Abstract
Introduction
Trichinellosis is a severe zoonosis involving the activation of inflammatory cells, accompanied by the prominent expressions of proinflammatory cytokines in the host. Semen vaccariae, the seeds of Vaccaria segetalis (Neck.) Garcke. ex Asch. (Caryophyllaceae), is a famous traditional herb that is rich in vaccaria n-butanol extract (VNE). Vaccarin is one major active component of VNE, and it is reported in the treatment of stranguria disease. Hypaphorine is another main active component of VNE and has good anti-inflammatory effect, whereas the potential bioactivity of VNE in trichinellosis treatment is still unknown.
Materials and methods
This study was designed to evaluate the potential anthelmintic and anti-inflammatory activity of VNE toward T. spiralis infection. ICR mice were used to assess the effect of VNE on repression larvae and adult worms in vivo. Immunohistochemistry analysis was performed to evaluate the expression levels of IL-1β, IL-6, TNF-α, and COX-2.
Results
Our results showed that VNE could effectively depress the expressions of proinflammatory cytokines, including IL-1β, IL-6, TNF-α, and COX-2. The adult worms were decreased by 79.53%, while the muscle larvae were diminished by 77.70% as compared to the control.
Conclusion
These results demonstrated that VNE may be a promising therapeutic agent against the inflammation and diseases caused by T. spiralis infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichinellosis is a worldwide severe zoonosis, which is transmitted mainly due to the ingestion of raw or undercooked pork as well as the meat of other animals containing infective Trichinella larvae [1, 33]. Trichinosis is not only a threat to the meat industry, especially the pig industry, but also seriously endangers human health [28]. Trichinosis infection shows main symptoms as fever and muscle inflammatory limb weakness [25]. Severe complications such as myocarditis can even lead to death [26]. During the infection, the larvae in the host can produce a variety of antigens, which directly induce the host immune system response against the antigens. It has been reported that China has suffered an outbreak of human trichinellosis with 11 deaths out of 828 cases during 2000–2004 [34]. In some developing countries in Africa, Central and South America and Asia, the risks of the outbreak of trichinellosis has been continuously increasing in the countryside [3].
Trichinellosis is an infectious disease that is caused by a parasite. It influences the host in the enteral and muscular phases [30]. In the muscle stage, the larvae induce a correlative inflammatory reaction when they invade the skeletal muscle bundles [1, 4]. Inflammation may be considered a response of the tissue immune system against the invasion. However, excessive regulation signals of inflammation can result in cellular destruction and tissue damage, which is responsible for inflammation-associated diseases [27]. In the host, Trichinella spiralis activate inflammatory cells to overexpress cyclooxygenase-2 (COX-2) [24], inducible nitric oxide synthase (iNOS) [11], tumor necrosis factor-α (TNF-α) [22], interleukin-1β (IL-1β) [17] and IL-6 [14].
Albendazole (ABZ) is a widely used commercial vermifuge for the treatment of human trichinellosis [12]. However, its low water solubility limited its absorption and bioactivity. Moreover, it is banned for pregnant women and children under 3 years old [37]. There is thus an urgent need to develop safe and efficient anti-trichinosis drugs with low side effects and high tolerance [2].
Traditional Chinese Medicine (TCM) as a complementary and alternative therapy has been clinically used for the treatment of various diseases for decades [9]. TCM is based on natural herbs; despite long-term treatment with TCM may cause liver or kidney damage, short-term treatment in a controlled dose range would be safe and effective. However, the active ingredients of the vast majority of the Chinese herbs are often unclear despite the tremendous efforts made so far [36, 38]. Our recent research showed that vaccarin, as one of the main constituents of VNE, demonstrated biological activity in the protection from oxidative stress-induced injury on endothelial cells via regulating Notch signaling [35]. Vaccarin is also believed to treat vascular diseases, as the increased level of inflammation factors in endothelial cells’ response to LPS was obviously prevented by vaccarin. Vaccaria total saponin (VTS) was another main component in Vaccaria segetalis (Neck.) Garcke. ex Asch. (Caryophyllaceae). Saponins from other traditional herbs may exert whole-body anti-inflammatory effects by negatively regulating NF-κB p65 activation [32], However, to the best of our knowledge, the bioactivity of VTS has been rarely reported. Hypaphorine, as an important active ingredient, has been proved to have good anti-inflammatory effect. In this study, the bioactivity of VNE toward trichinellosis in depressing the inflammation was investigated using T. spiralis-infected mice and we hypothesized that the anti-inflammation role of VNE might be a potential underlying mechanism of the insecticidal effect.
Materials and Methods
Experimental Animals and T. spiralis Infection
The ISS 534 strain of T. spiralis was originally obtained from a domestic pig in the Henan Province of China and was maintained by consecutive passages in ICR mice in Jiangsu Institute of Parasitic Diseases [19].
Six-week-old male ICR mice, weighing 25–30 g each, were experimented. The animals were obtained from Jiangsu Institute of Parasitic Diseases. During the research, the mice were housed in suitable environmental aspects with controlled humidity and temperature on a 12:12 light/dark cycle and fed a standard diet. Experimental mice were orally infected with 300 larvae in accordance with Dunn and Wright [10]. All animals handling procedures were performed on the basis of the national and institutional guidelines for the care and use of laboratory animals.
Drugs and Materials
Albendazole (Sigma-Aldrich, Shanghai, China) suspension was orally administered at a dose of 50 mg/kg day [2].
Plant Materials and Powder Preparation
Vaccaria was purchased from Wuxi Shanhe Group Chinese Medicine Slices Co., Ltd. Medicinal materials were crushed, sieved and extracted with ten volumes of 75% ethanol for 2 h twice. All ethanol extracts were pooled together, filtered, evaporated and spray dried. The total extracts were extracted in turn with petroleum ether, dichloromethane, ethyl acetate, n-butyl alcohol and spray dried again. The dried extracts were stored at + 4 °C for subsequent experiments. Vaccarin, hypaphorine and total saponins were contained in VNE, and their contents were about 12%, 1.2% and 86.8%, respectively, detected by HPLC. The VNE obtained as described above was orally administered at a dose of 5 mg/kg day. The group receiving ABZ was the positive control group.
Experimental Groups and Design
Mice infected with T. spiralis were randomly classified into five main groups—group I (control, n = 6): infected non-treated group; group II (intestinal phase, n = 6): received VNE starting from the 2nd dpi and lasting for 3 days; group III (intestinal phase, n = 6): received ABZ starting from the 2nd dpi and lasting for 3 days; group IV (muscular phase, n = 6): received VNE starting from the 7th dpi and lasting for 14 days; group V (muscular phase, n = 6): received ABZ starting from the 7th dpi and lasting for 14 days. Mice were killed by cervical dislocation on the 7th and 40th dpi, respectively. Muscle samples were obtained and reserved in 10% formalin for the histopathological analysis and immunohistochemical studies on the 40th dpi.
Assessment of Load of Muscle Larvae
Each carcass of the experimental mouse was decorticated, eviscerated, minced and digested in 250 mL prepared digestion solution with 1% concentrated HCl and 1% pepsin (1:10,000). Then the mixture was agitated at 37 °C for 2 h using a magnetic stirring apparatus. The digested mixture was filtered through an 80-mesh sieve. The liquid supernatant was discarded and the larvae were collected after sedimentation for 30 min, and then washed and counted using a McMaster counting chamber under a dissecting microscope (Nikon, Japan) [8].
Assessment of Burden of Adult Worms in the Intestine
The duodenum–ileum region of the small intestine was cut into small pieces 1 cm each and incubated at 37 °C in saline for 3 h; then the worms were collected. The saline was pipetted and the pieces of intestine were rinsed several times with saline. All of the fluid was collected in centrifuge tubes and centrifuged at 1500 rpm for 5 min. The supernatant was removed and the precipitate was resuspended with 2–3 mL saline and counted at a magnification of 10 × [31].
Histopathological Study
Histopathological evaluation of muscle tissue samples was conducted. Tissue specimens were fixed with 4% formaldehyde, flushed, dehydrated, cleared, wax immersed and then embedded in paraffin blocks. Paraffin blocks were sectioned at 5 μm thickness using microtome and dyeing with hematoxylin and eosin (H&E) and photographs taken under microscope (Nikon, Japan). For muscle specimens, histopathological analysis of the following parameters was taken on: the burden of muscle larvae per low power field (100 ×) and the magnitude of inflammatory reaction surrounding the capsuled larvae per high power field (400 ×).
Immunohistochemistry Analysis
Paraffin sections were baked at 65 °C for 1 h and then deparaffinized and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 15 min, then rinsed with PBS three times. Afterward, endogenous peroxidase activity was blocked using 3% hydrogen peroxide in PBS at room temperature for 10 min and then washed in PBS three times. After incubation with 10% goat serum for 30 min, an overnight incubation was done with the indicated primary antibody against IL-1, IL-6, TNF-α (1:100, Proteintech, China), and COX-2 (1:50, Abcam, UK) in a humidity chamber at 4 °C. Histologic sections were then incubated with HRP anti-rabbit IgG for half an hour. Sections were covered with 3-3′-diaminobenzidine tetrahydrochloride (DAB) chromogen for 5 min. Eventually, sections were redyed with hematoxylin, dehydrated and mounted. The signals were visualized by a microscope (Nikon, Japan). At least ten regions were selected and the integrated optical density (IOD) calculated with Image-Pro Plus software.
Insecticidal Experiment In vitro
The in vitro insecticidal experiment was conducted in nine groups according to the dose administered in vivo: respectively, the control group, ABZ low-dose group (0.625 μg/μL) and high-dose group (6.25 μg/μL), VNE low-dose group (0.0625 μg/μL) and high-dose group (0.625 μg/μL), vaccarin low-dose group (7.5 ng/μL) and high-dose group (75 ng/μL), hypaphorine low-dose group (0.75 ng/μL) and high-dose group (7.5 ng/μL). Muscle larvae-containing dilution (about 100 live larvae per group) was added to a 24-well plate, and incubated at 37 °C with drug treatment. The number of live larvae was then counted under a microscope after incubation for 30 min and 90 min, respectively. The insecticidal rate was then calculated.
Statistical Analysis
All results were expressed as mean ± SD. Comparisons were made by Student’s t test for two group comparisons. The probability of significant differences between groups was performed by ANOVA/Dunnet t test. The difference was deemed statistically significant when P < 0.05.
Results
The Amount of Main Components in VNE Measured by HPLC
VTS, vaccarin and hypaphorine are the major effective components in vaccaria n-butanol extract (VNE). We measured the amount of these effective components by HPLC (Fig. 1a–d). VNE contained the most abundant amount of VTS, followed by vaccarin (Fig. 1b) and hypaphorine (Fig. 1a). Three parallel experiments showed that the content of hypaphorine is 0.060 ± 0.006 mg, and the content of vaccarin is 0.596 ± 0.117 mg in the n-butanol extraction (Table 1). We have a complete extraction process, and the chemical substance content in each group is stable, which can be used as a research standard for quantitative administration in vivo.
HPLC profile of hypaphorine, vaccarin and VNE. a–c The HPLC spectrum of standard product hypaphorine, vaccarin and hypaphorine + vaccarin, respectively. d The fingerprint of vaccaria n-butanol extract (VNE). The x-axis represents time in minutes. The y-axis represents electrical signals in millivolts
VNE Reduced Both Adult and Muscle Larvae In Vivo
The mean number of adult worms in the small intestine was significantly reduced after administration of VNE (40 ± 8) and ABZ (37 ± 8) in comparison to the control infected untreated group (197 ± 15) (P < 0.001). A reduction of 79.53% in adult worms was observed in groups treated with VNE, which is 81.22% in the ABZ group, respectively. Similarly, total larvae counts were significantly lessened in both drug-received groups, as the mean number of total larvae in muscles in the VNE treated group was significantly reduced to (10,500 ± 3523) and (4500 ± 918) with ABZ treatment, with a percentage of reduction 77.7% and 90.7% in the VNE- and ABZ-treated group (Table 2), respectively, in comparison to the control infected untreated group (47,133 ± 3189).
Larvae Reduction Rate and Inflammatory Phenotype were Improved by VNE
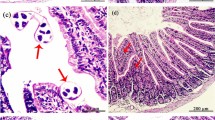
Histopathological changes were studied in both diaphragm and gastrocnemius sections of the infected mice without any treatment and the drug-treated groups. Both diaphragm and gastrocnemius of the control groups possessed a massive number of encysted larvae, accompanied by degeneration of the muscle fibers (Fig. 2a, b). Besides, a mass of inflammatory cellular infiltrations surrounding T. spiralis larvae and in between the muscle bundles were observed in groups without treatment (Fig. 2a, b). The inflammatory cells mainly included lymphocytes, eosinophils, plasma cells and histiocytes, while these phenotypes were markedly improved in either the VNE- or ABZ-treated group, with significant difference (P < 0.05) in comparison with the infected group without drug therapy (Fig. 2).
Histopathological changes of T. spiralis-infected mice (H&E) in striated muscular tissue of mice receiving VNE and ABZ on day 7 after T. spiralis infection. a Representative images showing the number of encysted larvae and the expression of inflammatory cell in diaphragm in different groups. b Representative images showing the number of encysted larvae and the expression of inflammatory cell in gastrocnemius in different groups. c Quantitative analysis of encysted larvae deposition of diaphragm and gastrocnemius in these groups. Values are presented as group mean ± SD. *P < 0.05 vs. infected non-treated group, n = 6 for each group. Tsp, T. spiralis; VNE, vaccaria n-butanol extract; ABZ, albendazole; Dia, diaphragm; Gas, gastrocnemius
VNE Reduced the Expression of Inflammatory Factors in the Muscle
It is well accepted that inflammatory factors are activated after T. spiralis infection [14, 17, 22]. VNE abolished the upregulation of inflammatory factors at the transcriptional level, as IL-1β, IL-6 and TNF-α significantly decreased in the muscle tissues (Fig. 3a) and ABZ was used as a positive control. VNE showed significant reduction effect on muscle larva, but less effective than ABZ (Fig. 3b).
The anti-inflammatory effect of VNE in vivo. Immunohistochemistry sections of diaphragm. a, c Representative expression levels of IL-1β, IL-6, TNF-α and COX-2 in different groups. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; Tsp, T. spiralis; VNE, vaccaria n-butanol extract; ABZ, albendazole; Dia, diaphragm; COX-2, cyclooxygenase-2. b, d Analysis of inflammatory cytokines expression levels in diaphragm. Values are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
COX-2 is overexpressed in T.spiralis-infected mice [24]. The infected control group demonstrated strong COX-2 expression. However, in VNE- and ABZ-receiving groups, the COX-2 expression was significantly reduced by VNE and ABZ (Fig. 3c, d). But it seemed like ABZ had a more effective role in insecticidal effect in the current concentration (Fig. 3b).
To verify whether VNE has direct insecticidal effect on T. spiralis, or by affecting the anti-inflammatory function of infected mice to achieve the insecticidal effect, we conducted in vitro insecticidal experiments. The results showed that neither VNE, vaccarin, nor hypaphorine had significant direct killing capacity toward T. spiralis muscle larvae in both standard concentration group and higher concentration group (Fig. 4). While it showed direct insecticidal effect only after 30 min of ABZ administration with standard concerntration to the muscle larvae. Furthermore, with 1.5 h ABZ treatment, both the standard concentration group and higher concentration group showed strong insecticidal effect, nearly leading to a reduction of 50%.
Discussion and Conclusion
An ever-increasing number of researchers have strong interest in the clinical applications of natural products including drug compounds from medicinal plants, which may have positive therapeutic effect on diseases caused by T. spiralis infection [6].
Accumulated evidences indicate that inflammatory mediators are involved in the muscular pathogenesis of T. spiralis infection and accelerate the development and progression of myositis associated with this phase [5]. In the present study, it was proved that VNE can be used against the disease caused by T. spiralis infection through lowering the parasite burden in the intestine and muscle tissues and alleviating muscular inflammatory reaction.
Monokines, such as IL-1, IL-6, IL-8, TNF-α and G-CSF, are immune molecules that are secreted mainly by monocyte and macrophages [18, 29]. Emerging researches suggest that monokines play a vital role in mediating and regulating immune and inflammatory reactions as reflected by overexpression of different proinflammatory mediators and cytokines [15, 16]. IL-1β is a crucial proinflammatory cytokine produced by mononuclear cells, endothelial cells, fibroblasts and other types of cells in response to infection. IL-1β is a pivotal immune mediator that responds to cellular infections and danger signals [20]. Previous studies have indicated that T. spiralis infection can induce the secretion and activation of IL-1β [21]. TNF-α is a potent proinflammatory factor, which performs its biological function in a pleiotropic way on multiple cell types and plays a pivotal role in the pathogenesis of chronic inflammatory diseases [13]. Consistent with these earlier studies, our present study displayed that T. spiralis infection obviously upregulated proinflammatory cytokines expressions of IL-1β, IL-6 and TNF-α, while both VNE and ABZ depressed the expressions. These results indicated that VNE has anti-inflammatory effect on the diseases caused by Trichinella infection.
COX-2 is an enzyme that mediates the production of prostaglandins (PGs) and thromboxane, resulting in pain and inflammation [7]. In turn, inflammation in many cells and tissues triggers considerable production of COX-2 [23]. In the present study, our results showed that VNE may be a potential anti-inflammatory agent in case of T. spiralis infection via depressing the expression of COX-2 in the muscle tissues. In both VNE- and ABZ-treated groups, the expressions of COX-2 were dramatically decreased as compared with the infected control group.
Herein, the experimental results clearly demonstrated that both VNE and ABZ were effective against intestinal adult worms and muscle larvae. In addition, IL-1β, IL-6, TNF-α and COX-2 were controlled at low levels due to the bioactivity of VNE, which indicated a powerful anti-inflammatory function of VNE in T. spiralis disease. Furthermore, in in vitro insecticidal experiments, the results showed that neither VNE, vaccarin nor hypaphorine had significant direct killing capacity toward T. spiralis muscle larvae compared with ABZ. Therefore, VNE may achieve anti-inflammatory effect by alleviating the inflammatory response of the infected mice and ultimately achieve the insecticidal effect of T. spiralis. The blood urea nitrogen of mice was significantly increased at high doses of 1000 mg/kg (as minimum lethal dose is 1500 mg/kg, data not shown) in vivo, suggesting VNE may cause damage to the kidney. But it should be mentioned that the usage of VNE at a low dose of 200 mg/kg (as minimum toxic dose is 100 mg/kg, data not shown) did not show significant injury in vivo. The VNE concentration used here to test its insecticidal effect was much lower than the minimum toxic dose. Notably, although ABZ plays a more effective function in reducing the burden of T. spiralis larvae, due to its limitation in clinical use VNE may be an ideal alternative solution to human trichinellosis.
In conclusion, our data demonstrated that vaccaria n-butanol extract from Vaccariae semen may be a promising anthelmintic against T. spiralis.
References
Abou Rayia DM et al (2017) Implication of artemisinin nematocidal activity on experimental trichinellosis: in vitro and in vivo studies. Parasitol Int 66:56–63. https://doi.org/10.1016/j.parint.2016.11.012
Attia RA et al (2015) Effect of myrrh and thyme on Trichinella spiralis enteral and parenteral phases with inducible nitric oxide expression in mice. Mem Inst Oswaldo Cruz 110:1035–1041. https://doi.org/10.1590/0074-02760150295
Bruschi F (2012) Trichinellosis in developing countries: is it neglected? J Infect Dev Ctries 6:216–222
Bruschi F, Chiumiento L (2011) Trichinella inflammatory myopathy: host or parasite strategy? Parasites Vectors 4:42. https://doi.org/10.1186/1756-3305-4-42
Bruschi F et al (2016) Matrix metalloproteinase (MMP)-9: a reliable marker for inflammation in early human trichinellosis. Vet Parasitol 231:132–136. https://doi.org/10.1016/j.vetpar.2016.04.011
Caner A et al (2008) Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella spiralis) in rats. Exp Parasitol 119:173–179. https://doi.org/10.1016/j.exppara.2008.01.012
Cannon CP, Cannon PJ (2012) COX-2 inhibitors and cardiovascular risk. Science 336:1386–1387. https://doi.org/10.1126/science.1224398
Denham DA (1965) Studies with methyridine and Trichinella spiralis. I. Effect upon the intestinal phase in mice. Exp Parasitol 17:10–14
Dong Y, Liao J (2017) Application of traditional Chinese medicine in treatment of atrial fibrillation. Hum Fertil 2017:1381732. https://doi.org/10.1155/2017/1381732
Dunn IJ, Wright KA (1985) Cell injury caused by Trichinella spiralis in the mucosal epithelium of B10A mice. J Parasitol 71:757–766
Falduto GH et al (2016) Regulatory parameters of the lung immune response during the early phase of experimental trichinellosis. Vet Parasitol 231:47–52. https://doi.org/10.1016/j.vetpar.2016.05.009
Gottstein B et al (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 22:127–145. https://doi.org/10.1128/cmr.00026-08 (Table of contents)
Horiuchi T et al (2010) Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology 49:1215–1228. https://doi.org/10.1093/rheumatology/keq031
Huang SW et al (2017) P2X7 receptor-dependent tuning of gut epithelial responses to infection. Immunol Cell Biol 95:178–188. https://doi.org/10.1038/icb.2016.75
Im SS et al (2011) Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab 13:540–549. https://doi.org/10.1016/j.cmet.2011.04.001
Imaizumi T et al (2010) IFN-gamma and TNF-alpha synergistically induce microRNA-155 which regulates TAB 2/IP-10 expression in human mesangial cells. Am J Nephrol 32:462–468. https://doi.org/10.1159/000321365
Keating C et al (2011) P2X7 receptor-dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol 187:1467–1474. https://doi.org/10.4049/jimmunol.1100423
Li Z et al (2016) Celastrol nanomicelles attenuate cytokine secretion in macrophages and inhibit macrophage-induced corneal neovascularization in rats. Int J Nanomedicine 11:6135–6148. https://doi.org/10.2147/ijn.s117425
Long SR, Tian XY, Wang ZQ, Liu RD, Liu LN et al (2015) Serodiagnosis of trichinellosis by ELISA using recombinant nudix hydrolase of Trichinella spiralis. Trop Biomed 32:669–675
Mariathasan S et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232. https://doi.org/10.1038/nature04515
Ming L et al (2016) Invasion by Trichinella spiralis infective larvae affects the levels of inflammatory cytokines in intestinal epithelial cells in vitro. Exp Parasitol 170:220–226. https://doi.org/10.1016/j.exppara.2016.10.003
Munoz-Carrillo JL et al (2017) Resiniferatoxin lowers TNF-alpha, NO and PGE2 in the intestinal phase and the parasite burden in the muscular phase of Trichinella spiralis infection. Parasites Immunol. https://doi.org/10.1111/pim.12393
Oliver KM et al (2009) Hypoxia. Regulation of NFkappaB signalling during inflammation: the role of hydroxylases. Arthritis Res Ther 11:215. https://doi.org/10.1186/ar2575
Othman AA et al (2016) Atorvastatin and metformin administration modulates experimental Trichinella spiralis infection. Parasitol Int 65:105–112. https://doi.org/10.1016/j.parint.2015.11.001
Park MK et al (2018) Effect of muscle strength by Trichinella spiralis infection during chronic phase. Int J Med Sci 15:802–807. https://doi.org/10.7150/ijms.23497
Puljiz I et al (2005) Electrocardiographic changes and myocarditis in trichinellosis: a retrospective study of 154 patients. Ann Trop Med Parasitol 99:403–411. https://doi.org/10.1179/136485905X36307
Ranganathan PV et al (2013) Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int 83:1087–1098. https://doi.org/10.1038/ki.2012.423
Rostami A et al (2017) Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol 64:65–71. https://doi.org/10.1016/j.fm.2016.12.012
Schultz HS et al (2016) OSCAR-collagen signaling in monocytes plays a proinflammatory role and may contribute to the pathogenesis of rheumatoid arthritis. Eur J Immunol 46:952–963. https://doi.org/10.1002/eji.201545986
Shalaby MA et al (2010) Effect of methanolic extract of Balanites aegyptiaca fruits on enteral and parenteral stages of Trichinella spiralis in rats. Parasitol Res 107:17–25. https://doi.org/10.1007/s00436-010-1827-9
Soliman GA et al (2011) Therapeutic efficacy of doramectin, ivermectin and levamisole against different stages of Trichinella spiralis in rats. Turk Parazitol Derg 35:86–91. https://doi.org/10.5152/tpd.2011.22
Su XD et al (2019) Anti-inflammatory potential of saponins from Aster tataricus via NF-kappaB/MAPK activation. J Nat Prod. https://doi.org/10.1021/acs.jnatprod.8b00856
Turiac IA et al (2017) Trichinellosis outbreak due to wild boar meat consumption in southern Italy. Parasites Vectors 10:107. https://doi.org/10.1186/s13071-017-2052-5
Wang SW et al (2011) Protein change of intestinal epithelial cells induced in vitro by Trichinella spiralis infective larvae. Parasitol Res 108:593–599. https://doi.org/10.1007/s00436-010-2102-9
Xie F et al (2015) Vaccarin attenuates the human EA.hy926 endothelial cell oxidative stress injury through inhibition of Notch signaling. Int J Mol Med 35:135–142. https://doi.org/10.3892/ijmm.2014.1977
Xie PS, Leung AY (2009) Understanding the traditional aspect of Chinese medicine in order to achieve meaningful quality control of Chinese materia medica. J Chromatogr A 1216:1933–1940. https://doi.org/10.1016/j.chroma.2008.08.045
Yadav AK, Temjenmongla (2012) Efficacy of Lasia spinosa leaf extract in treating mice infected with Trichinella spiralis. Parasitol Res 110:493–498. https://doi.org/10.1007/s00436-011-2551-9
Zhao X, Pang J (2017) Application of spontaneous photon emission in the growth ages and varieties screening of fresh Chinese herbal medicines. Evid Based Complement Altern Med 2017:2058120. https://doi.org/10.1155/2017/2058120
Acknowledgements
The authors acknowledge the funding from Wuxi Science and Technology Development Fund 2018 (Grant no. WX0302B010507180101 PB) and Jiangnan University Youth Fund 2018 (Grant no. K2050205). This work was also supported by the National First-Class Discipline Program of Food Science and Technology (JUFSTR20180101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
All experiments on human subjects published in this article were in accordance with the Helsinki Declaration of 1975.
Consent for publication
All animal procedures and protocols of this project were conformed to Good Publishing Practice in Acta Parasitologica. The study was approved by the Animal Care Committee of Jiangnan University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, F., Hou, B., Zhu, X. et al. Vaccaria n-Butanol Extract Lower the Production of Proinflammatory Cytokines and the Infection Risk of T. spiralis In Vivo. Acta Parasit. 64, 520–527 (2019). https://doi.org/10.2478/s11686-019-00064-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00064-6