Abstract
Neurons at sensory ganglia are widely known for their ability to transmit external or internal information toward the brain. A subpopulation of these neurons constitutively synthesizes the neuropeptide substance P (SP) and calcitonin gene-related peptide (CGRP). These peptides are related to pain transmission in the spinal cord, but in periphery, these peptides have a wide spectrum of biological effects. Noceffector is the term adopted to designate those peptidergic neurons at sensory ganglia which participate in maintaining tissue homeostasis. Here, we describe the role of noceffector on epithelial homeostasis both in noninjury conditions and during wound healing. Remarkably, peptidergic terminals seem to be an active participant of stem cell physiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The main function classically attributed to peripheral somatosensory system is to receive, transduce, and channel external or internal information toward central regions of the nervous system. However, there are numerous examples throughout the body of mammals which indicate that some neurons from sensory ganglia are not restricted to generate afferent impulses. This population of neurons is characterized by its capacity to release neuropeptides from their peripheral terminals. It is postulated that through this neurosecretory character, peptidergic neurons of dorsal root ganglia influence diverse processes in their targets (efferent function). This notion is also supported by the presence of receptor sites and degrading enzymes for neuropeptides in all tissues innervated by peptidergic neurons. Nevertheless, it is often assumed that efferent functions of sensory ganglia are only relevant in clearly pathological events (e.g., neurogenic inflammation). Indeed, it is the fact that sensory nerves participate in pathological events that explains a resurgence of the study and an effort to characterize the effects and mechanisms that govern the interaction between sensory nerves and their peripheral targets such as the skin.
The synthesis and transport of neuropeptides to the peripheral terminals of dorsal root ganglion (DRG) neurons have been documented in various species [1,2,3,4]. Thus, efferent functions of DRG neurons may represent a conserved mechanism for tissue renewal and functional maintenance during normal physiological conditions. There are systematic observations about the deleterious effects related to sensory denervation which provokes major changes of gene regulation on its targets [5, 6]. Moreover, the generation of antibodies to label fine terminals at the periphery has revealed that peptidergic terminals are in almost every part of the mammalian body, including the skin, muscle, bone, immune organs, teeth, blood vessels, and viscera. In these regions it has been observed both the existence of synaptic-like contacts between peptidergic endings and some target cells and the expression of neuropeptide receptors by different cell types [7,8,9,10]. Overall the anatomical and functional studies suggest that peptidergic innervation plays an active and continuous role on epithelial renewal, wound repair, glandular secretion, and mineralized tissue formation that is just beginning to be understood.
In this chapter, we will discuss several aspects of sensory innervation and the proposed mechanisms by which sensory terminals influence epithelial homeostasis. A brief survey of the main anatomical and neurochemical characteristics of the nerve terminals that innervate the skin will be made. All of this will be discussed under the context of the noceffector concept proposed by Kruger [11] which states that peptidergic neurons of DRG devote most of its biological existence to have an effector or trophic influence on its target.
2 Cytology and Neurochemistry of Dorsal Root Ganglion Neurons: An Overview
Broadly, two main classes of neurons have been described in sensory ganglia based on cell body size, cytoplasmic appearance, axonal diameter, and axonal myelin content. Due to light or dark appearance of its cytoplasm in electron and light microscopy studies, DRG neurons are subdivided into large light (also named A cells) and small dark neurons (also named B cells) [12, 13]. Furthermore, it has been determined by immunohistochemical studies that light appearance is given by a rich content of the 150 and 200 kDa neurofilament subunits [14]. Likewise, dark neurons have a cytoskeleton primarily constituted by the intermediate filament protein called peripherin [15, 16]. Besides these cytological features, it is known that electrical properties such as conduction velocity correlate with soma size and fiber diameter [17]. Thus, large light neurons (soma diameter > 35 mm) correspond to neurons with myelinated fibers [14, 18, 19]. These large-caliber and myelinated axons are the well-known A-fibers which are divided into three subgroups, namely, A, B, and C, from fastest to slowest. In addition, the small dark neurons (<20 mm) give rise to C-fibers which are unmyelinated fibers and, consequently, the thinnest and slowest fibers in sensory nerves [17, 20]. This relationship between anatomical parameters and functional properties does not necessarily apply to medium-sized neurons (20–35 mm). For instance, some A cells skewed toward the large population are neurofilament-negative, and, conversely, neurons skewed toward the small population are neurofilament-positive [18, 21]. Rather than a clear subdivision of neuronal populations, there is a perplexing scenario of subpopulations with overlapping phenotypic and functional properties.
Besides its afferent (i.e., sensory) role, C- and Aδ-fiber neurons are mainly implicated in tissue management [22,23,24]. Although neuropeptide content is associated with pain modulation, it has been recently documented that a fraction of peptidergic neurons does not process exclusively nociceptive stimuli [2, 23, 25,26,27]. Moreover, efforts to define a biochemical profile to predict receptive modalities have not been successful at all. Some DRG neurons have the intrinsic genetic program to express neuropeptides, and others acquire a peptidergic phenotype only after they have contacted a target in late embrionary stages [28,29,30]. Apparently, peptidergic phenotype is related to localization of peripheral terminals in the target tissue rather than to a sensory modality [2, 23, 25]. Indeed, it has been postulated that peptidergic neurons constitute a nocifensor system that probably lacks a sensory function [31]. In fact, there is still much debate about the existence of two separate populations for afferent and efferent role in dorsal root ganglion. For the sake of convenience, we will refer for those cranial and DRG neurons having an efferent role only as peptidergic neurons or noceffectors, assuming that if a neuron presents vesicles with peptides in the peripheral terminals, it conveys a specific message that helps maintain tissue homeostasis, regardless if this neuron transmits a sensory stimuli or whether it is a noxious/non-noxious stimuli [11].
In elegant studies using genetic axonal tracers, the peptidergic and non-peptidergic populations in mice are shown to be topographically segregated. For instance, in mouse epidermis, non-peptidergic fibers terminate in the stratum granulosum, while most of the peptidergic fibers terminate in the stratum spinosum [32]. Similarly, this segregation continues in the spinal cord and in ascending pathways. Peptidergic neurons project to spinal lamina I and the outer region of lamina II (IIo), and these spinal neurons project heavily to the brain stem (parabrachial nuclei) and thalamus, while non-peptidergic neurons connect with second-order interneurons in the internal region of lamina II (IIinner). These interneurons project to lamina V which then project to several limbic and striatal regions [32, 33]. The spatially segregated pathways suggest that these groups of neurons have at least different sensory processing capacities. If this anatomical separation could also be relevant for efferent functions of sensory neurons remains to be determined.
Neuropeptide content in DRG neurons has been reported in various vertebrates as rodents, primates, felines, birds, and reptiles [1,2,3,4]. The proportion of peptidergic neurons varies depending on the species, and inside a species varies according to the spinal cord level [34]. Regardless of the animal species, the peptidergic population is consistently composed by a subpopulation of C-fibers neurons and in smaller fraction by a subpopulation of A-fiber neurons [2, 3, 25, 35]. The major peptides synthesized by DRG neurons are substance P (SP) and calcitonin gene-related peptide (CGRP). In addition, DRG neurons also synthesize other peptides such as somatostatin, neuropeptide Y, galanin, vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide-38, and opioids.
3 CGRP and Substance P in Dorsal Root Ganglia: Synthesis, Release, and Receptors
3.1 CGRP
The calcitonin gene peptide superfamily consists of four members with potent vasoactive properties that include calcitonin, CGRP, adrenomedullin, and amylin [36, 37]. CGRP exists in two isoforms encoded by different genes, α and β in rat and I and II in human. While the rat isoforms differ in one amino acid residue, in humans they differ in three [38, 39]. The most noticeable site of synthesis of α-CGRP in the peripheral nervous system is the DRG, whereas β-CGRP is preferentially expressed by enteric neurons. The translation of I-CGRP mRNA generates a 121 and 128 amino acid precursor in rats and humans, respectively. The first 25 amino acids of this precursor correspond to the signal peptide, a sequence that assists the targeting of the messenger to the endoplasmic reticulum. The next 103 residues correspond to the proCGRP [40]. The final 37 amino acid peptide is created by proteolytic cleavage of flanking peptides in proCGRP [41].
CGRP receptor belongs to the G-protein-coupled receptor superfamily. A molecular, biological approach has revealed that CGRP receptor is a heterodimer composed of the calcitonin receptor-like receptor protein (CRLR or CLR) and receptor activity-modifying protein 1 (RAMP-1) [37]. The latter is required to transport CRLR to the plasma membrane and to control a specific pattern of glycosylation that determines the affinity for CGRP [42]. The CGRP receptor is associated with the formation of cAMP through the activation of adenylyl cyclase. The biological effects of CGRP end with a proteolytic cleavage by proteases as neutral endopeptidase, insulin-degrading enzyme, and endothelin-converting enzyme-1 [43, 44].
3.2 Substance P
SP is a member of the tachykinin family that includes peptides with a conserved FXGLM-NH2 C-terminal sequence. The mRNAs that encode SP, neurokinin A, neuropeptide K, and neuropeptide G are derived from the preprotachykinin 1 gene. In DRG neurons, alternative RNA splicing of the primary transcript results in the generation of four mRNAs called α-, β-, γ-, and δ-TAC1 [45, 46]. SP precursor sequences are encoded by all four TAC1 mRNAs, but what directs the alternative splicing in the range of tissues where tachykinins are expressed is still unknown [47,48,49]. Putatively, the posttranslational processing of all these precursors gives rise to the active form of substance P that consists of 11 amino acid residues [50, 51].
The effects of tachykinins are mediated through a group of three G-protein-coupled metabotropic receptors: neurokinin-1 (NK1), neurokinin-2 (NK2), and neurokinin-3 (NK3). Substance P binds preferentially to NK1 receptor [52, 53]. The activation of tachykinin receptor leads to inositol phosphate accumulation [54]. NK1 receptor stimulation in tracheal smooth muscle causes Ca2+ release from intracellular stores through the activation of both inositol triphosphate and ryanodine receptors. In muscle cells, the Ca2+ release from the sarcoplasmic reticulum in response to NK1 activation is coupled to Ca2+ influx through channels located in the plasma membrane [55]. Once released, SP is inactivated by the action of the neutral endopeptidase and the angiotensin-converting enzyme [56].
4 Release of SP and CGRP from Somatosensory Nerves
CGRP and SP are strongly expressed in normal DRG neurons, which suggests that they are ready to use whenever it is needed. A great portion of the neurons with capacity of peptide release are recognized for being capsaicin sensitive. Capsaicin is the pungent ingredient in hot chili peppers of the Capsicum genus, and it has been a valuable pharmacological and clinical tool, because it has allowed studying both afferent and efferent functions of DRG neurons [57]. The notion that C- and Aδ-fibers have a neurosecretory function dates from the early years of the twentieth century. Experiments by Bayliss [58] assigned an efferent role for the nerve fibers that emerge from posterior roots. They noticed that, when central ends of these fibers were excited at lumbar level, the impulse generated (i.e., antidromic process) provoked vascular dilatation at their peripheral ends in the hind limbs of various species. Nowadays it is known that antidromic stimulation of C and Aδ produces vasodilatation and increases plasma extravasation [59, 60]. Immunohistochemical and pharmacological experiments had revealed that CGRP induces arterial vasodilatation, whereas SP provokes an increase in vascular permeability [61, 62]. Overall, these vascular changes and concomitant activation of mast cells, lymphocytes, and neutrophils lead to what is called neurogenic inflammation. Thus, the main efforts to understand peptide release from peripheral terminals of peptidergic DRG neurons have been centered on factors involved in inflammation. In this regard, capsaicin is widely known for its capacity to induce neurogenic inflammation by releasing SP and CGRP from peripheral terminals. It is believed that capsaicin releases neuropeptides exclusively via activation of the vanilloid receptor 1 (TRPV1), but other members of TRPV family might be involved [63]. TRPV1 is a nonselective cation channel that allows entry of calcium and, besides capsaicin, is also gated by nociceptive stimuli such as low pH and heat [64, 65].Classical exocytosis occurs when Ca2+ influx into the terminals and initiates exocytotic mechanisms that release neuropeptides and/or other neurotransmitters [66]. The addition of capsaicin to nerve, skin, and mucosal explants induces peptide release, but it is prevented if explants are incubated in Ca-free medium containing EGTA [67,68,69]. The notion that this effect is partially mediated by TRPV1 is supported by the fact that a competitive antagonist of TRPV1, namely, capsazepine, diminished CGRP concentrations in eluates quantified by immunoassay or radioimmunoassays [68, 69]. Ruthenium red, a noncompetitive channel blocker of TRPV1, attenuates neuropeptide release in response to capsaicin [67]. In addition, acidic stimulation promotes CGRP release in the nerves and skin through TRPV1-dependent mechanism [70]. Noxious heat (40–50 °C) evokes CGRP release in a calcium-dependent manner, as shown that both incubating in calcium-free medium and skin loaded with (BAPTA) diminished CGRP release [71, 72]. However, it has been shown that neither capsazepine nor Ruthenium Red abolished completely peptide release from nerve and skin explants [71, 73]. It is proposed that other heat-activated channels of TRPV subfamily (V1–V4) might be involved in neuropeptide release from peripheral terminals [71, 73]. This is supported by the fact that neonatal capsaicin denervation does not eliminate all peptidergic fibers in different targets. Likewise, TRPV1 is not expressed by all peptidergic neurons, and its presence in fibers varies with the type of target [74]. It is noteworthy that TRPV members are coexpressed in DRG neurons and potentially different members may heteromultimerize, contributing to functional heterogeneity and a more complex pharmacology [75,76,77]. In considering TRPV channels as key elements for regulating peptide release from peripheral terminals, it must be taken into account that these channels are sensitized by vanilloids, temperature, and proinflammatory mediators, which results in distinct biophysical and regulatory properties [78]. TRP participation in peptide release on both patho- and physiological conditions awaits further investigation to define its precise contribution.
Regarding factors coming from a target, there are some inflammatory mediators capable to evoke or sensitize SP and CGRP release in certain tissues and conditions. For instance, bradykinin alone can induce neuropeptide release in the rat trachea and skin and in the heart of guinea pig [72, 79, 80]. Bradykinin evokes a significantly CGRP release only in the trachea, whereas in the skin, it only stimulates release of SP [72, 80]. The effects of bradykinin seem to be mediated through the activation of B2 receptor which activates phospholipase C, resulting in formation of diacylglycerol and activation of protein kinase C [72, 80, 81]. The sole action of histamine, serotonin, prostaglandin E2, or proinflammatory cytokines seems not to be sufficient to promote exocytosis in peripheral terminals [69, 79, 80, 82, 83]. The action of these mediators is favored by conditions such as acid pH or noxious heator in combination with other inflammatory mediators. The interaction of serotonin and histamine sensitizes bradykinin effect on CGRP and SP release [72, 80]. Near inflammation zones and tumors, leukocytes and thrombocytes produced proinflammatory cytokines. In this regard, stimulation of rat skin from hind paw with IL-1b and TNF-a augmented heat-induced release of CGRP in a dose-dependent manner [82]. As in the case of bradykinin, cytokines activate receptors coupled with kinases which may sensitize heat-activated ion channels by phosphorylation and lead to a major release of peptides [84]. It has also been observed that noceffector activity is also exerted to inhibitory modulation. Plasma extravasation in rat skin, bronchoconstriction of guinea pig and human, and contraction of the left atrium of guinea pig heart are blocked by the presence of nociceptin, an opioid-related peptide [85,86,87,88]. These processes require neuropeptides release from noceffector terminals. Indeed, release of substance P and CGRP from rat isolated trachea in response to electrical field stimulation was diminished by nociception [89]. It has been proposed that nociceptin stimulates the G-protein-coupled orphan receptor ORL1 to activate an inward-rectifier K+ channel. The latter reduces neuropeptide release from noceffector endings via a membrane hyperpolarization which probably counteracts TRPV1 gating [86]. Likewise, μ-/κ-/δ-opioid receptor agonist inhibited electrical-induced release from noceffector endings in several preparations, although not all agonists are effective in all sites tested [83, 90,91,92,93]. The actual effect of endogenous opioids and its physiological relevance for efferent functions remains to be elucidated. Apart from these factors that can be found in most tissues, apparently there are some tissue-specific signals capable to evoke peptide release. That is the case of the conversion of trans-urocanic acid to cis-urocanic acid by ultraviolet radiation in the stratum corneum of the skin. In rodents cis-urocanic acid may increment microvascular blood flow of hind paw and diminished contact hypersensitivity by means of releasing SP and CGRP [94].
As could be inferred for the depleting effects of capsaicin in neuropeptide contents in different preparations in vitro, long-term synthesis of neuropeptides is intimately related with the amount of these neuropeptides that are available for release from the noceffector endings. Several reports indicate that neuropeptide exocytosis can be achieved by two means: local factors that stimulate direct or indirectly TRP channels and antidromically stimulations of peripheral endings which rely in axonal conduction by activation of voltage-dependent calcium channels. Since much of the research has dealt with inflammatory conditions, little is known if the same factors could modulate synthesis and release of neuropeptides in noninjury conditions. Although capsaicin has helped to elucidate the pharmacology of noceffector terminals, it remains unclear which are the endogenous ligands that have similar effects as capsaicin and the dynamics of production and sources of such TRPV1 agonist in normal and pathophysiological conditions. Only a few molecules such as anandamide, arachidonate, and diacylglycerol have been shown to activate TRPV1 in a capsaicin-like manner [95, 96]. An intriguing issue that deserves further study is the role of antidromic process in vivo. It is known that a suprathreshold stimulus depolarizes primary afferents in the spinal cord (i.e., dorsal root reflex), which could trigger efferent action of noceffector [97]. Furthermore, dorsal rhizotomy, periaqueductal gray matter stimulation, and blockage in the spinal cord of GABAA, non-NMDA, or 5-HT3 receptors interfere with development of neurogenic cutaneous inflammation [98, 99]. This data implies that local mechanisms and/or central nervous mechanisms could modulate exocytotic release at periphery. The understanding of these mechanisms may clarify how noceffectors coordinate normal processes, such as hair growth, bone metabolism, gland secretion, and vascular tone.
5 Efferent Effects of Peptides Released by Somatosensory Nerves on Skin Physiology
Noceffectors establish synaptic-like contacts with Langerhans cells, melanocytes, and mast cells in the skin [7,8,9]. In other targets like the smooth muscle, epithelium, viscera, lymphoid organs, blood vessels, teeth, and bone, where noceffectors lack specialized contacts, they appear to establish a paracrine way of communication [10, 11, 100]. Cellular elements located in the aforementioned targets not only possess receptors for the peptides released by noceffectors from their C- and Aδ-fiber terminals but also express peptidases that terminate with the biological effects of such peptides [28, 29]. The anatomical and physiological evidence so far summarized suggests that, besides its ability to send information to the spinal cord (afferent role), the anatomical and functional organizations of ganglion sensory neurons render them capable of releasing the content of its vesicles and transmit a specific message to their peripheral targets (efferent role).
The skin receives innervation that originates from DRG and trigeminal ganglion. Nerve plexus of large caliber arrive at the deepest part of the dermis. As nerves ascend through the skin, they ramify in thinner plexuses. At the border between the dermis and the epidermis, individual fibers cross the basal membrane and terminate as free nerve endings in either stratum spinosum or stratum granulosum (Fig. 1). Free nerve endings also innervate structures immersed in the dermis like hair follicles, blood vessels, and sebaceous glands [101,102,103]. Many of these free nerve endings present immunoreactivity for SP and CGRP, and its distribution within the skin is conserved between individuals of the same species. The presence of SP and CGRP receptors in keratinocytes, fibroblasts, melanocytes, endothelial cells, and immune cells has been elucidated by immunohistochemical studies and functional assays [8, 104, 105].
General arrangement of sensory innervation in mammalian skin. In (a) glabrous skin and (b) hairy skin axons from dorsal root ganglia are grouped in the dermis as large plexuses. As axons reach the superficial layers of the skin, they travel in smaller plexuses. Sebaceous glands, blood vessels, and epidermis are innervated by ramified terminal fibers
A long-standing issue in the field of dermatology is related to the observation that cutaneous denervation is followed by trophic changes which are manifested as alterations in skin, nails, and subcutaneous tissues [106, 107]. Not until recent investigation, anecdotal observations have been replaced for a careful quantification of efferent activity of peptidergic DRG neurons. Recently, it has been established that skin noceffector is involved in modulating expression of genes of cytoskeleton, extracellular matrix, transcription factors, proteases, receptors, intracellular transducers, and adhesion molecules [5]. Taken altogether, these findings indicate that noceffector activity influences several kinds of cellular elements in its targets. Therefore, it is conceivable that malfunction of noceffectors may be a causal factor in some dermatological diseases.
In rodents and humans, epidermis becomes thinner after nerve injury [108, 109]. Sciatic nerve transection in rodents diminishes keratinocyte incorporation of analogs of thymidine up to 40% which suggest a reduction of keratinocyte proliferation [109,110,111]. Both epidermal thickness and proliferation are restored if reinnervation is permitted [108, 110, 111]. Due to alterations in motor innervation also affect keratinocyte proliferation, it is argued that lack of movement rather than neuropeptide secretion from noceffectors is the cause of skin atrophy. Dorsal rhizotomy or ganglionectomy, procedures that conserve normal gait, also produces epidermal thinning. Sensory denervation of dorsal skin, which does not support body weight, produces epidermal thinning [112, 113]. An insight into the mechanism of this phenomenon comes from in vitro and in vivo studies. In cultures of keratinocytes, fibroblasts, and endothelial cells, substance P promotes cell proliferation [114, 115], while CGRP promotes the proliferation of melanocytes and endothelial cells [8].
The hair follicles receive peptidergic innervation which shows immunoreactivity for substance P and CGRP (Fig. 1). Normal hair cycle is accompanied by substantial morphological, cellular, and biochemical changes in many skin compartments, such as changes in the thickness of the epidermis and dermis, reorganization of the skin vasculature and the extracellular matrix, as well as variations in the number and functional activity of major skin cell populations [116]. This tissue remodeling is associated with tightly regulated sprouting and regression of peptidergic fibers. The number of CGRP and SP fibers increases from telogen to anagen in the dermis and subcutis [117, 118]. Peptidergic nerve fibers are concentrated around and above the bulge region where one major population of epithelial hair follicle stem cells resides. Thus, it is conceivable that noceffectors participate actively in hair cycle modulation and concomitant tissue remodeling. SP-releasing microcapsules implanted at resting growth phase of hair (telogen) stimulate growth phase (anagen) in mice skin [119], while treatment with substance P in anagen induces a premature regression of hair follicles (catagen) [120]. CGRP subcutaneous implants failed to promote transition of telogen to anagen [118]. Further investigations are required to define the precise role of the combination of nerve-derived signals in hair cycle.
Overall, the evidence indicates that noceffectors interact with almost all cell populations in the skin. By means of this interaction, the optimum functioning of major physiological processes that maintain the skin in a healthy state is preserved. Neuropeptide release is involved in modulating epidermal renewal, hair growth, blood flow, and immunological priming. Accordingly, it is not surprising that alteration in the synthesis and release of neuropeptides may result in disturbance of skin homeostasis. That could be the case of some variants of dermatological diseases like atopic dermatitis, psoriasis, urticaria, or vitiligo whose etiology is unknown and sometimes attributed to a neurological origin. A common denominator in these diseases is an elevated number of nerve fibers in the dermis and epidermis containing SP and CGRP compared with healthy skin [121,122,123]. In addition, more frequent contacts of nerve fibers with mast cells and blood vessels are observed [124, 125]. Until now little is known if peripheral nerve fiber sprouting responds to a diminishing in peptide release which in turn evokes secretion of neurotrophic signals from a target organ. For instance, keratinocytes in psoriatic lesions have reduced expression of the transcription factor Jun B with concomitant augmented levels of mRNA of two chemotactic proteins, S100A8 and S100A9, which are involved in the onset of psoriasis [126]. Remarkably, sensory denervation leads to an upregulation of S100A8 and S1009 genes [5]. Likewise, psoriatic lesions contain an increased number of keratinocytes expressing NGF, whose synthesis is promoted by neuropeptide release [105, 127].
6 Role of Peptidergic Nerves on Epithelial Renewal and Wound Repair
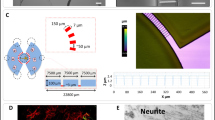
To get a better understanding on how sensory nerves influence epithelium physiology, we performed a series studies using neonatal capsaicin treatment. This chemical denervation model allowed us to reduce the amount of peptidergic terminals in the skin and to determine whether the reduction of peptidergic terminals affects epithelial homeostasis both in noninjury conditions and during wound repair [128, 129]. We employed design-based stereological methods to assure an unbiased quantification of biological structures (Fig. 2). Most of the dermatological research has relied on qualitative or 2D sampling which may overestimate or underestimate the magnitude of a certain cellular responses. For example, the data of cell number is usually expressed as a ratio quantity (i.e., cell/unit area) which can be misinterpreted if the reference space is not the same between experimental conditions. In contrast, stereological estimations of the number, length volume, or area of biological objects are performed by a systematic random sampling without any assumption of spatial distribution, size, shape, and object orientation. Stereological probes facilitate the comparison of experimental conditions by expressing the data of measured parameters as an absolute quantity (i.e., millions of cells). Rather than to offer a guide on how to design a stereological study, the main intention of this section is to show how this methodology was used to study the role of innervation during wound healing [130,131,132].
Stereological quantification of cell number and fiber length. Reliable and unbiased estimates of volume, number, area, and length of biological objects are obtained by design-based stereology methods. (a) Sections and counting sites are determined by a systematic random sampling which assures a representative sampling through the analyzed area. (b) The estimation of the total number of cells is performed by counting the cells inside a virtual box or optical dissector. The fiber length is obtained by counting the intersections of the nerve fibers with a stereological probe called space balls. Both procedures require thick tissue sections (>20 mm)
Although it is well-known that neonatal capsaicin treatment eliminates a great number of DRG neurons with C- and Aδ-axons, there was no quantitative data about the repercussion of capsaicin treatment on the development of epidermal innervation. For this purpose, we quantified the amount of intraepidermal nerve fibers (IENF) immunoreactive for protein gene product 9.5 (PGP+) and calcitonin gene-related peptide (CGRP+) in the glabrous skin of the rat [129]. In control animals, the total estimated length of PGP+ and CGRP+ fibers remained relatively constant at 1, 3, and 6 months. These findings suggest that nerve supply generated during development is only redistributed as animal ages. Moreover, we also observed changes on IENF morphology which indicate that nerve fibers undergo continual remodeling over time (Fig. 3). Accordingly, the arborization and location of sensory endings in the mouse cornea showed substantial changes over a 1-month period [133]. Capsaicin treatment reduced the total length of PGP+ fibers on average by 80%, and that of CGRP+ fibers was reduced by 55%. While IENF showed an intricate morphology in control rats, the nerve endings in the epidermis of treated animals had a straight thick morphology and were poorly ramified. Despite the reduction of the nerve supply to the epidermis, the keratinocyte proliferation was not altered in capsaicin-treated rats. Interestingly, the quantitative analysis of IENF on capsaicin-treated rats revealed that peptidergic fibers were the predominant type of fibers in the epidermis as was also confirmed by a double-immunofluorescence staining for CGRP and beta III tubulin (Fig. 3). Thus, we hypothesized that the remaining peptidergic innervation is sufficient to maintain adequate epithelial renewal in noninjury conditions, but in conditions of high cell demand, denervated epithelia are not able to generate the number of cells required for epithelial expansion.
Effects of neonatal capsaicin treatment on skin innervation. (a) Glabrous skin sections were immunostained for protein gene product 9.5. (b) Capsaicin treatment decreased the number of intraepidermal nerve fibers and fiber complexity. While in (c) control rats the most abundant type of nerve fibers was of non-peptidergic type (green arrows: immunoreactivity for beta III tubulin), (d) the epidermis of treated rats showed almost exclusively peptidergic fibers (red and yellow arrows: immunoreactivity for CGRP). Despite the reduction of intraepidermal nerve fibers in capsaicin-treated rats (F), the keratinocyte proliferation was similar in (e) control and (f) treated rats. (g) Sensory innervation in the epidermis of glabrous skin of control rats. (h) Summary of the changes induced by neonatal capsaicin treatment. Scale bar = 150 mm
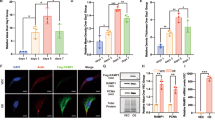
Until recently, all efforts to show a beneficial action of sensory nerves during skin wound repair have been limited to document the impact of denervation upon the time of wound closure [134,135,136,137,138]. Since the discovery of adult stem cells in different parts of the body, it became clear that the innervation is an essential part of the stem cell niche. Little is known about the exact interaction between neurons and progenitor cells. By using the neonatal capsaicin denervation, we explored whether sensory innervation was involved in the modulation of the epithelial progenitors that participate in reepithelialization of the hairy skin [128]. The hair follicle is an excellent model to study the signals and mechanisms that may govern the neural modulation of stem cells. Based on the anatomical location of sensory fibers in the bulge region of the hair follicle, we evaluated the possibility that nerve-derived signals may influence the activation or migration of epithelial progenitors (Fig. 4). During the first 47 h post-wound, the epidermal proliferation was reduced in the capsaicin-treated rats, while the proliferation in the hair follicles was the same as in control rats. To determine if the low number of bromodeoxyuridine-positive cells (BrdU+) in the epidermis of treated rats resulted from a reduced mobilization of transit amplifying cells from the hair follicle, we performed pulse and chase experiments with halogenated thymidine analogs (iododeoxyuridine, IdU; chlorodeoxyuridine, CldU). This procedure is based on the principle that cells in the hair follicle proliferate faster than the cells in the epidermis allowing to track the fate of the double-labeled cells in different skin compartments. Remarkably, the proportion of IdU+/CldU+ cells in the epidermis increased over time only in the control group. This finding suggests that the deficiency of sensory nerves hampers the traffic of cells from the follicle toward the epidermis. Although it has been shown that cells from the hair follicle are dispensable for reepithelialization, the migration of these cells accelerates the reestablishment of the epidermis [139, 140]. In capsaicin-treated animals, the efflux of hair follicle cells is diminished which correlates with an extended time for wound closure. Moreover, treated rats showed an extended recruitment of epithelial precursors as indicated by the broader area of epidermis expressing keratin 6, a marker of epidermal activation. Our results revealed that epithelial precursors must migrate more distance to reach the border of the wound in denervated rats. Taken together, our findings may explain the delay in reepithelialization observed in several models of denervation. From a clinical perspective, it would be desirable to understand the mechanism and signals behind the activation of distinct regions of the epithelium to better contend with chronic wounds. In this regard, it is noteworthy that the stem cell niche of the bulge showed the presence of receptors for substance P and CGRP (Fig. 5).
Effects of capsaicin treatment on wound healing. After 47h after wounding, the epidermis of (a) control rats was thicker and showed more BrdU+ nuclei than (b) capsaicin-treated rats. At 61h after wounding, the epidermis of (c) control rats presented more IdU/CldU labeled nuclei than (d) treated rats, which suggest that denervation is related to less migration of stem cell progeny from the hair follicle. Noteworthy, at 61 h after wounding, we observed an increased area of epidermis expressing keratin 6 in (f) capsaicin-treated rats than in (e) controls. epi epidermis, der dermis, sg sebaceous gland, b bulge, we wound edge, hf hair follicle. Scale bar = 200 mm. Modified from [128]
Neuropeptide receptors in the bulge region of the hair follicles. (a) Label-retaining cells were found in the bulge region of the rat hair follicles after 8 weeks of BrdU pulses in a region displaying expression of CD34. (b) By confocal microscopy, we found that (b) substance P receptor (NK-1) and (c, d) CGRP receptor (CLR and RAMP-1) were expressed by stem cells from the hair follicle. Scale bar = 20 mm. Modified from [128]
Conclusions
This chapter summarizes the evidence that sensory neurons of dorsal root ganglia are not restricted to transmit information toward the central nervous system. These neurons are thought to be crucial participants in the maintenance of tissue integrity and functionality. Nevertheless, we are just glimpsing the potential of neurosecretory function of the so-called sensory neurons for body health. Perhaps the notion that these neurons are merely transducers of noxious information has delayed advancement toward the understanding of efferent functions. Moreover, it is frequently assumed that peripheral release of neuropeptides is restricted to a noxious condition just because at spinal cord level, neuropeptides serves as cotransmitters of painful transmission. This view, however, responds in great extent to technical limitations for recording peripheral activity. Therefore, this field awaits for future improvement in procedures to investigate peripheral release in more physiological terms. This issue is extremely important because the available preparations only permit to study local factors that regulate noceffector activity and overlook systemic factors which may be more important during normal conditions. Although tissue homeostasis does not rely entirely on noceffectors, they seem to be an essential component because different cell populations express receptors and degradatory enzymes for neuropeptides. Accordingly, alterations in the communication between noceffectors and peripheral targets could lead to a variety of functional modifications in the innervated target. Although, at first glance, it could be considered that neuropeptide effects on different organs are non-related with each other, we think that such effects must be the manifestation whereby the brain interacts with the body regulating central issues for its homeostasis both in health and disease.
Regarding wound healing, dorsal root ganglion neurons are involved in processes such as reepithelialization, angiogenesis, and inflammation. Here we described a mechanism based on neural regulation of epithelial SC physiology. Peptidergic neurons seem to promote the mobilization of stem cell progeny from the hair follicle and to modulate the activation of epidermal progenitors (Fig. 6). The myriad of nerve-derived signals is not limited to neuropeptides. Both sympathetic and sensory neurons could act in concert to regulate diverse aspects of adult stem cells [141, 142]. Next research efforts should reveal the molecular pathways that the nervous system modulate to understand how neurons regulate the activation and differentiation of SC in different niches of the body and its possible implications for tumor formation.
Wound model explaining the effector function of sensory nerves. (Left) In normal skin, epithelial progenitor cells are activated both in the epidermis and the hair follicles which migrate toward the wound edge to promote reepithelialization. (Right) In partially denervated skin, there is less migration from stem cell progeny from the hair follicles toward the epidermis. Late on time, there is a recruitment of epidermal progenitors far from the wound edge. The lateness of this event and the longer distance of migration by epithelial progenitors to reach the wound edge may explain the delay in wound closure commonly observed in different denervation models
References
Holford LC, Case P, Lawson SN (1994) Substance P, neurofilament, peripherin and SSEA4 immunocytochemistry of human dorsal root ganglion neurons obtained from post-mortem tissue: a quantitative morphometric analysis. J Neurocytol 23(9):577–589
Lawson SN, Crepps BA, Perl ER (1997) Relationship of substance P to afferent characteristics of dorsal root ganglion neurons in guinea-pig. J Physiol Lond 505(1):177–191
McCarthy PW, Lawson SN (1989) Cell type and conduction velocity of rat primary sensory neurons with substance P-like immunoreactivity. Neuroscience 28(3):745–753
Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM (1984) Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4(12):3101–3111
Fundin BT, Rice FL, Ernfors P (2002) Patterned gene programs and target remodeling following axotomy at a major site for sensory innervation. J Neurobiol 53(3):370–380
Meyer MH, Etienne W, Meyer RA Jr (2004) Altered mRNA expression of genes related to nerve cell activity in the fracture callus of older rats: a randomized, controlled, microarray study. BMC Musculoskelet Disord 5(1):24
Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD (1993) Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature 363(6425):159–163
Hara M, Toyoda M, Yaar M, Bhawan J, Avila EM, Penner IR, Gilchrest BA (1996) Innervation of melanocytes in human skin. J Exp Med 184(4):1385–1395
Egan CL, Viglione-Schneck MJ, Walsh LJ, Green B, Trojanowski JQ, Whitaker-Menezes D, Murphy GF (1998) Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J Cutan Pathol 25(1):20–29
Hukkanen M, Konttinen YT, Rees RG, Gibson SJ, Santavirta S, Polak JM (1992) Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol 19(8):1252–1259
Kruger L (1996) The functional morphology of thin sensory axons: some principles and problems. Prog Brain Res 113:255–272
Lawson SN (1979) The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol 8(3):275–294
Lawson SN, Caddy KW, Biscoe TJ (1974) Development of rat dorsal root ganglion neurons. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res 153(3):399–413
Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH (1984) A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurons. J Comp Neurol 228(2):263–272
Lawson SN (1992) Morphological and biochemical cell types of sensory neurons. In: Scott SA (ed) Sensory neurons: diversity, development, and plasticity. Oxford University Press, New York, pp 27–59
Goldstein ME, House SB, Gainer H (1991) NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res 30(1):92–104
Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol 359:31–46
Lawson SN, Waddell PJ (1991) Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435:41–63
Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN (2005) trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci 25(19):4868–4878
Perl ER (1992) Function of dorsal root ganglion neurons: an overview. In: Scott SA (ed) Sensory neurons: diversity, development, and plasticity. Oxford University Press, New York, pp 3–23
Lee KH, Chung K, Chung JM, Coggeshall RE (1986) Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol 243(3):335–346
Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL (2006) Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain 120(3):244–266
Dux M, Sann H, Schemann M, Jancsó G (1999) Changes in fibre populations of the rat hairy skin following selective chemodenervation by capsaicin. Cell Tissue Res 296(3):471–477
Boilly B, Faulkner S, Jobling P, Hondermarck H (2017) Nerve dependence: from regeneration to cancer. Cancer Cell 31(3):342–354
Lawson SN, Crepps B, Perl ER (2002) Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurons in guinea-pigs. J Physiol Lond 540(3):989–1002
Fang X, McMullan S, Lawson SN, Djouhri L (2005) Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurons in the rat in vivo. J Physiol Lond 565(3):927–943
Djouhri L, Bleazard L, Lawson SN (1998) Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurons. J Physiol Lond 513(3):857–872
Goldstein ME, Grant P, House SB, Henken DB, Gainer H (1996) Developmental regulation of two distinct neuronal phenotypes in rat dorsal root ganglia. Neuroscience 71(1):243–258
Hall AK, Ai X, Hickman GE, MacPhedran SE, Nduaguba CO, Robertson CP (1997) The generation of neuronal heterogeneity in a rat sensory ganglion. J Neurosci 17(8):2775–2784
O’Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C (1989) Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 32(2):493–502
Holzer P, Maggi CA (1998) Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience 86(2):389–398
Zylka MJ, Rice FL, Anderson DJ (2005) Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45(1):17–25
Braz JM, Nassar MA, Wood JN, Basbaum AI (2005) Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47(6):787–793
Yang Y, Ozawa H, Lu H, Yuri K, Hayashi S, Nihonyanagi K, Kawata M (1998) Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res 791(1–2):35–42
McCarthy PW, Lawson SN (1990) Cell type and conduction velocity of rat primary sensory neurons with calcitonin gene-related peptide-like immunoreactivity. Neuroscience 34(3):623–632
Wimalawansa SJ (1997) Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol 11(2–3):167–239
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4):1099–1142
Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG (1985) Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science 229(4718):1094–1097
Steenbergh PH, Hoppener JW, Zandberg J, Lips CJ, Jansz HS (1985) A second human calcitonin/CGRP gene. FEBS Lett 183(2):403–407
Gkonos PJ, Born W, Jones BN, Petermann JB, Keutmann HT, Birnbaum RS, Fischer JA, Roos BA (1986) Biosynthesis of calcitonin gene-related peptide and calcitonin by a human medullary thyroid carcinoma cell line. J Biol Chem 261(31):14386–14391
Wimalawansa SJ (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17(5):533–585
McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393(6683):333–339
Kim YG, Lone AM, Nolte WM, Saghatelian A (2012) Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides. Proc Natl Acad Sci U S A 109(22):8523–8527
Hartopo AB, Emoto N, Vignon-Zellweger N, Suzuki Y, Yagi K, Nakayama K, Hirata K (2013) Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am J Respir Cell Mol Biol 48(4):465–476
Marchand JE, Zaccheo TS, Connelly CS, Kream RM (1993) Selective in situ hybridization histochemical analyses of alternatively spliced mRNAs encoding beta- and gamma-preprotachykinins in rat central nervous system. Brain Res Mol Brain Res 17(1–2):83–94
Harmar AJ, Hyde V, Chapman K (1990) Identification and cDNA sequence of delta-preprotachykinin, a fourth splicing variant of the rat substance P precursor. FEBS Lett 275(1–2):22–24
Carter MS, Krause JE (1990) Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci 10(7):2203–2214
Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD (1987) Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci 84(3):881–885
Page NM (2005) New challenges in the study of the mammalian tachykinins. Peptides 26(8):1356–1368
Carraway R, Leeman SE (1979) The amino acid sequence of bovine hypothalamic substance P. Identity to substance P from colliculi and small intestine. J Biol Chem 254(8):2944–2945
Chang MM, Leeman SE (1970) Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 245(18):4784–4790
Cascieri MA, Huang RR, Fong TM, Cheung AH, Sadowski S, Ber E, Strader CD (1992) Determination of the amino acid residues in substance P conferring selectivity and specificity for the rat neurokinin receptors. Mol Pharmacol 41(6):1096–1099
Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R, Kakizuka A, Ohkubo H, Nakanishi S (1989) Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem 264(30):17649–17652
Amadesi S, Moreau J, Tognetto M, Springer J, Trevisani M, Naline E, Advenier C, Fisher A, Vinci D, Mapp C, Miotto D, Cavallesco G, Geppetti P (2001) NK1 receptor stimulation causes contraction and inositol phosphate increase in medium-size human isolated bronchi. Am J Respir Crit Care Med 163(5):1206–1211
Lin YR, Kao PC, Chan MH (2005) Involvement of Ca2+ signaling in tachykinin-mediated contractile responses in swine trachea. J Biomed Sci 12(3):547–558
Okamoto A, Lovett M, Payan DG, Bunnett NW (1994) Interactions between neutral endopeptidase (EC 3.4.24.11) and the substance P (NK1) receptor expressed in mammalian cells. Biochem J 299(Pt 3):683–693
Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24:487–517
Bayliss WM (1901) On the origin of the vasodilator fibres of the hind limb, and on the nature of these fibers. J Physiol 26:173–209
Lembeck F, Holzer P (1979) Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedeberg’s Arch Pharmacol 310(2):175–183
Escott KJ, Brain SD (1993) Effect of a calcitonin gene-related peptide antagonist (CGRP8-37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. Br J Pharmacol 110(2):772–776
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313(5997):54–56
Brain SD, Newbold P, Kajekar R (1995) Modulation of the release and activity of neuropeptides in the microcirculation. Can J Physiol Pharmacol 73(7):995–998
Wang H, Woolf CJ (2005) Pain TRPs. Neuron 46(1):9–12
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21(3):531–543
De Camilli P, Jahn R (1990) Pathways to regulated exocytosis in neurons. Annu Rev Physiol 52:625–645
Sauer SK, Bove GM, Averbeck B, Reeh PW (1999) Rat peripheral nerve components release calcitonin gene-related peptide and prostaglandin E2 in response to noxious stimuli: evidence that nervi nervorum are nociceptors. Neuroscience 92(1):319–325
Kilo S, Harding-Rose C, Hargreaves KM, Flores CM (1997) Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain 73(2):201–207
Flores CM, Leong AS, Dussor GO, Hargreaves KM, Kilo S (2001) Capsaicin-evoked CGRP release from rat buccal mucosa: development of a model system for studying trigeminal mechanisms of neurogenic inflammation. Eur J Neurosci 14(7):1113–1120
Fischer MJ, Reeh PW, Sauer SK (2003) Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur J Neurosci 18(4):803–810
Petho G, Izydorczyk I, Reeh PW (2004) Effects of TRPV1 receptor antagonists on stimulated iCGRP release from isolated skin of rats and TRPV1 mutant mice. Pain 109(3):284–290
Kessler F, Habelt C, Averbeck B, Reeh PW, Kress M (1999) Heat-induced release of CGRP from isolated rat skin and effects of bradykinin and the protein kinase C activator PMA. Pain 83(2):289–295
Sauer SK, Reeh PW, Bove GM (2001) Noxious heat-induced CGRP release from rat sciatic nerve axons in vitro. Eur J Neurosci 14(8):1203–1208
Guo A, Vulchanova L, Wang J, Li X, Elde R (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X(3) purinoceptor and IB4 binding sites. Eur J Neurosci 11(3):946–958
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418(6894):186–190
Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem 276(30):28613–28619
Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K (2000) Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+−sensitive cation channels. J Biol Chem 275(36):27799–27805
Kichko TI, Reeh PW (2004) Why cooling is beneficial: non-linear temperature-dependency of stimulated iCGRP release from isolated rat skin. Pain 110(1–2):215–219
Franco-Cereceda A, Saria A, Lundberg JM (1989) Differential release of calcitonin gene-related peptide and neuropeptide Y from the isolated heart by capsaicin, ischaemia, nicotine, bradykinin and ouabain. Acta Physiol Scand 135(2):173–187
Hua XY, Yaksh TL (1993) Pharmacology of the effects of bradykinin, serotonin, and histamine on the release of calcitonin gene-related peptide from C-fiber terminals in the rat trachea. J Neurosci 13(5):1947–1953
Averbeck B, Reeh PW (2001) Interactions of inflammatory mediators stimulating release of calcitonin gene-related peptide, substance P and prostaglandin E2 from isolated rat skin. Neuropharmacology 40(3):416–423
Opree A, Kress M (2000) Involvement of the proinflammatory cytokines tumor necrosis Factor-alpha, IL-1beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci 20(16):6289–6293
Kress M, Guthmann C, Averbeck B, Reeh PW (1999) Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin in vitro. Neuroscience 89(1):303–310
Cesare P, McNaughton P (1996) A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. PNAS 93(26):15435–15439
Nemeth J, Helyes Z, Oroszi G, Than M, Pinter E, Szolcsanyi J (1998) Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur J Pharmacol 347(1):101–104
Faisy C, Naline E, Rouget CL, Risse PA, Guerot E, Fagon JY, Chinet T, Roche N, Advenier C (2004) Nociceptin inhibits vanilloid TRPV-1-mediated neurosensitization induced by fenoterol in human isolated bronchi. Naunyn Schmiedeberg’s Arch Pharmacol 370(3):167–175
Corboz MR, Rivelli MA, Egan RW, Tulshian D, Matasi J, Fawzi AB, Benbow L, Smith-Torhan A, Zhang H, Hey JA (2000) Nociceptin inhibits capsaicin-induced bronchoconstriction in isolated guinea pig lung. Eur J Pharmacol 402(1–2):171–179
Giuliani S, Maggi CA (1997) Prejunctional modulation by nociceptin of nerve-mediated inotropic responses in guinea-pig left atrium. Eur J Pharmacol 332(3):231–236
Helyes Z, Nemeth J, Pinter E, Szolcsanyi J (1997) Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br J Pharmacol 121(4):613–615
Bartho L, Ernst R, Pierau FK, Sann H, Faulstroh K, Petho G (1992) An opioid peptide inhibits capsaicin-sensitive vasodilatation in the pig’s skin. Neuropeptides 23(4):227–237
Ray NJ, Jones AJ, Keen P (1991) Morphine, but not sodium cromoglycate, modulates the release of substance P from capsaicin-sensitive neurons in the rat trachea in vitro. Br J Pharmacol 102(4):797–800
Yaksh TL (1988) Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res 458(2):319–324
Yonehara N, Imai Y, Chen JQ, Takiuchi S, Inoki R (1992) Influence of opioids on substance P release evoked by antidromic stimulation of primary afferent fibers in the hind instep of rats. Regul Pept 38(1):13–22
Khalil Z, Townley SL, Grimbaldeston MA, Finlay-Jones JJ, Hart PH (2001) Cis-Urocanic acid stimulates neuropeptide release from peripheral sensory nerves. J Invest Dermatol 117(4):886–891
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400(6743):452–457
Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB (2000) The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129(2):227–230
Willis WD (1999) Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124(4):395–421
Lin Q, Wu J, Willis WD (1999) Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol 82(5):2602–2611
Peng YB, Wu J, Willis WD, Kenshalo DR (2001) GABAA and 5-HT3 receptors are involved in dorsal root reflexes: possible role in periaqueductal gray descending inhibition. J Neurophysiol 86(1):49–58
Silverman JD, Kruger L (1987) An interpretation of dental innervation based upon the pattern of calcitonin gene-related peptide (CGRP)-immunoreactive thin sensory axons. Somatosens Res 5(2):157–175
Fundin BT, Arvidsson J, Aldskogius H, Johansson O, Rice SN, Rice FL (1997) Comprehensive immunofluorescence and lectin binding analysis of intervibrissal fur innervation in the mystacial pad of the rat. J Comp Neurol 385(2):185–206
Rice FL, Fundin BT, Arvidsson J, Aldskogius H, Johansson O (1997) Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol 385(2):149–184
Fundin BT, Pfaller K, Rice FL (1997) Different distributions of the sensory and autonomic innervation among the microvasculature of the rat mystacial pad. J Comp Neurol 389(4):545–568
Ruocco I, Cuello AC, Shigemoto R, Ribeiro-da-Silva A (2001) Light and electron microscopic study of the distribution of substance P-immunoreactive fibers and neurokinin-1 receptors in the skin of the rat lower lip. J Comp Neurol 432(4):466–480
Burbach GJ, Kim KH, Zivony AS, Kim A, Aranda J, Wright S, Naik SM, Caughman SW, Ansel JC, Armstrong CA (2001) The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 117(5):1075–1082
Thompson PD, Thomas PK (2005) Clinical patterns of peripheral neuropathy. In: Dyck PJ, Thomas PK (eds) Peripheral neuropathy, 4th edn. Elsevier Saunders, Philadelphia, pp 1137–1161
Paus R (2016) Exploring the “brain-skin connection”: leads and lessons from the hair follicle. Curr Res Transl Med 64(4):207–214
Hsieh ST, Choi S, Lin WM, Chang YC, McArthur JC, Griffin JW (1996) Epidermal denervation and its effects on keratinocytes and Langerhans cells. J Neurocytol 25(9):513–524
Stankovic N, Johansson O, Oqvist G, Hildebrand C (1999) Indirect effect of sciatic nerve injury on the epidermal thickness of plantar glabrous skin in rats. Scand J Plast Reconstr Surg Hand Surg 33(3):273–279
Hsieh ST, Lin WM (1999) Modulation of keratinocyte proliferation by skin innervation. J Invest Dermatol 113(4):579–586
Huang IT, Lin WM, Shun CT, Hsieh ST (1999) Influence of cutaneous nerves on keratinocyte proliferation and epidermal thickness in mice. Neuroscience 94(3):965–973
Burgess PR, English KB, Horch KW, Stensaas LJ (1974) Patterning in the regeneration of type I cutaneous receptors. J Physiol 236(1):57–82
Nurse CA, Macintyre L, Diamond J (1984) A quantitative study of the time course of the reduction in Merkel cell number within denervated rat touch domes. Neuroscience 11(2):521–533
Tanaka T, Danno K, Ikai K, Imamura S (1988) Effects of substance P and substance K on the growth of cultured keratinocytes. J Invest Dermatol 90(3):399–401
Kahler CM, Herold M, Reinisch N, Wiedermann CJ (1996) Interaction of substance P with epidermal growth factor and fibroblast growth factor in cyclooxygenase-dependent proliferation of human skin fibroblasts. J Cell Physiol 166(3):601–608
Peters EM, Botchkarev VA, Muller-Rover S, Moll I, Rice FL, Paus R (2002) Developmental timing of hair follicle and dorsal skin innervation in mice. J Comp Neurol 448(1):28–52
Botchkarev VA, Eichmuller S, Johansson O, Paus R (1997) Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J Comp Neurol 386(3):379–395
Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R (2001) Hair-cycle-associated remodeling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol 116(2):236–245
Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czarnetzki BM (1994) Hair growth induction by substance P. Lab Investig 71(1):134–140
Maurer M, Fischer E, Handjiski B, von Stebut E, Algermissen B, Bavandi A, Paus R (1997) Activated skin mast cells are involved in murine hair follicle regression (catagen). Lab Investig 77(4):319–332
Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM (1997) Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch Dermatol Res 289(11):611–616
Lazarova R, Hristakieva E, Lazarov N, Shani J (2000) Vitiligo-related neuropeptides in nerve fibers of the skin. Arch Physiol Biochem 108(3):262–267
Urashima R, Mihara M (1998) Cutaneous nerves in atopic dermatitis. Virchows Arch 432(4):363–370
Naukkarinen A, Jarvikallio A, Lakkakorpi J, Harvima IT, Harvima RJ, Horsmanheimo M (1996) Quantitative histochemical analysis of mast cells and sensory nerves in psoriatic skin. J Pathol 180(2):200–205
Jarvikallio A, Harvima IT, Naukkarinen A (2003) Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res 295(1):2–7
Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF (2005) Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437(7057):369–375
Raychaudhuri SP, Jiang WY, Farber EM (1998) Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol 78(2):84–86
Martinez-Martinez E, Galvan-Hernandez CI, Toscano-Marquez B, Gutierrez-Ospina G (2012) Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One 7(5):e36421
Martinez-Martinez E, Toscano-Marquez B, Gutierrez-Ospina G (2011) Long-term effects of neonatal capsaicin treatment on intraepidermal nerve fibers and keratinocyte proliferation in rat glabrous skin. Anat Rec (Hoboken) 294(1):173–184
Mouton PR (2002) Principles and practices of unbiased stereology: an introduction for bioscientists. Johns Hopkins University Press, Baltimore, p 214
Howard V, Reed MG (2005) Unbiased stereology: three-dimensional measurement in microscopy, 2nd edn. Garland Science, New York, NY, p 277
Martinez-Martinez E, Uribe-Querol E, Galvan-Hernandez CI, Gutierrez-Ospina G (2016) Stereological quantification of cell-cycle kinetics and mobilization of epithelial stem cells during wound healing. Methods Mol Biol 1453:93–107
Harris LW, Purves D (1989) Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci 9(6):2210–2214
Carr RW, Delaney CA, Westerman RA, Roberts RG (1993) Denervation impairs cutaneous microvascular function and blister healing in the rat hind limb. Neuroreport 4(5):467–470
Harsum S, Clarke JD, Martin P (2001) A reciprocal relationship between cutaneous nerves and repairing skin wounds in the developing chick embryo. Dev Biol 238(1):27–39
Westerman RA, Carr RW, Delaney CA, Morris MJ, Roberts RG (1993) The role of skin nociceptive afferent nerves in blister healing. Clin Exp Neurol 30:39–60
Fukai T, Takeda A, Uchinuma E (2005) Wound healing in denervated rat skin. Wound Repair Regen 13(2):175–180
Maggi CA, Borsini F, Santicioli P, Geppetti P, Abelli L, Evangelista S, Manzini S, Theodorsson-Norheim E, Somma V, Amenta F et al (1987) Cutaneous lesions in capsaicin-pretreated rats. A trophic role of capsaicin-sensitive afferents? Naunyn Schmiedeberg's Arch Pharmacol 336(5):538–545
Langton AK, Herrick SE, Headon DJ (2008) An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 128(5):1311–1318
Garcin CL, Ansell DM, Headon DJ, Paus R, Hardman MJ (2016) Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialization. Stem Cells 34(5):1377–1385
Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124(2):407–421
Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL (2011) Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8(5):552–565
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Martínez-Greene, J.A., Martínez-Martínez, E. (2018). Influence of Sensory Innervation on Epithelial Renewal and Wound Healing. In: Shiffman, M., Low, M. (eds) Vascular Surgery, Neurosurgery, Lower Extremity Ulcers, Antimicrobials, Wound Assessment, Care, Measurement and Repair. Recent Clinical Techniques, Results, and Research in Wounds, vol 5. Springer, Cham. https://doi.org/10.1007/15695_2018_130
Download citation
DOI: https://doi.org/10.1007/15695_2018_130
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10715-4
Online ISBN: 978-3-030-10716-1
eBook Packages: MedicineMedicine (R0)