Abstract
With advances in ultrasound technology, congenital diaphragmatic hernia (CDH) is easily diagnosed prenatally. In contrast, prenatal prognostication remains a challenge. In view of the current availability of fetal therapy this becomes even more important. To date, one of the most studied markers is the lung-area to head circumference ratio and has been shown in large studied to be predictive of postnatal outcome in terms of survival and also morbidity. Since the fetal lung is a fluid filled structure hence has high signal intensity on T2-WI, it can be easily discernible from the surrounding structures using fetal magnetic resonance imaging (MRI). This remains so even in case of oligohydramnios and obesity, conditions that make appropriate ultrasound evaluation difficult and inaccurate. The advantage of prenatal assessment in CDH using fetal MRI is that both lungs can be measured accurately, prediction can be adjusted to fetal biometry using the fetal body volume and intrathoracic position of the liver can be quantified, given a more accurate prediction of postnatal survival rather than the simple semiquantitative assessment of liver position. Further research using fetal MRI is directed towards the assessment of lungs at the microstructural level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apparent Diffusion Coefficient
- Congenital Diaphragmatic Hernia
- Congenital Diaphragmatic Hernia
- Pulmonary Hypoplasia
- Contralateral Lung
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Congenital diaphragmatic hernia (CDH) occurs in about 1/2,500–1/5,000 of newborns, depending on whether stillbirths are included or not (Butler and Claireaux 1962), and accounts for approximately 8% of all major congenital anomalies (Torfs et al. 1992). It is a surgically correctable, anatomical defect with unknown etiology. While the majority of cases are sporadic, less than 2% are familial (Enns et al. 1998), and recurrence rate of isolated CDH is known to be around 2% (Norio et al. 1984). The defect is believed to arise in the embryological period, and as a consequence lung development during further gestation is impaired (Keijzer et al. 2000). A genetic basis remains largely unidentified, but recent work points to the potential of gene discovery tools in CDH and also proves that the phenotypic spectrum of CDH may reflect mutations of different genes (Ackerman et al. 2005; Hara et al. 2005; Slavotinek 2005).
CDH is classified according to the location of the defect in the diaphragm. Most commonly, CDH are left-sided, however in 13% they are right-sided and in 2% they are bilateral (Torfs et al. 1992). Posterolateral defect, known as the Bochdalek hernia are the most common while the Morgagni hernia occurring at the anterior portion of the diaphragm are more rare.
In around 40% of the CDH patients, there are associated anomalies and these can be structural or chromosomal defects, and genetic syndromes. The structural anomalies associated with CDH are mainly congenital heart and central nervous system defects, but also include urogenital, gastrointestinal, musculoskeletal and respiratory anomalies (Crane 1979; Witters et al. 2001). The chromosomal anomalies associated with CDH are most commonly trisomy 21, 18 and 13. Other chromosomal abnormalities such as turner, tetrasomy 12p, partial trisomies 5 or 20 are rare. Genetic syndromes include Fryns syndrome, de Lange syndrome, Marfan syndrome and many others. Associated anomalies are independent predictors of survival, with less than 15% of babies surviving in this group (Skari et al. 2000; Stege et al. 2003).
Actual survival rates in isolated CDH are a matter of debate. Intuitively one would expect that, with advances in neonatal care, neonatal survival rates to improve accordingly. Larger surveys however rate mortality in antenatal diagnosed isolated and live born cases around 30%, still today (Stege et al. 2003; Colvin et al. 2005). Some series from postnatal surgical units report survival rates in excess of 80% (Bagolan et al. 2004; Downard et al. 2003). However, such results overlook the prenatal “hidden mortality” arising from fetal death or termination of pregnancies considered to have a poor prognosis (Harrison et al. 1978), or babies born in outreach hospitals and dying before referral for pediatric surgery (Scott 2004). A study from Western Australia, on 116 babies with CDH collected over a 10-year period, reported that prenatal diagnosis was made in 53% of cases and half of these underwent termination of pregnancy (Colvin et al. 2005). Although the survival in those cases that were selected for neonatal surgery was 92% and the overall survival of the prenatally diagnosed fetuses was only 16%. In another recent population-based study from France on 51 cases of CDH, the condition was isolated in 29 and 16 (55%) of these survived (Gallot et al. 2005).
Whatever the true mortality is, in the group with isolated CDH a high proportion of babies still die in the neonatal period due to pulmonary hypoplasia and/or hypertension. Antenatal prediction of likely postnatal outcome is a clinical need since we need to offer parents different management options including expectant management, termination of pregnancy and prenatal treatment. Nowadays, such a prediction is less of a challenge and the purpose of this chapter is to provide a review of the current imaging modalities available in the diagnosis and the assessment of prognosis of these cases.
2 Prenatal Diagnosis
The diaphragm is imaged by prenatal ultrasonography as an echo-free space between the thorax and abdomen. The diagnosis of CDH can be easily made on ultrasound by the demonstration of stomach and intestines (90% of the cases) or liver (50%) in the thorax and the associated mediastinal shift to the opposite side (Fig. 1). Herniated abdominal contents, associated with a left-sided diaphragmatic hernia, are easy to demonstrate because the echo-free fluid-filled stomach and small bowel contrast dramatically with the more echogenic fetal lung. In contrast, a right-sided hernia is more difficult to identify because the echogenicity of the fetal liver is similar to that of the lung, and visualization of the gall bladder in the right side of the fetal chest may be the only way of making the diagnosis. Polyhydramnios (usually after 25 weeks) is found in about 75% of cases and this may be the consequence of impaired fetal swallowing due to compression of the esophagus by the herniated abdominal organs. The main differential diagnosis is from cystic lung disease, such as cystic adenomatoid malformation (Fig. 2) where fetal magnetic resonance imaging (MRI) can be of help, or mediastinal cystic processes, e.g., neuroenteric cysts, bronchogenic cysts and thymic cysts (Table 1) (Graham and Devine 2005). In these cases, a fluid-filled structure causing mediastinal shift may be present within the chest. However, in contrast to diaphragmatic hernia, the upper abdominal anatomy is normal.
Ultrasound image in an axial view of a fetus at 26 weeks of gestation at the level of the 4-chamber view of the heart showing a left-sided congenital diaphragmatic hernia (CDH) with massive mediastinal shift caused by intrathoracic herniation of the left lobe of the liver (Li), small bowels (SB) and stomach (St). Note the remaining right lung (RL) and the heart (H) shifted towards the right part of the thorax. The RL area is measured by multiplying the longest axis (D1) and that perpendicular to it (D2)
3 Prenatal Prediction of Postnatal Survival
The main predictor of outcome is the presence of other major abnormalities that can be diagnosed by detailed ultrasonographic examination and fetal karyotyping.
In those with apparently isolated CDH prenatal prediction of outcome essentially relies on the assessment of the degree of lung compression by the herniated abdominal viscera. Many prognostic factors based on conventional 2D-ultrasound have been evaluated such as the left to right ventricle ratio (Sharland et al. 1992; Thebaud et al. 1997), lung diameter to thoracic circumference ratio (Bahlmann et al. 1999), amniotic fluid volume, mediastinal shift and stomach position (Hatch et al. 1992). A prognostic score combining sonographic features has also been proposed (Dommergues et al. 1996). Recently, new potential prognostic factors were reported, such as fetal pulmonary artery diameters (Sokol et al. 2002) or the use of Doppler ultrasound to measure impedance to flow in the pulmonary arteries (Fuke et al. 2003).
3.1 Prenatal Assessment with 2D-Ultrasound
3.1.1 The Lung to Head Ratio and Liver Herniation
The most widely studied prognostic factors using ultrasound are firstly, determination whether the herniated abdominal viscera in the fetal thorax include the liver and secondly, measurement of the contralateral lung area to head circumference ratio (LHR) (Fig. 1). The LHR was first proposed by Metkus et al., who observed a relationship to survival (Metkus et al. 1996). Prediction is improved by combining parameters with another anatomical observation, such as the presence of liver herniation as assessed by ultrasound (Fig. 3) and/or by MRI (Fig. 4). Liver herniation was earlier shown to be closely related to survival chances (Bootstaylor et al. 1995; Albanese et al. 1998). In practice the population investigated is stratified based on the position of the liver and the LHR, with pivotal points below or above LHR = 1.0 and 1.4 (Table 2) (Metkus et al. 1996; Lipshutz et al. 1997; Harrison et al. 1998; Flake et al. 2000; Sbragia et al. 2000; Laudy et al. 2003; Heling et al. 2005; Jani et al. 2006a).
T2-WI of fetuses with left-sided CDH: (a) in a sagittal view at 27 weeks of gestation showing massive intrathoracic herniation of the left lobe of the liver (Li) and (b) in an axial view at 26 weeks of gestation showing intrathoracic herniation of the small bowel (SB) and liver (Li). Note the remaining right lung (RL)
These considerations became clear in a recent study including several large referral centers. This retrospective chart review included 184 fetuses, diagnosed with isolated left-sided CDH and assessed between 22 and 28 weeks, expectantly managed and live born after 30 weeks between 1996 and 2004. Liver herniation had a clear effect on LHR. LHR on itself correlated to survival, but the predictive value of LHR became better when the liver was herniated. Using both variables, allows a clinical stratification of risk. In fetuses with liver herniation, which theoretically have a 50% chance to survive, survival can be predicted more accurately from LHR measured in the second trimester. When a fetus has a 1.0 ≤ LHR < 1.6 it has a 66% chance to survive. At 1.6 or higher this would increase to 83% or more (Jani et al. 2006 a). In the past LHR = 1.0 has been considered as a pivotal point, and this is still so today, however a further refinement of prediction might be possible for fetuses under this threshold. In this multicenter study not a single patient with a 0.4 ≤ LHR ≤ 0.7 survived. Three fetuses with 0.8 ≤ LHR < 1.0 survived (16%). This study certainly shows that the critical margin of viability in fetuses with liver herniation lies somewhere in the transition of 0.7–1.0, and while individual chances might vary from center to center, this might be the current limit. Furthermore, since LHR increased with gestation, we recently introduced the observed to expected (o/e) LHR which provides a gestation independent measurement of lung size (Jani et al. 2007 b). In fetuses with isolated CDH, the o/e LHR represents at this stage the most validated method of 2D assessment of lung size in the prediction of postnatal survival.
3.1.2 Blood Flow and Resistance Measurements
Fetal echocardiography is able to assess repercussion of pulmonary hypoplasia on right and left ventricular proportions. It can measure branch pulmonary artery diameters, later shown to correlate to lung mass. In CDH, a larger contralateral diameter and discrepancy between contra- and ipsilateral diameters have been shown to predict neonatal death and morbidity indicators such as later oxygen requirements (Sokol et al. 2002). Next to determination of the size of vessels, Doppler interrogation of the pulmonary circulation or ductus arteriosus may be a proxy of vascular resistance. Pulmonary vascular resistance is indirectly related to hypoplasia in some diseases (Laudy and Wladimiroff 2000; Fuke et al. 2003; Mahieu-Caputo et al. 2004). Although theoretically promising, it is not likely that large enough changes in peak systolic and end diastolic velocities would be detected, as they are already minimal during pregnancy to start with. This adds to other limitations, such as the difficulty to insonate at perpendicular angles to the vessel in the presence of abnormal anatomy, and the variability of the waveform along the proximal or distal vasculature.
3.2 Prenatal Assessment with 3D-Ultrasound
In the assessment of lung size by LHR only the contralateral lung area in a single transverse section of the thorax is measured. Therefore, it seemed logic to investigate whether the measurement of total lung volume either by 3D ultrasound (Fig. 5) or MRI (Fig. 6) may provide better prediction of outcome than the LHR measurement.
Three-dimensional technology is now widely available and several studies have published normograms for multiplan or rotational volume 3D ultrasound acquisitions. Moeglin et al. demonstrated that both 3D techniques are interchangeable in normal patients (Moeglin et al. 2005). Much less data are available on 3D volumetry in patients at risk for lung hypoplasia (Kalache et al. 2003; Ruano et al. 2004).
In a recent study involving 47 fetuses with CDH evaluated between 21 and 36 weeks of gestation, we found that the prediction obtained using the o/e LHR-tracing method was better than the contralateral lung volume measured by 3D ultrasound (Jani et al. 2007 c). A possible explanation for our finding that o/e LHR-tracing is better in predicting outcome than volume, is that maximum compression of the contralateral lung occurs laterally rather than longitudinally. Therefore, the knowledge of the third dimension of the contralateral lung is of no use in improving the prediction of postnatal survival. This has been shown in another study comparing LHR with contralateral lung volume in fetuses with CDH (Jani et al. 2006 b). Consequently, in fetuses with expectantly managed isolated CDH, there is currently less focus in 3D ultrasound volume assessment in the prediction of postnatal outcome.
3.3 Prenatal Assessment with Fetal MRI
3.3.1 Prenatal Assessment of Lungs Based on Gestational Age
Superior tissue contrast, a large field of view and relative operator independence enables fetal MRI to provide information that can supplement the information obtained by prenatal ultrasound examination. The advantage with MRI is that both the ipsilateral and contralateral lungs can be visualized and measured reliably, whereas with 3D ultrasound it is not possible to examine the ipsilateral lungs in nearly half of the cases (Jani et al. 2007a). Relatively few studies have examined the potential value of total fetal lung volume (TFLV) as measured by MRI, in the prediction of outcome (Table 3) (Cannie et al. 2006; Gorincour et al. 2005; Mahieu-Caputo et al. 2001; Paek et al. 2001; Walsh et al. 2000; Williams et al. 2004). So far, the number of patients examined was too small to draw definite conclusions, let be allowing assessment of confounding factors such as intrathoracic herniation of the liver or gestation at delivery which have a profound impact on survival. Furthermore, no studies have compared the value of the volume assessment by MRI as compared to LHR in the prediction of postnatal survival in fetuses with CDH.
None of all the previous studies has taken into account other variables than the TFLV in the prediction of postnatal survival, some of them being consistently shown to be major predictors such as intrathoracic liver herniation (Jani et al. 2006a; Kitano et al. 2005; Hedrick et al. 2007). The largest study by Gorincour el al., although being a prospective study on 77 fetuses with isolated CDH, failed to integrate intrathoracic liver herniation as a determinant of postnatal survival. Furthermore, in the same study, gestation at diagnosis was shown to be predictive of postnatal survival and the prediction by lung size was not corrected for it making the conclusions less reliable.
In a recent study on 148 fetuses with isolated CDH assessed at 22–38 weeks of gestation, the o/e TFLV was found to be a strong predictor of postnatal survival (Jani et al. 2008). Unlike many other studies, a large part of our population was assessed at the end of the second trimester which is important when considering termination of pregnancy where in some countries it becomes illegal after 24 weeks of gestation and most importantly fetal therapy that should better be offered before 29 weeks of pregnancy.
It was shown that both o/e TFLV and intrathoracic herniation of the liver as determined by MRI provided independent prediction of subsequent postnatal survival at discharge from the hospital but not side of CDH, gestation at diagnosis and delivery, nor year and institution at which the patient was managed.
In the group with intrathoracic herniation of the liver there was an inverse linear correlation between o/e TFLV and subsequent survival. Essentially, the survival rate increased from 12% for those with o/e TFLV of 25% or less, to about 40% for o/e TFLV of 26–35, 60% for o/e TFLV of 36–45% and more than 70% for o/e TFLV of 46% or more. In the group with no intrathoracic herniation of the liver, the survival rate was about three times higher in the group of o/e TFLV of 25% or less with a 40% survival rate; however this was substantially smaller than the survival rates of at least 80% in all groups with o/e TFLV above 25%.
Finally, in nearly half of the cases, fetuses were assessed within 2 weeks of each other with both MRI and 2D ultrasound with measurement of o/e LHR using the longest method. The study showed that there was a trend towards a better prediction with TFLV measurement by MRI rather than a 2D measurement with ultrasound, however it was underpowered to show any significance.
3.3.2 Quantification of Intrathoracic Liver
In fetuses with CDH, prenatal assessment of intrathoracic liver position using ultrasound and/or MRI is a strong predictor of subsequent survival in the neonatal period (Walsh et al. 2000; Kitano et al. 2005; Hedrick et al. 2007), and some authors have also suggested that it was better than estimation based on the lung size as measured by LHR (Hedrick et al. 2007). Most of the studies reporting on intrathoracic liver in the prediction of survival in CDH have done so in a nonquantitative manner. The position of the liver is reported as a categorical variable as “liver up” or “liver down.”
Although for all studies intrathoracic liver was defined the same way, this has not necessarily been the case with studies where only a fraction of the liver would qualify as liver up, whereas others would only consider massive liver herniation. It is clear that standardization of the definition for liver herniation would help, but more importantly a more quantitative method for liver herniation is required, which would also permit better comparison of studies. This concept was already introduced by Walsh et al. (2000) obtaining the liver/diaphragm ratio in a single dimension on MR images and unlike lung volumes, the liver/diaphragm ratio was shown to be predictive of postnatal survival.
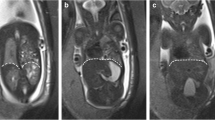
In a recent study and in fetuses with expectantly managed isolated CDH, the potential of volumetric quantification of intrathoracic liver herniation using MR imaging in the prediction of postnatal survival was evaluated (Cannie et al. 2008a). On axial T2-weighted images (T2-WI) the degree of intrathoracic liver herniation using the xyphoid process and thoracic apex as landmarks was defined (Fig. 7). Both of these are easily recognizable and unequivocal landmarks. The ratio of the liver to the thoracic cavity was calculated, further referred to as LiTR. It was demonstrated that measurement of LiTR is reproducible and that LiTR is larger in fetuses that subsequently died as compared to those that survived. Further, it was shown that LiTR provided independent prediction of survival from lung size as measured by o/e TFLV.
T2-WI of a fetus with left-sided CDH at 28 weeks of gestation (a) in a sagittal view showing the landmark at the xyphoid process (arrow) for the first plane of measurement (continuous line) and another plane higher into the thorax (interrupted line). Note the left lobe of the liver (Li) and stomach (St). Same fetus in an axial view at the level of the stomach (b) with delineation of the liver (continuous line) and the thoracic cavity (interrupted line)
3.3.3 Prenatal Assessment of Lungs Based on Fetal Biometry
To date, the most available normal ranges of TFLV are typically calculated based on gestational age and would exclude multiplets or fetuses under the fifth and above the 95th weight percentile as determined by ultrasound (Mahieu-Caputo et al. 2004; Rypens et al. 2001). Several biometric markers that can be obtained through the ultrasound or the MR examination, such as head circumference, femur length, liver volume or the combination of all markers have been proposed as an alternative to calculate relative lung volume in fetuses with normally developed lungs and those affected by pulmonary hypoplasia (Coakley et al. 2000; Williams et al. 2004). It was recently demonstrated that in normal fetuses, TFLV correlated better with such biometric markers than with GA. The advantage of the above biometric markers is their application irrespective of the actual fetal growth percentile or in multiple pregnancies.
As an alternative to such a complex process, we introduced the fetal body volume (FBV) as a single biometric variable which can be obtained throughout only one examination (Fig. 8). FBV has been earlier shown to be superior to ultrasound in predicting fetal weight at term, and our idea was to use it in an algorithm in order to improve postnatal prediction of survival in fetuses at risk for pulmonary hypoplasia.
In a recent study, we have built normal ranges of 200 fetuses between 16 and 40 weeks of gestation of TFLV vs. liver volume but also vs. FBV (Cannie et al. 2008 c). This study confirmed earlier studies from Coakley that TFLV correlated better with biometrical markers rather than with gestational age.
In another study and in fetuses with CDH, the benefit of predicting lung volume with FBV rather than with gestational age was shown (Cannie et al. 2008 b). The study included 53 fetuses with isolated CDH and showed that the prediction of survival based on gestational age vs. that based on FBV depends on fetal biometry and that there was a trend towards a better prediction when calculation was based on FBV rather than on gestational age. Clearly, correction for fetal biometry seems logical and very promising, but larger multicenter studies are still needed before wider use of fetal biometry in the prenatal prediction of postnatal survival in fetuses with CDH.
3.3.4 Prenatal Microstructural Assessment of Lungs
Microstructural assessment of the pulmonary system is less well developed. A leading study done by Osada et al. evaluated the lung/spinal fluid signal intensity ratio (Osada et al. 2004) and Brewerton et al. used another approach evaluating the lung/liver signal intensity ratio (Brewerton et al. 2005). However these studies are still small or methodologically flawed which needs to be solved before large implementation (Keller et al. 2004).
Studies on the evaluation of the human fetal lung using diffusion-weighted (DW) MRI are equally scarce. DW MRI is quantitatively expressed using the apparent diffusion coefficient (ADC). The first study by Moore et al. was conducted on 26 fetuses with normally developed lungs between 19 and 39 weeks of gestation (Moore et al. 2001) and showed an increase in the calculated ADC values with gestation. The second study by Balassy et al. was conducted on 53 fetuses with normally developed lungs between 20 and 37 weeks of gestation (Balassy et al. 2008) and was not able to identify a pattern of changes in the ADC values that correlate with lung maturation. They concluded that ADC cannot be used as an indicator of lung maturity. The most recent study was conducted by Manganaro et al. on 50 fetuses with normally developed lungs between 18 and 36 weeks of gestation (Manganaro et al. 2008). They found a significant correlation between ADC and gestational age and concluded that ADC can be considered as a new parameter for studying lung maturity.
The contradictory results between these studies confirms the fact that DWI in the fetal lungs needs certainly to be further explored in larger numbers and later on in the group of interest meaning fetuses at risk for pulmonary hypoplasia such as those with CDH.
Furthermore, both for DWI and T2 signal intensity of fetal lungs, results should be compared to functional studies performed using ultrasound on the vascular bed but also to histological findings in fetuses with termination of pregnancy in order to better understand the pathophysiology, behind the changes that are found.
4 Prenatal Prediction of Postnatal Morbidity
It is important to realize that survivors are not free of morbidity on the long term. They may suffer from serious long term morbidity, including chronic respiratory, feeding, hearing and neurodevelopmental problems (Muratore et al. 2001a, b; Jaillard et al. 2003; Trachsel et al. 2005; Crankson et al. 2006; Davis et al. 2004). Some newer neonatal strategies, like permissive hypercapnea and tolerance of lower oxygen saturation, may actually contribute to the latter, although they might improve survival. Morbidity may thus not be only related to the underlying condition but also to the postnatal therapy and can certainly not be ignored (Cortes et al. 2005; Stefanutti et al. 2004; Jaillard et al. 2003).
In severe cases, which are the targets of prenatal intervention (Deprest et al. 2004; Jani et al. 2005), one should be even more cautious not to substitute mortality by morbidity. For all these reasons, it is obvious that a proper long term multidisciplinary follow up program of CDH survivors is needed. High volume centers may be the best place to run such a program, caring for the kids and young adults even long time after discharge (West and Wilson 2005). Studies on prenatal prediction of postnatal morbidity in CDH are scarce although this became a clinical need for counseling parents, not only interested on the chances of their unborn child to survive but also on their quality of life.
A recent multicenter study on 100 survivors of expectantly managed isolated CDH was conducted to assess the prenatal measurement of o/e LHR and liver position in the prediction of postnatal morbidity events (Jani et al. 2009). O/e LHR and intrathoracic position of the liver were found to be independent significant predictors of the need for prosthetic patch repair. The incidence of gastroesophageal reflux was also related to the need for prosthetic patch repair. The o/e LHR predicted the need for postnatal assisted ventilation, supplemental O2 at 28 days – which may increase risk for longer term respiratory morbidity – and the postnatal age at full enteral feeding. This study established that in isolated CDH the prenatally assessed size of the contralateral lung is a significant predictor of the severity of the diaphragmatic defect, the functional consequences of impaired lung development and incidence of feeding problems. There are today no such studies based on fetal MRI in the prenatal prediction of postnatal morbidity in fetuses with isolated and expectantly managed CDH.
References
Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochillas L, Rao C, Pober BR, Babiuk RP, Epstein JA, Greer JJ, Beier DR (2005) Fog2 is required for normal diaphragm and lung development in mice and humans. PLos Genet 1:58–65
Albanese CT, Lopoo J, Goldstein RB, Filly RA, Feldstein VA, Calen PW, Jennings RW, Farrell JA, Harrison MR (1998) Fetal liver position and prenatal outcome for congenital diaphragmatic hernia. Prenat Diagn 18:1138–1142
Bagolan P, Casaccia G, Crescenzi F, Nahom A, Trucchi A, Giorlandino C (2004) Impact of a current treatment protocol on outcome of high-risk congenital diaphragmatic hernia. J Pediatr Surg 39:313–318
Bahlmann F, Merz E, Hallermann C, Stopfkuchen H, Kramer W, Hofmann M (1999) Congenital diaphragmatic hernia: ultrasonic measurement of fetal lungs to predict pulmonary hypoplasia. Ultrasound Obstet Gynecol 14:162–168
Balassy C, Kasprian G, Brugger PC, Csapo B, Weber M, Hörmann M, Bankier A, Bammer R, Herold CJ, Prayer D (2008) Diffusion-weighted MR imaging of the normal fetal lung. Eur Radiol 18:700–706
Bootstaylor BS, Filly RA, Harrison MR, Adzick NS (1995) Prenatal sonographic predictors of liver herniation in congenital diaphragmatic hernia. J Ultrasound Med 914:515–520
Brewerton LJ, Chari RS, Liang Y, Bhargava R (2005) Fetal lung-to-liver signal intensity ratio at MR imaging: development of a normal scale and possible role in predicting pulmonary hypoplasia in utero. Radiology 235:1005–1010
Butler N, Claireaux AE (1962) Congenital diaphragmatic hernia as a cause of perinatal mortality. Lancet 1:659–663
Cannie M, Jani JC, De Keyzer F, Devlieger R, Van Schoubroeck D, Witters I, Marchal G, Dymarkowski S, Deprest JA (2006) Fetal body volume: use at MR imaging to quantify relative lung volume in fetuses suspected of having pulmonary hypoplasia. Radiology 241:847–853
Cannie M, Jani J, Chaffiotte C, Vaast P, Deruelle P, Houfflin-Debarge V, Dymarkowski S, Deprest J (2008a) Quantification of intrathoracic liver herniation by magnetic resonance imaging and prediction of postnatal survival in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 32:627–632
Cannie M, Jani J, Meersschaert J, Allegaert K, Done’ E, Marchal G, Deprest J, Dymarkowski S (2008b) Prenatal prediction of survival in isolated diaphragmatic hernia with observed over expected total fetal lung volume determined by magnetic resonance imaging based either on gestational age or fetal body volume. Ultrasound Obstet Gynecol 32:633–639
Cannie M, Jani J, Van Kerkhove F, Meerschaert J, De Keyzer F, Lewi L, Deprest J, Dymarkowski S (2008c) Fetal body volume at MR imaging to quantify total fetal lung volume – normal ranges. Radiology 247:197–203
Coakley FV, Lopoo JB, Lu Y, Hricak H, Albanese CT, Harrison MR, Filly RA (2000) Normal and hypoplastic fetal lungs: volumetric assessment with prenatal single-shot rapid acquisition with relaxation enhancement MR imaging. Radiology 216:107–111
Colvin J, Bower C, Dickinson J, Sokol J (2005) Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 116:e356–e363
Cortes R, Keller R, Townsend T, Harrison MR, Farmer DL, Lee H, Piecuch RE, Leonard CH, Hetherton M, Bisgaard R, Nobuhara KK (2005) Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg 40:36–45
Crane JP (1979) Familial congenital diaphragmatic hernia: prenatal diagnostic approach and analysis of twelve families. Clin Genet 16:244–252
Crankson SJ, Al Jadaan SA, Namshan MA, Al-Rabeeah AA, Oda O (2006) The immediate and long-term outcomes of newborns with congenital diaphragmatic hernia. Pediatr Surg Int 22:335–340
Davis PJ, Firmin RK, Manktelow B, Goldman AP, Davis CF, Smith JH, Cassidy JV, Shekerdemian LS (2004) Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr 144:309–315
Deprest J, Gratacos E, Nicolaides KH, on behalf of the FETO task group (2004) Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 24:121–126
Dommergues M, Louis-Sylvestre C, Mandelbrot L, Oury JF, Herlicoviez M, Body G, Gamerre M, Dumez Y (1996) Congenital diaphragmatic hernia: can prenatal ultrasonography predict outcome? Am J Obstet Gynecol 174:1377–1381
Downard C, Jaksic T, Garza J, Dzakovic A, Nemes L, Jennings RW, Wilson JM (2003) Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg 38:729–732
Enns GM, Cox VA, Goldstein RB, Gibbs DL, Harrison MR, Golabi M (1998) Congenital diaphragmatic defects and associated syndromes, malformations and chromosomal anomalies. Am J Med Genet 79:215–225
Flake AW, Crombleholme TM, Johnson MP, Howell LJ, Adzick NS (2000) Treatment of severe congenital diaphragmatic hernia by fetal tracheal occlusion: clinical experience with fifteen cases. Am J Obstet Gynecol 183:1059–1066
Fuke S, Kanzaki T, Mu J, Wasada K, Takemura M, Mitsuda N, Murata Y (2003) Antenatal prediction of pulmonary hypoplasia by acceleration time/ejection time ratio of fetal pulmonary arteries by Doppler blood flow velocimetry. Am J Obstet Gynecol 188:228–233
Gallot D, Coste K, Francannet C, Laurichesse H, Boda C, Ughetto S, Vanlieferinghen P, Scheye T, Vendittelli F, Labbe A, Dechelotte PJ, Sapin V, Lemery D (2005) Antenatal detection and impact on outcome of congenital diaphragmatic hernia: a 12-year experience in Auvergne (France). Eur J Obstet Gynecol Reprod Biol 125:202–205
Gorincour G, Bouvenot J, Mourot MG, Sonigo P, Chaumoitre K, Garel C, Guibaud L, Rypens F, Avni F, Cassart M, Maugey-Laulom B, Bourliere-Najean B, Brunelle F, Durand C, Eurin D, Groupe Radiopediatrique de Recherche en Imagerie Foetale (GRRIF) (2005) Prenatal prognosis of congenital diaphragmatic hernia using magnetic resonance imaging measurement of fetal lung volume. Ultrasound Obstet Gynecol 26:738–744
Graham G, Devine PC (2005) Antenatal diagnosis of CDH. Semin Perinatol 29:69–76
Hara A, Chapin CJ, Ertsey R, Kitterman J (2005) Changes in fetal lung distension alter expression of vascular endothelial growth factor and its isoforms in the developing rat lung. Pediatr Res 58:30–37
Harrison M, Bjordal R, Langmark F, Knutrud O (1978) Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg 13:227–230
Harrison MR, Mychaliska GB, Albanese CT, Jennings RW, Farrell JA, Hawgood S, Sandberg P, Levine AH, Lobo E, Filly RA (1998) Correction of congenital diaphragmatic hernia in utero IX. Fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg 33:1017–1023
Hatch EI, Kendall J, Blumhagen J (1992) Stomach position as an in utero predictor of neonatal outcome in left-sided diaphragmatic hernia. J Pediatr Surg 27:778–779
Hedrick HL, Danzer E, Merchant A, Bebbington MW, Zhao H, Flake AW, Johnson MP, Liechty KW, Howell LJ, Wilson RD, Adzick NS (2007) Liver position and lung-to-head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. Am J Obstet Gynecol 197(422):e1–e4
Heling KS, Wauer RR, Hammer H, Bollmann R, Chaoui R (2005) Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetus with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 25:112–118
Jaillard SM, Pierrat V, Dubois A, Truffert P, Lequien P, Wurtz AJ, Storme L (2003) Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann Thorac Surg 75:250–256
Jani J, Gratacos E, Greenough A, Piero JL, Benachi A, Harrison M, Nicolaides K, Deprest J, FETO Task Group (2005) Percutaneous fetal endoscopic tracheal occlusion (FETO) for severe left sided congenital diaphragmatic hernia. Clin Obstet Gynecol N Am 48:910–922
Jani J, Keller RL, Benachi A, Nicolaides KH, Favre R, Gratacos E, Laudy J, Eisenberg V, Eggink A, Vaast P, Deprest J (2006a) Prenatal prediction of survival in isolated left-sided diaphragmatic hernia. Ultrasound Obstet Gynecol 27:18–22
Jani J, Peralta CFA, Van Schoubroeck D, Deprest J, Nicolaides KH (2006b) Relation between lung-to-head ratio and lung volume in normal fetuses and fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol 27:545–550
Jani J, Cannie M, Peralta CFA, Deprest J, Nicolaides KH, Dymarkowski S (2007a) Lung volumes in fetuses with congenital diaphragmatic hernia: comparison of 3D US and MR Imaging assessments. Radiology 244:575–582
Jani J, Nicolaides KH, Keller RL, Benachi A, Peralta CFA, Favre R, Moreno O, Tibboel D, Lipitz S, Eggink A, Vaast P, Allegaert K, Harrison M, Deprest J; on behalf of the antenatal-CDH-Registry group (2007b) Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 30:67–71
Jani J, Peralta CFA, Ruano R, Benachi A, Done E, Nicolaides KH, Deprest J (2007c) Comparison of fetal lung area to head circumference ratio with lung volume in the prediction of postnatal outcome in diaphragmatic hernia. Ultrasound Obstet Gynecol 30:850–854
Jani J, Cannie M, Sonigo P, Robert Y, Moreno O, Benachi A, Vaast P, Gratacos E, Nicolaides K, Deprest J (2008b) Prenatal prediction of survival in diaphragmatic hernia by fetal lung volume measured by magnetic resonance imaging. Ultrasound Obstet Gynecol 32:793–799
Jani J, Benachi A, Nicolaides KH, Allegaert K, Gratacós E, Mazkereth R, Matis J, Tibboel D, van Heijst A, Storme L, Rousseau V, Greenough A, Deprest JA; and the antenatal-CDH-Registry group (2009) Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol 33:64–69
Kalache KD, Espinoza J, Chaiworapongsa T, Londono J, Schoen ML, Treadwell MC, Lee W, Romero R (2003) Three-dimensional ultrasound fetal lung volume measurement: a systematic study comparing the multiplanar method with the rotational (VOCAL) technique. Ultrasound Obstet Gynecol 21:111–118
Keijzer R, Liu J, Deimling J, Tibboel D, Post M (2000) Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol 156:1299–1306
Keller TM, Rake A, Michel SC, Seifert B, Wisser J, Marincek B, Kubik-Huch RA (2004) MR assessment of fetal lung development using lung volumes and signal intensities. Eur Radiol 14:984–989
Kitano Y, Nakagawa S, Kuroda T, Honna T, Itoh Y, Nakamura T, Morikawa N, Shimizu N, Kashima K, Hayashi S, Sago H (2005) Liver position in fetal congenital diaphragmatic hernia retains a prognostic value in the era of lung-protective strategy. J Pediatr Surg 40:1827–1832
Laudy JA, Wladimiroff J (2000) The fetal lung 2: pulmonary hypoplasia. Ultrasound Obstet Gynecol 16:482–494
Laudy JAM, Van Gucht M, Van Dooren MF, Wladimiroff JW, Tibboel D (2003) Congenital diaphragmatic hernia. an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diag 23:634–639
Lipshutz GS, Albanese CT, Feldstein VA, Jennings RW, Housley HT, Beech R, Farrell JA, Harrison MR (1997) Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatric Surg 32:1634–1636
Mahieu-Caputo D, Sonigo P, Dommergues M, Fournet JC, Thalabard JC, Abarca C, Benachi A, Brunelle F, Dumez Y (2001) Fetal lung volume measurement by magnetic resonance imaging in congenital diaphragmatic hernia. BJOG 108:863–868
Mahieu-Caputo D, Aubry MC, El Sayed M, Joubin L, Thalabard JC, Dommergues M (2004) Evaluation of fetal pulmonary vasculature by power Doppler imaging in congenital diaphragmatic hernia. J Ultrasound Med 23:1011–1017
Manganaro L, Perrone A, Sassi S, Fierro F, Savelli S, Di Maurizio M, Tomei A, Francioso A, La Barbera L, Giancotti A, Ballesio L (2008) Diffusion-weighted MR imaging and apparent diffusion coefficient of the normal fetal lung: preliminary experience. Prenat Diagn 28:745–748
Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS (1996) Sonographic predictors of survival in fetal diaphragmatic hernia. J Ped Surg 31:148–151
Moeglin D, Talmant C, Duyme M, Lopez AC, CFEF (2005) Fetal lung volumetry using two- and three-dimensional ultrasound. Ultrasound Obstet Gynecol 25:119–127
Moore RJ, Strachan B, Tyler DJ, Baker PN, Gowland PA (2001) In vivo diffusion measurements as an indication of fetal lung maturation using echo planar imaging at 0.5T. Magn Reson Med 45:247–253
Muratore C, Kharasch V, Lund D, Sheils C, Friedman S, Brown C, Utter S, Jaksic T, Wilson J (2001a) Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J Pediatr Surg 36:133–140
Muratore C, Utter S, Jaksic T, Lund D, Wilson J (2001b) Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg 36:1171–1176
Norio R, Kaariainen H, Rapola J, Herva R, Kekomäki M (1984) Familial CDH defects: aspects of etiology, prenatal diagnosis and treatment. Am J Med Genet 17:471–483
Osada H, Kaku K, Masuda K, Iitsuka Y, Seki K, Sekiya S (2004) Quantitative and qualitative evaluations of fetal lung with MR imaging. Radiology 231:887–892
Paek BW, Coakley FV, Lu Y, Filly RA, Lopoo JB, Qayyum A, Harrison MR, Albanese CT (2001) Congenital diaphragmatic hernia: prenatal evaluation with MR lung volumetry-preliminary experience. Radiology 220:63–67
Ruano R, Benachi A, Joubin L, Aubry MC, Thalabard JC, Dumez Y, Dommergues M (2004) Three-dimensional ultrasonographic assessment of fetal lung volume as prognostic factor in isolated congenital diaphragmatic hernia. BJOG 111:423–429
Rypens F, Metens T, Rocourt N, Sonigo P, Brunelle F, Quere MP, Guibaud L, Maugey-Laulom B, Durand C, Avni FE, Eurin D (2001) Fetal lung volume: estimation at MR imaging-initial results. Radiology 219:236–241
Sbragia L, Paek B, Filly RA, Harrison MR, Farrell J, Farmer D, Albanese CT (2000) Congenital diaphragmatic hernia without herniation of the liver: does the lung-to-head ratio predict survival? J Ultrasound Med 19:845–848
Scott L (2004) Ontario Congenital Anomalies Study Group. Apparent truth about congenital diaphragmatic hernia: a population-based database is needed to establish benchmarking for clinical outcomes for CDH. J Pediatr Surg 39:661–665
Sharland GK, Lockhart SM, Heward AJ, Allan LD (1992) Prognosis in fetal diaphragmatic hernia. Am J Obstet Gynecol 166:9–13
Skari H, Bjornland K, Haugen G, Egeland T, Emblem R (2000) CDH: a meta-analysis of martoality factors. J Ped Surg 35:1187–1197
Slavotinek AM (2005) The genetics of congenital diaphragmatic hernia. Sem Perinatol 29:77–85
Sokol J, Bohn D, Lacro R, Ryan G, Stephens D, Rabinovitch M, Smallhorn J, Hornberger LK (2002) Fetal pulmonary artery diameters and their association with lung hypoplasia and postnatal outcome in congenital diaphragmatic hernia. Am J Obstet Gynecol 186:1085–1090
Stefanutti G, Filippone M, Tommasoni N, Midrio P, Zucchetta P, Moreolo GS, Toffolutti T, Baraldi E, Gamba P (2004) Cardiopulmonary anatomy and function in long-term survivors of mild to moderate congenital diaphragmatic hernia. J Pediatr Surg 39:526–531
Stege G, Fenton A, Jaffray B (2003) Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 12:532–535
Thebaud B, Azancot A, de Lagausie P, Vuillard E, Ferkadji L, Benali K, Beaufils F (1997) Congenital diaphragmatic hernia: antenatal prognostic factors. Does cardiac ventricular disproportion in utero predict outcome and pulmonary hypoplasia? Intensive Care Med 23:10062–10069
Torfs CP, Curry CJ, Bateson TF, Honoré LH (1992) A population-based study of congenital diaphragmatic hernia. Teratology 46:555–565
Trachsel D, Selvadurai H, Bohn D, Langer JC, Coates AL (2005) Long-term pulmonary morbidity in survivors of congenital diaphragmatic hernia. Pediatr Pulmonol 39:433–439
Walsh DS, Hubbard AM, Olutoye OO, Howell LJ, Crombleholme TM, Flake AW, Johnson MP, Adzick NS (2000) Assessment of fetal lung volumes and liver herniation with magnetic imaging in congenital diaphragmatic hernia. Am J Obstet Gynecol 183:1067–1069
West S, Wilson J (2005) Follow up of infants with congenital diaphragmatic hernia. Semin Perinatol 29:129–133
Williams G, Coakley FV, Qayyum A, Farmer DL, Joe BN, Filly RA (2004) Fetal relative lung volume: quantification by using prenatal MR imaging lung volumetry. Radiology 233:457–462
Witters I, Legius E, Moerman P, Deprest J, Van Schoubroeck D, Timmerman D, Van Assche FA, Fryns JP (2001) Associated malformations and chromosomal anomalies in 42 cases of prenatally diagnosed diaphragmatic hernia. Am J Med Genet 103:278–282
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Cannie, M., Jani, J. (2010). Diagnosis of Congenital Diaphragmatic Hernia. In: Prayer, D. (eds) Fetal MRI. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2010_21
Download citation
DOI: https://doi.org/10.1007/174_2010_21
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-73270-9
Online ISBN: 978-3-540-73271-6
eBook Packages: MedicineMedicine (R0)