Abstract
A simple, affordable, and safe (green chemistry) method is proposed for determining 17 quinolone antibiotics in waste and natural waters by the sorption-fluorimetric method using a smartphone as an analyte signal recorder. The method is based on static sorption of quinolones by silica gel from aqueous solutions. When the sorbate is irradiated with ultraviolet light (365 nm), blue or turquoise fluorescence is observed, the intensity of which is measured with a smartphone. The values of the basic components of the RGB colorimetric system are used as an analytical signal, followed by the calculation of the final color. The limits of detection and quantification are 0.0007–0.004 and 0.002–0.01 μg/mL, respectively, for all analyzed analytes. The range of the determined contents is 0.002 (0.01)–0.2 μg/mL. A procedure for the determination of quinolones in waste and natural waters is proposed. The relative standard deviation of the analysis results does not exceed 0.2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
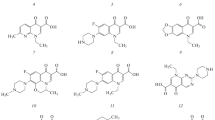
Quinolones (Fig. 1) are antimicrobial agents with a powerful antibacterial effect that totally blocks activity of ferments of pathogenic microorganisms. The bacteria did not reveal any mechanisms that might inactivate the action of quinolones; therefore, resistance to this group of medicines forms much slower. All derivatives of quinolone are divided into nonfluorinated preparations and fluoroquinolones. These classes differ fundamentally in structure, efficiency, as well as in indications for use. Four generations of antibacterial quinolone derivatives recorded in the Russian Federation are known. The main representatives of generation I are nalidixic, oxolinic, and pipemidic acids. Fluoroquinolones belong to generations II–IV.
Structural formulas of (1) sarafloxacin, (2) difloxacin, (3) levofloxacin, (4) lomefloxacin, (5) marbofloxacin, (6) moxifloxacin, (7) nalidixic acid, (8) norfloxacin, (9) oxolinic acid, (10) ofloxacin, (11) pefloxacin, (12) pipemidic acid, (13) danofloxacin, (14) flumequine, (15) ciprofloxacin, (16) enoxacin, and (17) enrofloxacin.
The widespread use of quinolones in veterinary medicine leads to their appearance in food products (meat, milk, fish) and environmental objects. Quinolones enter water bodies with the wastes from animal farms, poultry farms, and pharmaceutical enterprises. The occurrence of residual quantities of quinolones in water negatively affects ecological safety, causing resistance of aquatic microorganisms to antibiotics.
Molecules of quinolone antibiotics have a plane structure (Fig. 1); they can fluoresce upon irradiation with UV light, and the fluorescence intensity in dodecyl sulfate micelles is higher by a factor of 2–3 [2, 3]. In addition, quinolones form complexes with metal ions, in particular, with europium(III), terbium(III), and yttrium(III). In the latter case, this leads to the quinolone sensitized fluorescence of lanthanoid ions; this variant is used to determine quinolones in biological fluids (plasma, blood serum, and urine) [4–10]. It is proposed to determine levofloxacin in the natural water (the test solution, the Volga River) by the fluorescence of its complex with yttrium(III) in the presence of nanoparticles of silver and dodecyl sulfate micelles [11]. The range of the determined content of levofloxacin is 1 × 10–9–1 × 10–6 M, the limit of detection is 3.0 × 10–10 M.
To determine fluoroquinolone in waste and natural waters, the methods of high-performance liquid chromatography with a mass-spectrometry detector (HPLC-MS) are used. They make it possible to identify and determine particular quinolines [12–14]. The procedure for determining ciprofloxacin and norfloxacin together with other antibiotics in waste and surface waters was proposed in [12]. Solid-phase extraction on Oasis HLB cartridges was used for the concentration of analytes. The content of analytes in the sample was estimated by the calibration curves constructed on the matrices of waste and natural waters that do contain antibiotics. The limits of detection were 2–3 ng/L. The waste and natural waters were found to contain 99–410 and 22–54 ng/L of ciprofloxacin and norfloxacin, respectively.
It was proposed to determine cipro-, eno-, o-, and enrofloxacin in tap and river waters [13]. The analytes from a 100-mL sample were concentrated on the surface of wooden toothpicks treated with trimetoxi(7-octen-1-il)silan and sodium pyrosulfite. The desorption of analytes was done directly to the ionization source of the mass spectrometer. The detection limits of fluoroquinolones were 2.5–4.2 ng/L. The content of enrofloxacin and ofloxacin found in the river water was 18.3 and 16.6 ng/L, respectively. The molecularly imprinted polymers were used for concentrating 11 fluoroquinolones from 500 mL of waste waters [14]. The detection limits were 6–150 ng/L. However, complex sample preparation and complex purification of extracts do not allow using these methods widely.
In the works mentioned above, spectrofluorimeters and mass spectrometers were employed for recording an analyte signal. This approach does not provide the rapid determination of antibacterial preparations. An alternative is the use of simple and affordable rapid methods of analyses. One of the directions in this area is digital colorimetry, which has been used for solving different problems of analytical control with increasing frequency [15–17]. This group of methods is characterized by simple implementation and possible use of digital photo-, video-, and optical office equipment as color-recording devices. The considerable potential of colorimetry development is associated with the creation of modern-day smartphones and custom-made software [18, 19]. Due to the achievements in recent years, laboratory research can be done rapidly and on the go.
The purpose of this paper is to study a method for detecting quinolone antibiotics in waste and natural waters by their own fluorescence after their sorption on silica-gel using a smartphone as a color-recording device.
EXPERIMENTAL
Equipment. An Apple iPhone X smartphone (United States) with embedded RGBer custom-made software was used as a color-recording device to study optical and colorimetric characteristics. Fluorescence was excited by a monochromatic radiation source (365 nm) diagnostic fluorescent illuminator “Wood’s lamp OLDD-01” (Russia). The pH values were controlled with an Expert 001 ionometer (Russia).
The equipment in use included Ohaus PA 214C Pioneer-series analytical balance of a special precision class with a 0.1-mg weighing capacity range (United States), Proline Biohite single-channel mechanical pipettes with a variable volume of 2–20, 100–1000, and 1000–5000 μL (Finland), Pall polytetrafluoroethylene membrane filters, 25 mm, with a pore diameter of 0.45 μm (United States), and SPL Life Sciences 15- and 50-mL polypropylene tubes (Korea).
Reagents. The Sigma-Aldrich standard samples of quinolones (98–100%, United States) were used in this work. The stock standard solutions (1 mg/mL) were prepared by dissolving an accurate weight of preparations in methanol. The working solutions were prepared by diluting the stock solutions with deionized water (15–18 MOhm cm, OST 11 029.003-80).
We used silica gel for column chromatography (high purity grade, 70–230 mesh, 63–200 μm, SIAL, Sigma), tris(hydroxymethyl) aminomethane (99.8%, Sigma), EDTA (disodium ethylenediaminetetraacetate) (99%, Khimmed, Russia), hydrochloric acid (puriss., Khimreaktiv, Russia), and methanol (Fisher Scientific U).
Tris buffer was prepared by dissolving 10 g of tris(hydroximethyl)aminomethane and 0.1 g of EDTA in 100 mL of deionized water. The pH value of 8.0 was maintained with a 1 M HCl solution.
Construction of calibration curve. We filled a 50-mL tube with 5, 10, 50, 100, 200, 500, and 1000 μL stock solution of quinolones (10 μg/mL), 0.5 mL of a Tris buffer (pH 8.0), poured as much as 50 mL of deionized water, added 30 mg of silica gel, and mixed for 5 min. After the deposition of silica gel (5 min) and UV irradiation (365 nm), we measured the intensity of blue or turquoise fluorescence with a smartphone. The analyte signal (Ar) in the RGB system was estimated by the formula
where R0, G0, B0, Rx, Gx, and Bx are the digital values of intensities of the red, green, and blue colors of the blank sample and the sample of interest, respectively.
Analysis of natural waters. The water was filtered through a 0.45-μm membrane filter. We poured 50 mL of the studied water into a 50-mL polypropylene centrifuge tube; and added 0.5 mL of a Tris buffer (pH 8.0) and 30 mg of silica gel. Then the tube was covered with a lid and shaken by hand for 4–5 min. After the deposition of silica gel (5 min) and UV irradiation (365 nm), we measured the intensity of the blue or turquoise fluorescence of the sorbate with a smartphone. The content of quinolones was estimated using the calibration curves.
Analysis of waste water. The sample was diluted by a factor of 2 with deionized water and was filtered through a 0.45-μm membrane filter. We poured 50 mL of the prepared water into two 50-mL tubes, added 100 μL of the standard quinolone solution with a concentration of 10 μg/mL into one of the tubes and then repeated the procedure for the analysis of the natural waters. The content of quinolones was estimated by the formula
where cadd is the concentration of an addition in the sample, μg/mL; and Ax and Ax+add are analyte signals in the study sample and in the sample with an added analyte (0.02 μg/mL), respectively.
RESULTS AND DISCUSSION
From the variety of sorbents (polar and nonpolar silica gels, zeolite, silica), we selected silica gel with a particle size of 63–200 μm to implement the solid-phase fluorescence of quinolone antibiotics. The choice was conditioned by availability, safety, low cost, and ability to settle fast (in 3–5 min) on the bottom of the tube. In using silica gel with a smaller particle size (40 μm), centrifugation is required after sorption to separate it from the solution.
During the UV irradiation of the sorbate (365 nm), blue or turquoise fluorescence was observed; its intensity was measured with a smartphone (Fig. 2). As an example, Table 1 and Fig. 3 show the calculation of an analyte signal and the construction of the calibration curve for sarafloxacin.
pH effect. To create a certain pH effect, we used ammonium-acetate buffer solutions and a Tris buffer with the addition of a 1 M HCl solution. The highest intensity of the own fluorescence of quinolones on silica gel was recorded at pH 7.7–9.0 (Fig. 4).
Estimation of matrix effect. The matrix effect (ME) was estimated by the formula
where C and D are the values of the calibration curve slopes obtained on the matrix and the deionized water, respectively.
ME is determined by the effect of the fluorescent compounds and metals ions in the water that, being coadsorbed on the silica gel, are able to form complexes with quinolones. Under these conditions they can both intensify (+) and decrease (–) the intensity of an analyte signal. According to [20], ME can be neglected when its values are found within the range of ±20%. Table 2 shows that ME is insignificant for the natural water (the water in the Klyaz’ma River) and does not exceed 18.9%. To bind metal ions into strong complexes, we introduced 0.1% EDTA in the buffer solution. The study of the waste waters from the poultry farms and baytril-production enterprises revealed a significant matrix effect (30–40%). Its decrease was realized by diluting the water sample by a factor of 2 and by the method of additions for determining quinolones.
Limits of detection and quantification. The limit of detection (LOD) and limit of quantification (LOQ) were calculated by the formulas 3.3 × s/k and 10 × s/k, respectively (s is the standard deviation of the analyte signal for a blank experiment, k is the tangent of angle of the slope of the calibration curve). The standard deviation for Ar of the blank experiment was 0.21 ± 0.03 (n = 15, P = 0.95). The limits of detection and quantification were 0.0007–0.004 and 0.002–0.01 μg/mL, respectively, for all the considered analytes. The determined content varied within 0.002(0.01)–0.2 μg/mL. The calibration curves were nonlinear and the correlation coefficients were ≥0.98 (Table 2).
Analysis of actual samples. The validity and reproducibility of the results of determining quinolones were confirmed by the added-found method using the natural water (the Klyaz’ma River within the limits of the city of Vladimir) for two levels of concentration. The results of determination presented in Table 3 indicate the validity and good reproducibility of the procedure. The relative standard deviation of the results of the analysis does not exceed 0.2.
REFERENCES
Mashkovskii, M.D., Lekarstvennye sredstva: posobie dlya vrachei (Medicines: A Guide for Doctors), Moscow: Meditsina, 2010.
Gonzalez, J.A.O., Mochon, M.C., and de la Rosa, F.J.B., Talanta, 2000, vol. 52, p. 1149.
Ocana, J.A., Barragan, F.J., and Callejon, M., Talanta, 2004, vol. 63, p. 691.
Ocana, J.A., Barragan, F.J., and Callejon, M., J. Pharm. Biomed. Anal., 2005, vol. 37, p. 327.
Guo, C., Dong, P., Chu, Z., Wang, L., and Jiang, W., Luminescence, 2008, vol. 23, p. 7.
Shtykov, S.N., Smirnova, T.D., Bylinkin, Y.G., Kalashnikova, N.V., and Zhemerichkin, D.A., J. Anal. Chem., 2007, vol. 62, no. 2, p. 136.
Beltyukova, S., Teslyuk, O., Egorova, A., and Tselik, E., J. Fluoresc., 2002, vol. 12, no. 2, p. 269.
Egorova, A., Beltyukova, S., and Teslyuk, O., J. Pharm. Biomed. Anal., 1999, vol. 21, p. 585.
Rodriguez-Diaz, R.C., Aguilar-Caballos, M.P., and Gomez-Hens, A., Anal. Chim. Acta, 2003, vol. 494, p. 55.
Zhu, X., Gong, A., and Yu, S., Spectrochim. Acta, Part A, 2008, vol. 69, p. 478.
Smirnova, T.D., Danilina, T.G., Rusanova, T.Yu., and Simbireva, N.A., J. Anal. Chem., 2021, vol. 76, no. 1, p. 89.
Ngumba, E., Kosunen, P., Gachanja, A., and Tuhkanen, T., Anal. Methods, 2016, vol. 8, p. 6720.
Deng, J., Yu, T., Yao, Y., Qi, PengQ., Luo, L., Chen, B., Wang, X., Yang, Y., and Luan, T., Anal. Chim. Acta, 2017, vol. 954, p. 52.
Yu, R., Chen, L., Shen, R., Li, P., and Shi, N., Environ. Technol. Innovation, 2020, vol. 19, 100919.
Monogarova, O.V., Oskolok, K.V., and Apyari, V.V., J. Anal. Chem., 2018, vol. 73, no. 11, p. 1076.
Apyari, V.V., Gorbunova, M.V., Isachenko, A.I., Dmitrienko, S.G., and Zolotov, Yu.A., J. Anal. Chem., 2017, vol. 72, no. 11, p. 1127.
Ivanov, V.M. and Kuznetsova, O.V., Russ. Chem. Rev., 2001, vol. 70, no. 5, p. 357.
Huang, X., Xu, D., Chen, J., Liu, J., Li, Y., Song, J., Ma, X., and Guo, J., Analyst, 2018, vol. 143, p. 5339.
Rezazadeh, M., Seidi, Sh., Lid, M., Pedersen-Bjergaard, S., and Yamini, Y., TrAC, Trends Anal. Chem., 2019, vol. 118, p. 548.
Ferrer, C., Lozano, A., Aguera, A., Giron, A.J., and Fernandez, A.R., J. Chromatogr. A, 2011, vol. 1218, p. 7634.
Funding
This study was supported by Vladimir State University and the Federal Center for Animal Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
No supplementary materials are provided.
Additional information
Translated by L. Mukhortova
About this article
Cite this article
Amelin, V.G., Shogah, Z.A. & Bolshakov, D.S. Sorption-Fluorimetric Determination of Quinolones in Waste and Natural Waters with a Smartphone. Moscow Univ. Chem. Bull. 76, 262–268 (2021). https://doi.org/10.3103/S0027131421040027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131421040027