Abstract
The number of patients with left ventricular (LV) dysfunction due to coronary artery disease is increasing, as more patients now survive with acute myocardial infarction (MI) through primary reperfusion therapy. Severe LV dysfunction after MI, especially in combination with heart failure, is associated with a poor prognosis. Differentiation between reversible and irreversible LV dysfunction is important, as in the first situation, surgical revascularization improves prognosis. In case of reversible LV dysfunction, the myocardium can be stunned or hibernating. These principles are described. Myocardial viability assessment by noninvasive imaging techniques are indicated for this purpose. The different established imaging modalities for myocardial viability assessment are discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Single Photon Emission Compute Tomography
- Late Gadolinium Enhancement
- Left Ventricular Dysfunction
- Myocardial Viability
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Background
The number of patients with congestive heart failure (CHF) related to left ventricular (LV) dysfunction is rising. Also, the incidence of CHF-related mortality has increased considerably in the last decades (Deedwania 2003). An important cause of the rising prevalence of LV dysfunction is the improved ability to treat acute coronary syndrome (ACS), decreasing the initial mortality from ACS. In the developed world, about two-thirds of LV dysfunction cases result from coronary artery disease (CAD) and (chronic) ischemic heart disease (Gheorgiade and Bonow 1998). Following myocardial infarction (MI), a process of infarct expansion and subsequent increase in LV volume can be observed. LV remodeling occurs due to a progressive increase in end-diastolic and end-systolic volumes at an initially maintained ejection fraction. An increase in LV end-diastolic volume of at least 20 % compared to baseline is often used to define infarct remodeling (Bolognese et al. 2002; Savoy et al. 2006). This remodeling can affect the LV systolic function and the patient’s prognosis. The occurrence of severe LV dysfunction after MI, especially if combined with clinical CHF, is associated with a poor prognosis. These patients are at high risk of cardiac death and have high probability of recurrent hospitalizations due to CHF. In addition, they frequently have severe impairment of exercise capacity and daily activities. The estimated annual treatment cost for CHF in the United States is over 10 billion dollars (Abraham and Bristow 1997). In recent years, there have been considerable advances in medical therapy for LV dysfunction and the resulting symptoms of CHF (SOLVD investigators 1991; Pitt et al. 2000; Cohn et al. 1986; Pitt et al. 1999; Colucci et al. 2000; Abraham and Hayes 2003). However, the prognosis for CHF patients remains poor. For example, the Framingham Heart Study showed a 5-year mortality rate of 45 % in women and 59 % in men (Levy et al. 2002). Although medical therapy can be very beneficial, the best therapy in an appropriately selected patient is revascularization. Already by the 1970s, myocardial regions with abnormal wall motion by echocardiography were found to frequently recover function after coronary artery bypass grafting (CABG) (Chatterjee et al. 1973; Rees et al. 1971). Clinical trials have shown improved survival in patients with multivessel CAD and LV dysfunction after revascularization (Rahimtoola 1985).
LV dysfunction is not always the result of irreversible myocardial necrosis and scarring. After an initial ischemic injury, various processes can occur that lead to LV dysfunction, apart from myocyte death. These processes are to a certain extent reversible and include LV remodeling, impairment of energy metabolism, and myocyte dysfunction (Dilsizian 2003). In case of dysfunction due to myocardial necrosis and fibrotic replacement of myocardium, no recovery is to be expected after revascularization. However, if the LV dysfunction is due to myocardium that is jeopardized by ischemic injury but still viable, recovery may be possible. In these patients revascularization can result in better long-term survival, symptomatic improvement, and improved LV function (Baker et al. 1994; Bounous et al. 1988). Patients with LV dysfunction caused by CAD have, however, higher peri-operative risk compared to patients with normal ejection fraction. Therefore, appropriate selection of candidates for surgical revascularization is key: patients with dysfunctional but viable myocardium who are expected to show functional recovery (Blitz and Laks 1996). It is crucial to determine the risk-versus-benefit ratio for the individual patient with LV dysfunction due to CAD (Buckley and Di Carli 2011). Also, apart from viability, clinicians are often interested in whether there is presence of ischemia. If complete revascularization is not an option, detection of ischemia may assist in targeting the coronary artery that is likely to provide the most benefit. Noninvasive imaging of the myocardium provides essential information to derive the best clinical decision.
2 Concepts in Myocardial Viability
Prolonged ischemia of the myocardium triggers a cascade of events including edema and myocardial cell death (mainly necrosis). Infarcted myocytes lose cellular integrity by rupture of cell membranes. Infarcted myocardium does not benefit from revascularization. During the first days after acute coronary occlusion, the infarct volume can almost double in size, even without additional cell death. This is caused by an increase in edema and cellular elements (Reimer and Jennings 1979). Conversely, during the next 4–6 weeks after MI, infarct volume can diminish to about 25 % of its size in the acute phase as necrotic myocytes are replaced by scar tissue. In the months after acute MI, wall thinning of the infarct area and adjacent myocardium can be observed (Ganame et al. 2011). Even after restoring coronary flow in the acute phase, an area of residual myocardial perfusion abnormality may remain, called microvascular obstruction (MVO). The presence of MVO results in a more extensive final infarct size, LV remodeling, and lack of functional recovery (Bogaert et al. 2007), and is related to a worse prognosis (Ito et al. 1996; Wu et al. 1998; Lepper et al. 2000). In chronically infarcted myocardium, the necrotic and apoptotic myocytes have been replaced by collagenous scar tissue. This scar tissue has a small intracellular space and a large effective extracellular volume. Just like necrotic myocardium, scarred myocardium does not regain functionality after revascularization. The size of the final infarct is related to the extent of LV remodeling and LV dysfunction (Lund et al. 2007; Orn et al. 2007).
The outcome of ischemic injury in the typical clinical setting is not clear-cut. Not always does ischemic injury result in infarct and irreversible LV dysfunction. Sometimes the result is partly or completely reversible LV dysfunction. In those cases, revascularization can lead to improved contractile function. In such cases of reversible LV dysfunction, two states of the myocardium are important, myocardial stunning and hibernation.
2.1 Myocardial Stunning
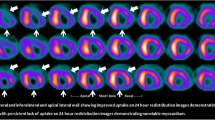
Myocardial stunning refers to reversible contractile dysfunction that can occur in the setting of restored coronary blood supply, after a brief period of impaired coronary perfusion (Heyndrickx et al. 1978; Braunwald and Kloner 1982). In myocardial stunning after reperfused ACS, coronary blood flow has been restored but contractility has not returned to baseline, which means that there is a mismatch in perfusion and contractility. Stunning can also develop after a period of unstable angina or exercise-induced ischemia. Episodes leading to stunning can be single or multiple, brief or prolonged, but by definition are not severe enough to cause myocardial necrosis. Stunned myocardium shows prolonged but transient dysfunction, that can last for hours to weeks (Kloner et al. 1998). In case of myocardial stunning, the myocardium is dysfunctional and viable. In the 1980s, small studies were already investigating myocardial functional recovery after thrombolytic treatment of ACS (plus coronary angioplasty in some cases) (Topol et al. 1985). After revascularization there was no immediate improvement in contractility. However, 10 days after the myocardial infarction, 85 % of reperfused infarct zone segments demonstrated improved wall motion versus 30 % of non-reperfused segments. The exact pathogenesis of myocardial stunning is still unclear. A variety of factors can be involved, including the presence of oxygen-free radicals (Schwaiger and Schricke 2000). In imaging, stunning is visible as a normally perfused, hypokinetic region that shows improvement under dobutamine.
2.2 Myocardial Hibernation
Myocardial hibernation is described as a condition of chronic contractile dysfunction due to severe CAD and chronically reduced rest perfusion (Rahimtoola 1989). The LV dysfunction associated with hibernation may be a protective response of the myocardium to meet the reduced supply of oxygen and substrates. Myocytes affected by this chronic low flow are thought to down-regulate their metabolic needs, and thus their energy demand. This limits cell death by preserving cell membrane integrity and glucose metabolism, but comes at the expense of contractile function (Rahimtoola 1982; Kloner et al. 1998; Baker et al. 1991; Gewirtz et al. 1994; Ragosta et al. 1993; Shavalkar et al. 1996; Dispersyn et al. 1999; Wilson 1999). A new balance in perfusion and contractility is reached (Braunwald and Rutherford 1986). Myocyte function can be restored to normal—partially or completely—if the myocardial oxygen supply–demand relationship is improved, either by increasing blood flow and/or by reducing demand (Rahimtoola 1989). The time course of functional recovery of hibernating myocardium may vary considerably and depends on several factors, such as the severity and duration of myocardial ischemia, the timing and completeness of myocardial revascularization, and the extent of microstructural changes in the dysfunctional myocardium (Vanoverschelde et al. 2000).
Recently, the concept that chronically reduced perfusion of the myocardium results in hibernation has been questioned. Studies have shown that resting blood flow in hibernating myocardium is not decreased to the extent that would account for the degree of contractile dysfunction. Rather, the myocardial perfusion reserve is significantly decreased (Conversano et al. 1996; Vanoverschelde et al. 1993; Marinho et al. 1996). The idea has arisen that stunning and hibernation may not be discrete entities but instead may exist on a continuum. Hibernation is likely a manifestation of chronic stunning due to repetitive, intermittent ischemic episodes (Braunwald and Kloner 1982; Marinho et al. 1996). Observations suggest that hibernation could develop during a time of repetitive stunning, with initial (near-) normal flow but reduced flow reserve, and with decreased resting flow in a later phase (Bax et al. 2003). Over time, microstructural changes in the myocardium can also occur, including changes in structural proteins, metabolism changes to a more fetal form, and apoptosis (Balliga et al. 2000; Vanoverschelde et al. 2000). In imaging, hibernating myocardium is diagnosed by an area of reduced contractility with improvement under low-dose dobutamine, normal or increased metabolism, and decreased perfusion.
3 Clinical Importance of Assessing Myocardial Viability
LV function is a well-established and strong prognostic factor after ACS (Burns et al. 2002). The development of LV systolic dysfunction after MI, especially if associated with clinical CHF, is associated with poor survival. For example, in the Coronary Artery Surgery Study, medically treated patients with an LV ejection fraction below 35 % had a 10-year survival of only 30 %, compared to a survival of 60 % for patients with an ejection fraction of 35–49 %, and a survival of approximately 90 % for those with an ejection fraction of at least 50 % (Emond et al. 1994). The surgical treatment for ischemic heart failure (STICH) trial studied 1,212 patients with an ejection fraction of 35 % or less and CAD amenable to CABG, who were randomized to optimal medical treatment alone or with CABG. The trial showed that CABG resulted in 19 % lower risk of cardiovascular death, although overall mortality was not lower compared to medical treatment alone (Velazquez et al. 2011).
As described above, LV dysfunction can be due to an irreversible process, such as infarction, necrosis, or scarring, but also due to myocardial hibernation or stunning, in which case the LV dysfunction is partly or completely reversible. Differentiation between reversible and irreversible causes of LV dysfunction has important implications. Revascularization can result in survival benefit, symptomatic improvement, and improved contractile function in patients with reversible causes of LV dysfunction, i.e., hibernating myocardium (Baker et al. 1994). The main goal of myocardial viability assessment is to identify patients whose symptoms and prognosis may improve after revascularization. Among patients with reduced LV function, those with hibernating myocardium have the worst prognosis if not referred for revascularization. In contrast, patients with LV dysfunction predominantly due to scarring do not seem to benefit from a revascularization procedure, but fare better with medical treatment. In a meta-analysis of more than 3,000 patients with reduced ejection fraction who underwent myocardial viability assessment, the annual mortality rate for patients with viable myocardium who were treated medically was 16 %, compared to 3 % for those undergoing revascularization (Allman et al. 2002). Revascularization improved survival of patients with viable myocardium by approximately 80 %. In contrast, there was a trend towards increased mortality in patients without viable myocardium who underwent revascularization, 7.7 % versus 6.2 %. No significant difference in predictive power was found between nuclear techniques and dobutamine echocardiography. In a nonrandomized subgroup of the STICH trial, myocardial viability was assessed by single-photon emission computed tomography (SPECT) and/or dobutamine stress echocardiography (DSE). In this selected cohort, a survival benefit of CABG on top of medical treatment in the case of viable myocardium could not be demonstrated (Bonow et al. 2011). Due to issues with the study design, conclusions need to be drawn with caution. The findings suggest that viability assessment on its own may not be the only determinant of outcome in patients with LV dysfunction.
In selecting patients for revascularization, the identification of potentially reversible LV dysfunction should not be the only consideration. Many factors influence the clinical outcome, such as comorbidity, patient frailty, prior revascularization, and extent of LV remodeling (Buckley and Di Carli 2011). The clinical decision to revascularize is generally easy in cases of severe LV dysfunction, debilitating anginal symptoms, mild LV remodeling, adequate target vessels for revascularization, and minimal comorbidity (Baker et al. 1994). Survival benefit in these patients likely results from revascularization of myocardial territories at risk, including areas of ischemia. However, in patients with estimated high-risk revascularization, clinical decision making can be more difficult, involving careful weighing of the risks and benefits of a revascularization procedure. In both settings, myocardial viability assessment is crucial to determine the optimal clinical management for the individual patient.
4 Noninvasive Imaging Approaches to Evaluate Myocardial Viability
According to current guidelines and appropriateness criteria documents, there is a choice in noninvasive imaging techniques that can be applied to assess myocardial viability (Klocke et al. 2003; Hendel et al. 2006, 2009; Beanlands et al. 2007a; Sicari et al. 2008; Douglas et al. 2011). Options include single photon emission computed tomography (SPECT), fluorine-18-labeled deoxyglucose (FDG)-positron emission tomography (PET), dobutamine echocardiography, and MRI. Different principles underlie the assessment of viability by these modalities. Nuclear imaging techniques rely on intact cellular membranes for uptake and retention of radiotracers (thallium-201, technetium-99m), as well as intact glucose uptake (FDG). Dobutamine echocardiography/MRI investigates contractile reserve of dysfunctional myocardium based on the inotropic effect of low-dose dobutamine. Delayed contrast enhancement MRI can assess the presence and extent of myocardial infarction in the acute and late phase after MI (dependent on rupture of cell membranes in necrosis and increased interstitial space in scar tissue, respectively). While all techniques above are accepted modalities for myocardial viability assessment, dobutamine echocardiography is the first choice technique for evaluating wall motion abnormalities in most patients. On the other hand, MRI provides optimal tissue characterization for assessment of infarct and scar composition. Computed tomography has only recently entered the stage of myocardial viability assessment. Computed tomography for this indication is the focus of the chapters by Kerl and by Ruszics.
It is important to consider the mechanism being targeted for viability assessment in order to understand advantages and limitations of each modality. In response to myocardial hypoperfusion, metabolic changes occur first, while subsequent steps lead to changes in myocardial contractility (Taegtmeyer 2010). Thus, modalities that make use of intact cell membrane function, a process that is affected early in the cascade, show a low probability of recovery post-revascularization if viability is absent (high sensitivity), while modalities that assess contractile function, a process that is affected only later, show a high probability of functional recovery if viability is present (high specificity). Apart from differences in diagnostic accuracy, modalities differ with regard to spatial resolution, availability, cost, radiation dose, and versatility. An overview of characteristics of the noninvasive modalities is provided in the included Table 1.
4.1 Nuclear Techniques
4.1.1 Single-photon Emission Computed Tomography
SPECT imaging uses single photon emitting radioisotopes to study the viability of the myocardium. The uptake of the radionuclide perfusion tracers depends on myocardial blood flow and the integrity of the cell membrane. Myocardial segments with maintained radiotracer uptake at rest are viable. However, segments with reduced radiotracer uptake may or may not be viable. In the latter cases, myocardial viability can be assessed by imaging myocardial metabolism or contractile reserve. A strong point of SPECT is the extensive clinical experience as well as the multitude of studies showing the ability of SPECT to predict viability. Also, SPECT imaging is widely available, easy to perform, and highly reproducible. However, due to the limited spatial resolution of SPECT, detection of small non-transmural infarcts is difficult. Additionally, both thallium-201 and technetium-99m studies are subject to attenuation artifacts from the diaphragm and breasts, although this can generally be solved by attenuation corrected SPECT. Finally, a disadvantage compared to MRI and DSE is the associated radiation burden.
4.1.1.1 Thallium-201
Thallium-201, one of the earliest radionuclide tracers, is actively extracted from the blood across the myocyte cell membrane via the sodium potassium-adenosine triphosphate pump. This transport system is unaffected by hypoxia unless irreversible injury is present. Images obtained early after radiotracer injection represent blood flow, whereas retention and redistribution of thallium over a 4–24 h period reflect intact cell membrane function, and thus, viability. Areas of LV dysfunction with thallium-201 activity >50 % of peak levels early after radiotracer injection are considered to be viable. Patients who have segments with < 50 % of peak levels undergo redistribution imaging after 4 or 24 h. Thallium-201 redistributes over time into viable cells independent of the extent of first-pass perfusion. It has been shown that imaging 4 h after stress injection can underestimate the presence of viable myocardium as compared to imaging results at 24 h (Perrone Filardi et al. 1996) and compared to metabolic imaging with FDG-PET (Brunken et al. 1992). Modified protocols that involve reinjection of radiotracer (Bonow et al. 1991; Dilsizian et al. 1990) were found to improve the detection of viable myocardium. Zimmerman et al. (1995) showed that regional thallium-201 activity in redistribution and reinjection images is proportional to the mass of preserved viable myocytes in jeopardized myocardium. Images are often interpreted visually, but relative quantification of regional radiotracer uptake may provide more objective and accurate results (Qureshi et al. 1997). The most common protocols used for viability detection with thallium-201 are: (1) stress-redistribution-reinjection imaging, which provides information about inducible ischemia and cellular viability (Dilsizian et al. 1990); and (2) rest-redistribution imaging, which provides information about myocardial blood flow at rest and cellular integrity (Ragosta et al. 1993). The probability of functional recovery post-revascularization decreases as regional thallium-201 uptake declines. Areas with little or no thallium-201 uptake are unlikely to recover function after revascularization (Perrone Filardi et al. 1996). In a meta-analysis of 40 studies, the weighted mean sensitivity of thallium-201 imaging was 87 %, specificity 54 %, positive predictive value (PPV) 67 %, and negative predictive value (NPV) 79 % (Schinkel et al. 2007). Thallium-201 was found to provide important long-term prognostic information in patients with severe LV dysfunction who underwent CABG (Gurserer et al. 2002). Disadvantages of thallium-201 include a long half-life (73 h) which leads to a relatively high radiation dose, and suboptimal image quality in the cases of obesity and large breasts, which can result in false-positives.
4.1.1.2 Technetium
In recent times, Technetium (Tc)-99m has become the preferred SPECT radiotracer. 99mTc-labeled agents emit higher energy photons than thallium-201, yielding better image quality. Also, the shorter half-life time of 99mTc allows the administration of a higher dose. Flow tracers such as 99mTc-sestamibi and 99mTc-tetrofosmin are lipophilic and positively charged. Unlike thallium-201, Tc-99m-sestamibi and Tc-99m-tetrofosmin are passively transported via the sarcolemmal membrane and bind to the inner membrane of mitochondria (Piwnicka-Worms et al. 1994). Uptake and retention of 99mTc-sestamibi and 99mTc-tetrofosmin is dependent on cell membrane integrity and mitochondrial function (Travin et al. 2005). Regional 99mTc-sestamibi and 99mTc-tetrofosmin activity is closely correlated with the results obtained in thallium-201 imaging (Udelson et al. 1994; Matsunari et al. 1997). The use of nitrates prior to the injection of Tc-99m-labeled tracers enhances collateral flow and thus myocardial uptake in areas of resting hypoperfusion (Aoki et al. 1991). This improves the evaluation of myocardial viability. Another method to enhance the detection of viability is simultaneous assessment of LV function using gated SPECT imaging, which allows assessment of contractility. An example patient is shown in Fig. 1. In combination with low dose dobutamine infusion, gated SPECT imaging can be used to evaluate contractile reserve (Iskandrian et al. 1998). Like in thallium-201 imaging, apart from visual analysis, quantification of tracer uptake can be performed in Tc-99m SPECT imaging. Dysfunctional myocardial segments with >50 % of peak levels are considered viable and have a good probability of functional recovery after revascularization. In contrast, segments showing <50 % tracer uptake at rest have poor viability and a much lower probability of improved function after revascularization. In addition to viability imaging, rest SPECT images can be used for assessment of infarct size (Gibbons et al. 2005). In the previously mentioned meta-analysis, the reported weighted mean sensitivity for technetium-99m SPECT was 83 %, specificity 65 %, PPV 74 %, and NPV 76 % for predicting regional functional improvement after revascularization (Schinkel et al. 2007). Most of the 26 studies in the meta-analysis applied Tc-99m-sestamibi as tracer. Tc-99m SPECT imaging with use of nitrates resulted in better specificity and NPV than without nitrates, with comparable sensitivity and PPV.
Gated single-photon emission computed tomography myocardial perfusion scintigram in a patient with a history of inferoseptal infarct. Left perfusion and endo-/epicardial contours, cross-sections of the left ventricle in end-diastole (ED) and end-systole (ES). Middle, above: bull’s eye views of the left ventricle of perfusion in ED and ES, wall motion and thickening. Middle, below: four-dimensional view of wall-motion in ED and ES. Color scale ranges from light yellow (normal perfusion/function) to green (no perfusion/function). On the right side the calculated cardiac parameters, including ED and ES volume and ejection fraction. Courtesy of Dr RHJA Slart, University Medical Center Groningen
4.1.2 Positron Emission Tomography
In PET imaging, radiotracers are used that emit positrons. Upon encountering an electron, the positron annihilates together with the electron, resulting in the production of a pair of 511 keV photons that travel at 180° from each other (Slart et al. 2006). PET imaging consists of detection of these photons when they hit the detectors within a pre-specified time interval (coincidence detection). The radiotracer is then assumed to be positioned directly between the two detectors. A low-resolution CT or a radionuclide transmission image is performed together with PET to correct for attenuation of photons. PET imaging can be applied to assess viability by the measurement of myocardial perfusion and/or metabolism (Fig. 2). Myocardial perfusion is assessed using rubidium-82 or N-13 ammonia. Commonly, myocardial metabolism is assessed by FDG, a glucose analog. The high temporal and spatial resolution of PET (Bacharach et al. 2003) combined with the attenuation correction allows quantification of small amounts of radiotracer uptake and estimation of myocardial blood flow (Al Mallah et al. 2010). Due to the very short half-life of most radiotracers (a few minutes), PET protocols are fast and radiation exposure is lower than for SPECT. FDG has a 2-h half-life that allows transport to sites without an on-site cyclotron. Disadvantages of PET include the high costs of the technology and the limited availability of PET scanners and radiotracers.
Positron emission tomography examination. Polarmaps of the left ventricle showing absolute quantification of myocardial perfusion and FDG metabolism. The upper-left polarmap shows rest 13N-ammonia perfusion, the upper right polarmap depicts dipyridamole stress 13N-ammonia perfusion, the lower polarmap shows FDG metabolism. The color scale of the polarmaps ranges from red (good perfusion/viability) to blue (no perfusion/viability). The stress 13N-ammonia polarmap shows a considerable perfusion defect of the inferior left ventricular wall, extending to the basolateral wall (arrows). This defect is largely reversible as shown on the rest 13N-ammonia polarmap (ischemia), with a small persistent perfusion defect (arrow). The persistent perfusion defect shows glucose metabolism on the FDG polarmap, indicating myocardial viability. Courtesy of Dr RHJA Slart, University Medical Center Groningen
The typical FDG PET viability study consists of FDG PET images paired with resting myocardial perfusion images, which can be obtained using SPECT or PET. In viable but dysfunctional myocardium, FDG uptake increases due to a shift to anaerobic metabolism and a preference for glucose rather fatty acid metabolism (Dilsizian et al. 2008). The specific pattern of regional perfusion and metabolism allows classification of myocardium as normal, hibernating, or scar. A myocardial area with a severe perfusion and metabolism defect (termed a flow-metabolism “match”), indicates a transmural or nearly transmural infarct. A territory with a less severe, matched perfusion and metabolism defect represents a non-transmural infarct without viability. Segments with reduced perfusion and normal or increased glucose metabolism (mismatch) indicate jeopardized, viable myocardium. In myocardial areas with repetitive stunning, myocardial perfusion is normal or nearly normal, FDG uptake is normal or reduced, but stress perfusion, if performed, is typically reduced. FDG imaging can theoretically miss viable tissue in regions of thinned myocardium due to partial volume effects (Kuhl et al. 2006).
PET has a high accuracy for the prediction of functional recovery after revascularization (Tillisch et al. 1986; vom Dahl et al. 1994). The accuracy of PET remains high even in patients with the most severe left ventricular dysfunction (LVEF < 25 %) (Marin-Neto et al. 1998). In a meta-analysis of 24 studies (Schinkel et al. 2007), PET had a weighted mean sensitivity of 92 %, specificity of 63 %, PPV of 74 %, and NPV of 87 %. FDG-PET has been considered the reference standard for viability imaging given the extensive clinical experience, the considerable research data, and its relatively high accuracy for predicting functional recovery following revascularization. Exercise and functional capacity have been found to improve to a greater extent in patients with multiple areas of intact viability by FDG-PET, compared to patients with less viable myocardium (Di Carli et al. 1995; Marwick et al. 1992). The extent of perfusion-metabolism PET mismatch, in particular, identifies patients who will have the largest improvement in heart failure symptoms (Di Carli et al. 1995). PET viability imaging can identify patients with LV dysfunction who will derive the most prognostic benefit from revascularization in terms of reduction of cardiovascular events and mortality (Eitzman et al. 1992; Rohatgi et al. 2001). The value of PET was recently assessed in a randomized trial, the PET and Recovery Following Revascularization (PPAR-2) trial. PET-guided management was compared to routine management of patients with ischemic cardiomyopathy (Beanlands et al. 2007b). In the PET arm, recommendations regarding revascularization were based on the amount of viable myocardium. The study found that the composite endpoint of cardiac events/hospitalizations did not occur significantly less in patients randomized into the PET-based approach compared to the routine care arm (30 vs. 36 %). However, in patients who underwent the treatment that was guided by PET results, there was a significant reduction in mortality rate compared with the routine care arm. In a sub-study, the number of viable, ischemic segments (with perfusion-metabolism mismatch) was strongly related to the prognostic benefit of revascularization (D’Egidio et al. 2009). The capability to perform quantitative assessment of perfusion and metabolism is a particular strength of PET.
4.2 Echocardiography
4.2.1 Morphological Assessment
With echocardiography, myocardial viability can be evaluated through measures of LV wall thickness or myocardial contractile reserve (response to dobutamine infusion). The simplest type of viability assessment by echocardiography concerns LV morphology. Patients with a severely dilated LV are unlikely to show functional recovery after revascularization. The higher the LV end-systolic volume, the less likely the LV is to show improvement of contractile function (Bax et al. 2004). For LV volume measurement, three-dimensional echocardiography is more accurate than two-dimensional echocardiography (Lang et al. 2006). Thinned myocardial segments in patients with chronic CAD typically represent non-viable scar. An LV end-diastolic wall thickness >6 mm has been used as a marker to predict functional recovery post-revascularization (Cwajg et al. 2000). Sensitivity was 94 %, specificity—48 %, indicating that patients who will not benefit from revascularization can be identified, but that end-diastolic wall thickness does not predict patients who will recover LV function. In a study by La Canna et al. (2000), patients with referral for CABG underwent echocardiography (morphological and dobutamine stress evaluation) and thallium-201 studies. LV end-diastolic wall thickness >5 mm had higher sensitivity but lower specificity than viability by dobutamine echocardiography or thallium-201 studies. Myocardial segments with LV end-diastolic wall thickness <6 mm very rarely have contractile reserve on dobutamine echocardiography (Schinkel et al. 2002). Thus, an LV end-diastolic wall thickness below 5–6 mm makes contractile recovery after revascularization very unlikely.
4.2.2 Dobutamine Stress Echocardiography
DSE has long been used to assess jeopardized myocardium for viability. Stress echocardiography relies on dynamic assessment of myocardial wall thickening and wall motion during administration of an inotropic agent. The most extensive experience is available with low-dose dobutamine (Pierard et al. 1990; Smart et al. 1993; Watada et al. 1994; Cigarroa et al. 1993; Perrone Filardi et al. 1995; Sicari et al. 2003; Pagano et al. 1998). Dobutamine is a synthetic catecholamine leading to a considerable increase in systolic blood pressure and heart rate, and an increase in myocardial oxygen demand. It has both a positive inotropic and a chronotropic action. The inotropic effect occurs before the chronotropic effect. This positive inotropic effect, which occurs at low doses of dobutamine, is applicable in myocardial viability assessment (Kuijpers et al. 2004; Nagel et al. 1999). DSE is a widely available technique and is relatively easy to implement. However, DSE involves subjective assessment of regional wall motion, which makes the accuracy of the technique operator dependent. Also, suboptimal echo windows limit its use in approximately 20 % of patients.
To assess myocardial contractile reserve, images are obtained at baseline and under increasing doses of dobutamine. Dobutamine infusion typically starts at 5 μg kg body weight−1 min−1 for 3 min, increasing every 3 min to 10, 20, and in some cases, 30, and 40 μcg kg body weight−1 min−1. In case of a low-dose dobutamine stress protocol, 20 μcg kg body weight−1 min−1 is the highest dose used. If ischemia is tested in the same examination, doses up to 40 μcg kg body weight−1 min−1 (high-dose) are infused. At each stage, echocardiographic images are reviewed to identify new wall motion abnormalities and worsening or improvement of pre-existing wall motion abnormalities.
Dysfunctional myocardial segments can present four different responses to dobutamine infusion (Nagueh et al. 1997): (1) progressive worsening of function. This likely represents hibernating myocardium, served by a critically stenosed coronary artery, or a significant scar. In this case there is no contractile reserve, and any increase in energy demand leads to ischemia. (2) No change in LV dysfunction, indicating scar. (3) Sustained improvement in contractility with increasing dobutamine doses; there is likely enough coronary flow even at high oxygen demands, for example in stunned myocardium. (4) A biphasic response in which a segment shows improvement in contractile function at low dose (5–10 μg kg−1 min−1) with worsening at a higher dose (at least 20 μg kg−1 min−1). Hibernating segments showing a biphasic response have contractile reserve, but this reserve is restricted usually due to concurrent coronary stenosis, resulting in ischemia at higher doses. The benefit of proceeding to higher doses of dobutamine, even if contractile reserve is demonstrated at lower doses, is to observe such a biphasic response.
The biphasic response has the best predictive value of the four possible responses to dobutamine in determining improvement in LV function after revascularization. Two studies in this field demonstrated that 72–75 % of dysfunctional segments with a biphasic response showed functional recovery following revascularization (Afridi et al. 1995; Cornell et al. 1998). Functional improvement post-revascularization is less likely in cases of worsening function (9–35 %) or sustained improvement (15–22 %), while recovery is not to be expected in case of no response to dobutamine (4–13 %). High-dose dobutamine protocols have a significantly higher sensitivity and a similar specificity to low-dose dobutamine protocols (Schinkel et al. 2007), and thus, are recommended if there are no contraindications to high-dose dobutamine. In a meta-analysis of 41 studies using DSE to predict improved ventricular function after revascularization (Schinkel et al. 2007), the sensitivity and specificity were 80 % and 78 %, respectively, and the PPV and NPV were 75 % and 83 %, respectively. Only eight of these studies used a high-dose protocol. The high-dose protocols yielded slightly higher sensitivity (83 versus 79 %) and NPV (85 versus 82 %) than the low-dose studies. In the comparison of DSE and nuclear techniques (Schinkel et al. 2007), nuclear imaging modalities had a higher sensitivity for prediction of regional LV functional recovery, while DSE had higher specificity. Nuclear techniques also had higher sensitivity of global contractile function compared to DSE, at similar specificity. In general, DSE has a tendency to underestimate viability while nuclear imaging modalities tend toward the overestimation of viability. A substantial number of non-viable segments by DSE will be interpreted as viable by nuclear imaging (Panza et al. 1995; Cornel et al. 1999). In the presence of significant myocardial viability on DSE, patients who undergo revascularization were found to have a much more favorable prognosis than those treated medically (Afridi et al. 1998; Chaudhry et al. 1999). Conversely, patients with mostly non-viable myocardium on DSE did not derive prognostic benefit from revascularization.
4.3 Magnetic Resonance Imaging
4.3.1 Imaging Findings in Acute and Chronic Situations
Viability imaging with MRI revolves around two approaches: morphology and function. In acute situations, early after primary percutaneous coronary intervention (PCI), function loss and edema are more pronounced; while in the chronic situation, more structural changes occur, ultimately also leading to function loss. Different indices play a role in the assessment of prognosis after myocardial infarction, such as infarct size, right ventricular involvement, papillary muscle involvement, pericarditis, and microvascular obstruction.
T2-weighted imaging (short-tau inversion recovery—STIR-imaging) can be used to assess the amount of edema, visible as high signal intensity. Edema is a sign of acute injury that becomes less pronounced in time. T2-weighted imaging, especially so-called T2* imaging, can also be used to detect hemorrhage in an area of high signal intensity due to edema. Intramyocardial hemorrhage is associated with more severe infarct-related injury (Kumar et al. 2011).
Another helpful technique is first-pass perfusion imaging. In acute or chronic MI, perfusion images can be normal. This implies that perfusion status has recovered, which is a favorable prognostic sign. Prognosis is worse when perfusion imaging is abnormal, specifically with an area of reduced myocardial enhancement after the injection of an intravenous contrast agent (see Fig. 3). This can imply that there is microvascular obstruction (MVO), also called the no-reflow phenomenon. Multiple factors have been suggested that play a role in the no-reflow phenomenon, including microvascular spasm, endothelial dysfunction, inflammation, edema, embolization of thrombus, and plaque (Krug et al. 1996; Kloner et al. 1974). To a certain extent, MVO can also be PCI-procedure related. Taylor et al. (2006) described that elective PCI immediately impaired resting function as assessed with cardiac MRI. Because first-pass perfusion imaging is a dynamic technique involving single-shot acquisition frames, the technique has relatively low signal- and contrast-to-noise ratios. A recent study showed that MVO is actually best detectable on delayed contrast enhancement MRI due to better contrast-to-noise ratio (see Fig. 3) (Nijveldt et al. 2009). However, the absence of a non-enhancing core on late enhancement images does not exclude the presence of MVO, as there is gradual filling-in of the MVO area with contrast in the minutes following contrast injection.
Magnetic resonance imaging examination in a patient with a partly reperfused infarction, 1 day after the acute coronary syndrome. Midventricular short-axis images. The left image shows a focal perfusion defect during first pass of contrast in the lateral wall, indicative of impaired perfusion and microvascular obstruction. The right image shows late gadolinium enhancement of this area, reflective of necrosis and edema, with a central hypointense area that represents microvascular obstruction. On invasive coronary angiography (not shown), patient had an occluded obtuse marginal branch of the left circumflex coronary artery that could not be reperfused
Functional cine imaging can be used to assess areas of hypokinesia, akinesia, or even dyskinesia as an expression of ischemic damage. In the setting of chronic MI, wall thinning can occur (Fig. 4). In the literature, an end-diastolic wall thickness of more than 5.5 or 6 mm is mentioned as the cut-off for myocardium that recovers function after revascularization (Romero et al. 2012). In a study by Stork et al. (2007), edema on T2-weighted images and wall thinning were accurate measures for differentiating acute from chronic MI, respectively. Delayed contrast enhancement and MVO did not play a role. On the other hand, T2-weighted imaging can substantially underestimate the extent of infarct in the presence of MVO.
Magnetic resonance imaging examination. Patient with history of myocardial infarction in right coronary artery territory. Upper row shows midventricular short axis cine slice images in end-diastole (left) and end-systole (right). Left ventricle dysfunction. Thinning of the inferior wall (<5.5 mm), without thickening/contractility in systole. Vertical long axis (left) and short axis (right) lower images show transmural late gadolinium enhancement in the inferior wall. Conclusion: Transmural infarction of the inferior wall without viable myocardium
4.3.2 Late Gadolinium Enhancement
One way to assess myocardial viability by cardiac MRI is the evaluation of late gadolinium enhancement (LGE). In the LGE technique, a T1-weighted imaging sequence is performed 8–10 min after the administration of the contrast agent, Gadolinium. Static imaging is performed, with more signal averaging and thus a higher signal-to-noise ratio than first-pass perfusion imaging. The signal from the myocardium is “nulled”, using an inversion recovery pulse. This results in normal myocardium appearing dark; areas with LGE will then appear relatively bright. The nulling ensures optimal visual contrast between normal and abnormal myocardium. The optimal inversion time for nulling of the normal myocardium differs per patient and sometimes has to be optimized during the acquisition of multiple slices.
The LGE technique aims to detect regions with delayed Gadolinium uptake. It is important to note that in ischemic cardiomyopathy, delayed enhancement can reflect different pathologies. In the acute phase of MI, hyperenhancing myocardium indicates the area of necrosis and edema (see Fig. 3). Similar to the decrease in infarct size during the first weeks after MI, the extent of LGE volume in MRI decreases during the weeks after acute MI in canine models and in patients (Rochitte et al. 1998; Fieno et al. 2004; Ibrahim et al. 2010). In the chronic situation, LGE identifies scarred myocardium—more factually, increased interstitial space. Thus, the statement, “bright is dead,” does not accurately reflect the meaning of LGE. LGE hardly ever only reflects cell death. In a landmark study by Kim et al. (2000), the pattern of LGE in ischemic cardiomyopathy was found to correspond to the myocardial perfusion territory of the specific coronary artery. It was also shown that recovery of myocardial function after revascularization depends on the transmural extent of infarction. Contractile function is very likely to recover if there is no late enhancement and unlikely to recover in the case of more than 50 % transmural late enhancement (Fig. 4). However, in cases with 1–25 % transmural late enhancement, the probability of functional recovery is approximately 65 %; the probability of functional recovery is 43 % if transmurality is 26–50 % (Kim et al. 2000; Dilsizian 2007). Recovery of function after revascularization appears to be related to the ratio of viable-to-scarred myocardium within dysfunctional myocardial segments. Different cut-off values for transmural extent of hyperenhancement have been applied to determine whether or not functional recovery post-revascularization can be expected, ranging from >0 to >75 % (Romero et al. 2012). Due to its superior spatial resolution, LGE by MRI is better than SPECT and PET at identifying regions of subendocardial scar (Klein et al. 2002).
Due to the fact that at least an 8 min waiting time is mandatory after contrast injection, the DCE technique is always combined with dynamic perfusion imaging (which also requires a contrast agent). After contrast administration and perfusion imaging, typically a stack of short-axis cine images of the LV are acquired for LV functional parameter assessment. Cine MRI is considered the reference standard for measurements of global LV function (Task Force European Society of Cardiology 1998).
4.3.3 Dobutamine MRI
Wall motion imaging by cardiac MRI provides important information about global and regional myocardial function. For adequate wall motion analysis, the entire cardiac cycle needs to be captured. Additionally, good contrast between the myocardial wall and the blood pool is needed. Fast imaging with steady-state free precession sequences results in improved image quality compared with gradient echo acquisition techniques (Barkhausen et al. 2001; Plein et al. 2001). The capture of the entire cardiac cycle can be obtained with retrospective electrocardiographic gating, allowing for cine-loops to be acquired. Parallel imaging allows for either reduced acquisition time or improvement of temporal resolution. The consistently high level of spatial and temporal resolution with which cine MRI images can be acquired enables the detection of small alterations of systolic wall motion up to heart rates of 200 beats per minute. This allows for analysis of regional function with dobutamine MRI in multiple slice positions. The rationale for the use and dosage of dobutamine for evaluation of myocardial viability is similar in MRI as in echocardiography (see Sect. 4.2). Often, improvement in systolic wall thickening of more than 2 mm is used as cut-off to predict functional recovery (Romero et al. 2012).
Visual evaluation of changes in myocardial contractility during infusion of dobutamine can be challenging. Myocardial tagging, a technique using non-selective radiofrequency pulses separated by spatial modulation of magnetization encoding gradients, can be helpful in regional LV functional analysis. The absence of inward movement of these grid lines can be used to diagnose absence of viability. Generally, improvement of a rest wall motion abnormality during low-dose dobutamine is a sign that there is still functional recovery possible, and can be used as a sign of viability. Use of myocardial tagging was shown to facilitate detection of wall motion abnormalities compared to non-tagged MRI images (Kuijpers et al. 2003).
4.3.4 Diagnostic and Prognostic Accuracy of MRI
While the validation for the DCE-MRI technique has been particularly extensive, the number of patient studies on diagnostic accuracy is smaller. A recent meta-analysis compared the diagnostic accuracy of the three described MRI methods for assessing viability (Romero et al. 2012). In total, 24 studies met the inclusion criteria, comprising 698 patients. End-diastolic wall thickness of more than 5.5 or 6 mm (in total four studies) had weighted sensitivity of 96 %, specificity of 38 %, PPV of 71 %, NPV of 85 %, and overall accuracy of 68 %. For late enhancement (more than 50 % transmurality, 11 studies), these parameters were 95, 51, 69, 90 and 70 %, respectively. For improved systolic wall thickening on low-dose dobutamine MRI (>2 mm increase, 9 studies), test characteristics were 81, 91, 93, 75, and 84 %, respectively. Thus, wall thickness and DCE showed the highest sensitivity, and low-dose dobutamine MRI had the highest specificity. Prediction of improvement of contractile function can be difficult when based solely on morphological information. Wellnhofer et al. (2004) showed that DCE and dobutamine MRI can provide complementary information in predicting functional recovery after revascularization.
So far, only a few studies have been published on the prognostic value of viability assessment by MRI. In studies comprising more than 300 patients each, extent and transmurality of scar on MRI was found to predict major cardiac adverse events beyond clinical and functional parameters (Kwong et al. 2006; Kwon et al. 2009). Gerber et al. (2012) recently showed that patient survival was considerably worse when dysfunctional but viable myocardium on DCE-MRI was treated medically instead of interventionally. Medically treated patients with dysfunctional but viable myocardium on DCE-MRI had a higher mortality than patients with non-viable myocardium. This is in line with meta-analysis results by Allman et al. (2002) for echocardiography and nuclear techniques. The worse prognosis in medically treated patients with viable versus non-viable myocardium is possibly related to increased arrhythmogenic vulnerability in still-viable myocardium, which can lead to cardiac death (Fallavollita et al. 2005). Lastly, in medically treated, chronic MI patients who underwent both DCE-MRI and dobutamine echocardiography, infarct size on MRI was a stronger prognostic factor than contractile reserve on echocardiography (Kelle et al. 2009). However, in the case of a large myocardial scar, contractile reserve was found to be more important as predictor of cardiac events.
5 Conclusion
Ischemic cardiomyopathy with LV dysfunction is a major burden in westernized societies, associated with high morbidity and mortality, and substantial costs. LV dysfunction can be reversible or irreversible, depending on whether underlying myocardium is viable or non-viable. Dysfunctional but viable myocardial segments with the potential for functional recovery are considered to be stunned or hibernating. In the case of reversible LV dysfunction, surgical revascularization can lead to improved prognosis in appropriately selected patients. Noninvasive imaging methods can be used to assess myocardial viability, to assist patient management optimization. The identification of viable myocardium differs for the discussed imaging modalities, and is generally based on morphology (mainly MRI) and/or function (nuclear techniques, dobutamine echocardiography, and MRI).
References
Abraham WT, Bristow MR (1997) Specialized centers for heart failure management. Circulation 96:2755–2757
Abraham WT, Hayes DL (2003) Cardiac resynchronization therapy for heart failure. Circulation 108:2596–2603
Afridi I, Kleiman NS, Raizner AE et al (1995) Dobutamine echocardiography in myocardial hibernation. Optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation 91:663–670
Afridi I, Grayburn PA, Panza JA, Oh JK, Zoghbi WA, Marwick TH (1998) Myocardial viability during dobutamine echocardiography predicts survival in patients with coronary artery disease and severe left ventricular systolic dysfunction. J Am Coll Cardiol 32:921–926
Allman KC, Shaw LJ, Hachamovitch R et al (2002) Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 39:1151–1158
Al-Mallah MH, Sitek A, Moore SC, Di Carli M, Dorbala S (2010) Assessment of myocardial perfusion and function with PET and PET/CT. J Nucl Cardiol 17:498–513
Aoki M, Sakai K, Koyanagi S, Takeshita A, Nakamura M (1991) Effect of nitroglycerin on coronary collateral function during exercise evaluated by quantitative analysis of thallium-201 single photon emission computed tomography. Am Heart J 121:1361–1366
Bacharach SL, Bax JJ, Case J et al (2003) PET myocardial glucose metabolism and perfusion imaging: Part 1—Guidelines for data acquisition and patient preparation. J Nucl Cardiol 10:543–556
Baker WB, Klein MS, Reardon MJ et al (1991) Reversible cardiac dysfunction (hibernation) from ischemia due to compression of the coronary arteries by a pseudoaneurysm. N Engl J Med 325:1858–1861
Baker DW, Jones R, Hodges J, Massie BM, Konstam MA, Rose EA (1994) Management of heart failure III. The role of revascularization in the treatment of patients with moderate or severe left ventricular systolic dysfunction. JAMA 272:1528–1534
Baliga RR, Schaper J, Narula JP (2000) Role of apoptosis in myocardial hibernation and myocardial stunning. In: Iskandrian AE, Van Der Wall EE (eds) Myocardial Viability, 2nd edn. Kluwer Academic Publishers, Dordrecht, pp 21–45
Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF (2001) MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology 219:264–269
Bax JJ, Wahba FF, Van Der Waal EE (2003) Myocardial viability/hibernation. In: Iskandrian AE, Verani MS (eds) Nuclear cardiac imaging. Oxford University Press, New York, pp 386–398
Bax JJ, Schinkel AFL, Boersma E et al (2004) Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation 110(suppl 1):II18–II22
Beanlands RS, Chow BJ, Dick A et al (2007a) CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease—executive summary. Can J Cardiol 23:107–119
Beanlands RSB, Nichol G, Huszti E et al (2007b) F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) (see comment). J Am Coll Cardiol 50:2002–2012
Blitz A, Laks H (1996) The role of coronary revascularization in the management of heart failure: identification of candidates and review of results. Curr Opin Cardiol 11:276–290
Bogaert J, Kalantzi M, Rademakers FE, Dymarkowski S, Janssens S (2007) Determinants and impact of microvascular obstruction in successfully reperfused ST-segment elevation myocardial infarction. Assessment by magnetic resonance imaging. Eur Radiol 17:2572–2580
Bolognese L, Neskovic AN, Parodi G et al (2002) Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation 106:2351–2357
Bonow RO, Dilsizian V, Cuocolo A, Bacharach SL (1991) Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction. Comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose (see comment). Circulation 83:26–37
Bonow RO, Maurer G, Lee KL et al (2011) Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med 364:1617–1625
Bounous EP, Mark DB, Pollock BG et al (1988) Surgical survival benefits for coronary disease patients with left ventricular dysfunction. Circulation 78:I151–I157
Braunwald E, Kloner RA (1982) The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 66:1146–1149
Braunwald E, Rutherford JD (1986) Reversible ischemic left ventricular dysfunction: evidence for the ‘‘hibernating myocardium’’. J Am Coll Cardiol 8:1467–1470
Brunken RC, Mody FV, Hawkins RA, Nienaber C, Phelps ME, Schelbert HR (1992) Positron emission tomography detects metabolic viability in myocardium with persistent 24-hour single-photon emission computed tomography 201Tl defects. Circulation 86:1357–1369
Buckley O, Di Carli M (2011) Predicting benefit from revascularization in patients with ischemic heart failure: imaging f myocardial ischemia and viability. Circulation 123:444–450
Burns RJ, Gibbons RJ, Yi Q et al (2002) The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 39:30–36
Chatterjee K, Swan HJ, Parmley WW et al (1973) Influence of direct myocardial revascularization on left ventricular asynergy and function in patients with coronary heart disease: with and without previous myocardial infarction. Circulation 47:276–286
Chaudhry FA, Tauke JT, Alessandrini RS, Vardi G, Parker MA, Bonow RO (1999) Prognostic implications of myocardial contractile reserve in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 34:730–738
Cigarroa CG, deFilippi CR, Brickner ME, Alvarez LG, Wait MA, Grayburn PA (1993) Dobutamine stress echocardiography identifies hibernating myocardium and predicts recovery of left ventricular function after coronary revascularization. Circulation 88:433–436
Cohn JN, Archibald DG, Ziesche S et al (1986) Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 314:1547–1552
Colucci WS, Elkayam U, Horton DP et al for the Nesiritide Study Group (2000) Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med 343:246–253
Conversano A, Walsh JF, Geltman EM et al (1996) Delineation of myocardial stunning an hibernation by positron emission tomography in advanced coronary artery disease. Am Heart J 131:440–450
Cornel JH, Bax JJ, Elhendy A, Maat AP, Kimman GJ, Geleijnse ML, Rambaldi R, Boersma E, Fioretti PM (1998) Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol 31:1002–1010
Cornel JH, Bax JJ, Elhendy A et al (1999) Agreement and disagreement between “metabolic viability” and “contractile reserve” in akinetic myocardium. J Nucl Cardiol 6:383–388
Cwajg JM, Cwajg E, Nagueh SF, He ZX, Qureshi U, Olmos LI et al (2000) End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: relation to restredistribution T1–201 tomography and dobutamine stress echocardiography. J Am Coll Cardiol 35:1152–1161
D’Egidio G, Nichol G, Williams KA et al (2009) Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC: Cardiovasc Imaging 2:1060–1068
Deedwania PC (2003) The key to unraveling the mystery of mortality in heart failure. An integrated approach. Circulation 107:1719–1721
Di Carli MF, Asgarzadie F, Schelbert HR, Brunken RC, Laks H, Phelps ME, Maddahi J (1995) Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation 92:3436–3444
Dilsizian V (2003) Myocardial viability: reversible left ventricular dysfunction. In: Dilsizian V, Narula J, Braunwald E (eds) Atlas of nuclear cardiology. Current Medicine, Philadelphia, pp 19–46
Dilsizian V (2007) Cardiac magnetic resonance versus SPECT: are all non-infarct myocardial regions created equal? J Nucl Card 14:9–14
Dilsizian V, Rocco TP, Freedman NM, Leon MB, Bonow RO (1990) Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med 323:141–146
Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Gropler RJ, Knuuti J, Schelbert HR, Travin M (2008) PET myocardial perfusion and metabolism clinical imaging. http://www.asnc.org/imageuploads/ImagingGuidelinesPETJuly2009.pdf
Dispersyn GD, Ausma J, Thone F et al (1999) Cardiomyocyte remodelling during myocardial hibernation and atrial fibrillation: prelude to apoptosis. Cardiovasc Res 43:947–957
Douglas PS, Garcia MJ, Haines DE et al (2011) ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr 24:229–267
Eitzman D, al-Aouar Z, Kanter HL et al (1992) Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography (see comment). J Am Coll Cardiol 20:559–565
Emond M, Mock M, Davis K et al (1994) Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation 90:2645–2657
Fallavollita JA, Riegel BJ, Suzuki G, Valeti U, Canty JM Jr (2005) Mechanism of sudden cardiac death in pigs with viable chronically dysfunctional myocardium and ischemic cardiomyopathy. Am J Physiol Heart Circ Physiol 289:H2688–H2696
Fieno DS, Hillenbrand HB, Rehwald WG et al (2004) Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol 43:2124–2131
Ganame J, Messalli G, Masci PG, Dymarkowski S, Abbasi K, Van de Werf F, Janssens S, Bogaert J (2011) Time course of infarct healing and left ventricular remodelling in patients with reperfused ST segment elevation myocardial infarction using comprehensive magnetic resonance imaging. Eur Radiol 21:693–701
Gerber BL, Rousseau MF, Ahn SA et al (2012) Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: impact of revascularization therapy. J Am Coll Cardiol 59:825–835
Gewirtz H, Fischman AJ, Abraham S et al (1994) Positron emission tomographic measurements of absolute regional myocardial blood flow permits identification of nonviable myocardium in patients with chronic myocardial infarction. J Am Coll Cardiol 23:851–859
Gheorgiade M, Bonow RO (1998) Chronic heart failure in the United States. A manifestation of coronary artery disease. Circulation 97:282–289
Gibbons RJ, Miller TD (2005) Tc-99m sestamibi infarct size as a surrogate endpoint. J Nucl Cardiol 12:12–19
Gursurer M, Emre A, Gercekoglu H, Uslubas S, Aksoy M, Ersek B (2002) Long-term prognostic value of stress-redistribution-reinjection Tl-201 imaging in patients with severe left ventricular dysfunction and coronary artery bypass surgery. Int J Cardiovasc Imaging 18:125–133
Hendel RC, Patel MR, Kramer CM et al (2006) ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging. A report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group. J Am Coll Cardiol 48:1475–1497
Hendel RC, Berman DS, Di Carli MF et al (2009) ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, The Society of Cardiovascular Computed Tomography, The Society for Cardiovascular Magnetic Resonance, and The Society of Nuclear Medicine. J Am Coll Cardiol 53:2201–2229
Heyndrickx GR, Baig H, Nellens P et al (1978) Depression of regional blood flow and wall thickening after brief coronary occlusions. Am J Physiol 234:H653–H659
Ibrahim T, Hackl T, Nekolla SG et al (2010) Acute myocardial infarction: serial cardiac MR imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology 254:88–97
Iskandrian AE, Acio E (1998) Methodology of a novel myocardial viability protocol. J Nucl Cardiol 5:206–209
Ito H, Maruyama A, Iwakura K et al (1996) Clinical implications of the “no reflow” phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93:223–228
Kelle S, Roes SD, Klein C et al (2009) Prognostic value of myocardial infarct size and contractile reserve using magnetic resonance imaging. J Am Coll Cardiol 54:1770–1777
Kim RJ, Wu E, Rafael A et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. New Engl J Med 343:1445–1453
Klein C, Nekolla SG, Bengel FM et al (2002) Assessment of myocardial viability with contrast enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 105:162–167
Klocke FJ, Baird MG, Lorell BH et al (2003) ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the clinical use of Cardiac Radionuclide Imaging). Circulation 108:1404–1418
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. Clin Investig 54:1496–1508
Kloner RA, Bolli R, Marban E et al (1998) Medical and cellular implications of stunning, hibernation, and preconditioning. An NHLBI workshop. Circulation 97:1848–1867
Krug A, de Rochemont WM, Korb G (1996) Blood supply of the myocardium after temporary coronary occlusion. Circ Res 19:57–62
Kuhl HP, Lipke CS, Krombach GA et al (2006) Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography-single photon emission computed tomography imaging protocol. Eur Heart J 27:846–853
Kuijpers D, Ho KY, van Dijkman PR, Vliegenthart R, Oudkerk M (2003) Dobutamine cardiovascular magnetic resonance for the detection of myocardial ischemia with the use of myocardial tagging. Circulation 107:1592–1597
Kuijpers D, van Dijkman PR, Janssen CH, Vliegenthart R, Zijlstra F, Oudkerk M (2004) Dobutamine stress MRI. Part II. Risk stratification with dobutamine cardiovascular magnetic resonance in patients suspected of myocardial ischemia. Eur Radiol 14:2046–2052
Kumar A, Green JD, Sykes JM, Ephrat P, Carson JJ, Mitchell AJ, Wisenberg G, Friedrich MG (2011) Detection and quantification of myocardial reperfusion hemorrhage using T2*-weighted CMR. JACC Cardiovasc Imaging 4:1274–1283
Kwon DH, Halley CM, Carrigan TP et al (2009) Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. J Am Coll Cardiol Imaging 2:34–44
Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB (2006) Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 113:2733–2743
La Canna G, Rahimtoola SH, Visioli O et al (2000) Sensitivity, specificity, and predictive accuracies of non-invasive tests, singly and in combination, for diagnosis of hibernating myocardium. Eur Heart J 21:1358–1367
Lang RM, Mor-Avi V, Sugeng L et al (2006) Three-dimensional echocardiography: the benefits of the added dimension. J Am Coll Cardiol 48:2053–2069
Lepper W, Hoffmann R, Kamp O et al (2000) Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty in patients with acute myocardial infarction. Circulation 101:2368–2374
Levy D, Kenchaiah S, Larson MG et al (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 93:1397–1402
Lund GK, Stork A, Muellerleile K et al (2007) Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology 245:95–102
Marinho NV, Keogh BE, Costa DC et al (1996) Pathophysiology of chronic left ventricular dysfunction: new insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation 93:737–744
Marin-Neto JA, Dilsizian V, Arrighi JA, Perrone-Filardi P, Bacharach SL, Bonow RO (1998) Thallium scintigraphy compared with 18F-fluorodeoxyglucose positron emission tomography for assessing myocardial viability in patients with moderate versus severe left ventricular dysfunction. Am J Cardiol 82:1001–1007
Marwick TH, Nemec JJ, Lafont A, Salcedo EE, MacIntyre WJ (1992) Prediction by postexercise fluoro-18 deoxyglucose positron emission tomography of improvement in exercise capacity after revascularization. Am J Cardiol 69:854–859
Matsunari I, Fujino S, Taki J et al (1997) Quantitative rest technetium-99m tetrofosmin imaging in predicting functional recovery after revascularization: comparison with rest-redistribution thallium-201. J Am Coll Cardiol 29:1226–1233
Nagel E, Lehmkuhl HB, Bocksch W et al (1999) Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 99:763–770
Nagueh SF, Vaduganathan P, Ali N et al (1997) Identification of hibernating myocardium: comparative accuracy of myocardial contrast echocardiography, rest-redistribution thallium-201 tomography and dobutamine echocardiography. J Am Coll Cardiol 29:985–993
Nijveldt R, Hofman MB, Hirsch A, Beek AM, Umans VA, Algra PR, Piek JJ, van Rossum AC (2009) Assessment of microvascular obstruction and prediction of short-term remodeling after acute myocardial infarction: cardiac MR imaging study. Radiology 250:363–370
Orn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K (2007) Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol 99:1109–1114
Pagano D, Bonser RS, Townend JN, Ordoubadi F, Lorenzoni R, Camici PG (1998) Predictive value of dobutamine echocardiography and positron emission tomography in identifying hibernating myocardium in patients with postischaemic heart failure. Heart 79:281–288
Panza JA, Dilsizian V, Laurienzo JM et al (1995) Relation between thallium uptake and contractile response to dobutamine: implications regarding myocardial viability in patients with chronic coronary artery disease and left ventricular dysfunction. Circulation 91:990–998
Perrone Filardi P, Pace L, Prastaro M, Piscione F, Betocchi S, Squame F et al (1995) Dobutamine echocardiography predicts improvement of hypoperfused dysfunctional myocardium after revascularization in patients with coronary artery disease. Circulation 91:2556–2565
Perrone-Filardi P, Pace L, Prastaro M et al (1996) Assessment of myocardial viability in patients with chronic coronary artery disease. Rest-4-hour-24-hour 201T1 tomography versus dobutamine echocardiography. Circulation 94:2712–2719
Pierard LA, De Landsheere CM, Berthe C, Rigo P, Kulbertus HE (1990) Identification of viable myocardium by echocardiography during dobutamine infusion in patients with myocardial infarction after thrombolytic therapy: comparison with positron emission tomography. J Am Coll Cardiol 15:1021–1031
Pitt B, Zannad F, Remme WJ et al (1999) Randomized aldactone evaluation study investigators: the effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341:709–717
Pitt B, Poole-Wilson PA, Segal R et al (2000) Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial—the losartan heart failure study ELITE II. Lancet 355:1582–1587
Piwnicka-Worms DP, Kronauge JF, LeFurgey A et al (1994) Mitochondrial localization and characterization of 99Tcsestamibi in heart cells by electron probe x-ray microanalysis and 99Tc-NMR spectroscopy. Magn Reson Imaging 12:641–652
Plein S, Bloomer TN, Ridgway JP, Jones TR, Bainbridge GJ, Sivananthan MU (2001) Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient-echo imaging. J Magn Reson Imaging 14:230–236
Qureshi U, Nagueh SF, Afridi I et al (1997) Dobutamine echocardiography and quantitative rest-redistribution 201Tl tomography in myocardial hibernation. Relation of contractile reserve to 201Tl uptake and comparative prediction of recovery of function. Circulation 95:626–635
Ragosta M, Beller GA, Watson DD et al (1993) Quantitative planar rest redistribution 201-Tl imaging in detection of myocardial viability and prediction of improvement in left ventricular function after coronary artery bypass surgery in patients with severely depressed left ventricular function. Circulation 87:1630–1641
Rahimtoola SH (1982) Coronary bypass surgery for chronic angina—1981: a perspective. Circulation 65:225–241
Rahimtoola SH (1985) A perspective on the three large multicenter randomized clinical trials of coronary bypass surgery for chronic stable angina. Circulation 72(suppl V):V123–V135
Rahimtoola SH (1989) The hibernating myocardium. Am Heart J 117:211–221
Rees G, Bristow JD, Kremkau EL et al (1971) Influence of aortocoronary bypass surgery on left ventricular performance. N Engl J Med 284:1116–1120
Reimer KA, Jennings RB (1979) The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 40:633–644
Rochitte CE, Lima JA, Bluemke DA et al (1998) Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 98:1006–1014
Rohatgi R, Epstein S, Henriquez J et al (2001) Utility of positron emission tomography in predicting cardiac events and survival in patients with coronary artery disease and severe left ventricular dysfunction. Am J Cardiol 87:1096–1099
Romero J, Xue X, Gonzalez W, Garcia MJ (2012) CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging 5:494–508
Savoye C, Equine O, Tricot O et al (2006) Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice [from the REmodelage VEntriculaire (REVE) study group]. Am J Cardiol 98:1144–1149
Schinkel AFL, Bax JJ, Boersma E et al (2002) Assessment of residual myocardial viability in regions with chronic electrocardiographic Q-wave infarction. Am Heart J 144:865–869
Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH (2007) Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 32:375–410
Schwaiger M, Schricke U (2000) Hibernating and stunned myocardium. Pathophysiological considerations. In: Iskandrian AE, Van Der Wall EE (eds) Myocardial viability, 2nd edn. Kluwer Academic Publishers, Dordrecht, pp 1–20
Shivalkar B, Maes A, Borgers M et al (1996) Only hibernating myocardium invariably shows early recovery after coronary revascularization. Circulation 94:308–315
Sicari R, Picano E, Cortigiani L, Borges AC, Varga A, Palagi C et al (2003) VIDA (Viability Identification with Dobutamine Administration) Study Group. Prognostic value of myocardial viability recognized by low-dose dobutamine echocardiography in chronic ischaemic left ventricular dysfunction. Am J Cardio 92:1263–1266
Sicari R, Nihoyannopoulos P, Evangelista A et al (2008) Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 9:415–437
Slart RHJA, Bax JJ, van Veldhuisen DJ et al (2006) Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging 22:63–80
Smart SC, Sawada S, Ryan T, Segar D, Atherton L, Berkovitz K et al (1993) Low-dose dobutamine echocardiography detects reversible dysfunction after thrombolytic therapy of acute myocardial infarction. Circulation 88:405–415
Stork A, Muellerleile K, Bansmann PM et al (2007) Value of T2-weighted, first-pass and delayed enhancement, and cine CMR to differentiate between acute and chronic myocardial infarction. Eur Radiol 17:610–617
Taegtmeyer H (2010) Tracing cardiac metabolism in vivo: one substrate at a time. J Nucl Med 51(Suppl 1):80S–87S
Task Force of the European Society of Cardiology, in collaboration with the Association of European Paediatric Cardiologists (1998) The clinical role of magnetic resonance in cardiovascular disease. Eur Heart J 19:19–39
Taylor AJ, Al-Saadi N, Abdel-Aty H et al (2006) Elective percutaneous coronary intervention immediately impairs resting microvascular perfusion assessed by cardiac magnetic resonance imaging. Am Heart J 151:891.e1–891.e7
The SOLVD investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325:293–302
Tillisch J, Brunken R, Marshall R et al (1986) Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 314:884–888
Topol EJ, Weiss JL, Brinker JA et al (1985) Regional wall motion improvement after coronary thrombolysis with recombinant tissue plasminogenactivator: importance of coronary angioplasty. J Am Coll Cardiol 6:426–433
Travin MI, Bergmann SR (2005) Assessment of myocardial viability. Semin Nucl Med 35:2–16
Udelson JE, Coleman PS, Metherall J et al (1994) Predicting recovery of severe regional ventricular dysfunction. Comparison of resting scintigraphy with 201Tl and 99mTc-sestamibi. Circulation 89:2552–2561
Vanoverschelde JLJ, Wijns W, Depre C et al (1993) Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation 87:1513–1523
Vanoverschelde JL, Depre C, Gerber BL et al (2000) Time course of functional recovery after coronary artery bypass graft surgery in patients with chronic left ventricular ischemic dysfunction. Am J Cardiol 85:1432–1439
Velazquez EJ, Lee KL, Deja MA et al (2011) Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 364:1607–1616
vom Dahl J, Eitzman DT, al-Aouar ZR et al (1994) Relation of regional function, perfusion, and metabolism in patients with advanced coronary artery disease undergoing surgical revascularization. Circulation 90:2356–2366
Watada H, Ito H, Oh H, Masuyama T, Aburaya M, Hori M et al (1994) Dobutamine stress echocardiography predicts reversible dysfunction and quantitates the extent of irreversibly damaged myocardium after reperfusion of anterior myocardial infarction. J Am Coll Cardiol 24:624–630
Wellnhofer E, Olariu A, Klein C et al (2004) Magnetic resonance low-dose dobutamine test is superior to scar quantification for the prediction of functional recovery. Circulation 109:2172–2174
Wilson JM (1999) Reversible congestive heart failure caused by myocardial hibernation. Tex Heart Inst J 26:19–27
Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP et al (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97:765–772
Zimmermann R, Mall G, Rauch B et al (1995) Residual 201Tl activity in irreversible defects as a marker of myocardial viability. Clinicopathological study. Circulation 91:1016–1021
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Vliegenthart, R., Lubbers, D. (2012). Why are We Interested in Viability?. In: Schoepf, U., Bamberg, F., Ruzsics, B., Vliegenthart, R., Bastarrika, G. (eds) CT Imaging of Myocardial Perfusion and Viability. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2012_774
Download citation
DOI: https://doi.org/10.1007/174_2012_774
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33878-6
Online ISBN: 978-3-642-33879-3
eBook Packages: MedicineMedicine (R0)