Abstract
One of the most sensitive process characteristics in the cultivation of filamentous biological systems is their complex morphology. In submerged cultures, the observed macroscopic morphology of filamentous microorganisms varies from freely dispersed mycelium to dense spherical pellets consisting of a more or less dense, branched and partially intertwined network of hyphae. Recently, the freely dispersed mycelium form has been in high demand for submerged cultivation because this morphology enhances the growth and production of several valuable products. A distinct filamentous morphology and productivity are influenced by the environment and can be controlled by inoculum concentration, spore viability, pH value, cultivation temperature, dissolved oxygen concentration, medium composition, mechanical stress or process mode as well as through the addition of inorganic salts or microparticles, which provides the opportunity to tailor a filamentous morphology. The suitable morphology for a given bioprocess varies depending on the desired product. Therefore, the advantages and disadvantages of each morphological type should be carefully evaluated for every biological system. Because of the high industrial relevance of filamentous microorganisms, research in previous years has aimed at the development of tools and techniques to characterise their growth and obtain quantitative estimates of their morphological properties. The focus of this review is on current advances in the characterisation and control of filamentous morphology with a separation of eukaryotic and prokaryotic systems. Furthermore, recent strategies to tailor the morphology through classical biochemical process parameters, morphology and genetic engineering to optimise the productivity of these filamentous systems are discussed.
Graphical Abstract

Robert Walisko, Judith Moench-Tegeder: Equal contribution.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Numerous industrial bioprocesses that use filamentous eukaryotic and prokaryotic microorganisms for the production of organic acids and antibiotics have been established within the last century. The oldest large-scale industrial process exploiting filamentous microorganisms is the production of citric acid by the filamentous fungus Aspergillus niger, which was established in 1919 in Belgium, even without an understanding of basic molecular and biochemical engineering principles [1, 2]. Since then, fungal expression and production systems have been developed for the production of pharmaceutical compounds, proteins and especially enzymes [3–5]. For example, A. niger has been shown to produce 0.9 g/L of immunglobulin G [6], and 1.6 million tons of citric acid is currently produced per year [7]. Filamentous fungi are superior to other industrially applied organisms because of their extraordinary production and secretion capacities. The ability of certain filamentous fungal organisms, such as A. niger and Trichoderma reesei, to secrete large quantities of biomass-degrading enzymes provides these organisms with potential applications in biorefineries [5]. Very high titres of hydrolytic enzymes, such as amylases and proteases, can be produced by these species; for example, titres of up to 30 g/L of glucoamylase can be achieved by A. niger [8], and industrial strains of T. reesei have been shown to secrete 40 g/L of cellulases into the cultivation medium [9]. Driven by advances in equipment and analytics, laboratory-scale fungal cultivation techniques have improved substantially over the last 10–15 years, enabling the completion of reproducible and repeatable bioreactor experiments [10].
Of the bacterial filamentous organisms, the Actinomycetes, mostly Streptomyces species, are of prime industrial interest because of their production of various secondary metabolites [11]. Despite some obvious differences, the morphological characteristics of Streptomyces species and their impact on culture processing are similar to those of fungi, such as A. niger [12]. Both grow filamentously, and in submerged cultures, they may both form pellets; therefore, their growth kinetics can be expressed using the same mathematical models [11]. Although there is no general consensus as to how the morphology and other biophysical parameters of Streptomyces in submerged cultivation correlate with secondary metabolite production, morphology is recognised as a parameter of prime importance [13] and is, therefore, worth investigating.
An obstacle for filamentous cultivation, however, is the complex morphology during submerged growth in a liquid medium, which can range from freely growing mycelium to clumps and dense circular pellets. The morphology of the mycelium has an enormous impact on the production of enzymes and primary or secondary metabolites [14].
The description of microscopic hyphal growth and macroscopic pellet growth of filamentous microorganisms on solid media and in submerged culture originates from the systematic investigations conducted by Emerson [15], resulting in the development of the cube root law, which describes pellet growth as it occurs in the outer nutrient-supplied shell of constant width. Additional important milestones include studies by Takahashi and Yamada [16] on coagulative and non-coagulative pellet formation, Pirt and Callow [17] and Pirt [18] on growth kinetics and the control of fungal morphology and Trinci [19, 20] and Trinci and Caldwell [21] on the introduction of the hyphal growth unit (HGU). Even at the early research stage, Robinson and Davidson [22] addressed large-scale cultivation of eukaryotic systems and Phillips [23] examined oxygen transfer in mycelia. These efforts in the field of filamentous systems were first reviewed by Metz and Kossen [24].
Beginning in the mid-1980s, advances in new analytical methods in molecular biology and biochemical engineering and increased computer capacity reinforced filamentous systems as the focus of various research groups. The Schügerl group characterised the oxygen supply of pellets using microelectrode and microtome techniques [25]. Furthermore, automated image analysis continued growing in importance in the research of filamentous systems. Adams and Thomas [26] innovated the research of filamentous systems by implementing automated image analysis for the characterisation of pellet- and mycelium-like structures [27–30]. Additionally, morphological growth kinetics via computer simulations were studied extensively during this time period [11, 31, 32]. During the early 1990s, the Wösten group pioneered the localisation of growth and protein secretion by the filamentous fungi A. niger [33] and addressed the issue of morphology affected by hydrophobin complexes [34, 35]. Engineering issues such as the influence of increased viscosity on the cultivation of filamentous organisms [36–38]; the relationship between morphology and metabolite production [39, 40]; and the impact of reactor geometry, stirrer shape and size, aeration rate and subsequent energy dissipation and mechanical particle stress [41–47] have been investigated extensively. In the mid-2000s, Papagianni [48] and Grimm et al. [49] summarised the influence, kinetics and control of fungal morphology on product formation with classical biochemical engineering parameters in their often cited review articles. Because the characterisation of mycelium-like systems through Euclidean, integral geometry parameters is limited, Papagianni [50] addressed the fractal nature of these aggregates.
Microparticle-enhanced cultivation (MPEC) was first applied by Kaup et al. [51] as a novel method to control fungal morphology development to improve productivity in the presence of aluminium oxide and hydrous magnesium silicate particles. The Braunschweig Collaborative Research Centre, From gene to product, expanded the range of supplements to talc, silicate and other types of microparticles to control the morphological development of A. niger spp. and increase product formation [52–54]. Another sophisticated method to tailor-make fungal morphology was found to be the manipulation of the osmolality of the culture medium; increasing NaCl concentrations led to mycelial growth and enhanced productivity in A. niger strains [55]. However, the mycelian growth form leads to a significant increase in culture broth viscosity with a reduced mass transfer and, therefore, requires more power input into the cultivation broth. As morphology, biomass and medium viscosity affect each other, dependencies and correlations are not easy to discover [56]. A comprehensive review on morphology, rheology and productivity has been published recently [53]. It is still not clear how morphology exactly affects productivity, but both parameters are clearly correlated [55].
Several recent reviews have focused on filamentous fungi, covering morphology and productivity [53, 54, 57–60], process design [61] and molecular characteristics [3, 10, 62]. Figure 1 highlights the most frequently cited key publications on bioprocesses involving filamentous microorganisms over the last 65 years.
The aim of this review is to focus on advances in the characterisation and control of filamentous morphology with particular regard to fungal (eukaryotic) and bacterial (prokaryotic) filamentous morphologies. Furthermore, recent strategies for the control of bioparticle shape and bioprocess performance for the optimal productivity of a filamentous system with classical biochemical process parameters, targeted morphology engineering and genetic engineering will be discussed.
2 Eukaryotic Filamentous Morphology
2.1 Interdependence Between Morphology and Productivity
One of the most important industrial aspects of fungal morphology is its relation to productivity because it would be convenient to select productivity levels of cultivation batches solely based on morphologic appearance or to determine early in a batch process how much product will be formed. A relationship between morphology and productivity was therefore the focus of many experiments. Often, optimal product yield corresponded with particular morphologic phenotypes [52, 63–74]. However, not all studies revealed such a link [75, 76]. Other sources confirm a strong relationship between morphological growth form and productivity, with the mycelial morphology being favourable for the production of various enzymes [51, 52, 55, 65, 68, 70]. Generally, the morphologic appearance depends on the sum of the environmental parameters of a cultivation, often summarised as the environome [53, 77].
There has been abundant research on how and why morphology and productivity in filamentous cultivations are correlated, but the mechanisms that regulate polarised growth are still only partially understood [78]. The macro-morphology determines the microenvironment of hyphae through effects on mixing, mass transfer and culture rheology, which in turn affect protein production [53]. Fungal pellets, for instance, may have dense and inactive cores due to poor nutrient diffusion, which may lead to cell lysis and, thus, a loss of the interior pellet structure [79]. Wongwicharn et al. [76] revealed a correlation between the active area of the biomass and protein secretion. Microscopic morphology has other indirect effects on productivity. Hyphal dimensions influence the secretion pathway [80], and protein secretion has been shown to be situated at the tips of fungal hyphae [33]. The branching frequency, in particular, is of importance, as several studies have shown that metabolite excretion is situated mostly at the hyphal tips [33, 81, 82]. A great quantity of hyphal tips creating a dense mycelium is, therefore, often linked to increased enzyme production [83].
Recent studies seem to suggest that in addition to morphology, culture heterogeneity has a notable impact on productivity. In two studies, two populations of pellets of different sizes and gene expressions were identified [84, 85]. The population of highly expressed genes of the produced enzymes was smaller than the population with a large pellet diameter, indicating that heterogeneity and pellet size are not related [86], which was confirmed by the finding that heterogeneity in gene expression is observed between pellets with one particular diameter [86]. Unfortunately, with the exception of pellet sizes, no further morphological characterisation was conducted; thus, it remains unclear whether the subpopulations of larger and smaller pellets might have been different in their morphological form as well. Additionally, the condition of conidia used as starter cultures contributes to the heterogeneity in a submerged cultivation, with some evidence that melanin content influences the size of later formed pellets [86, 87]. Further studies showed heterogeneity within the mycelium.
Studies with an abundance of novel and ever cheaper molecular tools available, including gene expression analysis, have shown that the RNA composition of the central and peripheral zones of plated colonies of A. niger [88] and A. oryzae [89] is different. Furthermore, in seven-day-old colonies of A. niger, a large percentage of active genes showed a significant difference in RNA accumulation between the inner and outer zone of the colony [88]. Further studies have shown the total amount of RNA per hyphae to be increased by approximately 50-fold at the periphery of a 1-mm pellet compared to the pellet centre [84]. Different RNA profiles, however, might be explained by the absence of RNA shown in a recent study covering intra- and intercompartmental streaming of green fluorescent protein (GFP) [90].
2.2 Advances in Morphology Characterisation and Whole-Broth Modelling
Fungal morphology is often a bottleneck of productivity in many industrial processes [91], and process optimisation requires the accurate characterisation of interdependencies between process parameters and performance [92]; therefore, from the very beginning of research with filamentous organisms, it has always been a goal to characterise and model morphology [93–95]. For the quantification of filamentous morphology, early studies relied on manual measurements of hyphal growth [96, 97]. Newer studies implement more or less automated steps of computer-aided image analysis [53, 98]. However, no image analysis parameter on its own is sufficient to discriminate between different morphological forms. With the introduction of a multifunctional shape parameter termed the morphology number (MN), Wucherpfennig et al. [55] were able to characterise a broad distribution of morphological forms of A. niger using just one dimensionless process parameter. A similar approach was proposed in a review about particle shape characterisation by Blott and Pye [99], who reported that no single quantitative measure could provide a full description of a three-dimensional shape, and the use of several shape indices in combination provides the best method of grouping and discriminating between particles. Describing mycelia quantitatively essentially involves the estimation of their space-filling capacity [50], and like many other natural structures, mycelia are approximately fractal [100]. The concept was introduced by Mandelbrot as an extension of the Euclidean geometry [101] and has since been used to measure complex geometric forms [102]. Fractal dimension and branching complexity were previously found to be positively correlated with phenol-oxidase expression in Pycnoporus cinnabarinus; both parameters were influenced by media composition [103]. Barry et al. [104] were also able to demonstrate a relationship between the fractal dimension and the HGU of a Penicillium and an Aspergillus strain. Furthermore, it was demonstrated that the branching behaviour of filamentous organisms “cultivated” in silico as “virtual mycelia” can be inferred from the fractal geometry of mycelial architecture, showing the potential application of fractal analysis in morphological quantification [105]. The benefit of the in silico approach is the large amount of mycelia that can be generated and analysed compared to the regular growth process in the laboratory. The compliance of fractals with conventional image analysis parameters was also proven in several studies [106, 107]. Another fractal value used for the characterisation of fungal morphology is lacunarity, which describes the heterogeneity of a structure or the degree of structural variance within a fractal object [102]. Lacunarity was found to be especially suited to analyse and discriminate between filaments or loose clumps of A. niger [56].
The exact manipulation and subsequent precise characterisation of filamentous morphology enables the establishment of holistic mathematical models. Schügerl et al. [108] noted the complex interrelationships among all process characteristics that are related to growth, morphology and physiology and, through this productivity, argued that no general conclusion could be drawn without considering all relevant factors [108, 109]. However, modelling approaches with that many variables tend to become confusing and, more importantly, often lack general applicability. Kossen suggested an approach for the characterisation of productivity considering only whole-broth properties, where productivity is assessed from morphological data, rheological data and mass transfer properties [109]. Because of general problems in measuring the viscosity of filamentous organisms, it was also deemed appropriate to omit the rheological properties of the cultivation broth. Several studies showed that the complex morphology of filamentous organisms is responsible for highly viscous cultivation broths, characterised by shear-rate-dependent viscosities and by yield stress [2, 38, 110, 111]. Furthermore, the consideration of morphology and the corresponding rheology is crucial for the explanation of mass transfer in multiphase systems, as these factors influence the bubble and drop sizes of suspended air and liquids [112]. The flow behaviour of cultivation broths of filamentous organisms differs distinctly from broths of other microorganisms; this matter has been thoroughly reviewed [53]. Tucker as well as Tucker and Thomas [113, 114] were the first authors studying the influences of biomass concentration and mycelial morphology on broth rheology using an industrial strain of Penicillium chrysogenum. The authors found the viscosity of the cultivation broth to be dependent on clump properties and studied the effect of biomass separately from that of morphology. The rheological parameter, mostly derived from the power law, could be related to biomass concentration as dry cell weight [114]. Riley et al. [38] later enhanced that model to include morphological parameters such as clump roughness and compactness, which were found to have a definite influence on rheology. The resulting correlations were highly successful at predicting culture rheology for P. chrysogenum and A. oryzae broths [115]. Wucherpfennig et al. [56] later refined the model for application to A. niger culture broth using the earlier introduced MN and a rheological parameter termed the biomass independent consistency coefficient (K BDW ).
Figure 2 shows the interrelationship among the parameters morphology, rheology and productivity [107]. Here, A. niger morphology is shown to directly influence productivity, and with the help of the image analytical generated dimensionless parameter MN, it was possible to directly correlate these parameters. Using K BDW, a distinct influence of morphology on culture broth rheology could be shown as well. Furthermore, in an unconventional approach, a correlation between the specific activity of the produced enzyme fructofuranosidase and rheological properties of the cultivation broth was displayed [107].
Established mathematical models relating fungal morphology, rheology and productivity [56]
2.3 Manipulation of Morphology
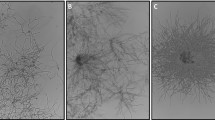
Distinct fungal morphologies must be generated to achieve optimum performance in biotechnological processes with filamentous organisms, and many different methods have been applied to date (Fig. 3). A classic way to direct filamentous morphology is to use defined and controlled environmental process conditions. The utilisation of different reactor types, such as stirred tank reactors (STRs), airlift loop reactors (ALRs) and disposable plastic reactors, extends this concept. However, genetic methods influence microscopic growth events, macroscopic morphology development and resulting process performance. New studies intended to tailor the fungal morphology for enhanced productivity investigated the addition of inorganic salts and microparticles.
2.3.1 Classical Biochemical Process Parameters
The direct interrelation of morphology and productivity of filamentous organisms has resulted in several attempts to control and regulate the morphology of different economically relevant filamentous organisms over the years. These investigations focused on variations in the environome, changing the most important operating parameters, and are still relevant [48].
Some of these process parameters (pH, temperature, shaking intensity and inoculum) were analysed recently in the context of applying brewery wastewater as a substrate for the production of fumaric acid with Rhizopus oryzae [116]. All of the investigated factors had a significant impact on the resulting morphology. Pellets were formed at pH values ranging from 5 to 7 with a shaking speed of 200 min−1 as an indirect parameter for the mechanical stress, a cultivation temperature of 25 °C and 5 % (v/v) inoculum size. Mycelia, however, resulted at pH values between 4 and 8 with shaking speeds of 100, 150 and 250 min−1; a cultivation temperature of 37 °C; and an inoculum size of 20 % (v/v). The highest concentration of fumaric acid (31 g/L) was obtained at pH 6, a cultivation temperature of 25 °C, a shaking speed of 200 min−1 and 5 % (v/v) inoculum.
Current cultivations of Penicillium griseoroseum in buffered and unbuffered mineral medium revealed improved production of polygalacturonase (EC 3.4.1.15) and significantly enhanced secretion of pectin lyase (EC 4.2.2.10) at pH values between 6.0 and 6.8, compared to more acidic conditions at pH 3 in the case of unbuffered medium [117]. Furthermore, the biomass increased, the biopellets reached a greater diameter and showed smaller hyphae on the surface in buffered medium compared to more acidic conditions in the unbuffered case.
Differences in power input and mechanical stress by varying stirring rates are a known source of morphology and productivity manipulation [118–121]. Yao et al. [122] reported significant improvements in product formation in response to power input through ultrasonication. After testing different cultivation times of A. fumigatus for a single treatment of irradiation (40 kHz, 500 W, 5 min), which all resulted in higher concentrations of the product fumigaclavin C, consecutive irradiations during a cultivation were applied and induced a twofold improvement in product concentration. This improvement was accompanied by a significant reduction in the average pellet diameter, longer hyphae on the pellet surface and even formation of more dispersed mycelia. This work supports previous findings with A. terreus and T. versicolor demonstrating that ultrasonication can have a positive effect on fungal morphology and productivity [123, 124].
2.3.2 Addition of Inorganic Salts or Microparticles
2.3.2.1 Effects of Inorganic Salts and Osmolality
The morphology of fungi is known to be affected by the addition of ions; polycations enhance pelleted growth, whereas polyanions suppress it, leading to the conclusion that ions influence the agglomeration behaviour of the naturally charged cell walls [39, 125, 126]. A sophisticated way to tailor fungal morphology was investigated by manipulation of osmolality within the culture medium. First experience was done by Bobowicz-Lassociska and Grajek [127] to increase protein secretion of A. niger mycelia by addition of KCl. Fiedurek [128] showed increased activity of A. niger expressed glucose oxidase twofold by adding NaCl, thus administering an osmotic shock to the fungus. Increased NaCl concentrations led also to stronger mycelial growth and enhanced productivity of two A. niger strains [55]. The morphology and productivity of both strains were shown to be influenced by osmotic pressure. The specific productivity of fructofuranosidase producing strain A. niger SKAn 1015 could be increased around 18-fold from 0.5 to 9 U/(mg h). The specific productivity of glucoamylase producing strain A. niger AB 1.13 could be elevated using the same procedure. Overall, it has to be noted that observed changes in productivity might not be due to the change in morphology alone. The osmolality might have affected fungal physiology and through this morphology independent of each other. The increase in the observed productivity at higher concentrations of NaCl, however, was shown to correlate with the active surface area of the fungal particles [54].
2.3.2.2 Microparticle-Enhanced Cultivations (MPEC)
A new and promising approach to control and engineer filamentous morphology without changing the original process parameters is the addition of rather insoluble microparticles to the cultivation medium [56]. Using this MPEC, Kaup et al. [51] were able to drastically improve the production of chloroperoxidase in the filamentous fungus Caldariomyces fumago. At the same time, the growth morphology of C. fumago was changed from pellet to mycelial growth by the addition of microparticles. This work suggested a universal mechanism occurring between microparticles and filamentous organisms because the same effect was observed for talc (3 MgO·SiO2·H2O) and aluminium oxide (Al2O3) particles.
Furthermore, a test of microparticle addition on the growth morphology of several filamentous organisms including Penicillium spp., Aspergillus spp., Streptomyces spp., (compare Sect. 3.2.3), Emericella spp., Acremonium spp., Pleurotus spp., Rhizopus spp. and Chaetomium spp. revealed the same effect as on C. fumago [51]. The authors suggested an improved supply of oxygen and other nutrients in the mycelium compared to the diffusion-limited pellet as a possible reason for the improved observed performance.
These results and conclusions were confirmed by the data from Driouch et al. [52, 65]. With talc microparticles, a fourfold and twofold enhancement of glucoamylase and β-fructofuranosidase was achieved, respectively. Furthermore, a GFP-producing strain of Aspergillus spp. supported the assumption that oxygen limitation might be one reason for the worse performance of pellets compared to mycelium. Thin pellet slices were analysed with a confocal laser scanning microscope (CLSM) and revealed that efficient GFP production only occurred in the oxygen-supplied outer boundary layer of the pellets. In the case of the mycelial growth morphology, which was induced by microparticle addition, biomass was more efficient in GFP production.
It is interesting to note that the effect induced by the microparticles on the one hand seems to be of a universal character—different particles lead to a change in morphology of different filamentous organisms (compare Table 1 in [60]), but the molecular interaction between microparticles and an organism, on the other hand, can be different. Whereas talc microparticles induce mycelial growth, titanate microparticles form small-sized pellets with an inner particle core surrounded by biomass, the so-called core–shell pellet [70].
Depending on the product to be produced, different morphologies of filamentous organisms can be favourable [40]. Whereas the production of enzymes, such as amylases, works best with a mycelial growth morphology of the producing strain [125, 129], the synthesis of secondary metabolites, such as citric acid or penicillin, is favourable with pellet morphology [130, 131]. Therefore, it is desirable to engineer the morphology according to the product type. Driouch et al. [52] managed to specifically fine tune the size of A. niger pellets by using different concentrations of talc (3 MgO·SiO2·H2O) and aluminium oxide (Al2O3) microparticles. The more particles were present during the cultivation, the smaller the pellet size became. With the highest applied concentrations, no pellets were observed, but mycelial growth was present. The authors also showed that the primary conidia agglomeration shortly after inoculation was probably obstructed by the microparticles, providing a potential explanation for the effect that is induced by the microparticles.
Recently, this technique was employed in the production of lovastatin by A. terreus [132]. Here, however, talc microparticles were added to the pre-culture instead of the main culture and were diluted below 5 % of the initial concentration. Nevertheless, an improvement of 60 % (with significant error bars) in the product amount was observed in the main culture at talc concentrations of 12 g/L in pre-culture. These results support the findings by Driouch et al. [52] concerning particle interference with conidia in the beginning of the cultivation.
A comprehensive screening of seventeen different types of microparticles was performed by Etschmann et al. [133] in the context of secondary metabolite production with filamentous fungi. In the case of 2-phenylethanol produced by A. niger DSM 821, nine types of the tested microparticles improved production, whereas in the case of 6-pentyl-α-pyrone produced by T. atroviride, thirteen different microparticle types were capable of increasing the product yield. Interestingly, the succession of the positively acting microparticles was different for both cases. Thus, despite the general character of the particle effect, there must be case-specific conditions that make one type of particle superior to the other.
Another current publication concerning secondary metabolites addresses an improved lipid accumulation of the fungus Mortierella isabellina upon the addition of magnesium silicate microparticles with an average diameter of 10 µm [134]. In addition, with increasing concentrations of the microparticles up to 10 g/L in the main culture, the average pellet size decreased and mycelium morphology was induced. Similar to the findings reported by Driouch et al. [70], the product distribution within the pellets was analysed by fluorescence and CLSM. The results are comparable to the GFP expression patterns noted above.
The increasing number of publications addressing the MPEC of filamentous organisms and productivity enhancement indicates the relevance of this relatively new but very simple and flexible technique.
2.3.3 Genetic Engineering
Genetic analyses include microscopic events such as hyphal tip extension [135, 136], branching [137–139] and hyphal development [140–146]. Rather, rarely is the genetic influence on macroscopic morphology development and the resulting process performance investigated. Recently, a study aimed at engineering a shear-resistant morphology of A. glaucus for the production of aspergiolide A, an antitumor polyketide, via the deletion of the growth genes AgkipA and AgteaR [147]. On the microscopic scale, these deletions resulted in meandering hyphae compared to the wild type and had a different impact on the macroscopic pellet morphology. Whereas the maximum product concentration remained unchanged for all three organisms in shaking flasks, production with the AgkipA mutant was 24 h faster. Cultivations with the same mutant in a 5 L STR resulted in an improved production of aspergiolide A by 82.2 %.
In another comprehensive study, the gene PcvelA from P. chrysogenum was found to be involved in the expression of biosynthesis and developmental genes and, as a result, was responsible for mycelial growth [148]. The deletion of PcvelA resulted in pellet formation after 72 h and was correlated with a down-regulation of genes involved in cell wall metabolism. The authors suggested a down-regulation of genes involved in chitin degradation by the deletion of PcvelA as a possible reason for the observed hyper-branching phenotype, pellet formation, and delayed fragmentation and autolysis. This possibility was confirmed by microarray analyses with a PcvelA knockout strain in which the gene PcchiB1, encoding for a class V chitinase, was down-regulated [149].
2.3.4 Different Types of Bioreactors
Instead of genetically creating a shear-resistant organism, attempts have also been made to reduce shear stress by using alternatives to STRs to manipulate growth morphology towards a favourable form. The identification and quantification of mechanical stress, which is related to a distinct morphology of filamentous elements within the process, is one of the main application fields of research in bioprocess engineering and industrial applications. The bioreactor geometry, stirrer shape and size, gassing rate and subsequent dissipated energy are among the fluid dynamic-related criteria that affect the morphology and productivity. The stirrer in STRs influences cultivation in several ways: lower stirring speeds lead to inadequate nutrient distribution and gas dispersion, whereas vigorous stirring can cause cell wall damage [26, 49] or sporulation [46]. Particle stress in an STR, ALR and bubble column reactor have been investigated by varying power input and impeller geometries [44]. For STRs, the stress produced by different agitators can be predicted with the aid of a geometrical correlation derived from experimental particle disintegration kinetics [54].
Disposable bag reactors, primarily developed for the cultivation of mammalian cells, were applied for the cultivation of mechanical stress-sensitive basidiomycetes Flammulina velutipes and Pleurotus sapidus and to produce esterases and peptidases [150]. In both cases, the authors report significant enhancements in product concentration compared to cultivations in regular STRs. At the same time, the morphology of the organisms in the disposable bag reactors was directed towards small and distinct pellets with average diameters of between 0.3 and 0.5 mm, in contrast to the large and inhomogeneous lumps of biomass in STRs. Another approach to reducing mechanical stress on the organism is to use an ALR. In a direct comparison of STR and ALR, the morphology of Pycnoporus sanguineus was directed from compact round pellets in the case of the STR towards a more hairy and loose morphology in the ALR [151]. The efficiency of exopolysaccharide production, however, was better in the STR.
3 Prokaryotic Filamentous Morphology
3.1 Development of Bacterial Morphology
Filamentous bacteria such as Streptomyces spp., Nocardia spp. and Lechevalieria spp. produce a wide range of products, including antibiotics, immunosuppressants and other classes of biologically active substances [152]. The group Actinomycetes produces at least two-thirds of the known microbial antibiotics, and 80 % of these are produced by members of the genus Streptomyces [153].
Although these microorganisms belong to the prokaryote family, they do not behave as such and they are able to form mycelium and pellets or produce spores.
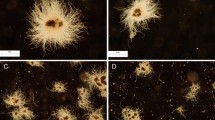
The life cycle of Streptomyces is marked by spore germination, the growth of vegetative mycelium and differentiation to reproductive (aerial) mycelium. The production of antibiotics and other biologically active substances mainly takes place in the stationary growth phase as secondary metabolites [152] (Fig. 4).
Life cycle of Streptomyces griseus [152]
Germination can be divided into three different morphological steps from the dormant to vegetative state: darkening, swelling and germ tube emergence. The darkening process depends on exogenous divalent ions (Ca2+, Mg2+ or Fe2+) and endogenous energy-providing processes. The swelling requires exogenous carbon sources and is mainly based on the rehydration of the cytoplasm [154]. Germ tube emergence is based on carbon and nitrogen sources. By adding different pairs of amino acids, different nitrogen sources were tested on S. viridochromogenes [155] and other carbon compounds on S. antibioticus [154]. Shortly after entering the germination phase, the synthesis of macromolecules was observed, starting with RNA followed by protein and DNA synthesis. The outgrowth is marked by the beginning of DNA synthesis [154].
On solid growth media, Streptomyces spp. shows two types of mycelia: vegetative and substrate mycelium and a reproductive aerial mycelium [156]. After germination of the spores, growth starts by tip extension and branching of the hyphae. New cell wall material is only synthesised at the tips of the hyphae [157]. A refined investigation showed that the development of vegetative mycelium can be further distinguished into different steps; a young, compartmentalised mycelium undergoes programmed cell death. Viable parts differentiate subsequently into multinucleated second mycelia divided by thin, infrequent and single-layer septa [158–160]. Some of these mycelia grow into the air to form aerial mycelia covered by a hydrophobic shell [161, 162]. Differentiation and septation of the apical cells lead to a chain of spores [163]. The growth of aerial mycelia is partly based on a second round of programmed cell death, where released nutrients are recycled and found in the spores [164]. Chater and Merrick [165] suggested the production of antibiotics as a defensive strategy that therefore takes place in such a late step to protect the lysed substrate against bacteria.
In submerged cultures, most Streptomyces spp. do not differentiate or produce spores [166]. Four morphological types have been distinguished depending on perimeter and convex perimeter, pellets, clumps, branched hyphae and unbranched hyphae [167]. The main difference between growth in submerged cultures and on solid medium is the absence of a second round of programmed death and sporulation [159]. A transition phase with transient growth arrest lies between the first vegetative compartmentalised mycelium and the second multinucleated differentiated mycelium accompanied by the first death round [159]. After the transition, the production of antibiotics occurs [168, 169].
Nevertheless, there are reports of sporulation in submerged cultures of several Streptomyces spp., including S. griseus [170] and others [171–175]. Daza et al. [166] showed the sporulation of several species after a nutritional downshift. The published record of spore survival is over 70 years [176].
According to Takahashi and Yamada [16], pellet formation for all filamentous organisms is generally described by two distinct mechanisms. The coagulative mechanisms involve early spore agglomeration; thus, several spores form one pellet. The non-coagulative mechanism is characterised by the lack of spore agglomeration; thus, one spore forms one pellet. However, regardless of the occurrence of spore agglomeration, grown out and branched hyphae tend to entangle and form clumps during the early stages of growth [27, 177]. Few filamentous bacteria species have been studied in terms of germination, agglomeration and pellet formation. For S. tendae and S. aureofaciens, non-coagulative pellet formation has been reported [178, 179], whereas S. hygroscopicus exhibits coagulative pellet formation [180].
Over the course of a submerged culture, hydrodynamic forces in the cultivation system lead to pellet fragmentation and erosion, thus affecting the pellet size and shape [81–184]. Furthermore, it was reported recently that submerged cultures of Streptomyces spp. consist of two pellet populations regardless of the examined strain and culture age: a population of pellets with small diameters of approximately 250 µm, which remained constant over time and was similar for all Streptomyces spp. under investigation; and a population of larger pellets with size fluctuations over time and that was dependent on the strain and media used. It was then suggested that classical parameters utilised for morphology engineering, such as pH, temperature, the composition of the medium and power input in the submerged culture, would only affect the population of large pellets, whereas the population of small pellets would remain unaffected [185].
The overall pellet stability depends on extracellular substances, which are still mostly unidentified. However, recently, it was suggested that DNA fragments from lysed cells, hyaluronic acid and β-glucan fibres play a major role in pellet cohesion and the architecture of S. coelicolor [185–187].
3.2 Similarities to and Differences from Eukaryotic Systems
3.2.1 Control of Morphology and Productivity
Similar to fungi, filamentous bacterial morphology is influenced by several process parameters, and there have been various attempts to correlate morphological characteristics with the productivity of enzymes and secondary metabolites in Actinomycetes. Earlier research activities in this field were summarised by Whitaker [188] and Junker et al. [189].
Interestingly, whereas the dependence between morphology and productivity was reported for several organisms and processes, different morphologies seem to be favourable for different products. Clumps and dispersed mycelia were found to be the favourable morphology for retamycin production in S. olindensis [190], nystatin production in S. noursei [191], tylosin production in S. fradiae [183], geldamycin production in S. hygroscopicus [192] and the expression of a recombinant Ala-Pro-rich O-glycoprotein in S. lividans [193]. However, productivities were higher in pellets than in dispersive growth forms when producing nikkomycin in S. tendae and avermectin in S. avermitilis, respectively [194, 195]. Furthermore, S. clavuligerus and S. virginiae exhibited similar productivity levels of clavulanic acid and virginiamycin, respectively, for different morphologies [177, 181]. When freely dispersed mycelia and clumps are favourable, the higher productivities can usually be attributed to an adequate supply of oxygen and other substrates, as the inner core of dense pellets is usually substrate limited. Regarding production systems which favour pelleted growth, Braun and Vecht-Lifshitz [39] postulated that the overproduction of antibiotics is affected by secreted metabolites, such as A-factor in S. griseus and factor 1 in S. aureofaciens, which can accumulate in pellets and, thus, increase productivity. Manteca et al. [159] developed this hypothesis further, suggesting that the production of secondary metabolites only occurs after mycelium differentiation from a young compartmentalised (first) mycelium to a syncytial (second) mycelium after a round of programmed cell death. This process is also supported by reports of changes in morphology during the course of cultivation, mainly during the transition from the exponential to stationary growth phase [182]. Hence, the correlation between morphology and productivity is strain and product dependent. Nevertheless, the knowledge of tools to morphologically engineer filamentous bacteria helps to further optimise product yields and process control.
3.2.2 Biochemical Process Parameters
Once a desirable morphology and, therefore, high productivity is established for a bioprocess involving filamentous organisms, the optimisation of classical environomal process parameters is a commonly utilised approach. Although several Streptomyces strains are used in industrial applications, mostly to produce secondary metabolites such as antibiotics, the effects of process parameters on Actinomycetes are not as well studied as their effects on filamentous fungi.
The impact of agitation and aeration on the morphology and productivity was demonstrated for several Actinomycetes. In general, high energy dissipations result in small, smooth and compact pellets, whereas lower energy dissipations lead to larger, fluffier pellets [196, 197]. Additionally, low dissolved oxygen levels promote mycelial morphologies, whereas high dissolved oxygen levels result mainly in pelleted growth [179, 195]. Therefore, the effects of agitation and aeration on Actinomycetes are comparable to those observed on fungi [198]. A comprehensive review on this subject was recently published by Olmos et al. [12].
In fungal morphology engineering, pH is a potent morphology-altering process parameter, as pellets are usually formed in more alkaline cultivation broths; thus, a decrease in pH results in mycelial growth [199]. Surprisingly, little is known about how changes in pH affect Streptomyces spp. morphology in submerged cultures. However, a study on S. akiyoshiensis suggests that filamentous bacteria react similarly to pH changes compared with fungi [200].
Another well-studied process parameter capable of altering filamentous morphology is the type and size of the inoculum. In the majority of cases, cultures are inoculated with a spore suspension. The number of viable spores in the inoculum can be directly correlated with the size of the filamentous agglomerate regardless of the strain used. Low inoculum sizes result in large, compact pellets, and pellet sizes decrease with increasing inoculum sizes [179, 201, 202]. Furthermore, vegetative mycelia and matured pellets are commonly used for inoculation in industrial cultivation processes. Such cultures tend to form clumps and pellets rather than freely dispersed hyphae [167]. These observations are consistent with those for filamentous fungi [124, 203].
The composition of growth media is known to be crucial for the development of the desired morphology. In general, complex media more often lead to dispersed mycelial morphology, whereas minimal media often promote pelleted growth [188].
The chosen carbon source has a great influence on the productivity and morphology of several Streptomyces spp., though no general conclusions can be drawn, as it mainly depends on the strain and the sought-after metabolite. Reviews on this rather broad field of research have been published in recent years [204, 205].
Yin et al. [195] reported that low nitrogen levels lead to decreased growth rates and therefore promote pelleted growth. Other investigations indicate that the morphology of several strains is also dependent on the type of complex nitrogen source [206–208], although admittedly, it is difficult to distinguish between effects based on the type and the concentration of nitrogen source, respectively, as the total amount of nitrogen in complex media components is usually unknown. Still, it was reported that fungal morphology is altered by different nitrogen sources [48].
Similar to the situation with fungi, a study proved that addition of ions has a distinct impact on the morphology of S. azureus, in which it was shown that the addition of excess Mg2+, Ca2+ and Mn2+ all induced pelleted growth, although supplementation with Ca2+ led to smaller pellets than supplementation with Mg2+. Additionally, Mn2+ induced pellet formation at much lower levels than Mg2+ and Ca2+ [209]. In contrast, Dobson and O’Shea [126] observed a pellet formation-enhancing effect for Ca2+ in cultivations of S. hygroscopicus but a dispersing effect for Mg2+.
Finally, the supplementation of various additives to the growth media is reportedly an easily applicable tool to tailor bacterial morphology. O’Cleirigh et al. [180] investigated the influence of xanthan gum on S. hygroscopicus var. geldanus. The pellet diameters decreased significantly, presumably because of reduced spore agglomeration. The growth of the same organism was also manipulated by surfactants, namely silicone antifoam, Tween 80 and Triton X-100, leading to decreasing mean pellet diameters with increasing concentrations of surfactant [126]. These observations are contradictory to earlier studies on S. tendae reporting increasing pellet sizes with increasing concentrations of Triton X-100, Pluronic F68 and Brij 58 [210]. These contradictory observations can presumably be attributed to the fact that S. tendae forms non-coagulative pellets, whereas S. hygroscopicus exhibits coagulative pellet formation. Furthermore, Hobbs et al. [211] investigated the effects of the uncharged polymer polyethylene glycol (PEG) 6000 and the negatively charged polymers agar, Junlon PW110 and carboyx-methylene (Carbopol) on S. lividans. All of these polymers were capable of directing growth to smaller and looser pellets, although Carbopol and Junlon PW110 were significantly more effective than agar and PEG 6000. Additionally, it was proven that Junlon PW110 would promote dispersed growth in several strains of S. lividans as well as S. coelicolor. In another approach, the chelating agents bacitracin and EDTA inhibited pellet formation in S. azureus [209].
In the case of industrial-scale applications, Actinomycetes are mainly cultivated in STRs and ALRs. Only a few attempts have been made to establish alternative cultivation systems. S. griseus was only recently deployed in a tubular biofilm reactor. The formed biofilm was characterised and engineered for use in continuous bioprocess applications [212].
3.2.3 Addition of Microparticles
Research in this field is not as prevalent as for eukaryotic systems, but some data indicate that microparticles are capable of changing the morphology of Streptomyces spp., as already shown for fungi [60]. Kaup et al. [51] were able to reduce the pellet size of S. aureofaciens ranging between 0.9 and 2.1 mm to a range of 0.06–0.5 mm by the addition of microparticles. In microtitre plates, the optimal influence of 3-mm-diameter glass beads was tested. The average pellet size was reduced from 0.33 to 0.058 mm with significantly improved productivity [213].
3.2.4 Genetic Engineering
There are several important regulators known for Streptomyces spp. development and secondary metabolite production. Two early discovered and widely studied regulators are A-factor and bldA [214–217].
The A-factor, discovered by Khokhlov in 1967 as a starting point of the A-factor regulatory cascade, leads to morphological development, sporulation and secondary metabolism. The A-factor binds the A-factor-dependent receptor protein ArpA. The gene adpA, encoding the central transcriptional regulator AdpA, is the target of ArpA. For S. griseus, it was proven that without the adpA gene, no aerial mycelium occurred and no streptomycin was produced. A clone strain with an overexpression of adpA showed large amounts of streptomycin at an earlier growth phase. The streptomycin productivity is therefore dependent on the A-factor concentration [152].
Strains with mutations in bldA also lack aerial mycelium and antibiotic production in S. coelicolor. The formation of aerial mycelium and spores can be restored by adding mannitol or maltose but not the production of antibiotics [217]. It was shown that bldA encodes a tRNA with the leucine anticodon UUA [218]. This codon is underrepresented in Streptomyces but occurs in adpA [219]. Most morphological effects of bldA mutations are based on the restricted expression of adpA but can be corrected by changing the TTA codon in AdpA [220].
Regulation in Streptomyces is still not fully understood, but further research can help understand the development of the morphology and, hence, develop tools derived to improve the productivity of secondary metabolites [220].
3.3 Morphology Characterisation
The morphology of filamentous bacteria not only influences the overall productivity but also affects the rheological properties of the cultivation broth [38]. Thus, the development of suitable methods to characterise the morphology of submerged cultures has been the subject of several studies. Whereas earlier efforts mainly involved digital image analysis [177, 182, 202, 221], more recent studies focused on different techniques, including laser diffraction and focused beam reflectance [222]. Microscopic image acquisition and the subsequent analysis of the pictures provide an extensive quantitative description of the fungal morphology, both micro- and macro-morphological, and, furthermore, the application of advanced imaging techniques such as CLSM [223].
Laser diffraction was introduced for the quantification of fungal morphology because of its rapid, unbiased and robust results for particle size distributions as manual sample preparation is eliminated [224]. However, this method fails to report any other descriptive factors such as the roughness or aspect ratio of the particles. The measurement principal relies on the light-scattering pattern produced by suspended particles that are illuminated by a laser. The light-scattering properties are converted to a size distribution by applying mathematical models, namely the Fraunhofer approximation for particles larger than 50 µm and the Mie theory for all particles. However, these mathematical models assume that the measured particles are spherical, which might lead to inaccurate results depending on the aspect ratio and flow orientation of the clumps and pellets [225].
Rønnest et al. [225] evaluated the feasibility of laser diffraction measurements for the characterisation of S. coelicolor pellets and clumps by comparing them to conventional image analysis results. It was found that laser diffraction resulted in similar particle size distributions to image analysis, whereas the standard deviations were generally higher, presumably because of discrepancies between the assumptions for the mathematical model and the biological sample and statistical artefacts based on the small number of particles analysed by image analysis. Studies on the application of laser diffraction for other filamentous organisms [55, 198, 224] as well as on other biological samples [226] indicate its applicability for the investigation of size distributions in process environments.
Additionally, there have been efforts to investigate the application of the focused beam reflectance method (FBRM) for monitoring the growth [222] and morphological characteristics [227]. A focused laser beam is rapidly rotated to scan the particle suspension, in this case, the culture broth. Particles backscatter the laser light, which is detected. Based on the scan speed, the chord length of the particle can be calculated from the duration of backscattering light pulses. To monitor changes in particle size, shape and concentration, a chord length distribution is calculated by plotting the number of detected particles per second as a function of chord length dimension. Different from most image analysis techniques, FBRM is suitable for online monitoring. Pearson et al. [227] compared FBRM with traditional image analysis techniques for cultivations of S. natalensis. It was found that whereas FBRM is not applicable for characterising filamentous morphology through shape descriptors such as roughness and aspect ratio, the obtained chord length distributions enable a characterisation of morphology to some extent. However, it is not possible to directly correlate FBRM data to image analysis results. Additionally, it was proven that FBRM is a valid measurement method for biomass concentrations in cultivations involving filamentous microorganisms [222]. Furthermore, Grimm et al. deployed FBRM for studying the agglomeration kinetics of spores [228, 229].
4 Conclusions and Future Perspectives
In recent decades, suitable and powerful high-performance control strategies have been developed for a tailor-made generation of product-optimised filamentous systems. Great achievements in the different fields of biotechnology provide tools to direct the morphology of filamentous systems towards high productivity.
The major efforts in the morphological characterisation of filamentous systems show a close connection among cultivation conditions, morphology and metabolic characteristics of the individual cells. Closing the gap between the metabolic processes and the engineering level is the most interesting aspect for future research. Still, the underlying metabolic and regulatory mechanisms that lead to the formation of a highly productive agglomerate shape are far from understood. Experimental and in silico techniques provide powerful tools to gain a better understanding of the complex biological and technical aspects of filamentous systems. Recent interesting approaches include morphology engineering by osmolality variation and microparticle addition. These promising and simple techniques are currently being evaluated for many different systems and have the potential to enhance the productivity of industrial filamentous biotechnological processes.
To direct a production strain towards a suitable production candidate in industrial use, a heterogeneous filamentous system must be analysed in its detailed subpopulations. Hence, the development of advanced, powerful and automated online image analysis techniques and modelling tools are desirable for the high-quality prediction of culture productivity with minor deviations.
The key to understanding and rationally improving eukaryotic and prokaryotic filamentous systems as a common platform technology depends on overcoming the existing collaboration barriers in the fields of genetic engineering, molecular biotechnology and bioprocess engineering.
Abbreviations
- ALR(s) :
-
Airlift loop reactor(s)
- CLSM :
-
Confocal laser scanning microscopy
- FBRM :
-
Focused beam reflectance
- GFP :
-
Green fluorescent protein
- HGU :
-
Hyphal growth unit
- K BDW :
-
Rheological parameter, biomass independent consistency coefficient
- MN :
-
Morphology number
- MPEC :
-
Microparticle-enhanced cultivation
- STR(s) :
-
Stirred tank reactor(s)
References
Sauer M, Porro D, Mattanovich D, Branduardi P (2008) Trends Biotechnol 26:100
Papagianni M (2007) Biotechnol Adv 25:244
Meyer V (2008) Biotechnol Adv 26:177
Lubertozzi D, Keasling JD (2009) Biotechnol Adv 27:53
Baker SE (2013) Ind Biotechnol 9:105
Ward M, Lin C, Victoria DC, Fox BP, Fox JA, Wong DL, Meerman HJ, Pucci JP, Fong RB, Heng MH, Tsurushita N, Gieswein C, Park M, Wang H (2004) Appl Env Microbiol 70:2567
Berovic M, Legisa M (2007) In: El-Gewely MR (ed) Biotechnology annual review. Elsevier, pp 303–343
Withers JM, Swift RJ, Wiebe MG, Robson GD, Punt PJ, van den Hondel CA, Trinci APJ (1998) Biotech Bioeng 4:407
Gusakov A (2011) Trends Biotechnol 29:419
Workman M, Andersen MR, Thykaer J (2013) Ind Biotechnol 9:337
Nielsen J (1996) Trends Biotechnol 14:438
Olmos E, Mehmood N, Haj Husein L, Goergen JL, Fick M, Delaunay S (2013) Bioprocess Biosyst Eng 36:259
Rioseras B, López-García MT, Yagüe P, Sánchez J, Manteca Á (2014) Bioresour Technol 151:191
Krijgsheld P, Bleichrodt R, van Veluw GJ, Wang F, Müller WH, Dijksterhuis J, Wösten HAB (2013) Stud Mycol 74:1
Emerson S (1950) J Bacteriol 60:221
Takahashi J, Yamada K (1959) Nippon Nōgeikagaku Kaishi 33:707
Pirt SJ, Callow DS (1959) Nature 184:307
Pirt SJ (1967) J Gen Microbiol 47:181
Trinci AP (1969) J Gen Microbiol 57:11
Trinci AP (1974) J Gen Microbiol 81:225
Caldwell IY, Trinci APJ (1973) Arch Microbiol 88:1
Robinson RF, Davidson RS (1959) The large-scale growth of higher fungi. In: Umbreit WW (ed) Advances in applied microbiology, vol 1. Academic Press, US, pp 261–278
Phillips DH (1966) Biotechnol Bioeng 8:456
Metz B, Kossen NWF (1977) Biotechnol Bioeng 14:781
Wittler R, Baumgartl H, Lübbers DW, Schügerl K (1986) Biotechnol Bioeng 28:1024
Adams HL, Thomas CR (1988) Biotechnol Bioeng 32:707
Packer HL, Thomas CR (1990) Biotechnol Bioeng 35:870
Cox PW, Thomas CR (1992) Biotechnol Bioeng 39:945
Tucker KG, Kelly T, Delgrazia P, Thomas CR (1992) Biotechnol Progr 8:353
Paul G, Thomas C (1998) In: Schügerl K (ed) Advances in Biochemical Engineering/Biotechnology, vol 60. Springer, Berlin, pp 1–59
Bartnicki-Garcia S, Hergert F, Gierz G (1989) Protoplasma 153:46
Prosser JI, Tough AJ (1991) Crit Rev Biotechnol 10:253
Wösten HAB, Moukha SM, Sietsma JH, Wessels JGH (1991) J Gen Microbiol 137:2017
de Vries OMH, Fekkes MP, Wösten HAB, Wessels JGH (1993) Arch Microbiol 159:330
Wösten HAB, Schuren FHJ, Wessels JGH (1994) EMBO J 13:5848
Grant Allen D, Robinson CW (1990) Chem Eng Sci 45:37
Olsvik ES, Tucker KG, Thomas CR, Kristiansen B (1993) Biotechnol Bioeng 42:1046
Riley GL, Tucker KG, Paul GC, Thomas CR (2000) Biotechnol Bioeng 68:160
Braun S, Vecht-Lifshitz SE (1991) Trends Biotechnol 9:63
Gibbs PA, Seviour RJ, Schmid F (2000) Crit Rev Biotechnol 20:17
Nienow AW (1990) Trends Biotechnol 8:224
Makagiansar HY, Ayazi Shamlou P, Thomas CR, Lilliy MD (1993) Bioprocess Eng 9:83
Jüsten P, Paul GC, Nienow AW, Thomas CR (1996) Biotech Bioeng 52:672
Henzler HJ (2000) In: Schügerl K, Kretzmer G (eds) Advances in Biochemical Engineering/Biotechnology, vol 67. Springer, Berlin, pp 35–82
Mahnke EU, Büscher K, Hempel DC (2000) Chem Eng Technol 23:509
Rocha-Valadez JA, Hassan M, Corkidi G, Flores C, Galindo E, Serrano-Carreón L (2005) Biotechnol Bioeng 91:54
Rocha-Valadez JA, Galindo E, Serrano-Carreón L (2007) J Biotechnol 130:394
Papagianni M (2004) Biotechnol Adv 22:189
Grimm LH, Kelly S, Krull R, Hempel DC (2005) Appl Microbiol Biotechnol 69:375
Papagianni M (2006) Microb Cell Fact 5:5
Kaup B-A, Ehrich K, Pescheck M, Schrader J (2008) Biotechnol Bioeng 99:491
Driouch H, Sommer B, Wittmann C (2009) Biotechnol Bioeng 105:1058
Wucherpfennig T, Kiep KA, Driouch H, Wittmann C, Krull R (2010) In: Allen SS, Laskin I, Geoffrey MG (eds) Advances in applied microbiology. Academic Press, US, pp 89–136
Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, Kampen I, Kwade A, Wittmann C (2013) J Biotechnol 163:112
Wucherpfennig T, Hestler T, Krull R (2011) Microb Cell Fact 10:58
Wucherpfennig T, Lakowitz A, Krull R (2013) J Biotechnol 163:124
Krull R, Cordes C, Horn H, Kampen I, Kwade A, Neu TR, Nörtemann B (2010) Adv Biochem Eng Biotechnol 121:1
Barry DJ, Williams GA (2011) J Microsc 244:1
Ward OP (2012) Biotechnol Adv 30:1119
Walisko R, Krull R, Schrader J, Wittmann C (2012) Biotechnol Lett 34:1975
Posch AE, Herwig C, Spadiut O (2013) Trends Biotechnol 31:37
Meyer V, Wu B, Ram A (2011) Biotechnol Lett 33:469
Papagianni M, Mattey M (2006) Microb Cell Fact 5:3
Ahamed A, Vermette P (2009) Bioresour Technol 100:5979
Driouch H, Roth A, Dersch P, Wittmann C (2010) Appl Microbiol Biotechnol 87:2011
Sitanggang AB, Wu H-S, Wang SS, Ho Y-C (2010) Bioresour Technol 101:3595
Choy V, Patel N, Thibault J (2011) Biotechnol Progr 27:1544
Driouch H, Roth A, Dersch P, Wittmann C (2011) Bioeng Bugs 2:100
Babič J, Pavko A (2012) J Ind Microbiol Biotechnol 39:449
Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C (2012) Biotechnol Bioeng 109:462
Liu Y-S, Wu J-Y (2012) J Ind Microbiol Biotechnol 39:623
Singh K, Wangikar P, Jadhav S (2012) J Ind Microbiol Biotechnol 39:27
Tepwong P, Giri A, Ohshima T (2012) Mycoscience 53:102
Wucherpfennig T, Lakowitz A, Driouch H, Krull R, Wittmann C (2012) J Vis Exp 61:e4023
Amanullah A, Blair R, Nienow AW, Thomas CR (1999) Biotechnol Bioeng 62:434
Wongwicharn A, McNeil B, Harvey LM (1999) Biotechnol Bioeng 65:416
Deckwer WD, Hempel DC, Zeng AP, Jahn D (2006) Eng Life Sci 6:455
Amicucci A, Balestrini R, Kohler A, Barbieri E, Saltarelli R, Faccio A, Roberson RW, Bonfante P, Stocchi V (2011) Fungal Genet Biol 48:561
Hille A, Neu TR, Hempel DC, Horn H (2009) Biotechnol Bioeng 103:1202
McIntyre M, Müller C, Dynesen J, Nielsen J (2001) In: Nielsen J (ed) Advances in Biochemical Engineering/Biotechnology, vol 73. Springer, Berlin, pp 103–128
Gordon CL, Archer DB, Jeenes DJ, Doonan JH, Wells B, Trinci APJ, Robson GD (2000) J Microbiol Meth 42:39
Müller C, McIntyre M, Hansen K, Nielsen J (2002) Appl Env Microbiol 68:1827
Spohr A, Carlsen M, Nielsen J, Villadsen J (1997) Biotechnol Lett 19:257
de Bekker C, van Veluw GJ, Vinck A, Wiebenga LA, Wösten HAB (2011) Appl Env Microbiol 77:1263
van Veluw GJ, Teerstra WR, de Bekker C, Vinck A, Müller WH, Arentshorst M, van der Mei HC, Ram AF, Dijksterhuis J, Wösten HAB (2013) Stud Mycol 74:47
Wösten HAB, Veluw GJ, Bekker C, Krijgsheld P (2013) Biotechnol Lett 35:1155
Priegnitz B-E, Wargenau A, Brandt U, Rohde M, Dietrich S, Kwade A, Krull R, Fleißner A (2012) Fungal Genet Biol 49:30
Levin AM, de Vries RP, Conesa A, de Bekker C, Talon M, Menke HH, van Peij NN, Wösten HAB (2007) Eukaryot Cell 6:2311
Masai K, Maruyama J, Sakamoto K, Nakajima H, Akita O, Kitamoto K (2006) Appl Microbiol Biotechnol 71:881
Bleichrodt R, Vinck A, Krijgsheld P, van Leeuwen MR, Dijksterhuis J, Wösten HAB (2013) Stud Mycol 74:31
Colin VL, Baigorí MD, Pera LM (2013) AMB Express 3:1
Posch AE, Herwig C (2014) Biotechnol Prog 30:689
Edelstein L, Hadar Y (1983) J Theor Biol 105:427
Aynsley M, Ward AC, Wright AR (1990) Biotechnol Bioeng 35:820
Tough AJ, Pulham J, Prosser JI (1995) Biotechnol Bioeng 46:561
van Suijdam JC, Metz B (1981) Biotechnol Bioeng 23:111
Metz B, de Bruijn EW, van Suijdam JC (1981) Biotechnol Bioeng 23:149
Papagianni M (2014) J Microb Biochem Technol 6:189
Blott SJ, Pye K (2008) Sedimentology 55:31
Obert M, Pfeifer P, Sernetz M (1990) J Bacteriol 172:1180
Mandelbrot BB (1982) The fractal geometry of nature. W. H. Freeman and Co., New York
Smith TG, Lange GD, Marks WB (1996) J Neurosci Meth 69:123
Jones CL, Lonergan GT (1997) Biotechnol Lett 19:65
Barry DJ (2009) ISAST Trans Electron Signal Process 4:71
Barry DJ (2013) Biotechnol Bioeng 110:437
Finkler L, Ginoris Y, Luna C, Alves T, Pinto J, Coelho M (2007) World J Microb Biot 23:801
Wucherpfennig T (2013) Cellular morphology: a novel process parameter for the cultivation of eukaryotic cells. Cuvillier Verlag, Göttingen
Schügerl K, Gerlach SR, Siedenberg D (1998) Adv Biochem Eng Biotechnol 60:195
Kossen NW (2000) Adv Biochem Eng Biotechnol 70:1
Charles M (1978) In: Ghose TK, Fiechter A, Blakebrough N (eds) Advances in Biochemical Engineering/Biotechnology, vol 8. Springer, Berlin, pp 1–62
Olsvik ES, Kristiansen B (1992) Biotechnol Bioeng 40:1293
Lucatero S, Larralde-Corona CP, Corkidi G, Galindo E (2003) Biotechnol Prog 19:285
Tucker KG (1994) Relationship between mycelial morphology biomass concentration and broth rheology in submerged fermentations. University of Birmingham, Birmingham
Tucker KG, Thomas CR (1993) Trans Inst Chem Eng 71:111
Riley GL, Thomas CR (2010) Biotechnol Lett 32:1623
Das RK, Brar SK (2014) Appl Biochem Biotechnol 172:2974
Teixeira JA, Correa TLR, de Queiroz MV, de Araujo EF (2014) J Basic Microbiol 54:133
König B, Seewald C, Schügerl K (1981) Eur J Appl Microbiol Biotechnol 12:205
Smith JJ, Lilly MD, Fox RI (1990) Biotechnol Bioeng 35:1011
Ujcová E, Fencl Z, Musílková M, Seichert L (1980) Biotechnol Bioeng 22:237
Gomez R, Schnabel I, Garrido J (1988) Enzyme Microb Technol 10:188
Yao L-Y, Zhu Y-X, Jiao R-H, Lu Y-H, Tan R-X (2014) Bioresour Technol 159:112
Sainz-Herran N, Casas-Lopez JL, Sanchez-Perez JA, Chisti Y (2008) J Chem Technol Biotechnol 83:593
Wang F, Ma A-Z, Guo C, Zhuang G-Q, Liu C-Z (2013) Ultrason Sonochem 20:118
Elmayergi H, Scharer JM, Moo-Young M (1973) Biotechnol Bioeng 15:845
Dobson LF, O’Shea DG (2008) Appl Microbiol Biotechnol 81:119
Bobowicz-Lassociska T, Grajek W (1995) Acta Biotechnol 15:277
Fiedurek J (1998) J Basic Microbiol 38:107
Ruohang W, Webb C (1995) Biotechnol Tech 9:55
Clark DS, Ito K, Horitsu H (1966) Biotech Bioeng 8:465
Schügerl K, Wittler R, Lorenz T (1983) Trends Biotechnol 1:120
Gonciarz J, Bizukojc M (2014) Eng Life Sci 14:190
Etschmann MMW, Huth I, Walisko R, Schuster J, Krull R, Holtmann D, Wittmann C, Schrader J (2014) Yeast. doi:10.1002/yea.3022
Gao D, Zeng J, Yu X, Dong T, Chen S (2014) Biotechnol Bioeng 111:1758
Smith DJ, Payton MA (1994) Mol Cell Biol 14:6030
Tinsley JH, Lee IH, Minke PF, Plamann M (1998) Mol Gen Genet 259:601
Brunt SA, Borkar M, Silver JC (1998) Fungal Genet Biol 24:310
Sone T, Griffiths AJF (1999) Fungal Genet Biol 28:227
Veses V, Casanova M, Murgui A, Domínguez Á, Gow NAR, Martínez JP (2005) Eukaryot Cell 4:1088
Borgia PT, Iartchouk N, Riggle PJ, Winter KR, Koltin Y, Bulawa CE (1996) Fungal Genet Biol 20:193
Goh J, Kim KS, Park J, Jeon J, Park S-Y, Lee Y-H (2011) Fungal Genet Biol 48:784
Ichinomiya M, Motoyama T, Fujiwara M, Takagi M, Horiuchi H, Ohta A (2002) Microbiol 148:1335
Lettner T, Zeidler U, Gimona M, Hauser M, Breitenbach M, Bito A (2010) PLoS ONE 5:e11993
Wang G, Wang C, Hou R, Zhou X, Li G, Zhang S, Xu JR (2012) PLoS ONE 7:e38324
Zhao P-B, Ren A-Z, Xu H-J, Li D-C (2010) J Microbiol Biotechnol 20:208
Zhong YH, Wang TH, Wang XL, Zhang GT, Yu HN (2009) Fungal Genet Biol 46:255
Cai M, Zhang Y, Hu W, Shen W, Yu Z, Zhou W, Jiang T, Zhou X, Zhang Y (2014) Microb Cell Factories 13:73
Hoff B, Kamerewerd J, Sigl C, Mitterbauer R, Zadra I, Kurnsteiner H, Kuck U (2010) Eukaryot Cell 9:1236
Kamerewerd J, Zadra I, Kürnsteiner H, Kück U (2011) Microbiol 157:3036
Jonczyk P, Takenberg M, Hartwig S, Beutel S, Berger RG, Scheper T (2013) J Biotechnol 167:370
Cao J, Zhang HJ, Xu CPJ (2014) J Taiwan Inst Chem Eng 45:2075
Ohnishi Y, Yamazaki H, Kato J-Y, Tomono A, Horinouchi S (2005) Biosci Biotechnol Biochem 69:431
Bibb MJ, Buttner KF, Chater KF, Hopwood DA (eds) (2000) Practical streptomyces genetics. John Innes Foundation, Norwich
Hardisson C, Manzanal MB, Salas JA, Suárez JE (1978) J Gen Microbiol 105:203
Hirsch CF, Ensign JC (1976) J Bacteriol 126:13
Hodgson DA (1992) Prokaryotic structure and function. Cambridge University Press, Cambridge, pp 407–440
Flärdh K (2003) Curr Opin Microbiol 6:564
Manteca A (2005) Microbiol 151:3689
Manteca A, Alvarez R, Salazar N, Yague P, Sanchez J (2008) Appl Environ Microbiol 74:3877
Yagüe P, López-García MT, Rioseras B, Sánchez J, Manteca A (2013) FEMS Microbiol Lett 342:79
Hopwood DA, Glauert AM (1961) J Gen Microbiol 26:325
Wildermuth H (1972) Arch Mikrobiol Arch 81:309
Flärdh K, Buttner MJ (2009) Nat Rev Microbiol 7:36
Miguélez EM, Hardisson C, Manzanal MB (1999) J Cell Biol 145:515
Chater KF, Merrick MJ (1979) Devolpment biology of prokaryotes. In: Parish JH (ed) Studies in Microbiology, vol 1. University of California Press, Berkeley, pp 93–114
Daza A, Martín JF, Dominguez A, Gil JA (1989) J Gen Microbiol 135:2483
Denser Pamboukian CR, Guimarães LM, Facciotti MCR, Braz J (2002) J Microbiol 33:17
Huang J (2001) Genes Dev 15:3183
Neumann T, Piepersberg W, Distler J (1996) Microbiol 142:1953
Kendrick KE, Ensign JC (1983) J Bacteriol 155:357
Glazebrook MA, Doull JL, Stuttard C, Vining LC (1990) J Gen Microbiol 136:581
Kuimova TF, Soina VS (1981) Hindustan Antibiot Bull 23:1
Novella IS, Barbés C, Sánchez J (1992) Can J Microbiol 38:769
Rho YT, Lee KJ (1994) Microbiol Read Engl 140(Pt 8):2061
Rueda B, Miguélez EM, Hardisson C, Manzanal MB (2001) Can J Microbiol 47:1042
Hopwood DA (2007) Streptomyces in nature and medicine: the antibiotic makers. Oxford University Press, Oxford, New York
Yang YK, Morikawa M, Shimizu H, Shioya S, Suga K-I, Nihira T, Yamada Y (1996) J Ferment Bioeng 81:7
Tresner HD, Hayes JA, Backus EJ (1967) Appl Microbiol 15:1185
Vecht-Lifshitz SE, Magdassi S, Braun S (1990) Biotechnol Bioeng 35:890
O’Cleirigh C, Casey JT, Walsh PK, O’Shea DG (2005) Appl Microbiol Biotechnol 68:305
Belmar-Beiny MT, Thomas CR (1991) Biotechnol Bioeng 37:456
Treskatis SK, Orgeldinger V, Wolf H, Gilles ED (1997) Biotechnol Bioeng 53:191
Tamura S, Park Y, Toriyama M, Okabe M (1997) J Ferment Bioeng 83:523
Pinto LS, Vieira LM, Pons MN, Fonseca MMR, Menezes JC (2004) Bioprocess Biosyst Eng 26:177
van Veluw GJ, Petrus MLC, Gubbens J, de Graaf R, de Jong IP, van Wezel GP, Wösten HAB, Claessen D (2012) Appl Microbiol Biotechnol 96:1301
Kim Y-M, Kim J-H (2004) J Microbiol 42:64
Xu H, Chater KF, Deng Z, Tao M (2008) J Bacteriol 190:4971
Whitaker A (1992) Appl Biochem Biotechnol 32:23
Junker BH, Hesse M, Burgess B, Masurekar P, Connors N, Seeley A (2004) Appl Biochem Biotechnol 119:241
Pamboukian CRD, Facciotti MCR (2004) Process Biochem 39:2249
Jonsbu E, McIntyre M, Nielsen J (2002) J Biotechnol 95:133
Dobson LF, O’Cleirigh C, O’Shea DG (2008) Appl Microbiol Biotechnol 79:859
Gamboa-Suasnavart RA, Valdez-Cruz NA, Cordova-Dávalos LE, Martínez-Sotelo JA, Servín-González L, Espitia C, Trujillo-Roldán MA (2011) Microb Cell Factories 10:110
Vecht-Lifshitz SE, Sasson Y, Braun S (1992) J Appl Bacteriol 72:195
Yin P, Wang Y-H, Zhang S-L, Chu J, Zhuang Y-P, Chen N, Li X-F, Wu Y-B (2008) J Chin Inst Chem Eng, 39:609
Tough AJ, Prosser JI (1996) Microbiol 142:639
Bellgardt KH (1998) Adv Biochem Eng Biotechnol 60:153
Lin P-J, Scholz A, Krull R (2010) Biochem Eng J 49:213
Papagianni M, Mattey M, Berovǐ M, Kristiansen B (1999) Food Technol Biotechnol 37:165
Glazebrook MA, Vining LC, White RL (1992) Can J Microbiol 38:98
El-Enshasy HA, Farid MA, El-Sayed A (2000) J Basic Microbiol 40:333
O’Cleirigh C, Walsh PK, O’Shea DG (2003) Biotechnol Lett 25:1677
Tucker KG, Thomas CR (1992) Biotechnol Lett 14:1071
Ruiz B, Chávez A, Forero A, García-Huante Y, Romero A, Sánchez M, Rocha D, Sánchez B, Rodríguez-Sanoja R, Sánchez S, Langley E (2010) Crit Rev Microbiol 36:146
Sánchez S, Chávez A, Forero A, García-Huante Y, Romero A, Sánchez M, Rocha D, Sánchez B, Valos M, Guzmán-Trampe S, Rodríguez-Sanoja R, Langley E, Ruiz B (2010) J Antibiot (Tokyo) 63:442
Šťastná J, Čáslavská J, Wolf A, Vinter V, Mikulík K (1977) Folia Microbiol (Praha) 22:339
Choi DB, Park EY, Okabe M (1998) J Ferment Bioeng 86:413
Choi DB, Park EY, Okabe M (2000) Biotechnol Prog 16:525
Okba AK, Ogata T, Matsubara H, Matsuo S, Doi K, Ogata S (1998) J Ferment Bioeng 86:28
Vecht-Lifshitz SE, Magdassi S, Braun S (1989) J Dispers Sci Technol 10:265
Hobbs G, Brown CM, Gardner DCJ, Cullum JA, Oliver SG (1989) Appl Microbiol Biotechnol 31:272
Winn M, Casey E, Habimana O, Murphy CD (2014) FEMS Microbiol Lett 352:157
Sohoni S, Bapat P, Lantz A (2012) Microb Cell Factories 11:9
Chater KF (1972) J Gen Microbiol 72:9
Horinouchi S, Beppu T (1992) Annu Rev Microbiol 46:377
Khokhlov AS, Tovarova II, Borisova LN, Pliner SA, Shevchenko LN, Kornitskaia EI, Ivkina NS, Rapoport IA (1967) Dokl Akad Nauk SSSR 177:232
Merrick MJ (1976) J Gen Microbiol 96:299
Lawlor EJ, Baylis HA, Chater KF (1987) Genes Dev 1:1305
Chater KF, Chandra G (2008) J Microbiol Seoul Korea 46:1
McCormick JR, Flärdh K (2012) FEMS Microbiol Rev 36:206
Reichl U, King R, Gilles ED (1992) Biotechnol Bioeng 39:164
Pearson AP, Glennon B, Kieran PM (2004) J Chem Technol Biotechnol 79:1142
Park Y, Tamura S, Koike Y, Toriyama M, Okabe M (1997) J Ferment Bioeng 84:483
Petersen N, Stocks S, Gernaey KV (2008) Biotechnol Bioeng 100:61
Rønnest NP, Stocks SM, Lantz AE, Gernaey KV (2012) Biotechnol Lett 34:1465
Wucherpfennig T, Schilling JV, Sieblitz D, Pump M, Schütte K, Wittmann C, Krull R (2012) Eng Life Sci 12:595
Pearson AP, Glennon B, Kieran PM (2003) Biotechnol Progr 19:1342
Grimm LH, Kelly S, Hengstler J, Göbel A, Krull R, Hempel DC (2004) Biotechnol Bioeng 87:213
Grimm LH, Kelly S, Völkerding II, Krull R, Hempel DC (2005) Biotechnol Bioeng 92:879
Acknowledgments
The authors gratefully acknowledge financial support from Alliance Industrial Research within the project Micro-particle based cultivation of filamentous fungi (No. 16926/N, R. Walisko); the Lower Saxony Ministry for Science and Culture for the PhD scholarships in the Graduate Schools Novel synthesis and formulation methods for poorly soluble drugs and sensitive biopharmaceuticals (SynFoBia) (J. Moench-Tegeder) within the Center for Pharmaceutical Process Engineering (PVZ) and Microbial Natural Products (MINAS) (J. Blotenberg) at the Technische Universität Braunschweig, Germany.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Walisko, R., Moench-Tegeder, J., Blotenberg, J., Wucherpfennig, T., Krull, R. (2015). The Taming of the Shrew - Controlling the Morphology of Filamentous Eukaryotic and Prokaryotic Microorganisms. In: Krull, R., Bley, T. (eds) Filaments in Bioprocesses. Advances in Biochemical Engineering/Biotechnology, vol 149. Springer, Cham. https://doi.org/10.1007/10_2015_322
Download citation
DOI: https://doi.org/10.1007/10_2015_322
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20510-6
Online ISBN: 978-3-319-20511-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)