Abstract
The use of coal pitch as a binder in anode production for the aluminum industry is an outdated approach. Such pitch has a high content of carcinogens, polycyclic aromatic hydrocarbons, and tar phenols, and its environmental impact is unacceptable. A possible alternative binder, which is free of these problems, is environmentally benign petroleum pitch from heavy petroleum residues. Current petroleum pitch must meet requirements on factors such as its physicochemical parameters, production characteristics, and the conditions of transportation and storage. Environmental constraints are especially important on account of current global conditions. These requirements are met by a new product: petroleum pitch for the production of anode mass. This pitch has similar properties to coal pitch of grades A and B1. In the present study, it is investigated by up-to-date physicochemical methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Attempts to obtain industrial batches of electrode pitch in Russia ended unsuccessfully, for various reasons: a lack of appropriate industrial processes; imperfect technological systems; unconditioned petroleum derivatives; and miscommunication between purchasers and consumers [1–4]. Today, a high-quality, environmentally benign, alternative binder is needed for anode production.
For many years, researchers at the Irkutsk National Research Technical University, in collaboration with the Kataliz group of companies, have studied catalytic liquid-phase oxidative cracking, in which the residue is highly aromatic fuel oil with parameters similar to those of heavy pyrolytic tar in ethylene production (the best starting point for the production of petroleum pitch) [5, 6]. Catalytic liquid-phase oxidative cracking employs both homogeneous and heterogeneous catalysts (produced in-house), which are essential in order to obtain petroleum pitch with characteristics matching or surpassing those of coal pitch.
EXPERIMENTAL MATHODS AND EQUIPMENT
The best raw materials for the production of petroleum pitch are petroleum distillation residues of high density and aromatic content and low sulfur content. We use highly aromatic fuel oil from the process of catalytic liquid-phase oxidative cracking developed by the Kataliz-Proekt group. For the production of petroleum pitch, we consider heavy petroleum residues (highly aromatic fuel oil) from a system for catalytic liquid-phase oxidative cracking.
We introduce a special activating additive (up to 2%) at room temperature (20–25°C) in the highly aromatic fuel oil, with thorough homogenization for 10 min (Fig. 1). The resulting mixture is placed in an autoclave preheated to 80°C, which is sealed. Nitrogen is introduced, and the pressure is increased to 0.5 MPa. A thermal-regulation system controls the heating. The light vapor is captured through an upper valve and is condensed in a cooling system, where it is collected. The excess pressure in the autoclave is regulated by a valve and monitored by means of a manometer. After releasing the pressure, live steam is introduced, to ensure effective removal of the light components. The supply of steam (ml/min) is regulated as necessary. On reaching the oxidation temperature, air is supplied to the autoclave, and polycondensation occurs.
Laboratory apparatus for producing petroleum pitch: (1) air cylinder; (2) valve; (3) steam heater; (4) three-way valve; (5) autoclave; (6) manometer; (7) thermosensor; (8) lower valve; (9) upper valve; (10) autoclave jacket; (11) thermal regulation unit; (12) heat exchanger; (13) intake tank; (14) baffle; (15) gas meter.
Thermolysis of the initial petroleum derivative is studied in the sealed autoclave, with variation in the temperature (420–450°C), pressure (1–2 MPa), and duration of the process (heat treatment for 5–20 min at the solution stage). The α fraction (coke residue) is formed from the low-molecular fraction of the petroleum residue in the relatively high-molecular fraction. The reaction occurs at high speed, with gradual heating of the initial mixture.
Then the autoclave is slowly cooled, with continuous mixing, to 260°C. The cooled petroleum pitch is removed from the autoclave.
Practically no clear criteria for assessing petroleum residues as raw materials on the basis of laboratory analysis may be found in the literature on the production of petroleum pitch [7–12]. In order to produce pitch of high quality, we must find optimal means of improving the content of the α fraction in petroleum pitch without markedly changing the content of the α1 fraction or the softening temperature [13–16]. By stopping the process at any stage—that is, by regulating the conversion of heavy petroleum residues—we may obtain products with the required aromatic content and density and with specified content of tar, asphaltenes, carbenes, and carboids.
RESULTS AND DISCUSSION
The quality of the petroleum-pitch samples is assessed in terms of the RUSAL guidelines [11]. By standard methods, we determine the softening temperature, yield of volatiles, coke residue, sulfur content, sodium content, carcinogenic content, ash content, and content of toluene- and quinoline-insoluble materials (Table 1).
The experimental pitch samples are assessed in accordance with the characteristics of coal pitch (State Standard GOST 10200–83). We see in Table 1 that the petroleum-pitch samples lack benzo[a]pyrene; that is an environmental benefit. We also investigate the thermogravimetric characteristics of the petroleum-pitch samples and the qualitative composition of the gases liberated on heating in an oxidative atmosphere. The following methods are employed:
— synchronous thermal analysis: investigation of the thermal effects and mass change of the sample on heating and melting;
— mass spectrometric determination of the composition of the gaseous products in thermogravimetric analysis.
The STA 449 F1 Jupiter instrument is used for synchronous thermal analysis. The quantitative and qualitative composition of the gaseous products is monitored by means of the QMS 403 C Aeolos quadrupole mass spectrometer, with an electron-impact energy of 70 eV.
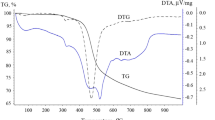
The thermogravimetric data (Figs. 2 and 3) indicate that the thermal decomposition of petroleum pitch in an oxidative atmosphere has three stages. At the end of the experiment, the petroleum-pitch sample has burned up completely.
In the first stage (325–415°C), we see a double endothermal effect with a peak at 396°C. The mass loss is 17.44%. This may be attributed to the rupture of weak chemical bonds to form low-molecular products, as confirmed by the mass spectra.
In the second stage (415–525°C), we note an exothermal effect. The mass loss is 18.10%. This may be attributed to the rupture of stronger chemical bonds to form low-molecular products, as confirmed by the mass spectra.
In the third stage (525–800°C), we also note an exothermal effect. The mass loss is 66.43%. This may be attributed to the rupture of strong chemical bonds (including C–C bonds) to form mainly CO2 and H2O, as confirmed by the mass spectra.
In the oxidation of petroleum-pitch samples at 290–800°C, sulfur-bearing compounds break down, releasing SO2. The quantity of SO2 formed is five moles or 0.15% of the sample mass. This process also occurs in three stages. We compare petroleum pitch and coal pitch by 1H and 13C NMR spectroscopy. From the 1H and 13C NMR spectra recorded on a Bruker DPX250 instrument, we obtain the distributions of hydrogen and carbon atoms in different hydrocarbon structures for petroleum pitch and coal pitch. We analyze the fraction soluble in CDCl3. Because of its complex chemical composition, pitch is usually characterized by the group composition of its fractions with different solubility in isooctane (petroleum ester), toluene, and quinoline.
In terms of selective solution, pitch may be divided into the following fractions.
1. The γ fraction is soluble in petroleum ester. These are aromatic and heterocyclic compounds. With increase in temperature, the γ fraction becomes an isotropic liquid and determines the steeping properties of pitch.
2. The β fraction is soluble in toluene but not in petroleum ester. These are high-molecular aromatic compounds and determine the binding properties of pitch.
3. The α fraction is insoluble in toluene and may be subdivided into two parts:
— the α2 fraction is soluble in quinoline but insoluble in toluene; the properties of the insoluble component are typical for crystalline graphite (coke);
— the α1 fraction is insoluble in quinoline. It has polymer properties, but with different temperature transitions. This set of high-molecular compounds determines the possibility of mesophase properties and is associated with the graphitizing properties of the pitch.
On the basis of the 1H and 13C NMR spectra, the pitch composition may be assessed without division into fractions. In combination with the accuracy of the method and the rate at which 1H NMR spectra are recorded, this permits rapid analysis.
Samples of petroleum pitch produced by catalytic liquid-phase oxidative cracking from different raw materials are investigated; pitch samples from two producers are compared. The samples are as follows: 1) petroleum pitch produced by catalytic liquid-phase oxidative cracking; 2) pitch from heavy tar obtained by pyrolysis in ethylene production (catalytic liquid-phase oxidative cracking); 3) coal pitch (Zaporozhe, Ukraine); 4) coal pitch (Russia). By analysis of the 13C NMR spectra, we determine the structural characteristics of the components in pitches 1–4. In particular, there are no signals in the range 150–200 ppm. That clearly shows the lack of carbonyl- and carboxyl-bearing fragments in the pitch structure. We may also note the absence of O-alkyl and O-aryl functional groups, corresponding to signals at 50–60 ppm and 145–160 ppm, respectively (Fig. 4).
For petroleum pitch 1, the 13C NMR spectrum contains intense signals at 10–30 ppm, corresponding to saturated linear aliphatic chains. According to the relative integral intensity of these signals, the length of such fragments will be 7–8 carbon atoms. The integral intensity of all the signals in the 13C NMR spectra is normalized to 100. For petroleum pitch samples 1 and 2, signals in the range 130–150 ppm are more pronounced than for coal pitch. This spectral range corresponds to resonances of quaternary atoms in aromatic structures directly linked to aliphatic fragments (Car–alkyl groups).
The 13C NMR spectra of coal pitch samples 3 and 4 are more clearly resolved. That would be consistent with simpler composition of the pitch components not susceptible to polymerization.
Comparison of the 13C NMR spectra for petroleum pitch and coal pitch samples (Table 2) reveals a significant difference in the content of aromatic and aliphatic hydrocarbons. The proportion of aromatic and aliphatic fragments in any samples may be assessed on the basis of the ratio Car/Cal.
To determine the impurity content, we employ X‑ray diffraction analysis of pitch samples on a Shimadzu XRD-7000 instrument using a tube with a copper anode and CuKα radiation. Diffraction patterns are recorded at a rate of 1°/min at 0.02° intervals within the range 5°–70°. IPS FI software is used to identify the phase composition.
The composition established is as follows: 28 ppm Na; 182 ppm Fe; 270 ppm V; and 164 ppm other elements (total).
To determine the influence of additives on the structure and rheological properties of the pitch, we analyze electron microscope images of the surface of the petroleum pitch by means of a Tesla BS-242E scanning electron microscope (resolution ×1500). As is evident from Fig. 5, additives significantly change the pitch surface. The formation of coke particles is clearly evident on the images.
Chromatography and mass spectrometry are used to determine the content of 3,4-benzopyrenes and polycyclic aromatic hydrocarbons in the petroleum pitch (low-voltage mass spectrometry). We know that the hydrocarbons in petroleum are characterized by different ionization potentials: for example, arenes 7.00–9.24 eV; alkanes 13 keV and above; and naphthene hydrocarbons more than 11 eV. Therefore, the energy range of the ionizing electrons employed begins at 9 eV or less. The mass spectra only include peaks of molecular ions in aromatic compounds. In Figs. 6 and 7, we show the results of chromatography and mass spectrometry for samples of coal pitch and petroleum pitch. We clearly see that the molecular ion corresponding to a mass of 252 m/e (3,4-benzopyrene) is of intensity 30% in coal pitch but less than 1% in petroleum pitch.
CONCLUSIONS
1. Petroleum pitch with characteristics matching or exceeding those of coal pitch of grades A and B1 has been produced for the first time. The petroleum pitch itself and the anode mass derived from it were successfully tested in the laboratory of the Engineering Technology Center at RUSAL, Krasnoyarsk.
2. The characteristics of the petroleum pitch clearly indicate the potential for producing environmentally benign pitch (with no benzo[a]pyrene and minimal sulfur content). Its use does not require modernization or reconstruction of the production line at aluminum plants employing Soderberg technology (self-baking anodes).
3. The ash content of the petroleum pitch is low; and the content of mechanical impurities is minimal. No water is present.
4. The content of crystalline sodium is minimal relative to coal pitch. High sodium content always increases anode consumption in aluminum production.
5. The production of petroleum pitch is technologically and environmentally superior to the production of coal pitch.
6. The structure of the petroleum pitch has been investigated by thermogravimetry, NMR spectroscopy, X-ray methods, and also chromatography and mass spectrometry. We have developed a rapid method of qualitative and quantitative analysis of 3,4-benzo[a]pyrenes and also polycyclic aromatic hydrocarbons in petroleum pitch and coal pitch. This method is based on molecular mass spectrometry with low energy of the ionizing electrons.
7. The production of petroleum pitch has obvious benefits relative to other methods of producing petroleum binder. In particular, it is simple and effective. The petroleum pitch produced is characterized by constant physicochemical composition and stable operational characteristics. It may be used in liquid or granulated form.
8. Organizing the production of petroleum pitch permits considerably more efficient use of petroleum coke and makes available additional resources of quality and stability on account of the use of filler and binder of the same type.
REFERENCES
Khairudinov, I.R., Opyt proizvodstva i primeneniya neftyanykh pekov (Production and Use of Petroleum Pitches), Ufa: Bashkir. Nauchno-Issled. Inst. Pererab. Nefti, 1994.
Syunyayev, Z.I., Neftyanoi uglerod (Oil Carbon), Moscow: Khimiya, 1980.
Dolmatov, L.V., Production of pitches in exhaustive crude oil processing schemes, Chem. Technol. Fuels Oils, 1987, vol. 23, no. 3, pp. 313–315.
Doshlov, I.O. and Konovalov, N.P., Adgeziya i adgezivy: Teoriya adgezii, svoistva i kharakteristiki organicheskikh adgezivov, ikh modifikatsiya (Adhesion and Adhesives: The Theory of Adhesion, Properties and Characteristics of Organic Adhesives, and Their Modification), Saarbrucken: LAP LAMBERT Academic, 2017.
Smakova, U.M., Fedoseeva, M.V., and Budnik, V.A., Structure and types of petroleum pitches. Prospective production of pitches, Neftepererab. Neftekhim. (Moscow), 2019, no. 1, pp. 19–22.
Doshlov, I., Novel technology for production of petroleum pitches for non-ferrous metallurgy, Proc. Int. Conf. “Aviamechanical Engineering and Transport (AviaENT 2018)”, Amsterdam: Atlantis, 2018, vol. 158, pp. 94–99.
Varfolomeev, D.F., et al., Perspektivy proizvodstva i primeneniya NSD pri poluchenii metallurgicheskogo koksa iz shikht s povyshennym soderzhaniem slabospekayushchikhsya i nespekayushchikhsya uglei (Production and Use of Petroleum Sintering Additives for the Production of Metallurgical Coke from Charges with a High Content of Low-Caking and Non-Caking Coals), Moscow: Tsentr. Nauchno-Issled. Inst. Inf. Tekh.-Ekon. Issled. Neftepererab. Neftekhim. Prom., 1990.
Vershinina, E.P., Gil’debrandt, E.M., and Selina, E.A., Development of the production of anode binder for aluminum electrolyzers, Zh. Sib. Fed. Univ., Tekh. Tekhnol., 2012, vol. 2, no. 5, pp. 752–759.
Nciri, N., Kim, J., Song, S., and Kim, N., Chemical and physical properties of petroleum pitch, Proc. 2014 Annual Int. Offshore and Polar Engineering Conf., Cupertino, CA: Int. Soc. Offshore Polar Eng., 2014. P. 92–99.
Kuzora, I.E., Doshlov, O.I., Moiseev, V.M., and Doshlov, I.O., Prospective production of petroleum binders in JSC Angarsk Petrochemical Company, Mir Nefteprod., 2015, no. 5, pp. 14–20.
Suprunov, V.V., Production of sintering materials from heavy oil residues, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Sverdlovsk, 1969.
GOST (State Standard) 10200-83: Electrode Coal-Tar Pitch. Specifications, Moscow: Izd. Standartov, 1985.
Shcherbakov, B.V., Doshlov, I.O., Lubinskii, I.V., Lebedeva, I.P., Chizhik, K.I., Lazarev, D.G., et al., RF Patent 2415972, Byull. Izobret., 2011, no. 10.
Khairudinov, I.R., et al., Use of petroleum sintering additive in coke production, Koks Khim., 1988, no. 9, pp. 11–12.
Dolmatov, L.V., Khairudinov, I.R., and Galeev, R.G., Production of petroleum pitch by combined processing scheme, Chem. Technol. Fuels Oils, 1988, vol. 24, no. 3, pp. 5–7.
Doshlov, I.O., Advanced technology for the production of petroleum binders, Trudy 72-i Mezhdunarodnoi molodezhnoi nauchnoi konferentsii “Neft’ i gaz 2018” (Proc. 72nd Int. Youth Sci. Conf. “Oil and Gas 2018”), Moscow: Ross. Gos. Univ. Nefti Gaza im. I.M. Gubkina, 2018, pp. 110–120.
Mann, V., The Soderberg technology: problems or advantages in the future, Proc. Conf. “Light Metals 2006,” San Antonio, Texas, Warrendale, PA: Miner., Met. Mater. Soc., 2006. P. 181–184.
Elshin, N.A., Doshlov, I.O., Osipov, D.I., Lubinskii, I.V., Lebedeva, I.P., Lazarev, D.G., et al., RF Patent 2397276, 2010.
Doshlov, I.O. and Ushakov, I.A., Analysis of petroleum and coal tar pitches by 1H and 13C NMR spectroscopy, in Pererabotka prirodnogo i tekhnogennogo syr’ya (Processing of Natural and Technogenic Raw Materials), Voronezh: Inst. Vys. Tekhnol., 2018, pp. 128–132.
Kovalev, M.S., Simonenko, D.V., and Polikarpova, D.D., Classification of binders by the example of coal and petroleum pitches, Molodezhnyi Vestn. Irkutsk. Gos. Tekh. Univ., 2019, vol. 9, no. 1, pp. 86–88.
Doshlov, I.O., Novel technology for production of petroleum pitches for non-ferrous metallurgy, Proc. Int. Conf. “Aviamechanical Engineering and Transport (AviaENT 2018)”, Amsterdam: Atlantis, 2018.
Chockalingam, K., Saravanan, U., and Murali Krishnan, J., Characterization of petroleum pitch using steady shear experiments, Int. J. Eng. Sci., 2010, vol. 48, no. 11, pp. 1092–1109.
Shouhui, J. and Guo, A., Effects of olefins on mesophase pitch prepared from fluidized catalytic cracking decant oil, Fuel, 2020, vol. 262, no. 12, art. ID 116671.
Smakova, U.M., Fedoseeva, M.V., and Budnik, V.A., Structure and types of oil pitches. Prospects for the production of pitches, Neftepererab. Neftekhim. (Moscow), 2019, no. 1, pp. 19–22.
Funding
Financial support was provided within the federal program devoted to the creation of innovative technology capable of producing petroleum pitch (as an alternative to imported pitch) for nonferrous metallurgy and electrode production; and the targeted research and development program for Russian science and technology between 2014 and 2020 (measure 1.2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by B. Gilbert
About this article
Cite this article
Doshlov, I.O., Kondratev, V.V. & Diachkova, S.G. Petroleum Pitch from Highly Aromatic Fuel Oil for Nonferrous Metallurgy. Coke Chem. 64, 332–339 (2021). https://doi.org/10.3103/S1068364X21070048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X21070048