Abstract
Thin films of Zn1–xMnxO (x = 1–4 at %) were applied onto a glass substrate by low-cost chemical bath deposition (CBD). The films were characterized by XRD, UV–VIS absorption spectra, SEM, and vibrating sample magnetometry—with special emphasis on the influence of dopant concentration x. Structural and optical properties of deposited films imply that the Mn2+ ions substituted Zn2+ ions without changing the wurtzite structure of ZnO. No impurity phases were detected in XRD patterns. The band gap in ZnO was found to increase with increasing x. Magnetic measurements showed that Mn-doped ZnO samples exhibited ferromagnetic behavior at room temperature, which tentatively was associated with the replacement of Zn2+ ions by Mn2+ ions in the ZnO lattice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
Over the past two decades, diluted magnetic semiconductor materials have attracted much attention as an excellent media in which both the charge (electron and holes) and spin related phenomena take place in a one and the same substance, which is an important property for spintronic devices [1]. Due to a large exciton binding energy of ZnO, some zinc ions can be replaced by magnetic ions capable of ferromagnetic coupling [2]. Among other transition metals, ZnO doping with Mn seems most encouraging because Mn has a highest possible magnetic moment [3], and the first half of the d-band is fully occupied, thus promoting the formation of a stable fully polarized state. In addition, Mn is isomorphic to Zn and exhibits variable oxidation state and acceptor properties in a ZnO matrix since the ionic radius of Zn2+ (0.74 Å) is smaller than that of Mn2+ (0.80 Å) and larger than that of Mn3+ (0.66 Å).
There are continuing efforts to elucidate the origin of the observed ferromagnetism in Mn-doped ZnO [4] and the Mn2+-induced room-temperature ferromagnetism and spin-glass behavior in hydrothermally grown Mn-doped ZnO nanorods [5] where the ferromagnetic ordering got stabilized upon an increase in hole concentration. Wojnarowicz et al. [6] studied the structural and magnetic properties of Co–Mn co-doped ZnO nanoparticles obtained by microwave solvothermal synthesis. Yan et al. [7] described Mn-doped ZnO nanorod arrays prepared by CVD method with ferromagnetic ordering above room temperature. Droubay et al. [8] synthesized Mn-doped ZnO epitaxial thin films at different temperatures and achieved room temperature ferromagnetism in the samples synthesized at 950°C. Sample preparation and annealing effects on the ferromagnetism in Mn-doped ZnO can be found in [9].
To date, bulk samples and thin films of Mn-doped ZnO have been prepared by different methods such as sol–gel [10], radiofrequency magnetron sputtering [11], pulsed laser deposition [12], and spray pyrolysis [13]. Ferromagnetism in different (ZnO)1 – x(MnO2)x systems still remains controversial and the magnetic properties of oxide films were found highly sensitive to preparation method [14, 15].
In this communication, we report on the preparation of thin Zn1–xMnxO films (x = 1–4 at %) by low-cost chemical bath deposition (CDD) method and their characterization, with special emphasis on the influence of dopant concentration x.
2 EXPERIMENTAL
CBD method is based on heating the alkaline (pH 10.3) bath of zinc salt. Zinc nitrate Zn(NO3)2⋅ 2H2O and manganese nitrate Mn(NO3)2⋅2H2O were used as a source of Zn and Mn, respectively. Each formula unit contained one Mn2+ cation and two NO3– anions. Starting solution was prepared by dissolution of 0.08 M zinc nitrate in deionized water. To this solution, varied amounts of manganese nitrate were added and pH was adjusted to 10.3 with aqueous solution of ammonia. Dopant concentration was varied between 1.0 and 4.0 at %. Prior to deposition, glass substrates were cleaned with acetone, isopropyl alcohol, and triply distilled water successively for 15 min each in an ultrasonic sonicator and finally dried with a flux of high-pressure dioxygen. Cleaned glass substrates were immersed in the above alkaline bath heated to 80°C. Heterogeneous reaction on the substrate surface resulted in deposition of Mn-doped ZnO on the substrate surface. The deposition process lasted for 2 h. Deposited films (100–150 nm thick) were annealed in air at 65–85°C for 2 h.
Deposited films were characterized by XRD (Rigaku diffractometer, Cu Kα radiation), SEM (JEOL JSM-7001 microscope), absorption spectra (Shimadzu-1800 spectrophotometer), and vibrating sample magnetometry (SQUID magnetometer).
3 RESULTS AND DISCUSSION
3.1 XRD Spectra
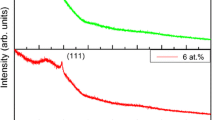
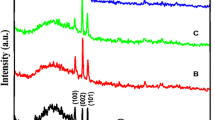
The diffraction patterns of undoped and Mn-doped ZnO films are presented in Figs. 1a and 1b. The spectra confirm the crystalline nature of the films: identified (100), (002), (101), (110), (200), and (004) reflections belong to hexagonal ZnO (JCPDS card no. 89-1397).
No other crystalline by-products such as manganese and its oxides were found within the XRD detection limit, thus indicating that the as-prepared thin films have a purely wurtzite structure. This implies that Mn2+ ions substituted for Zn2+ of the ZnO host without changing the wurtzite structure. The intensity of the (002) plane in pure ZnO film is much stronger than that of Mn-doped ZnO films. With increasing x, the intensity of ZnO (002) peaks is getting weaker, which indicates that the film crystallinity is deteriorated. We also observed a slight shift in position of the (002) peak (Fig. 1c). The weaker intensity and slight shift of the (002) peak may imply that Mn2+ incorporates into the ZnO lattice and substitutes the Zn2+ site. Similar behavior was also reported by other researchers [16].
Mean crystallite size D was calculated using the Scherrer formula
where D is the crystallite size, k = 0.89 is a coefficient, β is the full width at half-maximum (FWHM) of diffraction peak, and λ is the wavelength of X-rays. The D values derived from (002) peak widths were estimated as 35.4, 34.1, 32.4, 30.5, and 29.6 nm, respectively. Our XRD results show that Mn doping does not change the wurtzite structure of ZnO for all x. Small (002) peak broadening indicates a decrease in crystal quality in terms preferred grain growth with an increase in Mn content [17].
Figure 2 shows the SEM images of Zn1 – xMnxO films deposited onto a Si (100) substrate. Instead of transparent oxide glass, silicon was used to achieve better durability of thin film in photovoltaic measurements. In all cases, deposited films have grown in the form of nanorods along the [002] direction. The SEM micrographs also reveal that Mn-doping of ZnO reduces the average crystallite size, which agrees with the XRD results. An increase in x is seen to decrease the diameter of nanorods and increase film randomness.
3.2 Absorption Spectra
Figure 3 presents the absorption spectra and band gaps for undoped and Mn-doped ZnO films with different x. Zinc oxide is a direct band gap material and the energy gap (Eg) can thus be estimated by assuming direct transition between the conduction and valence bands. Theory gives the following relationship between absorption coefficients α and photon energy hν:
As is seen in Fig. 3a, the absorption edges of Zn1 ‒ xMnxO films (x = 0–4 at %) are at 450, 430, 410, 3185 and 375 nm, respectively. With increasing x, the absorption edge is seen to shift toward lower wavelengths. Figure 3b shows that band gap Eg grows from 2.8 to 3.2 eV with increasing concentration of Mn2+ dopant [18–20]. The blue shift of Eg can be explained in terms of the Burstein–Moss effect [21].This is the phenomenon of which the Fermi level merges into the conduction band upon an increase in carrier concentration, so that the low-energy transitions are blocked. Our results are in good agreement with those reported by Sakai and Rekha [22, 23].
3.3 Magnetic Behavior
The room-temperature M–H curves for Mn-doped ZnO thin films under study (Fig. 4) clearly indicate the occurrence of ferromagnetic ordering [24]. Remanent magnetization Mr of Zn1 – xMnxO films is seen to grow with increasing x. Saturation magnetization Ms attains a value of 0.062, 0.086, 0.24 and 0.238 for x = 1.0, 2.0, 3.0 and 4.0 at %, respectively. These values well agree with the reported ones [25–27].
We have also found that saturation magnetization Ms initially increases up to x = 3.0 and then decreases upon further increase in x, reaching it maximum at x = 3.0. Sharma et al. [27] observed a maximum ferromagnetic moment at x = 2.2 at % in their Mn-doped samples and found that the ferromagnetic ordering decreases with increasing Mn concentration. In our case, we also observed a maximum of Ms at x = 4.0.
Since we did not notice the presence of Mn-related phases in our XRD patterns, it can be concluded that the room-temperature ferromagnetism in our samples is not due to presence of manganese oxides or clusters [28].
Therefore, we consider that the observed room-temperature ferromagnetism most likely is due to the substitution of Mn ions (Mn2+) for Zn ions in the ZnO lattice, which is evidenced by XRD results. According to Ruderman–Kittel–Kasuya–Yosida theory [29, 30], the magnetism originates from the exchange interaction between local spin-polarized electrons (such as the electrons of Mn2+ions) and conduction electrons. This interaction leads to the spin polarization of conductive electrons and subsequently, the spin-polarized conductive electrons undergo exchange interaction with local spin-polarized electrons of other Mn2+ ions. Because of the long-range exchange interaction, almost all Mn2+ ions exhibit the same spin direction. The conduction electrons are regarded as a medium to “connect” all Mn2+ ions. As a result, the material exhibits ferromagnetiс behavior [31].
CONCLUSIONS
Thin films of Zn1 – xMnxO (x = 1–4 at %) were applied onto a glass substrate by low-cost chemical bath deposition (CBD) and characterized by XRD, UV–VIS absorption spectra, SEM, and vibrating sample magnetometry—with special emphasis on the influence of dopant concentration x. XRD results imply that Mn doping does not change the wurtzite structure of ZnO in all samples. Slight (002) peak broadening with an increase in x indicates a gradual growth in crystallite size. Mn doping affects the absorption spectra and optical band gap. For deposited films, saturation magnetization Ms, remanent magnetization Mr, and coercive field Hc (Oe) attain a value of 0.345 emu/g, 0.152 A/m, and 170 kA/m, respectively. Mn doping produces marked influence on the structural, optical, and magnetic properties of ZnO films.
REFERENCES
Kazakova, O., van der Meulen, M.I., Petkov, N., and Holmes, J.D., Magnetic properties of single crystalline Ge1–xMnx nanowires, IEEE Trans. Magn., 2009, vol. 45, no. 10, pp. 4085–4088. https://doi.org/10.1109/TMAG.2009.2023073
Nasir, A., Vijaya, A.R., Zahir, A.K., Tarafder, K., Kumar, A., Wadhwa, M.K., Singh, B., and Ghosh, S., Ferromagnetism from non-magnetic ions: Ag-doped ZnO, Sci. Rep., 2019, vol. 9, 20039. https://doi.org/10.1038/s41598-019-56568-8
Shatnawi, M., Alsmadi, A.M., Bsoul, I., Salameh, B., Mathai, M., Alnawashi, G., Alzoubi, G.M., Al-Dweri, F., and Bawa’aneh, M.S., Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles, Res. Phys., 2016, vol. 6, pp. 1064–1071. https://doi.org/10.1016/j.rinp.2016.11.041
Yuan, K., Yu, Q.X., Gao, Q.Q., Wang, J., and Zhang, X.T., A threshold of Vo+/Vo++ to room temperature ferromagnetism of hydrogenated Mn doped ZnO nanoparticles, Appl. Surf. Sci., 2012, vol. 258, no. 8, pp. 3350–3353. https://doi.org/10.1016/j.apsusc.2011.08.080
Vinod, R., Junaid Bushiri, M., Sajan, P., Achary, S.R., and Sanjose, V.M., Mn2+-induced room-temperature ferromagnetism and spin-glass behavior in hydrothermally grown Mn-doped ZnO nanorods, Phys. Status Solidi A, 2014, vol. 211, no. 5, pp. 1155–1161. https://doi.org/10.1002/pssa.201330394
Wojnarowicz, J., Omelchenko, M., Szczytko, J., Chudoba, T., Gierlotka, S., Majhofer, A., Twardowski, A., and Lojkowski, W., Structural and magnetic properties of Co‒Mn codoped ZnO nanoparticles obtained by microwave solvothermal synthesis, Crystals, 2018, vol. 8, no. 11, 410. https://doi.org/10.3390/cryst8110410
Yan, H.L., Zhong, X.L., Wang, J.B., Huang, G.J., Ding, S.L., Zhou, G.C., and Zhou, Y.C., Cathodoluminescence and room temperature ferromagnetism of Mn-doped ZnO nanorod arrays grown by chemical vapor deposition, Appl. Phys. Lett., 2007, vol. 90, no. 8, 082503. https://doi.org/10.1063/1.2460297
Droubay, T.C., Keavney, D.J., Kaspar, T.C., Heald, S.M., Wang, C.M., Johnson, C.A., Whitaker, M., Gamelin, D.R., and Chambers, S.A., Correlated substitution in paramagnetic Mn2+-doped ZnO epitaxial films, Phys. Rev. B, 2009, vol. 79, no. 15, pp.155203–155208. https://doi.org/10.1103/PhysRevB.79.155203
Kluth, O., Schope, G., Hupkes, J., Agashe, C., Muller, J., and Rech, B., Modified Thornton model for magnetron sputtered zinc oxide: Film structure and etching behavior, Thin Solid Films, 2003, vol. 442, nos. 1–2, pp. 80–85. https://doi.org/10.1016/S0040-6090(03)00949-0
Huang M.H., Mao S., Feick H., Yan H., Wu Y., Kind H., Weber E., Russo R., and Yang P., Room-temperature ultraviolet nanowire nanolasers, Science, 2001, vol. 292, no. 5523, pp. 1897–1899, https://doi.org/10.1126/science.1060367
Maiti, U.N., Ghosh, P.K., Nandy, S., and Chattopadhyay, K.K., Effect of Mn doping on the optical and structural properties of ZnO nano/micro-fibrous thin film synthesized by sol–gel technique, Physica B: Condens. Matter, 2007, vol. 387, nos. 1–2, pp. 103–108. https://doi.org/10.1016/j.physb.2006.03.090
Lu, J.G., Huang, K., Zhu, J.B., Chen, X.M., Song, X.P., and Sun, Z.Q., Preparation and characterization of Na-doped ZnO thin films by sol–gel method, Physica B, 2010, vol. 405, no. 15, pp. 3167–3171. https://doi.org/10.1016/j.physb.2010.04.045
Yılmaz, S., McGlynn, E., Bacaksız, E., Ozcan, Ş., Byrne, D., Henry, M.O., and Chellappan, R.K., Effects of Cu diffusion-doping on structural, optical, and magnetic properties of ZnO nanorod arrays grown by vapor phase transport method, Appl. Phys., 2012, vol. 111, no. 1, pp. 013903-013905. https://doi.org/10.1063/1.3673861
Yılmaz, S., Bacaksız, E., McGlynn, E., Polat, İ., and Ozcan, Ş., Structural, optical and magnetic properties of Zn1–xMnxO micro-rod arrays synthesized by spray pyrolysis method, Thin Solid Films, 2012, vol. 520, no. 16, pp. 5172–5178. https://doi.org/10.1016/j.tsf.2012.04.002
Yan, X., Ho, D., Li, H., Li, L., Chong, X., and Wang, Y., Nanostructure and optical properties of M doped ZnO (M = Ni, Mn) thin films prepared by sol–gel process, Physica B: Condens. Matter, 2011, vol. 406, no. 20, pp. 3956–3962. https://doi.org/10.1016/j.physb.2011.07.037
Yang, S. and Zhang, Y., Structural, optical and magnetic properties of Mn-doped ZnO thin films prepared by sol–gel method, J. Magn. Magn. Mater., 2013, vol. 334, pp. 52–58. https://doi.org/10.1016/j.jmmm.2013.01.026
Lee, J.H., Ko, K.H., and Park, B.O., Electrical and optical properties of ZnO transparent conducting films by the sol–gel method, J. Cryst. Growth, 2003, vol. 247, nos. 1–2, pp. 119–125. https://doi.org/10.1016/S0022-0248(02)01907-3
Deka, S. and Joy, P.A., Synthesis and magnetic properties of Mn doped ZnO nanowires, Solid State Commun., 2007, vol. 142, no. 4, pp. 190–194. https://doi.org/10.1016/j.ssc.2007.02.017
Hao, Y.M., Lou, S.Y., Zhou, S.M., Yuan, R.J., Zhu, G.Y., and Hao, N.L., Fabrication and characterization of Mn-doped CuO thin films by the SILAR method, Nanoscale Res. Lett., 2012, vol. 7, p. 100. https://doi.org/10.1186/1556-276X-7-100
Gulen, Y., Bayansal, F., Sahin, B., Cetinkara, H.A., and Guder, H.S., Fabrication and characterization of Mn-doped CuO thin films by the SILAR method, Ceram. Int., 2013, vol. 39, no. 6, pp. 6475–6480. https://doi.org/10.1016/j.ceramint.2013.01.077
Kharroubi, B., Baghdad, R., Abdiche, A., Bousmaha, M., Bousquet, M., Zeinert, A., Marssi, M.E., Zellama, K., and Hamzaoui, S., Mn doping effect on the structural properties of ZnO-nanostructured films deposited by the ultrasonic spray pyrolysis method, Phys. Scr., 2007, vol. 86, no. 1, 015805. https://doi.org/10.1088/0031-8949/86/01/015805
Sakai, K., Kakeno, T., Ikari, A., Shirakata, S., Sakemi, T., Awai, K., and Yamamoto, T., Defect centers and optical absorption edge of degenerated semiconductor ZnO thin films grown by a reactive plasma deposition by means of piezoelectric photothermal spectroscopy, J. Appl. Phys., 2006, vol. 99, no. 4, 043508. https://doi.org/10.1063/1.2173040
Rekha, K., Nirmala, M., Nair, M., and Anukaliani, A., Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles, Physica B, 2010, vol. 405, no. 15, pp. 3180–3185. https://doi.org/10.1016/j.physb.2010.04.042
Arvind, A., Jayara, M.K., Kumar, M., and Chandra, R., Structural, optical and magnetic properties of Mn doped ZnO thin films prepared by pulsed laser deposition, Mater. Sci. Eng., B, 2012, vol. 177, no. 13, pp. 1017–1020. https://doi.org/10.1016/j.mseb.2012.05.005
Diaconu, M., Schmidt, H., Hochmuth, H., Lorenz, M., Benndorf, G., Lenzer, J., Spemann, D., Setzer, A., Nielsen, K.W., Esquinazi, P., and Grundmann, M., UV optical properties of ferromagnetic Mn-doped ZnO thin films grown by PLD, Thin Solid Films, 2005, vol. 486, nos. 1–2, pp. 117–121. https://doi.org/10.1016/j.tsf.2004.11.211
Theodoropoulou, N., Misra, V., Philip, J., Leclair, P., Berera, G.P., Moodera, J.S., Satpati, B., and Som, T., High-temperature ferromagnetism in Zn1–xMnxO semiconductor thin films, J. Magn. Magn. Mater., 2006, vol. 300, no. 2, pp. 407–411. https://doi.org/10.1016/j.jmmm.2005.05.039
Sharma, P., Gupta, A., Rao, K.V., Owens, F.J., Sharma, R., Ahuja, R., Guillen, J.M.O., Johansson, B., and Gehring, G.A., Ferromagnetism above room temperature in bulk and transparent thin films of Mn-doped ZnO, Nat. Mater., 2003, vol. 2, pp. 673–677. https://doi.org/10.1038/nmat984
Baik, J.M. and Lee, J.L., Fabrication of vertically well-aligned (Zn,Mn)O nanorods with room temperature ferromagnetism, Adv. Mater., 2005, vol. 17, no. 22, pp. 2745–2748. https://doi.org/10.1002/adma.200500776
Ruderman, M.A. and Kittel, C., Indirect exchange coupling of nuclear magnetic moments by conduction electrons, Phys. Rev., 1954, vol. 96, pp. 99–102. https://doi.org/10.1103/PhysRev.96.99
Lin, Y.B., Xu, J.P., Zou, W.Q., Lu, L.Y., Lu, Z.H., Zhang, F.M., Du, Y.W., Huang, Z.G., and Zheng, J.G., Effects of annealing and hydrogenation on the properties of ZnMnO polycrystalline films synthesized by plasma enhanced chemical vapor deposition, J. Phys. D: Appl. Phys., 2007, vol. 40, no. 12, 3674. https://doi.org/10.1088/0022-3727/40/12/019
Theodoropoulou, N., Misra, V., Philip, J., Leclair, P., Berera, G.P., Moodera, J.S., Satpati, B., and Som, T., High-temperature ferromagnetism in Zn1 − xMnxO semiconductor thin films, J. Magn. Magn. Mater., 2006, vol. 300, no. 2, pp. 407–411. https://doi.org/10.1016/j.jmmm.2005.05.039
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Patil, G.R., Shelar, M.B., Kambale, N.J. et al. Thin Zn1 – xMnxO Films (x = 1–4 at %) by Chemical Bath Deposition: Influence of Dopant Concentration. Int. J Self-Propag. High-Temp. Synth. 30, 100–105 (2021). https://doi.org/10.3103/S1061386221020096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386221020096