovascular outcomes.
The vast majority of RRI measurements reported in literature are carried out in hypertensives on different pharmacological combinations without a wash-out period; this could result in confounding factors for the study of determinants of RRI.Remarkably, scarce data are available in literature (Leoncini et al. 2002; Watanabe et al. 2006) about the effect of pharmacological therapy on RRI values; whether and how the decrease in RRI values could result in an improvement of renal damage and in renal and CV outcomes is unknown. This fact is mainly responsible for the limited use of RRI in clinical practice and need dedicated studi.
4.2.5 RRI and Renal Damage in Diabetes
RRI can detect early renal damage in patients with diabetes type 1 and 2: when renal function is normal and albuminuria is absent; increased RRI predicts the occurrence of albuminuria (Hamano et al. 2008; Nosadini et al. 2006). Most importantly, in patients without microalbuminuria RRI values >0.70 independently predicted the occurrence of diabetic nephropathy. In diabetic subjects with albuminuria and reduced creatinine clearance, RRI >0.80 predicts a worse renal outcome (Boddi et al. 2015).
Newly diagnosed Type 2 diabetic patients show higher baseline RRI and lower vasodilatation induced by nytroglicerin than those observed in newly diagnosed hypertensive subjects (Bruno et al. 2011). Pulse pressure proved to be a strong predictor of impaired RRI decrease in hypertensives and diabetics, but only in diabetic subjects was impaired vasodilatation significantly related to glycated haemoglobin and systolic pressure. Indeed,in patients with diabetic nephropathy the postglomerular vessels were the major contributor to increased resistance, whereas the pathognomonic histological sign of hypertensive nephropathy is preglomerular arteriolar hyalinosis disease.
These findings suggest that in diabetic patients renal vasculature might be compromised even in the presence of early glucose metabolism impairment, as in pre-diabetic condition where systemic vascular dysfunction and increased arterial stiffness are already present.
Accordingly, in hypertensive patients with no albuminuria and normal renal function, the coexistence of diabetes was associated with higher RRI values despite similar PWV in hypertensives with and without diabetes (Maestripieri et al. 2012).
4.2.6 RRI and Renal Damage in Chronic Renal Disease
In 2002 Radermacher et al. reported that in patients with chronic renal disease of any cause, an increased (>0.80) RRI correlates with the rate of decline in renal function and predicts the course of the disease (Radermacher et al. 2002). During a mean 3 years of follow up in these patients proteinuria (>1 g/day) and creatinine clearance (<40 ml/min) were also important indicators of disease progression, but in terms of positive and negative prediction RRI demonstrated superior utility. High RRI values were not secondary to differences in pulse rate or in the use of antihypertensive medication (Radermacher et al. 2002). Sugiura and Wada (2011) showed that high (>0.70) RRI as well as proteinuria, low GFR and hypertension, are independent risk factors for the progression of CKD (follow-up 4 years) and reinforced the feeling that RRI could be used as an additional tool for predicting the progression of CKD. High RRI could identify patients at high risk of end stage renal disease, because the initial measurements of RRI in patients with various nephropathies at the time of renal biopsy is associated with severe interstitial fibrosis and arteriolosclerosis and a worse glomerular filtration rate at 18 months (Bigè et al. 2012). In the high (>0.70) RRI group of 202 patients with CKD who underwent renal biopsies, RI ≥ 0.7, hypertension, proteinuria, and low eGFR at diagnosis were independent risk factors for predicting worse renal dysfunction.
In conclusion, according to the above reported findings RRI in CKD patients can be considered an independent predictor of renal failure, histological damage, and worse renal prognosis, as well as a possible determinant of the response to steroid therapy.
In middle aged and elderly hypertensive subjects Doi et al. (2013) confirmed the relationship between high RRI and worse cardiovascular and renal outcomes and that the combination of (<40 ml/min) eGFR and RRI was a powerful independent predictor of worse outcome, even when adjusted for traditional cardiovascular risk factors. The independent role of RRI in outcomes was maintained also for subjects with a GRF <60 mL/min. It is noteworthy that patients with both decreased eGFR and increased RRI had a significant burden of CV risk factors and a higher risk of the primary composite end points compared with those with either isolated decreased eGFR or increased RRI. Although both eGFR and increased RRI reflect renal dysfunction, the pathophysiological mechanisms leading to these abnormalities may, at least in part, be different. (Radermacher et al. 2002; Boddi et al. 2015; Doi et al. 2013).
Increased RI could be considered a marker of systemic atherosclerotic vessel damage, and compounded with reduced eGFR it may significantly increase the cardiovascular and renal risk. Data obtained from renal transplant recipients strongly supported that the predictive role of RRI for renal and CV outcome was the expression of systemic and not renal determinants (Chirinos and Townsend 2014; O’Neill 2014).
5 Resistant Hypertension
We have already mentioned that patients with treatment-resistant hypertension showed high RRI (Mahfoud et al. 2012). In these patients renal denervation was proposed as an attractive opportunity but so far only invasive procedures have been tested with conflicting results. Recently an approach for delivering externally focused ultrasound specifically targeting the perirenal artery tissues has been proposed. The application of acoustic energy creates a thermal field which is capable of ablating renal nerves around the renal artery, up to 1 cm beyond the lumen. In 69 patients with treatment-resistant hypertension who underwent renal denervation with externally delivered focused ultrasound, a good reduction (24/10 mmHg) after 6 months was observed without major side effects (Neuzil et al. 2016). Further studies are needed to confirm these first promising results.
6 Ultrasound Diagnostics of Renal Artery Stenosis
Eligibility for ultrasound screening for renal artery stenosis is based on clinical criteria (Meola et al. 2016a, b; Schaberle et al. 2016). Screened subjects are mostly adults (especially elderly subjects) with atherosclerotic vascular disease involving multiple districts and stage 2 and 3 CKD without a documented history of renal disease. During a routine ultrasound examination a small kidney (length <9 cm) can suggest ischemic damage due to renal artery stenosis. The Doppler parameters used to define stenosis as hemodinamically significant are well standardized and can be divided into “major or direct” and “minor or indirect”, or even “intrarenal or extrarenal” parameters. The criteria adopted by Zierler and Strandnes, published in the American Journal of Hypertension 1996 (Zierler et al. 1996), are still in use. Currently, RRI assay is the only Doppler parameter that provides information on the total vascular impedance of the parenchymal circle (Meola et al. 2016b; Schaberle et al. 2016).
Direct criteria are peak systolic velocity (PSV) and the ratio between PSV at renal stenosis and PSV in the aorta (Fig. 4); renal aortic ratio (RAR) a- PSV determines the degree of stenosis according to the continuity equation, because PSV is inversely proportional to the cross-sectional area of stenosis. However, PSV is also influenced by current blood pressure, wall vessel compliance, tortuosity of renal arteries and chronic renal parenchymal damage. Hyper-dynamic circle as observed in young people, hyperthyroidism and anemia, can also affect PSV. b-RAR compares the increased intrastenotic flow velocity in the renal arteries with the reference value measured in the aorta, and permits the decrease of the influence of the above mentioned systemic factors on PSV, measured at renal artery. Under physiological conditions the PSV along the main renal artery ranges between 60 and 120 cm/sec.
We want to remind readers that since eccentric stenosis results in a lower hemodynamic effect at the same angiographic diameter reduction, compared with concentric stenosis, (50 % of diameter reduction in concentric stenosis = 75 % of area reduction, whereas = 50 % in eccentric stenosis), PSV can rise twice as high at the same diameter reduction in eccentric stenosis. Compared with gold standard angiography, PSV measured by Color Duplex ultrasound shows sensitivities of 71–98 % and specificities of 62–98 %. Studies usually set the PSV cut-off value for >60 % renal artery stenosis at 180–200 cm/s, but they are determined by each author using receiver operating characteristics (ROC) curves, and different values are reported by different authors. It is to be noticed that selecting higher PSV cut-off values results in lower sensitivity and greater specificity in ROC curves compared with angiography (Meola et al. 2016b; Schaberle et al. 2016).
The combined use of PSV with RAR allows the increase in sensitivity and specificity of Doppler renal ultrasound to detect severe renal artery stenosis (Schaberle et al. 2016).
End-diastolic peak velocity was reported as stenosis criteria for the grading of carotid stenosis, but is markedly influenced by peripheral resistance which increases early in renal parenchymal damage and its use in the grading of renal artery stenosis is discussed.
We would like to point out that only >70–75 % RAS causes a relevant post-stenotic pressure drop, activating the renin angiotension system and requiring treatment. Only in high grade drop in post stenotic pressure can the severity of renal artery stenosis be calculated as validated for iliac arteries (Meola et al. 2016b; Schaberle et al. 2016).
Indirect criteria are based on the analysis of post-stenotic Doppler frequency spectra found distally to a >70 % renal artery stenosis, that depend also on intrarenal wall vessel and extra-vascular compliance and parenchyma function (Fig. 4) 1- RRI assayed in the kidney distally to renal artery stenosis shows a decreased difference between maximum and minimum flow velocity with a tardus-parvus spectrum and is lateralized with a difference in RRI >0.05 between the two kidneys, 2-Delayed acceleration time (AT) i.e. delay in the systolic rise from end diastole up to PSV on RRI spectral analysis. These ultrasound findings suggest that the ischemic kidney is protected by marked vasodilation, modulated by the self-regulating intrarenal mechanisms (Meola et al. 2016b; Zierler et al. 1996) which predict a good outcome of revascularization in terms of blood pressure control and recovery of renal function.
Renal artery stenosis due to fibromuscular dysplasia., usually discovered in young female, is characterized by specific renal vascular modifications and a normal renal function. For this kind of renal artery stenosis has been clearly shown the utility of doppler findings (PSV, RRI) in evaluating the severity of stenosis and the presence of intrarenal hemodynamic modifications before and after interventional procedures when compared to those obtained from the gold standard such as selective renal arteriography (Schaberle et al. 2016; Zierler et al. 1996).
In subjects with atherosclerotic renal artery stenosis, the typical post-stenotic criteria can be well evident in patients aged <60, with normal renal function, but not always in older patients with combined arteriolosclerosis and renal damage. These older subjects show high and symmetric RRI. The concurrence of chronic renal disease independently increasing RRI can hide the hemodynamic effect of renal artery stenosis and limit the information obtainable through Doppler ultrasound. Moreover, when parenchymal renal damage is asymmetrical as in pielonephritis, the bias for RRI measurement as marker of severe renal artery stenosis further increases (Boddi et al. 2015) (Fig. 4).
Recently, RRI >0.73 measured in the kidney controlateral to renal artery stenosis was the strongest predictor of renal function, worsening after renal revascularization also adjusted for male sex, regional angioplasty without stenting, obesity, pulse pressure >75 mmHg and serum creatinine >1.8 mg/dl (Bruno et al. 2014).
When hypoperfusion due to renal arterial stenosis persists for a long time and becomes chronic, damage of renal parenchyma develops, with a progressive reduction of renal volume and increase in interstitial and vascular resistance that results in high RRI (Bommart et al. 2010). High RRI (>0.75), especially when associated wih renal interpolar diameter < 9 cm and low renal volume, predicts a bad outcome of revascularization (Radermacher et al. 2001). An increased RRI value >80 is a strong predictor of renal functional decline in patients with renal artery stenosis, despite correction of the stenosis. As a whole data available in literature can be summarized as follows:
-
(a)
Asymmetric low RRI distal to renal artery stenosis is a good marker of the hemodynamic impact of renal artery stenosis on renal parenchyma.
-
(b)
When parenchymal disease concurs to renal artery stenosis and causes a symmetrical increase in RRI values, scarce or no information can be obtained on the hemodynamic impact of arterial stenosis on renal parenchyma.
-
(c)
High asymmetric RRI values (≥0.80) distal to renal artery stenosis, with low interpolar diameter and volume of the ischemic kidney, are associated to bad outcome after revascularization.
-
(d)
In subjectcs with renal artery stenosis and high symmetric RRI values can be also the mirror of systemic rather than renal parameters; in these subjects the predictive role of RRI for good revascularization outcome is under debate.
In the absence of direct or indirect signs of renal artery stenosis, increases in the intraparenchymal RRI (RI > 0.75 e/o > 0.80; PI > 1.50) associated with systemic atherosclerotic disease are indicative of microcirculatory damage related to nephroangiosclerosis or atheroembolic disease (Meola et al. 2016a).
7 Conclusions
The use of RRI in clinical practice is limited by the incomplete knowledge of all renal and extra-renal pathophysiological determinants that can concur to modulate RRI value in a different way in different subjects. In acute conditions such as hydronephrosis and AKI, renal determinants have a major role and RRI can directly monitor renal damage. In vascular and parenchymal nephropathies, the role of renal and extra-renal determinants must be analyzed singly, according to the subject’s clinical characteristics and questions put to RRI by the internist, who searches for an early marker of targeted organ damage in hypertension or diabetes, or for an independent predictor of renal and CV outcome (Lennartz et al. 2016). To summarize, from the literature: (a) In hypertensives with normal renal function and no albuminuria, RRI is an early marker of renal damage and could improve current clinical scores used to stratify early renal damage. Especially in younger hypertensive and diabetics subjects. In older subjects RRI increases in accordance with the increase in systemic vascular stiffness and the role of renal determinants can weaken; because of this close relationship, RRI is also a marker of systemic atherosclerotic burden but the clinical relevance was not specifically investigated. (b) In transplant kidney and in chronic renal disease high (>0.80) RRI values can mark renal damge and independently predict renal failure. (c) Doppler ultrasound allows diagnosis and grading of renal stenosis in both fibromuscolar dysplastic and atherosclerotic diseases and can indirectly measure the hemodynamic impact of renal artery stenosis on the homolateral kidney. However, in elderly subjects with atherosclerotic renal artery stenosis coexisting renal diseases can independently increase RRI by the augmentation in renal vascular stiffness and tubulo-interstitial pressure and partially or completely hidden changes due to renal artery stenosis.
How and whether RRI assay could allow for improving the prediction of renal damage and of cardiovascular risk in asymptomatic subjects remains a matter of debate.
References
Bardelli M, Jensen G, Volkmann R, Caidahl K, Aurell M (1992) Experimental variations in renovascular resistance in normal man as detected by means of ultrasound. Eur J Clin Investig 22(9):619–624
Berni A, Boddi M, Fattori EB et al (2010) Serum uric acid levels and renal damage in hyperuricemic hypertensive patients treated with renin angiotensin system blockers. Am J Hypertens 23:675–680
Berni A, Ciani E, Bernetti M, Cecioni I, Berardino S, Poggesi L, Abbate R, Boddi M (2012) Renal resistive index and low-grade inflammation in patients with essential hypertension. J Hum Hypertens 26(12):723–730
Bigè N, Levy PP, Callard P, Faintuch J-M, Chigot V, Jousselin V et al (2012) Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic disease. BMC Nephrol 25(13):139. doi:10.1186/1471-2369-13-139
Blacher J, Halimi JM, Hanon O et al (2014) Management of hypertension in adults: the 2013 French society of hypertension guidelines. Fundam Clin Pharmacol 28:1–9
Boddi M, Sacchi S, Lammel RM, Mohseni R, Neri Serneri GG (1996) Age-related and vasomotor stimuli-induced changes in renal vascular resistance detected by Doppler ultrasound. Am J Hypertens 9(5):461–466
Boddi M, Cecioni I, Poggesi L, Fiorentino F, Olianti K, Berardino S, La Cava G, Gensini GF (2006) Renal resistive index early detects chronic tubulointerstitial nephropathy in normo- and hypertensive patients. Am J Nephrol 26(1):16–21
Boddi M, Natucci F, Ciani E (2015) The internist and the renal resistive index: truths and doubts. Intern Emerg Med 10(8):893–905
Boddi M, Bonizzoli M, Chiostri M, Begliomini D, Molinaro A, Tadini Boninsegni L, Gensini GF, Peris A (2016) Renal resistive Index and mortality in critical patients with acute kidney injury. Eur J Clin Investig 46(3):242–251
Bommart S, Cliche A, Therasse E et al (2010) Renal artery revascularization: predictive value of kidney length and volume weighted by resistive index. AJR Am J Roentgenol 194(5):1365–1372
Bruno RM, Daghini E, Landini L et al (2011) Dynamic evaluation of renal resistive index in normoalbuminuric patients with newly diagnosed hypertension or type 2 diabetes. Diabetologia 54(9):2430–2439
Bruno RM, Daghini E, Versari D, Sgrò M, Sanna M, Venturini L et al (2014) Predictive role of renal resistive index for clinical outcome after revascularization in hypertensive patients with atherosclerotic renal artery stenosis: a monocentric observational study. Cardiovasc Ultrasound 12:9. doi:10.1186/1476-7120-12-9
Bude RO, Rubin JM (1999) Relationship between the resistive index and vascular compliance and resistance. Radiology 211(2):411–417
Bude RO, Di Pietro MA, Platt JF, Rubin JM, Miesowicz S, Lundquist C (1992) Age dependency of the renal resistive index in healthy children. Radiology 184(2):469–473
Butterly DW, Schwab SJ (2000) Renal artery stenosis: the case for conservative management. Mayo Clin Proc 75(5):435–436
Calabia J, Tourguet P, Garcia I et al (2014) The relationship between renal resistive index, arterial stiffness, and atherosclerotic burden: the link between macrocirculation and microcirculation. J Clin Hyperens (Greenwhich) 16(3):186–191
Chiang C-E, Wang T-D, Li Y-H et al (2010) 2010 guidelines of the Taiwan society of cardiology for the management of hypertension. J Formos Med Assoc 109:740–773
Chirinos JA, Townsend RR (2014) Systemic arterial hemodynamics and the “renal resistive index”: what is in a name? J Clin Hypertens (Greenwich) 16(3):170–171. doi:10.1111/jch.12276
Ciccone MM, Iacoviello M, Gesualdo L, Puzzovivo A, Antoncecchi V, Doronzo A, Monitillo F, Citarelli G, Paradies V, Favale S (2014) The renal arterial index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail 16(2):210–216
Darmon M, Schortgen F, Vargas F et al (2011) Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 37(1):68–76
Dasgupta K, RR Q, KB Z et al (2014) The 2014 Canadian Hypertension Education Program (CHEP) recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention and treatment of hypertension. Can J Cardiol (30):485–501
Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J (2007) Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med 33(9):1557–1562 Epub 2007 May 8
Dewitte A (2013) Jrenal Doppler in the management of the acute kidney injury in intensive care unit. J Crit Care 28(3):314. doi:10.1016/j.jcrc.2013.01.003
Doi Y, Iwashima Y, Yoshihara F, Kamide K, Takata H, Fujii T et al (2012) Association of renal resistive index with target organ damage in essential hypertension. Am J Hypertens 25(12):1292–1298
Doi Y, Iwashima Y, Yoshinhara F, Kamide K, Hayashi S, Kubota Y et al (2013) Response to renal resistive index and cardiovascular and renal outcomes in essential hypetension. Hypertension 61(2):e23
Geraci G, Mulè G, Mogavero M, Geraci C, D’Ignoti D, Guglielmo C (2015) Renal haemodynamics and severity of carotid atherosclerosis in hypertensive patients with and without impaired renal function. Nutr Metab Cardiovasc Dis 25(2):160–166
Geraci G, Mulè G, Mogavero M, Geraci C, Nardi E, Cottone S (2016) Association between uric acid and renal haemodinamics: pathophysiological implications for renal damage in hypertensive patients. J Clin Hypertens 2:1–8
Go AS, Bauman M, King SMC et al (2014) An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 63:878–885
Gosling RG, Lo PT, Taylor MG (1991) Interpretation of pulsatility index in feeder arteries to low-impedance vascular beds. Ultrasound Obstet Gynecol 1(3):175–179
Granata A, Zanoli L, Clementi S, Fatuzzo P, Di Nicolò P, Fiorini F (2014) Resistive intrarenal index: myth or reality? Br J Radiol 87(1038):20140004
Hamano K, Nitta A, Ohtake T, Kobayashi S (2008) Associations of renal vascular resistance with albuminuria and other macroangiopathy in type 2 diabetic patients. Diabetes Care 31:1853–1857
Hashimoto J, Hito S (2011) Central pulse pressure and aortic stiffness determine renal haemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58(5):839–846
Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H et al (2005) Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46(4):603–609
Jacobson HR (1988) Ischemic renal disease: an overlooked clinical entity. Kidney Int 34:729–743
James PA, Oparil S, Carter BL et al (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 311:507–520
Jensen G, Bardelli M, Volkmann R, Caidahl K, Rose G, Aurell M (1994) Renovascular resistance in primary hypertension: experimental variations detected by means of Doppler ultrasound. J Hypertens 12(8):959–964
Le Dorze M, Bouglè A, Deruddre S, Duranteau J (2012) Renal Doppler ultrasound: a nex tool to asses renal perfusion in critical illness. Shock 37(4):360–365
Lennartz CS, Pickering JW, Seiler-Mußler S, Bauer L, Untersteller K, Emrich IE et al (2016) External validation of the kidney failure risk equation and re-calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol 11(4):609–615. doi:10.2215/CJN.08110715.Epub
Leoncini G, Martinoli C, Viazzi F, Ravera M, Parodi D, Ratto E et al (2002) Changes in renal resistive index and urinary albumin excretion in hypertensive patients under long-term treatment with lisinopril or nifedipine GITS. Nephron 90(2):169–173
Liu LS (2010) Chinese guidelines for the management of hypertension. zhonghua Xin Xue Guan Bing Za Zhi 39:579–615
Maestripieri V, Pacciani G, Tassinari I et al (2012) Hypertensive patients with diabetes mellitus and normal arterial stifness show an early increase in renal resistive index. Eur Heart J 33(N 17/201):70–75
Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Böhm M (2012) Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patient with resistant hypertension. Hypertension 60(2):419–424
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34:2159–2219
Meola M, Samoni S, Petrucci I (2016a) Imaging in chronic kidney disease. Contrib Nephrol 188:69–80
Meola M, Samoni S, Petrucci I (2016b) Clinical scenario in chronic kidney disease: vascular chronic disease. Contrib Nephrol 188:81–88
Miyoshi K, Okura T, Tanino A, Kukida M, Nagao T, Higaki J (2016) Usefulness of renal resistive index to predict an increase in urinary albumin excretion in patients with essential hypertension. J Hum Hypertens. Online Publication. doi: 10.1038/jhh.2016.38
Modesti PA, Ferrari A, Bazzini C, Boddi M (2015) Time sequence of autonomic changes induced by daily slow-breatinghing sessions. Clin Auton Res 25(2):95–104
Naesens M, Heylen L, Lerut E et al (2013) Intrarenal resistive index after renal transplantation. N Engl J Med 369(19):1797–1806. doi: 10.56/NEJMoa1301064
National Institute for Health and Care Excellence (2014) Hypertension: clinical management of primary hypertension in adults (Clinical guideline 127). http://guidance.nice.org.uk/CG127. Accessed 19 June 2014
Neuzil P, Ormiston J, Brinton TJ, Starek Z, Esler M, Dawood O et al (2016) Externally delivered focused ultrasound for renal denervation. ACC Cardiovasc Interv 9(12):1292–1299. doi:10.1016/j.jcin.2016.04.013 Epub 2016 Jun 20
Norris CS, Barnes RW (1984) Renal artery flow velocity analysis: a sensitive measure of experimental and clinical renovascular resistance. J Surg Res 36(3):230–236
Nosadini R, Velussi M, Brocco E et al (2006) Increased renal arterial resistance predicts the course of renal function in type 2diabetes with microalbuminuria. Diabetes 55:234–239
O’Neill WC (2014) Renal resistive index: a case of mistaken identity. Hypertension 64(59):915–917
Okura T, Jotoku M, Irita J, Enomoto D, Nagao T, Desilva VR, Yamane S, Pei Z, Kojima S, Hamano Y, Mashiba S, Kurata M, Miyoshi K, Higaki J (2010) Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol 14(6):584–588
Platt JF, Rubin JM, Ellis JH (1989) Distinction between obstructive and nonostructive pyelocaliectasis duplex Doppler sonography. AJR Am J Roentgenol 153(5):997–1000
Platt JF, Ellis JH, Rubin JM, Di Pietro MA, Sedman AB (1990) Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol 154(6):1223–1227
Ponte B, Pruijm M, Ackermann D, Vuistiner P, Eisenberger U, Guessons I et al (2014) Reference values and factors associated with renal resistive index in a family-based population study. Hypertension 63(1):136–142
Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V, Leoncini G, Ruello N, Zagami P, Bezante GP, Derchi LE, Deferrari G (1999a) Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant 14(2):360–365
Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V et al (1999b) Increased renal resistive index in patients with essential hypertension: a marker of organ damage. Nephrol Dial Transplant 14:360–365
Pourcelot L (1974) Applications Clinique de l’examen Doppler transcutane. In: Peronneau P (ed) Symposium: Velocimetric Ultrasonnordoppler. Inserm, Paris, pp 213–240
Radermacher J, Chavan A, Bleck J, Vitzthum A, Stoess B, MJ G et al (2001) Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med 344(6):410–417
Radermacher J, Ellis S, Haller H (2002) Renal resistance index and progression of renal disease. Hypertension 2(2 Pt2):699–703
Raff U, Ott C, John S, Schmidt BM, Fleischmann EH, Schmieder RE (2010) Nitric oxide and reactive hyperemia: role of location and duration of ischemia. Am J Hypertens 23(8):865–869. doi:10.1038/ajh.2010.72
Ratto E, Leoncini G, Viazzi F, Vaccaro V, Falqui V, Parodi A, Conti N, Tomolillo C, Deferrari G, Pontremoli R (2006) Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens 24(10):2033–2038
Sanchez-Losada LG, Tapia E et al (2005) Mild hyperuricemia induces vasoconstriction and maintaings glomerular hypertension in normal and remnant kidney rat. Kidney Int 67(1):237–247
Schaberle W, Leyerer L, Schierlinq W, Pfister K (2016) Ultrasound diagnostics of renal artery stenosis: stenosis criteria, CEUS and recurrent in-stent-stenosis. Gefässchirurgie 21:4–13
Schnell D, Deruddre S, Harrois A et al (2012) Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 38(6):592–597
Sugiura T, Wada A (2011) Resistive index predicts renal prognosis in chronic kidney disease: results of a 4-year follow-up. Clin Exp Nephrol 15(1):114–120
Textor SC, Wilcox CS (2001) Renal artery stenosis: a common treatable cause of renal failure? Annu Rev Med 52:421–442
Tublin ME, Tessler FN, Murphy ME (1999) Correlation between renal vascular resistance, pulse pressure, and resistive index in isolated perfused kidneys. Radiology 213(1):258–564
Tublin ME, Bude RO, Platt JF (2003) The resistive index in renal Doppler sonography: where do we stand? Am J Roentgenol 180:885–892
Vegar Zubović S, Kristić S, Sefić Pašić I (2016) Relationship between ultrasonographically determined kidney volume and progression of chronic kidney disease. Med Glas (Zenica) 13(2):90–94
Viazzi F, Leoncini G, Derchi LE, Pontremoli R (2014) Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens 32:149–153
Watanabe S, Okura T, Kurata M, Irita J, Manabe S, Miyoshi K et al (2006) Valsartan reduces serum cystatin C and the renal vascular resistance in patients with essential hypertension. Clin Exp Hypertens 28(5):451–461
Weber MA, Schiffrin EL, White WB et al (2014) Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens 32:3–15
Zierler RE, Bergelin RO, Davidson RC et al (1996) A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens 9(11):1055–1061
Aknowledgements
I thank Ms. Susan Seeley for her precious help in revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this chapter
Cite this chapter
Boddi, M. (2016). Renal Ultrasound (and Doppler Sonography) in Hypertension: An Update. In: Islam, M.S. (eds) Hypertension: from basic research to clinical practice. Advances in Experimental Medicine and Biology(), vol 956. Springer, Cham. https://doi.org/10.1007/5584_2016_170
Download citation
DOI: https://doi.org/10.1007/5584_2016_170
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44250-1
Online ISBN: 978-3-319-44251-8
eBook Packages: MedicineMedicine (R0)