Abstract
Receptor antagonist and knockout studies have demonstrated that blockade of signalling via nociceptin/orphanin FQ (N/OFQ) and its receptor (NOP) has antidepressant-like effects in mice submitted to the forced swimming test (FST). The aim of the present study was to explore further the antidepressant-like properties of the NOP antagonist UFP-101 in different species (mouse and rat) and using different assays [FST and tail suspension test (TST)], and to investigate the mechanism(s) involved in its actions.
UFP-101 (10 nmol i.c.v.) reduced immobility time of Swiss mice in the TST (mean±SEM) from 179±11 to 111±10 s. N/OFQ (1 nmol i.c.v.) was without effect per se, but fully prevented the effect of UFP-101. The spontaneous immobility time of NOP−/− CD1-C57BL/6J-129 mice in the TST was much lower than that of wild-type (NOP+/+) littermates (75±11 vs. 144±17 s) or of Swiss mice. UFP-101 (10 nmol i.c.v.) decreased immobility time (−65%) and increased climbing time (71%) in rats submitted to the FST. In rat brain slices, N/OFQ (100 nM) triggered robust K+-dependent hyperpolarizing currents in locus coeruleus and dorsal raphe neurons. UFP-101 (3 µM) fully prevented N/OFQ-induced currents, but was inactive per se. Fluoxetine, desipramine (both 30 mg/kg i.p.) and UFP-101 (10 nmol i.c.v.) reduced immobility time of mice in the FST. The serotonin synthesis inhibitor p-chlorophenylalanine methylester (PCPA, 4×100 mg/kg per day i.p.) prevented the antidepressant-like effects of fluoxetine and UFP-101 (but not desipramine), whereas N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4, neurotoxic for noradrenergic neurons; 50 mg/kg i.p., 7 days beforehand), suppressed only the effect of desipramine. Neither pretreatment affected spontaneous immobility time per se.
Thus, UFP-101 exhibits pronounced antidepressant-like effects in different species and animal models, possibly by preventing the inhibitory effects of endogenous N/OFQ on brain monoaminergic (in particular serotonergic) neurotransmission. Participation of the N/OFQ-NOP receptor system in mood modulation sets new potential targets for antidepressant drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nociceptin/orphanin FQ (N/OFQ) and its receptor (NOP) (Cox et al. 2000) constitute a novel peptide-receptor system that modulates several central and peripheral functions and is pharmacologically distinct from the classical opioid systems, despite the close genetic, structural and transductional similarities (Calo’ et al. 2000b; Mogil et al. 2001). At the brain level, N/OFQ reportedly stimulates food intake, produces anxiolytic-like effects, modulates locomotor activity, impairs learning and memory, controls cardiovascular parameters and modulates pain threshold and opioid-induced analgesia (Calo’ et al. 2000b; Mogil et al. 2001). N/OFQ and NOP are expressed widely in the mammalian brain and are particularly abundant in various areas of the limbic system involved in the control of emotional processes (Mollereau and Mouledous 2000; Reinscheid et al. 2000).
Two new selective NOP antagonists, the peptide [Nphe1]N/OFQ(1–13)-NH2 (Calo’ et al. 2000a) and the non-peptide J-113397 (Ozaki et al. 2000) evoke antidepressant-like effects in the forced swimming test (FST) in mice (Redrobe et al. 2002). This initial observation has since been extended to another highly selective peptide NOP antagonist, [Nphe1,Arg14,Lys15]N/OFQ-NH2 (UFP-101) (Calo’ et al. 2002; Gavioli et al. 2003). This peptide binds with high subnanomolar affinity to NOP and behaves as a potent and selective antagonist at human recombinant NOP expressed in Chinese hamster ovary cells (Calo’ et al. 2002; McDonald et al. 2003) as well as at native receptors expressed in animal peripheral tissues and brain preparations (Calo’ et al. 2002; Marti et al. 2003; Mela et al. 2004).
In the mouse FST, UFP-101 produces antidepressant-like effects that are prevented by the co-injection of N/OFQ (Gavioli et al. 2003). Consistent with these pharmacological data, mice lacking the NOP gene (NOP−/−) display a reduced immobility time in the FST compared with their wild-type (NOP+/+) littermates. In addition, UFP-101 antidepressant-like effects are no longer evident in NOP−/− mice (Gavioli et al. 2003). Based on this evidence, the current study was undertaken to investigate further the antidepressant-like effects of the NOP antagonist UFP-101 in different species and employing different behavioural tests, as well as to provide some insight into the possible mechanisms underlying these effects. To this end, we evaluated the effects of UFP-101 on Swiss mice and characterized the spontaneous behaviour of NOP+/+ and NOP−/− mice submitted to the tail suspension test (TST), a behavioural despair test used to screen antidepressant drugs (Cryan et al. 2002a). We also evaluated the effect of UFP-101 in rats submitted to the FST. Furthermore, we examined how pharmacological manipulations of brain serotonergic and noradrenergic systems affect the antidepressant-like effects of UFP-101 in the mouse FST, and provide electrophysiological evidence, in rat brain slices, for a selective action of this NOP antagonist against N/OFQ-induced activation of K+ currents in the dorsal raphe (Vaughan and Christie 1996) and the locus coeruleus (Connor et al. 1996).
Materials and methods
Animals
Experiments were conducted using male Swiss mice (20–25 g), CD1-C57BL/6J-129 NOP+/+ and NOP−/− mice (20–25 g) and male Wistar rats (250–300 g). NOP+/+ and NOP−/− mice were genotyped by PCR. These animals were obtained by backcrossing C57BL/6J-129 mice (Nishi et al. 1997) with CD1 animals for nine generations. Mice and rats were kept under standard animal housing conditions (12 h light/dark cycle, lights on at 7 a.m., food and water ad libitum). All experiments and experimental procedures adopted for in vivo studies were conducted in accordance with the standards set in the directives of the European Community Council (86/609/ECC) and with Italian national regulations (D.L. 116/92). The minimum number of animals and duration of observation required to obtain consistent data were employed. Each animal was used only once and experimental groups consisted of 8–12 mice or 6–10 rats. Rat and mouse FST experiments were performed between 9 a.m. and noon, while mouse TST experiments were conducted between 3 and 6 p.m.
Swiss mice were injected intracerebroventricularly (i.c.v., injection volume 2 µl) under light ether anaesthesia (i.e. just sufficient to cause the loss of the righting reflex) using the “free-hand” technique (Laursen and Belknap 1986). In brief, a 27-G needle attached via a polyethylene tube to a 10-μl Hamilton syringe was used for the injection at angle of approximately 45°, 2 mm lateral to the bregma midline. Each animal only received one i.c.v. injection. Upon termination of the experiment each mouse was decapitated and the brain examined a fresco. Results from mice presenting cannula misplacement or any signs of cerebral haemorrhage (<5%) were discarded from statistical analysis.
NOP+/+ and NOP−/− mice were not injected, only their spontaneous behaviour in the TST was assessed. In rats, a 22-G, stainless-steel guide cannula was implanted into the lateral ventricle (AP +0.8 mm; ML ±1.5 mm; DV −3.5 mm) under anaesthesia (ketamine 87 mg/kg plus xylazine 13 mg/kg i.p.). The cannula was fixed with polyacrylic cement, anchored to the skull with stainless-steel screws and plugged with a stainless-steel stylet. The experiments began 5 days after surgery. At the end of the experimental procedures, all animals were sacrificed with an overdose of pentobarbitone sodium, perfused transcardially with saline solution followed by 10% formalin and the brains removed immediately and fixed using a 10% formalin/20% sucrose solution. The brains were then frozen and 30-μm-thick serial sections cut in the frontal plane. The sections were mounted on gelatine-coated slides and stained with thionin. The placement of the cannula was verified under a light microscope. Results from animals in which the cannula was not correctly placed (<10%) were not included in the study.
Drugs and treatment protocols
UFP-101 and N/OFQ were synthesised in house and purified as previously described (Guerrini et al. 1997), while N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), p-chlorophenylalanine methylester (PCPA) and desipramine were purchased from Sigma (St. Louis, Mo., USA). Methionine enkephalin (Met-enkephalin) was obtained from Auspep (Parkville, Vic., Australia). Fluoxetine, UK14304 and idazoxan were purchased from Tocris Cookson (Bristol, UK). All compounds were dissolved in 0.9% saline just prior to use.
UFP-101 and N/OFQ were administered, either alone or in combination, by slow i.c.v. injection over 1 min, using a 10-µl Hamilton syringe, 5 min before the experiment. Control groups were treated with 0.9% saline injected in a volume of 2 μl. In pilot experiments in mice submitted to the FST and TST, dose-response curves (3, 10 and 30 mg/kg i.p.) for the standard antidepressants fluoxetine and desipramine were constructed (data not shown). Both produced significant effects only at the highest dose tested, i.e. 30 mg/kg. Treatments with fluoxetine and desipramine (in both cases 30 mg/kg i.p.) were made 30 min before starting behavioural evaluations.
To deplete neuronal serotonin stores, mice were pretreated with the serotonin synthesis inhibitor PCPA (100 mg/kg i.p.) once a day for 4 consecutive days, the last injection being given 45 min before the experiment (Sanders-Bush et al. 1972). To destroy noradrenergic neurones selectively, mice were given a single injection (50 mg/kg i.p.) of the neurotoxin DSP-4 7 days prior to the experiments (Jaim-Etcheverry and Zieher 1980). These animals were pretreated with fluoxetine (10 mg/kg i.p.), a blocker of the serotonin transporter 30 min before the DSP-4 injection to protect serotonergic neurons from DSP-4 neurotoxicity (Jonsson et al. 1981). DSP-4 administration (50 mg/kg i.p., 7 days prior to start the experiments) in rats reduces the noradrenaline content in the cerebral cortex significantly (−69%; Cryan et al. 2002b) whilst PCPA reduces the brain levels of serotonin by about 70% (Page et al. 1999). Similar monoamine depletion by these agents has been reported in mice (Redrobe et al. 1998).
All experimental procedures, including control groups, treated with 0.9% saline, followed the same schedule described for treated groups. Behavioural observations were made by an experienced observer who was blind with respect to the treatment conditions.
Mouse tail suspension test
The TST was carried out according to Steru et al. (1987). Briefly, mice were isolated acoustically and visually and suspended, 50 cm above the floor, by an adhesive tape placed 1 cm from the tip of the tail. The total amount of time each animal remained immobile (i.e. did not struggle) during the 5-min session in which the animal remained suspended was recorded (in seconds) as immobility time. To improve response reliability, the mice were submitted to an initial 5-min training session of tail suspension 24 h before the actual test session.
Rat forced swimming test
The FST consisted of placing rats, individually, in Plexiglas cylinders (46 cm high, 20 cm in diameter) containing water (24–26 °C, 30 cm deep), for two swimming sessions: an initial 15-min training session, which was followed, 24 h later, by a 5-min test session. At the end of each swimming session, the animal was removed from the cylinder, dried with paper towels, placed in an individual cage to rest and recover for 15 min and was then returned to its collective home cage.
Three behavioural parameters, previously shown to be reliable and validated for the detection of antidepressant drug effects in the rat FST (Detke et al. 1995), were scored cumulatively in the second (test) swimming session only: (i) immobility time (i.e. the time spent floating in the water without struggling, making only those movements necessary to keep the head above the water), (ii) swimming time (i.e. the time spent making active swimming motions to move around in the cylinder) and (iii) climbing time (i.e. the time spent making active movements with its forepaws in and out of the water, directed specifically to the cylinder wall). To assess possible effects of UFP-101 on spontaneous locomotor activity in the rat the open-field test was used: 5 min after drug injection, each rat was placed into a Plexiglas open field (50×50×30 cm) for 5 min and the number of crossings (i.e. number of floor sections crossed) recorded.
Mouse forced swimming test
The mouse FST was performed as described by Porsolt et al. (1977). The procedure was the same as that in the rat FST, except that the first 15-min training session and the 5-min test session (carried out 24 h later) were carried out in a smaller polyethylene cylinder (18.5 cm high, 12.5 cm diameter, water 13.5 cm deep), and only the immobility time was recorded (exclusively during the test session).
Electrophysiological studies
Sprague-Dawley rats (14–24 days old) were anaesthetised with halothane, decapitated and four to five coronal pontine/midbrain slices containing the locus coeruleus or dorsal raphe cut (250 µm). The slices were cut in ice-cold artificial cerebrospinal fluid (ACSF, in mM): NaCl 126, KCl 2.5, NaH2PO4 1.4, MgCl2 1.2, CaCl2 2.4, glucose 11, NaHCO3 25. Slices were maintained at 34 °C in a submerged chamber containing ACSF equilibrated with 5% CO2/95% O2. The brain slices were then transferred to a chamber and superfused continuously (1.8 ml/min) with ACSF (34 °C). Locus coeruleus or dorsal raphe neurons were visualized using infra-red Nomarski optics on an upright microscope (Olympus BX51). Whole-cell voltage clamp recordings of trans-membrane currents were made using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, Calif., USA) with patch electrodes (2–5 MΩ) filled with an internal solution containing (mM) 115 K-gluconate, 25 KCl 25; 15 NaCl, 1 MgCl2, 10 HEPES, 11 EGTA, 2 Mg-ATP, 0.25 Na-GTP, pH 7.3, osmolarity 280–285 mosmol/l. Series resistance (<20 MΩ) was compensated to 80%. Postsynaptic currents (holding potential −60 mV, with a liquid junction potential correction of −10 mV) were filtered (50 Hz low-pass filtered) and sampled (200 Hz) for analysis (Axograph 4, Axon Instruments). Stock solutions of all drugs were diluted to working concentrations using ACSF immediately before use and applied by superfusion.
Statistical analysis
All data are expressed as mean±SEM for n experiments. Data were analysed using Student’s t-test or one-way ANOVA followed by Dunnett’s test, as specified in the figure legends. Differences were considered significant when P<0.05.
Results
Mouse tail suspension test
In the TST, i.p. saline-treated Swiss mice displayed an average immobility time of 131±19 s (n=12), whereas those treated with the classical antidepressant drug desipramine (30 mg/kg i.p.) exhibited a significant reduction of immobility time (61±18 s; n=8).
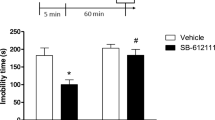
N/OFQ (0.01–1 nmol i.c.v.) in the Swiss mice did not modify the immobility time (Fig. 1A; n=9–13 animals per group). In contrast, UFP-101 (1, 3 and 10 nmol) reduced the immobility time dose-dependently by 18, 24 and 38%, respectively (Fig. 1B; n=12–16). Co-administration of 1 nmol N/OFQ fully prevented the antidepressant-like effect elicited by 10 nmol UFP-101 (Fig. 1C; n=8–12). Untreated NOP+/+ CD1-C57BL/6J-129 mice displayed an immobility time (144±17 s; n=9) similar to that of control Swiss mice, while NOP−/− mice were significantly less immobile than their wild-type control (75±11 s; n=12; P<0.05 vs. wild-type; Fig. 1D).
Effects of i.c.v. injections of nociceptin/orphanin FQ (N/OFQ, A), N/OFQ receptor (NOP) antagonist UFP-101 (B) and N/OFQ plus UFP-101 (C) on immobility time in Swiss mice submitted to the tail suspension test (TST). D Influence of NOP knockout on immobility time in CD1-C57BL/6J-129 mice submitted to the TST. Means±SEM, n=9–12 animals. *P<0.05 vs. control (ANOVA followed by Dunnett’s test, in B, or Student’s t-test for unpaired data in C and D)
Rat forced swimming test
In the rat FST, desipramine (30 mg/kg i.p.) given 30 min before the test session significantly reduced immobility time (saline 168±7 s, desipramine 39±13 s; n=6–8) and increased climbing time (saline 83±11 s, desipramine 206±27 s). This treatment did not significantly modify the swimming time of the animals (saline 49±7 s, desipramine 55±18 s). Injection of UFP-101 (3 nmol i.c.v) had no significant affect on any of the behavioural parameters assessed in the rat FST, whilst 10 nmol fully mimicked the effects of desipramine, reducing both immobility and climbing times, without modifying active swimming time (Fig. 2). The same dose of antagonist had no significant effect on spontaneous locomotor activity measured in the open field in 5 min (saline 53±2; UFP-101 10 nmol 54±9; n=8–10).
Electrophysiology
In dorsal raphe neurons, superfusion of N/OFQ (100 nM) produced an outwards current that was reduced by 87±6% following addition of UFP-101 (3 µM, Fig. 3A). The mean N/OFQ-induced outwards current was 27±4 pA before and 4±2 pA after the addition of UFP-101 (n=6). In N/OFQ-naive dorsal raphe slices, superfusion with UFP-101 (3 µM) alone produced no change in the mean holding current (0±2 pA, n=3).
Effects of N/OFQ and UFP-101 on membrane currents in dorsal raphe (n=6 and 3, respectively) and locus coeruleus (n=8 and 4, respectively) neurons. Typical current traces from dorsal raphe (A) and locus coeruleus neurons (B, C) during superfusion with N/OFQ (100 nM), UFP-101 (3 µM), 5-hydroxytryptamine (5-HT, 30 µM), met-enkephalin (ME, 10 µM), UK14304 (UK, 3 µM) and idazoxan (Idaz, 3 µM). Current traces in A–C are from different neurons which were voltage clamped at −60 mV
In locus coeruleus neurons, superfusion with met-enkephalin (10 µM) produced an outwards current of 207±22 pA (Fig. 3B, n=8). In four of these neurons, superfusion with N/OFQ (100 nM) produced an outwards current that was reduced by 82±6% following addition of UFP-101 (3 µM, Fig. 3b). The reduction in the N/OFQ induced current was reversed following washout of UFP-101 (Fig. 3B). The mean outwards current elicited by N/OFQ was 117±14 and 22±8 pA in the absence and presence of UFP-101, respectively. Subsequent superfusion with the α2-adrenergic agonist UK-14304 (3 µM) produced an outwards current of 108±16 pA that was reversed by the α2-adrenergic antagonist idazoxan (3 µM, Fig. 3B). Moreover, superfusion with UFP-101 (3 µM) alone produced no change in the holding current (Fig. 3C, mean current 2±5 pA; n=4) and did not modify currents evoked by 5-HT (data not shown).
Mouse forced swimming test
Mice pretreated with PCPA and DSP-4 did not show any obvious deficits in sensory or motor function and their behaviour in the FST was indistinguishable from that of the respective saline-treated control groups. Figure 4 summarizes the results obtained in the mouse FST with desipramine (30 mg/kg i.p.), fluoxetine (30 mg/kg i.p.) and UFP-101 (10 nmol i.c.v.) in mice pretreated with saline, PCPA or DSP-4. In the saline-pretreated controls, desipramine, fluoxetine or UFP-101 were each effective in reducing the immobility time, with the largest effect being obtained with the NOP antagonist (Fig. 4; n=9–12). Pretreatment with the 5-HT synthesis inhibitor PCPA, which had no effect per se, was also ineffective in changing the antidepressant-like effect of desipramine. Conversely, this treatment fully prevented the action of fluoxetine and partially counteracted that of UFP-101 (Fig. 4). Finally, DSP-4, which had no effect per se nor modified the reduction in immobility time elicited by either fluoxetine or UFP-101, fully prevented the effect of desipramine (Fig. 4; n=9–12).
Influence of pretreatment with p-chlorophenylalanine methylester (PCPA), N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) or vehicle (saline) on the antidepressant-like effects induced by fluoxetine, desipramine (both 30 mg/kg i.p.) or UFP-101 (10 nmol i.c.v.) in Swiss mice submitted to the FST. Data shown are relative to immobility time during the 5-min test swimming session and are mean±SEM of 9–12 animals. *P<0.05 vs. the corresponding control saline-treated group (ANOVA followed by Dunnett’s test)
Discussion
The present results confirm and extended our previous findings (Gavioli et al. 2003; Redrobe et al. 2002) demonstrating that the blockade of N/OFQ-NOP signalling has antidepressant-like effects in rodents. The present data, obtained in the mouse TST and in the rat FST, together with those obtained previously in mouse FST experiments (Gavioli et al. 2003; Redrobe et al. 2002) indicate that the antidepressant-like effect of NOP-selective antagonists is a robust phenomenon in different species (mouse and rat) and tests used to screen antidepressant drugs (FST and TST). In addition, we obtained preliminary evidence for the involvement of brain monoaminergic (in particular serotonergic) systems in this action of NOP antagonists.
The peptide UFP-101 was used in the present study as a pharmacological tool for blocking endogenous N/OFQ signalling. The pure antagonistic and NOP-selective properties of this peptide have been documented in previous studies in vitro using different techniques, e.g. bioassay in isolated tissues (Calo’ et al. 2002), cAMP- and GTPγS-binding experiments in CHO cells expressing the recombinant human NOP receptor (Calo’ et al. 2002; McDonald et al. 2003) and neurotransmitter release experiments in rat brain slices (Marti et al. 2003), and are further strengthened in the present study by electrophysiological data showing that UFP-101 acted as pure NOP antagonist in both the dorsal raphe and locus coeruleus. Moreover, UFP-101 reportedly prevents various N/OFQ actions in vivo such as inhibition of locomotor activity and pro-nociceptive action in mice (Calo’ et al. 2002), stimulation of food intake in rats (M. Massi, personal communication), bradycardia, hypotension and reduction of noradrenaline blood levels in guinea-pigs (Hashiba et al. 2003), stimulation of glutamate release in the rat substantia nigra (M. Morari, personal communication) and spinal antinociceptive effects in rats (S. Candeletti, personal communication). Finally, UFP-101 has antidepressant-like effects that are reversed by N/OFQ in mice submitted to the FST, but is completely inactive in this regard in NOP−/− mice (Gavioli et al. 2003). Similar results were obtained in the present study in the mouse TST, where UFP-101 produced a dose-dependent reduction of the immobility time which was prevented fully by co-administration of the natural NOP agonist N/OFQ. Moreover, the immobility time of untreated CD1-C57BL/6J-129 NOP−/− mice was significantly lower than that of their wild-type counterparts, but very similar to that of UFP-101-treated Swiss mice. Collectively, this pharmacological evidence and behavioural phenotype differences, allied to the fact that i.c.v. N/OFQ does not enhance immobility time per se in mice submitted to either the TST (present study) or FST (Gavioli et al. 2003), strongly suggest that NOP activation by endogenous N/OFQ contributes decisively to the expression of passive coping behaviour in both behavioural tests. To determine whether species differences exist in the antidepressant-like effects of UFP-101, a separate series of experiments was performed in rats submitted to the modified FST (Detke et al. 1995). i.c.v. injection of this NOP antagonist closely mimicked the effects of the classical antidepressant drug desipramine, promoting substantial reduction of immobility time allied to an increase in climbing behaviour. Thus, UFP-101 has a similar antidepressant-like profile of action in both mice (Gavioli et al. 2003) and rats (present data) submitted to the FST.
In the FST and TST, false-positive results can be obtained with certain drugs, in particular psychomotor stimulants, that decrease immobility time by stimulating locomotor activity (Bourin et al. 2001). This is not the case for UFP-101, which (at the doses investigated) does not modify spontaneous locomotor activity either in mice (Calo’ et al. 2002; Gavioli et al. 2003) or in rats (present data). On the contrary, a clear inhibitory effect on locomotion has been reported for N/OFQ (Reinscheid et al. 1995; Rizzi et al. 2001). However, the i.c.v. administration of N/OFQ does not seem to affect behaviour in either the FST (Gavioli et al. 2003) and the TST (present data); the only action of the peptide in these assays was the reversal of UFP-101-induced reduction of immobility time.
Most, if not all, of the antidepressant drugs currently in clinical use have mechanisms of action that ultimately raise monoamine levels in the synaptic cleft (Nutt 2002). There is substantial background evidence that NOP antagonists might achieve a similar endpoint by acting at different levels. In rat brain slices, N/OFQ exerts direct post-synaptic inhibitory effects on the activity of noradrenergic neurons of the locus coeruleus (Connor et al. 1996) and serotonergic neurons of the dorsal raphe (Vaughan and Christie 1996) and also depresses the release of both noradrenaline and serotonin from pre-synaptic terminals in the cerebral cortex (Schlicker and Morari 2000). Furthermore, not only does UFP-101 selectively antagonise this N/OFQ-induced pre-synaptic inhibition of noradrenaline (Marti et al. 2003) and serotonin (Calo’ et al. 2002) release in cortical preparations, but it also prevents the K+-channel-mediated hyperpolarisation triggered by N/OFQ in both locus coeruleus and dorsal raphe neurons (present data).
To assess the contribution of monoaminergic systems to the antidepressant-like effects elicited by UFP-101 in the mouse FST, we tested the possible influences of prior treatment with the inhibitor of serotonin synthesis PCPA or the noradrenergic-selective neurotoxin DSP-4. In line with previous findings (Page et al. 1999), pre-treatment with PCPA did not modify the immobility time in saline-injected animals or the antidepressant-like effects of the noradrenaline-uptake blocker desipramine, but fully prevented the action of the selective serotonin reuptake inhibitor fluoxetine. Importantly, the antidepressant-like effects of UFP-101 were also clearly reduced in PCPA-treated animals. On the other hand, prior treatment with DSP-4 (in addition to fluoxetine to protect serotonergic neurons from degeneration) fully prevented the antidepressant action of desipramine, thus confirming previous findings (Danysz et al. 1986), while leaving unchanged the effects elicited either by fluoxetine or by UFP-101.
Collectively, these experiments implicate the serotonergic system strongly in the antidepressant-like effects induced by NOP antagonists, which might be brought about by their ability to prevent the inhibitory actions of N/OFQ on the activity of dorsal raphe neurones and/or their terminals in the cerebral cortex. However, we cannot completely rule out the involvement of noradrenergic pathways in the antidepressant-like actions of NOP antagonists, at least in the rat. Indeed, the pattern of effects produced by UFP-101 in rats submitted to FST is very similar to that reported for noradrenaline reuptake inhibitors (i.e. reduction of immobility time, increase of the climbing behaviour, no changes of the swimming behaviour), but different from that elicited by selective serotonin reuptake inhibitors e.g. reduction of immobility time, increase of the swimming behaviour and no change in climbing behaviour (Cryan et al. 2002a). Moreover, DSP-4 reportedly prevents the effects of desipramine (Danysz et al. 1986), but not those produced by the selective noradrenaline reuptake inhibitor reboxetine (Cryan et al. 2002b). Thus, additional studies are clearly required to characterise further the relative contributions of the noradrenergic and serotonergic systems to the antidepressant-like actions of NOP receptor antagonists and the possible involvement of other neurotransmitter systems.
In conclusion, the present study demonstrates that the selective NOP antagonist UFP-101 elicits pronounced antidepressant-like effects in distinct species and behavioural models used to screen antidepressant drugs. This activity of the antagonist is strictly dependent on specific blockade of NOP, as mice lacking the NOP gene were insensitive to the antidepressant-like actions of UFP-101 and displayed an innate antidepressant-like behavioural phenotype. Moreover, it is likely to involve reversal of N/OFQ-induced inhibition of brain monoaminergic systems, particularly (but perhaps not exclusively) those involving serotonin. Altogether, these results suggest that the N/OFQ-NOP system might be important in mood regulation and that NOP blockade may constitute a novel and mechanistically distinct target for development of antidepressant drugs. However to define firmly the therapeutic potential of selective NOP antagonists as new antidepressant agents further experiments assessing the effects of repeated administrations of systemically active compounds (i.e. non-peptide agents) in these and in other behavioural models are clearly mandatory.
References
Bourin M, Fiocco AJ, Clenet F (2001) How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16:9–21
Calo’ G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D (2000a) Characterization of [Nphe1]nociceptin(1–13)NH2, a new selective nociceptin receptor antagonist. Br J Pharmacol 129:1183–1193
Calo’ G, Guerrini R, Rizzi A, Salvadori S, Regoli D (2000b) Pharmacology of nociceptin and its receptor—a novel therapeutic target. Br J Pharmacol 129:1261–1283
Calo’ G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, Salvadori S, Regoli D (2002) [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol 136:303–311
Connor M, Vaughan CW, Chieng B, Christie MJ (1996) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119:1614–1618
Cox BM, Chavkin C, Christie MJ, Civelli O, Evans C, Hamon MD, Hoellt V, Kieffer B, Kitchen I, McKnight AT, Meunier JC, Portoghese PS (2000) Opioid Receptors. In: Girdlestone D (eds) The IUPHAR compendium of receptor characterization and classification. IUPHAR Media, London, pp 321–333
Cryan JF, Markou A, Lucki I (2002a) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Cryan JF, Page ME, Lucki I (2002b) Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol 436:197–205
Danysz W, Kostowski W, Kozak W, Hauptmann M (1986). On the role of noradrenergic neurotransmission in the action of desipramine and amitriptyline in animal models of depression. Pol J Pharmacol Pharm 38:285–298
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121:66–72
Gavioli EC, Marzola G, Guerrini R, Bertorelli R, Zucchini S, De Lima TC, Rae GA, Salvadori S, Regoli D, Calo’ G (2003) Blockade of nociceptin/orphanin FQ-NOP receptor signaling produces antidepressant-like effects: pharmacological and genetic evidences from the mouse forced swimming test. Eur J Neurosci 17:1987–1990
Guerrini R, Calo’ G, Rizzi A, Bianchi C, Lazarus LH, Salvadori S, Temussi PA, Regoli D (1997) Address and message sequences for the nociceptin receptor: a structure-activity study of nociceptin-(1–13)-peptide amide. J Med Chem 40:1789–1793
Hashiba E, Hirota K, Kudo T, Calo’ G, Guerrini R, Matsuki A (2003) Effects of nociceptin/orphanin FQ receptor ligands on blood pressure, heart rate and plasma catecholamine concentrations in guinea pigs. Naunyn Schmiedeberg’s Arch Pharmacol 367:342–347
Jaim-Etcheverry G, Zieher LM (1980) DSP-4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain Res 188:513–523
Jonsson G, Hallman H, Ponzio F, Ross S (1981) DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)—a useful denervation tool for central and peripheral noradrenaline neurons. Eur J Pharmacol 72:173–188
Laursen SE, Belknap JK (1986) Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods 16:355–357
Marti M, Stocchi S, Paganini F, Mela F, De Risi C, Calo’ G, Guerrini R, Barnes TA, Lambert DG, Beani L, Bianchi C, Morari M (2003) Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol 138:91–98
McDonald J, Calo’ G, Guerrini R, Lambert DG (2003) UFP-101, a high affinity antagonist for the nociceptin/orphanin FQ receptor: radioligand and GTPgamma35S binding studies. Naunyn Schmiedeberg’s Arch Pharmacol 367:183–187
Mela F, Marti M, Ulazzi L, Vaccari E, Zucchini S, Trapella C, Salvadori S, Beani L, Bianchi C, Morari M (2004) Pharmacological profile of nociceptin/orphanin FQ receptors regulating 5-hydroxytryptamine release in the mouse neocortex. Eur J Neurosci 19:1317–1324
Mogil JS, Pasternak GW (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53:381–415
Mollereau C, Mouledous L (2000) Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides 21:907–917
Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T (1997) Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J 16:1858–1864
Nutt DJ (2002) The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol 17:S1–S12
Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H (2000) In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol 402:45–53
Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I (1999) Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 147:162–167
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Redrobe JP, Bourin M, Colombel MC, Baker GB (1998) Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology (Berl) 138:1–8
Redrobe JP, Calo’ G, Regoli D, Quirion R (2002) Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedeberg’s Arch Pharmacol 365:164–167
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science 270:792–794
Reinscheid RK, Nothacker H, Civelli O (2000) The orphanin FQ/nociceptin gene: structure, tissue distribution of expression and functional implications obtained from knockout mice. Peptides 21:901–906
Rizzi A, Bigoni R, Marzola G, Guerrini R, Salvadori S, Regoli D, Calo’ G (2001) Characterization of the locomotor activity-inhibiting effect of nociceptin/orphanin FQ in mice. Naunyn Schmiedeberg’s Arch Pharmacol 363:161–165
Sanders-Bush E, Bushing JA, Sulser F (1972) P-Chloroamphetamine inhibition of cerebral tryptophan hydroxylase. Biochem Pharmacol 21:1501–1510
Schlicker E, Morari M (2000) Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 21:1023–1029
Steru L, Chermat R, Thierry B, Mico JA, Lenegre A, Steru M, Simon P, Porsolt RD (1987) The automated tail suspension test: a computerized device which differentiates psychotropic drugs. Prog Neuropsychopharmacol Biol Psychiatry 11:659–671
Vaughan CW, Christie MJ (1996) Increase by the ORL1 receptor (opioid receptor-like1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol 117:1609–1611
Acknowledgements
This study was supported by funds from the University of Ferrara (60% grant to GC), the Italian Ministry of University (2002 Cofin grant to DR), and the Brazilian Ministry of Education (CAPES, research fellowship grant to ECG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gavioli, E.C., Vaughan, C.W., Marzola, G. et al. Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidence from rats and mice. Naunyn-Schmiedeberg's Arch Pharmacol 369, 547–553 (2004). https://doi.org/10.1007/s00210-004-0939-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-0939-0