Abstract
The effect of solid dispersions (SDs) on the solubility of furazolidone is studied. Furazolidone and its SDs with polyvinylpyrrolidone (PVP), PVP-10 000, PVP-12 600, and PVP-24 000 are examined. When using SDs, the solubility and dissolution rate of the nitrofuran derivative is increased. The solubility of furazolidone from SD is increased by a factor of 1.1 to 1.6. The dissolution rate of SDs is increased by a factor of 1.8 to 2.8. The complex of physicochemical methods of investigation suggests that an increase in solubility from SDs is caused by the loss of crystallinity and the formation of a solid solution of furazolidone during the preparation of SDs, as well as the solubilizing effect of the polymer during the subsequent dissolution of the SD in water. The results can be used in the development of hydrophilic soft and fast dissolving solid dosage forms of furazolidone with increased release and bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Furazolidone (Fig. 1) is a synthetic derivative of nitrofuran widely used in clinical practice as an antibacterial and antiprotozoal agent for both internal (dysentery, paratyphoid, foodborne toxic infections, trichomoniasis (when nitroimidazoles are inefficient), shigellosis, and giardiasis) and external (infected wounds and burns) applications [1–3]. It is a yellow or yellow with a greenish tint, odorless fine crystalline powder. It is not hygroscopic, slightly soluble in dimethylformamide, very slightly soluble in acetone, practically insoluble in water, and 96% alcohol [4, 5], which limits the possibility of its use in the form of soft hydrophilic (gels) and solid fast dissolving (granules and tablets) dosage forms (DFs).
To increase the solubility and release of active substances (ASs), the method for the preparation of solid dispersions (SDs), namely, bi- or multicomponent systems consisting of an AS and a carrier, which are a highly dispersed solid phase of AS or molecularly dispersed solid solutions with the partial formation of complexes of variable composition with carrier material, can be used [6–8]. Various polymers can be used as a carrier in the preparation of SDs.
One of the reasons for increasing the dissolution rate of ASs from SDs can be complexation with polymers in a solution, which was previously described in a number of works [9, 10]. From the point of view of chemistry, an increase in the amount of the AS dissolved in water is caused by the formation of hydrogen bonds. The main problem in studying this process by IR spectroscopy is a decrease in the intensity of a large number of characteristic bands of the AS in the composition of the SD, which is associated with a significant shielding effect of polymer. The stoichiometry of complexes consisting of AS and polymer requires a separate study.
The purpose of the work is to study the solubility of furazolidone used in the form of an SD with polyvinylpyrrolidone (PVP).
EXPERIMENTAL
We used furazolidone (Irbit Chemical Pharmaceutical Plant, Russia), which meets the requirements of regulatory documentation (State Pharmacopoeia XIV of the Russian Federation). PVPs with different molecular weights were used as carrier polymers for SDs: 10 000 ± 2000 (Sigma Aldrich, United States), 12 600 ± 2700 (AK Sintvita, Russia), and 24 000 ± 2000 (Sigma Aldrich, United States).
Procedure for the preparation of solid dispersions. The calculated amounts of AS and polymer were dissolved in 96% ethanol (analytical grade), then the solvent was evaporated under reduced pressure in a water bath at a temperature of 95 ± 1°С.
Procedure for preparation of mixtures. Mixtures of furazolidone and PVP were prepared by cogrinding the components in a mortar for 1 min in the same amounts as the corresponding SDs.
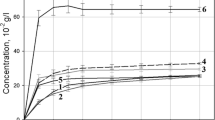
Study of furazolidone dissolution. The dissolution of furazolidone was studied according to the procedure described in [7, 8] at a wavelength of the maximum absorption of furazolidone 367 ± 2 nm. The results of measuring the concentration of furazolidone in the studied solutions are presented in Table 1 and Fig. 2.
Microcrystalloscopic analysis was performed using a Levenhuk D50LNG digital microscope (China), according to the technique described in [7, 8]. Statistical data processing was performed according to OFS.1.1.0013.15 (General Pharmacopoeia Article) (State Pharmacopoeia XIV) (p = 95%, n = 5).
RESULTS AND DISCUSSION
SDs of furazolidone with PVP are homogeneous solid masses of a yellow color or yellow with a greenish tint, glassy in the process of preparation, and prone to sticking. For the study, weighed portions of the samples were taken in an amount sufficient to obtain saturated solutions of the AS. The relative error of the average values of the furazolidone concentration given in Table 1 and Fig. 2 ranged from 2.6 to 4.1%.
The increase in the solubility of furazolidone was calculated as the ratio of the concentration of the saturated solution obtained by dissolving the SD to a concentration of the saturated solution obtained by dissolving its substance in 60 min after the start of dissolution.
As can be seen from the data presented in Table 1 and Fig. 2, furazolidone dissolves better in the form of an SD (in comparison with the active substance); on average, its solubility increases from 1.08 to 1.65 times, depending on the molecular weight of the polymer and the ratio of components in SD.
The greatest increase in the solubility of furazolidone was observed from an SD with PVP-24 000 in the ratio of 1 : 6 by weight (Fig. 2). In the fifth minute of the experiment, the AS concentration in the solution was 3.65 × 10–2 g/L, while for the starting substance in the same time point this value was 1.23 × 10–2 g/L. During the experiment, the AS concentration increased and 60 min after the start of dissolution it reached 4.74 × 10–2 g/L; i.e., the solubility increased by 1.65 times compared with the AS. An increase in the polymer content in SD to a ratio of 1 : 8 and 1 : 10 by weight does not lead to a significant increase in the solubility of furazolidone, increasing it by 1.20 and 1.37 times, respectively.
In order to justify the use of the technological method using solid dispersions, we studied a mixture of furazolidone and PVP-24 000 in a ratio of 1 : 6 by weight (the composition that provides the greatest increase in the AS solubility). The maximum AS concentration in the solution of a mixture was reached 60 min after the start of dissolution and amounted to 2.86 × 10–2 g/L, which is almost identical to the concentration in the furazolidone substance solution at the 60th minute of the experiment, 2.87 × 10–2 g/L (Table 1, Fig. 2). Recrystallization of furazolidone leads to a slight decrease in the solubility of the AS (by a factor of 0.89).
When comparing the dissolution profiles of the substance of furazolidone and its mixture with PVP-24 000, starting from the 50th minute from the beginning of dissolution, there are practically no differences in the course of the curves. This allows us to conclude that the main role in increasing the solubility and dissolution rate of furazolidone is played by the use of SDs obtained by removing the solvent.
It has been found that SDs also affect the dissolution rate of furazolidone. Thus, as compared with the substance, in the first 5 min of the experiment, the dissolution rate of furazolidone from SDs AS : PVP-10 000 (in the ratio of 1 : 4 by weight), AS : PVP-12 600 and AS : PVP-24 000 (in the ratio of 1 : 6 by weight) increases by 2.88 times on average; by the 10th minute of the experiment, this indicator increases by 1.96 times; and to the 15th and 20th minutes, 1.68 and 1.83 times, respectively. Thus, we can conclude that the effect of the quantitative PVP content on the increase in the solubility of furazolidone is pronounced. For each individual case, there are general trends that are sometimes difficult to describe. This is due to the fact that different processes such as crystallization (precipitation), solubilization, the formation of various types of complexes, as well as the formation of a colloidal solution, colloidal protection, and salting out, affect the concentration of furazolidone in the SD solutions over time [9].
Based on the results of the microcrystalloscopic analysis, we can conclude that the observed increase in the solubility of furazolidone from SDs with PVP-24 000 (in the ratio of 1 : 6 by weight) is due to the loss of the crystalline structure, i.e., the disintegration of the AS molecules in PVP, and the preparation of a solid solution of furazolidone in PVP before the stage of dissolution in water, as well as the solubilizing effect of PVP during the dissolution process.
CONCLUSIONS
The results obtained during the study indicate an increase in the solubility and dissolution rate of furazolidone in water from SDs with PVP obtained by the removal of the solvent. To obtain SDs, it is preferable to use a PVP-24 000 carrier in a ratio with AS of 6 : 1 by weight. This ratio provides the greatest increase in the AS’s solubility and dissolution rate.
The results of microcrystalloscopic analysis suggest that the increase in the solubility of furazolidone from SDs is associated with the disintegration of the AS molecules in PVP, the loss of the crystalline structure, the formation of a solid solution of furazolidone in PVP, and the solubilizing effect of the polymer.
A solution of the solid dispersion is a combined system consisting of the true AS solution in the form of molecules and a colloidal solution solubilized by polymer molecules.
The results obtained during this study can be used in the development of hydrophilic soft and fast-dissolving solid DFs of furazolidone with increased release and bioavailability.
REFERENCES
Mashkovskii, M.D., Lekarstvennye sredstva (Medicinal Preparations), Moscow: Novaya Volna, 2016.
Vidal, Medicines Handbook. www.vidal.ru/drugs/ molecule/447. Accessed March 12, 2019.
Russian Medicines Register. www.rlsnet.ru/mnn_ index_id_1371.htm. Accessed March 12, 2019.
United States Pharmacopeia USP40–NF35, 2017, p. 2856.
Gosudarstvennaya farmakopeya Rossiiskoi Federatsii (State Pharmacopoeia of the Russian Federation), Moscow, 2018, 14th ed., vol. 3, p. 5004.
Krasnyuk, I.I., Jr., Beliatskaya, A.V., Krasnyuk, I.I., et al., BioNanoSci, 2017, vol. 7, no. 2, p. 340. https://doi.org/10.1007/s12668-016-0342-6
Belyatskaya, A.V., Krasnyuk, I.I., Jr., Krasnyuk, I.I., et al., Moscow Univ. Chem. Bull. (Engl. Transl.), 2019, vol. 74, no. 1, p. 93.
Belyatskaya, A.V., Krasnyuk, I.I., Jr., Krasnyuk, I.I., et al., Pharm. Chem. J., 2019, vol. 52, no. 12, p. 1001. https://doi.org/10.1007/s11094-019-01941-0
Krasnyuk, I.I., Extended Abstract of Doctoral (Pharm.) Dissertation, Moscow: Sechenov Univ., 2010.
Nikulina, O.I., Krasnyuk, I.I., Belyatskaya, A.V., et al., Pharm. Chem. J., 2012, vol. 46, no. 12, p. 745.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Levit
About this article
Cite this article
Beliatskaya, A.V., Krasnyuk, I.I., Elagina, A.O. et al. Study of the Solubility of Furazolidone from Solid Dispersions with Polyvinylpyrrolidone. Moscow Univ. Chem. Bull. 75, 43–46 (2020). https://doi.org/10.3103/S0027131420010046
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0027131420010046