Abstract
Voltage-sensitive Ca2+ (CaV) channels are the primary route of depolarization-induced Ca2+ entry in neurons and other excitable cells, leading to an increase in intracellular Ca2+ concentration ([Ca2+]i). The resulting increase in [Ca2+]i activates a wide range of Ca2+-dependent processes in neurons, including neurotransmitter release, gene transcription, activation of Ca2+-dependent enzymes, and activation of certain K+ channels and chloride channels. In addition to their key roles under physiological conditions, CaV channels are also an important target of alcohol, and alcohol-induced changes in Ca2+ signaling can disturb neuronal homeostasis, Ca2+-mediated gene transcription, and the function of neuronal circuits, leading to various neurological and/or neuropsychiatric symptoms and disorders, including alcohol withdrawal induced–seizures and alcoholism.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In neurons, voltage-sensitive Ca2+ (CaV) channels serve as the primary route of Ca2+ entry in response to membrane depolarization, driving a localized increase in intracellular Ca2+ concentration ([Ca2+]i). The driving force for Ca2+ entry arises from the steep electrochemical gradient maintained between extracellular and intracellular Ca2+ concentrations, which are typically on the order of 1 mM and 100 nM, respectively; thus Ca2+ entry can change membrane potential and can therefore affect neuronal excitability. In neurons, low [Ca2+]i is maintained by a variety of mechanisms and processes, including Ca2+ efflux via a Na+/Ca2+ exchange protein and a Ca2+-ATPase located at the plasma membrane, as well as the sequestration of intracellular Ca2+ into in Ca2+ stores (e.g., via the sarco-endoplasmic reticular ATPase pump) or by Ca2+-buffering proteins (Berridge 2012).
The CaV-mediated localized increase in [Ca2+]i in neurons activates a variety of downstream processes, including Ca2+-induced Ca2+ release from intracellular Ca2+-gated Ca2+ stores, activation of Ca2+-activated K+ channels, Ca2+-activated chloride channels and Ca2+-dependent enzymes, and other Ca2+-dependent processes such as gene transcription and neurotransmitter release. In addition, Ca2+ entry following relatively mild membrane depolarization (e.g., depolarization induced by activation of N-methyl-d-aspartate receptors) can give rise to low-threshold Ca2+ spikes, which can further depolarize the plasma membrane, causing voltage-gated Na+ channels to open and initiating the repetitive firing of action potentials (Cain and Snutch 2010). Thus, CaV channels play a wide range of important roles under both physiological and pathophysiological conditions, including a variety of diseases associated with neuronal excitability. In the central nervous system (CNS), CaV channels are also an important molecular target for alcohol, and changes in neuronal Ca2+ signaling induced by alcohol exposure and subsequent withdrawal can lead to alcoholism and alcohol withdrawal–induced seizures, (AWSs).
2 Structure, Diversity, and Localization of Voltage-Sensitive Ca2+ Channels in the CNS
2.1 Structure and Diversity of CaV Channels
CaV channels are large protein complexes comprised of a pore-forming α1 subunit and up to three auxiliary β, α2/δ, and γ subunits (Simms and Zamponi 2014). In addition to providing the pore through which Ca2+ flows, the α1 subunit of CaV channels also confers the channel’s electrophysiological and pharmacological properties; in contrast, the auxiliary subunits modulate the channel’s biophysical properties and regulate the channel’s trafficking to the plasma membrane. In human, nine distinct genes encode the α1 subunits (designated α1A through α1I), all of which are expressed in the CNS (Simms and Zamponi 2014). Based on their responsiveness to changes in membrane potential, these nine CaV channels are broadly classified as either low voltage–activated (LVA, comprising the CaV3 family) channels or high voltage–activated (HVA, which include the CaV1 and CaV2 families) channels. Activation of LVA channels and HVA channels produced transient and sustained currents, respectively.
HVA CaV channels have both distinct and overlapping voltage dependence and kinetics, making it difficult to differentiate HVA CaV currents based solely on their biophysical properties. Fortunately, however, HVA CaV channels have unique pharmacological profiles, which have been used to confirm the heterogeneity of the channels expressed in the CNS. Moreover, based largely on their sensitivity to various CaV channel blockers, HVA CaV channels currents have been further classified into the following five types: L-type CaV1.2 (α1C), L-type CaV1.3 (α1D), N-type CaV2.2 (α1B), P/Q-type Cav2.1 (α1A), and R-type CaV2.3 (α1E) channels, encoded by the CACNA1C, CANA1D, CANA1B, CANA1A, and CACNA1E genes, respectively (Ertel et al. 2000; Randall and Tsien 1995). In the CNS, P/Q-type Cav2.1 channels can give rise to both P-type and Q-type currents; this distinction is likely due to a combination of factors, including the CaV-β subunit and/or alternative splicing of the CACNA1A gene that encodes the channels (Richards et al. 2007).
Molecular analyses revealed that the LVA family of CaV channels consists of three distinct α1 pore-forming subunits, namely CaV3.1 (α1G), CaV3.2 (α1H), and CaV3.3 (α1I), encoded by the CACNA1G, CANA1H, and CACNA1I genes, respectively (Cribbs et al. 1998; Lee et al. 1999; Perez-Reyes et al. 1998). Interestingly, unlike HVA CaV channels, the α1 subunit of LVA CaV channels does not require auxiliary subunits to form a fully functional channel, although LVA CaV channels can be regulated by auxiliary subunits (Klöckner et al. 1999). Finally, the three genes that encode the CaV3.x subunits can undergo alternative splicing, giving rise to a wide diversity of functional LVA CaV channels (Swayne and Bourinet 2008). The CaV-α1 subunit is comprised of four transmembrane domains, which are connected by cytoplasmic linkers (Simms and Zamponi 2014; Turner and Zamponi 2014). The N and C termini are located in the cytoplasmic side and they contained important sites for protein–protein interactions such as with G-protein and protein kinases (Simms and Zamponi 2014; Turner and Zamponi 2014). Interestingly, phosphorylation by PKA or PKC alters the voltage dependence and kinetics of CaV currents (Gray and Johnston 1987; Nagao and Adachi-Akahane 2001; Sculptoreanu et al. 1993; Stea et al. 1995).
2.2 Localization and Function HVA CaV1 Channels
Although L-type CaV1.x channels are expressed widely throughout brain, each channel subtype has a unique cellular and subcellular distribution. For example, L-type CaV1.3 channels are distributed relatively evenly, whereas L-type CaV1.2 channels are localized in clusters (Hell et al. 1993; Tippens et al. 2008). Moreover, L-type CaV1.2 and CaV1.3 channels are located predominantly on the cell soma (where they regulated depolarization and Ca2+-dependent pathways that control gene expression), proximal dendrites, and in some interneurons in the olfactory bulb, cerebral cortex (pyramidal neurons), hippocampus (pyramidal neurons in the CA1–CA3 areas), dentate gyrus (granule neurons), amygdala, inferior colliculus, cerebellum (granule layer, molecular layer, Purkinje cells), and spinal cord (Hell et al. 1993). Unlike L-type CaV1.3 channels, CaV1.2 channels are expressed in astrocytes in the CA3 area of the hippocampus (Tippens et al. 2008; Westenbroek et al. 1990). The distribution of CaV1.2 and CaV1.3 channels throughout the CNS has been confirmed by RT-PCR analysis, which shows that the levels of CACNA1C and CACNA1D mRNA matches the protein levels of CaV-α1C and CaV-α1D subunits, respectively (Sinnegger-Brauns et al. 2009; Schlick et al. 2010). In the striatum, CACNA1C and CACNA1D mRNA are co-expressed in medium-sized spiny neurons (Olson et al. 2005). Interestingly, L-type CaV1.3a (but not CaV1.3b) isoform co-localizes with Shank protein and the synaptic protein PSD-95 in medium spiny neurons at excitatory synapses (Olson et al. 2005). In the CNS, approximately 80% and 20% of L-type CaV1 channels are CaV1.2 and CaV1.3 channels, respectively (Hell et al. 1993; Sinnegger-Brauns et al. 2009). With respect to function, evidence suggests that L-type CaV1.3 channels activate with less depolarization and inactivate more slowly than CaV1.2 channels (Koschak et al. 2001; Xu and Lipscombe 2001). Given their unique set of biophysical properties, L-type CaV1.3 channels likely play an important role in controlling Ca2+-dependent firing; moreover, L-type CaV1.3 channels help sustain Ca2+ influx at membrane potentials at which CaV1.2 channels are closed.
CaV2.1, CaV2.2, and CaV2.3 channels (i.e., P/Q-type, N-type, and R-type, respectively) are also expressed throughout the CNS. P/Q-type CaV2.1 channels are primarily concentrated in presynaptic terminals and dendritic shafts, N-type CaV2.2 are found mainly in dendrites and some cell bodies of neurons, and R-type CaV2.3 channels are found mainly in the cell soma in most sites with variable expression in dendrites (Westenbroek et al. 1992, 1995; Yokoyama et al. 1995). These CaV channels are found primarily in the olfactory bulb, cerebral cortex (pyramidal neurons), striatum (medium-sized spiny neurons), amygdala, hippocampus (pyramidal neurons in CA1–CA3 areas), dentate gyrus (granule neurons), thalamus, globus pallidus, hypothalamus, inferior colliculus, and cerebellum (Purkinje cells) (Hillman et al. 1991; Westenbroek et al. 1992, 1995; Volsen et al. 1995; Yokoyama et al. 1995; Day et al. 1996; Xu et al. 2010). In the cortex and hippocampus, there is barely detection of R-type CaV2.3 channels in proximal dendrites, while other structures such as olfactory bulb, amygdala, and cerebellum have intense expression of these channels in the dendrites, the prominent sites of Ca2+ entry, causing transient increase in cytosolic Ca2+. Molecular and biochemical analyses have confirmed that mRNA levels match the corresponding protein for CaV2.1(α1A), CaV2.2(α1B), and CaV2.3 (α1E) (Mori et al. 1991; Soong et al. 1993; Day et al. 1996; Ludwig et al. 1997; Schlick et al. 2010).
At synaptic terminal, the rapid release of neurotransmitters requires tight coupling between presynaptic CaV2.x channels to the release machinery. In addition to regulating vesicle fusion, members of the CaV2.x channels also control neuronal excitability. For example, P/Q-type CaV2.1 and N-type CaV2.2 channels interact both physically and functionally with large-conductance, Ca2+-activated K+ channels, providing the Ca2+ influx needed to activate these channels (Faber and Sah 2003; Berkefeld et al. 2010); thus, P/Q-type CaV2.1 and N-type CaV2.2 channels control neuronal excitability by regulating K+ conductances.
2.3 Localization and Function LVA CaV3 Channels
Like HVA CaV channels, LVA CaV3 channels are also distributed throughout the CNS; however, their expression is restricted to the cell body and dendrites of neurons primarily in the olfactory bulb (granule layer), cerebral cortex (pyramidal neurons, GABAergic interneurons), striatum, amygdala, hippocampus (CA1–CA3 pyramidal neurons), dentate gyrus (granule cells), thalamus (large neurons, GABAergic interneurons), substantia nigra, inferior colliculus, superior colliculus, inferior olive, cerebellum (granule layer, molecular layer, Purkinje cells), and spinal cord (Craig et al. 1999; Talley et al. 1999; Yunker et al. 2003; McKay et al. 2006; Kovács et al. 2010; Liu et al. 2011; Kanyshkova et al. 2014).
As discussed above, LVA CaV3 channels are activated upon weak depolarization and carry depolarizing currents; therefore, similar to L-type CaV1.3 channels, LVA CaV3.x channels also play an important role in controlling neuronal excitability. LVA CaV3.x channels also inactivate at a fast rate. Thus, a combination of low threshold of activation with fast inactivation kinetics results in transient Ca2+ influx, giving rise to the so-called “low-threshold Ca2+ potentials,” which initiate the burst-firing process (Cain and Snutch 2010; Contreras 2006; Jahnsen and Llinas 1984; Lee et al. 2003; Yazdi et al. 2007; Xu and Clancy 2008). The burst-firing mode in the CNS contributes to the generation of physiological events such as sleep spindles, and pathological conditions such as epileptic seizures (Cain and Snutch 2010, 2012). In addition, LVA CaV3.x channels generate a so-called “window current” near the neuron’s resting membrane potential, thereby regulating Ca2+ homeostasis (Dreyfus et al. 2010). In the CNS, LVA CaV3.x channels are also associated both with voltage-gated K+ channels and with Ca2+-activated K+ channels (Anderson et al. 2010; Rehak et al. 2013), giving LVA CaV3.x channels the ability to activated K+ channels and regulate neuronal firing.
3 Effects of Acute Alcohol Exposure on the Expression and Function of CaV Channels
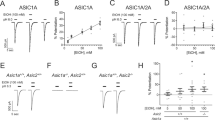
Oakes and Pozos (1982a, b) reported that alcohol exposure decreased CaV currents (and voltage-gated K+ currents but not voltage-gated Na+ currents) in dorsal root ganglia neurons. This effect was not associated with change in the resting membrane potential and spike amplitude. However, the duration of the action potential (AP) was decreased, and AP threshold was increased (Oakes and Pozos 1982a, b). A large body of experimental evidence indicates that acute alcohol exposure suppresses K+ depolarization–induced and AP–evoked Ca2+ transients in several CNS neurons including inferior colliculus, cerebellar, and hippocampal neurons (Gruol et al. 1997; Mah et al. 2011; Morton and Valenzuela 2016; our unpublished data). Consistent with these findings, we found that acute alcohol exposure inhibits the current carried by HVA CaV channels in inferior colliculus neurons (our unpublished data). Furthermore, acute alcohol exposure suppresses currents through L-type CaV1.x channels at neurohypophysial terminals, in supraoptic neurons, and hippocampal neurons (Wang et al. 1991, 1994; Widmer et al. 1998; Zucca and Valenzuela 2010). On the other hand, P-type CaV2.1 channels in Purkinje cells are unaffected by acute alcohol exposure (Hall et al. 1994). Thus, in the CNS, L-type CaV1.x channels appear to be particularly sensitive to the acute effects of alcohol exposure.
Interestingly, LVA CaV3.x channels are also an important target for alcohol. For example, acutely exposing rodent thalamic neurons to a low or high alcohol concentration increases or decreases, respectively, LVA CaV3.x currents (Mu et al. 2003; Joksovic et al. 2005). Furthermore, the inhibitory effect of alcohol on LVA CaV3.x currents appears to be mediated by protein kinase C (Shan et al. 2013). In contrast, acute exposure to either low or high alcohol concentration inhibits LVA CaV3.x currents in the inferior olive in primates (Welsh et al. 2011). Thus, the increase in LVA CaV3.x currents in response to low alcohol concentration in rodents – but not in primates – suggests species-specific differences in the underlying mechanisms.
The inhibition of HVA CaV channels and LVA CaV channels (Fig. 1), and downstream Ca2+-related signaling following acute alcohol exposure suggests that this mechanism may induce a compensatory upregulation of HVA CaV channels and LVA CaV channels during chronic alcohol intoxication; this upregulation would be masked by the inhibitory effect alcohol, but would then be revealed during alcohol withdrawal.
4 Effects of Chronic Alcohol Exposure on the Expression and Function of CaV Channels
Several lines of evidence indicate that chronic alcohol exposure alters Ca2+ signaling in the CNS. For example, chronic alcohol exposure increases AP–evoked Ca2+ transients in hippocampal neurons (Mulholland et al. 2015), possibly by upregulating of CaV channels. Consistent with this notion, P-type CaV2.1 current is increased in the cerebellum during chronic alcohol exposure (Gruol and Parsons 1994). On the other hand, chronic alcohol intoxication by inhalation did not alter the protein levels of P/Q-type CaV2.1 (α1A) protein levels in cortical neurons (Katsura et al. 2005). Similarly, the protein levels of the P/Q-type α1A subunit were unchanged in the central nucleus of the amygdala following chronic intermittent alcohol exposure (Varodayan et al. 2017a). Increased protein levels of L-type CaV1.3 (α1D) channels were measured in cortical neurons in mice following chronic alcohol exposure by inhalation (Katsura et al. 2005). However, in the model of chronic intermittent alcohol exposure, the protein levels of the L-type CaV1.2 (α1C) subunit were decreased in the central nucleus of the amygdala (Varodayan et al. 2017b). The dihydropyridine binding sites, which represent L-type CaV1.x channels, were increased in ethanol-dependent brains (Dolin et al. 1987). Accordingly, chronic alcohol exposure increased total CaV currents including L-type CaV1.x in hippocampal neurons in ethanol-tolerant long-sleep mice compared to short-sleep mice; this effect was not associated with changes in the biophysical properties of the channels, suggesting an increase in the number of functional L-type CaV1.x channels (Huang and McArdle 1993). L-type CaV1.x channels are also implicated in alcohol-mediated neurodegeneration, as inhibition of these channels attenuated cytotoxicity related to chronic alcohol exposure of neocortical cell cultures (Ruhe and Littleton 1994).
Finally, the protein levels of N-type CaV2.2 (α1B) channels were unchanged in cortical neurons following chronic alcohol administration (Katsura et al. 2005), whereas McMahon et al. (2000) reported an increase in the number of N-type CaV2.2 channels in the frontal cortex and hippocampus in AWS-prone mice following chronic alcohol administration. Thus, the increase in N-type CaV2.2 channel expression may be specific to certain brain structures, and this increase may be related to the genetic predisposition of AWS-prone mice to these seizures. Importantly, mice that lack functional N-type CaV2.2 channels have reduced alcohol consumption (Newton et al. 2004). Similarly, mice treated with blockers and/or agonists of L-type CaV1.x channels have reduced alcohol consumption (Rezvani and Janowsky 1990; Rezvani et al. 1991; De Beun et al. 1996a, b). These findings suggest that the anti-alcohol effect may not be related to antagonistic activity at L-type CaV1.x channels; alternatively, the anti-alcohol effect may be restricted to specific brain sites. The amygdala appears to be one of the brain sites underlying this behavioral effect, as blocking of L-type CaV1.x channels in the central nucleus of the amygdala reduces alcohol intake in rodents (Varodayan et al. 2017b). Taken together, these findings suggest that both L-type CaV1.x channels and N-type CaV2.2 channels might serve as viable therapeutic targets for treating of alcoholism. The mechanisms underlying changes in L-type CaV1.x channels and N-type CaV2.2 channels are not fully understood (Fig. 2). Nevertheless, chronic alcohol exposure increases the expression of protein kinase C (PKC) isoforms, including PKC delta (PKCδ) and PKC epsilon (PKCε); moreover, chronic alcohol exposure upregulated L-type CaV1.x channels and N-type CaV2.2 channels via PKCδ- and PKCε-dependent mechanism, respectively (Gerstin et al. 1998; McMahon et al. 2000).
Chronic alcohol intoxication upregulates CaV channels in the brain. During chronic alcohol exposure, a compensatory mechanism to the inhibitory effect of alcohol exposue occurs leading to an upregulation of CaV channels including L-type CaV1.3 (but downregulation of CaV1.2); moreover, the function and/or expression of these channels is masked by the inhibitory effect of alcohol. LVA CaV channels are either upreglated or downregulated in various brain sites
Interestingly, in primates, chronic alcohol exposure decreases and increases LVA CaV3.x in the thalamus and inferior olive, respectively (Carden et al. 2006; Welsh et al. 2011). In contrast, no changes in the mRNA levels or current density of LVA CaV3.x channels were seen in thalamic neurons in a mouse model of chronic alcohol exposure (Graef et al. 2011); however, the steady-state inactivation of LVA CaV3.x channels was altered in these neurons during alcohol intoxication suggesting a change in Ca2+ currents carried by these channels (Graef et al. 2011).
5 Effects of Alcohol Withdrawal on the Expression and Function CaV Channels
Alcohol withdrawal triggers increase in the expression of early gene c-fos throughout the CNS at the time at which the seizure susceptibility peaked (Bouchenafa and Littleton 1998). The increased expression of c-fos was prevented by inhibition of L-type CaV1.x channels, suggesting an important role of Ca2+ influx in the mechanisms underlying AWS susceptibility (Bouchenafa and Littleton 1998). In addition, withdrawal from chronic alcohol exposure induced neuronal hyperexcitability in the hippocampus; this epileptiform activity was mediated, in part, by L-type CaV1.x channels (Riplet et al. 1996; Whittington and Little 1991, 1993; Whittington et al. 1992, 1995). Seizures are usually the most severe symptoms associated with alcohol withdrawal syndrome. Typically, these AWSs are generalized tonic-clonic seizures, which are initiated in the brainstem. In our model of acoustically evoked AWSs, neurons in the IC play a critical role in initiating AWSs, whereas the cortex, hippocampus, and amygdala play a role in propagating these seizures (Faingold et al. 1998; Takao et al. 2006; Faingold 2008; Newton and N’Gouemo 2017). In this model, K+ depolarization–induced Ca2+ transients were increased in inferior colliculus neurons when the susceptibility to AWS peaks (our unpublished data). The influx of Ca2+ into neurons plays an important role in the neuronal hyperexcitability that underlies seizures, as [Ca2+]i rises – and extracellular [Ca2+] decreases – during epileptiform activity (Heinemann et al. 1977; Albowitz et al. 1997; Delorenzo et al. 2005). Thus, inhibition of Ca2+ influx into neurons is a promising therapeutic approach for various types of seizures, including AWSs. Interestingly, pharmacologically blocking L-type CaV1.x channels suppressed acoustically evoked AWSs (Little et al. 1986). These findings suggest that altered L-type CaV1.x channels – at least in the IC – play a key role in initiating these seizures. Consistent with this notion, currents through HVA CaV channels are increased before the onset of AWS susceptibility and when the prevalence of AWSs peaks, but they returned to control levels after AWS susceptibility has returned to baseline (N’Gouemo 2015; N’Gouemo and Morad 2003). Thus, the increase in HVA CaV currents measured in IC neurons prior to the onset of AWS susceptibility cannot be a consequence of seizure activity. Interestingly, alcohol withdrawal increased HVA CaV currents in dentate granule neurons in AWS-prone mice but not in AWS-resistant mice (Perez-Velazquez et al. 1994), suggesting that genetic differences in the genes encoding HVA CaV channels may contribute to differences in AWS susceptibility and the expression of HVA CaV channels.
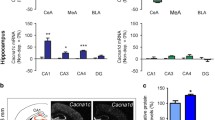
Alcohol withdrawal-induced upregulation of L-type CaV1.x channels in the brain was also reported in a mouse model (Brennan et al. 1990; Guppy et al. 1995; Watson and Little 1999). In our rat model of acoustically evoked AWSs, the increased Ca2+ current density in IC neurons mediated by L-type CaV1.x channels and P-type CaV2.1 channels occurs during peak AWS susceptibility (N’Gouemo 2015; N’Gouemo and Morad 2003). These findings suggest a possible causal relationship between the upregulation of L-type CaV1.x channels and P-type CaV2.1 channels in IC neurons and the occurrence of AWSs. L-type CaV1.x channels and P-type CaV2.1 channels play important roles in synaptic plasticity and glutamate release, respectively (Thiagarajan et al. 2005; Ermolyuk et al. 2013). Thus, an increase in currents through L-type CaV1.x channels and/or P-type CaV2.1 channels in IC neurons is likely to increase both firing and transmitter release, leading to increased AWS susceptibility. Consistent with this notion, blocking L-type CaV1.x channels in the IC suppressed AWS susceptibility, whereas inhibiting P-type CaV2.1 channels only reduced AWS severity (N’Gouemo 2015). Moreover, the protein levels of L-type CaV1.3 (α1D) channels – but not L-type CaV1.2 (α1C) channels or P/Q-type CaV2.1(α1A) channels – are upregulated in IC neurons when AWS susceptibility peaks (Fig. 3), but not prior to the onset of AWS susceptibility (N’Gouemo et al. 2015; Newton et al. 2018). However, it is important to note that the lack of change in protein levels of P/Q-type CaV2.1 (α1A) channels reflects all P/Q-type channel phenotypes and may therefore masks any increase in the selective expression of P-type CaV2.1 channels occurring in some selective neuronal subtypes.
Alchol withdrawal upregulates CaV channels. Alcohol withdrawal following chronic alcohol exposure unmasks the upregulation of CaV channels mainly L-type CaV1.3 channels and downregulation of L-type CaV1.2 and N-type CaV2.2 channels. LVA CaV channels are either upreglated or downregulated in various brain sites
Interestingly, although mRNA expression of CACNA1D and CACNA1A (which encode the L-type α1D and P/Q-type α1D subunits, respectively) is increased in IC neurons prior to the onset of AWS susceptibility, their corresponding total protein levels are unchanged in these neurons (N’Gouemo et al. 2015; Newton et al. 2018). Thus, changes in cell surface expression and/or phosphorylation of these HVA CaV channels may account for the increased current density in IC neurons prior to the onset of AWS susceptibility. In support of this notion, the activity and expression of protein kinase A are increased in IC neurons prior to the onset of AWS susceptibility (Akinfiresoye et al. 2016). Under normal conditions, phosphorylation by protein kinase A enhances L-type CaV1.x and P-type CaV2.1 currents (Fournier et al. 1993; Mogul et al. 1993; Davare and Hell 2003), while activation of PKC inhibits the activity of N-type CaV2.2 channels, but increases other types of CaV currents (Diversé-Pierluissi and Dunlap 1993; Rane and Dunlap 1986; Rane et al. 1989). Interestingly, alcohol acts on L-type CaV1.x channels by inhibiting calmodulin-dependent activity of the channel (Canda et al. 1995). Thus, increase in L-type CaV1.x currents prior to the onset of AWS susceptibility may be due to phosphorylation of the channels. Similarly, downregulation of N-type CaV2.2 channels seen in IC neurons at the time at which AWS susceptibility peaks may be due to enhanced PKC activity.
On the other hand, the protein levels of N-type CaV2.2 (α1B) subunit are decreased in IC neurons when AWS susceptibility peaks (N’Gouemo et al. 2006) (Fig. 3). Interestingly, activation of PKC inhibits the activity of N-type CaV2.2 channels, but increases other types of CaV currents (Diversé-Pierluissi and Dunlap 1993; Rane and Dunlap 1986; Rane et al. 1989), suggesting increased PKC activity in the IC following alcohol withdrawal at the time at which the susceptibility to AWS peaked. The downregulation of N-type CaV2.2 channels may contribute to AWS susceptibility by reducing Ca2+-dependent inhibitory mechanisms, as Ca2+ influx contributes to the activation of Ca2+-activated K+ current, which initiates repolarization and underlies the afterhyperpolarization, an intrinsic neuronal inhibitory mechanism (Faber and Sah 2003; Loane et al. 2007; Berkefeld et al. 2010; N’Gouemo and Morad 2014). Interestingly, some Ca2+ channel types have been shown to provide the necessary Ca2+ influx required to activate small-conductance, and/or large-conductance, Ca2+-activated K+ channels in the brain (Faber and Sah 2003; Berkefeld et al. 2010). Thus, there appear to be significant differences in coupling between Ca2+ channels and Ca2+-activated K+ channels, suggesting a functional role for the Ca2+ channels in driving the activity of Ca2+ microdomains.
In primates, alcohol withdrawal decreases LVA CaV3.x currents in inferior olive neurons (Welsh et al. 2011). In a mouse model of alcohol withdrawal, thalamic neurons have increased mRNA levels of the genes encoding the LVA CaV3.2 and CaV3.3 channel subtypes, but not CaV3.1 channel subtype (Graef et al. 2011). Despite these changes in mRNA levels and in the steady-state inactivation of LVA CaV3.1x channels, alcohol withdrawal does not cause a change in LVA CaV3.1x currents in thalamic neurons (Graef et al. 2011). However, ethosuximide, a potent blocker of LVA CaV3.x channels commonly used to treat absence seizures, suppresses susceptibility to AWSs in a mouse model (Riegle et al. 2015), suggesting these channels may have therapeutic applications beyond the treatment of absence seizures.
6 Conclusion
In the CNS, CaV channels play an important role in regulating neuronal excitability, and changes in their activity and/or expression contribute to a wide variety of pathological conditions, including seizures. In keeping with their central role in CNS excitability, CaV channels are also an important target for alcohol, and both acute and chronic alcohol exposure, as well as alcohol withdrawal, can alter the function of CaV channels, giving rise to an array of symptoms and disorders, including alcohol abuse, alcoholism, and AWSs. Paradoxically, there is a positive relationship between increased CaV channel function/expression and increased susceptibility to AWSs, yet downregulating CaV channels can also cause seizures, as some CaV channels are functionally coupled to K+ channels and/or chloride channels. From this review, it becomes clear that HVA CaV1.x (i.e., L-type) channels and HVA CaV2.2 (i.e., N-type) channels are promising targets for treating alcohol abuse and alcoholism; in contrast, L-type CaV1.3 – and to some extent LVA CaV3.x (i.e., T-type) – channels are promising targets for treating AWSs. Moreover, the alcohol-related changes in the function and/or expression of various CaV channels vary among brain structures, suggesting the need for targeted therapeutic approaches, reflecting the notion that localized changes in specific CaV channels induce distinct sets of symptoms associated with alcoholism and the alcohol withdrawal syndrome.
References
Akinfiresoye LR, Miranda C, Lovinger DM, N’Gouemo P (2016) Alcohol withdrawal increases protein kinase A activity in the rat inferior colliculus. Alcohol Clin Exp 40:2359–2367
Albowitz B, König P, Kuhnt U (1997) Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J Neurophysiol 77:491–501
Anderson D, Rehak R, Hameed S, Mehaffey WH, Zamponi GW, Turner RW (2010) Regulation of the KV4.2 complex by CaV3.1 calcium channels. Channels (Austin) 4:163–167
Berkefeld H, Fakler B, Schulte U (2010) Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90:1437–1459
Berridge MJ (2012) Calcium signalling remodelling and disease. Biochem Soc Trans 40:297–309
Bouchenafa O, Littleton JM (1998) Expression of c-Fos protein immunoreactivity in rat brain during ethanol withdrawal is prevented by nifedipine. Alcohol 15:71–77
Brennan CH, Crabbe J, Littleton JM (1990) Genetic regulation of dihydropyridine-sensitive calcium channels in brain may determine suscpetibility to physical dependence on alcohol. Neuropharmacology 29:429–432
Cain SM, Snutch TP (2010) Contributions of T-type calcium channel isoforms to neuronal firing. Channels 4:44–51
Cain SM, Snutch TP (2012) Voltage-gated calcium channels in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies, 4th edn. Oxford University Press, Bethesda, pp 66–84
Canda A, Yu BH, Sze PY (1995) Biochemical characterization of ethanol actions on dihydropyridine-sensitive calcium channels in brain synaptosomes. Biochem Pharmacol 50:1711–1718
Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, Godwin DW (2006) Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res 1089:92–100
Contreras D (2006) The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets 5:571–585
Craig PJ, Beattie RE, Folly EA, Banerjee MD, Reeves MB, Priestley JV, Carney SL, Sher E, Perez-Reyes E, Volsen SG (1999) Distribution of the voltage-dependent calcium channel alpha1G subunit mRNA and protein throughout the mature rat brain. Eur J Neurosci 11:2949–2964
Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E (1998) Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res 83:103–109
Davare MA, Hell JW (2003) Increased phosphorylation of neuronal L-type Ca2+ channel CaV1.2 during aging. Proc Natl Acad Sci U S A 100:16018–16023
Day NC, Shaw PJ, McCormack AL, Craig PJ, Smith W, Beattie R, Williams TL, Ellis SB, Ince PG, Harpold MM, Lodge D, Volsen SG (1996) Distribution of alpha 1A, alpha 1B and alpha 1E voltage-dependent calcium channel subunits in the human hippocampus and parahippocampal gyrus. Neuroscience 71:1013–1024
De Beun R, Schneider R, Klein A, Lohmann A, De Vry J (1996a) Effects of nimodipine and other calcium channel antagonists in alcohol-preferring AA rats. Alcohol 13:263–171
De Beun R, Schneider R, Klein A, Lohmann A, Schreiber R, De Vry J (1996b) The calcium channel agonist BAY k 8644 reduces ethanol intake and preference in alcohol-preferring AA rats. Psychopharmacology 127:302–310
Delorenzo RJ, Sun DA, Deshpande LS (2005) Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintenance of epilepsy. Pharmacol Ther 105:229–266
Diversé-Pierluissi M, Dunlap K (1993) Distinct, convergent second messenger pathways modulate neuronal calcium currents. Neuron 10:753–760
Dolin S, Little H, Hudspith M, Pagonis C, Littleton J (1987) Increased dihydropyridine-sensitive calcium channels in rat brain may underlie ethanol physical dependence. Neuropharmacology 26:275–279
Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N (2010) Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 30:99–109
Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM, Volynski KE (2013) Differential triggering of spontaneous glutamate release by P/Q-, N-, and R-type Ca2+ channels. Nat Neurosci 16:1754–1763
Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe Y, Birnbauner L, Tsien RW, Catterall WA (2000) Nomenclature of voltage-gated calcium channels. Neuron 25:533–535
Faber ES, Sah P (2003) Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9:181–194
Faingold CL (2008) The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci. Chapter 9: Unit 9.28
Faingold CL, N’Gouemo P, Riaz A (1998) Ethanol and neurotransmitter interaction-from molecular to integrative effects. Prog Neurobiol 55:509–535
Fournier F, Bourinet E, Nargeot J, Charnet P (1993) Cyclic AMP-dependent regulation of P-type calcium channels expressed in Xenopus oocytes. Pflugers Arch 423:173–180
Gerstin EH, McMahon T, Dadgar J, Messing RO (1998) Protein kinase Cδ mediates ethanol-induced upregulation of L-type calcium channels. J Biol Chem 273:16409–16414
Graef JD, Huitt TW, Nordskog BK, Hammarback JH, Godwin DW (2011) Disrupted thalamic T-type Ca2 channel expression and function during ethanol exposure and withdrawal. J Neurophysiol 105:528–540
Gray R, Johnston D (1987) Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature 327:620–622
Gruol DL, Parsons KL (1994) Chronic exposure to alcohol during development alters the calcium currents of cultured cerebellar Purkinje neurons. Brain Res 624:283–290
Gruol DL, Parsons KL, DiJulio N (1997) Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res 773:82–89
Guppy LJ, Crabbe JC, Littleton JM (1995) Time course and genetic variation in the regulation of calcium channel antagonist binding sites in rodent tissues during the induction of ethanol physical dependence and withdrawal. Alcohol Alcohol 30:607–615
Hall AC, Lieb WR, Franks NP (1994) Insensitivity of P-type calcium channels to inhalation and intravenous general anesthetics. Anesthesiology 81:117–123
Heinemann U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in the cerebral cortex of the cat. Exp Brain Res 27:237–243
Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA (1993) Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol 123:949–962
Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR (1991) Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A 88:7076–7080
Huang G-J, McArdle JJ (1993) Chronic ingestion of ethanol increases the number of Ca2+ channels of hippocampal neurons of long-sleep but not short-sleep mice. Brain Res 615:328–330
Jahnsen H, Llinas R (1984) Voltage-dependent burst-to-tonic switching of thalamic cell activity: an in vitro study. Arch Ital Biol 122:73–82
Joksovic PM, Brimelow BC, Murbartián J, Perez-Reyes E, Todorovic SM (2005) Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant Ca(v)3.3 channels. Br J Pharmacol 144:59–70
Kanyshkova T, Ehling P, Cerina M, Meuth P, Zobeiri M, Meuth SG, Pape HC, Budde T (2014) Regionally specific expression of high-voltage-activated calcium channels in thalamic nuclei of epileptic and non-epileptic rats. Mol Cell Neurosci 61:110–122
Katsura M, Torigoe F, Hayashida S, Honda T, Tsujimura A, Ohkuma S (2005) Ethanol physical dependence is accompanied by up-regulated expression of L-type high voltage-gated calcium channel alpha1 subunits in mouse brain. Brain Res 1039:211–215
Klöckner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T (1999) Comparison of the Ca2+ currents induced by expression of three cloned alpha1 subunits, alpha1G, alpha1H and alpha1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci 11:4171–4178
Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J (2001) Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem 276:22100–22106
Kovács K, Sík A, Ricketts C, Timofeev I (2010) Subcellular distribution of low-voltage activated T-type Ca2+ channel subunits (Ca(v)3.1 and Ca(v)3.3) in reticular thalamic neurons of the cat. J Neurosci 88:448–460
Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöckner U, Schneider T, Perez-Reyes E (1999) Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci 19:1912–1921
Lee Y, Han J-H, Lim C-S, Chang D-J, Lee Y-S, Soh H, Park CS, Kaang BK (2003) Impairment of a parabolic bursting rhythm by the ectopic expression of a small conductance Ca2+-activated K+ channel in Aplysia neuron R15. Neurosci Lett 349:53–57
Little HJ, Dolin SJ, Halsey MJ (1986) Calcium channel antagonists decrease the ethanol withdrawal syndrome. Life Sci 39:2059–2065
Liu XB, Murray KD, Jones EG (2011) Low-threshold calcium channel subunit Cav3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol 519:1181–1195
Loane DJ, Lima PA, Marrion NV (2007) Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci 120:985–995
Ludwig A, Flockerzi V, Hofmann F (1997) Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J Neurosci 17:1339–1349
Mah SJ, Fleck MW, Lindsley TA (2011) Ethanol alters calcium signaling in axonal growth cones. Neuroscience 189:384–396
McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW (2006) CaV3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci 24:2581–2594
McMahon T, Andersen R, Metten P, Crabbe JC, Messing RO (2000) Protein kinase C epsilon mediates up-regulation of N-type calcium channels by ethanol. Mol Pharmacol 57:53–58
Mogul DJ, Adams ME, Fox AP (1993) Differential activation of adenosine receptors decreases N-type currents an potentiates P-type Ca2+ currents in hippocampal CA3 neurons. Neuron 10:327–334
Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S (1991) Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350:398–402
Morton RA, Valenzuela CF (2016) Further characterization of the effect of ethanol on voltage-gated Ca2+ channel function in developing CA3 hippocampal pyramidal neurons. Brain Res 1633:19–26
Mu J, Carden WB, Kurukulasuriya NC, Alexander GM, Godwin DW (2003) Ethanol influences on native T-type calcium current in thalamic sleep circuitry. J Pharmacol Exp Ther 307:197–204
Mulholland PJ, Spencer KB, Hu W, Kroener S, Chandler LJ (2015) Neuroplasticity of A-type potassium channel complexes induced by chronic alcohol exposure enhances dendritic calcium transients in hippocampus. Psychopharmacology 232:1995–2006
N’Gouemo P (2015) Altered voltage-gated calcium channels in rat inferior colliculus neurons contribute to alcohol withdrawal seizures. Eur Neuropsychopharmacol 25:1342–1352
N’Gouemo P, Morad M (2003) Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ channels currents in rat inferior colliculus neurons. Neuropharmacology 45:429–437
N’Gouemo P, Morad M (2014) Alcohol withdrawal is associated with a downregulation of large-conductance Ca2+-activated K+ channels in rat inferior colliculus neurons. Psychopharmacology 231:2009–2018
N’Gouemo P, Yasuda RP, Morad M (2006) Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Res 1108:216–220
N’Gouemo P, Akinfiresoye LR, Allard JS, Lovinger DM (2015) Alcohol withdrawal-induced seizure susceptibility is associated with an upregulation of CaV1.3 channels in the rat inferior colliculus. Int J Neuropsychopharmacol 18:pyu123. https://doi.org/10.1093/ijnp/pyu123
Nagao NI, Adachi-Akahane S (2001) Ser1901 of alpha(1c) subunit is required for PKA mediated enhancement of L-type Ca2+ channels currents but not for the negative shift of activation. FEBS Lett 489:87–91
Newton J, N’Gouemo P (2017) Withdrawal seizures. In: Pitkänen A, Buckmaster P, Galanopoulou AS, Moshé SL (eds) Models of seizures and epilepsy, 2nd edn. Academic, San Diego, pp 911–931
Newton PM, Orr CJ, Wallace MJ, Kim C, Shin HS, Messing RO (2004) Deletion of N-type calcium channels alters ethanol reward and reduces ethanol consumption in mice. J Neurosci 24:9862–9869
Newton J, Suman S, Akinfiresoye LR, Datta K, Lovinger DM, N’Gouemo P (2018) Alcohol withdrawal upregulates mRNA encoding for CaV2.1-α1 subunit in the rat inferior colliculus. Alcohol 66:21–16
Oakes SG, Pozos RS (1982a) Electrophysiologic effects of acute ethanol exposure. I. Alterations in the action potentials of dorsal root ganglia neurons in dissociated culture. Brain Res 281:243–249
Oakes SG, Pozos RS (1982b) Electrophysiologic effects of acute ethanol exposure. II. Alterations in the calcium component of action potentials from sensory neurons in dissociated culture. Brain Res 281:251–255
Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ (2005) G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci 25:1050–1062
Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH (1998) Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391:896–900
Perez-Velazquez JL, Valiante TA, Carlen PL (1994) Changes in calcium currents during ethanol withdrawal in a genetic mouse model. Brain Res 649:305–309
Randall A, Tsien RW (1995) Pharmacological dissection of multiple type of Ca2+ channels in rat cerebellar granule neurons. J Neurosci 15:2995–3012
Rane SG, Dunlap K (1986) Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A 83:184–188
Rane SG, Walsh MP, McDonald JR, Dunlap K (1989) Specific inhibitors of protein kinase C block transmitter-induced modulation of sensory neuron calcium current. Neuron 3:239–245
Rehak R, Bartoletti TM, Engbers JD, Berecki G, Turner RW, Zamponi GW (2013) Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS One 8:e61844
Rezvani AH, Janowsky DS (1990) Decreased alcohol consumption by verapamil in alcohol preferring rats. Prog Neuro-Psychopharmacol Biol Psychiatry 14:623–631
Rezvani AH, Grady DR, Janowsky DS (1991) Effect of calcium-channel blockers on alcohol consumption in alcohol-drinking monkeys. Alcohol Alcohol 26:161–167
Richards KS, Swensen AM, Lipscombe D, Bommert K (2007) Novel CaV2.1 clone replicates many properties of Purkinje cell CaV2.1 current. Eur J Neurosci 26:2950–2961
Riegle MA, Masicampo ML, Shan HQ, Xu V, Godwin DW (2015) Ethosuximide reduces mortality and seizure severity in response to pentylenetetrazole treatment during ethanol withdrawal. Alcohol Alcohol 50:501–508
Riplet TL, Whittington MA, Butterworth AR, Little HJ (1996) Ethanol withdrawal hyperexcitability in vivo and in isolated mouse hippocampal slices. Alcohol Alcohol 31:347–357
Ruhe CA, Littleton JM (1994) The possible role of voltage-operated calcium channels in the enhancement of excitatory amino acid toxicity following chronic ethanol exposure in vitro. Alcohol Alcohol Suppl 2:217–221
Schlick B, Flucher BE, Obermair GJ (2010) Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience 167:786–798
Sculptoreanu A, Scheuer T, Catterall WA (1993) Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364:240–243
Shan HQ, Hammarback JA, Godwin DW (2013) Ethanol inhibition of a T-type Ca2+ channel through activity of protein kinase C. Alcohol Clin Exp Res 37:1333–1342
Simms BA, Zamponi GW (2014) Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82:24–45
Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J (2009) Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 75:407–414
Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP (1993) Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 260:1133–1136
Stea A, Soong TW, Snutch TP (1995) Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron 15:929–940
Swayne LA, Bourinet E (2008) Voltage-gated calcium channels in chronic pain: emerging role of alternative splicing. Pflugers Arch 456:459–466
Takao T, Murakami H, Fukuda M, Kawaguchi T, Kakita A, Takahashi H, Kudoh M, Tanaka R, Shibuki K (2006) Transcranial imaging of audiogenic epileptic foci in the cortex of DBA/2J mice. Neuroreport 17:267–271
Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA (1999) Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19:1895–1911
Thiagarajan TC, Lindskog M, Tsien RW (2005) Adaptation to synaptic inactivity in the hippocampal neurons. Neuron 47:725–737
Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A (2008) Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol 506:569–583
Turner RW, Zamponi GW (2014) T-type channels buddy up. Pflugers Arch 466:661–675
Varodayan FP, Logrip ML, Roberto M (2017a) P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology 125:197–206
Varodayan FP, de Guglielmo G, Logrip ML, George O, Roberto M (2017b) Alcohol dependence disrupts amygdalar L-type voltage-gated calcium channel mechanisms. J Neurosci 37:4593–4603
Volsen SG, Day NC, McCormack AL, Smith W, Craig PJ, Beattie R, Ince PG, Shaw PJ, Ellis SB, Gillespie A, Harpold MM, Lodge D (1995) The expression of neuronal voltage-dependent calcium channels in human cerebellum. Mol Brain Res 34:271–282
Wang X, Dayanithi G, Lemos JR, Nordmann JJ, Treistman SN (1991) Ca2+ currents and peptide release from neurohypophysial terminals are inhibited by ethanol. J Pharmacol Exp Ther 259:705–711
Wang X, Wang G, Lemos JR, Treistman SN (1994) Ethanol directly modulates gating of a dihydropyridine-sensitive Ca2+ channels in neurohypophysial terminals. J Neurosci 14:5453–5460
Watson WP, Little HJ (1999) Correlation between increases in dihydropyridine binding in vivo and behavioural signs of ethanol withdrawal in mice. Alcohol Alcohol 34:35–42
Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA (2011) Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci U S A 108:10314–10319
Westenbroek RE, Ahlijanian MK, Catterall WA (1990) Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347:281–284
Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA (1992) Biochemical properties and distribution of N-type calcium channel α1 subunit. Neuron 9:1099–1115
Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TVB, Snutch TP, Catterall WA (1995) Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J Neurosci 15:6403–6418
Whittington MA, Little HJ (1991) Nitrendipine, given during drinking, decreases the electrophysiological changes in the isolated hippocampal slice, seen during ethanol withdrawal. Br J Pharmacol 103:1677–1684
Whittington MA, Little HJ (1993) Changes in voltage-operated calcium channels modify ethanol withdrawal hyperexcitability in mouse hippocampal slices. Exp Physiol 78:347–370
Whittington MA, Butterworth AR, Dolin SJ, Patch TL, Little HJ (1992) The effects of chronic treatment with dihydropyridine, Bay K 8644, on hyperexcitability due to ethnaol withdrawal, in vivo and in vitro. Br J Pharmacol 105:285–292
Whittington MA, Lambert JD, Little HJ (1995) Increased NMDA receptor and calcium channel activity underlying ethanol withdrawal hyperexcitability. Alcohol Alcohol 30:105–114
Widmer H, Lemos JR, Treistman SN (1998) Ethanol reduces the duration of single evoked spikes by a selective inhibition of voltage-gated calcium currents in acutely dissociated supraoptic neurons of the rat. J Neuroendocrinol 10:399–406
Xu J, Clancy CE (2008) Ionic mechanisms of endogenous bursting in CA3 hippocampal pyramidal neurons: a model study. PLoS One 3:e2056
Xu W, Lipscombe D (2001) Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21:5944–5951
Xu JH, Long L, Wang J, Tang YC, Hu HT, Soong TW, Tang FR (2010) Nuclear localization of Ca(v)2.2 and its distribution in the mouse central nervous system, and changes in the hippocampus during and after pilocarpine-induced status epilepticus. Neuropathol Appl Neurobiol 36:71–85
Yazdi HH, Janahmadi M, Behzadi G (2007) The role of small-conductance Ca2+-activated K+ channels in the modulation of 4-aminopyridine-induced burst firing in rat cerebellar Purkinje cells. Brain Res 1156:59–66
Yokoyama CT, Westenbroek RE, Hell JW, Soong TW, Snutch TP, Catterall WA (1995) Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J Neurosci 15:6419–6432
Yunker AM, Sharp AH, Sundarraj S, Ranganathan V, Copeland TD, McEnery MW (2003) Immunological characterization of T-type voltage-dependent calcium channel CaV3.1α1G and CaV3.3α1I isoforms reveal differences in their localization, expression, and neural development. Neuroscience 117:321–335
Zucca S, Valenzuela CF (2010) Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci 30:6778–6781
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
N’Gouemo, P. (2018). Voltage-Sensitive Calcium Channels in the Brain: Relevance to Alcohol Intoxication and Withdrawal. In: Grant, K., Lovinger, D. (eds) The Neuropharmacology of Alcohol . Handbook of Experimental Pharmacology, vol 248. Springer, Cham. https://doi.org/10.1007/164_2018_93
Download citation
DOI: https://doi.org/10.1007/164_2018_93
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-96522-2
Online ISBN: 978-3-319-96523-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)