Abstract
Background
Magnetic resonance imaging (MRI) assesses pulmonary hypoplasia in fetal congenital diaphragmatic hernia (CDH). Neonatal mortality may occur with CDH.
Objective
To quantify MRI parameters associated with neonatal survival in fetuses with isolated CDH.
Materials and methods
Fetal MRI for assessing CDH included region of interest (ROI) measurements for total lung volume (TLV), herniated liver volume, herniated other organ volume and predicted lung volume. Ratios of observed lung volume and liver up volume to predicted lung volume (observed to predicted TLV, percentage of the thorax occupied by liver) were calculated and compared to neonatal outcomes. Analyses included Wilcoxon rank sum test, multivariate logistic regression and receiver operating characteristic (ROC) curves.
Results
Of 61 studies, the median observed to predicted TLV was 0.25 in survivors and 0.16 in non-survivors (P=0.001) with CDH. The median percentage of the thorax occupied by liver was 0.02 in survivors and 0.22 in non-survivors (P<0.001). The association of observed to predicted TLV and percentage of the thorax occupied by liver with survival for gestational age (GA) >28 weeks was greater compared to GA ≤28 weeks. The ROC analysis demonstrated an area under the curve of 0.96 (95% confidence interval 0.91–1.00) for the combined observed to predicted TLV, percentage of the thorax occupied by liver and GA.
Conclusion
The percentage of the thorax occupied by liver and observed to predicted TLV was predictive of neonatal survival in fetuses with CDH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) affects approximately 1–2 in 5,000 births [1,2,3]. Overall mortality frequencies range from 61–68% [2, 4] and 44–47.9% for live born infants [1, 2, 4, 5]. There is a significant burden on the health care system due to associated morbidity, mortality and long-term sequelae [6,7,8,9].
In isolated CDH, mortality and morbidity have been shown to be related to pulmonary hypertension and the degree of pulmonary hypoplasia [10, 11]. Prenatal imaging has demonstrated utility in predicting lung volumes and may be used to predict the degree of pulmonary hypoplasia [12,13,14,15,16]. Ultrasound is the primary modality utilized in fetal imaging, and one of the first imaging parameters to predict pulmonary volume was the ultrasound lung–head ratio [10, 17,18,19]. However, studies have demonstrated variability in the utility of the lung–head ratio, and the lung–head ratio has been shown to increase with increasing gestational age (GA) [20,21,22]. Fetal magnetic resonance imaging (MRI) may provide a useful adjunct in the evaluation of lung volumes. With the advent of fast MRI sequences, MRI has increasingly been used in prenatal evaluation of CDH. Multiple MRI parameters to evaluate pulmonary hypoplasia in patients with CDH have previously been investigated [14, 18, 19, 23,24,25,26]. However, many of the previous studies evaluating fetal MRI in CDH relied on lung volume nomograms to generate observed to expected lung volume ratios, rather than calculating expected lung volumes within each fetus. Additionally, although previous studies have evaluated the presence of liver within the hernia, there are limited studies evaluating the quantified volume of liver herniation [19, 26,27,28]. We sought to combine both lung and liver volumes within the chest along with GA at the time of fetal MRI to offer prognostic findings, specifically, neonatal survival. Overall survival rates have not significantly changed over time [1, 2]. However, more recent studies suggest better neonatal outcomes, perhaps in improving morbidity, with more precise prenatal evaluation as well as referral to a tertiary care center [1, 23].
Our objective was to identify the MRI parameters associated with neonatal survival in isolated CDH. We predict that calculating the degree of liver herniation as well as actual lung volumes will improve the prediction of neonatal survival.
Materials and methods
We performed an institutional review board (IRB)-approved retrospective observational study of fetuses with CDH evaluated with fetal MRI in a single center.
Patients
Inclusion criteria included patients undergoing fetal MRI for evaluation of left-side CDH between August 2000 and December 2018 with survival data available. This data was obtained from the fetal MRI clinical database at our institution. Exclusion criteria included right-side or bilateral CDH and/or antenatal diagnosis of aneuploidy or additional complex congenital anomalies that would preclude surgical correction. Data collected included GA at the time of MRI exam; side of diaphragmatic defect; MRI-based measurement of fetal lung volume, hernia volume and herniated liver volume; neonatal survival; and the use of extracorporeal membrane oxygenation (ECMO). Neonatal survival was defined as discharge from the neonatal intensive care unit.
Management protocol
During the study period, CDH management did not significantly change at our institution. In general, management consisted of ventilation to support oxygenation followed by operative repair after the neonate demonstrated evidence of his or her ability to oxygenate blood. Surgical approach largely also did not change during the study period and consisted of reducing intrathoracic abdominal contents into the abdomen and closing the defect with either primary closure, muscle flap or mesh based on the size of the defect. Additionally, the decision to operate was based solely on the patient’s hemodynamic stability.
Magnetic resonance imaging protocol
All fetal MRI exams were performed on a 1.5-tesla (T) system (Siemens Magnetom Avanto; Siemens Healthineers, Erlangen, Germany; or GE Signa; GE Medical Systems, Waukesha, WI) utilizing a multi-channel body coil. Pregnant mothers were placed in a supine or decubitus position. No sedation or contrast administration was used during image acquisition. Free-breathing T2-weighted single-shot fast spin echo (SSFSE) sequences (repetition time [TR]/echo time [TE] 1,100/115 ms, flip angle 150°, matrix size 192×256 mm, section thickness 3 mm, no intersection gap) were obtained in transverse, sagittal and coronal planes. Free-breathing T2-weighted short tau inversion recovery (STIR) sequences (TR/TE 1,600/89 ms, flip angle 150°, matrix size 179×256 mm, slice thickness 5 mm, no intersection gap) were obtained in transverse, sagittal and coronal planes. Breath-hold T1-weighted gradient recalled echo (GRE) sequences (TR/TE 117/4.75 ms, flip angle 70°, matrix size 192×256 mm, slice thickness 5.5 mm, intersection gap 0.6 mm) were obtained in the coronal plane. Balanced steady-state free precession (bSSFP) free-breathing sequences (TR/TE 6.07/2.45 ms, flip angle 54°, matrix size 256×256 mm, slice thickness 5 mm, no intersection gap) were obtained in the coronal plane at 5-mm slice thickness.
Magnetic resonance imaging assessment and interpretation
Images were evaluated by two investigators in concert: one fellowship-trained, board-certified radiologist (D.M.T.) with more than 25 years of experience in obstetrics imaging including antenatal ultrasound and fetal MRI and one fellowship-trained, board-certified radiologist (A.A.B.) with 5 years of experience in fetal MRI. All measurements were obtained on Philips IntelliSpace PACS Radiology version 4.4 workstations (Philips Healthcare, Brisbane, CA). Measurements obtained from MR images included actual observed lung volume, intrathoracic liver volume and herniated organ volume. The diaphragm location was estimated at the level of the defect to obtain these volumes (Fig. 1). Volumes of the visualized lung parenchyma (Fig. 2), herniated organs (Fig. 2) and intrathoracic liver (Fig. 3) were obtained by drawing a region of interest (ROI) measurement around the area of interest (i.e. visualized lung parenchyma, herniated organs or intrathoracic liver) for each slice, adding the ROI measurements, and then multiplying the summed measurements by the slice thickness. Mediastinum was excluded from the volume measurements. Visualized lung parenchyma (observed total lung volume [TLV]) was defined as the volume of lung parenchyma present. Herniated organ volume (mass) was defined as the volume of herniated contents in the thoracic cavity (e.g., stomach and bowel). Intrathoracic liver volume (liver up) was defined as the volume of liver present within the thoracic cavity. ROI measurements were completed in the coronal plane. The total thoracic cavity volume, not including the mediastinum, was the summation of observed TLV, volume of herniated liver, and volume of herniated organs, referred to as the predicted TLV. This was preferred over nomograms of expected TLV because of the large variability of fetal weights and, therefore, lung volume measurements. The measurements were then used to calculate the percentage of the thoracic cavity occupied by lung (observed to predicted TLV) and the percentage of thoracic cavity occupied by liver (% of thorax occupied by liver).
Diaphragm location. a–c Sequential T2-W single-shot fast spin echo coronal images obtained at 32 weeks’ gestation through the posterior (a), mid (b) and anterior (c) fetal chest with left congenital diaphragmatic hernia demonstrating diaphragm location (dashed lines). The diaphragm’s location on the side of the defect was estimated using the contralateral lung as reference. Although the hemidiaphragms are not necessarily at the same level, the contralateral diaphragm was favored to be the best approximation of diaphragm level on the side of the defect
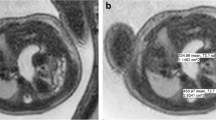
Region of interest (ROI) measurements of a left congenital diaphragmatic hernia. a–c Sequential T2-W single-shot fast spin echo coronal images obtained at 32 weeks’ gestation through the posterior (a), mid (b) and anterior (c) fetal chest demonstrate a left congenital diaphragmatic hernia. The dotted line indicates ROI of residual lung, the dashed line indicates ROI of hernia contents and the solid line indicates ROI of the mediastinum. The liver is located in the right upper quadrant of the abdomen, and no intrathoracic liver is identified
Region of interest (ROI) measurements of the intrathoracic liver. a–c Coronal T2-W short tau inversion recovery (a), breath-hold T1 gradient recalled echo (b) and balanced steady-state free precession (c) images obtained at 27 weeks’ gestation through the fetal thorax demonstrate left congenital diaphragmatic hernia with the intrathoracic liver. The dotted line indicates ROI of residual lung, the dashed line indicates ROI of mediastinum and the solid line indicates ROI of liver
Statistical analysis
Statistical analyses included the Wilcoxon rank sum test for continuous measures, receiver operating characteristic (ROC) curves, area under the curve (AUC) and multivariate regression analysis. The Wilcoxon rank sum test was chosen because of the size of the statistical sample.
Results
One hundred and ten MRI studies were performed for CDH from 2000 to 2018. Neonatal survival data were available for 61 of these exams. Eleven patients were found to have additional complex fetal anomalies and were excluded from evaluation (Table 1). Ten patients were found to have either right-side CDH (8) or bilateral CDH (2) and were also excluded from evaluation. Forty patients with survival data met inclusion criteria. Of the study patients, there were 21/40 male fetuses (52.5%) and 19/40 female fetuses (47.5%). There were 21/40 survivors for a survival frequency of 52.5%. The median GA at the time of MRI exam was 32 weeks; 30 exams were performed after 28 weeks’ GA, and 10 exams were performed before or at 28 weeks’ GA. The observed to predicted TLV ranged from 0.06 to 1.00 and the % of thorax occupied by liver ranged from 0 to 0.67. Eleven patients required ECMO.
The medians and quartiles of observed to predicted TLV and % of thorax occupied by liver were compared between survivors and non-survivors (Table 2). Medians and quartiles were compared due to the nonstandardized distribution of the patient population. Using the Wilcoxon rank sum test, there was a significantly higher observed to predicted TLV for survivors compared to non-survivors (P=0.001), and there was a significantly lower % of thorax occupied by liver for survivors compared to non-survivors (P<0.001). The data were further evaluated based on GA at the time of study (Table 3). For exams performed after 28 weeks’ GA, the P-values decreased for both observed to predicted TLV and % of thorax occupied by liver.
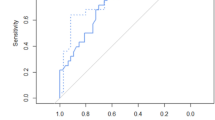
An ROC curve was generated to predict neonatal survival using GA, MRI-derived observed to predicted TLV and % of thorax occupied by liver (Fig. 4). ROC analysis demonstrated an AUC of 0.96 (95% confidence interval [CI]: 0.91, 1.00). ROC curves were also generated to predict survival using observed to predicted TLV only or % of thorax occupied by liver only (Fig. 5). ROC analysis for observed to predicted TLV demonstrated an AUC of 0.80 (95% CI: 0.66, 0.94). ROC analysis for % of thorax occupied by liver demonstrated an AUC of 0.88 (95% CI: 0.77, 0.99). Comparing the three ROC curves demonstrated that a combination of factors (GA, observed to predicted TLV and % of thorax occupied by liver) generated the largest AUC and had the greatest strength of prediction. Multivariable analysis evaluating observed to predicted TLV, % of thorax occupied by liver and GA at the time of MRI exam demonstrated significant differences between survivors and non-survivors (Fig. 6).
The receiver operator characteristic (ROC) curve predicts neonatal survival using gestational age, MRI-derived observed to predicted total lung volume (TLV) and percentage of thorax occupied by liver. ROC analysis demonstrates the value of using observed to predicted TLV, percentage of thorax occupied by liver and gestational age at the time of the exam in predicting survival in isolated congenital diaphragmatic hernia. AUC area under the curve, CI confidence interval, GA gestational age
Receiver operator characteristic (ROC) curves compare a combination of factors with observed to predicted total lung volume (TLV) alone or percentage of thorax occupied by liver alone. ROC analysis demonstrates that a combination of factors generated the largest area under the curve (AUC) with the greatest strength of prediction. Ninety-five percent confidence intervals are given in parentheses. GA gestational age
About one-fourth of our patient population received ECMO (11/40; 27.5%). An ROC curve generated to predict the need for ECMO based on observed to predicted TLV, % of thorax occupied by liver and GA demonstrated an AUC of 0.67 (95% CI: 0.51, 0.83). Both observed to predicted TLV and % of thorax occupied by liver were not significantly different in predicting the need for ECMO. Patients requiring ECMO had higher mortality with 10/11 (90.9%) dying.
Discussion
CDH is a complex medical problem with significant societal cost [6, 8, 11] and stagnant survival rates over the past several decades despite advances in both detection and management [1, 2, 9]. Studies have demonstrated that outcomes in isolated CDH are related to both the degree of pulmonary hypoplasia and the presence of pulmonary hypertension [10, 11]. Imaging provides assessment of pulmonary hypoplasia [14, 29]. Further research into the imaging parameters has the potential to significantly impact management of this disease.
The present study demonstrated that observed to predicted TLV and % of thorax occupied by liver evaluated in conjunction with GA at the time of the exam were most predictive of neonatal survival in isolated left-side CDH. Furthermore, both the % of thorax occupied by liver and the observed to predicted TLV were independent predictors of neonatal survival in isolated CDH. The strength of prediction (e.g., greater differences in medians and lower P-values) improved with MRI exams performed after 28 weeks’ GA. This may indicate weaker evidence when the MRI exam is performed earlier than 28 weeks and, furthermore, more confidence of prediction of survival when the exam is performed at a later GA [3, 30]. However, fewer fetuses were evaluated before 28 weeks in our study, and therefore, higher P values may be related to the smaller sample size and weaker power. This may influence practice guidelines regarding when fetal MRI for CDH should be performed.
The observed to predicted TLV, % of thorax occupied by liver and the GA at the time of the exam were not found to predict the need for ECMO use in our study. However, only a small number of patients required ECMO use (n=11), and smaller sample size may result in weaker, more limited data.
Our study had several limitations. Evaluation was retrospective and our patient population was predominantly from outside referents. Therefore, despite having a large total number of patients who were evaluated with CDH, outcome data were not available in a percentage of patients. GA at birth was not available for a large percentage of our imaged patients, including study participants. Planning a prospective evaluation would improve this aspect of the study. The sample size was small, in part due to the lack of outcome data, specifically for evaluations before 28 weeks’ GA. Additionally, as a referral center, it may not be possible to extrapolate results to the general population. Ultrasound imaging parameters were not included in evaluation or comparison. Additionally, other parameters, such as GA at delivery, birth weight, Apgar scores and the time of CDH repair were also not included in the evaluation.
The use of antenatal imaging in the management of CDH is variable across centers. This is, in part, due to differences in access and resources available to centers [31]. Furthermore, most of the current data about CDH are related to single-center experiences. Single-center experiences are difficult to generalize and reproduce as outcomes are variable due to differences in center protocols, experience and resource availability. Theoretically, antenatal imaging data may better triage patients to centers capable of managing CDH as well as select the appropriate patients for fetal intervention. However, this does not consistently occur.
Antenatal imaging parameters generally do not dictate postnatal management of CDH. The ultimate goal is to use antenatal data to initially identify those potentially high-risk fetuses requiring care in a tertiary referral center, aid in delivery management, more expeditiously effect bedside decision-making after delivery and possibly reduce mortality and morbidity related to delays in care. Standardized management guidelines in CDH have not been developed, largely due to differences in the resources available to centers. However, limited work has been initiated to develop practice guidelines [32, 33]. Future directions include a large multicenter trial to improve sample size and power of the study as well as reproducibility. Prospective analysis using data from our study and additional data comparing ultrasound with MRI would be useful to further extrapolate and validate study outcomes.
Conclusion
Fetal MRI in the setting of CDH provides discriminating quantitative evaluation of actual lung volumes and liver up in association with survival during the neonatal period. This data may assist prenatal counseling and clinical decisions regarding delivery, surgery and neonatal intervention.
References
Balayla J, Abenhaim HA (2014) Incidence, predictors and outcomes of congenital diaphragmatic hernia: a population-based study of 32 million births in the United States. J Matern Fetal Neonatal Med 27:1438–1444
Colvin J, Bower C, Dickinson JE, Sokol J (2005) Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 116:e356–e363
Kotecha S, Barbato A, Bush A et al (2012) Congenital diaphragmatic hernia. Eur Respir J 39:820–829
Stege G, Fenton A, Jaffray B (2003) Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 112:532–535
Brownlee EM, Howatson AG, Davis CF, Sabharwal AJ (2009) The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg 44:317–320
Faraoni D, Nasr VG, DiNardo JA, Thiagarajan RR (2016) Hospital costs for neonates and children supported with extracorporeal membrane oxygenation. J Pediatr 169:69–75.e61
Hollinger LE, Harting MT, Lally KP (2017) Long-term follow-up of congenital diaphragmatic hernia. Semin Pediatr Surg 26:178–184
Metkus AP, Esserman L, Sola A et al (1995) Cost per anomaly: what does a diaphragmatic hernia cost? J Pediatr Surg 30:226–230
Zamora IJ, Oluyinka OO, Cass DL et al (2014) Prenatal MRI fetal lung volumes and percent liver herniation predict pulmonary morbidity in congenital diaphragmatic hernia (CDH). J Pediatr Surg 49:688–693
Metkus AP, Filly RA, Stringer MD et al (1996) Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 31:148–151
Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB (2009) Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the congenital diaphragmatic hernia study group. J Pediatr Surg 44:1315–1321
Busing KA, Kilian AK, Schaible T et al (2008) Reliability and validity of MR image lung volume measurement in fetuses with congenital diaphragmatic hernia and in vitro lung models. Radiology 246:553–561
Jani JC, Cannie M, Peralta CFA et al (2007) Lung volumes in fetuses with congenital diaphragmatic hernia: comparison of 3D US and MR imaging assessments. Radiology 244:575–582
Ruano R, Aubry M-C, Barthe B et al (2009) Three-dimensional ultrasonographic measurements of the fetal lungs for prediction of perinatal outcome in isolated congenital diaphragmatic hernia. J Obstet Gynaeco Res 35:1031–1041
Walleyo A, Debus A, Kehl S et al (2013) Periodic MRI lung volume assessment in fetuses with congenital diaphragmatic hernia: prediction of survival, need for ECMO, and development of chronic lung disease. AJR Am J Roentgenol 201:419–426
Gerards FA, Twisk JWR, Bakker M et al (2007) Fetal lung volume: three-dimensional ultrasonography compared with magnetic resonance imaging. Ultrasound Obstet Gynecol 29:533–536
Jani J, Keller RL, Benachi A et al (2006) Prenatal prediction of survival in isolated left-sided diaphragmatic hernia. Ultrasound Obstet Gynecol 27:18–22
Oluyomi-Obi T, Kuret V, Puligandla P et al (2017) Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH). J Pediatr Surg 52:881–888
Ruano R, Lazar DA, Cass DL et al (2014) Fetal lung volume and quantification of liver herniation by magnetic resonance imaging in isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 43:662–669
Arkovitz MS, Russo M, Devine P et al (2007) Fetal lung-head ratio is not related to outcome for antenatal diagnosed congenital diaphragmatic hernia. J Pediatr Surg 42:107–110
Snoek KG, Peters NCJ, van Rosmalen J et al (2017) The validity of the observed-to-expected lung-to-head ratio in congenital diaphragmatic hernia in an era of standardized neonatal treatment; a multicenter study. Prenat Diagn 37:658–665
Yang SH, Nobuhara KK, Keller RL et al (2007) Reliability of the lung-to-head ratio as a predictor of outcome in fetuses with isolated left congenital diaphragmatic hernia at gestation outside 24-26 weeks. Am J Obstet Gynecol 197:30.e1–30.e7
Bebbington M, Victoria T, Danzer E et al (2014) Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 43:670–674
Deprest J, Jani J, Cannie M et al (2006) Prenatal intervention for isolated congenital diaphragmatic hernia. Curr Opin Obstet Gynecol 18:355–367
Paek BW, Coakley FV, Lu Y et al (2001) Congenital diaphragmatic hernia: prenatal evaluation with MR lung volumetry — preliminary experience. Radiology 220:63–67
Worley KC, Dashe JS, Barber RG et al (2009) Fetal magnetic resonance imaging in isolated diaphragmatic hernia: volume of herniated liver and neonatal outcome. Am J Obstet Gynecol 200:318.e1-6
Akinkuotu AC, Cruz SM, Abbas PI et al (2016) Risk-stratification of severity for infants with CDH: prenatal versus postnatal predictors of outcome. J Pediatr Surg 51:44–48
Mullassery D, Ba'ath ME, Jesudason EC, Losty PD (2010) Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol 35:609–614
Spaggiari E, Stirnemann JJ, Sonigo P et al (2015) Prenatal prediction of pulmonary arterial hypertension in congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 45:572–577
Jani J, Nicolaides KH, Benachi A et al (2008) Timing of lung size assessment in the prediction of survival in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol 31:37–40
Lally PA, Skarsgard ED (2017) Congenital diaphragmatic hernia: the role of multi-institutional collaboration and patient registries in supporting best practice. Semin Pediatr Surg 26:129–135
Jancelewicz T, Brindle ME, Guner YS et al (2019) Toward standardized management of congenital diaphragmatic hernia: an analysis of practice guidelines. J Surg Res 243:229–235
Canadian Congenital Diaphragmatic Hernia Collaborative, Puligandla PS, Skarsgard ED et al (2018) Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ 190:E103-e112
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, A.A., Furey, E.A., Bailey, A.A. et al. Fetal liver and lung volume index of neonatal survival with congenital diaphragmatic hernia. Pediatr Radiol 51, 1637–1644 (2021). https://doi.org/10.1007/s00247-021-05049-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05049-0