Abstract
The discovery of nociceptin/orphanin FQ (N/OFQ) marks the genuine start of the reverse pharmacology era, when systematic hunting for ligands of orphan receptors began. The choice of this particular target was no coincidence as the orphan receptor ORL-1 displayed high similarity to known opioid receptors, and thus its elusive ligand held promise to find more than a ligand but a missing opioid peptide. N/OFQ indeed turned out to belong to the opioid peptide family, but with significant pharmacological and functional distinctions. The quest for understanding N/OFQ’s physiological functions has produced some novel insights into stress regulation and many other body functions but is still ongoing almost 25 years after its discovery. This chapter highlights the early steps of orphan receptor research and some of the protagonists who helped to advance the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Analgesia
- Bioassay
- G protein-coupled receptor
- Nociceptin/orphanin FQ
- Opioid peptide
- Orphan receptor
- Stress
1 Introduction
In the beginning stood a pivotal question for neuroscience: Do we know all the transmitters in the brain, or are the numerous sequences of orphan receptors suggesting that we are still missing many? When on January 31, 1995, we saw the sequence of orphanin FQ/nociceptin, we had an answer. Preceding this moment, the quest had all the ingredients of science: doubts, rejection, competition, and of course hard work.
The notion that the G protein-coupled receptor (GPCR) family had many more members than expected arose, for us, in 1987 when we used a known GPCR probe to identify related but novel gene sequences by low stringency screening (Bunzow et al. 1988). This observation was greatly supported by the landmark study of Libert et al. (1989), who used degenerate primers for conserved regions of known GPCRs to amplify a series of novel receptor sequences. These “homology cloning” approaches were rapidly applied to identify novel but sequentially closely related GPCRs and led to the discovery of most of the receptors in specific families such as the adrenergic, dopamine, and serotonin receptors. Some had been predicted by pharmacology; many were not. The obvious limitation of this approach was, of course, that only GPCRs for known ligands could be discovered.
However there were also a number of putative GPCRs which did not belong to the known GPCR families. These receptors had obviously not been conserved in the genome without matching ligands, thus immediately suggesting that many more ligands remained to be identified. Since these novel GPCRs stayed “alone” until identification of their cognate ligands, they were termed “orphan receptors.”
Until 1995, no truly novel ligand had been identified for any of the growing number of orphan receptors. Using an orphan GPCR as bait to identify its natural ligand from tissue extracts was later termed “the orphan receptor strategy” or “reverse pharmacology,” promising the towering prize of discovering an entirely new ligand-receptor-physiology system that might even offer novel therapeutic targets. Few believed it was possible; even fewer tried.
Half way to this goal came the discovery of the first cannabinoid receptor using the plant-derived ligand Δ9-tetrahydrocannabinol and related cannabinoid compounds as tools (Matsuda et al. 1990). The researchers noticed that certain cell lines and particular brain regions – both previously reported to express cannabinoid binding sites – showed overlapping expression of the novel GPCR. Similar to the homology cloning approaches, this study represents a good example of the so-called matching strategy, using libraries of known ligands together with anatomical information to identify ligands for orphan GPCRs. Most importantly, the discovery of the first cannabinoid receptor opened the door to finding its natural mammalian ligands anandamide and 2-arachidonoylglycerol a few years later (Devane et al. 1992; Mechoulam et al. 1995; Sugiura et al. 1995), using in essence the orphan receptor strategy but with a synthetic ligand as a critical aide.
2 The “Orphan Receptor Strategy” Launches the Era of “Reverse Pharmacology”
The main steps of the orphan receptor strategy can be summarized as follows:
-
1.
An unknown GPCR sequence with variable homology (high-moderate-none at all) to known GPCRs, including anatomical information about sites of expression.
-
2.
By definition, not even synthetic ligands are available to test expression or functionality. Thus no binding assays are available, and second messenger coupling is unknown or can only be postulated by homology to closely related GPCRs.
-
3.
Heterologous expression of the orphan GPCR produces a cell-based assay tool.
-
4.
Second messenger responses can sometimes be guided to a common readout by co-expression of promiscuous or engineered G proteins, such as Gα16 or Gαqi3.
-
5.
Fractionated tissue extracts suspected to contain the natural ligand(s) are tested for specific activity at orphan GPCR-expressing cells vs. non-transfected cells.
-
6.
Purification of activity to (near) homogeneity and determination of its structure by physicochemical methods.
It is easily conceivable that this strategy contains many unknowns. For example, functional expression of the orphan GPCR cannot be verified in the absence of any ligand. Tagging of receptor proteins at either the N- or C-terminus carries the risk of accidental interference with functionality. The presence of a natural ligand in a given tissue cannot always be inferred by anatomical vicinity, especially for GPCRs mainly expressed in peripheral tissues. And finally, the chemical nature of the sought-after ligand can only be predicted for orphan GPCRs with closely related family members. In addition, tissue content of highly potent ligands that naturally act in the nanomolar range can be incredibly low, challenging the detection limits of even the most advanced analytical methods. Considering this long list of uncertainties, “deorphanizing” an orphan receptor was an extremely high-risk project which needed to be carried out in a scientific environment that was not risk-averse. Consequently, most of the pioneering breakthroughs on orphan receptors were made in the pharmaceutical industry as well as the European and Japanese university systems, which are less dependent on short-term funding cycles.
3 The Quest for the Endogenous Ligand of ORL1 (and Other Orphan GPCRs)
3.1 Uncertainties Setting the Stage
Out of all the uncertainties that we faced in 1993, the one we were most concerned with was the issue of predicting the second messenger response of a GPCR. There were no generally applicable rules, as it is still now. There were no automated activity measurement tools. There was, however, an instrument that monitored pH changes (lactic acid, bicarbonate) around cultured cells, called the “microphysiometer,” which in principle should be able to monitor any second messenger response, since GPCR activation “consumes” energy leading to increased cellular metabolism. Using this “general” assay tool, we embarked on searching for the ligands of several orphan GPCRs, which included a novel opioid receptor, GPR7 and 8, and a number of GPCRs with poor homology to any known family members.
The stage for the first successful isolation of a natural ligand for an orphan GPCR was set in 1994 when numerous groups reported the cloning of a fourth member of the opioid receptor family that did not bind any natural or synthetic opioid ligands at reasonable concentrations (Mollereau et al. 1994; Bunzow et al. 1994; Chen et al. 1994; Fukuda et al. 1994; Wang et al. 1994; Lachowicz et al. 1995). The three main subtypes of opioid receptors (mu, delta, and kappa) had just been cloned 2 years earlier (Kieffer et al. 1992; Evans et al. 1992; Yasuda et al. 1993; Chen et al. 1993). Given the inherent fascination and long history of opioid research (starting with Sertürner 1806) together with the untypically large research community in this field, it was even more surprising that a fourth opioid receptor had eluded discovery for so long. The many simultaneous reports of this unexpected opioid receptor immediately produced a Babylonian multiplicity in nomenclature. For simplicity reasons, the term ORL1 (for “opioid receptor-like”) proposed in the first report by Mollereau and colleagues should serve as a synonym. Efforts to match ORL1 to previously postulated opioid receptor subtypes, such as an enigmatic kappa3 subtype, contained little convincing evidence (Pan et al. 1995), so that ORL1 remained a scientific and intellectual challenge.
3.2 In the Eye of the Storm: It Is Back to cAMP
During the annual meeting of the Society for Neuroscience in Miami in the fall of 1994, a perplexed opioid research community presented more than ten posters on ORL1 without an answer about its natural ligand. On a memorable evening in the midst of a tropical storm, the first author of this article who had attempted to deorphanize ORL1 as well GPR7 and 8 using the microphysiometer (with little success and major technical obstacles) came to the conclusion that it should be possible to find the ligand of ORL1 by monitoring inhibition of adenylyl cyclase in analogy to all the other opioid receptors. That launched the project back. Fortunately we were at the time in the CNS Department of Hoffmann-La Roche in Basel, Switzerland, which would not resist at providing the funds necessary to carry out such a screening project using numerous and expensive cAMP assays.
In the case of ORL1, its high similarity to the three known opioid receptors held a few advantages that increased the likelihood of success for finding its natural ligand. First of all, the ligand should be a peptide in analogy to all other endogenous opioids. Second, as presented above, the receptor was likely coupling to Gi-type G proteins, thus predicting an inhibition of adenylate cyclase and consequently inhibition of cAMP accumulation. Third, the endogenous ligand was most likely synthesized in the brain, in particular the hypothalamus, as this brain region showed highest levels of ORL1 expression. We could therefore devise a purification strategy that was based on traditional protocols for peptide isolation, which had been developed in the 1970s and 1980s. Nevertheless, peptides are known to occur at notoriously low quantities, even in enriched preparations.
3.3 The Isolation
We started with collecting a large amount (close to 10 kg) of porcine hypothalamic tissue at the local Basel slaughterhouse. Special thanks for this effort goes to Robert A. Henningsen, who overcame more than one natural inhibition during that long morning and the ensuing isolation. A batch of 4.5 kg porcine hypothalamic tissue was frozen and then homogenized in acetic acid using a kitchen blender. The combined supernatants were supposed to contain all soluble material, including peptides, and we further enriched peptides by batch adsorption on C18 reversed-phase silica. This step also depleted most small and highly water-soluble molecules while irreversibly trapping lipids on the reversed-phase matrix. The concentrated peptide extract then underwent the first fractionation using preparative cation-exchange chromatography. Since almost all natural peptides carry at least one positive charge under mildly acidic conditions, we employed a gradient of increasing salt for separating differently charged molecules. Due to the inherent chemical complexity of the crude homogenates or even the enriched peptide concentrate, it was not possible to test any of the previous steps for biological activity that would indicate an ORL1-activating molecule. Only at the stage of well-separated cation-exchange fractions the first and most critical proof-of-concept could be obtained in a functional ORL1 assay. Using small aliquots, we monitored inhibition of cAMP accumulation in cells stably expressing ORL1 and wildtype cells as controls. Positive controls for the presence of endogenous opioid peptides were kappa opioid receptor (KOR)-expressing cells. After a few pilot experiments, we noticed that ORL1-specific activity was found only in fractions eluting at high salt concentrations, indicating a peptide carrying multiple positive charges. These fractions also contained dynorphin-like material as they robustly activated KOR-expressing cells.
ORL1-specific activity “survived” when we further purified the cation-exchange fractions by reversed-phase HPLC and remained intact during a reluctant Christmas break. Using a total of five reversed-phase purification steps, a single peak was finally isolated that contained the only biological activity from porcine brain to produce profound inhibition of cAMP accumulation in ORL1-expressing cells. Fortunately, the isolated amount was more than sufficient for Sanger peptide sequencing, as we later calculated that we had purified 200 pmol of active peptide. When we saw the sequence on January 31, 1995, we immediately knew that we had not only found a ligand for ORL1 but also the missing fourth member of the opioid peptide family.
4 The Novel Opioid Peptide from Basel...
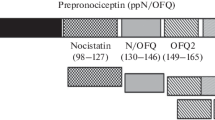
All natural opioid peptides start with the canonical sequence YGGF (Tyr-Gly-Gly-Phe), and this motif is considered to be critically required for opioid receptor activity, with highest stringency for the amino terminal tyrosine residue (Fig. 1). Instead, the new peptide sequence started with FGGF (Phe-Gly-Gly-Phe), or in other words, one single oxygen as the difference between phenylalanine and tyrosine. The evolutionary relatedness to the known opioid peptides is obvious, while the subtle deviation from the conserved opioid motif immediately offers an intuitive explanation for the pharmacological separation. Structure-activity studies later confirmed our early hypotheses: This ligand still looks like an opioid peptide but is pharmacologically distinct, founded in its structure. Included in this thought is another important postulate: There must be a biological reason for the separation from classical opioids.
Because of its ancestry and structural features, we termed this peptide “orphanin FQ,” marking its relation to a former orphan receptor and its first and last amino acids as unique identifiers. The naming was a courageous guess, since we did not know at the time that the first and last amino acids of this peptide are indeed conserved across all vertebrate animals (Sundström et al. 2010). Later structure-activity relationship studies identified the structural components that provide and ensure functional separation between the classical opioids and this fourth member of the ligand family (Reinscheid et al. 1996, 1998; Shimohigashi et al. 1996; Mollereau et al. 1999). In one of the first experiments following our discovery, we observed that changing the N-terminal phenylalanine to tyrosine was not sufficient to render orphanin FQ into a functional opioid ligand, as Tyr1-orphanin FQ was unable to activate classical opioid receptors while remaining a full agonist at ORL1.
5 ... Is Simultaneously Discovered in Toulouse
As is often the case in science, you are never alone with a good idea for long. In June 1995, we learned about an upcoming presentation at the International Narcotics Research Conference (INRC) that announced the identification of an endogenous ligand for ORL1. At the meeting, a team consisting of the group of Jean-Claude Meunier from the University of Toulouse, France, and the group of Gilbert Vassart from the University of Brussels, Belgium, presented data showing that they had isolated a peptide ligand for ORL1 from rat brain. They named their peptide “nociceptin” since they had early evidence that the novel transmitter was producing hyperalgesia-like behaviors in vivo. Although they did not show the sequence (since their manuscript was still under review), one of their graphs showed that a Tyr1-nociceptin analogue had equal potency as the native peptide. This detail told us that we had found the same sequence.
6 Race to the Finish Line
What followed was a frantic race to the finish line by both teams: As an example, the first complete version of our later paper in Science was written in a single night in June 1995. Since our discovery in January, we had accumulated data about tissue distribution, initiated extensive structure-activity studies, launched a project to clone the orphanin FQ precursor protein (which took until September, after submission and acceptance of our manuscript), and, importantly, collected the first in vivo data about behavioral responses. We found that central administration of orphanin FQ profoundly reduced locomotor activity in rats. More importantly, we also saw an apparent increase in pain responsiveness after central orphanin FQ administration, similar to the data reported at INRC. However, we opted against naming the new peptide after a physiological effect since we could not exclude that later investigations might discover a more dominant or entirely different function (there are some examples in the orphan receptor field where a first-glance functional effect of a newly discovered ligand was used for naming but later turned out to be less important). The multiplicity of names, however, has remained to this date, as both reports appeared almost simultaneously in October and November of 1995. Meunier’s paper in Nature beat ours in Science by 3 weeks (Meunier et al. 1995; Reinscheid et al. 1995). Since then, the novel peptide has been alternatingly referred to as nociceptin/orphanin FQ (N/OFQ) or orphanin FQ/nociceptin (OFQ/N). For the remainder of this text, we will refer to the natural ligand of ORL1 as N/OFQ, giving credit to the earlier publication date of the paper by Jean-Claude Meunier’s team. It is also important to mention that a third team around Seiji Itoh at Kansai Medical University in Japan successfully isolated the endogenous ligand of ORL1 from bovine brain at the time of the first two publications (Okuda-Ashitaka et al. 1996).
7 Early Steps to Uncover the Physiological Functions of N/OFQ
Surprisingly, and although both teams came from a background of opioid research, both original publications lacked an important control experiment in their studies on nociceptive effects of N/OFQ: There were no uninjected control animals correcting for the effects of intracerebroventricular (ICV) injections on basal pain perception. If we and Meunier’s team had included such animals, we both would have noticed that ICV injections alone produce profound stress-induced analgesia, an effect well-known in the field. Instead of causing pronociceptive effects, N/OFQ merely reversed this procedure-induced analgesia, as later studies demonstrated (Mogil et al. 1996). Rather than modulating pain sensitivity on its own, central N/OFQ reversed a number of stress-related behavioral effects, including most notably anxiety and fear responses (Jenck et al. 1997; Köster et al. 1999). Since stress is a natural trigger for release of endogenous opioid peptides, N/OFQ can indeed be viewed as a functional anti-opioid peptide as it reverses the initial protective analgesic effects of classical opioid peptides. At the same time, N/OFQ produces profound anxiolysis that may be required to initiate defensive behaviors in situations of severe stress. In fact, the reversal of some opioid effects may constitute the physiological reason for the pharmacological separation of classical opioids from the N/OFQ system. But they all serve the same goal: to preserve the individual’s ability to respond to a potentially life-threatening challenge.
8 Hopes for Clinical Applications
It is probably the dream of every neuroscientist to discover a new transmitter in the brain. To discover an endogenous opioid peptide has essentially happened only four times in history, and we feel honored to have been part of this scientific milestone. But part of our dreams was also the hope to see new therapeutic drugs being developed based on our discovery. Since our work occurred in the midst of a large pharmaceutical company, it was probably the first time in history that a drug discovery program was launched even before publication of the target. Synthetic ORL1 agonists with potent anxiolytic and anti-stress profiles were indeed identified in preclinical research efforts (Wichmann et al. 1999; Jenck et al. 2000; Ciccocioppo et al. 2002), but unfortunately never went into clinical trials, despite their lack of reinforcing effects in contrast to the prototypical benzodiazepine anxiolytics. In the meantime, potential applications have also emerged for ORL1 antagonists as possible adjuvants during chronic morphine therapy in order to prevent or attenuate development of analgesic tolerance (Ueda et al. 1997, 2000; Lutfy et al. 2001; Chung et al. 2006). However, none of these promising targets has been pursued in clinical trials thus far. More progress has been made on the somewhat unexpected finding that ORL1 antagonists can produce antidepressant-like effects. Early studies in animal models (Gavioli et al. 2003, 2004; Gavioli and Calo 2013) were recently followed up by the first human clinical trials with promising results (Post et al. 2016). More recently, renewed interest in the N/OFQ system has been resurrected by identification of bifunctional compounds such as cebranopadol that target both mu-opioid receptors and ORL1 (recently renamed by IUPHAR into “NOP receptor,” standing for “nociceptin/orphanin FQ peptide receptor”) to produce analgesia in chronic pain conditions but with limited abuse liabilities (Linz et al. 2014; Günther et al. 2018). Results from phase II clinical trials with cebranopadol appear promising (Scholz et al. 2018), and we hope that one not too-distant day real patients will ultimately benefit from our work.
9 Reverse Pharmacology Success Stories
In the end, it was possible to find the natural ligand of an orphan GPCR, against all the odds and doubts. Since 1995, numerous ligands for other orphan GPCRs have been discovered, using the orphan receptor strategy. Most productive and successful proved to be a team around Shuji Hinuma and Masahiko Fujino at Takeda Pharmaceuticals in Tsukuba, Japan, who discovered more than a dozen of new ligands for orphan receptors (Hinuma et al. 1998, 2000; Tatemoto et al. 1998; Shimomura et al. 1999; Mori et al. 1999; Fujii et al. 2000, 2002; Ohtaki et al. 2001; Masuda et al. 2002; Kawamata et al. 2003; Itoh et al. 2003; Fukusumi et al. 2003; Sugo et al. 2003; Shinohara et al. 2004). Other big successes were the isolation of ghrelin (Kojima et al. 1999) as a major regulator of food intake and the discovery of the orexins/hypocretins (de Lecea et al. 1998; Sakurai et al. 1998) together with their genetic link to narcolepsy (Chemelli et al. 1999; Lin et al. 1999). The orexin/hypocretin system is currently the first and only example of a former orphan GPCR with a drug on the market. Since 2015, the nonselective orexin/hypocretin receptor 1/2 antagonist suvorexant is marketed as a treatment for insomnia under the name of Belsomra®. More examples are certainly going to follow, as drug development speed is lagging notoriously far behind basic science.
10 Conclusion
This should serve as a final remark: Risk taking and tropical storms can have benefits, some even long lasting.
References
Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O (1988) Cloning and expression of a rat D2 dopamine receptor cDNA. Nature 336(6201):783–787. PubMed PMID: 2974511
Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347(2–3):284–288. PubMed PMID: 8034019
Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98(4):437–451. PubMed PMID: 10481909
Chen Y, Mestek A, Liu J, Hurley JA, Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 44(1):8–12. PubMed PMID: 8393525
Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L (1994) Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett 347(2–3):279–283. PubMed PMID: 8034018
Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK (2006) Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther 318(1):262–267. Epub 2006 Apr 4. PubMed PMID: 16595734
Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M (2002) Reversal of stress- and CRF-induced anorexia in rats by the synthetic ociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology (Berl) 161(2):113–119. Epub 2002 Mar 22. PubMed PMID: 11981590
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95(1):322–327. PubMed PMID: 9419374; PubMed Central PMCID: PMC18213
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949. PubMed PMID: 1470919
Evans CJ, Keith DE Jr, Morrison H, Magendzo K, Edwards RH (1992) Cloning of a delta opioid receptor by functional expression. Science 258(5090):1952–1955. PubMed PMID: 1335167
Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Habata Y, Hinuma S, Onda H, Nishimura O, Fujino M (2000) Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J Biol Chem 275(28):21068–21074. PubMed PMID: 10783389
Fujii R, Yoshida H, Fukusumi S, Habata Y, Hosoya M, Kawamata Y, Yano T, Hinuma S, Kitada C, Asami T, Mori M, Fujisawa Y, Fujino M (2002) Identification of a neuropeptide modified with bromine as an endogenous ligand for GPR7. J Biol Chem 277(37):34010–34016. Epub 2002 Jul 12. PubMed PMID: 12118011
Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343(1):42–46. PubMed PMID: 8163014
Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M (2003) A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem 278(47):46387–46395. Epub 2003 Sep 5. PubMed PMID: 12960173
Gavioli EC, Calo G (2013) Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol Ther 140(1):10–25. https://doi.org/10.1016/j.pharmthera.2013.05.008. Epub 2013 May 24. Review. PubMed PMID: 23711793
Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo G (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signalling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci 17(9):1987–1990. PubMed PMID: 12752799
Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo G (2004) Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn Schmiedeberg’s Arch Pharmacol 369(6):547–553. Epub 2004 May 25. PubMed PMID: 15197534
Günther T, Dasgupta P, Mann A, Miess E, Kliewer A, Fritzwanker S, Steinborn R, Schulz S (2018) Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol 175(14):2857–2868. https://doi.org/10.1111/bph.13809. Epub 2017 May 26. Review PubMed PMID: 28378462; PubMed Central PMCID: PMC6016677
Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M (1998) A prolactin-releasing peptide in the brain. Nature 393(6682):272–276. Erratum in: Nature 1998 Jul 16;394(6690):302. PubMed PMID: 9607765
Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M (2000) New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2(10):703–708. PubMed PMID: 11025660
Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422(6928):173–176. Epub 2003 Feb 23. PubMed PMID: 12629551
Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ Jr, Nothacker HP, Civelli O (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A 94(26):14854–14858. PubMed PMID: 9405703; PubMed Central PMCID: PMC25127
Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A 97(9):4938–4943. PubMed PMID: 10758169; PubMed Central PMCID: PMC18336
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M (2003) A G protein-coupled receptor responsive to bile acids. J Biol Chem 278(11):9435–9440. Epub 2003 Jan 10. PubMed PMID: 12524422
Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG (1992) The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A 89(24):12048–12052. Erratum in: Proc Natl Acad Sci U S A 1994 Feb 1;91(3):1193. PubMed PMID: 1334555; PubMed Central PMCID: PMC50695
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660. PubMed PMID: 10604470
Köster A, Montkowski A, Schulz S, Stübe EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK (1999) Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A 96(18):10444–10449. PubMed PMID: 10468628; PubMed Central PMCID: PMC17908
Lachowicz JE, Shen Y, Monsma FJ Jr, Sibley DR (1995) Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem 64(1):34–40 PubMed PMID: 7798930
Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, Simons MJ, Dumont JE, Vassart G (1989) Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science 244(4904):569–572. PubMed PMID: 2541503
Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98(3):365–376. PubMed PMID: 10458611
Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schröder W, Kögel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349(3):535–548. https://doi.org/10.1124/jpet.114.213694. Epub 2014 Apr 8. PubMed PMID: 24713140
Lutfy K, Hossain SM, Khaliq I, Maidment NT (2001) Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol 134(3):529–534. PubMed PMID: 11588106; PubMed Central PMCID: PMC1572978
Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M (2002) Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 293(1):396–402. PubMed PMID: 12054613
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564. PubMed PMID: 2165569
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50(1):83–90. PubMed PMID: 7605349
Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B et al (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377(6549):532–535. PubMed PMID: 7566152
Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK (1996) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75(2):333–337. PubMed PMID: 8930999
Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341(1):33–38. PubMed PMID: 8137918
Mollereau C, Mouledous L, Lapalu S, Cambois G, Moisand C, Butour JL, Meunier JC (1999) Distinct mechanisms for activation of the opioid receptor-like 1 and kappa-opioid receptors by nociceptin and dynorphin A. Mol Pharmacol 55(2):324–331. PubMed PMID: 9927625
Mori M, Sugo T, Abe M, Shimomura Y, Kurihara M, Kitada C, Kikuchi K, Shintani Y, Kurokawa T, Onda H, Nishimura O, Fujino M (1999) Urotensin II is the endogenous ligand of a G-protein-coupled orphan receptor, SENR (GPR14). Biochem Biophys Res Commun 265(1):123–129
Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M (2001) Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411(6837):613–617
Okuda-Ashitaka E, Tachibana S, Houtani T, Minami T, Masu Y, Nishi M, Takeshima H, Sugimoto T, Ito S (1996) Identification and characterization of an endogenous ligand for opioid receptor homologue ROR-C: its involvement in allodynic response to innocuous stimulus. Brain Res Mol Brain Res 43(1–2):96–104. PubMed PMID: 9037523
Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW (1995) Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol 47(6):1180–1188. PubMed PMID: 7603458
Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, Wafford K, McCarthy A, Barth V, Witkin JM (2016) A selective nociceptin receptor antagonist to treat depression: Evidence from preclinical and clinical studies. Neuropsychopharmacology 41(7):1803–1812. https://doi.org/10.1038/npp.2015.348 Epub 2015 Nov 20. PubMed PMID: 26585287; PubMed Central PMCID: PMC4869049
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270(5237):792–794. PubMed PMID: 7481766
Reinscheid RK, Ardati A, Monsma FJ Jr, Civelli O (1996) Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J Biol Chem 271(24):14163–14168. PubMed PMID: 8662940
Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ Jr, Civelli O (1998) Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem 273(3):1490–1495. PubMed PMID: 9430687
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92(4):573–585. PubMed PMID: 9491897
Scholz A, Bothmer J, Kok M, Hoschen K, Daniels S (2018) Cebranopadol: a novel, first-in-class, strong analgesic: results from a randomized phase IIA clinical trial in postoperative acute pain. Pain Physician 21(3):E193–E206. PubMed PMID: 29871387
Sertürner FWA (1806) Darstellung der reinen Mohnsäure (Opiumsäure) nebst einer chemischen Untersuchung des Opiums mit vorzüglicher Hinsicht auf einen darin neu entdeckten Stoff und die dahin gehörigen Bemerkungen. J Pharm XIV:47–93. Leipzig: S.L. Crusins
Shimohigashi Y, Hatano R, Fujita T, Nakashima R, Nose T, Sujaku T, Saigo A, Shinjo K, Nagahisa A (1996) Sensitivity of opioid receptor-like receptor ORL1 for chemical modification on nociceptin, a naturally occurring nociceptive peptide. J Biol Chem 271(39):23642–23645. PubMed PMID: 8798582
Shimomura Y, Mori M, Sugo T, Ishibashi Y, Abe M, Kurokawa T, Onda H, Nishimura O, Sumino Y, Fujino M (1999) Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem Biophys Res Commun 261(3):622–626
Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S (2004) Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem 279(22):23559–23564. Epub 2004 Mar 22. PubMed PMID: 15037633
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215(1):89–97. PubMed PMID: 7575630
Sugo T, Murakami Y, Shimomura Y, Harada M, Abe M, Ishibashi Y, Kitada C, Miyajima N, Suzuki N, Mori M, Fujino M (2003) Identification of urotensin II-related peptide as the urotensin II-immunoreactive molecule in the rat brain. Biochem Biophys Res Commun 310(3):860–868. PubMed PMID: 14550283
Sundström G, Dreborg S, Larhammar D (2010) Concomitant duplications of opioid peptide and receptor genes before the origin of jawed vertebrates. PLoS One 5(5):e10512. https://doi.org/10.1371/journal.pone.0010512 PubMed PMID: 20463905; PubMed Central PMCID: PMC2865548
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251(2):471–476. PubMed PMID: 9792798
Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H (1997) Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett 237(2–3):136–138. PubMed PMID: 9453234
Ueda H, Inoue M, Takeshima H, Iwasawa Y (2000) Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci 20(20):7640–7647. PubMed PMID: 11027224
Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348(1):75–79. PubMed PMID: 8026588
Wichmann J, Adam G, Röver S, Cesura AM, Dautzenberg FM, Jenck F (1999) 8-Acenaphthen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one derivatives as orphanin FQ receptor agonists. Bioorg Med Chem Lett 9(16):2343–2348. PubMed PMID: 10476866
Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI (1993) Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci U S A 90(14):6736–6740. PubMed PMID: 8393575; PubMed Central PMCID: PMC47007
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Reinscheid, R.K., Civelli, O. (2018). The History of N/OFQ and the NOP Receptor. In: Ko, MC., Caló, G. (eds) The Nociceptin/Orphanin FQ Peptide Receptor. Handbook of Experimental Pharmacology, vol 254. Springer, Cham. https://doi.org/10.1007/164_2018_195
Download citation
DOI: https://doi.org/10.1007/164_2018_195
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20185-2
Online ISBN: 978-3-030-20186-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)