Abstract
The paper is concerned with the results of 137Cs monitoring in the irrigation ponds of the Okuma town in the Fukushima Dai-ichi Nuclear Power Plant (FDNPP) exclusion zone. The 137Cs activity concentrations in the ponds appeared to be higher than those in the rivers and dam reservoirs in the region. The study has revealed a trend for a decline in 137Cs activity concentrations, both particulate and dissolved. The rate of particulate 137Cs decline was much higher than that of dissolved. The total distribution coefficient \(K_\mathrm{d}(^{137}\mathrm{Cs}\)) in the suspended sediment–water system in the studied ponds was decreasing in time with the rate constant of 0.12–0.18 year–1. Assuming that the decrease in \(K_\mathrm{d}\) is associated with decomposition of hot glassy particles, the time scale of 137Cs leaching from them in these water bodies was estimated to be 5–8 years. These estimates are consistent with the findings of recent laboratory experiments on the subject. With respect to seasonal variations, the highest levels of dissolved 137Cs in the studied ponds were observed from June to October as a function of specific pond and monitoring year. Based on data about 137Cs speciation in the bottom sediment top layer of the ponds and its distribution in the sediment–water system, the exchangeable radiocesium interception potential \(\mathit{RIP}^\mathrm{ex}(K)\) for the ponds sediments was estimated to be 1650–2250 mg-eq/kg, which is within the range of values measured by laboratory studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The Fukushima Dai-ichi Nuclear Power Plant (FDNPP) accident caused by the tsunami following the Great East Japan Earthquake in March 2011 resulted in the contamination of extensive territories on the Honshu Island (Japan) by radioactive cesium isotopes 134Cs (half-life \(T_{1/2}= 2.06\) years) and 137Cs (\(T_{1 / 2 }= 30.17\) years), which rekindled the interest in the behavior of radiocesium in specific geoclimatic conditions of Japan. Radiocesium was deposited northwest of the NPP, forming a footprint about 20 km wide and 50–70 km long [9, 12, 18] and leading to the contamination of both terrestrial and water ecosystems. The initial ratio of cesium isotopes 134Cs/ 137Cs in the Fukushima fallout was about unity [12]. With time, the contribution of 134Cs to radiation contamination was decreasing as compared to 137Cs due to faster decay, and as of today the main radionuclide of dose significance is 137Cs.
After the Chernobyl NPP (ChNPP) accident, closed water bodies such as lakes, ponds and stagnant reservoirs were found to be more sensitive to radioactive contamination [3, 6, 16]. On the territory of Fukushima prefecture there are more than 3700 ponds of varying size, many of which are used for paddy water supply. Irrigation ponds were created over the course of centuries in Japan for rice cultivation. These ponds are also a concern because they are used for fishing and watering of agricultural fields, which can cause crop contamination [11, 24, 26].
The purpose of the present paper is to study the distribution of Fukushima-derived radiocesium and its dynamics in closed and semi-closed ponds of the FDNPP exclusion zone and to compare with the radionuclide behavior in similar objects in the ChNPP zone.
MATERIALS AND METHODS
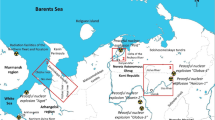
The total of 137 small and medium-size ponds occur in the Okuma town in the FDNPP 10-km exclusion zone. Of them, three ponds Inkyozaka, Suzuuchi and Funazawa were selected as objects for study (Fig. 1). Here are the characteristics of the considered ponds in the FDNPP exclusion zone:
...
Pond | Inkyozaka | Suzuuchi | Funazawa |
Northern latitude | \(37^\circ25.499'\) | \(37^\circ24.950'\) | \(37^\circ24.363'\) |
Eastern longitude | \(141^\circ01.05'\) | \(140^\circ58.791'\) | \(140^\circ59.173'\) |
Distance from FDNPP, km | 0.24 | 3.75 | 3.5 |
137Cs deposition, kBq/m2 | \(2250 \pm 1350\) | \(6850 \pm 1870\) | \(2370 \pm 495\) |
Water surface area, m2 | 6500 | 4100 | 10700 |
Maximum depth, m | 2.0 | 1.0 | 2.5 |
The selected ponds represent the main pond types in the exclusion zone, where the 137Cs deposition is varying from 2 to 7 MBq/m2 [4, 21, 22]. Water sampling from the ponds was conducted once a month in 2015–2017, and at least once every two months in 2018–2019. Water samples of 4 l were collected from the surface layer and then filtered through 0.45 \(\mu\)m membrane filters in laboratory. The activity concentrations of 134Cs and 137Cs were determined in filtrate and on suspended material separately by gamma spectrometry using the high-purity germanium detector (HPGe) CANBERRA GC3018. The concentrations of the main cations K+, NH\(_4^+\), Na+, Ca2+, and Mg2+ were determined by the ion chromatography using Thermo Scientific DIONEX 1100. The stable \(^{133}\)Cs was determined by the inductively coupled plasma spectrometry ICP-MS (Perkin Elmer ELAN DRC 2).
RESULTS AND DISCUSSION
Figure 2 shows the time dependence of the mean annual particulate and dissolved 137Cs activity concentrations from 2015 to 2019 in the ponds Inkyozaka, Suzuuchi, and Funazawa. In all three ponds, the dissolved and particulate 137Cs activity concentrations were decreasing. Table 1 includes the quantitative characteristics of 137Cs decline in the Okuma ponds. Here \(\lambda_\mathrm{ef}\) is the effective rate constant for decline in 137Cs activity concentration; \(\lambda_\mathrm{env}\) is the rate constant for decline in 137Cs activity concentration due to natural attenuation processes: \(\lambda _\mathrm{env}=\lambda _\mathrm{ef} -\lambda\ \), where \(\lambda\ \) is the rate constant for 137Cs decay equal to 0.023 year-1; \(T_{1 / 2 \mathrm{env}}\) is the 137Cs concentration half-reduction due to natural attenuation processes.
It follows from Fig. 2 and Table 1 that the particulate 137Cs activity concentration is declining faster than the dissolved 137Cs. The partitioning of the radionuclide between the sediment and solution is described by the distribution coefficient \(K_\mathrm{d}\) (l/kg) equal to the ratio of the particulate radionuclide activity concentration (Bq/kg) to its dissolved activity concentration (Bq/l) at equilibrium [15]. For research purposes the notions of the total distribution coefficient \(K_\mathrm{d}\) and the exchangeable distribution coefficient \(K_\mathrm{d}^\mathrm{ex}\) are used [15]. \(K_\mathrm{d}\) is equal to the ratio of the total activity concentration of the radionuclide in the solid phase to its activity concentration in solution, whereas \(K_\mathrm{d}^\mathrm{ex}\) is equal to the ratio of the exchangeable radionuclide activity concentration in the solid phase to its activity concentration in solution. The advantage of the exchangeable distribution coefficient is that its value can be calculated easily based on data about sorption capacity of the solid phase and water cation composition [2, 15].
A faster reduction in the particulate 137Cs concentration, as compared to the dissolved one, is expected to result in a decrease of the \(K_\mathrm{d}\) value for the given time period, which is corroborated by Fig. 3 showing time dependence of the 137Cs total distribution coefficient in the ponds. The rate constants of \(K_\mathrm{d}\) decline for the ponds under study are in the range 0.12–0.18 year–1.
Figure 4 illustrates seasonal variations of dissolved 137Cs concentration in the FDNPP exclusion zone ponds for 2016 and 2017. Obviously, 137Cs concentrations grow significantly in the summer–early autumn. In the ponds Inkyozaka and Funazawa the highest concentrations were observed from July to October, while in the pond Suzuuchi the highest concentration of 137Cs occurred in June.
Table 2 includes results of determining the fraction of 137Cs exchangeable forms in the 3-cm top sediment layer in comparison with similar data for soils on the catchments [7, 8, 22]. As seen, soils are characterized by extremely low values of exchangeable 137Cs fraction, even compared with Chernobyl data. At the same time, the bottom sediment top layer formed as a result of sedimentation, is characterized by the high fraction of exchangeable 137Cs (up to 40% in Inkyozaka).
The above data about 137Cs speciation allow us to calculate the total (\(K_\mathrm{d}\)) and exchangeable (\(K_\mathrm{d}^\mathrm{ex}\)) distribution coefficients [2] in Okuma ponds (Table 2), and on this basis to calculate Radiocesium Interception Potential RIP \(^\mathrm{ex}\) in accordance with equation [5]:
where \([\mathrm{K}^+]\) and \([\mathrm{NH}_4^+]\) are potassium and ammonium concentrations in mg-eq/l.
On the territories contaminated after the Chernobyl accident, the values of the radiocesium distribution coefficient in the closed lakes and ponds were much lower than those reported for the large open lakes [3, 6, 16]. For example, in the closed lake Svyatoe in the Bryansk oblast (Russia) in 1993–1995, 7–9 years after the Chernobyl accident \(K_\mathrm{d}\) was \((3.5 \pm 0.7)\times 10^3\) l/kg [3]. Similar values of \(K_\mathrm{d}\) (\(8.1\times 10^3\) l/kg) were reported for the closed lake Vorsee in Baden-Wurttemberg (Germany) in the same time period [16]. On the other hand, in large open Lake Constance (Germany/Austria/Switzerland) having high self-purification capacity with respect to radiocesium the \(K_\mathrm{d}(^{137}\)Cs), values were \((1{-}10)\times 10^5\) l/kg [16], which is close to \(K_\mathrm{d}(^{137}\mathrm{Cs})\) in the FDNPP zone water bodies.
The \(K_\mathrm{d}\) values in the studied ponds of Okuma (\({>}10^5\) l/kg) appeared to be about two orders of magnitude higher than those in closed lakes Svyatoe and Vorsee, and close to the values in Lake Constance [16], as well as the rivers and dam reservoirs of the FDNPP contaminated area [25]. In our view, this can be attributed to two reasons. The first is associated with the occurrence of insoluble glassy hot particles in the Fukushima fallout [13] and their higher content in the vicinity of the FDNPP. The second reason consists in the high binding ability of soils sediments in the Fukushima contaminated area (Table 2). The obtained high estimates of \(\mathit{RIP}^\mathrm{ex}(K)\) showing the ability of sediment particles to adsorb 137Cs selectively are consistent with the values measured in laboratory experiments [17, 23] for the Fukushima area soils. The sediments of the studied ponds in terms of their ability to adsorb radiocesium selectively are even superior to those of Lake Constance in Europe [16].
The trend for a decline in the total distribution coefficient \(K_\mathrm{d}\) reported for all three studied ponds (Fig. 3) can be due to 137Cs leaching from glassy hot particles the proportion of which in the near zone of FDNPP can be as high as 30% [14]. In this respect, the rate constant of \(K_\mathrm{d}\) decline in the ponds is a characteristic of weathering of glassy hot particles. Then the time scale of 137Cs leaching from particles (the value reverse to the rate constant) is estimated to be 5–8 years. After the limiting stage of 137Cs leaching from a glassy hot particle as a result of its decomposition or weathering, radiocesium gets fixed quite quickly by micaceous clay minerals with the time scale of 2–3 months. The derived estimates are in agreement with the outcomes of laboratory experiments to study radiocesium leaching from glassy hot particles in various salt solutions [19].
CONCLUSIONS
The studies of 137Cs behavior in the irrigation ponds of the city Okuma in the vicinity of FDNPP conducted in 2015–2019 have demonstrated that the concentrations of this radionuclide in the ponds are higher than those in the rivers and dam reservoirs of the region. The highest levels of dissolved 137Cs in the studied ponds were observed from June to October as a function of pond and monitoring year.
A trend for decline in both particulate and dissolved 137Cs activity concentrations was revealed. The reduction rate of the particulate 137Cs activity concentrations was notably higher than of the dissolved one.
This manifested itself in the trend for decline of the total distribution coefficient \(K_\mathrm{d}(^{137}\mathrm{Cs})\) in the sediment–water system with the rate constant 0.12–0.18 year–1. Assuming that the decrease in \(K_\mathrm{d}\) is associated with decomposition of hot glassy particles, the time scale of 137Cs leaching from them in the ponds under study is estimated to be 5–8 years. The obtained estimates are consistent with the findings of recent laboratory experiments on the subject.
In absolute magnitude, the value of \(K_\mathrm{d}(^{137}\mathrm{Cs})\) in the studied ponds appeared to be much higher than the corresponding values in the closed lakes of the Chernobyl contaminated area and comparable to the values characteristic of the rivers and reservoirs of the FDNPP contaminated area.
Based on the data on the 137Cs speciation in the bottom sediment top layer of the ponds and its distribution in the sediment–water system, the exchangeable radiocesium interception potential \(\mathit{RIP}^\mathrm{ex}(K)\) for the ponds sediments was estimated to be 1.7–2.3 g-eq/kg, which is within the range of values measured in laboratory.
FUNDING
The study was supported by the Japan Society for the Promotion of Science (JSPS), project number 18H03389.
REFERENCES
A V. Konoplev, “Distribution of Radiocesium of Accidental Origin between the Suspended Matter and Solution in Rivers: Comparison of Fukushima and Chernobyl” Radiochemistry, No. 5, 57 (2015).
A. V. Konoplev and A. A. Bulgakov, “90Sr and 137Cs Exchange Distribution Coefficient in Soil–Water Systems,” Atomic Energy, No. 2, 88 (2000).
A. V. Konoplev, A. A. Bulgakov, V. G. Zhirnov, Ts. I. Bobovnikova, I. V. Kutnyakov, A. A. Siverina, V. E. Popov, and E. P. Virchenko, “Study of the 137Cs and 90Sr Behavior in Lakes Svyatoe and Kozhanovskoe, Bryansk Region,” Meteorol. Gidrol., No. 11 (1998) [Russ. Meteorol. Hydrol., No. 11 (1998)]
A. V. Konoplev, Y. Wakiyama, T. Wada, V. N. Golosov, K. Nanba, and T. Takase, “Radiocesium in Ponds in the Near Zone of Fukushima Dai-ichi NPP,” Water Res., No. 4, 45 (2018).
A. V. Konoplev and I. V. Konopleva, “Characteristics of Steady-state Selective Sorption of Radiocesium on Soils and Floor Sediments,” Geochemistry International, No. 2, 37 (1999).
A. V. Konoplev, L. P. Kopylova, Ts. I. Bobovnikova, A. A. Bulgakov, and A. A. Siverina, “Distribution of 90Sr and 137Cs within the System of Bottom Sediments–Water of the Reservoir in the Areas Adjacent to the Chernobyl NPP,” Sov. Meteorol. Hydrol., No. 1 (1992) [in Russian].
I. Byrnes, Radiocesium Dynamics in Irrigation Ponds in Okuma, Japan, Thesis for the Degree of Master of Science (Colorado State University, Fort Collins, 2017).
M. Carradine, Vertical Distribution of Radiocesium in Soil Deposits on the Contaminated Areas after the Fukushima Dai-ichi Nuclear Power Plant Aqccident, Thesis for the Degree of Master of Science (Colorado State University, Fort Collins, 2017).
M. Chino, H. Nakayama, H. Nagai, H. Terada, G. Katata, and H. Yamazawa, “Preliminary Estimation of Release Amounts of 131I and 137Cs Accidentally Discharged from the Fukushima Daiichi Nuclear Power Plant into the Atmosphere” J. Nucl. Sci. Technol., 48 (2011).
R. N. J. Comans, J. J. Middelburg, J. Zonderhuis, J. R. W. Woittiez, G. J. De Lange, H. A. Das, and C. H. Van der Weijden, “Mobilization of Radiocaesium in Pore Water of Lake Sediments,” Nature, 339 (1989).
T. Fukushima and H. Arai, “Radiocesium Contamination of Lake Sediments and Fish Following the Fukushima Nuclear Accident and Their Partition Coefficient,” Inland Waters, 4 (2014).
K. Hirose, “2011 Fukushima Dai-ichi Nuclear Power Plant Accident: Summary of Regional Radioactive Deposition Monitoring Results,” J. Environ. Radioact., 111 (2012).
Y. Igarashi, T. Kogure, Y. Kurihara, H. Miura, T. Okumura, Y. Satou, Y. Takahashi, and N. Yamaguchi,” A Review of Cs-bearing Microparticles in the Environment Emitted by the Fukushima Dai-ichi Nuclear Power Plant Accident,” J. Environ. Radioact., 205–206 (2019).
R. Ikehara, M. Suetake, T. Komiya, G. Furuki, A. Ochiai, S. Yamasaki, W. R. Bower, G. T. W. Law, T. Ohnuki, B. Grambow, R. C. Ewing, and S. Utsunomiya, “Novel Method of Quantifying Cesium-rich Microparticles (CsMPs) in the Environment from the Fukushima Daiichi Nuclear Power Plant,” Environ. Sci. Technol., 52 (2018).
A. Konoplev, “Mobility and Bioavailability of Chernobyl-derived Radionuclides in Soil-water Environment: Review,” in Behavior of Radionuclides in the Environment II: Chernobyl, Ed. by A. Konoplev, K. Kato, and S. N. Kalmykov (SPRINGER Nature, 2020).
A. Konoplev, S. Kaminski, E. Klemt, I. Konopleva, R. Miller, and G. Zibold, “Comparative Study of 137Cs Partitioning between Solid and Liquid Phases in Lakes Constance, Lugano, and Vorsee,” J. Environ. Radioact., 58 (2002).
A. Nakao, S. Ogasawara, O. Sano, T. Ito, and J. Yanai, “Radiocesium Sorption in Relation to Clay Mineralogy of Paddy Soils in Fukushima, Japan,” Sci. Total Environ., 468–469 (2014).
MEXT (Ministry of Education, Culture, Sports, Science and Technology of Japan). Results of the (i) Fifth Airborne Monitoring Survey and (ii) Airborne Monitoring Survey Outside 80 km from the Fukushima Dai-ichi NPP (Tokyo, 2012) (http://radioactivity.nsr.go.jp/en/contents/6000/5790/24/ 203_0928 14e.pdf).
T. Okumura, N. Yamaguchi, T. Dohi, K. Iijima, and T. Kogure, “Dissolution Behavior of Radiocesium-bearing Microparticles Released from the Fukushima Nuclear Plant,” Sci. Reports, 9 (2019).
K. Saito, I. Tanihata, M. Fujiwara, T. Saito, S. Shimoura, T. Otsuka, Y. Onda, M. Hoshi, Y. Ikeuchi, F. Takahashi, N. Kinouchi, J. Saegusa, H. Takemiya, and T. Shibata, “Detailed Deposition Density Maps Constructed by Large Scale Soil Sampling for Gamma-ray Emitting Radioactive Nuclides from the Fukushima Dai-ichi Nuclear Power Plant Accident,” J. Environ. Radioact., 139 (2015).
Y. Wakiyama, A. Konoplev, T. Wada, T. Takase, I. Byrnes, M. Carradine, and K. Nanba, “Behavior of 137Cs in Ponds in the Vicinity of the Fukushima Dai-ichi Nuclear Power Plant,” J. Environ. Radioact., 178–179 (2017)
Y. Wakiyama, A. Konoplev, T. Wada, T. Takase, Y. Igarashi, K. Nanba, and I. Byrnes, “Temporal Trends of 137Cs Activity Concentration in Pond Waters in the Vicinity of Fukushima Dai-ichi Nuclear Power Plant,” Proc. IAHS, 381 (2019).
N. Yamaguchi, H. Tsukada, K. Kohyama, Y. Takata, A. Takeda, S. Isono, and I. Taniyama, “Radiocesium Interception Potential of Agricultural Soils in Northeast Japan,” Soil Sci. Plant Nutr., No. 2, 63 (2017).
N. Yoshikawa, H. Obara, M. Ogasa, S. Miyazu, N. Harada, and M. Nonaka, “ 137Cs in Irrigation Water and Its Effect on Paddy Fields in Japan after the Fukushima Nuclear Accident,” Sci. Total Environ., 481 (2014).
K. Yoshimura, Y. Onda, A. Sakaguchi, M. Yamamoto, and Y. Matsuura, “An Extensive Study of the Concentrations of Particulate/Dissolved Radiocaesium Derived from the Fukushima Dai-ichi Nuclear Power Plant Accident in Various River Systems and Their Relationship with Catchment Inventory,” J. Environ. Radioact., 139 (2015).
M. Yoshimura and T. Yokoduka, “Radioactive Contamination of Fishes in Lake and Streams Impacted by the Fukushima Nuclear Power Plant Accident,” Sci. Total Environ., 482–483 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Meteorologiya i Gidrologiya, 2021, No. 5, pp. 38-45. https://doi.org/10.52002/0130-2906-2021-5-38-45.
About this article
Cite this article
Konoplev, A.V., Wakiyama, Y., Wada, T. et al. Transformation of Radiocesium Speciation in Ponds at the Vicinity of Fukushima Dai-ichi Nuclear Power Plant and Dynamics of Its Distribution in Sediment–Water System. Russ. Meteorol. Hydrol. 46, 312–318 (2021). https://doi.org/10.3103/S1068373921050058
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068373921050058