Abstract
A procedure for measuring the rhenium content in radioactive ore rocks is tested. Samples of rhenium-containing rocks are irradiated with neutrons from a photoneutron source. Analysis of activation gamma spectra shows that rhenium can be identified against a background of accompanying elements, making neutron activation analysis an effective way of determining rhenium content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rhenium is a rare trace element (abundance of Re = 1 mg t–1). One of its important properties is a very high melting point, 3180°С, which results in its use in high-technology industries. Rhenium has also high corrosion resistance and relative chemical inertness. Under ordinary conditions, it is poorly soluble in hydrochloric, sulfuric, and hydrofluoric acids [1].
Due to its extremely low concentrations in the Earth’s crust and geochemical features [2], the detection of rhenium is a complicated analytical problem. Existing chemical means are characterized by poor determination of rhenium content due to competition from other elements. Independent ways of analyzing rhenium-containing rocks must therefore be developed.
Three procedures are currently used to analyze rhenium: X-ray fluorescence analysis (XRF), inductively coupled plasma mass spectrometry (ICP-MS), and kinetic analysis (Re). Each of has its own limits, which are discussed below. Along with the indicated authors, we tested the procedure for neutron activation analysis (NAA) [3], the sensitivity of which is affected little by the polycomponent composition of hydrogenous uranium ores or by coal saturation of the samples.
BRIKETNO–ZHELTUKHINSKOE DEPOSIT
The world’s main rhenium producers are Chile (Chuquicamata et al., >50% of the world potential) and the United States (Bingcham et al.) where rhenium is mined from copper–molybdenum ores in copper–porphyry deposits [4]. The second industrial source of Re is deposits of cupriferous sandstones in Poland and other countries. The Russian Federation’s reserves of such deposits are either not large (porphyric) or not mined (Udokan copper sandstones), and it is hard to extract rhenium from them. At the same time, the demand for rhenium has grown considerably due to the expansion of aerospace industry. In 1992, elevated concentrations of Re were recorded in gas emanations from Kudryavyi volcano, Iturup Island (South Kuril Islands) where rhenium coming from deep fluids is confined to a high-temperature fumarole field [5].
Estimates of the Re raw material base in the Russian Federation show that the main ore-forming types of deposits include an infiltration-polymetallic (hydrogenous) type (in which rhenium is recovered along with uranium via in situ leaching) that has the largest resource potential [6]. Three Re-containing uranium provinces are known: Moscow, Trans-Ural, and Trans-Baikal. The most promising (~80% of the Re from this type of resource) is the Moscow province [7], where the U–Mo–Re Briketno–Zheltukhinskoe deposit of this type was explored in 2013–2015 surveys and included in the official balance sheet in 2016 [8]. This deposit is now being prepared for development.
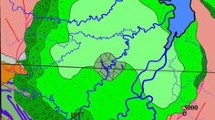
The Briketno–Zheltukhinskoe deposit is on the territory of the Skopin district of Ryazan oblast, 254 km from the city of Moscow (Fig. 1, inset). Drilling revealed a single ore deposit with a Re content of >0.1 g t−1. Re productivity per area is as high as 57 g m−2 (Fig. 1). The ribbon-like ore deposit, which has a cap delineated along flank concentration CRe = 0.5 g t−1, is as high as 42 m, and its lateral dimension can be as great as 500 m [8, 9].
Position of the deposit (inset) and isolines of the productivity of Re mineralization in the Bobrikov formation of the Briketno–Zheltukhinskoe deposit in the lithofacies scheme (plan) [8, 9]: (1) Briketno–Zheltukhinskoe deposit (inset); (2) sands with bands and lenses of coal clays, coals, and aleurites; (3) inequigranular sands with gravel and rare shale interbeds; (4) boundaries for the main channel (noncarboniferous) or flood plain, oxbow, and lagoon (carboniferous) facies; (5) numbers of boreholes from which core samples were taken.
Re mineralization at this deposit is localized in paleo-channel deposits of the Bobrikov horizon represented by fine- to medium-grained sands ranging from light to dark grey of different shades [8, 9]. The nature of the predominant grey and dark grey color is due to the deposits being saturatrated with carbonaceous material and an abundance of iron disulfides [10].
We identified two types of ore-bearing rocks with Re mineralization. The first contained sulfide-rich silty varieties of sandstone; the second, carbonaceous detritus that also contained sulfides. Samples of P9-50 and D5-6 ores (Table 1) were classified as fine- to coarsely-grained sands (>95 wt % SiO2) brownish-grey to dark grey in color, with carbonaceous detritus and sulfide mineralization. They were distinguished by enhanced contents of Fe, S, U, Mo, and Re associated with zones of pyritization and the presence of carbonaceous material. Sample G1-47 was grey medium-grained sand with rare bands of dark grey fine-grained sand and contained 1.85 g ⋅ t−1 of Re.
Our rock materials were collected during surveys for rhenium and secondary components, where a number of core hole profiles were drilled to depths of ~90–100 m with complete core recovery. Horizontal Neogene–Quaternary deposits and the productive horizons of sands of the Visean stage of the Lower Carboniferous were developed 1–2 m deep into the underlying Famennian limestones [11].
PROCEDURES FOR ANALYZING AND COMPARING COMPOSITIONS
The mineral composition of the ores was studied in detail via electron microscopy at the Russian Academy of Sciences’ Institute of the Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry [10]. It was established that uranium in the ores was in the form of an oxide (uraninite, or nasturan), which is contained in pyritic mass in the form of rare micron-sized inclusions and is found in carbonaceous particles. Molybdenum in the ores was in the form of amorphous sulfide Mo with Fe impurities and was identified as jordisite (MoS2). Molybdenum was also detected as molybdenite in crystal form (MoS2). Rhenium was contained in micron-sized particles of Mo(Fe)-sulfide composition (jordisite mass) and in molybdenite particles (≤1 wt % Re).
After breaking and grinding, the chemical composition of the samples was studied via XRF (analysts O.A. Nabelkin, Institute of Mineralogy, Geochemistry, and the Crystal Chemistry of Rare Elements, and A.I. Yakushev of the Russian Academy of Sciences’ Institute of the Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry); ICP-MS (analyst I.A. Pichugin, Institute of Mineralogy, Geochemistry, and the Crystal Chemistry of Rare Elements); and kinetic analysis (for Re) (analyst G.G. Lebedeva, Institute of Mineralogy, Geochemistry and Crystal the Chemistry of Rare Elements).
ICP-MS was used to determine the concentrations of elements Re, Mo, U, V, Cr, Co, Ni, Cu, Zn, As, Se, Ag, Cd, Sb, Tl, Pb, Bi, Th, and Mn. Quantitative chemical (kinetic) analysis of rhenium content in the rocks and sulfide-containing ores was performed photometrically. The procedure was based on the catalytic action of rhenium on the reaction between sodium tellurate and stannous chloride. It was certified in accordance with GOST 8.563–2009 and OST 41-08-205–04. The weight of samples ground into powder was 0.5–2 g. The procedure allowed us to determine Re contents of 0.002 to 200 g t−1 in rocks and sulfide ores [12].
Detecting rhenium by means of kinetic analysis, ICP-MS, and XRF has certain limits. Along with the difficulties of detecting rhenium associated with its tendency toward hydrolysis and disproportionation, its variety of developed ionic forms, and the effect of interfering chemical elements always present in considerable amounts in Re-containing ores (molybdenum, tungsten, vanadium), heavy demands are placed on the conditions for performing analyses (e.g., thermostating of the solution) and the equipment that is used, which requires an analytical chemist to be highly qualified.
Sample preparation during ICP-MS analysis is usually done via the microwave decomposition of samples in an autoclave using a mixture of two acids (nitric and hydrofluoric) with additions of hydrogen peroxide. Though this technique is quite simple, considerable time is needed to prepare samples. The limits on its use for rich ores saturated with carbonaceous matter (Corg ≤ 10 wt %) are due to uncontrolled losses of Re (and platinoids) associated with its removal from the gaseous phase during sample preparation.
Although sample preparation for direct X-ray fluorescence analysis is fairly simple, the technique has poor sensitivity (the lower limit of detection is 50–100 g t−1 of Re) due to the strong overlap of the interfering ZnKα line of zinc and the analytical ReLα line. The analytical Re\({{L}_{{{{\beta }_{1}}}}}\) line is strongly absorbed by the K-edge of zinc, the content of which is usually 2–3 times greater in Re-bearing ores.
NEUTRON ACTIVATION ANALYSIS OF SAMPLE COMPOSITIONS
Neutron activation analysis [13], especially with thermal neutrons of a photoneutron source, has several important advantages over the above techniques. As was noted above, its sensitivity is affected little by the polycomponent composition of uranium ores and the coal saturation of the samples. This analysis does not require special measures for sample preparation. It is also important that when irradiating bulk samples with neutron fluxes of 107–108 neutron/cm2 s, most of the samples can be used in unchanged form for further studies after 1–2 weeks. This is especially important where unique or expensive samples are concerned. The radioactivity of the samples will be so low that the irradiated material can be classified as nonradioactive waste and not require disposal. This makes the proposed procedure of nondestructive analysis immeasurably less of a radiation hazard than nuclear reactor analysis.
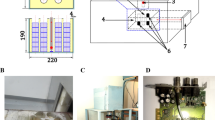
Neutron activation analysis of the samples was performed on the activation measuring complex at the Institute for Nuclear Research [3]. It consists of the IN-LUE photoneutron W–Be source based on the LUE-8-5 electron linear accelerator and a low-background gamma spectrometer [14]. Our ~2 g samples were activated over ~1–3 h with a thermal neutron flux of ~107 neutron/cm2 s and held for 10–30 min before measurements. The activation γ spectra were measured for ~20–44 h. Pure Re samples weighing 0.1–0.25 g were irradiated simultaneously as reference samples.
The SpectraLineGP software collected activation spectra [15] by consecutively recording them on a computer disc every 100 s. Special software converted the spectra from the half-binary to the text format that was used later in software that determines a nuclide’s half-life according to a fixed peak. The peaks were identified in the spectra by software containing a database on nuclides activated upon neutron irradiation in the (n, γ), (n, n'γ), and (n, p) reactions. The software found the corresponding variants of nuclide lines according to the fixed peaks in the spectrum and indicated half-life and intensity. The half-life data obtained in processing the spectra were used for final identification of the peaks.
Activation spectra of the samples after irradiation by thermal neutrons are presented in Figs. 2а, 2b. When identifying rhenium, we are interested in the energy range of ≤750 keV. Figure 2b presents spectra of the samples after they were activated by neutrons in this range of energies, where peaks from the states of rhenium can appear.
(а) Activation gamma spectra of (1) P9, (2) D5, and (3) G1 samples after irradiation by thermal neutrons, reduced to the same period of measurement (44 h) and sample weight (1 g). The periods of irradiation are 1–3 h, and those of holding are 10–30 min. (b) The same spectrum on a different scale, in the energy range of Re isotopes.
All of the spectra exhibit numerous peaks corresponding to the activated states of rock-forming elements. At the same time, there are clear peaks corresponding to Re isotopes. To consider them suitable for analytical purposes, we must be certain they are not overlapped by background lines from natural radioactivity, or by lines of other elements contained in these ore rocks.
The natural radioactivity of the samples was also analyzed using the activation measuring complex at the Institute for Nuclear Research. The provided samples were placed into the low-background gamma spectrometer [14]. Data were collected using the SpectraLineGP software [15]. The natural background of the samples was measured for 10–20 h. The spectra of natural radioactivity of the samples in the energy ranges of Re isotopes are presented in Figs. 3c, 4c, and 5c. The main peaks of U–Th series isotopes can be seen.
Figures 3b, 4b, and 5b show the gamma spectra of the Re samples. Rhenium is found in nature in the form of two isotopes: 185Re (37.4%) and 187Re (62.6%). Interaction with neutrons in (n, γ) reactions can result in the formation of Re isotopes: 186Re (principal lines, 122 and 137 keV; half-life period, 89.24 h), 188Re (155, 478, and 633 keV; 17 h) and 188mRe (92, 106 keV; 18.6 m), the decay γ-peaks of which are seen in the spectra in Figs. 3b, 4b, and 5b.
Comparative analysis of the spectra in Figs. 3–5 in the energy range of 50–200 keV showed they were much simpler in the range of ≤200 keV than in Fig. 2. There is a notable peak at 155 keV that corresponds to the 188Re isotope with a half-life period of 17 h. It is not overlapped by background peaks or peaks from other elements, and can therefore be chosen as an analytical peak.
Since the samples were taken at different boreholes and horizons, and were therefore of different elemental compositions, we can say that the presence of a well-defined line of 155 keV from 188Re is a characteristic feature of the rocks at the Briketno–Zheltukhinskoe deposit, and neutron activation analysis is an effective way of determining rhenium content.
CONCLUSIONS
We considered the composition of the Briketno–Zheltukhinskoe Re deposit, which is characterized by enhanced natural radioactivity, due to the presence of uranium, and by complex mineral compositions with increased contents of Fe, S, Se, and Mo.
Several procedures for analyzing Re were compared: X-ray fluorescence analysis, inductively coupled plasma mass spectrometry, and kinetic analysis. Neutron activation analysis was also tested.
Using an activation measuring complex consisting of a photoneutron W–Be source of neutrons and a low-background gamma-spectrometer, measurements were made via neutron activation analysis. The procedure for measuring the Re content in radioactive ore rocks was tested using samples from the Briketno–Zheltukhinskoe deposit. The samples of Re-containing rocks were irradiated by photoneutron source neutrons. Analysis of the activation gamma spectra demonstrated the possibility of identifying rhenium against a background of accompanying elements, making neutron activation analysis an effective way of determining rhenium content.
REFERENCES
Lebedev, K.B., Renii (Rhenium), Moscow: Metallurgizdat, 1963.
Ivanov, V.V., Poplavko, E.M., and Gorokhova, V.N., Geokhimiya reniya (Rhenium Geochemistry), Moscow: Nauka, 1969.
Andreev, A.V., Burmistrov, Yu.M., Zuyev, S.V., et al., Bull. Russ. Acad. Sci.: Phys., 2017, vol. 81, no. 6, p. 748.
Kremenetskii, A.A., Luneva, N.V., and Kulikova, I.M., Razved. Okhr. Nedr, 2011, no. 6, p. 33.
Znamenskii, V.S., Korzhinskii, M.A., Shteinberg, G.S., et al., Zap. Ross. Mineral. O-va, 2005, vol. 134, no. 5, p. 32.
Kul’tin, Yu.V., Novgorodtsev, A.A., Fomenko, A.E., et al., Gorn. Zh., 2007, no. 6, p. 19.
Trach, G.N. and Beskin, S.M., Razved. Okhr. Nedr, 2011, no. 6, p. 26.
Karas’, S.A., Kremenetskii, A.A., Orlov, S.Yu., et al., Razved. Okhr. Nedr, 2017, no. 8, p. 20.
Vikentyev, I.V. and Kailachakov, P.E., Lithol. Miner. Resour., 2020, vol. 55, no. 3, p. 177.
Kailachakov, P.E., Doynikova, O.A., Belousov, P.E., and Vikentyev, I.V., Lithol. Miner. Resour., 2020, vol. 55, no. 4, p. 286.
Kailachakov, P.E., Vest. RUDN, Ser. Inzh. Issled., 2017, no. 3, p. 353.
Metodika kolichestvennogo khimicheskogo analiza. Renii. Opredelenie massovykh dolei reniya v gornykh porodakh i sul’fidnykh rudakh fotometricheskim metodom (Quantitative Chemical Analysis Procedure. Rhenium. Determination of Mass Fractions of Rhenium in Rocks and Sulfide Ores by Photometric Method), Moscow: Inst. Mineral., Geokhim., Kristallokhim. Redk. Elementov, 2015.
Gut’ko, V.I., Aktivatsionnyi analiz (Activation Analysis), Minsk: Mezhdunar. Gos. Ekol. Univ., 2008.
Andreev, A.V., Burmistrov, Yu.M., Zuev, S.V., et al., Yad. Fiz. Inzh., 2013, vol. 4, nos. 9–10, p. 879.
SpectraLineGP. http://lsrm.ru/products/detail.php? ELEMENT_CODE=SpectraLineGP.
ACKNOWLEDGMENTS
We are grateful to the senior staff of the Institute of Mineralogy, Geochemistry and Crystal Chemistry of Rare Elements for providing our rock samples. We also thank laboratory personnel O.A. Nabelkin, V.A. Ivanov, I.A. Pichugin, G.G. Lebedeva, and A.I. Yakushev of the Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry for performing our chemical analyses.
Funding
This work was supported by the RF Ministry of Science and Higher Education, research program of the Institute of Geology of Ore Deposits, Petrography, Mineralogy and Geochemistry (IGEM) of the Russian Academy of Sciences, and by the Russian Foundation for Basic Research, project no. 19-35-90095, “Post-Graduate Competition.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Mukhortova
About this article
Cite this article
Afonin, A.A., Burmistrov, Y.M., Vikentyev, I.V. et al. Studying the Possibility of Neutron Activation Determination of the Content of Rhenium in Radioactive Rocks. Bull. Russ. Acad. Sci. Phys. 85, 1059–1064 (2021). https://doi.org/10.3103/S106287382110004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S106287382110004X