Abstract—
Exposure to β-lactam, fluoroquinolone, and aminoglycoside antibiotics caused an increase in the production of hydrogen peroxide and the expression of OxyR-regulon of the oxidative stress response in Escherichia coli cells. Under the conditions of microaeration, the attenuation of secondary oxidative stress due to the addition of the antioxidant thiourea influenced the antibacterial effect of fluoroquinolones. Thiourea potentiated the effect of sublethal (not decreasing the number of colony-forming units below 103/mL) doses of the antibiotic and increased the viability of cells exposed to lethal doses. The addition of thiourea reduced the expression of OxyR-regulon activated by the sublethal action of the antibiotic to the level of antibiotic-free culture. When exposed to lethal doses, a decrease in the antibiotic-mediated expression of oxidative stress response genes in the presence of thiourea was also observed; however, the expression level remained higher compared to antibiotic-free culture. This may indicate the dual role of ROS under antibiotic treatment as the damaging agents contributing to cell death and as signal molecules activating stress responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
One of the problems facing modern medicine is the reduced efficacy of antibiotic therapy for bacterial infections due to the drastic increase in the number of resistant forms of microorganisms. This problem can be solved by searching for compounds with new mechanisms of antibacterial action and for factors enhancing the antimicrobial activity of the known drugs as well as by studying the mechanisms of formation of antibiotic resistance in order to prevent the emergence of resistant forms.
It has previously been demonstrated that the oxidative stress markers, such as elevated intracellular levels of reactive oxygen species (ROS), activation of antioxidant protection systems, or oxidative damage to macromolecules, appear in bacterial cells exposed to different antibacterial agents [1, 2]. In view of the increase in antibiotic resistance, the potential involvement of ROS in bactericidal effects of antibiotics attracts special attention and has been widely discussed throughout the past decade. It is supposed that antibacterial effects of the known drugs can be enhanced due to potentiation of intracellular ROS production or attenuation of bacterial antioxidant defense.
The active discussion of this issue was initiated by the work of Collins et al., who demonstrated the increased ROS production in bacterial cells exposed to bactericidal antibiotics, including fluoroquinolones, aminoglycosides, and β-lactams. Attenuation of the accompanying oxidative stress on the addition of antioxidants resulted in the higher survival rate of bacteria [3]. Hence, Collins’ team supposed that bacterial death caused by bactericidal antibiotics was determined, apart from specific effects of drugs on the target, by a common mechanism based on oxidative damage to cell components.
In spite of the great number of works devoted to this subject, the role of ROS in bactericidal effects of antibiotics, in particular, under natural conditions of existence of microorganisms, remains a subject of debate [4–6]. Taking into account the limitations of each of the methods used in these studies, including the comparative evaluation of bacterial viability under aerobic and anaerobic conditions, with the addition of chemical antioxidants and in the presence/absence/overexpression of antioxidant genes [3, 7–9], it can be concluded that ROS are involved in cell death caused by antibiotics, at least under laboratory conditions.
As a rule, the contribution of ROS to bacterial death under antibiotic exposure has been demonstrated for fast-growing cells cultivated under conditions of intense aeration [3, 8, 9], while natural habitats are rather characterized by microaeration and slow growth. Activation of antioxidant protection systems under microaeration conditions in response to antibiotics [10], as well as the elevated levels of ROS in biofilm cells in the presence of the latter [11], also suggests the involvement of the common mechanism of oxidative damage in bactericidal effects of antibiotics under these conditions.
At the same time, it has been shown that endogenous oxidative stress promotes genetic diversity in biofilm cells, which may enhance the adaptive capacity of a bacterial population exposed to antibiotics [12]. A similar case scenario was observed under the sublethal antibiotic exposure [13]. In addition to mutagenic effects, ROS can also perform the signaling function by activating the systems of adaptive responses. In Escherichia coli, most antioxidant genes are combined into two regulons: the SoxRS-regulon, with its expression being induced in response to the high level of superoxide anions and the effects of redox-cycling compounds, and the OxyR-regulon activated in response to the increasing concentration of hydrogen peroxide [14].
One of the proofs for the involvement of ROS in bactericidal effects of antibiotics under natural conditions is the fact that the basal level of expression of the SoxRS-regulon genes increases in some clinical isolates of enterobacteria resistant to fluoroquinolones [15]. However, the mechanisms of limitation of antibiotic influx and the activity of multidrug efflux pumps, which is also under control of this regulon, can also make their contributions here, together with the activity of antioxidant enzymes. In addition, it has been shown that the clinical isolates of Acinetobacter baumanii characterized by multiple drug resistance exhibit the enhanced activity of catalase responsible for the decomposition of hydrogen peroxide [16].
Thus, the role of ROS in bactericidal effects of antibiotics is not so unambiguous and depends on conditions, drug dose, and the state of a microorganism.
In the present work, we have studied the level of hydrogen peroxide production and expression of the genes comprising the OxyR-regulon and assessed the contribution of ROS to the death of E. coli cells exposed to β-lactam, fluoroquinolone, and aminoglycoside antibiotics under microaeration conditions.
MATERIALS AND METHODS
Microorganisms and cultivation conditions. E. coli BGF931 (MC4100 λ katG’::lacZ) and BGF940 (MC4100 λ oxyR’::lacZ) were kindly provided by Professor B. Demple [17].
The cells of E. coli maintained on LB agar slants (Sigma, United States) were placed into the tubes with 5 mL of LB broth (Amresco, United States) and cultivated for 4–6 h at 37°C without stirring. Bacterial cells were then transferred (1 : 1000) to Erlenmeyer flasks containing 50 mL of the M9 medium with 0.4% glucose and cultivated at 37°C with stirring at 100 rpm (thermostatic shaker 1092 (GFL, Germany)) for 14–16 h. An overnight culture was diluted in a fresh medium to the optical density of 0.1 and cultivated under the conditions described above. The tested antibiotics were added after the culture had reached an optical density A600 = 0.3 (Figs. 1, 2).
Effects of antibiotics on hydrogen peroxide production by the E. coli BGF931 cells. Bacterial cells were cultivated in the absence of antibiotics (control) and in the presence of 15 µg/mL of Amikacin, 0.02 µg/mL of Levofloxacin, or 3 µg/mL of Cefotaxime. The data are presented as the mean ± standard error. DW is absolutely dry weight. *Statistically significant difference between the culture with Cefotaxime and the control; **statistically significant difference between the culture with Levofloxacin and the control; ***statistically significant difference between the culture with Amikacin and the control (t-test, p < 0.05).
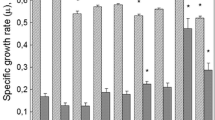
Effects of antibiotics on the katG gene expression in the E. coli BGF931 cells. The level of expression was determined 4 h after the addition of antibiotics. The data are presented as the mean ± standard error. n.s. is the absence of statistically significant difference from the control (t-test, p < 0.05).
In experiments aimed at studying the effect of thiourea (Sigma, United States), an overnight culture was diluted in a fresh M9 medium with the addition of 0.4% glucose (50 mL) to optical density A600 = 0.1 and cultivated without stirring until the culture reached A600 = 0.3. Equal volumes of the culture were then transferred into the tubes with thiourea/water, where the tested antibiotics (at a final volume of 5 mL) were added after 10-min incubation. The tubes were incubated at 37°C without stirring (Table 1).
The optical density of bacterial cultures (A600) was measured by absorption at 600 nm with an UV-1650PC spectrophotometer (Shimadzu, Japan).
Gene expression. The level of gene expression was assessed by the activity of β-galactosidase in the cells carrying the fusion of the promoter of the gene under study and the structural part of the lacZ gene. Activity was measured in the cells pretreated with a sodium dodecyl sulfate and chloroform mixture, with the addition of ortho-nitrophenyl-β-galactopyranoside (Sigma, United States) as a substrate [18].
Bacterial viability. Several serial dilutions of the bacterial culture in normal saline solution were prepared and the cell suspension was inoculated by 10 µL onto LB agar in Petri dishes. The dishes were incubated at 37°C for 16–18 h and then the colony-forming units (CFU) were counted.
H2O2 production by bacterial cells. The amount of H2O2 in the cultivation medium was determined using the Amplex red fluorescent dye (Invitrogen, United States) in the reaction with horseradish peroxidase (Sigma, United States). 0.5 mL of the culture was centrifuged at 16 000g for 2 min, and the supernatant was frozen. The reaction was reproduced according to the manufacturer’s instructions. Fluorescence (530/590 nm) was measured with an Infinity M200 multimode plate reader (Tecan, Switzerland).
Statistical processing of the results was performed with Statistica 6.0 (StatSoft Inc., United States). Statistical significance of the differences between the mean values of the compared groups was assessed by the unpaired Student’s t-test. The differences were considered significant at p < 0.05.
RESULTS
Antibiotics cause an increase in hydrogen peroxide production and expression of the katG gene in bacterial cells. Oxidative stress markers were studied in E. coli cultures exposed to the sublethal action of β-lactam, fluoroquinolone, and aminoglycoside antibiotics, including the expression of the OxyR-regulon genes and the amount of hydrogen peroxide in the cultivation medium. As is known, β-lactams (Cefotaxime) inhibit cell wall synthesis, fluoroquinolones (Levofloxacin) influence DNA synthesis and integrity, and aminoglycosides (Amikacin) inhibit protein synthesis. In addition, the oxidation of bacterial cell components as a result of increased ROS production is considered to be the common mechanism of antibacterial action for all three groups of antibiotics [3]. The sublethal doses of the antibiotics (sublethal action) are antibiotic concentrations not causing the death of all cells of bacterial culture (in particular, the decrease in optical density of the culture compared to the starting point at the moment of antibiotic addition and/or decline in CFU number below 103/mL). The subsequent experiments were also carried out with inhibitory doses, when CFU number in the culture was maintained at the same level as at the moment of adding the antibiotic, and lethal doses causing a decline in CFU number below 103/mL.
The increase in the density of E. coli batch culture in the absence of antibiotics (control conditions) was accompanied by H2O2 accumulation in the cultivation medium followed by an increase (by approximately 15%) in the level of expression of the katG gene encoding catalase, which catalyzes the decomposition of hydrogen peroxide. Activation of/Growth of expression of katG was recorded after the concentration of H2O2 in the medium had exceeded 0.1 µM. Further increase in the H2O2 concentration (to 0.7 µM) was not accompanied by an increase in gene expression. The decrease in the growth rate as the culture began to enter the stationary phase was accompanied by stabilization of H2O2 concentration in the medium and decrease in the katG expression to the initial level. The increase in H2О2 concentration with the culture growth could be due to the increase in population density but not in ROS production by bacterial cells. In order to eliminate this point when measuring ROS generation by the cells exposed to antibiotics, the rate of H2О2 production was calculated with allowance for the parameters of biomass.
After 90-min exposure to β-lactam and fluoroquinolone antibiotics, the H2О2 content in the medium was above 0.1 µM; in the culture exposed to aminoglycoside, this limit was not exceeded throughout the whole time of observation. Nevertheless, in the first hour of exposure to Amikacin, the level of ROS production was 5–10-fold higher compared to the control (Fig. 1). A considerable increase in the level of H2О2 production by the cells exposed to β-lactam and fluoroquinolone was observed 2 h after the addition of antibiotics (Fig. 1).
The sublethal actions of the fluoroquinolone Levofloxacin and the β-lactam Cefotaxime caused an increase in expression of the katG gene by the dose-dependent manner, though the stimulating effect was observed to decrease after reaching a certain threshold (Fig. 2). The higher level of expression of the genes of the OxyR-regulon occurred during the cultivation both under conditions of more intense aeration (with stirring) (Fig. 2) and under conditions of microaeration (without stirring) (Table 1). The comparatively lower doses of Amikacin (5 µg/mL) caused a decrease in the level of the katG gene expression relative to the control; the minor excess of the control level under the exposure to higher concentrations is determined by the decrease in katG expression in the control culture rather than by the increase of it in response to an antibiotic (Fig. 2).
Decrease in the intensity of secondary oxidative stress due to addition of the antioxidant thiourea changes the susceptibility of E. coli to fluoroquinolone. One of the approaches to evaluation of the involvement of ROS in antibacterial action is to compare the sensitivity of bacterial cells to antibiotics in the presence and absence of an antioxidant. Since this study is focused on the peroxide-induced oxidative stress, the antioxidant was thiourea capable of H2O2 neutralization [19]. In addition, thiourea was used in the previous works devoted to elucidation of the role of ROS in bactericidal effects of antibiotics [3, 4].
In preliminary experiments, we selected the concentration of thiourea sufficient to neutralize the lethal effect of exogenous hydrogen peroxide (6 mM) but having no effect on the growth rate and CFU number during the cultivation under control conditions. Under microaeration conditions, the addition of thiourea changed the susceptibility of E. coli to fluoroquinolones but had no statistically significant effect on the antibacterial effects of β-lactam and aminoglycoside (Table 1). The antioxidant did not influence the inhibitory effect of fluoroquinolone, intensified its sublethal effect, and reduced the effects of lethal doses.
Together with the influence on bacterial cell viability, thiourea exerted an effect on the level of expression of the OxyR-regulon genes. The antioxidant effect of thiourea was manifested by a decrease in the oxyR gene expression activated by the antibiotic (Table 1).
DISCUSSION
The present work has shown the intensification of intracellular production of H2O2 under conditions of medium-intensity aeration and microaeration in bacterial cells treated by antibiotics. In case of exposure to the fluoroquinolone Levofloxacin and the β-lactam Cefotaxime, the elevated level of hydrogen peroxide production was maintained throughout the entire time of observation and was accompanied by the activation of expression of the genes comprising the OxyR-regulon (Figs. 1, 2). Our results differ from the previously published data demonstrating that the effects of the β-lactam Ampicillin and the fluoroquinolone Norfloxacin do not cause accumulation of H2О2 in the amount sufficient for activation of the OxyR-regulon [4]. Divergence of results may be associated both with the limited period of observation in the cited work (2 h) and with different cultivation conditions, including the higher aeration intensity and cell death rate compared to the respective parameters in our experiments.
The involvement of endogenous ROS in the bactericidal effects of antibiotics can be assessed using antioxidants. If ROS production contributes to antibiotic-induced cell death, an antioxidant is supposed to enhance the viability of bacteria by neutralizing ROS. The antioxidant effect of thiourea used in the present work is based on its ability to neutralize hydrogen peroxide [19]. It has also been shown that thiourea can reduce hydroxyl radical production in vitro and in vivo [3, 20], probably due to its ability to interact with H2O2, which is a substrate in the Fenton reaction resulting in the production of hydroxyl radicals. In addition, it has been demonstrated that thiourea is able to neutralize superoxide anions [21].
In contrast to the approaches used in the previously published works, our experiments aimed at studying the effect of thiourea on the antibiotic sensitivity of E. coli were performed under microaeration conditions similar to the conditions of natural habitats of these bacteria, where they are exposed to antibiotics. The protective effect of thiourea was observed only in the cells treated with the fluoroquinolone antibiotic (Table 1). It has been shown previously that the addition of thiourea under conditions of intense aeration protects bacterial cells from the lethal doses of both fluoroquinolones and β-lactams/aminoglycosides [3]. This can be regarded as confirmation of the fact that the contribution of secondary oxidative stress to antibacterial action is proportional to oxygen availability (intensity of aeration of the medium). The latter is true for both antibacterial agents and other stresses [22]. In addition, it can be supposed that ROS generation makes the greatest contribution to the antibacterial effect of fluoroquinolones among the three groups of antibiotics under study.
It should be noted that the effect of thiourea depended on the concentration of the antibiotic (severity of antibiotic stress). The antioxidant did not influence the inhibitory effect of the antibiotic but increased and decreased its sublethal and lethal action, respectively. The measurement of the level of gene expression under these conditions has shown that the addition of thiourea reduces the response of the antioxidant protection system (OxyR-regulon) activated by the antibiotic (Table 1). In case of moderate sublethal action, the oxyR expression upon the addition of thiourea decreased almost to the control level; in the cells exposed to the higher doses of antibiotics, the expression of the antioxidant regulon remained at a comparatively high level. This is evidence of the important role of activation of the OxyR-regulon in the protection of bacterial cells from antibiotic-induced secondary oxidative stress. In addition, the findings can be interpreted as evidence of not only the damaging activity but also the signaling role of ROS under stress conditions. At the same time, such a role is not confined to activation of the oxidative stress response/antioxidant defense mechanisms but can also extend to the general stress response regulator rpoS, the multidrug efflux pumps, the regulation of cell wall permeability, etc. [23–25].
Thus, ROS, the accumulation of which accompanies the effects of β-lactam, aminoglycoside, and fluoroquinolone antibiotics, only influence the antibacterial effect of fluoroquinolones under conditions of micraeration. The decrease in the intensity of secondary oxidative stress and, accordingly, the response of antioxidant protection systems due to addition of the antioxidant (thiourea) increases the sublethal action of the antibiotic and reduces the efficacy of its lethal doses.
REFERENCES
Albesa, I., Becerra, M., Battán, P., and Páez, P., Oxidative stress involved in the antibacterial action of different antibiotics, Biochem. Biophys. Res. Commun., 2004, vol. 317, no. 2, pp. 605–609.
Becerra, M., Paez, P., Larovere, L., and Albesa, I., Lipids and DNA oxidation in Staphylococcus aureus as a consequence of oxidative stress generated by ciprofloxacin, Mol. Cell. Biochem., 2006, vol. 285, nos. 1–2, pp. 29–34.
Kohanski, M., Dwyer, D., Hayete, B., Lawrence, C., and Collins, J., A common mechanism of cellular death induced by bactericidal antibiotics, Cell, 2007, vol. 130, no. 5, pp. 797–810.
Liu, Y. and Imlay, J., Cell death from antibiotics without the involvement of reactive oxygen species, Science, 2013, vol. 339, no. 6124, pp. 1210–1213.
Van Acker, H. and Coenye, T., The role of reactive oxygen species in antibiotic-mediated killing of bacteria, Trends Microbiol., 2017, vol. 25, no. 6, pp. 456–466.
Hong, Y., Zeng, J., Wang, X., Drlica, K., and Zhao, X., Post-stress bacterial cell death mediated by reactive oxygen species, Proc. Natl. Acad. Sci. U.S.A., 2019, vol. 116, no. 20, pp. 10064–10071.
Goswami, M., Mangoli, S., and Jawali, N., Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli, Antimicrob. Agents Chemother., 2006, vol. 50, no. 3, pp. 949–954.
Wang, X. and Zhao, X., Contribution of oxidative damage to antimicrobial lethality, Antimicrob. Agents Chemother., 2009, vol. 53, no. 4, pp. 1395–1402.
Dwyer, D., Belenky, P., Yang, J., et al., Antibiotics induce redox-related physiological alterations as part of their lethality, Proc. Natl. Acad. Sci. U.S.A., 2014, vol. 111, no. 20, pp. 2100–2109.
Akhova, A. and Tkachenko, A., ATP/ADP alteration as a sign of the oxidative stress development in Escherichia coli cells under antibiotic treatment, FEMS Microbiol. Lett., 2014, vol. 353, no. 1, pp. 69–76.
Battan, P., Barnes, A., and Albesa, I., Resistance to oxidative stress caused by ceftazidime and piperacillin in a biofilm of Pseudomonas, Luminescence, 2004, vol. 19, no. 5, pp. 265–70.
Boles, B. and Singh, P., Endogenous oxidative stress produces diversity and adaptability in biofilm communities, Proc. Natl. Acad. Sci. U.S.A., 2008, vol. 105, no. 34, pp. 12503–12508.
Kohanski, M., DePristo, M., and Collins, J., Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis, Mol. Cell, 2010, vol. 37, no. 3, pp. 311–320.
Imlay, J., Pathways of oxidative damage, Annu. Rev. Microbiol., 2003, vol. 57, pp. 395–418.
Koutsolioutsou, A., Pena-Llopis, S., and Demple, B., Constitutive soxR mutations contribute to multiple-antibiotic resistance in clinical Escherichia coli isolates, Antimicrob. Agents Chemother., 2005, vol. 49, no. 7, pp. 2746–2752.
Sato, Y., Unno, Y., Miyazaki, C., Ubagai, T., and Ono, Y., Multidrug-resistant Acinetobacter baumannii resists reactive oxygen species and survives in macrophages, Sci. Rep., 2019, vol. 9, no. 1, 17462.
Ding, H. and Demple, B., In vivo kinetics of a redox-regulated transcriptional switch, Proc. Natl. Acad. Sci. U.S.A., 1997, vol. 94, no. 16, pp. 8445–8449.
Miller, J.H., Experiments in Molecular Genetics, New York: Cold Spring Harbor Lab. Press, 1972.
Randall, L., Reaction of thiol compounds with peroxidase and hydrogen peroxide, J. Biol. Chem., 1946, vol. 164, no. 2, pp. 521–527.
Anbar, M. and Neta, P., A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution, Int. J. Appl. Radiat. Isot., 1967, vol. 18, no. 7, pp. 493–523.
Kelner, M., Bagnell, R., and Welch, K., Thioureas react with superoxide radicals to yield a sulfhydryl compound. Explanation for protective effect against paraquat, J. Biol. Chem., 1990, vol. 265, no. 3, pp. 1306–1311.
Mols, M., Pier, I., Zwietering, M., and Abee, T., The impact of oxygen availability on stress survival and radical formation of Bacillus cereus, Int. J. Food Microbiol., 2009, vol. 135, no. 3, pp. 303–311.
Fraud, S. and Poole, K., Oxidative stress induction of the MexXY multidrug efflux genes and promotion of aminoglycoside resistance development in Pseudomonas aeruginosa, Antimicrob. Agents Chemother., 2011, vol. 55, no. 3, pp. 1068–1074.
Wang, X., Kim, Y., Hong, S., Ma, Q., Brown, B., Pu, M., Tarone, A., Benedik, M., Peti, W., Page, R., and Wood, T., Antitoxin MqsA helps mediate the bacterial general stress response, Nat. Chem. Biol., 2011, vol. 7, no. 6, pp. 359–366.
Tkachenko, A., Stress responses of bacterial cells as mechanism of development of antibiotic tolerance, Appl. Biochem. Microbiol., 2018, vol. 54, no. 2, pp. 108–127.
ACKNOWLEDGMENTS
We would like to thank Professor Bruce Demple (Stony Brook University Medical School, Stony Brook, N.Y.) for providing E. coli strains.
Funding
The study was conducted within the framework of the State Order, topic state registration number AAAA-A19-119112290009-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
ADDITIONAL INFORMATION
Akhova ORCID https://orcid.org/0000-0002-3477-750X
Tkachenko ORCID https://orcid.org/0000-0002-8631-8583
Additional information
Translated by E. V. Makeeva
About this article
Cite this article
Akhova, A.V., Tkachenko, A.G. Role of Secondary Oxidative Stress in the Bactericidal Action of Antibiotics. Moscow Univ. Biol.Sci. Bull. 75, 218–223 (2020). https://doi.org/10.3103/S009639252004001X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S009639252004001X