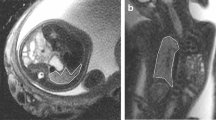
Abstract
Perinatal survival crucially depends on a sufficiently sized and functionally well-developed cardiopulmonary system. Especially, the stage of lung development and the biochemical and structural maturity of the fetal lung are the most important determinants for survival and after birth. Since our understanding of the complexity of the processes influencing lung growth has substantially improved, this knowledge can now be translated into clinical science. Fetal MR has opened a new exciting field in the prenatal assessment of lung maturity, since it noninvasively provides important data on the size, structure, and biochemical maturity of the fetal lung. In order to confidently diagnose developmental pathologies of the growing respiratory system and evaluate their impact on extrauterine life, it is of utmost importance to be fully aware of the potential and limitations of new MR imaging methods in the characterization of normal fetal lung development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Normal Fetal Lung Development
As most of the fetal organs, the structure and morphology of the fetal lung change during development. The tissue composition of this organ is variable even at later stages of intrauterine life. This is mirrored by the changing appearance of fetal lung tissue on different MR sequences. Currently, the principles of human fetal lung development are understood and the microscopic appearance of the organ well described. However, since it is impossible to perform correlations between MR and histology in normally developing fetuses, the microstructural background for the MR appearance of the fetal lung is rather hypothetical. Still, by knowing the principles of fetal lung development on a histological and anatomical basis, a reasonable interpretation of fetal MR images and the confident detection of abnormalities are possible. Figure 1 schematically illustrates the overlapping histological stages of fetal lung development and the major changes seen on different MR sequences.
During the first trimester, the developing lung resembles structurally and functionally more an exophytic gland than a parenchymatous organ. By 26 embryonic days, two lung buds bulge out of the ventrolateral wall of the primitive foregut (Burri 1997) and mark the onset of growth of the primordial airways. Shortly after this event, the first vascular structures appear and surround the tubular appearing airways (Hall et al. 2002). By 6 GW, all lobar bronchi are generated and the trachea is coated by a single layer of smooth muscle. The further dividing primordial pathways serve as a template for the accompanying pulmonary vasculature. Besides the early developing pulmonary veins and arteries, the bronchial arteries appear later at 8 GW.
Dichotomous airway branching mainly occurs in the following pseudoglandular stage of lung development between 10 and 14 GW, with all conducting airways developed by 16 GW (Reid 1977; Thurlbeck 1988). At this stage, the fetal lung appears as tubular structure with poorly differentiated cellular lining (Fig. 1).
The processes influencing lung growth during the following canalicular stage substantially determine the postnatal viability of the developing fetus. Structurally, the canalicular stage is characterized by widening of the airway lumina, growth of the respiratory airways, and most importantly, flattening and massive angiogenesis at the future gas-exchanging developing alveolar structures, accompanied by rapid growth of the distal pulmonary vasculature. These structural changes are driven by a variety of factors, of which mechanical forces begin to play an increasingly important role. In the past decades these factors have been identified in several animal studies. Now this knowledge serves as important scientific background for clinical prenatal MR imaging studies, since the canalicular stage is the first stage of lung development, which can be routinely assessed by fetal MR (after 18 GW).
The developmental physiology of the fetal lung in the second trimester, and especially before 24 GW, is dominated by the mechanical effects of permanent secretion of lung fluid by the bronchopulmonary epithelium by means of a chloride-dependent transport mechanism (McCray et al. 1992) (Fig. 1). The large amount of lung fluid produced daily (48–100 mL/day), contributing to 30% of amniotic fluid volume (Brace et al. 1994), requires well-regulated clearance mechanisms (Kotecha 2000). The clearance of lung fluid is granted through fetal breathing movements, cyclic contractions of the diaphragm (Harding and Hooper 1996), and phasic contractions of the fetal airways, which are present throughout pregnancy (Schittny et al. 2000) and generated by pacemaker cells in the bronchial smooth muscle (Jesudason et al. 2005). However, lung fluid efflux is limited by a high laryngeal pressure during phases of fetal apnea. According to the long periods of fetal apnea during the second trimester, the retained lung fluid accumulates and builds up a transpulmonary pressure gradient. This mechanical key stimulus induces proliferation and differentiation of alveocytes (Scott et al. 1993) and additionally leads to apoptosis of mesenchymal fibroblasts. Additionally, mechanical stretch on alveocytes (Sanchez-Esteban et al. 2002), mainly induced by the pressure gradient in combination with fetal breathing movements (Inanlou et al. 2005), facilitates later surfactant production and release.
Concordantly with the differentiation of the epithelial lung structures, the pulmonary vasculature forms. The development of the vessel wall of fetal pulmonary arteries, which is twice as thick as in the adult (Hislop and Reid 1973), indirectly controls the high vascular resistance of the pulmonary vasculature during fetal development. The muscle cells of the pulmonary artery muscle layer are derived from three sources – the bronchial smooth muscle during the embryonal stage, the pulmonary mesenchyme during later stages of lung growth, and finally from endothelial cells, whereas the muscular wall of the pulmonary veins is exclusively derived from the pulmonary mesenchyme (Hall et al. 2002).
By 24 GW, the fetal lung has reached a degree of structural maturity, which allows extremely preterm children to survive under intensive respiratory support. As a consequence of the aforementioned complex mechanisms, the terminal airways have further developed, and histologically, the saccular stage of lung development has been reached. Still, the important process of “biochemical” lung maturation has not yet begun. The advanced differentiation of type 2 alveocytes enables their cellular organelles to produce surfactant, which can be readily detected in the amniotic fluid of the majority of fetuses by 30 GW (Odom and Ballard 1997) (Fig. 1).
By 30 GW, the structural development of the fetal lung is by far incomplete, since the first alveolar structures are now appearing (Fig. 1). Alveoles are uniformly present in the lungs at 36 GW (Langston et al. 1984), but continue to increase in number, with over 85% of alveoles formed (Thurlbeck 1982), particularly during the first 2 years (Burri 1997) after birth.
2 MRI of the Developing Fetal Lung
Since the beginning of fetal MR imaging (Smith et al. 1983), the assessment of the maturity and size of the developing respiratory system has been of great interest (Powell et al. 1988). Particularly the advance of ultrafast MR imaging sequences has attracted the scientific and clinical interest in this technique. Nowadays, MR offers a detailed visualization of the thoracic organs irrespective of conditions, which are known to interfere with sonographic image quality, such as maternal obesity, advanced rib ossification, or oligo/anhydramnios. According to the ability to characterize chemical tissue properties, fetal MR gains insights into the biochemical and microstructural maturation of the lung. Further improvements in the acquisition speed of MR sequences and in the correction of motion artifacts by advanced postprocessing methods promise to boost the diagnostic impact of MRI on the perinatal management of fetuses at risk for lethal or life-threatening pulmonary hypoplasia.
2.1 MR Assessment of Fetal Lung Structure
During development, the pulmonary tissue is subject to substantial structural changes. Most notably, the mesenchyme and interstitial tissues between the forming saccules and alveoles undergo apoptotic changes and continuously disappear (Fig. 1). Together with angiogenesis, this leads to an approximation of future air spaces and pulmonary capillaries. Subsequently, the alveolar-capillary barrier thins and guarantees an efficient and sufficient gas exchange after birth.
These developmental changes of lung structure can be visualized by ultrasound and MRI. According to the decrease in tissue density, especially during the canalicular and saccular stages of lung development, the sonographic appearance of the fetal lung parallels this process by changes in the lung tissue echogenicity. At 22 and 23 GW, the mean gray value of the fetal lung on T2-weighted and T1-weighted sequences equals that of the fetal liver (Fig. 2). Between 24 and 31 GW, the lungs linearly become darker and less echogenic, and finally, increase in echogenicity in the last weeks before birth (Tekesin et al. 2004). However, no single sonographic feature has been found to significantly correlate with the biochemical maturity of the fetal lung (Cayea et al. 1985).
Axial T2-weighted sequences of the fetal thorax, at the level of the tracheal bifurcation at 15 (a), 21 (b), 25 (c), and 31 (d) GW. Note the initial intermediate signal intensity of the fetal lung, decreasing during the canalicular stage with the lowest signal intensity between 19 and 23 GW (b). Afterwards, the T2-weighted signal increases substantially
Image examples of the six evaluated sequences of fetuses of three different age groups: (a) 23–26 weeks, (b): 32+1–32+ 6 weeks, and (c): 34–37 weeks of gestation. The increasing signal intensities on all T2-weighted sequences and on FLAIR and the decreasing signal intensity on T1-weighted sequence are demonstrated during the course of pregnancy. (Copyright Balassy et al. 2007)
Since MRI is based on the visualization of magnetic properties of molecules and tissues, particularly those rich in protons, it is an ideal tool to gain data on the chemical composition of a certain organ or tissue type. Thus, MR allows us to noninvasively examine the correlates of the aforementioned structural and biochemical developmental processes leading to a functionally mature lung. This is mainly achieved by the use of a variety of different imaging sequences.
Nowadays, the T2-weighted single-shot fast spin echo sequence is the most commonly used MR sequence in the assessment of the fetus in general. Using an optimized field of view and a rapid acquisition (approximately 20 s) by implementing acceleration factors, this sequence can be acquired with a slice thickness of as low as 3 mm in fetuses aged 17 GW and older.
At the beginning of the canalicular stage (17 GW), the fetal lung is visualized by T2-weighted sequences with an intermediate signal intensity, modestly hypointense to amniotic fluid and clearly hyperintense to muscle and to liver (Figs. 2–4). Using T2-weighted sequences, the anatomy of the lungs, grossly showing their characteristic morphological organ shape, the trachea and hilar structures, which are mostly fluid-filled at this stage, are successfully visualized (Figs. 5–7). Occasionally and inconsistently, the fluid-filled esophagus can be seen as well as fluid-filled structure, especially at the level of the hiatus (Fig. 5).
The changing imaging characteristics of the fetal lung and liver between 21 and 28 GW in a longitudinal study of the same fetus: increase of the lung signal intensity and slight decrease of the liver signal intensity, visible on T2- (left row) and less evidently on echo-planar sequences (right row). The signal changes occur vice versa on T1-weighted sequences (middle)
Comparative scheme of axial T2-weighted and balanced gradient echo sequences in the visualization of the fetal thorax at 21 GW: the fetal respiratory tract is visualized from laryngeal level (black arrowheads) to the tracheal bifurcation (white arrow) and the fluid-filled main bronchi. The esophagus can be frequently seen on T2-weighted sequences at the level of the hiatus (circle). Note the less clear delineation between lung and liver tissue on balanced gradient echo sequences
Whereas T1-weighted sequences are less efficient in depicting the pulmonary anatomy, they initially provide a stable, intermediate signal intensity of the pulmonary tissue (Figs. 3, 4, and 6). These imaging characteristics may be related to the high amount of protein and lipid rich interstitial mesenchyme and the comparably low amount of free intraluminal lung fluid.
It is commonly accepted that the brightness of the fetal lung parenchyma on T2-weighted sequences increases with gestational age (Duncan et al. 1999a, b; Kuwashima et al. 2001; Levine et al. 2003; Balassy et al. 2004; Keller et al. 2004; Osada et al. 2004; Brewerton et al. 2005). This effect can be detected at different echo times, but its visual perception is greatest using echo times longer than 120 ms (Fig. 2) (Balassy et al. 2007).
In the last decade it was hoped that the properties of FLAIR (long tau inversion recovery) sequences, to attenuate the signal of the unbound water fraction, can be used to evaluate the level of lung maturity and potentially elaborate the contribution of surfactant to the lung signal. As with T2-weighted sequences, the signal of the lung parenchyma was found to increase (Balassy et al. 2007). Unfortunately, FLAIR imaging is very sensitive to motion and thus often provides artifact degraded data with abnormally high or low lung signals. Additionally, the effect of age-dependent signal increase is less perceptible on these sequences.
T1-weighted sequences can often be successfully acquired during maternal breath hold providing a robust signal, which can be most consistently quantified. It has been concordantly demonstrated that the T1-weighted signal of the fetal lung is declining during the canalicular and saccular stage of lung growth (between 20 and 30 GW, Figs. 3, 4, and 6) (Duncan et al. 1999a, b; Balassy et al. 2007).
Before birth, the fetal lung displays a very characteristic homogenous bright appearance on T2-weighted images and profound hypointensity on T1-weighted sequences.
The physiologic background behind the changing MR signal properties of the growing lung is complex and may not be attributed to a single maturational mechanism. Actually it is appreciated that the MR characteristics of the pulmonary parenchyma relate to a variety of structural and biochemical developmental processes and indirectly reflect the maturational state of the fetal lung (Fig. 1).
Currently, the theoretic concept assumes that the lung signal increase on T2-weighted sequences and decrease on T1 weighted sequences is mainly influenced by the changing amount of lung fluid and the larger fraction of unbound protons at later gestational ages (Sedin et al. 2000). As fetal lung fluid production serves as a key stimulus for lung growth (described above and proven by a MR study in fetal sheep (Wedegaertner et al. 2004)), its noninvasive evaluation by fetal MR allows an estimation of the actual growth potential of the fetal lung.
The decreasing amount of interstitial tissue and mesenchyme (Fig. 1) is leading to a lower amount of the overall lipid and protein content of the lung, mirroring the process of alveolar-capillary barrier thinning. This may particularly affect the signal on T1-weighted sequences resulting in a lower signal in structurally more mature lungs (Sedin et al. 2000; Osada et al. 2004; Balassy et al. 2007). Besides microstructural changes, the generation of surfactant after 24 GW may influence the lung signal. According to the chemical composition of surfactant (90% phospholipids and 10% proteins), it affects the MR appearance of the fetal lung parenchyma; however, it is still unclear if its quantity and concentration are high enough to visually contribute to the MR signal of the fetal lung on any of the used sequences.
Vasculogenesis and growth of the distal capillary bed importantly affect the state of maturity of the fetal cardiopulmonary system. Still, the exact impact of these processes on the signal intensity of the fetal lung is not yet known. Basically, the fetal lung remains a low flow, high resistance vascular network throughout pregnancy. By the 20th week of gestation, only 13% of the cardiac output supplies the lung, doubling until 30 GW and then remaining constant (Rasanen et al. 1996).
Recently, diffusion-weighted sequences have also been used to monitor lung maturation (Fig. 8). Some works found a significant linear increase in the apparent diffusion coefficient (ADC) during pregnancy and related this finding mainly to hemodynamic changes and vasculogenesis (Moore et al. 2001, Manganaro et al. 2008). Others did not find any age-dependent changes of lung diffusion parameters, but detected their regional variation as expression of the local heterogeneity of fetal lung tissue (Fig. 8) (Balassy et al. 2008), possibly reflecting the apicobasal gradient of structural lung maturity reported in animal studies (Zeltner et al. 1990) and human fetuses (Laudy and Wladimiroff 2000). Since diffusion-weighted imaging is highly susceptible to motion, the high variability of diffusion measurements may be explained by technical limitations. Before no more consistent data on this subject are available, diffusion-weighted imaging of the fetal lung remains of theoretical use.
Coronal diffusion-weighted sequence with b-values of 0 and 700 s/mm². The signal characteristics of the fetal lungs on diffusion-weighted images are inconsistent in their developmental appearances as well as in their anatomic variation. Note the low ADC values of the cerebral cortex, the kidneys, and the side differences in ADC and diffusivity values between the right and left lung
Basically, a variety of developmental processes are contributing to the appearance of the fetal lung on MRI. As yet, not enough animal data are available to confidently link a certain maturational factor to the lung signal changes of the lungs. However, an abnormally low signal on T2-weighted sequences and an abnormally high signal on T1-weighted sequences is always an indicator of abnormal or even immaturity.
2.2 Quantification of Fetal Lung Signal Intensities
Several attempts have been made to quantify the MR signal changes of the fetal lung in order to objectify abnormal signal properties of the fetal lung. After the first relaxation time measurements by Duncan et al. (Duncan et al. 1999a, b) using time- consuming multiecho sequences, more recent studies focused on the quantification of lung signals by direct measurement of the lung signal intensity. Since the absolute SI values showed a high variability, lung signal intensity ratios between lung and liver signals were calculated in order to achieve more stable values (Keller et al. 2004; Osada et al. 2004; Balassy et al. 2007). As hematopoietic organ, the fetal liver does not provide a stable MR signal during the second and third trimester (Balassy et al. 2004; Keller et al. 2004), and therefore, cannot serve as an optimal reference organ. Thus, none of the following studies in larger cohorts and especially in younger fetuses (under 24 GW) was successful in defining age-dependent absolute reference values, which could be clinically used to identify abnormal lung growth (Keller et al. 2004; Osada et al. 2004; Brewerton et al. 2005). Still, several experiences show that the quantification of fetal lung signal intensity increases sensitivity in the detection of pulmonary hypoplasia, especially when combined with fetal lung volumetry (Ikeda et al. 2000; Kuwashima et al. 2001, Kasprian et al. 2006; Balassy et al. 2004; Brewerton et al. 2005). Therefore, future work will aim to develop fast multiecho sequences, which allow the absolute organ-independent lung signal measurement techniques.
2.3 Surfactant Detection by MR Spectroscopy
Surfactant, chemically, is mainly composed of proteins (10%) and phospholipids (90%), including mainly phosphatidylcholine (lecitihin, 70%) and lesser amounts of phosphytidylglycerol, phosphatidylethanolamine, and phosphatidylinositol. So far only invasive testing of the lecithin/sphingomyelin ratio in amniotic fluid samples after amniocentesis could provide in vivo and in utero data on the state of surfactant production and “biochemical” fetal lung maturity (Krieglsteiner et al. 1976). Due to the ability of MR spectroscopy to noninvasively detect and quantify the chemical compounds of fluids and tissues in vivo, the potential of this technique has been realized early (Nelson et al. 1987) and, despite great technical challenges, it is still hoped to be of great value in the assessment of fetal lung maturity.
Using single voxel H1 spectroscopy, the quantification of surfactant in vivo basically followed two strategies: either the point-resolved spectroscopy (PRESS) box was placed in amniotic fluid pockets or directly in the pulmonary tissue of the fetus (Fenton et al. 2001; Clifton et al. 2006; Kim et al. 2008).
The attempt of detecting surfactant in amniotic fluid has the advantage of being less affected by fetal motion. In earlier ex vivo studies, a broad profile of chemical compounds has been found in amniotic fluid samples, comprising creatinine, glucose, organic acids (acetate, citrate, and lactate), and several amino acids (valine, alanine, histidine, tyrosine, phenylalanine, leucine, and isoleucine) (McGowan et al. 1993; Sims et al. 1993, Nelson et al., 1987). However, according to the low concentrations of choline in amniotic fluid, the possibility of detecting surfactant in vivo at a clinical 1.5 T scanner has been doubted (McGowan et al. 1993; Sims et al. 1993).
A recent 11.5 T ex vivo MR spectroscopy study managed to consistently detect the main compound of surfactant – phosphatidylcholine – in amniotic fluid with the characteristic peak at 3.2 ppm and found a progressive increase in the choline/creatine ratio during the second and third trimester of pregnancy (Clifton et al. 2006). Actually, only sporadic experiences of in utero and in vivo choline detection in amniotic fluid and in the fetal lung at clinical 1.5 T MR scanner are available. They have been successfully performed exclusively in late pregnancy (>35 GW), thus being of almost no clinical use at this developmental stage (Fenton et al. 2001; Clifton et al. 2006; Kim et al. 2008).
Generally, caution is necessary when interpreting in utero spectroscopy results. Apart from the low signal-to-noise ratio in clinical scanning, the signals arising from the target tissues may be contaminated especially by lipids from maternal or fetal fatty tissue and consequently cause false-positive or -negative results (Kok et al. 2002, Kim, et al 2008). Therefore, clinical in utero spectroscopy is rather a matter of ongoing MR research (particularly at higher field strengths), then a reliable instrument in the assessment of fetal lung maturity.
Although different approaches in the noninvasive assessment of fetal lung maturity are currently under investigation, the subjective assessment of the fetal lung signals on different MR sequences by a physician with experience in fetal MR imaging seems to be the most reliable strategy to assess fetal lung development.
The visual qualitative assessment of the signal intensities of the fetal lung should be part of each fetal MR examination, since it allows a quick and sensitive marker for the detection of pulmonary hypoplasia.
2.4 Quantification of Fetal Pulmonary Volume
The postnatal viability of a fetus requires a sufficient surface area of the gas-exchanging alveolar epithelium. Currently, there is no in vivo method available to directly or indirectly assess this parameter. The lung volume of a fetus does not necessarily correlate with the actual potential of pulmonary oxygen uptake, thus its quantification does only allow an estimate of future respiratory outcome. Actually, it constitutes the most important prognostic parameter for postnatal respiratory outcome, measureable by noninvasive prenatal imaging techniques.
As clinical tool, quantification fetal lung volume is essentially feasible in the age period between 17 and 30 GW. Earlier than 17 GW, the organ size is very small and the utility as a prognostic parameter is limited. As pregnancy advances, the fetal lung usually reaches a certain level of microstructural and biochemical maturity, which seems to compensate for a small organ size. This is reflected by the fact that later than 30 GW normal fetal lung volume shows a wide range of individual variability without any major impact on postnatal lung function.
Estimation of fetal lung size is helpful in any condition, where pulmonary hypoplasia must be suspected (see Chap. 27). Both methods – prenatal sonography and fetal MR – technically allow the 2D and 3D assessment of fetal lung size. As the postmortem quantification of the pulmonary volume is hampered by postmortem lung collapse and tissue degradation, it cannot serve as reasonable “gold standard.” Therefore, the direct comparison of the accuracy of these methods is hardly possible.
2.4.1 D Fetal Lung Biometry
2D Biometry is the most timesaving way to assess fetal lung size and has been frequently applied by ultrasound studies in order to detect pulmonary hypoplasia. Sonographic measurements were initially confined to biometry of the fetal thorax, by measuring the thoracic circumference (Thompson and Makowski 1971; Nimrod et al. 1986; Fong et al. 1988; Songster et al. 1989). As the quantification of thoracic circumference includes contribution from all thoracic organs, this method was associated with a low specificity, which could not be improved by determination of the fetal thoracic area and subtraction of the measured heart area (Vintzileos et al. 1989). Finally, it had to be noted that the measurement of chest circumference without any additional diameter of the fetal lung was not sufficiently accurate to predict pulmonary hypoplasia (Merz et al. 1999). Thus, it became clear that the only way to raise the sensitivity and specificity of prenatal biometry methods was to restrict the measurements to the fetal lung and avoid the inclusion of other thoracic organs.
The value of linear 2D measurements of certain diameters of the fetal lung (mostly at the level of the four-chamber view) seems to depend on the etiology of pulmonary hypoplasia. Several studies could show that the lung-to-head ratio (see paragraph CDH) is a quick and useful parameter in predicting lethality of left-sided CDH (Metkus et al. 1996; Sbragia et al. 2000; Jani et al. 2006) and has been used in the inclusion criteria for FETO. Still, the clinical evidence for this tool as independent predictor of outcome in CDH is unclear (Heling et al. 2005; Ba’ath et al. 2007).
In pulmonary hypoplasia secondary to other conditions, such as PROM, hydrothorax, thoracic lesions, and renal failure, the results of 2D lung biometry (lung length, transverse diameter, and sagittal diameter) are not reliable (Heling et al. 2001; Gerards et al. 2008).
Therefore, and according to the multiplanar capabilities of fetal MR, the examiner should take advantage of the possibility of 3D organ volume quantification and avoid linear measurements in any case or situation where pulmonary hypoplasia is suspected.
2.4.2 Clinical Evidence for Fetal Lung Volumetry as Diagnostic Test
Depending on the indication, the technique of fetal lung volumetry is currently in stage II and III of Gluud and Gluud’s four-stage assessment of diagnostic tests (Gluud and Gluud 2005), meaning that normal reference values have been established, but evaluations of the accuracy of the test are still ongoing. Clinical evidence that a method is suitable to predict perinatal survival in certain conditions requires the assessment in all four stages including randomized intervention trials and large cohort studies, which determine the clinical effects of the respective test (stage IV). Due to the traditionally small cohort sizes in fetal MR studies, it will require some time to establish fetal lung volumetry as reliable test for pulmonary hypoplasia in a variety of conditions, although the most recent results in certain pathologies (such as CDH) are promising.
2.4.3 Method of Fetal Lung Volumetry
2.4.3.1 Background
Recently, several groups reported normal reference values of fetal lung volumes between 18 and 37 GW (Table 1). Except for some specialized prenatal care centers, this method has not been accepted as standard in the detection and quantification of pulmonary hypoplasia. This is mainly due to the complicated and time-consuming postprocessing, as well as the need for the involvement of an examiner, experienced in fetal anatomy and the technique of MR volumetry. Automated, computerized lung volume quantification (Thayyil et al. 2008) would help to overcome some of these problems. Currently, it is difficult to automatically create appropriate masks, which accurately define the organ boundaries and prevent the inclusion of other organs, mainly due to the inhomogeneous signal properties of the fetal lung, which are often overlapping with extrapulmonary structures. Additionally, the changing signal characteristics of the fetal lungs, especially in the condition of hypoplasia, would lead to inconsistent and unreliable results. Therefore, the manual tracing method is actually the only and most reliable tool in the fetal MR biometry of fetal lung volumes. Still, its accuracy and diagnostic value depend on skill and experience of the examiner. This can be easily and quickly achieved, as the anatomy of the fetal lung is visualized in an unequivocal way (Figure 4, 5, 6).
2.4.3.2 Procedure of Fetal Lung Volumetry
2.4.3.2.1 The Sequences
The consistency and validity of MR volumetry data crucially depend on the quality of the image raw data. In case of suspected pulmonary hypoplasia, at least one high-quality MR sequence covering the entire fetal thorax, without evident fetal motion artifacts, must be acquired. Generally, it has been shown that the axial slice orientation offers the most accurate measurement data (Tanigaki et al. 2004; Jani et al. 2005; Ward et al. 2006; Busing et al. 2008). Sagittal and coronal measurement planes are acceptable as well (Ward et al. 2006), but according to their smaller slice number, they may underestimate the total lung volume (Jani et al. 2005; Busing et al. 2008). In order to compare the measured individual data with the fetal lung volumetry reference data available in literature, it has to be acknowledged that the majority of studies chose the axial slice orientation as reference for the volumetry procedure.
Another important factor is the choice of the imaging sequence. Accurate lung volumetry demands a low slice thickness and a small field of view, respecting a reasonable acquisition time to avoid movement artifacts. In literature, two types of sequences are mainly described as a basis for fetal MR volumetry: T2w (single-shot turbo or fast spin echo/HASTE or RARE sequences) and balanced gradient echo (steady-state free precession/true FISP/FIESTA) sequences. In Fig. 5, the differences in the visualization of the fetal lung are graphically compared. Both have advantages and limitations:
T2w Sequences (Fig. 5) have the advantage to:
-
(a)
Provide an excellent contrast between the high-signal lung tissue and the surrounding structures (particularly, when using long echo times – >120 ms).
-
(b)
Offer an exact delineation between pulmonary tissue and vascular structures at the hilum.
-
(c)
Allow the reliable identification of fetal pulmonary parenchyma even in cases of severe pulmonary hypoplasia and in pathological conditions, where the fetal thoracic anatomy is unclear.
Have the disadvantage to:
-
(a)
Be limited by a rather greater slice thickness. However, a slice thickness between 3 and 4.5 mm should be achieved (Kasprian et al. 2006) and is commonly used in the reference literature (Jani et al. 2005; Cannie et al. 2006; Busing et al. 2008).
Balanced gradient echo sequences (steady-state free precession/true FISP/FIESTA) (Fig. 5) have the advantage to:
-
(a)
Be more robust against motion artifacts.
-
(b)
Offer a smaller slice thickness (consistently 3 mm, see Chap. 29).
-
(c)
Show the anatomy of thoracic blood vessels and the fetal heart in more detail, than T2-w sequences.
and have the disadvantage to
-
(a)
Allow a less distinct delineation of the pulmonary parenchyma, which appears more isointense to the thoracic wall and mediastinal structures. Consequently, they seem to rather underestimate the “real” fetal lung volume (Busing et al. 2008)
-
(b)
Prevent the detailed differentiation between pathological and normal lung tissue (for instance, in cases of congenital cystic adenomatoid malformations –CCAM)
Mainly, the choice of the sequence has to follow practical considerations. Basically, T2-weighted sequences may be currently the most reasonable fetal lung imaging sequence choice, as most of the reference data are based on T2-weighted data. In cases of considerable motion artifacts, balanced gradient echo sequences may be more efficient in providing an artifact-free image dataset, but are limited in the comparison with available reference data. According to the low resolution (large field of view and the longer acquisition times), T1- and T2-FLAIR weighted sequences currently play no role in MR lung volumetry.
In addition to the choice of sequence type, the matrix and slice thickness have to be optimized (see Chap. 29 and Table 1). Currently, a matrix of 256 and a slice thickness between 3 and 5 mm should be achieved.
Almost always occurring motion artifacts substantially limit image data quality, and in certain cases, the use of maternal and fetal sedation constitutes the only possibility to reduce them. As any kind of in utero pharmacological intervention should be used in a restrictive way, other strategies to limit fetal motion have to be exploited. Especially, sequence acquisition using maternal breath hold does not only limit maternal motion, but also reduce fetal breathing movements (Meyberg-Solomayer et al. 2007).
2.4.3.3 Data Preparation and Measurement Procedure
The postprocessing of fetal imaging data is always a debate of personal habits and individual opinions. There are many software packages (commercially or freely) available (see Table 1) which allow a quantification of body or organ volumes. According to the semiautomated technique, the postprocessing software does not impact the volumetry data quality, but may influence the speed of the postprocessing workflow.
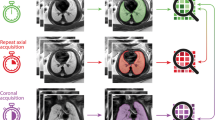
In order to achieve the most reliable results, it is important to rule out movement as a major source of error in fetal lung volumetry (Fig. 9). For obvious reasons, the detection of motion-degraded images should be already performed by the time of sequence acquisition as this may guarantee at least one axial sequence of acceptable quality for volumetry. This mainly applies to artifacts caused by fetal motion, as they are easily detectable.
The problem of fetal motion in fetal lung volumetry: multiplanar reconstruction of the original data sensitively detects fetal and/or maternal motion, even if the in-plane/axial image appears of quite good quality (upper row). Repeated acquisition of T2-weighted sequences increases the chance of a nonmotion-degraded dataset with a smooth reconstruction (lower row)
However, maternal breathing movements may result in significant motion of the fetus in the craniocaudal axis and thereby to the repeated acquisition of one slice position. Especially, while assessing axial images, this bias is difficult to realize. A fast and secure way to prevent this error is the reconstruction of the sequence in the coronal and sagittal plane (Fig. 9). Most of the available image processing software packages provide a multiplanar reconstruction tool, which is easily accessible. The appearance of large “steps” of the fetal thoracic body contour in the reconstructed image may serve as a good indicator of maternal or fetal motion (Fig. 9). In this way, misregistrations of the volumetry data may be avoided and accuracy of the measurements improved.
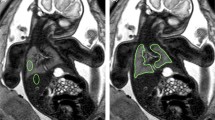
The manual delineation of the pleural contours of the fetal lung is still the most reliable way to segment this organ. The clear delineation of fetal pulmonary tissue may be challenging in certain anatomical regions. In order to compare the volumetry data with the reference literature, the hilar structures (pulmonary vessels, bronchial airways) should be avoided (Fig. 10). Segmentation of the lung base is often challenged by partial volume artifacts (due to the diaphragmatic domes). Additionally, the hyperintense adrenal cortex and retroperitoneal fat make it difficult to differentiate between the bordering tissue types (Fig. 5). A consistent way to measure in this area is important as incorrect segmentation at the lung base causes the greatest error.
2.4.4 Reference Data of Fetal Lung Volumes
Table 1 provides an overview of the currently available reference data of fetal lung volumes. Fig. 11 shows the (quadratic regression) growth curves of three MR studies with a study population over 100 cases (Rypens et al. 2001; Cannie et al. 2006; Kasprian et al. 2006). These studies used comparable methods concerning imaging parameters and postprocessing methods. The major limitation in these studies is the inclusion of fetuses with (mostly) cerebral abnormalities.
In order to apply the volumetry results in the assessment of pulmonary hypoplasia, the volumetry results have to be correlated to a reference, such as gestational age (Rypens et al. 2001; Kasprian et al. 2006) or fetal body volume (Cannie et al. 2008). As total body volume shows the closest correlation with fetal lung volume (Cannie et al. 2008), this additional measurement may be acquired as well. However, total body volume quantification is time-consuming and currently it is not clear if this additional effort has a clinical impact. Nevertheless, especially in conditions in which total body volume is reduced as well (IUGR, syndromes, complex malformations), this approach is beneficial and more sensitive in the detection of lethal pulmonary hypoplasia.
Figure 11 shows a comparison of the mean values of fetal lung volumes between 18 and 38 GW, determined by 3 MR studies (Rypens et al. 2001; Kasprian et al. 2006; Cannie et al. 2008) in at least more than 106 (Kasprian et al. 2006) and a maximum of 215 (Rypens et al. 2001) cases and a overall case number of 521 cases. All studies used a comparable measurement and imaging technique (Table 1) leading to a standard variation between the age-related mean values of overall and between 18 and 32 GW. These data may be used as initial reference data.
For a number of reasons, the assessment of lung volume as a prognostic determinant of later pulmonary function is especially valuable before 27 GW. First, variations in lung volume can be physiologically compensated more easily after and the functional consequences of a small lung size are more detrimental before this fetal age. Second, the impact of these data on perinatal management is much greater at earlier gestational ages. Third, at 18–27 GW, the anatomical variability of lung size is much less evident compared to older fetuses leading to a lower standard deviation. However, volumetry is generally more inaccurate in small organ sizes leading to a certain examiner-dependent variability.
The diagnostic utility of fetal lung volumetry reference data in different pathologies is discussed in Chap. 26.
3 MR Assessment of Fetal Breathing Movements
Over 120 years ago, Ahlfeld (1888) and Weber (1888) described see-saw movements in the umbilical region of the mother and attributed them to fetal breathing movements. Nowadays, these movements have been widely noticed as early expression of the maturing central nervous system and are frequently used as biological marker of fetal wellbeing (Trudinger et al. 1979). Moreover, they importantly contribute to the structural and biochemical maturation of the fetal lung. In the complete absence of fetal breathing movements, type 2 pneumocytes are unable to compile, store, and release surfactant, and type 1 pneumocytes are unable to flatten and optimize gas exchange (Inanlou et al. 2005).
Using sonography, fetal breathing movements can be observed from 10 GW onwards (de Vries et al. 1982). Initially, fetal breathing movements are observed infrequently (Natale et al. 1988). During second and third trimester development, their incidence, frequency, and pattern change. Before 20 GW, fetal breathing movements are dominated by abdominal movements with typical “see-saw” configurative changes of the fetal trunk, then followed by more extensive chest movements, and finally nasal fluid flow is detected sonographically (Cosmi et al. 2003), constituting the sonographic equivalent of frequent diaphragmatic contractions or the result of contractions of the airway smooth muscle (Badalian et al. 1996). Before 32 GW, the breathing cycle is short and periodic and finally gets more uniform and regular before birth (Trudinger and Knight 1980).
When encountered during fetal MR studies, fetal breathing movements are mostly causing motion artifacts and thereby reducing image quality and the accuracy of fetal lung volumetry. However, using dynamic sequences (Prayer et al. 2006), fetal breathing movements can be visualized during repeated intervals of 200 s of observation/image acquisition time. Since comparably long periods of apnea (14 min (Natale et al. 1988) to 122 min (Rayburn 1995)) have been reported in normal fetuses, the current observational window offered by MRI is too small to allow their conclusive assessment. However, in fetal MR studies complicated by severe fetal motion, the application of dynamic MR sequences adds information on the biophysical state of the fetus. Mainly two types of abnormal fetal breathing movements have been reported in various fetal pathologies: hyperkinetic and hypokinetic movements (de Vries and Fong 2007). Poor/hypokinetic fetal breathing movements seem to be a nonspecific movement pattern encountered in numerous fetal pathologies. Conversely, hyperkinetic breathing motion with high-speed, large amplitudes may be a more specific phenomenon as it has been observed in tracheal atresia (Baarsma et al. 1993).
Although the importance of fetal MR in monitoring fetal breathing movements is definitely limited by the short observation time, the dynamic MR examination of the fetus offers insights into the fetal breathing pattern, which may be used in the holistic assessment of fetal abnormalities.
References
Ahlfeld F (1888) Über bisher noch nicht beschriebene intrauterine Bewegungen des Kindes. Verhandlungen der Deutschen Gesellschaft für Gynäkologie, 2. Versammlung, Halle
Ba’ath ME, Jesudason EC, Losty PD (2007) How useful is the lung-to-head ratio in predicting outcome in the fetus with congenital diaphragmatic hernia? A systematic review and meta-analysis. Ultrasound Obstet Gynecol 30:897–906
Baarsma R, Bekedam DJ, Visser GH (1993) Qualitative abnormal fetal breathing movements, associated with tracheal atresia. Early Hum Dev 32:63–69
Badalian SS, Fox HE, Zimmer EZ, Fifer WP, Stark RI (1996) Patterns of perinasal fluid flow and contractions of the diaphragm in the human fetus. Ultrasound Obstet Gynecol 8:109–113
Balassy C, Brugger PC, Csapo B, Mittermayer C, Kasprian G, Prayer D (2004) In vivo investigation of fetal lung maturation with MRI. Eur Radiol 14:220–221
Balassy C, Kasprian G, Brugger PC, Weber M, Csapo B, Mittermayer C, Hormann M, Prayer D (2007) MRI investigation of normal fetal lung maturation using signal intensities on different imaging sequences. Eur Radiol 17:835–842
Balassy C, Kasprian G, Brugger PC, Csapo B, Weber M, Hormann M, Bankier A, Bammer R, Herold CJ, Prayer D (2008) Diffusion-weighted MR imaging of the normal fetal lung. Eur Radiol 18:700–706
Brace RA, Wlodek ME, Cock ML, Harding R (1994) Swallowing of lung liquid and amniotic fluid by the ovine fetus under normoxic and hypoxic conditions. Am J Obstet Gynecol 171:764–770
Brewerton LJ, Chari RS, Liang Y, Bhargava R (2005) Fetal lung-to-liver signal intensity ratio at MR imaging: development of a normal scale and possible role in predicting pulmonary hypoplasia in utero. Radiology 235:1005–1010. Epub 2005 Apr 1021
Burri PH (1997) Structural Aspects of Prenatal and Postnatal Development and Growth of the Lung. In: McDonald JA (ed) Lung growth and development. Marcel Dekker, New York, pp 1–35
Busing KA, Kilian AK, Schaible T, Debus A, Weiss C, Neff KW (2008) Reliability and validity of MR image lung volume measurement in fetuses with congenital diaphragmatic hernia and in vitro lung models. Radiology 246:553–561
Cannie M, Jani JC, De Keyzer F, Devlieger R, Van Schoubroeck D, Witters I, Marchal G, Dymarkowski S, Deprest JA (2006) Fetal body volume: use at MR imaging to quantify relative lung volume in fetuses suspected of having pulmonary hypoplasia. Radiology 241:847–853
Cannie MM, Jani JC, Van Kerkhove F, Meerschaert J, De Keyzer F, Lewi L, Deprest JA, Dymarkowski S (2008) Fetal body volume at MR imaging to quantify total fetal lung volume: normal ranges. Radiology 247:197–203
Cayea PD, Grant DC, Doubilet PM, Jones TB (1985) Prediction of fetal lung maturity: inaccuracy of study using conventional ultrasound instruments. Radiology 155:473–475
Clifton MS, Joe BN, Zektzer AS, Kurhanewicz J, Vigneron DB, Coakley FV, Nobuhara KK, Swanson MG (2006) Feasibility of magnetic resonance spectroscopy for evaluating fetal lung maturity. J Pediatr Surg 41:768–773
Coakley FV, Lopoo JB, Lu Y, Hricak H, Albanese CT, Harrison MR, Filly RA (2000) Normal and hypoplastic fetal lungs: volumetric assessment with prenatal single-shot rapid acquisition with relaxation enhancement MR imaging. Radiology 216:107–111
Cosmi EV, Anceschi MM, Cosmi E, Piazze JJ, La Torre R (2003) Ultrasonographic patterns of fetal breathing movements in normal pregnancy. Int J Gynaecol Obstet 80:285–290
de Vries JI, Fong BF (2007) Changes in fetal motility as a result of congenital disorders: an overview. Ultrasound Obstet Gynecol 29:590–599
de Vries JI, Visser GH, Prechtl HF (1982) The emergence of fetal behaviour. I. Qualitative aspects. Early Hum Dev 7:301–322
Duncan KR, Gowland PA, Freeman A, Moore R, Baker PN, Johnson IR (1999a) The changes in magnetic resonance properties of the fetal lungs: a first result and a potential tool for the non-invasive in utero demonstration of fetal lung maturation. Br J Obstet Gynaecol 106:122–125
Duncan KR, Gowland PA, Moore RJ, Baker PN, Johnson IR (1999b) Assessment of fetal lung growth in utero with echo-planar MR imaging. Radiology 210:197–200
Fenton BW, Lin CS, Macedonia C, Schellinger D, Ascher S (2001) The fetus at term: in utero volume-selected proton MR spectroscopy with a breath-hold technique–a feasibility study. Radiology 219:563–566
Fong K, Ohlsson A, Zalev A (1988) Fetal thoracic circumference: a prospective cross-sectional study with real-time ultrasound. Am J Obstet Gynecol 158:1154–1160
Gerards FA, Twisk JW, Bakker M, Barkhof F, van Vugt JM (2007) Fetal lung volume: three-dimensional ultrasonography compared with magnetic resonance imaging. Ultrasound Obstet Gynecol 29:533–536
Gerards FA, Twisk JW, Fetter WP, Wijnaendts LC, van Vugt JM (2008) Predicting pulmonary hypoplasia with 2- or 3-dimensional ultrasonography in complicated pregnancies. Am J Obstet Gynecol 198(140):e141–e146
Gluud C, Gluud LL (2005) Evidence based diagnostics. BMJ 330:724–726
Hall SM, Hislop AA, Haworth SG (2002) Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol 26:333–340
Harding R, Hooper SB (1996) Regulation of lung expansion and lung growth before birth. J Appl Physiol 81:209–224
Heling KS, Tennstedt C, Chaoui R, Kalache KD, Hartung J, Bollmann R (2001) Reliability of prenatal sonographic lung biometry in the diagnosis of pulmonary hypoplasia. Prenat Diagn 21:649–657
Heling KS, Wauer RR, Hammer H, Bollmann R, Chaoui R (2005) Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 25:112–118
Hislop A, Reid L (1973) Lung development in relation to gas exchange capacity. Bull Physiopathol Respir (Nancy) 9:1317–1343
Ikeda K, Hokuto I, Mori K, Hayashida S, Tokieda K, Tanigaki S, Tanaka M, Yuasa Y (2000) Intrauterine MRI with single-shot fast-spin echo imaging showed different signal intensities in hypoplastic lungs. J Perinat Med 28:151–154
Inanlou MR, Baguma-Nibasheka M, Kablar B (2005) The role of fetal breathing-like movements in lung organogenesis. Histol Histopathol 20:1261–1266
Jani J, Breysem L, Maes F, Boulvain M, Roubliova X, Lewi L, Vaast P, Biard JM, Cannie M, Deprest J (2005) Accuracy of magnetic resonance imaging for measuring fetal sheep lungs and other organs. Ultrasound Obstet Gynecol 25:270–276
Jani J, Keller RL, Benachi A, Nicolaides KH, Favre R, Gratacos E, Laudy J, Eisenberg V, Eggink A, Vaast P, Deprest J (2006) Prenatal prediction of survival in isolated left-sided diaphragmatic hernia. Ultrasound Obstet Gynecol 27:18–22
Jesudason EC, Smith NP, Connell MG, Spiller DG, White MR, Fernig DG, Losty PD (2005) Developing rat lung has a sided pacemaker region for morphogenesis-related airway peristalsis. Am J Respir Cell Mol Biol 32:118–127. Epub 2004 Dec 2002
Kasprian G, Balassy C, Brugger PC, Prayer D (2006) MRI of normal and pathological fetal lung development. Eur J Radiol 57:261–270
Keller TM, Rake A, Michel SC, Seifert B, Wisser J, Marincek B, Kubik-Huch RA (2004) MR assessment of fetal lung development using lung volumes and signal intensities. Eur Radiol 14:984–989. Epub 2004 Mar 2011
Kim DH, Vahidi K, Caughey AB, Coakley FV, Vigneron DB, Kurhanewicz J, Mow B, Joe BN (2008) In vivo (1)H magnetic resonance spectroscopy of amniotic fluid and fetal lung at 1.5 T: technical challenges. J Magn Reson Imaging 28:1033–1038
Kok RD, van den Berg PP, van den Bergh AJ, Nijland R, Heerschap A (2002) MR spectroscopy in the human fetus. Radiology 223:584; author reply 584–585
Kotecha S (2000) Lung growth for beginners. Paediatr Respir Rev 1:308–313
Krieglsteiner P, Schneider R, Kopcke H, Tolle W, Johannigmann J, Blumel G (1976) Prenatal prediction of respiratory distress syndrome. Measurement of surface properties and Lecithin/Sphingomyelin ratio in human amniotic fluid. J Perinat Med 4:261–270
Kuwashima S, Nishimura G, Iimura F, Kohno T, Watanabe H, Kohno A, Fujioka M (2001) Low-intensity fetal lungs on MRI may suggest the diagnosis of pulmonary hypoplasia. Pediatr Radiol 31:669–672
Langston C, Kida K, Reed M, Thurlbeck WM (1984) Human lung growth in late gestation and in the neonate. Am Rev Respir Dis 129:607–613
Laudy JA, Wladimiroff JW (2000) The fetal lung. 1: Developmental aspects. Ultrasound Obstet Gynecol 16:284–290
Levine D, Barnewolt CE, Mehta TS, Trop I, Estroff J, Wong G (2003) Fetal thoracic abnormalities: MR imaging. Radiology 228:379–388. Epub 2003 Jun 23
Manganaro L, Perrone A, Sassi S, Fierro F, Savelli S, Di Maurizio M, Tomei A, Francioso A, La Barbera L, Giancotti A, Ballesio L (2008) Diffusion-weighted MR imaging and apparent diffusion coefficient of the normal fetal lung: preliminary experience. Prenat Diagn 28: 745–748
McCray PB Jr, Bettencourt JD, Bastacky J (1992) Developing bronchopulmonary epithelium of the human fetus secretes fluid. Am J Physiol 262:L270–L279
McGowan PE, Reglinski J, Wilson R, Walker JJ, Wisdoms S, McKillop JH (1993) Quantitative 1H-NMR analysis of amniotic fluid. J Pharm Biomed Anal 11:629–632
Merz E, Miric-Tesanic D, Bahlmann F, Weber G, Hallermann C (1999) Prenatal sonographic chest and lung measurements for predicting severe pulmonary hypoplasia. Prenat Diagn 19:614–619
Metkus AP, Filly RA, Stringer MD, Harrison MR, Adzick NS (1996) Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 31:148–151; discussion 151–142
Meyberg-Solomayer GC, Wallwiener D, Solomayer E (2007) Maternal breath-holding and the valsalva maneuver: methods to overcome fetal breathing movements during Doppler sonography. Ultrasound Med Biol 33:1586–1591
Moore RJ, Strachan B, Tyler DJ, Baker PN, Gowland PA (2001) In vivo diffusion measurements as an indication of fetal lung maturation using echo planar imaging at 0.5T. Magn Reson Med 45:247–253
Natale R, Nasello-Paterson C, Connors G (1988) Patterns of fetal breathing activity in the human fetus at 24 to 28 weeks of gestation. Am J Obstet Gynecol 158:317–321
Nelson TR, Gillies RJ, Powell DA, Schrader MC, Manchester DK, Pretorius DH (1987) High resolution proton NMR spectroscopy of human amniotic fluid. Prenat Diagn 7:363–372
Nimrod C, Davies D, Iwanicki S, Harder J, Persaud D, Nicholson S (1986) Ultrasound prediction of pulmonary hypoplasia. Obstet Gynecol 68:495–498
Odom MW, Ballard PL (1997) Developmental and hormonal regulation of the surfactant system. In: McDonald JA (ed) Lung growth and development. Marcel Dekker, New York, pp 495–575
Osada H, Kaku K, Masuda K, Iitsuka Y, Seki K, Sekiya S (2004) Quantitative and qualitative evaluations of fetal lung with MR imaging. Radiology 231:887–892. Epub 2004 Apr 2029
Powell MC, Worthington BS, Buckley JM, Symonds EM (1988) Magnetic resonance imaging (MRI) in obstetrics. II. Fetal anatomy. Br J Obstet Gynaecol 95:38–46
Prayer D, Kasprian G, Krampl E, Ulm B, Witzani L, Prayer L, Brugger PC (2006) MRI of normal fetal brain development. Eur J Radiol 57:199–216
Rasanen J, Wood DC, Weiner S, Ludomirski A, Huhta JC (1996) Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation 94:1068–1073
Rayburn WF (1995) Fetal movement monitoring. Clin Obstet Gynecol 38:59–67
Reid L (1977) 1976 Edward B.D. Neuhauser lecture: the lung: growth and remodeling in health and disease. AJR Am J Roentgenol 129:777–788
Rypens F, Metens T, Rocourt N, Sonigo P, Brunelle F, Quere MP, Guibaud L, Maugey-Laulom B, Durand C, Avni FE, Eurin D (2001) Fetal lung volume: estimation at MR imaging-initial results. Radiology 219:236–241
Sanchez-Esteban J, Wang Y, Cicchiello LA, Rubin LP (2002) Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 282:L448–L456
Sbragia L, Paek BW, Filly RA, Harrison MR, Farrell JA, Farmer DL, Albanese CT (2000) Congenital diaphragmatic hernia without herniation of the liver: does the lung-to-head ratio predict survival? J Ultrasound Med 19:845–848
Schittny JC, Miserocchi G, Sparrow MP (2000) Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants. Am J Respir Cell Mol Biol 23:11–18
Scott JE, Yang SY, Stanik E, Anderson JE (1993) Influence of strain on [3H]thymidine incorporation, surfactant-related phospholipid synthesis, and cAMP levels in fetal type II alveolar cells. Am J Respir Cell Mol Biol 8:258–265
Sedin G, Bogner P, Berenyi E, Repa I, Nyul Z, Sulyok E (2000) Lung water and proton magnetic resonance relaxation in preterm and term rabbit pups: their relation to tissue hyaluronan. Pediatr Res 48:554–559
Sims CJ, Fujito DT, Burholt DR, Dadok J, Giles HR, Wilkinson DA (1993) Quantification of human amniotic fluid constituents by high resolution proton nuclear magnetic resonance (NMR) spectroscopy. Prenat Diagn 13:473–480
Smith FW, Adam AH, Phillips WD (1983) NMR imaging in pregnancy. Lancet 1:61–62
Songster GS, Gray DL, Crane JP (1989) Prenatal prediction of lethal pulmonary hypoplasia using ultrasonic fetal chest circumference. Obstet Gynecol 73:261–266
Tanigaki S, Miyakoshi K, Tanaka M, Hattori Y, Matsumoto T, Ueno K, Uehara K, Nishimura O, Minegishi K, Ishimoto H, Shinmoto H, Ikeda K, Yoshimura Y (2004) Pulmonary hypoplasia: prediction with use of ratio of MR imaging-measured fetal lung volume to US-estimated fetal body weight. Radiology 232:767–772
Tekesin I, Anderer G, Hellmeyer L, Stein W, Kuhnert M, Schmidt S (2004) Assessment of fetal lung development by quantitative ultrasonic tissue characterization: a methodical study. Prenat Diagn 24:671–676
Thayyil S, Schievano S, Robertson NJ, Jones R, Chitty LS, Sebire NJ, Taylor AM (2008) A semi-automated method for non-invasive internal organ weight estimation by post-mortem magnetic resonance imaging in fetuses, newborns and children. Eur J Radiol 72:321–326
Thompson HE, Makowski EL (1971) Estimation of birth weight and gestational age. Obstet Gynecol 37:44–47
Thurlbeck WM (1982) Postnatal human lung growth. Thorax 37:564–571
Thurlbeck WM (1988) Lung growth. In: Thurlbeck WM (ed) Pathology of the lung. Georg Thieme, Stuttgart, pp 1–10
Trudinger BJ, Knight PC (1980) Fetal age and patterns of human fetal breathing movements. Am J Obstet Gynecol 137:724–728
Trudinger BJ, Gordon YB, Grudzinskas JG, Hull MG, Lewis PJ, Arrans ME (1979) Fetal breathing movements and other tests of fetal wellbeing: a comparative evaluation. Br Med J 2:577–579
Vintzileos AM, Campbell WA, Rodis JF, Nochimson DJ, Pinette MG, Petrikovsky BM (1989) Comparison of six different ultrasonographic methods for predicting lethal fetal pulmonary hypoplasia. Am J Obstet Gynecol 161:606–612
Ward VL, Nishino M, Hatabu H, Estroff JA, Barnewolt CE, Feldman HA, Levine D (2006) Fetal lung volume measurements: determination with MR imaging–effect of various factors. Radiology 240:187–193
Weber H (1888) Über physiologische Atmungsbewegungen des Kindes im Uterus. Thesis. Marburg/Lahn, University of Marburg
Wedegaertner U, Tchirikov M, Habermann C, Hecher K, Deprest J, Adam G, Schroeder HJ (2004) Fetal sheep with tracheal occlusion: monitoring lung development with MR imaging and B-mode US. Radiology 230:353–358. Epub 2003 Dec 2029
Williams G, Coakley FV, Qayyum A, Farmer DL, Joe BN, Filly RA (2004) Fetal relative lung volume: quantification by using prenatal MR imaging lung volumetry. Radiology 233:457–462. Epub 2004 Sep 30
Zeltner TB, Bertacchini M, Messerli A, Burri PH (1990) Morphometric estimation of regional differences in the rat lung. Exp Lung Res 16:145–158
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kasprian, G. (2010). MRI of the Normal Fetal Lung. In: Prayer, D. (eds) Fetal MRI. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2010_19
Download citation
DOI: https://doi.org/10.1007/174_2010_19
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-73270-9
Online ISBN: 978-3-540-73271-6
eBook Packages: MedicineMedicine (R0)