Abstract
Experiments on the melting of samples of fluorite-containing ongonites from the Ary-Bulak massif at 700–800°C at a pressure of 100 MPa and oxygen fugacity corresponding to the Ni–NiO and Mt–Hem buffers were carried out. In all experiments, the limit of the fluorine content in the aluminosilicate melt and saturation with respect to fluorite and topaz were reached. The change in oxygen fugacity did not qualitatively affect the phase relations in the studied samples but led to a slight increase in the solubility of topaz and a decrease in the solubility of fluorite in the silicate melt. In the composition of the silicate melt, a corresponding change occurred in the value of the agpaite coefficient Ka and the content of CaO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Fluorite-bearing ongonites were described (Antipin, 2009; Kovalenko V.I. and Kovalenko N.I., 1976; Peretyazhko and Savina, 2010) as one of the varieties of the Ary-Bulak subvolcanic massif rocks in Eastern Transbaikalia. In terms of chemical composition, these rocks differ significantly in Ca content from the classical ongonites of the Ongon-Khaierkhan deposit in Mongolia. While the classical ongonites are characterized by an extremely low (<0.5 wt %) content of CaO (Kovalenko V.I. and Kovalenko N.I., 1976; Kovalenko et al., 1971), in the fluorite-bearing ongonites of the Ary-Bulak massif the amount of CaO can reach 10 wt % or more.
Based on the results of the study of melt and fluid inclusions in the phenocrysts of these rocks, the authors of (Peretyazhko and Savina, 2010) concluded that in the ongonite magma of the massif that coexisted with crystalline phases and silicate melt, water-salt fluids and various fluoride melts occurred that were similar in composition to fluorite, sellaite, cryolite, or chiolite. In the same work, mineral associations characterized the coexistence in the subliquidus region of a system of two immiscible melts, that is, silicate and Ca–F salt. Signs of the existence of a Ca–F melt were described for alkaline granites of Mongolia (Peretyazhko et al., 2018) and granite rare-metal pegmatites of Canada (Vasyukova and Williams-Jones, 2014).
The formation of fluoride melts at the magmatic stage of formation of massifs can affect the distribution of ore components and, as a result, be one of the decisive factors in the formation of deposits (Alferyeva et al., 2019, 2020; Gramenitskiy et al., 2005). Numerous attempts were made to experimentally model the equilibrium coexistence of quartz-standard silicate and Ca–F salt melts; however, the immiscibility of silicate and mainly calcium fluoride melts were reliably obtained only in the region of high supra-liquidus temperature (Suk et al., 2018). It has been shown that for fluorite-containing trachyrhyolites of Mongolia a Ca–F salt melt coexists with a silicate melt at 1250°C. The equilibrium silicate melt–Ca–F salt melt will most likely also occur in the studied samples at a temperature exceeding the crystallization temperature of the fluorite melt. At a lower temperature for similar systems in all experimental studies without exception, a stable silicate melt–fluorite phase association was obtained (Alferyeva et al., 2018a; Dolejš and Baker, 2004, 2006; Gramenitskiy et al., 2005; Lukkari and Holtz, 2007; Price et al., 1999; Scaillet and Macdonald, 2004).
From the point of view of the generally accepted model, high-fluorine granite melts are formed as residuals from crystallization of ordinary granites. The liquidus temperature of a granite melt saturated with water at P = 100 MPa is not more than 800°C (Holtz et al., 2001). The addition of fluorine to the system leads to a significant decrease in its crystallization temperature (Holtz et al., 1993; Kovalenko, 1979; Manning, 1981). According to the data of (Syritso et al., 2012), the crystallization temperature of various massifs of ongonites in Transbaikalia lies in the range of 600–700°C. The liquidus temperature of the Ary-Bulak ongonite melt corresponds to 600–750°C (Antipin et al., 2009; Peretyazhko and Savina, 2010). Based on these data, the existence in nature of a fluorine-rich granite melt at a temperature of 1250°C, which is higher than the liquidus temperature of an ordinary granite melt, seems impossible. One of the objectives of this experimental work was an attempt to obtain a Ca–F melt at a low temperature that meets the conditions of the subliquidus of the granite system.
One characteristic feature that distinguishes the composition of the Ca–F melt from fluorite is its significant oxygen content (4–10 wt %). We propose that an increase in O2 fugacity in the system can lead to an increase in the amount of the oxygen impurity in fluorite and, due to an increase in impurities, possibly in its transformation into Ca–F melt at the subliquidus temperature of the granite system. The aim of our work was to determine the possible effect of oxygen fugacity on the phase relations and the composition of phases in the system corresponding to fluorite–containing ongonite at T = 700–800°C and a pressure of 100 MPa.
MATERIALS AND METHODS
Natural samples of porphyry fluorite-bearing ongonites (no. ARB–24) and aphyric rocks of the endo–contact facies (no. ARB-19) on the southwestern flank of the Ary-Bulak massif were used as the initial compositions. All samples for experimental study in the form of powders were provided by I.S.Peretyazhko. The contents of the main components in the rock samples are presented in Table 1. A detailed petrographic description and complete data on the chemical composition of the samples were given in (Kovalenko V.I. and Kovalenko N.I., 1976; Peretyazhko and Savina, 2010). Data on the geological structure of the Ary-Bulak massif can be found in (Antipin et al., 2009).
Porphyry fluorite-containing ongonite (sample no. ARB-24) consists of 20–30% porphyry phenocrysts represented by quartz, albite, and sanidine. Topaz and mica of the zinnwaldite series are present in small quantities. The micro-grained groundmass is composed of the same minerals and glass; the presence of fluorite (up to 5%) was noted. The rock of the endocontact facies (sample no. ARB-19) has a similar mineral composition and aphyric structure with rare (up to 1–2%) phenocrysts in the cryptocrystalline groundmass. In these two varieties of rocks, numerous areas up to several millimeters in size were described (Peretyazhko and Savina, 2010), consisting of “F–Ca glass” and crystals of fluorite and dickite; their formation is attributed to the liquid immiscibility of silicate and Ca–F salt melts.
The rock samples were ground in jasper mortars. The resulting powder was loaded into platinum ampoules; 10 wt % water was added to each ampoule. The ampoules were then hermetically sealed. The length of the obtained ampoules was approximately 1.5 cm, the outer diameter was 3 mm, and the wall thickness was 0.2 mm.
In the first series of experiments, the oxygen fugacity was set by the composition of the exoclave reactor; it corresponded to the Ni–NiO buffer. The experiments were carried out at 800 and 700°C, a pressure of 100 MPa on a high-pressure hydrothermal installation with external heating and a cold seal at the Chair of Petrology and Volcanology of the Department of Geology of Moscow State University. The duration of the experiments at 800°C was 3 days, at 700°C it was 7 days. The accuracy of temperature maintenance was 10°С and the pressure was 10 MPa. The hardening time was approximately 10 min.
In the second series of experiments, the conditions of increased oxygen fugacity were set using a buffer Mt–Hem mixture. The temperature regime and the duration of the experiments in this case were limited by the time of the reaction between the components of the buffer pair. The experiments were carried out at the Institute of Experimental Mineralogy (IEM) RAS (Chernogolovka) on a UVGD10000 high gas pressure unit under the direction of V.Yu. Chevychelova.
The buffer mixture was made up of magnetite and hematite, mixed in a ratio of 1 : 9. The sealed ampoules with crushed rock samples and the required amount of water were loaded into another ampoule with a volume of approximately 0.8 cm3. Buffer and water were added to this vial. The ampoule was also welded. To increase the buffer run time, this double ampoule was placed in another external ampoule with a volume of approximately 1.9 cm3, which was also filled with the buffer mixture and water and sealed. Each stage of ampoule preparation was accompanied by taking the sample weight with an accuracy of ±10–5 g.
The temperature of the second series of experiments was 750°C, the pressure was 100 MPa, and the duration was 2 days. The hardening time was approximately 20 min.
According to the results of the analysis of the buffer mixture phase composition at the end of the experiment it consisted of 100% magnetite in the outer ampoule, the Mt : Hem ratio in the middle ampoule was ~4 : 1.
The determination of the chemical composition of the samples was performed in the laboratory of local methods for the study of matter at the Chair of Petrology and Volcanology of the Department of Geology of Moscow State University using an energy-dispersive microanalyzer based on a Jeol JSM-6480LV scanning electron microscope (INCA-Energy 350 spectrometer). The dispersion characterizing the detection threshold for F was 0.05 wt %, while for Na, K, Ca, Al, and Si it was 0.02 wt %. The determination accuracy was ±10 rel. % at a content of up to 1 wt %; ±5 rel. % at a content from 1 to 5 wt %; and ±2 rel. % at a content from 5 to 10 wt %.
RESULTS AND DISCUSSION
As a result of the experiments, approximately 2–10 mm samples were obtained, which mainly consist of aluminosilicate glass and various crystalline phases. The chemical composition of glasses according to EDS analysis is given in Tables 2 and 3. The Ca–F salt melt was not found in the studied samples. The change in oxygen fugacity corresponding to the Ni–NiO and Mt–Hem buffers did not lead to a qualitative change in the phase relations in the system.
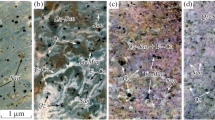
For the Ni–NiO buffer T = 800–700°С. Porphyry fluorite-containing ongonite at 800°С (experiment No. 24NN800) consists of 85% aluminosilicate melt. Crystalline phases are represented by fluorite (10%) and topaz (5%). Fluorite forms small (up to 10 μm) isometric well-faceted or rounded crystals. Topaz is represented by grains ranging in size from 2 to 10 μm, with elongated short-columnar or isometric outlines (Fig. 1).
When the temperature was decreased to 700°С (experiment no. 24NN700), plagioclase (~3%) occurred in the sample, which consisted of elongated subidiomorphic or skeletal crystals of andesine composition up to 20 μm. The size of fluorite crystals increased to 15–20 μm. According to the results of EDS analysis, it has the following composition (at %): Ca, 31.3; F, 66.5; O, 2; Si, 0.2; Al, 0.1; and K, 0.1.
In aphyric rocks at 800°C (experiment no. 19NN800), stable phases are represented by silicate melt (55%) and approximately equal amounts of quartz, topaz, and fluorite. Fluorite forms isometric faceted or rounded grains of up to 10 μm. Topaz is represented by small (up to 5 μm) elongated short-columnar crystals, quartz is represented by round irregular grains up to 50 μm in size. They often contain inclusions of aluminosilicate glass or crystalline phases.
When the temperature was decreased to 700°C (experiment no. 19NN700), plagioclase also occurred in the sample; its composition corresponds to bitownite. It is represented by elongated idiomorphic crystals of up to 10 μm. Fluorite consists of (at %) Ca (30.8), F (65.7), Si (0.4), Al (0.3), and O (2.7). Topaz forms small (up to 5 microns) grains of an irregular shape. Quartz is represented by large (up to 70 microns) segregations of an irregular rounded shape. They often contain inclusions of glass, plagioclase, and topaz. The chemical composition of quartz is characterized by the presence of impurities (at %): Al, 0.6; F, 0.5; K and Ca, 0.1.
Mt–Hem buffer, T = 750°C. An increase in oxygen fugacity in the studied samples does not lead to a qualitative change in phase equilibria. As in the previous series of experiments, with the specified parameters (experiments no. 24MH and no. 19MH), the samples mostly consisted of silicate glass, 85 and 55%, respectively (Fig. 2). In addition, fluorite (10 and 15%) and topaz (5 and 15%) are present. In sample no. 19MH, quartz was formed. The fluorite of both samples consists in (at. %) Ca (31), F (65), Si (0.4), O (3.7), K (0.1), and Na (0.1).
The obtained experimental data are fully consistent with the results of previous studies of phase relations in fluorine-containing magmatic systems (Alferyeva et al., 2011, 2018a, 2018b; Dolejš and Baker, 2004, 2006; Gramenitskiy et al., 1993, 2005; Lukkar and Holtz, 2007; Price et al., 1999; Scaillet and Macdonald, 2004; Shchekina et al., 2013). Both in model systems and during melting of natural samples under subliquidus conditions of a deeply differentiated granite system, immiscible silicate and Ca–F salt melts are not formed.
The Ca–F phase in all the obtained samples under the experimental parameters is a stable equilibrium crystalline fluorite, and not a Ca–F salt melt. Most of the grains of this phase in the samples have characteristic crystallographic shapes with well-formed crystal edges and vertices. In some cases, the crystal vertices are rounded, but, as a rule, even in these cases, facets or their traces are clearly visible in the grains. In the grains of this phase, no characteristic morphological forms characterizing the coalescence of droplets or the formation of a layer of molten salt were found. On the contrary, between individuals in the case of their joint growth, acute, right, or obtuse angles with a well-defined apex are formed. Skeletal forms are absent among the grains, which could indicate the crystallization of fluorite from the supercooled melt during the quenching of the samples. The chemical composition of rounded and faceted fluorite varieties is the same within the same sample. The ratio of Ca and F in grains is close to stoichiometric. It remains constant and does not depend on the composition of the coexisting silicate melt.
According to the data we obtained, an increase in oxygen fugacity promotes a slight increase in the content of oxygen impurities in fluorite: from 2—3 at % with the Ni–NiO buffer and approximately up to 4 at % with the Mt–Hem buffer. This correlation between the oxygen content in fluorite and the oxygen fugacity requires detailed verification by other analytical methods. The measured content of impurities does not lead to the destruction of the crystal structure of fluorite and to the expansion of the stability field of the Ca–F salt melt into the subliquidus region of the granite system.
Our experiments simulated the equilibrium phase relations in these rocks at the given parameters. The composition of the glass in the samples (excluding the content of volatile components) reflects the composition of the silicate melt under the parameters of the experiment. The change in the composition of the glass of the samples with decreasing temperature shows the direction of the change in the composition of the residual melt during the crystallization differentiation of these rocks.
Figure 3 shows the trends of changes in the content of SiO2 and F in the glasses with decreasing temperature. Since the liquidus minerals of these samples are represented by high-fluoride fluorite and topaz, with a decrease in temperature from 800 to 700°C, their residual melt is depleted in fluorine. The absence of high-silicon minerals in samples 24NN800 and 24MH led to a significant increase in the silica content in the melt of sample 24NN700 at 700°C. Despite the formation of quartz, in samples no. 19NN800, no. 19MH and no. 19NN700, the silica content in the residual melt also increased with a decrease in temperature from 800 to 700°C.
The obtained inverse proportional relationship between the contents of SiO2 and F in the residual melt is in accordance with the data of (Kovalenko, 1979; Kovalenko V.I. and Kovalenko N.I., 1976; Manning, 1981). An increase in the fluorine content in the system leads to an expansion of the quartz stability field and a corresponding decrease in the silica content in the residual melt. The increase in oxygen fugacity has practically no effect. The points of the glasses composition obtained in the conditions of the Mt–Hem buffer fall within the limits of errors on the trends of changes in the content of these elements in the conditions of the Ni–NiO buffer.
The change in oxygen fugacity affects the agpaiticity coefficient of silicate glasses. Figure 4 shows that considering the values of the absolute measurement error the composition of the silicate glass obtained in experiment no. 24MH under the conditions of the Mt–Hem buffer is more plumazitic than that of the glasses obtained in samples no. 24NN800 and no. 24NN700 under the conditions of the Ni –NiO buffer. The only crystalline phase that affects the melt agpaiticity coefficient in experimental samples 24NN800, 24NN700, and 24MH is topaz. Therefore, a decrease in the agpaite coefficient and an increase in the Al2O3 content in the silicate melt in this case are most likely due to an increase in the solubility of topaz in it. In experimental samples based on the rock of the endocontact facies (sample no. ARB-19) this effect, considering the measurement errors, is almost not manifested.
In the glasses of experiments with increased oxygen fugacity, a reduced CaO content was noted. Figure 5 shows that the compositions of these glasses are significantly lower than the trend lines obtained for systems with fugacity corresponding to the Ni–NiO buffer. The only Ca-containing phase in these samples (except for silicate melt) is fluorite. Therefore, apparently, a decrease in the calcium content in the melt indicates a decrease in the solubility of fluorite in it. This effect is more pronounced for experimental samples based on the rock sample no. ARB-19 than for specimen no. ARB-24.
The experimental data we obtained indicate that the composition of the silicate melt and the solubility of fluorine-rich phases in it can vary significantly depending on the oxygen fugacity value. This effect is most likely based on the principle of the acid–base interaction of system components. An increase in oxygen fugacity can lead to a redistribution of cations between fluorine and oxygen and, as a result, contribute to a change in the stability fields of various phases. In natural systems, a change in the regime of volatiles can cause partial dissolution of already formed high-fluoride minerals or, conversely, the beginning of crystallization of new ones.
CONCLUSIONS
In all the experiments, the maximum limit of the fluorine content in the silicate melt of the given composition and the saturation of this melt with respect to fluorite and topaz were reached.
No signs of silicate–fluoride liquid immiscibility were found in the products of the experiments. A change in the oxygen fugacity within the Ni–NiO and Mt–Hem buffers in the studied samples does not lead to the formation of a Ca–F melt.
The increase in oxygen fugacity promotes a slight increase in the aluminum content and a decrease in the calcium content in the silicate melt. Such variations in the composition of the quartz-standard fluorine-containing melt are possible due to a change in the solubility of topaz and fluorite in it.
REFERENCES
Alferyeva, Ya.O., Gramenitskii, E.N., and Shchekina, T.I., Experimental study of phase relations in a lithium bearing fluorine rich haplogranite and nepheline syenite system, Geochem. Int., 2011, vol. 49, no. 7, pp. 676–690.
Alferyeva, Ya.O., Novikova, A.S., and Dmitrieva, A.S., Experimental study of phase relations in crystallization of ongonite melt from the Ary-Bulak intrusion, in Tr. Vseross. ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Trans. All-Russ. Annu. Sem. Experiment. Mineral., Petrol., Geochem.), Moscow: GEOKhI RAN, 2018a, pp. 93–97.
Alferyeva, Ya.O., Shchekina, T.I., and Gramenitskiy, E.N., The limiting contents of fluorine and water in highly differentiated granite melts, Moscow Univ. Geol. Bull., 2018b, vol. 73, no. 4, pp. 390–396.
Alferyeva, Ya.O., Gramenitskiy, E.N., Shchekina, T.I., and Zinov’eva, N.G., Variations of Ta and Nb contents in peraluminous granite high-fluorine melt in connection with association of liquidus phases, Moscow Univ. Geol. Bull., 2019, vol. 74, no. 4, pp. 393–400.
Alferyeva, Ya.O., Gramenitskii, E.N., and Shchekina, T.I., Changes in the Ta/Nb ratio in successively formed differentiates of granite melt (calculations based on experimental data), Russ. Geol. Geophys., 2020, vol. 61, no. 1, pp. 26–35.
Antipin, V.S., Andreeva, I.A., and Kovalenko, V.I., Kuznetsov. V.A. Geochemical specifics of ongonites in the Ary-Bulak Massif, eastern Transbaikalia, Petrology, 2009, vol. 17, no. 6, pp. 558–569.
Dolejš, D. and Baker, D.R., Thermodynamic analysis of the system Na2O–K2O–CaO–Al2O3–SiO2–H2O–F2O–1: Stability of fluorine-bearing minerals in felsic igneous suites, Contrib. Mineral. Petrol., 2004, vol. 146, pp. 762–778.
Dolejš, D. and Baker, D.R., Fluorite solubility in hydrous haplogranitic melts at 100 MPa, Chem. Geol., 2006, vol. 225, pp. 40–60.
Gramenitskiy, E.N. and Shchekina, T.I., Phase relationships in the liquidus part of a granitic system containing fluorine, Geochem. Int., 1994, no. 31, pp. 52–70.
Gramenitskii, E.N., Shchekina, T.I., and Devyatova, V.N., Fazovye otnosheniya vo ftorsoderzhashchikh granitnoi i nefelin-sienitovoi sistemakh i raspredelenie elementov mezhdu fazami (Phase Relations in the Fluorine-Bearing Granite and Nepheline–Syenite Systems and Element Partitioning between Phases), Moscow: GEOS, 2005.
Holtz, F., Dingwell, D.B., and Behrens, H., Effects of F, B2O3, and P2O5 on the solubility of water in haplogranite melts compared to natural silicate melts, Contrib. Mineral. Petrol., 1993, vol. 113, no. 4, pp. 492–501.
Holtz, F., Johannes, W., Tamic, N., and Behrens, H., Maximum and minimum water contents of granitic melts generated in the crust: A reevaluation and implications, Lithos, 2001, vol. 56, no. 1, pp. 1–14.
Kovalenko, N.I., Eksperimental’noe issledovanie obrazovaniya redkometall’nykh litii-ftoristykh granitov (Experimental Studies of the Formation of Rare-Metal Li–F Granites), Moscow: Nauka, 1979.
Kovalenko, V.I. and Kovalenko, N.I., Ongonity – subvulkanicheskie analogi redkometall’nykh litii-ftoristykh granitov (Ongonites – Subvolcanic Analogues of the Rare Metal Lithium-Fluorine Granites), Moscow: Nauka, 1976.
Kovalenko, V.I., Kuz’min, M.I., Antipin, V.S., and Petrov, L.L., Topaz-bearing quartz keratophyre (ongonite) – A new variety of subvolcanic vein magmatic rocks, Dokl. Akad. Nauk SSSR, 1971, vol. 199, no. 2, pp. 430–433.
Lukkari, S. and Holtz, F., Phase relations of F-enriched peraluminous granite: an experimental study of the Kymi topaz granite stock, southern Finland, Contrib. Mineral. Petrol., 2007, vol. 153, pp. 273–288.
Manning, D.A.C., The effect of fluorine on liquidus phase relationships in the system Qz–Ab–Or with excess water at 1 kb, Contrib. Mineral. Petrol., 1981, vol. 76, pp. 206–215.
Peretyazhko, I.S. and Savina, E.A., Fluid and magmatic processes in the formation of the Ary-Bulak ongonite massif (Eastern Transbaikalia), Russ. Geol. Geophys., 2010, vol. 51, no. 10, pp. 1110–1125.
Peretyazhko, I.S., Zagorskii, V.E., Tsareva, E.A., and Sapozhnikov, A.N., Immiscibility of calcium fluoride and aluminosilicate melts in ongonite from the Ary-Bulak intrusion, Eastern Transbaikal region, Dokl. Earth Sci., 2007, vol. 413, no. 2, pp. 315–320.
Peretyazhko, I.S., Savina, E.A., Karmanov, N.S., and Dmitrieva, A.S., Immiscibility of fluoride–calcium and silicate melts in trachyrhyolitic magma: Data on acidic volcanic rocks from the Nyalga Basin, Central Mongolia, Petrology, 2018, vol. 26, no. 4, pp. 389–413.
Price, J.D., Hogan, J.P., Gilbert, M.C., et al., Experimental study of titanite-fluorite equilibria in the A-type Mount Scott granite: Implications for assessing f contents of felsic magma, Geology, 1999, vol. 27, pp. 951–954.
Scaillet, B. and Macdonald, R., Fluorite stability in silicic magmas, Contrib. Mineral. Petrol., 2004, vol. 147, pp. 319–329.
Shchekina, T.I., Gramenitskiy, E.N., and Alferyeva, Ya.O., Leucocratic magmatic melts with the maximum fluorine concentrations: Experiment and relations in nature, Petrology, 2013, vol. 21, no. 5, pp. 454–470.
Suk, N.I., Kotel’nikov, A.R., Peretyazhko, I.S., and Savina, E.A., Evolution of trachyrhyolite melt as evidenced from experimental data, in Tr. Vseross. Ezhegodnogo seminara po eksperimental’noi mineralogii, petrologii i geokhimii (Trans. All-Russ. Annual Sem. Experiment. Mineral., Petrol., Geochem.), Moscow, 2018, pp. 129–132.
Syritso, L.F., Badanina, E.V., Abushkevich, V.S., et al., Volcanoplutonic association of felsic rocks in the rare-metal ore units of Transbaikalia: Geochemistry of rocks and melts, age, and P–T conditions of their crystallization, Petrology, 2012, vol. 20, no. 6, pp. 567–592.
Vasyukova, O. and Williams-Jones, A.E., Fluoride-silicate melt immiscibility and its role in REE ore formation: Evidence from the Strange Lake rare metal deposit, Quebec-Labrador, Canada, Geochim. Cosmochim. Acta, 2014, vol. 139, pp. 110–130.
Funding
Analytical data were obtained at the Laboratory of Local Methods of Material Research (Chair of Petrology and Volcanology, Department of Geology of Moscow State University) using a JEOL JXA-8230 electron probe microanalyzer purchased with funds from the Moscow University Development Program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Nickolsky
About this article
Cite this article
Alferyeva, Y.O., Novikova, A.S. The Composition of a Silicate Melt of Fluorite-Containing Ongonites at 700–800°C, 100 MPa, and Different Oxygen Fugacities. Moscow Univ. Geol. Bull. 76, 415–422 (2021). https://doi.org/10.3103/S0145875221040025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0145875221040025