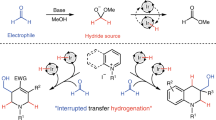
Abstract
In this chapter, we describe the development in homogeneous Ir-catalyzed asymmetric hydrogenation with particular emphasis on the achievements made during the last 10 years. We also present their application to the synthesis of complex molecules. The first section deals with the hydrogenation of unfunctionalized olefins or with poorly coordinative groups. The second section includes the advances made in the hydrogenation of functionalized olefins. The last two sections cover the hydrogenation of imines and ketones, respectively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Metal-catalyzed asymmetric hydrogenation (AH) offers some of the most sustainable and straightforward reactions for producing pharmaceuticals, flavors, fragrances, agrochemicals, and fine chemicals due to its perfect atom economy and operational simplicity [1,2,3,4,5]. It is estimated that around 10% of all chemical steps in the synthesis of these compounds are hydrogenations. Despite the extensive research dedicated to the asymmetric hydrogenation and the important progress reached, some issues still need to be solved. Most catalysts only work with a limited number of substrates, and each type of substrates needs a specific catalyst for optimal enantioselectivity. For example, the asymmetric hydrogenation of functionalized alkenes is mostly carried out by Ru- and Rh-diphosphine catalysts (see, e.g., [6,7,8,9]), while the asymmetric hydrogenation of unfunctionalized olefins or with poorly coordinative groups is mainly carried out with Ir-P,N catalysts (for reviews, see [10,11,12,13,14,15]). A broad substrate scope is desirable to reduce the time dedicated to ligand/catalyst design and preparation. A desired additional condition is that the catalyst family should be synthesized from available starting materials and be easy to handle.
The number and types of functionalized substrates have been remarkably expanded, and their use is commonplace, as illustrated in the commercial production of the Parkinson’s L-DOPA drug [16, 17], the broad-spectrum antibiotics levofloxacin [18] and sitagliptin [19], and the pesticide (S)-metolachlor [20]. The success of catalysts relies on the ability of the substrate to form a metal chelate involving the double bond and a donor atom. Although the reduction of functionalized olefins has been thoroughly studied for decades, there are some substrate types that are still a challenge. Among them it can be found the cyclic β-enamides, which have recently attracted attention because their hydrogenation products are found in many pharmaceutically and biologically active products. Two representative examples are rotigotine, used to treat Parkinson’s disease, and alnespirone, a selective 5-HT1A receptor with antidepressant and anxiolytic properties [21,22,23,24]. In the last decade, it has been found that Ir-containing catalysts [25,26,27] can be used in their reduction with results that surpass the most studied Rh- and Ru-catalysts [28,29,30,31,32,33,34,35,36,37,38]. Other challenging substrates where Ir-catalysts have shown to be superior or complementary to the Rh-/Ru-catalysts are unsaturated carboxylic acids and nitroolefins, among others (see Sects. 3–5).
The absence of a coordinative group in the olefins makes their hydrogenation a great challenge. So, compared to the AH of functionalized olefins, the reduction of unfunctionalized alkenes or with poor coordinative groups is much less mature [10,11,12,13,14,15]. The best catalysts have two characteristics in common: (1) they mainly contain P,N-ligands [39,40,41] and (2) their optimal structure is highly dependent on the geometry and substitution pattern of the olefin [10,11,12,13,14,15]. The consequence is that for each particular olefin type, a different ligand family needs to be developed. It is also important to notice that catalysts have been developed in different grades for each olefin substitution pattern. The most successful cases have been reported for trisubstituted olefins and, to a less extent, for disubstituted. The asymmetric hydrogenation of tetrasubstituted unfunctionalized substrates is still underdeveloped.
In this book chapter, we describe the development in Ir-catalyzed asymmetric hydrogenation with particular emphasis on the achievements made during the last 10 years. We also present their applications to the synthesis of complex molecules. Most of the work has been devoted to the hydrogenation of nonfunctionalized olefins or with poorly coordinative groups (Sect. 2), with significant advances in both substrate scope and mechanistic studies. However, also notable advances have been made in improving the catalytic performance in the reduction of relevant and more challenging functionalized substrates, such as unsaturated carboxylic acids, nitroolefins, cyclic β-enamides, imines, etc. (see Sects. 3–5).
2 Ir-Catalyzed Asymmetric Hydrogenation of Unfunctionalized Olefins or with Poorly Coordinative Groups
Among the most challenging substrates to date are the unfunctionalized olefins or olefins with poorly coordinative groups [10,11,12,13,14,15]. A breakthrough in the hydrogenation of this type of substrates came in 1997 when Pfaltz et al. used phosphine-oxazoline PHOX ligands L1 (Fig. 1) to design [Ir(L1)(cod)]PF6 (cod = 1,5-cyclooctadiene), a chiral analogue of Crabtree’s catalyst ([Ir(py)(PCy3)(cod)]PF6) that enantioselectively hydrogenated imines [42]. Although this catalyst also hydrogenated unfunctionalized olefins highly enantioselectively, it was unstable to the reaction conditions. Pfaltz and co-workers overcame this problem by changing the catalyst anion to [(3,5-(F3C)2-C6H3)4B]−([BArF]−). The result was [Ir(L1)(cod)]BArF (Fig. 1), an active, enantioselective, and stable catalyst library for olefin hydrogenation. Despite this success, its scope was limited to mainly E-trisubstituted olefins [43]. It was also seen that the optimal catalyst was highly dependent on the geometry and substitution pattern of the olefin. This triggered the search for new catalysts that would reach a wider substrate scope.
In this respect, Pfaltz group continued to develop new versions of the PHOX complexes, modifying the ligand backbone, with the discovery of very efficient ligand libraries [44,45,46,47,48,49,50,51,52]. Successive work incorporated pyridine and quinoline rings instead of the oxazoline, which allowed the successful reduction of challenging purely alkyl-substituted substrates in high ee [53,54,55]. A notable application was the total synthesis of γ-tocopherol as a single diastereoisomer in 98% ee, controlling two stereocenters in one reductive step (see below) [54]. The various developed ligands also enabled the reduction of various type of substrates, such as allylic alcohols, α,β-unsaturated esters [56], furan derivatives [57], boronic esters [58], and tetrasubstituted olefins [59].
Other groups also provided new successful ligand libraries, by modifying the chiral backbone and by replacing either the P-group by a N-heterocyclic carbene moiety or the oxazoline moiety by other N-donor groups (such as oxazole, thiazole, and imidazole) and by O- and S-donor groups [14, 15, 60, 61]. All these modifications allow to further extend the substrate scope.
Concerning mechanistic aspects, although the mechanism of olefin hydrogenation by Rh-catalysts is well understood, the mechanism when Ir-catalysts are used has not been fully determined until recently. In this context, computational and experimental research with P,N- and C,N- ligands have shown that the hydrogenation of minimally functionalized olefins proceeds via an IrIII/IrV migratory-insertion/reductive-elimination catalytic cycle (Fig. 2) [62,63,64,65,66,67]. Very recently, Pfaltz’s group, based on mechanistic studies under hydrogenation conditions, was able to detect the Ir(III) dihydride alkene intermediates responsible for the catalytic performance for the first time [68]. They found that, similar to the classical Halpern mechanism for asymmetric hydrogenation with Rh-catalysts, the minor intermediate, which is less stable, is converted to the major product enantiomer.
In the next sections, we collect the catalytic results on the asymmetric hydrogenation of unfunctionalized olefins or with poorly coordinative groups.
2.1 Di- and Trisubstituted Unfunctionalized Olefins or with Poorly Coordinative Groups
Aryl/alkyl trisubstituted alkenes have become the model substrates for evaluating the efficiency of new catalytic systems. In general, the hydrogenation of 1,2-diarylalkenes (i.e., trans α-methylstilbene) proceeded with higher enantioselectivities than monoarylated ones (such as E-2-(4-methoxyphenyl)-2-butene) for which only a limited number of catalysts provided high enantioselectivities [10,11,12,13,14,15]. The geometry of the olefin also affects the catalytic performance. Z-Trisubstituted olefins are usually hydrogenated less enantioselectively than the related E-trisubstituted olefins. The lower enantioselectivities can be mainly attributed to a Z/E isomerization process to form the more stable E-alkene, which gives the opposite enantiomer of the hydrogenated product [10,11,12,13,14,15]. Z-2-(4-Methoxyphenyl)-2-butene and dihydronaphthalenes (i.e., 7-methoxy-4-methyl-1,2-dihydronaphthalene) are frequently used to study the ligand scope in the hydrogenation of Z-alkenes. Dihydronaphthalenes have recently received much attention because the corresponding chiral tetraline motif is found in numerous natural products [69]. Trialkyl substituted alkenes have been much less studied. This is due in part to the difficulty in developing methods for ee determination and also the lack of an aryl group that could direct the reaction via π-stacking interaction between the substrate and the chiral catalyst. The best results have been reported in the reduction of 1-methoxy-4-(3-methyl-pent-3-enyl)-benzene (ees up to 95%) [54].
Nowadays, Ir-catalysts have also been able to reduce olefins with a variety of relevant poorly coordinative groups such as α,β-unsaturated esters, ketones, and lactames and vinyl boronates, among others [10,11,12,13,14,15]. The effective hydrogenation of such a range of olefins is of great importance since their reduced products are key structural chiral units found in many high-value chemicals (e.g., α- and β-chiral ketones and carboxylic acid derivatives are ubiquitous in natural products, fragrances, agrochemicals, and drugs). Substrate scope has also been extended to 1,1-diaryl or 1,1,2-triaryl substituted substrates (i.e., 1-(1,2-diphenyl-vinyl)-3,5-dimethyl-benzene) and more recently to 1,4- and cyclic dienes (i.e., 1,5-dimethyl-cyclohexa-1,4-diene), linear and cyclic sulfones, and alkyl fluorides, which are present in several important drugs and natural products.
Unlike trisubstituted olefins, a large range of 1,1-disubstituted olefins have not been successfully asymmetrically hydrogenated until very recently [10,11,12,13,14,15]. This is because the catalyst has the added difficulty of controlling not only the face selectivity coordination (only two substituents compared with the three of trisubstituted olefins, Scheme 1a) but also the isomerization of the olefins to form the more stable E-trisubstituted substrates, which are hydrogenated to form the opposite enantiomer (Scheme 1b).
Next we compile the most representative catalytic results in the hydrogenation of di- and trisubstituted olefins organized by the type of ligands.
2.1.1 Phosphine-Oxazoline Ligands
Inspired by the work of Pfaltz et al. with PHOX ligands, many other phosphine-oxazoline ligands have been developed. Künding, Pfaltz et al. reported a modification in the oxazoline moiety with the phosphine-benzoxazine analogues L2 (Fig. 3, R = tBu, iPr) [46]. The enantioselectivities were lower than those recorded with PHOX ligands. The presence of a bulky substituent, a tBu, at the oxazine group provided good enantioselectivities for E-trisubstituted olefins (ees up to 89%), except for trisubstituted allylic alcohols, but low for Z-trisubstituted olefins, 1,1′-di- and tetrasubstituted olefins.
The rest of the new developments in the ligand design were based on modifications of the ligand backbone. Ligands L3 (Fig. 3, R1 = Ph, o-Tol and R2 = Me, tBu, 1-Ad, CPh3), developed by Burgess et al., were applied in the hydrogenation of several aryl-alkyl alkenes [70]. These ligands proved to be superior to the PHOX ligands in the hydrogenation of Z-trisubstituted alkenes, while ees for E-trisubstituted alkenes were lower. The best enantioselectivities for Z-olefins were obtained with a tBu group at the oxazoline and a diphenylphosphanyl group, while for E-olefins a bis(o-tolyl)phosphanyl group was needed (ees up to 80%). A further modification of ligands L3 was to introduce again the ortho-phenylene motif of the PHOX ligands. New ligands L4 (Fig. 3, R1 = Ph, Cy and R2 = tBu, 1-Ad, CHPh2, 3,5-tBu2-C6H3) provided excellent results in the reduction of trans-α-methylstilbene derivatives and trisubstituted α,β-unsaturated esters (ees up to 99%) [71]. Again bulky groups in oxazoline and phosphine moieties were needed (R1 = Cy; R2 = tBu).
Later, Cozzi’s group developed ligands L5, in which the phenyl ring of the PHOX ligands was replaced by a thiophene group (Fig. 3, R1 = Ph, o-Tol, Cy and R2 = iPr, tBu) [72]. This modification also led to high enantioselectivities but only in the hydrogenation of trans-α-methylstilbene (ees up to 99%). Hou et al. developed phosphine-oxazoline ligands L6 in which the flat ortho-phenylene group in the PHOX ligands was replaced by a benzyl group (Fig. 3, R1 = Ph, o-Tol, p-Tol and R2 = Me, iPr, tBu) [73, 74]. These ligands allow to extend the type of substrates successfully hydrogenated. High enantioselectivities were achieved with E-trisubstituted aryl-alkyl alkenes, allylic alcohols, and α,β-unsaturated esters and ketones (ees up to 98%). The best enantioselectivities were obtained with an iPr oxazoline group and a diphenylphosphanyl functionality.
Another modification was to introduce a ferrocenyl group (ligands L7, Fig. 3, R2 = Me, iPr, tBu, Ph, Bn). The best results were obtained with the ligand that contains a small methyl substituent in the oxazoline group that proved to be superior than PHOX in the Z-substrates (89% ee), while ees for E-alkenes were lower (ees up to 89%) [75].
Pfaltz et al. also further modified PHOX ligands by replacing the ortho-phenylene tether by a branched alkyl chain (ligands L8; Fig. 3, R1 = Ph, o-Tol, Xyl and R2 = iPr, tBu, Bn) [69]. These ligands provided higher enantioselectivities in the hydrogenation of trisubstituted E- and Z-aryl alkenes than the PHOX ligands (ees up to 98%). The best results were achieved with the ligand that contains bulky substituents at both phosphine and oxazoline groups (R1 = Xyl and R2 = tBu). The authors showed its applicability with the synthesis of (R)-7-demethyl-2-methoxycalamenene, an antitumor natural product.
The spirocyclic phosphine-oxazoline ligands L9 (Fig. 3, R1 = o-Tol, Ph and R2 = Ph, Bn) were also successfully used in the hydrogenation of α,β-unsaturated Weinreb amides [76] and α,α′-bis(2-hydroxyarylidene) ketones [77].
Afterward, Zhang et al. developed phosphine-oxazoline ligands L10 with a biphenyl backbone (Fig. 3, R1 = Ph, 3,5-tBu2-C6H3, 3,5-tBu2-4-MeO-C6H2 and R2 = iPr, tBu, Ph, Me) which successfully hydrogenated exocyclic α,β-unsaturated carbonyl compounds (including ketones, lactones, and lactams) [78], 3-substituted 2,5-dihydropyrroles [79], and 2,5-dihydrothiophene 1,1-dioxides [79].
2.1.2 Aminophosphine-Oxazoline Ligands
Some aminophosphine-oxazoline ligands have also showed comparable high efficiency than phosphine-oxazolines in the reduction of unfunctionalized olefins or with poorly coordinative groups. In this context, Pfaltz et al. modified the PHOX ligands by replacing the ortho-phenylene group by a pyrrole group leading to ligands L11 (Fig. 4, R1 = Ph, o-Tol, Cy and R2 = iPr, tBu) [80]. Enantiomeric excesses surpassed those previously obtained with the PHOX ligands, with ligands bearing a bulky tert-butyl oxazoline substituent and either an ortho-tolyl or cyclohexyl P-group. Nevertheless, the enantioselectivities for Z-trisubstituted olefins were not above 80% ee. Then, Gilbertson et al. developed the proline-based aminophosphine-oxazoline ligands L12 (Fig. 4, R1 = Ph, o-Tol and R2 = iPr, tBu), related to previous ligands L11; however, they provided lower enantioselectivities [81]. The best result was obtained with the ligand bearing a bulky tert-butyl oxazoline substituent.
Andersson et al. developed ligands L13 and L14 (Fig. 4, L13; R1 = Ph, o-Tol, Cy; R2 = H, tBu, Ph and R3 = H, Ph; and L14; R1 = Ph; R2 = H, iPr, Ph and R3 = H, iPr, Ph) [82,83,84,85,86,87,88,89,90,91]. Ligands L13, which are based on a rigid bicyclic backbone, provided higher enantioselectivities than ligands L14, with a more flexible backbone. Ir/L13 catalyst (with R1 = R2 = R3 = Ph) afforded, for first time, high enantioselectivities in the hydrogenation of enol phosphinates [84, 85], vinylsilanes [86], fluorinated olefins [87], vinyl boronates [88], α,β-unsaturated acyclic esters [89], α,β-unsaturated lactones [90] and γ,γ-disubstituted and β,γ-disubstituted allylic alcohols [91] (Fig. 5).
2.1.3 Phosphinite-Oxazoline Ligands
It can be highlighted the family of phosphinite-oxazoline ligands L15 (Fig. 6, R1 = Ph, o-Tol, Cy; R2 = tBu, Ph, ferrocenyl, 2-Naph; R3 = H, Me, 3,5-Me2-C6H3 and R4 = Me, iPr, tBu, Bn), developed by Pfaltz et al. [47, 49, 92,93,94]. Ligands L15 constitute one of the most privileged ligands for this process. They provided excellent enantioselectivities in the reduction of a broad range of both E- and Z-trisubstituted olefins, including α,β-unsaturated esters and for the first time, in a limited range of more challenging terminal olefins as well as in the reduction of 1,1′-disubstituted enamines (ees up to 99%, Fig. 7) [92,93,94,95]. More recently, Ir-catalysts containing ligands L15 have also been successfully applied in the reduction of α,β-unsaturated nitriles (ees up to 98%, Fig. 7) [94]. The best enantioselectivities were achieved with ligands containing a methyl substituent at R3, a benzyl substituent at R4, and a phenyl at R1. However, the appropriate substituent at the oxazoline and the configuration of the carbon of R3 depend on the substrate to be hydrogenated. For E-trisubstituted olefins, ees are best with ligands containing a Ph or a 3,5-Me2-Ph and a S-configuration, while for Z-olefins the highest enantioselectivities were achieved using ligands with Ph and a R-configuration. In addition, these catalysts work efficiently in propylene carbonate as an environmentally friendly solvent, and this allowed the Ir-catalysts to be reused maintaining the excellent enantioselectivities [96, 97]. Based on ligands L15, Pfaltz group developed ligands L16 (Fig. 6, R1 = Ph, o-Tol and R2 = iPr, tBu) where the alkyl chain is bonded in the C-2 instead of the C-4 of the oxazoline moiety, which shifts the chirality from the alkyl chain to the oxazoline moiety [50, 75]. The scope of these ligands is narrower than with the privileged phosphinite-oxazoline ligands L15; however, they are complementary. Ligands L16 provided high enantioselectivities for allylic alcohols and alkenes with heteroaromatic substituents.
Kazmeier et al. synthetized ligands L17 (Fig. 6) that provided excellent enantioselectivities for linear and cyclic α,β-unsaturated ketones (ees up to >99%) [95].
2.1.4 Phosphite-Oxazoline Ligands
Phosphite-containing ligands have shown to be particularly useful for asymmetric catalysis. They have a greater resistance to oxidation than phosphines and phosphinites, they are easily synthesized from readily available chiral alcohols, and their modular constructions are easy (for reviews, see [98,99,100]). Despite this, it was not until 1999 that a publication reported their use in the reduction of unfunctionalized olefins using Ir-TADDOL-based phosphite-oxazoline catalysts (L18, Fig. 8). However, their substrate scope and enantioselectivities were lower than their related Ir-phosphinite/phosphine-oxazoline catalysts. Additionally, the use of high pressures (100 bars) and high catalyst loadings (4 mol%) was required to obtain full conversions [51].
Our group contributed to the asymmetric Ir-catalyzed hydrogenation of unfunctionalized olefins with a new series of air-stable ligands that are applicable to a wide variety of substrates (di- and trisubstituted). The key was the introduction of a flexible biaryl phosphite group in the ligand. We first developed the pyranoside phosphite-oxazoline ligand library L19 (Fig. 8, R = Me, iPr, tBu, Ph, Bn) synthetized from D-glucosamine, an inexpensive natural feedstock, that contains several biaryl phosphite groups [67, 101, 102]. It was found that for enantioselectivities to be high, the presence of bulky substituents in the biaryl phosphite group and less sterically demanding substituents in the oxazoline moiety was required. Thus, it was possible to identify two general ligands (L19c and L19e with R = Ph) that provided high enantioselectivities. For comparative purposes, the related phosphinite-oxazoline analogues were also tested, but with lower success [102]. With ligands L19c and L19e, high enantioselectivities and activities (ees up to >99%) in many trisubstituted olefins (25 examples, Fig. 9), even in the reduction of the more challenging Z-isomers, could be reached and triarylsubstituted substrates, which provide an easy entry point to diarylmethine chiral centers that are present in several important drugs and natural products [103,104,105,106]. High enantioselectivities could also be achieved in the reduction of many trisubstituted substrates with poorly coordinative groups, such as α,β-unsaturated esters and ketones, vinylsilane, allylic alcohol, and acetates. Also, it should be noted the excellent enantioselectivities obtained in the hydrogenation of vinyl boronates (ees ranging from 92% to >99%). Their hydrogenation provides chiral borane compounds, which are useful building blocks in organic synthesis because the C-B bond can be readily converted to C-O, C-N, and C-C bonds with retention of the chirality. Even more remarkable were the high enantioselectivities obtained for the first time in the reduction of a broad range of 1,1′-disubstituted olefins (19 examples, Fig. 9). It was found that the Ir/19e system was robust against variations in the electronic nature of the substrate aryl substituents (ees up to 99%). Also high levels of enantioselectivity were obtained in the reduction of heteroaromatic terminal olefins (ees up to 99%). Nevertheless, the enantioselectivities were affected by the nature of the alkyl chain and diminished in 1,1′-diaryl alkenes due to an isomerization process.
With the aim of understanding the catalytic performance of Ir/L19 catalysts, a DFT computational study was performed in collaboration with Norrby et al. [67]. It was found that the preferred reaction path is an Ir(III/V) cycle with migratory insertion of a hydride as the selectivity-determining step. In addition, the effect of the ligand parameters could be rationalized by using a simple quadrant model (Fig. 10), where the phenyl oxazoline’s substituent occupies the upper left quadrant and one of the aryls of the biaryl phosphite moiety partly blocks the lower right quadrant (Fig. 10a). The other two quadrants are free. The calculated structure had a chiral pocket that fits perfectly E-olefins (Fig. 10b). This quadrant model also explains the change of ligand (from L19e to L19c) to obtain a high enantioselectivity in Z-olefins (Fig. 10c). Ligand L19c has bulky substituents in the para position, which increases the dihedral angle of the biaryl group and results in lower occupancy of the lower right quadrant than with ligand L19e. Therefore, the substituent in the biphenyl group can tune the occupancy of the lower right quadrant, and therefore Z-alkenes can also be successfully hydrogenated (Fig. 10c). The same explanation accounts for the triaryl- and disubstituted substrates. In conclusion, the DFT studies confirm that the flexibility of the biaryl phosphite group is a crucial parameter in the achievement of high enantioselectivities for substrates with different geometries and steric requirements.
Following this contribution comes the developments of new biaryl phosphite-oxazoline ligand libraries with the aim to increase even further the range of substrates successfully hydrogenated [27, 107,108,109,110]. Among them, we can highlight the application of phosphite-oxazoline ligands (L20, Fig. 8) [107, 109], which were inspired in one of the best families developed for this transformation (previous ligands L15, Fig. 6) by replacing the phosphinite groups by several biaryl phosphite moieties. Selecting the ligand parameters’ high enantioselectivities has been reported for trisubstituted olefins. Whereas Ir/L20g catalyst provided the best enantioselectivities for linear and cyclic olefins and a α,β-unsaturated ester, the best ees for the more demanding Z-isomers and allylic alcohol and acetate were obtained with Ir/20a catalyst. This high catalytic performance was also extended to the hydrogenation of the more challenging 1,1′-disubstituted olefins (29 compounds, Fig. 11), surpassing the previous family L19 and becoming one of the best catalysts for the reduction of this type of substrates. High enantioselectivities were achieved in a broad range of aryl-alkyl (ees up to >99%), even with substrates bearing decreasingly sterically alkyl substituents, and heteroaromatic-alkyl (ees up to >99%) olefins. These catalyst precursors also tolerate very well the presence of neighboring polar groups. High enantioselectivities were achieved in the reduction of allylic alcohols and an allylic silane. Interestingly, the reaction showed no loss of enantioselectivity when dichloromethane was replaced by propylene carbonate. In addition, the use of propylene carbonate allowed the catalysts to be recycled up to five times by a simple two-phase extraction maintaining the excellent enantioselectivities [107].
2.1.5 Phosphorus-Other Nitrogen Donor Ligands
In the recent years, the research has also focused on the design of ligands containing more robust groups than oxazolines. A collection of the most representative phosphorus-other nitrogen donor ligands will be next presented.
As an alternative to P-oxazoline ligands, pyridine-containing ligands have attracted interest due to the robustness and the easy incorporation of pyridine group. Despite this, few pyridine-containing ligands have provided outstanding results in terms of enantioselectivity and substrate versatility. For selected examples, see Fig. 12 [53, 57, 111,112,113,114,115,116,117,118,119,120,121,122,123]. Among them, we can highlight the first pyridine-containing ligand developed by Pfaltz et al. (phosphinite-pyridine ligands L21; Fig. 12, R1 = Ph, o-Tol, Cy, tBu and R2 = Me, tBu, Ph, CPh3), which was successfully used in a limited range of alkenes [57]. The performance was subsequently further improved by the same group introducing a more rigid chiral bicyclic ligand backbone (ligands L22, Fig. 12, R1 = Ph, o-Tol, Cy, tBu; R2 = H, Ph, Me and R3 = H, Me). This ligand family with high rigidity was successfully applied in several kinds of trisubstituted olefins, including purely alkyl trisubstituted alkenes, furans, and benzofurans as well as trisubstituted pinacol derivatives, α,β-unsaturated lactones, and N-protected indoles [57, 114, 116, 117]. The enantioselectivity was highest with a Ph substituent at the R2 and bulky substituents at the phosphinite moiety (tBu or o-Tol). To obtain excellent enantiocontrol in the reduction of 7-methoxy-4-methyl-1,2-dihydronaphthalene, the introduction of a large aryl substituent at R2 (2,4,6-tri-Me-Ph) was needed. Its applicability was demonstrated in the reduction of γ-tocotrienyl acetate to obtain γ-tocopherol, a principal component of vitamin E [118], resulting in enantioselectivity >98% for the RRR enantiomer. Another synthetic application can be found in the diastereo- and enantioselective hydrogenation of farnesol stereoisomers. By changing the bond’s geometry, these catalysts give access to the four stereoisomers of the product in high selectivity.
To benefit from the advantages of phosphite and pyridine moieties, our group replaced in ligands L21 the phosphinite moieties by several biaryl phosphite groups increasing even further the substrate scope (Fig. 12; ligands L23, R1 = H, Me, Br, Ph and R2 = Me, tBu, Ph) [122]. Excellent enantioselectivities (ees up to 99%) were obtained in a wide range of E- and Z-trisubstituted alkenes, including more demanding triarylsubstituted olefins and dihydronaphthalenes, and also terminal disubstituted olefins and alkenes containing neighboring polar groups.
Another interesting change in the nitrogen donor group is the replacement of the oxazoline by imidazole, oxazole, thiazole, thiazoline, and sulfoximine [124] groups. For selected examples, see Fig. 13. The first application was reported by Pfaltz et al. with the phosphine-imidazole ligands L24 (Fig. 13, R1 = Ph, o-Tol; R2 = iPr, tBu and R3 = iPr, tBu, Cy, Ph, Bn, p-Tol) [48]. One advantage of the imidazoline group over the oxazoline is the possibility to introduce a new substituent R3 at the nitrogen that could serve as a linker to attach the ligand to a solid support. Ligands L24 provided better enantioselectivities in the hydrogenation of Z-trisubstituted olefins (ees up to 88%) than PHOX ligands (ees up to 42%). The best results were achieved with ligands containing bulky substituents at both R1 and R2 positions, while the substituent at R3 had to be optimized for each substrate. Andersson group also developed the phosphine-imidazole ligands L25 (Fig. 13, R1 = Ph, o-Tol, 3,5-diMe-Ph) that gave high enantioselectivities for E-aryl/alkyl trisubstituted olefins (ees up to 98%) [125, 126] and cyclic dienes (ee’s up to >99% for the trans isomer) [127,128,129], but was only moderate in the reduction of Z-olefins (ees up to 72%) [126]. Interestingly, the hydrogenation of dienes was also found to be regioselective, and by controlling the reaction conditions, selective hydrogenation of one of the two trisubstituted olefins was achieved. In addition, trisubstituted olefins were selectively hydrogenated in the presence of tetrasubstituted olefins. Thus, enantioselectivities were best with ligand containing a bisphenylphosphanyl group except for the reduction of trans-α-methylstilbene for which a bis-(o-tolyl)phosphanyl group was needed. More recently, they also showed its applicability in the enantioconvergent formal deoxygenation of racemic alcohols (Fig. 13). This methodology was successfully used in the total synthesis of antidepressant sertraline and σ2 receptor PB [75] [35j].
Several classes of other P,N-ligands have been developed by Andersson et al. (Fig. 13, ligands L26-L29; R1 = Ph, o-Tol … and R2 = Ph, tBu …). The investigation of different bicyclic heteroaromatic rings led to highly enantioselective iridium catalysts containing oxazoles [69] and thiazoles [70] (Fig. 13). These catalysts perform excellently on the typically tested trisubstituted nonfunctionalized olefins and also allow extending the substrate scope to vinyl allylsilanes [86], fluorinated olefins [87, 126, 130], vinyl boronates [88], enol phosphinates [84], and E- and Z-chiral sulfones [131, 132], allylic alcohols [90, 133], and the monohydrogenation of 1,4-dienes [134]. Among the hydrogenation of dienes, many purely alkyl-substituted were successfully hydrogenated (ees up to 99%). In addition, they are able to selectively hydrogenate only one of the double bonds, leaving room for further synthetic manipulations. In this respect, Andersson’s group use this methodology for the total synthesis of (-)-juvabione, a natural sesquiterpene exhibiting juvenile hormone activity using Ir/L28 catalyst [135].
Another interesting example of ligand design was the phosphite-thiazoline ligand L30 (Fig. 13), in which the oxazoline group in ligands L20 was replaced by a thiazoline moiety. The introduction of a thiazoline moiety has not only provided enantioselectivities up to >99% for a range of α,β-unsaturated ketones, vinylsilane, and trifluoromethyl olefins but also has increased the enantioselectivities of Z-trisubstituted olefins while maintaining the excellent enantioselectivities for a range of E-trisubstituted and 1,1-disubstituted minimally functionalized olefins [136].
2.1.6 Carbene-Nitrogen Ligands
Another type of effective catalysts is the Ir/carbene-nitrogen complexes. An important advantage of N-heterocyclic carbene (NHC) catalysts compared to their phosphine analogues concerns their better tolerance for acid-sensitive substrates. In 2001, Burgess’ group reported for the first time that NHC-oxazoline-based Ir-catalysts (ligand L31, Fig. 14, R1 = 2,6-iPr2-Ph and R2 = 1-Ad) can also be applied in the hydrogenation of unfunctionalized olefins with results comparable to the commonly used Ir-P,N catalysts [137, 138]. These catalysts afforded high enantioselectivities (up to 98% ee) in a limited group of unfunctionalized olefins, mainly trisubstituted, and for the more challenging disubstituted olefins, only one example was reported with low enantioselectivity. Since then, a few more carbene-N ligands have been developed but with less success [139,140,141,142,143], except for the family of Ir-NHC-pyridine catalysts [144] developed by Pfaltz’s group (with ligands L32, Fig. 14, R = 2,6-diisopropylbenzene) that showed similar enantioselectivities to the Burgess ones. So, high enantioselectivities (>90% ee) were observed, even for Z-trisubstituted (94% ee) and endocyclic substrates (96% ee).
2.1.7 Application of P-O/S Ligands
In contrast to other catalytic processes and to the Rh-/Ru-hydrogenation, for the reduction of unfunctionalized olefins, the possibility of changing the nature of the N-donor atom in the ligand design of heterodonor ligands was not contemplated until recently. In 2011, Pfaltz successfully reported the application of proline-based P,O ligands L33 in the asymmetric hydrogenation of trisubstituted alkenes (Fig. 15, R1 = Ph, tBu, Cy, o-Tol and R2 = tBu, 1-Ad, CPh3, 1Ad-NH, MesNH, CPh3NH) [145,146,147]. Phosphines bearing either a bulky amide or urea groups at the pyrrolidine N-atom formed efficient Ir-catalysts for the asymmetric hydrogenation of several minimally functionalized olefins (ees up to 99%).
At the same time, our group reported the application of a highly modular furanoside phosphite-thioether ligand library (ligands L34, Fig. 15) [148, 149]. By selecting the ligand components in these furanoside-based ligands (position of the thioether group at either C-5 or C-3 of the furanoside backbone, the configuration of C-3, the thioether substituent, and the substituents/configuration in the biaryl phosphite moiety), we found that the best enantioselectivities were obtained using ligands with a 5-deoxy-ribofuranoside backbone L34. Excellent enantioselectivities were obtained (ees up to 99%) in the reduction of a range of trisubstituted alkenes, including relevant examples with poorly coordinative groups (such as α,β-unsaturated esters and vinyl boronates). The results are comparable to the best ones reported in the literature except for the hydrogenation of 1,1′-disubstituted aryl/alkyl olefins. For this substrate class, our results indicated that enantioselectivity is dependent on the nature of the alkyl substrate substituent and much less affected by the electronic nature of the aryl ring. This has been attributed to an isomerization process that was supported by the fact that the hydrogenation of substrates bearing a tert-butyl group, for which isomerization cannot occur, provides high levels of enantioselectivity (ees up to 98%). We also studied the effect on catalytic performance of introducing either phosphinite or phosphine moieties with lower success.
Since then, several other P-thioether ligands have been developed [150,151,152]. From them, we can highlight the modular phosphite/phosphinite-thioether ligand library L35 (Fig. 15; R1 = Ph, Tol, Cy, Mes; R2 = Ph, 2,6-Me2-Ph, 4-MeOPh, 2-Naph, tBu, Ad, Cy; R3 = Me, Tr, Mes). In a simple three-step procedure, several ligand parameters were easily tuned to maximize the enantioselectivities for each substrate (ees up to 99% in 43 hydrogenated products, Fig. 16) [153]. In contrast to the furanoside-based ligands mentioned above (L34), the best enantioselectivities were obtained with the phosphinite-S ligands, while results achieved with the phosphite-S analogues were less optimal. The crystal structures of the Ir-catalyst precursors indicate an equatorial disposition of the thioether group for the phosphite-based ligands, while an axial disposition of the thioether group is found in the analogues phosphinite ligands. The modularity of the ligands together with DFT studies were crucial to find which ligand parameters could be modified to generate more selective catalysts. In this respect, the use of a bulky mesityl group instead of a phenyl group in the ligand backbone improved enantioselectivity. With catalyst Ir/L35, with R1 = Tol, R2 = 2,6-Me2-Ph, and R3 = Me, excellent enantioselectivities (ees up to >99%) were recorded for many trisubstituted olefins, including olefins with relevant neighboring polar groups such as α,β-unsaturated esters, ketones, vinyl boronates, and allylic alcohols (Fig. 16). High enantioselectivities were also achieved in the hydrogenation of 1,1′-disubstituted alkenes. Excellent enantioselectivities were also maintained by using propylene carbonate as an environmentally benign solvent, which allowed the Ir-catalyst to be reused up to three times. DFT studies also confirmed that the preferred reaction path is an IrIII/IrV cycle where the selectivity-determining step is the migratory insertion of a hydride. DFT results also allowed the formulation of a quadrant model which explains the effect of the ligand parameters on selectivities. In this quadrant model, the thioether substituent occupies the upper left quadrant, and one of the P-substituents partly occupies the lower right quadrant, while the other two quadrants are free. This explains the high enantioselectivities obtained with the DFT-optimized guided design of thioether-phosphinite ligands in the reductions of (E)-olefins. In the case of the analogous phosphite-thioether ligands, the upper left quadrant is not enough blocked due to the equatorial disposition of the thioether group, which explains that they provided lower enantioselectivities than the related phosphinites.
2.2 Tetrasubstituted Unfunctionalized Olefins or with Poorly Coordinative Groups
Despite the advances during the last 10 years in the asymmetric hydrogenation of unfunctionalized olefins with the development of ligand libraries that allowed a significant increase in the range of substrates that can been successfully hydrogenated, the reduction of tetrasubstituted olefins remains a challenge. The range of such substrates that can be efficiently hydrogenated is still narrow [154].
In 1999, Buchwald’s group reported the first successful asymmetric hydrogenation of tetrasubstituted unfunctionalized olefins [155]. Although high enantioselectivities were achieved for substituted indenes using the zirconocene catalyst 1 (Fig. 17; ee’s in the range 52–99%), the high catalyst loading (8 mol%), the high H2 pressure (typically >110 bar), and the low stability of the catalyst hampered their broad use. Much more recently, Zhang’s group reported a Rh-catalyst 2 (Fig. 17), containing a P-stereogenic diphosphine ligand synthesized in nine steps that provided 85–95% ees in the reduction of some indenes [156]. But it still required high catalyst loading (10 mol%), 60 °C, and longer reaction times (4 days). Again, Pfaltz’s group made an important breakthrough in this field. In 2007, they found that the stability and/or the harsh reaction condition issues of the Zr/Rh-catalysts can be overcome with Ir/P,N catalysts. Another important finding was that the optimum ligand structures for tri- and tetrasubstituted olefins differed strongly [59]. Using Ir-catalysts 3 (Fig. 17), containing ligands that form a 5-membered chelate ring, a wide range of indenes were hydrogenated with ees in the range of 94–96%, under milder reaction conditions and low catalyst loading (typically 1–2 mol%). Nevertheless, ees diminished for noncyclic olefins and for 1,2-dihydro-napthalenes (ees between 89 and 97% and up to 77%, respectively). It should be mentioned that these catalysts provided low enantioselectivities in the hydrogenation of trisubstituted olefins.
This finding prompted the interest in the design of new specific ligands for Ir-hydrogenation of unfunctionalized tetrasubstituted olefins. In 2013, Busacca’s group found that the Ir-catalyst 4 could hydrogenate two cyclic substrates with ees up to 96% at low catalyst loading. An inconvenience was that low temperature (0 °C) was required [157].
Several more successful reports have appeared in the last few years. Notably, Andersson’s group showed the efficient asymmetric hydrogenation of the challenging acyclic tetrasubstituted olefins. They successfully hydrogenated a broad range of tetrasubstituted vinyl fluorides using one of their privileged Ir-P,N catalysts for the reduction of trisubstituted olefins, with a small modification in the P-group (ligand L29 with R1 = o-EtPh; R2 = iPr, see above Fig. 13) [158]. The challenge of these substrates is that the catalyst must not only control de face selectivity but also avoid the side defluorination reaction. Advantageously, the reaction proceeded smoothly without defluorination in high diastereo- and enantioselectivities. Various aromatic, aliphatic, and heterocyclic systems with a variety of functional groups were efficiently hydrogenated. The successful asymmetric hydrogenation of these substrates opens up a direct, atom-efficient path to synthesize chiral fluorine molecules with two contiguous stereogenic centers. However, the catalyst was not successful for other classes of cyclic and acyclic tetrasubstituted olefins and also required the use of high H2 pressure (20–100 bar).
Our group in collaboration with Riera’s group reported the application of an Ir/P-stereogenic aminophosphine-oxazoline catalyst library L36 (Fig. 18, R = Ph, iPr, tBu), with a simple, modular architecture, in the asymmetric hydrogenation of a broad range of different types of unfunctionalized tetrasubstituted olefins [159]. Improving previous results reported until now, the same family of catalysts is able to efficiently reduce indenes and the challenging 1,2-dihydro-napthalene derivatives (ees up to 96%) and also a broad range of the elusive acyclic olefins with enantioselectivities up to 99% under mild reaction conditions. Moreover, the excellent catalytic performance is maintained for a range of aryl and alkyl vinyl fluorides (drs > 99% and ees up to 98%), where two vicinal stereogenic centers are created.
Then, by substitution of the aminophosphine group by simple readily available phosphinite groups (Ir/L37 catalyst, R1 = Ph, o-Tol, Cy; R2 = Ph, iPr, tBu, Fig. 18), we could also efficiently reduce many unfunctionalized tetrasubstituted olefins (ees up to 98%) under mild reaction conditions [27]. It should be noted that the more rigid the tetrasubstituted olefin is, the less bulky phosphinite moieties are required to reach the maximum enantioselectivity. For the more rigid cyclic indene derivatives, the best catalytic performance is therefore reached with the phosphinite-based ligand with Ph phosphinite substituent, while for the less rigid cyclic substrate, the phosphinite ligand with a bulkier o-tolyl group is needed. Finally, the even less rigid acyclic substrates require the ligand with the bulkiest cyclohexyl phosphinite group. Even more interestingly, maintaining the same skeleton of the ligand by simply changing the phosphinite functionality by the right phosphite group (ligands L37a-c, Fig. 19), we could also efficiently reduce many unfunctionalized tri- and disubstituted olefins (ees up to 98%, Fig. 19). In summary, from a common simple skeleton, the correct choice of either phosphite or phosphinite groups gives for the first time ligands that are suitable for di-, tri-, and tetrasubstituted unfunctionalized substrates and also for cyclic β-enamides (62 examples, with ees up to 99%).
A notable last contribution is the identification of an Ir-catalyst that is able to successfully hydrogenate a very broad range of diverse acyclic unfunctionalized tetrasubstituted olefins (around 30 examples) [160]. A first parallel screening of a set of 34 different Ir-catalysts found previous Ir-catalyst 3 (Fig. 17, R1 = o-Tol; R2 = iPr) to be the best candidate. A subsequent optimization of its structure (phosphine and oxazoline substituent) identified 3 with R1 = Cy and R2 = 3,5-bis-tBu-Ph as the optimal catalyst.
3 Ir-Catalyzed Asymmetric Hydrogenation of Olefins Containing Coordinating Groups
As already mentioned, the hydrogenation of olefins with strongly coordinating groups has been predominantly performed using Rh- or Ru-catalysts bearing chiral diphosphine ligands. They still constitute the optimal choice for the synthesis of optically active α-amino acids and many pharmaceutically relevant compounds. Nowadays, excellent enantioselectivities can be achieved for N-acyl α-dehydroamino acid derivatives, enamides and acrylates or itaconates, among others [6,7,8,9]. However, in the last decade, Ir-based catalysts appear as a good alternative in the reduction of challenging functionalized olefins, providing higher catalytic performance than the Rh- and Ru-catalysts. In this respect, we next show the improved catalytic performance in the reduction of carboxylic acids and nitroolefins using Ir-catalyst. Chiral carboxylic acids are important intermediates for the preparation of biologically active compounds ([161], for a review, see [162]; for selected examples, see [163,164,165,166]). On the other hand, enantiomerically pure nitroalkanes can be easily converted to other versatile building blocks, such as amines, aldehydes, carboxylic acids, nitrile oxides, and denitrated compounds [167, 168].
3.1 Ir-Catalyzed Asymmetric Hydrogenation of Carboxylic Acids
Although only few Ir-catalysts have been studied for this transformation, they have allowed to overcome the limitations of most studied Rh- and Ru-catalysts. Indeed, most of the reported Rh- and Ru-catalysts showed a scope limited to acrylic and cinnamic acids, and especially with Ru-catalysts, high pressures and catalyst loadings are usually needed [6,7,8,9]. Scrivanti and co-workers explored for the first time the Ir-PHOX catalyst in the hydrogenation of 2-phenethylacrylic acid, recording enantioselectivities not higher than 81% ee [169]. Later, Burgess applied a chiral N-heterocyclic carbene-oxazoline L31 (Fig. 14) in the hydrogenation of tiglic acid with only 55% ee [170]. The inefficiency of these iridium catalysts was partly attributed to their tendency to aggregate into inactive trimers under a hydrogen atmosphere [171].
Zhou et al. showed for the first time that Ir-catalysts, the spiro phosphine-oxazoline (SIPHOX) Ir-catalysts (Fig. 20a) [172], could efficiently hydrogenate unsaturated carboxylic acids with the presence of a base [173]. The addition of a base results in the formation of a carboxylate anion, which act as a strong coordinating group. Under mild reaction conditions, excellent yields (90–97%) and enantioselectivities (96−>99% ee) could be achieved for a broad range of α-aryloxy- and α-alkyloxy-substituted α,β-unsaturated acids, with TONs up to 10,000 (Fig. 20a). It was found that the best catalysts contained a ligand with a bulky P-aryl group (Ar = 3,5-tBu2Ph), which was the best choice for most of the substrates studied afterward. The hydrogenation protocol was efficiently used for the preparation of α-benzyloxy-carboxylic acid, a key intermediate in the syntheses of the rhinovirus protease inhibitor rupintrivir (Fig. 20b) [174]. In contrast to previous Ir-catalysts, the rigidity and bulkiness of the spiro scaffold on SIPHOX ligands seemed to prevent the Ir-catalysts to trimerize under hydrogenation conditions. The authors also found that while Ir-SIPHOX catalysts were not effective for the hydrogenation of α,β-unsaturated esters [175], the Ir-PHOX analogue did provide excellent enantioselectivities [11]. Thus, both catalyst types have complementary substrate scope.
With the use of Ir-SIPHOX catalysts, Zhou and co-workers have largely unblocked the amount of unsaturated carboxylic acid derivatives that can be hydrogenated enantioselectively [175, 176] and for which Rh- and Ru-catalysts showed no success. Thus, they further expanded its application to the reduction of other trisubstituted α,β-unsaturated carboxylic acids, such as α-aryl- and α-oxymethyl-substituted cinnamic acids (Fig. 21). The hydrogenation of both types of substrates were used as a key step for the total synthesis of two natural products. Thus, the enantioselective reduction of α-arylcinnamic acid (Ar1 = 2,4-(MeO)2Ph, Ar2 = 4-MeOPh) [177] and α-oxymethylcinnamic acid (Ar = 4-MeOPh, R = 3,4-(OCH2O)Ph) was used for preparing (S)-equol and (S)-(+)-homoisoflavone [178]. Similarly, a range of N- and O-heterocycles of different ring sizes could be also hydrogenated with enantioselectivities ranging from 89 to 99% ee (Fig. 21). The methodology allowed the direct preparation of (R)-nipecotic acid and (R)-tiagabine in excellent yields and enantioselectivities [179]. Again, with a ligand containing a 3,5-tBu2Ph substituent in the phosphine, a range of tetrasubstituted acrylic acids with α-aryl, α-alkyl, α-aryloxy, or α-alkyloxy substituents were reduced in high enantioselectivities (90–99%) (Fig. 21). It should be noted that some of the hydrogenated products are key intermediates of chiral drugs, such as mibefradil and fenvalerate [180].
The excellent results of spiro phosphine-oxazoline ligands (SIPHOX) are not only limited to α,β-unsaturated acids. The authors also explored the reduction of several β,γ-unsaturated acids, which gives access to molecules with a chiral center at the γ-position. A range of 4-alkyl-4-aryl-3-butenoic acids could be hydrogenated in up to 97% ee with a ligand containing an α-naphthylmethyl group on the oxazoline ring and a 3,5-Me2Ph as a phosphine substituent (Fig. 22). With this asymmetric hydrogenation as the key step, the concise total syntheses of the natural products (R)-aristelegone-A, (R)-curcumene, and (R)-xanthorrhizol were accomplished [181]. It should be noted that β,γ-unsaturated ester (E)-methyl 4-phenylpent-3-enoate was inert under hydrogenation conditions, thus indicating that the functional carboxy group is crucial for the reaction by acting as a directing group. The use of a carboxy directing group was also extended to the hydrogenation of terminal 1,1-dialkyl, 1,1-diaryl and 1-aryl-1-alkyl γ,δ-unsaturated acids (Fig. 22, ees up to 99%) [182, 183]. This strategy is particularly useful for the reduction of 1,1-diaryl and 1,1-dialkylethenes, in which most of the catalysts fail in differentiating the Re- and Si-faces due to the similarity on size of both substituents of the olefin. In addition, it was shown that the directing carboxy group on these substrates can be subsequently removed or easily transformed to other useful functional groups if desired [182]. A range of α-alkyl-α-aryl terminal olefins were also reduced in excellent enantioselectivities (98–>99%; Fig. 22), yielding valuable compounds with a chiral benzylmethyl center. The developed hydrogenation process was also used as a key step for preparing (S)-curcudiol and (S)-curcumene in excellent enantioselectivities and overall yields [183]. Finally, the authors further confirmed the role of the carboxylate moiety as a directing group by showing that when no free carboxylic acid was present or no basic conditions were used, the reaction didn’t proceed [182]. Moreover, it was found that for substrates having an extra C=C double bond in the alkyl side chain, the presence of the carboxy directing group makes the reaction chemoselective toward the α-alkyl-α-aryl double bond (Fig. 22), even when the additional double bond was placed in the terminal position [183].
As found with Rh- and Ru-catalysts, the Ir-SIPHOX catalysts showed unsatisfactory results for the hydrogenation of 2-substituted α-arylacrylic acids. To overcome this limitation, Zhou and co-workers developed a new series of spiro P,N-ligands (SpiroBAP), with a benzylamino moiety instead of the oxazoline group (Fig. 23a). The new generation of ligands exhibited extremely high reaction rates (TOFs up to 6,000 h−1) and excellent enantioselectivities (94–98% ee) in the reduction of α-aryl and α-alkyl acrylic acids to the corresponding chiral carboxylic acids, including ibuprofen, naproxen, and flurbiprofen, which are widely used nonsteroidal anti-inflammatory drugs (Fig. 23a). As for SIPHOX ligands, ligands with a bulky P-aryl group (Ar = 3,5-tBu2Ph) gave the best catalytic results [184].
Zhou et al. have also recently developed a neutral version of spiro-based Ir-catalysts (Ir-SpiroCAP, Fig. 23b), by replacing the oxazoline moiety on SIPHOX ligands by an anionic carboxy group. The resulting Ir-complexes do not require the use of a tetrakis[3,5-bis(tri-fluoromethyl)phenyl]borate (BArF−) counterion, which is necessary for stabilizing chiral cationic Crabtree-type catalysts, while remaining highly stable for a long time in air. These new generations of catalysts exhibited an unprecedented high enantioselectivity (up to >99% ee) in the hydrogenation of the challenging 3-alkyl-3-methylenepropionic acids (Fig. 23b). To demonstrate its potential application in organic synthesis, the synthesis of (S)-14-methyloctadec-1-en, a female sex pheromone of the peach leaf miner moth (Lyonetia clerkella), was carried out. The new catalysts were also effective (ees up to 99.4%) in the reduction of other α-methyl cinnamic acid, tiglic acid, and α-substituted acrylic acids, among others [176].
The authors also performed a mechanistic study including DFT studies that strongly supported an Ir(III)/Ir(V) cycle. The high stability of the chiral spiro-iridium catalysts under reaction conditions [172] allowed the trapping of the active intermediates and facilitated the mechanistic study [185]. To mimic the basic conditions used in the hydrogenation reactions, the authors used sodium (E)-2-methyl-3-phenyl acrylate as a model substrate. The isolation of the monohydride intermediate 5 (Fig. 24), resulting from the migratory insertion of 6 (Fig. 24), was key to understand the mechanism. Dimeric species 7 and 8 (Fig. 24), which are off-cycle species, were also isolated and characterized by X-ray diffraction. Both dinuclear intermediates have the carboxy group acting as a bridge of the two Ir-centers. The isolation of intermediates 5–8 confirms the coordination of carboxy group to Ir when the reaction is performed under basic conditions. It should be noted that in contrast to Ir-hydrogenation of unfunctionalized olefins, the Ir-dihydride olefin complex 5 can undergo migratory insertion in the absence of H2 [68]. This mechanistic divergence could indicate that the mechanism of Ir-catalyzed hydrogenation of alkenes may vary depending on the type of substrate and/or catalyst.
Two other Ir-catalysts have been studied for this transformation. Already in 2010, Ding et al. tested the spiro-based P,N-ligands L9 (Fig. 3) in the reduction of α-aryl-β-substituted acrylic acids. Enantioselectivities up to 96% ee were achieved, leading to the production of a series of biologically interesting carboxylic acids, such as those containing a β-tetrahydro-2H-pyran-4-yl moiety [186]. These ligands were also applied in the hydrogenation of (E)-2-(hydroxymethyl)-3-arylacrylic acids in good-to-high enantioselectivities (Fig. 25a) [187].
Recently Zhang et al. reported the application of the phosphine-oxazoline ligands L38 (Fig. 6b), with a biphenyl moiety, which has the advantage of a simple synthesis from the readily available (S)-(+)-2-phenylglycinol. Ir/L38 (Ar = 3,5-tBu2Ph, R = Ph) provided high yields and enantioselectivities in the widely studied α-methyl cinnamic acids (Fig. 25b, up to 97% ee, 98% yield, 2.000 TON) [78].
3.2 Ir-Catalyzed Asymmetric Hydrogenation of Nitroolefins
Despite the synthetic utility of reduced chiral nitroalkanes, the direct asymmetric hydrogenation of these types of substrates was not achieved until quite recently, by using diphosphine-based Rh-catalysts [188,189,190]. Although these catalysts were quite efficient in the hydrogenation of β,β-disubstituted nitroolefins, they were sensitive to the steric hindrance of the substrate. Hou and co-workers developed an Ir-(R,R)-f-SpiroPhos complex for the reduction of β-acylamino nitroolefins (Fig. 26). This newly developed Ir-catalysts allowed the preparation of a range of β-amino nitroalkanes in high yields and excellent optical purities (up to >99% ee), including substrates with ortho-substituted phenyl groups in the β-position [191]. The substrates studied contained a NH-acyl group which by chelation could facilitate the enantioselective hydrogenation. However, the authors showed later that Ir-(R,R)-f-SpiroPhos-catalyst was also highly enantioselective without the presence of this additional chelating group. Thus, excellent enantioselectivities (up to 98% ee, Fig. 26) were also achieved in the reduction of β,β-disubstituted nitroalkenes, including nitroalkenes with an ortho-substituted phenyl ring and thus, overcoming the limitations of the catalytic systems developed by Zhang and co-workers [192]. After this, the groups of Zhang and Zhou have also explored the enantioselective Ir-catalyzed reduction of nitroolefins without an extra chelating group, obtaining also high enantioselectivities [193, 194].
3.3 Ir-Catalyzed Asymmetric Hydrogenation of Enamines, Enamides, and Allylic Amines
Ir-catalysts have also been used in the hydrogenation of amino-functionalized alkenes, albeit to a lesser extent than other alkenes [111, 195,196,197,198,199]. Nevertheless, they proved to be useful in the asymmetric hydrogenation of very attractive and challenging substrates, such as enamide esters, cyclic β-enamides, etc. In this context, the asymmetric hydrogenation of β-enamine esters is a straightforward way to prepare enantiopure β-amino acids and their derivatives. However, the methodology has been largely limited to the involvement of an N-acyl group that assists the reaction by chelation to the metal and facilitates to achieve high reactivity and enantioselectivity. The direct hydrogenation of unprotected enamine esters would be a more high-atom economical approach to obtain these valuable building blocks. Some Ir-catalysts modified with chiral monophosphoroamidites or diphosphine ligands have been applied in the hydrogenation of some unprotected NH- and N-aryl enamine esters with good-to-high enantioselectivities (ees up to 97%) (see, for instance, [200, 201]). Recently, Dong and Zhang et al. disclosed the highly effective asymmetric hydrogenation of tetrasubstituted α-fluoro-β-enamino esters using bisphosphine-thiourea ZhaoPhos ligand (Fig. 27) [202]. A series of valuable chiral α-fluoro-β-amino esters containing two adjacent tertiary stereocenters were afforded with high yields and excellent diastereo- and enantioselectivities (drs up to >25:1, ees up to >99% ee, and TON values up to 8,600). Importantly, no defluorinated by-product was detected.
Ir-catalysts have also shown to be very useful for the enantioselective hydrogenation of cyclic β-aryl enamides. The reduction of this type of substrates constitutes a direct route to 2-aminotetralines and 3-aminochromanes, which are key structural units found in numerous therapeutic agents and biologically active natural products [21,22,23,24]. However, their hydrogenation has provided unsatisfactory results, and only few Rh- and Ru-catalysts have been successful [28,29,30,31,32,33,34,35,36,37,38]. In 2012, a neutral Ir-complex, with a sulfonimidamido-based phosphoramidite (SIAPhos) ligand, catalyzed the reduction of three cyclic β-enamides with promising enantioselectivities (up to 92% ee; Fig. 28). However, this catalyst showed low activities (55–81% conversion after 18 h at rt. and at PH2 = 50 bar) [203].
It has not been until very recently that Ir-catalysts have shown their high efficiency in the reduction of this type of challenging substrates. In 2016, two reports appeared demonstrating the potential of Ir-catalysts modified with P,N-ligands and showing that Ir-P,N catalysts can be also efficient in the reduction of alkenes bearing metal-coordinating groups [25, 26]. Riera and Verdaguer et al. found that bulky P-stereogenic phosphine-oxazoline ligands L36 (Fig. 18) provided the highest selectivity ever reported for the reduction of cyclic enamides derived from α- and β-tetralones (Fig. 29a, ees up to 99% at only 3 bars of H2), surpassing the results obtained with the widely studied Rh- and Ru-catalysts [25]. In the same year, Diéguez and co-workers also reported the successful application of PHOX-derived phosphite ligands L39 (Fig. 29b) to the hydrogenation of cyclic β-enamides [26]. A range of 2-aminotetralines and 3-aminochromanes were obtained in high yields and excellent enantioselectivities (ees up to 99%, Fig. 29b). The hydrogenation of cyclic α-enamides also proceeded in high enantioselectivities (ees up to 96%). In addition, the reactions could be carried out in environmentally friendly solvents, propylene carbonate, with no loss of selectivity.
The already mentioned ligands L37 (Fig. 18) that were successful for disubstituted, trisubstituted, and tetrasubstituted unfunctionalized olefins also provided excellent enantioselectivities in the hydrogenation of various cyclic β-enamides (6 examples, ees up to 99%) [27]. Moreover, both enantiomers of the reduced products could be accessed with the correct choice of the phosphite-based ligand.
Finally, it has been recently shown that Ir-phosphite-thioether catalysts can also successfully catalyze the hydrogenation of cyclic β-enamides. The sugar-derived phosphite-thioether ligands (L40, Fig. 30) provided a range of 2-aminotetralines and 3-aminochromanes with excellent enantioselectivities (Fig. 30, ees up to 99%) [204]. Interestingly, both enantiomers of the hydrogenated products were obtained by simply switching from Rh to Ir. Moreover, low hydrogen pressure (10 bar) and environmentally friendly propylene carbonate could be used, with no loss of selectivity.
Very recently, it has been reported that Ir-catalysts can also successfully catalyze the hydrogenation of N-sulfonyl allyl amines [205] and aryl allyl phthalimides [206]. The hydrogenation of both types of substrates is another way to produce valuable chiral amines, such as β-aryl propanamines, which are important precursors for the synthesis of several pharmaceutical drugs (see, for instance, [207,208,209,210,211]). The commercially available threonine-derived phosphinite (UbaPHOX) iridium catalysts were found to be the best candidates for the hydrogenation of several N-sulfonyl allyl amines (Fig. 31a). A range of β-methyl amines were afforded with good to excellent ees of up to 94%. The synthetic potential of this methodology was shown with the synthesis of the biologically active compounds (R)-lorcaserin and LY-404187.
Concerning the reduction of aryl allyl phthalimides, the previously mentioned Ir-L36 catalysts were the best choice. Various enantioenriched β-aryl-β-methyl amines were yielded with excellent enantiomeric excess values (up to >99% ee) and using a low catalyst loading (1 mol%) and low hydrogen pressure (1 bar H2) (Fig. 31b). The utility of the methodology was exemplified with the formal synthesis of (R)-lorcaserin, OTS514, and enantiomerically enriched 3-methyl indolines.
4 Asymmetric Ir-Catalyzed Hydrogenation of Imines
The asymmetric hydrogenation of imines is a high-atom economical way to prepare chiral amines. Despite all the advances made, their hydrogenation still remains a challenging task, and most of the reported catalysts present low reactivity and enantioselectivity, harsh reaction conditions, and narrow substrate scope. The reason is probably due to instability of certain imines; coordination of substrates, which can take place through both the nitrogen donor atom and the double bond; and E/Z imine interconversion in the case of acyclic imines [212, 213]. The first catalysts developed for this reaction were based mostly on Rh-diphosphine catalysts, and they showed only moderate enantioselectivities (60–70%) [214]. Later, some Ir-, Ru-, and Ti-catalysts also appeared improving enantioselectivities. Among the early applications, it should be highlighted the iridium-Xyliphos catalyst, which led to the large-scale production of the amine herbicide (S)-metolachlor (Fig. 32) [215]. Later Pfaltz’s PHOX ligand (L1) [42] and Zhang’s (S,S)-f-binaphane ligand [216] also exhibited excellent results for catalytic enantioselective hydrogenation of imines. This inspired several groups to explore the asymmetric hydrogenation of imines, and to date, Ir-complexes are among the most efficient catalysts for this transformation [212, 213].
Both cyclic and acyclic imines provide useful chiral amines, but usually, specific catalysts are required for each type of substrates. The reason is that acyclic imines might exist as Z/E mixtures, while cyclic imines usually have a fixed configuration imposed by the cycle. For the asymmetric hydrogenation of acyclic imines, in general, two types of iridium precursors are used, neutral and cationic complexes. Neutral complexes are generated from [{Ir(μ-Cl)(cod)}2] precursor and are usually combined with diphosphine, monophosphoramidite, or phosphoramidite-N ligands. Some additives are also usually added, such as I2 and KI. Cationic complexes are prepared from [Ir(cod)2]X catalyst precursor and P-oxazoline ligands. Although cheaper counterions (X−) were studied [217], usually BArF has been the most used counterion.
4.1 Asymmetric Hydrogenation Using Cationic Catalyst Precursors
Phosphine-oxazoline PHOX ligands L1 (Fig. 1) and L8 (Fig. 3) are among the most efficient ligands for the enantioselective hydrogenation of acyclic N-aryl imines. Excellent activities and enantioselectivities (up to 96% ee; Fig. 33) were obtained by using low catalyst loadings (0.1–0.5 mol%) at −20 °C and 5–50 bar hydrogen pressure in the reduction of aryl/alkyl N-aryl ketimines (Fig. 33a) [218]. Other related P,N-ligands, such as L6 (Fig. 3) and L16 (Fig. 6), have also been applied albeit with moderate success [73, 122]. A few years later, the success of PHOX-based catalytic systems was extended to dialkyl ketimines [219]. A key to this improvement was the addition of the appropriate imine as additive. Mechanistic investigation revealed that the active species is a cyclometalated complex 9 (Fig. 33b). Thus, a range of N-aryl dialkyl imines were hydrogenated in high enantioselectivities (up to 92% ee; Fig. 33a). Nevertheless, N-alkyl aliphatic imines were only hydrogenated in moderate ees (up to 77%; Fig. 33a).
Recently, cyclometalated complex 10 containing the P-stereogenic-oxazoline ligand L36 proved to be successful in the asymmetry of a wide range of N-alkyl amines, including the more challenging N-methylated ones (ees up to 94%; Fig. 34) [220]. Interestingly, high ees (up to 96%) were also achieved in the reduction of N-aryl ketimines (Fig. 34) [221].
Cationic iridium complexes based on the already mentioned L9 ligands (Fig. 3) were found to be highly efficient in the hydrogenation of a broad range of aryl alkyl N-benzyl imines (ees up to 93%). Importantly, even higher enantioselectivities were obtained for various exocyclic N-alkyl imines (ees up to 98%) [222]. The protocol was successfully employed in the synthesis of the antidepressant chiral drug sertraline [223]. High enantioselectivities in the reduction of some exocyclic N-aryl-dihydronaphthalene imines were also achieved using P-chiral dihydrobenzooxaphosphole-oxazoline LalithPhos ligand (S)-2-((2R,3R)-3-(tert-butyl)-4-methoxy-2,3-dihydrobenzo[d][1,3]oxaphosphol-2-yl)-4-phenyl-4,5-dihydrooxazole (ees up to 99%) [224].
4.2 Asymmetric Hydrogenation Using Neutral Catalyst Precursors
Neutral catalyst modified with Xyliphos and (R,R)-f-binaphane ligands early proved to be suited for the AH of sterically hindered N-aryl imines [215, 216]. More recently, Hu et al. improved previous results with the use of phosphine-phosphoroamidite ligand L41 (Fig. 35) [225]. A range of sterically hindered N-aryl imines were therefore hydrogenated featuring high ees (up to 99%) and turnover numbers (up to 100,000; Fig. 35). Later, the same group disclosed that the steric effect of substituents on the o-positions of the binaphthyl showed a significant influence on the enantioselectivity and found that L42 (Fig. 35) was also highly enantioselective in the Ir-hydrogenation of sterically hindered N-aryl imines (ees up to 98%) [226]. The utility of this methodology was demonstrated in the synthesis of the chiral herbicide (S)-metolachlor and the chiral fungicide (R)-metalaxyl.
Neutral Ir-catalysts have also shown excellent enantioselectivities in the asymmetric hydrogenation of diarylmethanimines, whose hydrogenation products are found in numerous biologically active compounds of pharmaceutical relevance [227,228,229,230,231,232,233]. In this context, two catalytic systems should be highlighted. Ir/L43 catalytic system provided high enantioselectivities in the reduction of benzophenone N-H iminium salts with one of the aryl groups ortho-substituted (ees up to 98%; Fig. 36a) [234]. More recently, (R,R)-f-SpiroPhos ligand (Fig. 26) allowed the enantioselective hydrogenation of N-alkylester-substituted diarylimines under mild reaction conditions (ees up to >99%; Fig. 36b) [235]. A feature of this catalyst was that the presence of an ortho-substituent in one of the aryl groups was not required to achieve high enantioselectivities.
Hu et al. have also disclosed the Ir-hydrogenation of α-imino esters. A range of optically active α-aryl glycines were synthesized using Ir-L44 catalytic system (ees up to 96%; Fig. 37a) [236]. Very recently a high-throughput experimentation (HTE) at AstraZeneca enabled the identification of highly enantioselective catalytic systems for the hydrogenations of N-alkyl α-aryl ketimines containing a furyl moiety [237]. After an extensive screening of Ru-, Rh-, and Ir- catalysts, Ir-catalytic system bearing the (S,S)-f-binaphane ligand was found to be the most enantioselective (ees up to 90%; Fig. 37b).
Neutral complexes generated from [Ir(I)(COD)Cl]2 and activated by addition of halogen-based oxidants such as iodine are among the most successful systems for the asymmetric hydrogenation of cyclic amines. Among the cyclic imines studied, the hydrogenation of isoquinolines and 3,4-dihydroisoquinolines is the most desired since they provide a straightforward synthetic route toward valuable chiral compounds with a 1,2,3,4-tetrahydroisoquinoline motif, which is present in several natural alkaloids and pharmaceutical molecules. However, the strong coordination ability of isoquinolines and their enhanced stability due to their aromaticity make them less reactive toward hydrogen. In this context, Zhou et al. developed the first example of highly enantioselective hydrogenation of quinoline derivatives with a (R)-MeO-BIPHEP [6,6′-dimethoxy-2,2′-bis(diphenylphosphino)-1,1′-biphenyl] as the ligand [238,239,240]. However, these systems were restricted only to quinolines. Importantly, the protocol was later expanded to isoquinolines, which is a very challenging class of substrates, but stoichiometric amounts of chloroformate as a substrate activator were needed [241]. In 2012, a more efficient Ir(COD)Cl]2/(R)-SynPhos catalytic system was disclosed, which used 1-bromo-3-chloro-5,5-dimethylhydantoin (BDCMH) as a catalyst activator, and therefore, only catalytic amounts of activator were required [242]. A range of chiral 3,4-disubstituted tetrahydroisoquinoline derivatives were obtained with ee values as high as 96% (Fig. 38). The scope of this catalytic system was extended to other aromatic imines, such as polycyclic nitrogen-containing heteroaromatics pyrrolo/indolo[1,2-a]quinoxalines and phenanthridines [243], sulfur-containing dibenzo[b,f][1,4]thiazepines [244], activated N-benzyl-pyridinium bromides [245], and fluorinated isoquinoline derivatives [246] (Fig. 38). Finally, the authors also reported the use of (R)-SynPhos ligand in the deracemization of secondary and tertiary amines with a tetrahydroisoquinoline core [247]. The process consisted in a redox N-bromosuccinimide oxidation of the amines and the subsequent Ir-catalyzed asymmetric hydrogenation. A range of chiral 1-substituted 1,2,3,4-tetrahydroisoquinolines were generated with up to 98% ee in 93% yield.
Other diphosphine-based catalysts have also been successfully used in the asymmetric hydrogenation of aromatic iminium salts. Thus, for instance, a range of 1- and 3-substituted isoquinolinium salts [248], 2,6-disubstituted pyridinium hydrochloride (3·HCl) [249], and pyrrolo[1,2-a]pyrazines [250] were successfully hydrogenated using (S,S,Rax)-C3*-TunePhos ligand (ees up to 96%, Fig. 39). Ir/(R,Sp)-Josiphos catalyst also provided excellent ees in the hydrogenation of a range of pyrazinium salts (ees up to 96%, Fig. 39) [251]. It should be noticed that recently, an efficient catalytic system for the hydrogenation of pyrrolo[1,2-a]pyrazines without the need of formation of the corresponding salts or the addition of any additive has been reported using Ir/(R)-BTFM-Garphos catalyst (ees up to 96%, Fig. 39) [252]. A highly enantioselective hydrogenation of heteroaromatics bearing a hydroxyl group, 3-hydroxypyridinium salts, has been successfully developed using Ir/(S,S)-f-binaphane catalyst, providing a direct access to trans 6-substituted piperidin-3-ols with up to 95% ee [253]. Another interesting example can be found in the successful hydrogenation of iminium salts of N-alkyl tetrahydroisoquinolines (ees up to 96%, Fig. 39) [254] and of N-alkyl-2-arylpyridium salts (ees up to 98%, Fig. 39) [255] using SegPhos-type ligands. Recently, a new strategy has been developed, in which pyridinium and isoquinolinium salts are generated in situ by employing halogenide trichloroisocyanuric acid as a traceless activation reagent. Mechanistic studies indicated that hydrogen halide generated in situ acted as an activator. This method allowed the Ir/(R)-SegPhos-mediated hydrogenation of a range of isoquinolines and pyridines in excellent yields and enantioselectivities (up to 99% ee, Fig. 39) while avoiding tedious steps of installation and removal of the activating groups [256]. Finally, it should be mentioned that not only Ir/diphosphine catalysts are able to catalyze the reduction of iminium salts. Thus, it should be highlighted the recent works of Qu’s group in the use of dihydrobenzooxaphosphole-pyridine ligand MeO-BoQPhos for the asymmetric hydrogenation of 2-alkyl-pyridinium salts including examples containing an α-heteroaryl substituent [257, 258].
Similarly, the successful hydrogenation of 6-membered ring cyclic imines has not been only limited to the use of SynPhos-based system. Thus, for instance, a range of quinoline derivatives have been successfully hydrogenated with diphosphines and monophosphoroamidites. (S,S)-f-Binaphane ligand has been also efficiently used in the reduction of 1-substituted 3,4-dihydroisoquinolines (ees up to >99%) [259]. Similarly, a range of 1-aryl-substituted tetrahydroisoquinolines were obtained in ees of up to >99% and good TON (up to 4,000) using Josiphos-type binaphane ligand [260]. Higher TONs (up to 43,000) were achieved in the reduction of a range of quinolines using both Ir/(R)-Difluorphos [261] and Ir/(R)-P-Phos [262] catalytic systems (Fig. 40). A successful example of the use of monophosphoramidite ligand can be found in the use of (Rax,S,S)-Siphos-pe ligand (Fig. 40) in the hydrogenation of 1-alkyl-dihydroisoquinolines with ees of up to 96%. The usefulness of the latter reaction was demonstrated with the synthesis of the tetracyclic alkaloid (S)-xylopinine in 85% yield and 96% ee [263].
Besides quinoline derivatives, the range of 6-membered ring cyclic amines successfully hydrogenated has been extended. Thus, for instance, Ir-SegPhos catalyst was successfully used in the hydrogenation of 1,4-benzoxazines [264] and quinazolines [265] (ees up to 98%, Fig. 41). The utility of the method was demonstrated with the synthesis of the bioactive compounds Eg5 inhibitor and (−)-SDZ 267-489 in excellent enantioselectivities (>99% and 99% ee, respectively) [265]. Another example can be found in the use of cationic dinuclear iridium(III) chloride catalyst {[IrH((S)-Difluorphos)]2(μ-Cl)3}Cl for the reduction of 2-alkyl and 2-aryl- substituted dihydroquinoxalines [266,267,268] and tosylamido-substituted pyrazines [269] (ees up to 95%, Fig. 41). Ir-catalyst modified with phosphine-phosphite ligand L45 proved to be highly efficient in the asymmetric hydrogenation of benzoxazines, benzoxazinones, benzothiazones, and quinoxalinones (ees up to 99%; Fig. 41) [270, 271].
Most of the literature dealing with the hydrogenation of cyclic imines reports examples on 6-membered ring systems. Examples on the successful hydrogenation of 7-membered cyclic imines are rare. In particular, Ir/C3*-TunePhos catalysts proved to be highly efficient in the asymmetric hydrogenation of substituted dibenzo[b,f][1,4]oxazepines, benzodiazepinones, and benzodiazepines (ees up to 96%, Fig. 42a) [272, 273]. More recently, Zhou et al. reported the asymmetric hydrogenation of 6-substituted 5H-benzo[d]benzofuro[3,2-b]azepines using a BIPHEP-type ligand L46 (ees up to 91%, Fig. 42b) [274]. 2,4-Diaryl-1,5-benzodiazepines and 2,4-diaryl-3H-benzo[b]azepines has been also successfully hydrogenated (ees up to 99% and drs up to >20:1) using aminophosphine-pyridine ligand L47 (Fig. 42c) [275] and a dendritic PHOX derivative [276, 277]. Benzoxazinone derivatives have also been recently reduced using ZhaoPhos ligand (Fig. 27) achieving excellent enantioselectivities (up to 99% ee) [278].
There are very few examples on the asymmetric hydrogenation of 5-membered ring cyclic imines. In this context, the use of iodine-bridged dimeric [{Ir(H)[(S,S)-(f)-binaphane]}2(μ-I)3]I complex catalyzed the asymmetric hydrogenation of a series of 2-aryl-1-pyrrolines in good ees (up to 86%) and high turnover numbers (TONs up to 5,000) [279]. More recently, the use of (R,R)-f-SpiroPhos ligand (Fig. 26) improved enantioselectivities to up to 98% (Fig. 43) [280]. In contrast to Ir/(S,S)-f-binaphane catalyst, the enantioselectivity was only affected when a 2-alkyl substituent was present on the imine instead of an aryl group (ees up to 77%). Moreover, this method was successfully applied to the synthesis of (+)-(6S,10bR)-McN-4612-Z, a potent inhibitor for the uptake of important central neurotransmitters norepinephrine, dopamine, and serotonin into nerve cells. Very recently a bifunctional bisphosphine-thiourea ZhaoPhos ligand (Fig. 27) was successfully applied in the Ir-catalyzed asymmetric hydrogenation of cyclic sulfamidate imines (ees up to 99%; Fig. 43) [281].
5 Ir-Catalyzed Asymmetric Hydrogenation of Ketones
Over the last decades, the Ir-catalyzed asymmetric hydrogenation of ketones has experienced a huge advance. Thus, a range of Ir-catalysts have demonstrated to be highly efficient, achieving excellent enantioselectivities and activities for a range of ketones. Such progress has made Ir-catalysts good alternatives to the most commonly used Ru-catalysts. Most of the key ligands are phosphine-based, although there are some important examples that do not contain a P-donor group.
5.1 P-Donor-Based Ligands
One of the key P-containing ligands was disclosed by Poli’s group [282]. They developed a ferrocenyl-based phosphine-thioether ligand L48 that provided high enantioselectivities (ees up to 99%) for a range of alkyl aryl ketones (Fig. 44), albeit activities (TOFs up to 250 h−1) do not compare well with the current state of the art.
In 2010, Xie and Zhou et al. developed chiral spiro aminophosphine ligands L49 (Fig. 44) [283, 284]. The use of Ir/L49 catalyst provided high ees (up to 97%) and activities (TOFs up to 3.7 × 104 h−1) in the hydrogenation of aryl alkyl ketones and exo-cyclic α,β-unsaturated ketones. Nevertheless, the catalyst deactivates under hydrogenation conditions. Mechanistic investigations indicated that the deactivation was due to the formation of [IrH2(L49)2]+ complex [284].
To prevent the formation of such inactive species, the same group introduced in L49 a pyridine group as a third coordinating position, leading to the tridentate P,N,N spiro pyridine-aminophosphine ligands SpiroPAP (Fig. 43) [285,286,287]. Ir/SpiroPAP (Ar = 3,5-tBu2-C6H3 and R = 3-Me) system proved to be highly efficient in the AH of aryl alkyl ketones and aryl β- and δ-ketoesters (ees up to 99.8% and TONs up to 4.550.000; Fig. 45) [285,286,287]. The excellent performance of SpiroPAP ligands has been further extended to acyclic α,β-unsaturated acyclic ketones [288]; α-, γ-, and δ-keto acids [289, 290]; α-amino ketones [291]; and α,β-unsaturated-α-ethoxy-carbonyl-β-substituted cyclic ketones [292] (Fig. 45). This catalytic system has been also used in the deracemization of α-substituted lactones [293, 294] (Fig. 45) via dynamic kinetic resolution (DKR) as well as in the kinetic resolution of aliphatic alcohols [295]. The synthetic versatility of Ir/SpiroPAP catalyst was demonstrated with the synthesis of drugs and natural products such as (−)-mesembrine, rivastigmine, and (−)-Hamerigan B [288, 296,297,298,299,300].
Xie’s and Zhou’s group developed a P,N,S variant (SpiroSAP; Fig. 44) with a 1,3-dithiane group instead of the pyridine group [301]. Ir/SpiroSAP catalyst proved to be highly efficient in the asymmetric hydrogenation of β-alkyl-β-ketoesters [301] as well as in the dynamic kinetic resolution (DKR) of β-ketolactams [302] (Fig. 46). Ir/SpiroSAP catalyst was also used in the formal total synthesis of (−)-cyanolide A and (−)-doluculine [303, 304]. Recently, a new SpiroPAP variant has been developed by replacing the pyridine group by an oxazoline moiety (SpiroOAP; Fig. 43) [305]. SpiroOAP ligands proved to be efficient in the reduction of α-ketonamides (ees up to 98%).
Yin and Zhang et al. developed a variant of SpiroPAP ligands containing an oxa-spirocyclic ligand L50 (Fig. 44) [306]. Ir/L50 catalyst was successfully used in the asymmetric hydrogenation of Bringmann’s lactones via DKR to yield enantiopure chiral biaryl diols in high ees (up to >99%; Scheme 2).
Dong and Zhang et al. developed novel tridentate ferrocene aminophosphoxazoline ligands (f-Amphox, Fig. 44). Ir/f-Amphox catalyst proved to be highly efficient in the reduction of simple aryl alkyl and dialkyl ketones (ees up to >99% and TONs up to 1,000,000; Fig. 47) [307]. f-Amphox-based catalytic systems were also successfully used in the hydrogenation of α-, β-, γ-, and δ-keto amides [308, 309], α-amino ketones [310], α-hydroxy ketones [311], β-ketoesters [312], styrylglyoxylamides [313], and halohydrins [314] as well as in the deracemization of α-amino β-unfunctionalized ketones via DKR [315] (Fig. 47). Hou’s group also developed a modification in which Uggi’s amine was replaced by phenethylamine [316]. However, only moderate ees were achieved in the reduction of β-ketoesters.
Dong’s and Zhang’s groups developed a P,N,O variant (f-Ampha; Fig. 44) with a chiral carboxylic acid instead of the oxazoline moiety [317]. f-Ampha ligands exhibited excellent catalytic performance for a range of aryl alkyl ketones [317] and α-ketoesters [318] as well as in the desymmetrization of cyclic 1,3-diketones [319]. For these catalytic systems, the hydroxyl group of the carboxylic acid group is involved with the formation of O-H···substrate interaction with a new catalytic bifunctional mode. At the same time, a new ferrocene-based amino-phosphine-alcohol was developed (f-Amphol; Fig. 44) [320]. Ir/f-Amphol catalysts also showed excellent enantioselectivities for simple aryl alkyl ketones [320,321,322], albeit the turnover numbers are lower than for Ir/f-Amphox catalysts. Ir/f-Amphol ligands were also successfully used in the asymmetric hydrogenation of β-keto sulfones [323] and of α-substituted β-ketoesters via DKR [324]. For these catalytic systems, DFT studies indicated that the hydroxyl group of the f-Amphol ligands plays a key role in the reduction process.
Hou’s group developed a sterically hindered ferrocenyl P,N,N-ligands in which the oxazoline in the f-Amphox ligands was replaced by a pyridinylmethyl group with an extra coordinating stereogenic center (ligands L51; Fig. 44) [325]. Ir/L51 (R = 2-tolyl) catalyst demonstrated to be highly efficient in the asymmetric hydrogenation of α-alkyl-substituted β-aryl-β-ketoesters via DKR yielding the corresponding alcohols in high diastereo- and enantioselectivities (drs up to >95/5 and ees up to 99%).
Zhong’s group developed tridentate ferrocene-based diamine-phosphine sulfonamide ligands (f-Diaphos; Fig. 44) [326]. The f-Diaphos ligands provided excellent reactivity and enantioselectivities in the Ir-catalyzed hydrogenation of diaryl and 2-pyridyl aryl ketones (ees up to >99%; TONs up to 19,600) [326, 327].
5.2 Non-P-Donor-Based Ligands
Ir-complexes bearing chiral phosphine-free ligands have also been successfully used in the asymmetric hydrogenation of ketones. Albeit such catalytic systems are able to induce high enantioselectivities, the turnover numbers are still lower than the most active P-based ligands such as SpiroPAP and f-Amphox.
In this context Ohkuma’s group demonstrated that Cp*Ir(III) complex containing MsDPEN ligand L52 (Fig. 48) efficiently hydrogenated α-hydroxy ketones [328]. A range of 1-aryl-1,2-ethanediols were therefore achieved in high ees (up to 99%) with TONs as high as 6,000. Later their high performance was extended to the use of aromatic (hetero)cyclic ketones (e.g., indanones, benzofuranones, chromanones, etc.) achieving an excellent enantiocontrol (ees up to >99%) [329]. At the same time, Ikariya’s group developed a bifunctional triflylamide-tethered CpIr version of [Cp*Ir(OTf)L52], albeit it provided only good ees (up to 93%) in the reduction of benzophenone [330].
Kempe’s group introduced an imidazo[1,5-b]pyridazine-type ligand L53 (Fig. 47) for the Ir-catalyzed hydrogenation of simple ketones [331]. A range of aryl alkyl and aryl aryl ketones were therefore efficiently hydrogenated providing excellent ees (up to >99%) and TONs (up to 200,000) using Ir/L53 catalyst. It should be pointed out that the catalyst precursor [Ir(cod)L53] rapidly evolves under catalytic conditions to the formation of Ir-K catalyst complex 11 in which L53 is coordinated thought the amino-alcohol (Fig. 49). In 2016, Kempe et al. developed the pyridylalkylamine ligands L54 (Fig. 48) [332]. Nevertheless, the efficiency of Ir/L54 catalysts proves to be somewhat lower than Ir/L53 catalyst in the reduction of simple ketones (ees up to 96%).
6 Conclusions
Due to perfect atom economy and simplicity, the metal-catalyzed asymmetric hydrogenation continues to be one of the most sustainable and straightforward reactions for creating stereogenic centers in target molecules. In the asymmetric hydrogenation of unfunctionalized olefins or with poorly coordinative groups, Ir containing heterodonor P,X ligands continues to be the catalysts of choice. Examining the last progress in this field, the substrate scope has been largely extended, with the successful hydrogenation of challenging and relevant substrates such as polyene substrates, with the formation of multiple stereocenters, olefins that contain non-coordinating groups such as halides avoiding the dehalogenation side reaction, and allylsilanes. A lot of progress has also been achieved in the regio- and stereoselective monohydrogenation, as an efficient route for preparing natural products and complex organic molecules (e.g., juvabione). This has been achieved, thanks to the recent development of powerful catalytic systems that have also allowed to advance in the search of a single catalysts able to tolerate a large number of olefin types. However, some limitations have still to be solved such as the necessity to work with pure geometrical isomers (e.g., E- and Z-trisubstituted alkene produced opposite enantiomers of the hydrogenated products). Other demanding substrates are hindered tetrasubstituted olefins, whose hydrogenation can generate two vicinal stereocenters in a single reaction and thus give rise to products of higher stereochemical complexity. The first significant progress has been made in the past 5 years, but the scope of the substrate needs to be further improved, and this area of research is likely to expand in the future as well. The advances of Ir-catalysts in the last 10 years have not only been limited to unfunctionalized olefins, but the development of Ir-catalysts has also notably expanded the field of the asymmetric hydrogenation of unresolved functionalized substrates, providing in some cases complementarity to Rh- and Ru-catalysts and in other cases surpassing the widely used Rh- and Ru-catalysts.
References
Blaser H-U, Federsel H-J (2010) Asymmetric catalysis in industrial scale: challenges, approaches and solutions.2nd edn. Wiley, Weinheim
Shang G, Li W, Zhang X (2000) Ojima I (ed) Catalytic asymmetric synthesis3rd edn. Wiley, Hoboken, p 343
Brown JM (1999) Jacobsen EN, Pfaltz A, Yamamoto H (eds) Comprehensive asymmetric catalysis, vol 1. Springer, Berlin, p 121
Noyori R (1994) Asymmetric catalysis in organic synthesis. Wiley, New York
Cornils B, Herrmann WA (2002) Applied homogeneous catalysis with organometallic compounds.2nd edn. Wiley-VCH, Weinheim
Genêt JP (2008) Andersson PG, Munslow IJ (eds) Modern reduction methods. Wiley-VCH, Weinheim, p 3
Tang W, Zhang X (2003) Chem Rev 103:3029
Kitamura M, Noyori R (2004) Murahashi S-I (ed) Ruthenium in organic synthesis. Wiley-VCH, Weinheim, p 3
Weiner B, Szymański W, Janssen DB, Minnaard AJ, Feringa BL (2010) Chem Soc Rev 39:1656
Cui X, Burgess K (2005) Chem Rev 105:3272
Roseblade S, Pfaltz A (2007) Acc Chem Res 40:1402
Woodmansee DH, Pfaltz A (2011) Chem Commun 47:7912
Zhu Y, Burgess K (2012) Acc Chem Res 45:1623
Verendel JJ, Pàmies O, Diéguez M, Andersson PG (2014) Chem Rev 114:2130
Margarita C, Andersson PG (2017) J Am Chem Soc 139:1346
Knowles WS, Sabacky MJ, Vineyard BD (1972) J Chem Soc Chem Commun 10
Knowles WS (2002) Angew Chem Int Ed 41:1998
Noyori R (2002) Angew Chem Int Ed 41:2008
Shultz CS, Krska SW (2007) Acc Chem Res 40:1320
Blaser HU (2002) Adv Synth Catal 344:17
Pharm DQ, Nogid A (2008) Clin Ther 30:813. (Rotigotine)
Osende JI, Shimbo D, Fuster V, Dubar M, Badimon JJ (2004) J Thromb Haemost 2:492. (Terutroban)
Ross SB, Thorberg SO, Jerning E, Mohell N, Stenfors C, Wallsten C, Milchert IG, Ojteg GA (1999) CNS Drug Rev 5:213. (Robalzotan)
Astier B, Lambás Señas L, Soulière F, Schmitt P, Urbain N, Rentero N, Bert L, Denoroy L, Renaud B, Lesourd M, Muñoz C, Chouvet G (2003) Eur J Pharmacol 459:17. (Alnespirone)
Salom E, Orgué S, Riera A, Verdaguer X (2016) Angew Chem Int Ed 55:7988
Magre M, Pàmies O, Diéguez M (2016) ACS Catal 6:5186
Biosca M, Magre M, Pàmies O, Diéguez M (2018) ACS Catal 8:10316
Renaud JL, Dupau P, Hay AE, Guingouain M, Dixneouf PH, Bruneau C (2003) Adv Synth Catal 345:230
Hoen R, van den Berg M, Bernsmann H, Minnaard AJ, de Vries JG, Feringa BL (2004) Org Lett 6:1433
Jiang XB, Lefort L, Goudriaan PE, de Vries AHM, van Leeuwen PWNM, Reek JNH (2006) Angew Chem Int Ed 45:1223
Sandee AJ, van der Burg AM, Reek JNH (2007) Chem Commun:864
Revés M, Ferrer C, León T, Doran S, Etayo P, Vidal-Ferran A, Riera A, Verdaguer X (2010) Angew Chem Int Ed 49:9452
Wu Z, Ayad T, Ratovelomanana-Vidal V (2011) Org Lett 13:3782
Pignataro L, Boghi M, Civera M, Carboni S, Piarulli U, Gennari C (2012) Chem Eur J 18:1383
Frank DJ, Franzke A, Pfaltz A (2013) Chem Eur J 19:2405
Bravo MJ, Ceder RM, Muller G, Rocamora M (2013) Organometallics 32:2632
Arribas I, Rubio M, Kleman P, Pizzano A (2013) J Org Chem 78:3997
Liu G, Liu X, Cai Z, Jiao G, Xu G, Tang W (2013) Angew Chem Int Ed 52:4235
Ohta T, Ikegami H, Miyake T, Takaya H (1995) J Organomet Chem 502:169
Schmid R, Broger EA, Cereghetti M, Crameri Y, Foricher J, Lalonde M, Müller RK, Scalone M, Schoettel G, Zutter U (1996) Pure Appl Chem 68:131
Forman GS, Ohkuma T, Hems WP, Noyori R (2000) Tetrahedron Lett 41:9471
Schnider P, Koch G, Prétôt R, Wang G, Bohnen FM, Krüger C, Pfaltz A (1997) Chem Eur J 3:887
Lightfoot A, Schnider P, Pfaltz A (1998) Angew Chem Int Ed 37:2897
Hilgraf R, Pfaltz A (1999) Synlett:1814
Blackmond DG, Lightfoot A, Pfaltz A, Rosner T, Schnider P, Zimmermann N (2000) Chirality 12:442
Bernardinelli GH, Kündig EP, Meier P, Pfaltz A, Radkowski K, Zimmermann N, Neuburger-Zehnder M (2001) Helv Chim Acta 84:3233
Blankenstein J, Pfaltz A (2001) Angew Chem Int Ed 40:4445
Menges F, Neuburger M, Pfaltz A (2002) Org Lett 4:4713
Menges F, Pfaltz A (2002) Adv Synth Catal 344:40
Smidt SP, Menges F, Pfaltz A (2004) Org Lett 6:2023
Hilgraf R, Pfaltz A (2005) Adv Synth Catal 347:61
Schönleber M, Hilgraf R, Pfaltz A (2008) Adv Synth Catal 350:2033
Drury WJ, Zimmermann N, Keenan M, Hayashi M, Kaiser S, Goddard R, Pfaltz A (2004) Angew Chem Int Ed 43:70
Bell S, Wüstenberg B, Kaiser S, Menges F, Netscher T, Pfaltz A (2006) Science 311:642
Woodmansee DH, Müller MA, Neuburger M, Pfaltz A (2010) Chem Sci 1:72
Woodmansee DH, Müller MA, Tröndlin L, Hörmann E, Pfaltz A (2012) Chem Eur J 18:13780
Kaiser S, Smidt SP, Pfaltz A (2006) Angew Chem Int Ed 45:5194
Ganič A, Pfaltz A (2012) Chem Eur J 18:6724
Schrems MG, Neumann E, Pfaltz A (2007) Angew Chem Int Ed 46:8274
Biosca M, Pàmies O, Diéguez M (2020) Cat Sci Technol 10:613
Pàmies O, Magre M, Diéguez M (2016) Chem Rec 16:1578
Brandt P, Hedberg C, Andersson PG (2003) Chem Eur J 9:339
Fan Y, Cui X, Burgess K, Hall MB (2004) J Am Chem Soc 126:16688
Cui X, Fan Y, Hall MB, Burgess K (2005) Chem Eur J 11:6859
Church TL, Rasmussen T, Andersson PG (2010) Organometallics 29:6769
Hopmann KH, Bayer A (2011) Organometallics 30:2483
Mazuela J, Norrby PO, Andersson PG, Pàmies O, Diéguez M (2011) J Am Chem Soc 133:13634
Gruber S, Pfaltz A (2014) Angew Chem Int Ed 53:1896
Schrems MG, Pfaltz A (2009) Chem Commun:6210
Hou DR, Reibenspies J, Colacot TJ, Burgess K (2001) Chem Eur J 7:5391
Liu D, Tang W, Zhang X (2004) Org Lett 6:513
Cozzi PG, Menges F, Kaiser S (2003) Synlett:833
Lu WJ, Chen YW, Hou XL (2010) Adv Synth Catal 352:103
Lu WJ, Chen YW, Hou XL (2008) Angew Chem Int Ed 47:10133
Li X, Li Q, Wu X, Gao Y, Xu D, Kong L (2007) Tetrahedron Asymmetry 18:629
Shang J, Han Z, Li Y, Wang Z, Ding K (2012) Chem Commun 48:5172
Wang X, Han Z, Wang Z, Ding K (2012) Angew Chem Int Ed 51:936
Wang Q, Zhang Z, Chen C, Yang H, Han Z, Dong XQ, Zhang X (2017) Org Chem Front 4:627
Meng K, Xia J, Wang Y, Zhang X, Yang G, Zhang W (2017) Org Chem Front 4:1601
Cozzi PG, Zimmermann N, Hilgraf R, Schäffner S, Pfaltz A (2001) Adv Synth Catal 343:450
Xu G, Gilbertson SR (2003) Tetrahedron Lett 44:953
Trifonova A, Diesen JS, Andersson PG (2006) Chem Eur J 12:2318
Chakka SK, Peters BK, Andersson PG, Maguire GEM, Kruger HG, Govender T (2010) Tetrahedron Asymmetry 21:2295
Cheruku P, Diesen J, Andersson PG (2008) J Am Chem Soc 130:5595
Cheruku P, Gohil S, Andersson PG (2007) Org Lett 9:1659
Källström K, Munslow IJ, Hedberg C, Andersson PG (2006) Adv Synth Catal 348:2575
Engman M, Diesen JS, Paptchikhine A, Andersson PG (2007) J Am Chem Soc 129:4536
Paptchikhine A, Cheruku P, Engman M, Andersson PG (2009) Chem Commun:5996
Verendel JJ, Li JQ, Quan X, Peters B, Zhou T, Gautun OR, Govender T, Andersson PG (2012) Chem Eur J 18:6507
Li JQ, Liu J, Krajangsri S, Chumnanvej N, Singh T, Andersson PG (2016) ACS Catal 6:8342
Zheng J, Jongcharoenkamol J, Peters BBC, Guhl J, Ponra S, Ahlquist MSG, Andersson PG (2019) Nat Catal 2:1093
McIntyre S, Hörmann E, Menges F, Smidt SP, Pfaltz A (2005) Adv Synth Catal 347:282
Baeza A, Pfaltz A (2009) Chem Eur J 15:2266
Müller MA, Pfaltz A (2014) Angew Chem Int Ed 53:8668
Maurer F, Huch V, Ullrich A, Kazmaier U (2012) J Org Chem 77:5139
Verevkin S, Preetz A, Börner A (2007) Angew Chem Int Ed 46:5971
Verevkin SP, Emelyanenko VN, Bayardon J, Schäffner B, Baumann W, Börner A (2011) Ind Eng Chem Res 51:126
van Leeuwen PWNM, Kamer PCJ, Claver C, Pàmies O, Diéguez M (2011) Chem Rev 111:2077
Diéguez M, Pàmies O (2010) Acc Chem Res 43:312
Diéguez M, Pàmies O (2012) Isr J Chem 52:572
Diéguez M, Mazuela J, Pàmies O, Verendel JJ, Andersson PG (2008) J Am Chem Soc 130:7208
Mazuela J, Pàmies O, Diéguez M (2013) Eur J Inorg Chem:2139
Rovner ES, Wein A (2002) J Eur Urol 41:6
Wefer J, Truss MC, Jonas U (2001) World J Urol 19:312
Hills CJ, Winter SA, Balfour JA (1998) Drugs 55:813
McRae AL, Brady KT (2001) Expert Opin Pharmacother 2:883
Mazuela J, Verendel JJ, Coll M, Schäffner B, Börner A, Andersson PG, Pàmies O, Diéguez M (2009) J Am Chem Soc 131:12344
Krajangsri S, Wu H, Liu J, Rabten W, Singh T, Andersson PG (2019) Chem Sci 10:3649
Diéguez M, Mazuela J, Pàmies O, Verendel JJ, Andersson PG (2008) Chem Commun:3888
Biosca M, Magre M, Coll M, Pàmies O, Diéguez M (2017) Adv Synth Catal 359:2801
Bunlaksananusorn T, Polborn K, Knochel P (2003) Angew Chem Int Ed 42:3941
Liu QB, Zhou YG (2007) Tetrahedron Lett 48:2101
Zalubovskis R, Hörmann E, Pfaltz A, Moberg C (2008) ARKIVOC 14:58
Wang A, Fraga RPA, Hörmann E, Pfaltz A (2011) Chem Asian J 6:599
Liu QB, Yu CB, Zhou YG (2006) Tetrahedron Lett 47:4733
Netscher T (1996) Chimia 50:563
Verendel JJ, Andersson PG (2007) Dalton Trans:5603
Meng X, Li X, Xu D (2009) Tetrahedron Asymmetry 20:1402
Chelucci G, Marchetti M, Malkov AV, Friscourt F, Swarbrick ME, Kočovský P (2011) Tetrahedron 67:5421
Li X, Kong L, Gao Y, Wang X (2007) Tetrahedron Lett 48:3915
Han Z, Wang Z, Zhang X, Ding K (2010) Tetrahedron Asymmetry 21:1529
Mazuela J, Pàmies O, Diéguez M (2013) Adv Synth Catal 355:2569
Margalef J, Lega M, Ruffo F, Pàmies O, Diéguez M (2012) Tetrahedron Asymmetry 23:945
Biosca M, Pàmies O, Diéguez M (2019) J Org Chem 84:8259
Tolstoy P, Engman M, Paptchikhine A, Bergquist J, Church TL, Leung AWM, Andersson PG (2009) J Am Chem Soc 131:8855
Kaukoranta P, Engman M, Hedberg C, Bergquist J, Andersson PG (2008) Adv Synth Catal 350:1168
Paptchikhine A, Itto K, Andersson PG (2011) Chem Commun 47:3989
Rabten W, Margarita C, Eriksson L, Andersson PG (2018) Chem Eur J 24:1681
Peters BK, Liu J, Margarita C, Rabten W, Kerdphon S, Orebom A, Morsch T, Andersson PG (2016) J Am Chem Soc 138:11930
Ponra S, Yang J, Kerdphon S, Andersson PG (2019) Angew Chem Int Ed 58:9282
Zhou T, Peters B, Maldonado MF, Govender T, Andersson PG (2012) J Am Chem Soc 134:13592
Peters BK, Zhou T, Rujirawanich J, Cadu A, Singh T, Rabten W, Kerdphon S, Andersson PG (2014) J Am Chem Soc 136:16557
Liu J, Krajangsri S, Yang J, Li JQ, Andersson PG (2018) Nat Catal 1:438
Liu J, Krajangsri S, Singh T, De Seriis G, Chumnanvej N, Wu H, Andersson PG (2017) J Am Chem Soc 139:14470
Zheng J, Margarita C, Krajangsri S, Andersson PG (2018) Org Lett 20:5676
Mazuela J, Pàmies O, Diéguez M (2013) ChemCatChem 5:2410
Powell MT, Hou DR, Perry MC, Cui X, Burgess K (2001) J Am Chem Soc 123:8878
Perry MC, Cui X, Powell MT, Hou DR, Reibenspies JH, Burgess K (2003) J Am Chem Soc 125:113
Bolm C, Focken T, Raabe G (2003) Tetrahedron Asymmetry 14:1733
Källström K, Andersson PG (2006) Tetrahedron Lett 47:7477
Nanchen S, Pfaltz A (2006) Chem Eur J 12:4550
Chen D, Banphavichit V, Reibenspies J, Burgess K (2007) Organometallics 26:855
Khumsubdee S, Fan Y, Burgess K (2013) J Org Chem 78:9969
Schumacher A, Bernasconi M, Pfaltz A (2013) Angew Chem Int Ed 52:7422
Rageot D, Woodmansee DH, Pugin B, Pfaltz A (2011) Angew Chem Int Ed 50:9598
Rageot D, Pfaltz A (2012) Helv Chim Acta 95:2176
Elías-Rodríguez P, Borràs C, Carmona AT, Faiges J, Robina I, Pàmies O, Diéguez M (2018) More recently another example of P,O ligand has been reported with successful application in the Ir-catalyzed asymmetric hydrogenation of unfunctionalized olefins. ChemCatChem 10:5414
Coll M, Pàmies O, Diéguez M (2011) Chem Commun 47:9215
Coll M, Pàmies O, Diéguez M (2013) Adv Synth Catal 355:143
Borràs C, Biosca M, Pàmies O, Diéguez M (2015) Organometallics 34:5321
Margalef J, Borràs C, Alegre S, Alberico E, Pàmies O, Diéguez M (2019) ChemCatChem 11:2142
Biosca M, Coll M, Lagarde F, Brémond E, Routaboul L, Manoury E, Pàmies O, Poli R, Diéguez M (2016) Tetrahedron 72:2623
Margalef J, Caldentey X, Karlsson EA, Coll M, Mazuela J, Pàmies O, Diéguez M, Pericàs MA (2014) Chem Eur J 20:12201
Kraft S, Ryan K, Kargbo RB (2017) J Am Chem Soc 139:11630
Troutman MV, Appella DH, Buchwald SL (1999) J Am Chem Soc 121:4916
Zhang Z, Wang J, Li J, Yang F, Liu G, Tang W, He W, Fu JJ, Shen YH, Li A, Zhang WD (2017) J Am Chem Soc 139:5558
Busacca CA, Qu B, Grět N, Fandrick KR, Saha AK, Marsini M, Reeves D, Haddad N, Eriksson M, Wu JP, Grinberg N, Lee H, Li Z, Lu B, Chen D, Hong Y, Ma S, Senanayake CH (2013) Adv Synth Catal 355:1455
Ponra S, Rabten W, Yang J, Wu H, Kerdphon S, Andersson PG (2018) J Am Chem Soc 140:13878
Biosca M, Salomó E, de la Cruz-Sánchez P, Riera A, Verdaguer X, Pàmies O, Diéguez M (2019) Org Lett 21:807
Bigler R, Mack KA, Shen J, Tosatti P, Han C, Bachmann S, Zhang H, Scalone M, Pfaltz A, Denmark SE, Hildbrand S, Gosselin F (2020) Angew Chem Int Ed 59:2844
Shen TY (1972) Angew Chem Int Ed Engl 11:460
Lednicer D, Mitscher LA (1977) The organic chemistry of drug synthesis. Wiley, New York
Lu Y, Nguyen TMD, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PWJ (2001) Med Chem 44:3048
Churcher I, Ashton K, Butcher JW, Clarke EE, Harrison T, Lewis HD, Owens AP, Teall MR, Williams S, Wrigley JDJ (2003) Bioorg Med Chem Lett 13:179
Henke BR (2004) J Med Chem 47:4118
Kasuga J, Makishima M, Hashimoto Y, Miyachi H (2006) Bioorg Med Chem Lett 16:554
Ono N, The Nitro Group (2001) Organic synthesis. Wiley-VCH, New York
Ballini R, Petrini M (2004) Tetrahedron 60:1017
Scrivanti A, Bovo S, Ciappa A, Matteoli U (2006) Tetrahedron Lett 47:9261
Zhou J, Ogle JW, Fan Y, Banphavichit V, Zhu Y, Burgess K (2007) Chem Eur J 13:7162
Smidt SP, Pfaltz A, Martínez-Viviente E, Pregosin PS, Albinati A (2003) Organometallics 22:1000
Zhu SF, Xie JB, Zhang YZ, Li S, Zhou QL (2006) SIPHOX ligands were developed for Ir-catalyzed asymmetric imine hydrogenation. J Am Chem Soc 128:12886
Li S, Zhu SF, Zhang CM, Song S, Zhou QL (2008) J Am Chem Soc 130:8584
Li S, Zhu SF, Xie JH, Song S, Zhang CM, Zhou QL (2010) J Am Chem Soc 132:1172
Zhu SF, Zhou QL (2017) Acc Chem Res 50:988
Yang S, Che W, Wu HL, Zhu SF, Zhou QL (2017) Chem Sci 8:1977
Yang S, Zhu SF, Zhang CM, Song S, Yu YB, Li S, Zhou QL (2012) Tetrahedron 68:5172
Li ZY, Song S, Zhu SF, Guo N, Wang LX, Zhou QL (2014) Chin J Chem 32:783
Song S, Zhu SF, Pu LY, Zhou QL (2013) Angew Chem Int Ed 52:6072
Song S, Zhu SF, Li Y, Zhou QL (2013) Org Lett 15:3722
Song S, Zhu SF, Yang S, Li S, Zhou QL (2012) Angew Chem Int Ed 51:2708
Song S, Zhu SF, Yu YB, Zhou QL (2013) Angew Chem Int Ed 52:1556
Yang S, Zhu SF, Guo N, Song S, Zhou QL (2014) Org Biomol Chem 12:2049
Zhu SF, Yu YB, Li S, Wang LX, Zhou QL (2012) Angew Chem Int Ed 51:8872
Li ML, Yang S, Su XC, Wu HL, Yang LL, Zhu SF, Zhou QL (2017) J Am Chem Soc 139:541
Zhang Y, Han Z, Li F, Ding K, Zhang A (2010) Chem Commun 46:156
Xu L, Zhaobin H, Zheng W, Kuiling D (2014) Acta Chim Sin 72:849
Li S, Huang K, Cao B, Zhang J, Wu W, Zhang X (2012) Angew Chem Int Ed 51:8573
Li S, Huang K, Zhang J, Wu W, Zhang X (2013) Chem Eur J 19:10840
Zhao Q, Li S, Huang K, Wang R, Zhang X (2013) Org Lett 15:4014
Yan Q, Liu M, Kong D, Zi G, Hou G (2014) Chem Commun 50:12870
Liu M, Kong D, Li M, Zi G, Hou G (2015) Adv Synth Catal 357:3875
Yu YB, Cheng L, Li YP, Fu Y, Zhu SF, Zhou QL (2016) Chem Commun 52:4812
Li S, Xiao T, Li D, Zhang X (2015) Org Lett 17:3782
Xie JH, Zhu SF, Zhou QL (2012) Chem Soc Rev 41:4126
Maire P, Deblon S, Breher F, Geier J, Böhler C, Rüegger H, Schönberg H, Grützmacher H (2004) Chem Eur J 10:4198
Giacomina F, Meetsma A, Panella L, Lefort L, de Vries AHM, de Vries JG (2007) Angew Chem Int Ed 46:1497
Enthaler S, Erre G, Junge K, Schröder K, Addis D, Michalik D, Hapke M, Redkin D, Beller M (2008) Eur J Org Chem:3352
Erre G, Enthaler S, Junge K, Addis D, Beller M (2009) Adv Synth Catal 351:1437
Hou G, Li W, Ma M, Zhang X, Zhang X (2010) J Am Chem Soc 132:12844
Busscher GF, Lefort L, Cremers JGO, Mottinelli M, Wiertz RW, de Lange B, Okamura Y, Yusa Y, Matsumura K, Shimizu H, de Vries JG, de Vries AHM (2010) Tetrahedron Asymmetry 21:1709
Han Z, Guan YQ, Liu G, Wang R, Yin X, Zhao Q, Cong H, Dong XQ, Zhang X (2018) Org Lett 20:6349
Patureau FW, Worch C, Siegler MA, Spek AL, Bolm C, Reek JNH (2012) Adv Synth Catal 354:59
Margalef J, Pàmies O, Diéguez M (2017) Chem Eur J 23:813
Cabré A, Verdaguer X, Riera A (2019) Adv Synth Catal 361:4196
Cabré A, Romagnoli E, Martínez-Balart P, Verdaguer X, Riera A (2019) Org Lett 21:9709
Lazarevski G, Kobrehel G, Metelko B, Duddeck H (1996) J Antibiot 49:1066
Nicholas GM, Molinski TF (2000) Tetrahedron 56:2921
Davies HML, Ni A (2006) Chem Commun:3110
Harris RN, Stabler RS, Repke DB, Kress JM, Walker KA, Martin RS, Brothers JM, Ilnicka M, Lee SW, Mirzadegan T (2010) Bioorg Med Chem Lett 20:3436
Ramesh P, Suman D (2018) Synthesis 50:211
Fleury-Brégeot N, de la Fuente V, Castillón S (2010) ChemCatChem 2:1346
Xie JH, Zhu SF, Zhou QL (2011) Chem Rev 111:1713
James BR (1997) Catal Today 37:209
Blaser HU, Buser HP, Loers K, Hanreich R, Jalett HP, Jelsch E, Pugin B, Schneider HD, Spindler F, Wagmann A (1999) Chimia 53:275
Xiao D, Zhang X (2001) Angew Chem Int Ed 40:3425
Mršić N, Panella L, Ijpeij EJ, Minnaard AJ, Feringa BL, De Vries JG (2011) ChemCatChem 3:1139
Baeza A, Pfaltz A (2010) Chem Eur J 16:4003
Schramm Y, Barrios-Landeros F, Pfaltz A (2013) Chem Sci 4:2760
Salomó E, Gallen A, Sciortino G, Ujaque G, Grabulosa A, Lledós A, Riera A, Verdaguer X (2018) J Am Chem Soc 140:16967
Salomó E, Rojo P, Hernández-Lladó P, Riera A, Verdaguer X (2018) J Org Chem 83:4618
Han Z, Wang Z, Zhang X, Ding K (2009) Angew Chem Int Ed 48:5345
Welch WM, Kraska AR, Sarges R, Koe BK (1984) J Med Chem 27:1508
Qu B, Samankumara LP, Ma S, Fandrick KR, Desrosiers JN, Rodriguez S, Li Z, Haddad N, Han ZS, McKellop K, Pennino S, Grinberg N, Gonnella NC, Song JJ, Senanayake CH (2014) Angew Chem Int Ed 53:14428
Hou CJ, Wang YH, Zheng Z, Xu J, Hu XP (2012) Org Lett 14:3554
Li Q, Hou CJ, Liu XN, Huang DZ, Liu YJ, Yanga RF, Hu XP (2015) RSC Adv 5:13702
Bishop MJ, McNutt RW (1995) Bioorg Med Chem Lett 5:1311
Spencer CM, Foulds D, Peters DH (1993) Drugs 46:1055
Sakurai S, Ogawa N, Suzuki T, Kato K, Ohashi T, Yasuda S, Kato H, Ito Y (1996) Chem Pharm Bull 44:765
Tulshian D, Ho GD, Silverman L, Matasi JJ, McLeod RL, Hey JA, Chapman RW, Bercovici A, Cuss FM, WO 01/07050 A1 (Schering-Plough)
Jolidon S, Narquizian R, Pinard E. WO 2008/022938 A1 (Hoffman-LaRoche)
Ducray P, Cavaliero T, Lorhmann M, Bouvier J WO 2008/062006 A1 (Novartis)
Baker RK, Hale JJ, Maio S, Rupprecht KM. WO 2007/062193 A1 (Merck). Cetirizine HCl, a istamine H1-receptor inverse agonist is commercialized as Zyrtec/Reactine by Pfizer
Hou G, Tao R, Sun Y, Zhang X, Gosselin F (2010) J Am Chem Soc 132:2124
Kong D, Li M, Zi G, Hou G, He Y (2016) J Org Chem 81:6640
Hu XH, Hu XP (2019) Adv Synth Catal 361:5063
Mazuela J, Antonsson T, Knerr L, Marsden SP, Munday RH, Johansson MJ (2019) Adv Synth Catal 361:578
Wang WB, Lu SM, Yang PY, Han XW, Zhou YG (2003) J Am Chem Soc 125:10536
Yang PY, Zhou YG (2004) Tetrahedron Asymmetry 15:1145
Wang DW, Wang XB, Wang DS, Lu SM, Zhou YG, Li YX (2009) J Org Chem 74:2780. Later, it was shown that an electronically deficient ligand synthesized from (S)-MeO-BiPhep showed an increase of the activity and enantioselectivity: Zhang DY, Wang DS, Wang MC, Yu CB, Gao K, Zhou YG (2011) Synthesis 2796
Lu SM, Wang YQ, Han XW, Zhou YG (2006) Angew Chem Int Ed 45:2260
Shi L, Ye ZS, Cao LL, Guo RN, Hu Y, Zhou YG (2012) Angew Chem Int Ed 51:8286
Hu SB, Zhai XY, Shen HQ, Zhou YG (2018) Adv Synth Catal 360:1334
Guo RN, Gao K, Ye ZS, Shi L, Li Y, Zhou YG (2013) Pure Appl Chem 85:843
Ye ZS, Chen MW, Chen QA, Shi L, Duan Y, Zhou YG (2012) Angew Chem Int Ed 51:10181
Guo RN, Cai XF, Shi L, Ye ZS, Chen MW, Zhou YG (2013) Chem Commun 49:8537
Ji Y, Shi L, Chen MW, Feng GS, Zhou YG (2015) J Am Chem Soc 137:10496
Ye ZS, Guo RN, Cai XF, Chen MW, Shi L, Zhou YG (2013) Angew Chem Int Ed 52:3685
Chen MW, Ye ZS, Chen ZP, Wu B, Zhou YG (2015) Org Chem Front 2:586
Huang WX, Yu CB, Shi L, Zhou YG (2014) Org Lett 16:3324
Huang WX, Liu LJ, Wu B, Feng GS, Wang B, Zhou YG (2016) Org Lett 18:3082
Hu SB, Chen ZP, Song B, Wang J, Zhou YG (2017) Adv Synth Catal 359:2762
Huang WX, Yu CB, Ji Y, Liu LJ, Zhou YG (2016) ACS Catal 6:2368
Ji Y, Feng GS, Chen MW, Shi L, Duc H, Zhou YG (2017) Org Chem Front 4:1125
Chang M, Huang Y, Liu S, Chen Y, Krska SW, Davies IW, Zhang X (2014) Angew Chem Int Ed 53:12761
Chen MW, Ji Y, Wang J, Chen QA, Shi L, Zhou YG (2017) Org Lett 19:4988
Qu B, Mangunuru HPR, Wei X, Fandrick KR, Desrosiers JN, Sieber JD, Kurouski D, Haddad N, Samankumara LP, Lee H, Savoie J, Ma S, Grinberg N, Sarvestani M, Yee NK, Song JJ, Senanayake CH (2016) Org Lett 18:4920
Qu B, Mangunuru HPR, Tcyrulnikov S, Rivalti D, Zatolochnaya OV, Kurouski D, Radomkit S, Biswas S, Karyakarte S, Fandrick KR, Sieber JD, Rodriguez S, Desrosiers JN, Haddad N, McKellop K, Pennino S, Lee H, Yee NK, Song JJ, Kozlowski MC, Senanayake CH (2018) Org Lett 20:1333
Chang M, Li W, Zhang X (2011) Angew Chem Int Ed 50:10679
Nie H, Zhu Y, Hu X, Wei Z, Yao L, Zhou G, Wang P, Jiang R, Zhang S (2019) Org Lett 21:8641
Tang W, Sun Y, Xu L, Wang T, Fan O, Lamc KH, Chan ASC (2010) Org Biomol Chem 8:3464
Tang WJ, Tan J, Xu LJ, Lam KH, Fan QH, Chan ASC (2010) Adv Synth Catal 352:1055
Xie JH, Yan PC, Zhang QQ, Yuan KX, Zhou QL (2012) ACS Catal 2:561
Gao K, Yu CB, Wang DS, Zhou YG (2012) Adv Synth Catal 354:483
Feng GS, Zhao ZB, Shi L, Zhou YG (2019) Org Chem Front 6:2250
Cartigny D, Nagano T, Ayad T, Genêt JP, Ohshima T, Mashima K, Ratovelomanana-Vidal V (2010) Adv Synth Catal 352:1886
Cartigny D, Berhal F, Nagano T, Phansavath P, Ayad T, Genêt JP, Ohshima T, Mashima K, Ratovelomanana-Vidal V (2012) J Org Chem 77:4544
Nagano T, Iimuro A, Schwenk R, Ohshima T, Kita Y, Togni A, Mashima K (2012) Chem Eur J 18:11578
Higashida K, Nagae H, Mashima K (2016) Adv Synth Catal 358:3949
Núñez-Rico JL, Fernández-Pérez H, Benet-Buchholz J, Vidal-Ferran A (2010) Organometallics 29:6627
Núñez-Rico JL, Vidal-Ferran A (2013) Org Lett 15:2066
Gao K, Yu CB, Li W, Zhou YG, Zhang X (2011) Chem Commun 47:7845
Gao K, Wu B, Yu CB, Chen QA, Ye ZS, Zhou YG (2012) Org Lett 14:3890
Shen HQ, Gao X, Liu C, Hu SB, Zhou YG (2016) Org Lett 18:5920
Liu Y, Chen F, He YM, Lia C, Fan QH (2019) Org Biomol Chem 17:5099
Ma B, Ding Z, Liu J, He Y, Fan QH (2013) Chem Asian J 8:1101
Miao T, Ma B, Ding Z, Liu Y, He YM, Fan QH (2017) Asian J Org Chem 6:1219
Han Z, Liu C, Wang R, Dong XQ, Zhang X (2019) Chem Sci 10:4328
Chang M, Li W, Hou G, Zhang X (2010) Adv Synth Catal 352:3121
Zhang Y, Kong D, Wang R, Hou G (2017) Org Biomol Chem 15:3006
Liu Y, Huang Y, Yi Z, Liu G, Dong XQ, Zhang X (2019) Adv Synth Catal 361:1582
Roux EL, Malacea R, Manoury E, Poli R, Gonsalvi L, Peruzzini M (2007) Adv Synth Catal 349:309
Xie JB, Xie JH, Liu XY, Kong WL, Li S, Zhou QL (2010) J Am Chem Soc 132:4538
Xie JB, Xie JH, Liu XY, Zhang QQ, Zhou QL (2011) Chem Asian J 6:899
Xie JH, Liu XY, Xie JB, Wang LX, Zhou QL (2011) Angew Chem Int Ed 50:7329
Xie JH, Liu XY, Yang XH, Xie JB, Wang LX, Zhou QL (2012) Angew Chem Int Ed 51:201
Yang XH, Xie JH, Liu WP, Zhou QL (2013) Angew Chem Int Ed 52:7833
Zhang QQ, Xie JH, Yang XH, Xie JB, Zhou QL (2012) Org Lett 14:6158
Yan PC, Xie JH, Zhang XD, Chen K, Li YQ, Zhou QL, Che DQ (2014) Chem Commun 50:15897
Hua YY, Bin HY, Wei T, Cheng HA, Lin ZP, Fu XF, Li YQ, Xie JH, Yan PC, Zhou QL (2020) Org Lett 22:818
Yuan ML, Xie JH, Yang XH, Zhou QL (2014) Synthesis 46:2910
Liu YT, Chen JQ, Li LP, Shao XY, Xie JH, Zhou QL (2017) Org Lett 19:3231
Gu XS, Yu N, Yang XH, Zhu AT, Xie JH, Zhou QL (2019) Org Lett 21:4111
Yang XH, Yue HT, Yu N, Li YP, Xie JH, Zhou QL (2017) Chem Sci 8:1811
Yang XH, Wang K, Zhu SF, Xie JH, Zhou QL (2014) J Am Chem Soc 136:17426
Yan PC, Zhu GL, Xie JH, Zhang XD, Zhou QL, Li YQ, Shen WH, Che DQ (2013) Org Process Res Dev 17:307
Lin H, Xiao LJ, Zhou MJ, Yu HM, Xie JH, Zhou QL (2016) Org Lett 18:1434
Zhu GL, Zhang XD, Yang LJ, Xie JH, Che DP, Zhou QL, Yan PC, Li YQ (2016) Org Process Res Dev 20:81
Zuo XD, Guo SM, Yang R, Xie JH, Zhou QL (2017) Chem Sci 8:6202
Bin HY, Wang K, Yang D, Yang XH, Xie JH, Zhou QL (2019) Angew Chem Int Ed 58:1174
Bao DH, Wu HL, Liu CL, Xie JH, Zhou QL (2015) Angew Chem Int Ed 54:8791
Bao DH, Gu XS, Xie JH, Zhou QL (2017) Org Lett 19:118
Che W, Li YZ, Liu JC, Zhu SF, Xie JH, Zhou QL (2019) Org Lett 21:2369
Che W, Wen DC, Zhu SF, Zhou QL (2019) Helv Chim Acta 102:e1900023
Zhang FH, Wang C, Xie JH, Zhou QL (2019) Adv Synth Catal 361:2832
Chen GQ, Lin BJ, Huang JM, Zhao LY, Chen QS, Jia SP, Yin Q, Zhang X (2018) J Am Chem Soc 140:8064
Wu W, Liu S, Duan M, Tan X, Chen C, Xie Y, Lan Y, Dong XQ, Zhang X (2016) Org Lett 18:2938
Gu G, Yang T, Yu O, Qian H, Wang J, Wen J, Dang L, Zhang X (2017) Org Lett 19:5920
Hu Y, Yin X, Chen Z, Dong XQ, Zhang X (2018) Org Chem Front 5:2000
Hu Y, Wu W, Dong XQ, Zhang X (2017) Org Chem Front 4:1499
Wu W, Xie Y, Li P, Li X, Liu Y, Dong XQ, Zhang X (2017) Org Chem Front 4:555
Qin C, Chen XS, Hou CJ, Liu H, Liu YJ, Huang DZ, Hu XP (2018) Synth Comun 48:672
Wang S, Yu Y, Wen J, Zhang X (2018) Synlett 29:2203
Yin C, Wu W, Hu Y, Tan X, You C, Liu Y, Chen Z, Dong XQ, Zhang X (2018) Adv Synth Catal 360:2119
Wu W, You C, Yin C, Liu Y, Dong XQ, Zhang X (2017) Org Lett 19:2548
Wei DQ, Chen XS, Hou CJ, Hu XP (2019) Synth Commun. https://doi.org/10.1080/00397911.2018.1550203
Yu J, Long J, Yang Y, Wu W, Xue P, Chung LW, Dong XQ, Zhang X (2017) Org Lett 19:690
Gu G, Yang T, Lu J, Wen J, Dang J, Zhang X (2018) Org Chem Front 5:1209
Gong Q, Wen J, Zhang X (2019) Chem Sci 10:6350
Yu J, Duan M, Wu W, Qi X, Xue P, Lan Y, Dong XQ, Zhang X (2017) Chem Eur J 23:970
Yin C, Dong XQ, Zhang X (2018) Adv Synth Catal 360:4319
Liang Z, Yang T, Gu G, Dang L, Zhang X (2018) Chin J Chem 36:851
Tao L, Yin C, Dong XQ, Zhang X (2019) Org Biomol Chem 17:785
Gu G, Lu J, Yu O, Wen J, Yin Q, Zhang X (2018) Org Lett 20:1888
Hou CJ, Hu XP (2016) Org Lett 18:5592
Ling F, Nian S, Chen J, Luo W, Wang Z, Lv Y, Zhong W (2018) J Org Chem 83:10749
Nian S, Ling F, Chen J, Wang Z, Shen H, Yi X, Yang YF, She Y, Zhong W (2019) Org Lett 21:5392
Ohkuma T, Utsumi N, Watanabe M, Tsutsumi K, Arai N, Murata K (2007) Org Lett 9:2565
Utsumi N, Tsutsumi K, Watanabe M, Murata K, Arai N, Kurono N, Ohkuma T (2010) Heterocycles 80:141
Ito M, Endo Y, Tejima N, Ikariya T (2010) Organometallics 29:2397
Irrgang T, Friedrich D, Kempe R (2011) Angew Chem Int Ed 50:2183
Kumar P, Irrgang T, Kostakis GE, Kempe R (2016) RSC Adv 6:39335
Acknowledgments
We gratefully acknowledge financial support from the Spanish Ministry of Economy and Competitiveness (CTQ2016-74878-P and PID2019-104904GB-I00), the European Regional Development Fund (AEI/FEDER, UE), the Catalan Government (2017SGR1472), the ICREA Foundation (ICREA Academia award to MD) and from “La Caixa” Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Margalef, J., Pàmies, O., Diéguez, M. (2020). Iridium-Catalyzed Asymmetric Hydrogenation. In: Oro, L.A., Claver, C. (eds) Iridium Catalysts for Organic Reactions. Topics in Organometallic Chemistry, vol 69. Springer, Cham. https://doi.org/10.1007/3418_2020_64
Download citation
DOI: https://doi.org/10.1007/3418_2020_64
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69082-3
Online ISBN: 978-3-030-69083-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)