Abstract
Background and Objectives: Ticagrelor, the first reversibly binding oral P2Y12 receptor antagonist, improves outcomes in patients with acute coronary syndromes (ACS) compared with clopidogrel. In the ONSET-OFFSET study (parallel group trial) and the RESPOND study (crossover trial), the pharmacodynamic effects of ticagrelor were compared with clopidogrel in patients with coronary artery disease (CAD). We now report the pharmacokinetic analyses of ticagrelor, and the exposure-inhibition of platelet aggregation (IPA) relationships from these studies.
Patients and Methods: Patients were treated with ticagrelor (180 mg loading dose, 90 mg twice daily maintenance dose) or clopidogrel (600 mg loading dose, 75 mg once daily maintenance dose) in addition to aspirin (acetylsalicylic acid) [75–100 mg once daily]. Ticagrelor administration was for 6 weeks in ONSET-OFFSET. In RESPOND, ticagrelor was given for 14 days before or after 2 weeks of clopidogrel in patients classified as clopidogrel responders or non-responders. Pharmacokinetics and IPA were evaluated following the loading and last maintenance doses. Exposure-IPA relationships were evaluated using a sigmoid maximum effect (Emax) model.
Outcome Measures: The outcome measures were ticagrelor and AR-C124910XX (active metabolite) pharmacokinetics and exposure-IPA relationships in both trials, including the effect of prior clopidogrel exposure, and effects in clopidogrel responders and non-responders in RESPOND.
Results: In ONSET-OFFSET, maximum (peak) plasma concentration (Cmax), time to Cmax (tmax) and area under the plasma concentration-time curve from time 0 to 8 hours (AUC8) for ticagrelor were 733 ng/mL, 2.0 hours and 4130 ng · h/mL, respectively; and for AR-C124910XX were 210 ng/mL, 2.1 hours and 1325 ng · h/mL, respectively. Emax estimates were IPA > 97%. Trough plasma ticagrelor (305 ng/mL) and AR-C124910XX (121 ng/mL) concentrations were 5.2 and 7.7 times higher than respective concentration producing 50% of maximum effect (EC50) estimates. In RESPOND, ticagrelor mean Cmax and AUC8 following 2-week dosing were comparable between clopidogrel responders (724 ng/mL and 3983 ng · h/mL, respectively) and non-responders (764 ng/mL and 3986 ng · h/mL, respectively). Pharmacokinetics of ticagrelor were unaffected by prior clopidogrel dosing. Emax estimates were IPA > 96% for both responders and non-responders. Trough plasma concentrations were sufficient to achieve high IPA.
Conclusions: Ticagrelor pharmacokinetics in stable CAD patients were comparable to previous findings in stable atherosclerotic and ACS patients, and were not affected by prior clopidogrel exposure or clopidogrel responsiveness. Ticagrelor effectively inhibited platelet aggregation, and trough plasma concentrations of ticagrelor and AR-C124910XX were sufficient to result in high IPA in stable CAD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Ticagrelor, the first reversibly binding oral P2Y12 receptor antagonist,[1–4] was recently approved for use in the EU[5] and the US[6] for adult patients with acute coronary syndromes (ACS); ticagrelor has been approved in more than 40 countries. Phase III data from the PLATO (PLATelet inhibition and patient Outcomes) trial in ACS patients, with or without ST-segment elevation, showed that ticagrelor treatment (180 mg loading dose then 90 mg twice daily) significantly reduced the rate of the composite of myocardial infarction, stroke and death from vascular causes compared with clopidogrel (300–600 mg loading dose then 75 mg once daily).[7] This composite endpoint occurred in 9.8% (ticagrelor group) and 11.7% (clopidogrel group) of patients (hazard ratio 0.84, 95% CI 0.77, 0.92; p < 0.001).[7]
Healthy volunteer studies have provided extensive information on ticagrelor pharmacokinetics and pharmacodynamics. Ticagrelor exhibited linear and predictable pharmacokinetics with single doses up to 1260 mg.[8,9] Following multiple doses, maximum ticagrelor plasma concentrations were achieved within 1.5–3.0 hours, and the elimination half-life (t1/2) ranged from 6.2 to 13.1 hours.[10] Ticagrelor (100–400 mg) produced near-complete inhibition of platelet aggregation (IPA) [88–95%] by 2 hours post-dosing.[8,9]
Clopidogrel requires metabolic activation via a multistep process involving several cytochrome P450 (CYP) isoenzymes.[11,12] In contrast, ticagrelor can directly bind to the P2Y12 receptor, thereby inhibiting adenosine diphosphate (ADP)-induced platelet aggregation (PA).[13] In vitro studies have demonstrated that ticagrelor binds reversibly to P2Y12 receptors, with rapid receptor kinetics (e.g. mean ± standard error of the mean dissociation constant [Koff]: 8.7 ± 1.4 × 10-4 s-1; time to achieve 50% Koff [t1/2(off)] 13.5 ± 1.9 min).[13] In a mass balance study, ticagrelor was metabolized to at least ten metabolites, with AR-C124910XX being the major component.[14] This active metabolite, formed mainly by CYP3A4/5 isoenzymes,[15] has a similar potency to ticagrelor and has plasma concentrations approximately one-third that of the parent compound.[9,14]
Two phase II ticagrelor studies, DISPERSE (Dose-finding Investigative Study to assess the Pharmacodynamic Effects of AZD6140 in atheRoSclErotic disease) and DISPERSE-2 (Dose confIrmation Study assessing anti-Platelet Effects of AZD6140 versus clopidogRel in non–STsegment Elevation myocardial infarction), have confirmed the consistent and predictable pharmacokinetic profiles of ticagrelor and AR-C124910XX in patients with either stable atherosclerosic disease[3] or ACS.[16] Furthermore, both trials demonstrated that IPA was greater and less variable with ticagrelor than with standard-dose clopidogrel (300 mg load/75 mg once-daily maintenance) in such patients.
Two randomized, multicentre, clinical trials have evaluated the antiplatelet activity, safety and tolerability of ticagrelor versus high-loading dose clopidogrel (both with concomitant aspirin [acetylsalicylic acid; 75–100 mg once daily] as background therapy) in patients with stable coronary artery disease (CAD). In these trials, the PLATO trial dose of ticagrelor was evaluated. In the randomized, double-blind ONSET-OFFSET (ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease [NCT00528411; parallel group trial]) trial, IPA with ticagrelor was more rapid and greater than with clopidogrel; mean ± standard deviation final-extent IPA at 2 hours after first dose: 88 ± 15% versus 38 ± 33%, respectively (p < 0.0001).[17] In the RESPOND (REsponse to ticagrelor in clopidogrel non-responders and ReSPONDers and the effect of switching therapies [NCT00642811; crossover trial]) study, the ticagrelor antiplatelet effect was similar in patients responsive or non-responsive to clopidogrel.[18]
Secondary objectives of ONSET-OFFSET and RESPOND included evaluation of ticagrelor pharmacokinetics and the exposure-IPA relationships of ticagrelor and AR-C1249XX in patients with stable CAD. Results of these pre-planned subanalyses from both studies are reported herein.
Methods
Detailed methodology for ONSET-OFFSET and RESPOND has been published previously.[17,18]
Patients
Patients were enrolled in the US and Europe. Key inclusion criteria were ≥18 years of age with documented stable CAD and stable aspirin therapy (75–100 mg once daily). In both studies, key exclusion criteria were ACS within 12 months of screening; a history of bleeding diathesis or severe pulmonary disease; pregnancy; concomitant therapy with moderate or strong CYP3A inhibitors or strong inducers in the prior 14 days; atrial fibrillation, coronary stent, mitral stenosis or prosthetic heart valve requiring antithrombotic treatment; and platelet count <100 000/mm3 or haemoglobin <10 g/dL. In addition, smokers were excluded in ONSET-OFFSET, whereas in RESPOND patients who were currently smoking >1 pack per day were excluded. Both studies were performed in accordance with standard ethical principles; written consent was obtained from all patients.
Study Designs and Treatments
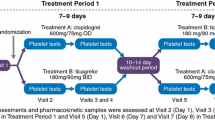
ONSET-OFFSET was a randomized, double-blind, double-dummy, parallel-group multicentre study to evaluate the onset and offset of ticagrelor antiplatelet effect versus clopidogrel or placebo. Patients treated with ticagrelor received a single oral loading dose (180 mg) in the morning on day 1, followed by a maintenance dose (90 mg) 12 hours later. For 6 weeks thereafter, ticagrelor was administered at 90 mg twice daily (figure 1a).
Study designs of (a) ONSET-OFFSET and (b) RESPOND. The N values are the number of patients randomized to treatments indicated, and the n values are the number of patients with evaluable pharmacokinetic/pharmacodynamic data and may differ from the number of patients who were randomized and treated. Clop = clopidogrel; PD = pharmacodynamic; PK = pharmacokinetic; Tic = ticagrelor.
RESPOND was a multicentre, randomized, double-blind, double-dummy crossover study to compare the antiplatelet effects of ticagrelor with clopidogrel in patients previously identified as either responsive or non-responsive to clopidogrel (figure 1b). Non-responsiveness to clopidogrel was defined as ≤10% absolute change in maximum-extent PA, induced by 20 μmol/L ADP,[18] between pre-dose and 6–8 hours post-dosing with clopidogrel 300 mg at screening.
In period 1, responders and non-responders received either ticagrelor (180 mg loading dose then 90 mg twice daily) or clopidogrel (600 mg loading dose then 75 mg once daily) for 14 days. For period 2, all non-responders switched treatments and were treated for a further 14 days. In period 2, half of the responders switched treatments, with the remaining patients continuing with treatment as in period 1. These patients did not receive a loading dose of study drug at the start of period 2.
All patients in both ONSET-OFFSET and RESPOND received concomitant aspirin (75–100 mg once daily).
Pharmacokinetic Evaluations
Blood sampling times were 0 (pre-dose), 0.5, 1, 2, 4, 8 and 24 hours after a single loading dose (180 mg) of ticagrelor on day 1 in ONSET-OFFSET (figure 1a), and at 0 (pre-dose), 0.5, 1, 2, 4 and 8 hours post-dosing on day 1 in periods 1 and 2 in RESPOND (figure 1b). Following maintenance dosing with ticagrelor (90 mg twice daily), blood samples were collected at 0 (pre-dose), 2, 4, 8, 24 and 48 hours post-dosing after the last dose at 6 weeks in ONSET-OFFSET (figure 1a). In RESPOND, blood sampling times, after ticagrelor maintenance dosing, were 0 (pre-dose), 2, 4 and 8 hours post-dosing on day 14 in periods 1 and 2 (figure 1b).
For ticagrelor and AR-C124910XX evaluations, venous blood samples (2 mL) were collected into lithium-heparinized tubes, mixed and placed on ice immediately. Within 30 minutes of collection, blood samples were centrifuged (10 min, 4°C, 1500 × g) and the plasma stored at -20°C until analysis.
Plasma concentrations of ticagrelor and AR-C124910XX were quantified using a validated reverse-phase liquid chromatography/tandem mass spectrometry method. Mean intra-batch accuracy was 91.9–109.0% (ticagrelor) and 86.8–109.2% (AR-C124910XX); intra-batch precision was 4.0–8.4% and 5.2–16.9%, respectively. Lower limits of quantification were 5 ng/mL (ticagrelor) and 2.5 ng/mL (AR-C124910XX).[19]
Pharmacodynamic Assessment
Blood sampling times for IPA assessment included 0 (pre-dose), 0.5, 1, 2, 4 and 8 hours after a single loading dose (180 mg) of ticagrelor on day 1 in both trials (figure 1). After the last ticagrelor dose at 6 weeks in ONSET-OFFSET, blood samples were collected for 10 days (figure 1a). IPA (20 μmol/L ADP-induced, final extent) in platelet-rich plasma was assessed, as described previously.[18,20]
Data Analyses
Pharmacokinetic parameters were estimated by noncompartmental methods using WinNonlin Professional (Pharsight Corporation, Mountain View, CA, USA). Key pharmacokinetic parameters calculated for ticagrelor and AR-C124910XX were maximum (peak) plasma concentration (Cmax); time to Cmax (tmax); area under the plasma concentration-time curve (AUC) from time 0 to 8 hours (AUC8; calculated by the linear trapezoidal rule); and trough (minimum) plasma drug concentration over the dosing interval (Ctrough) [ONSET-OFFSET].
Pharmacokinetic parameters and plasma concentration data for each moiety (ticagrelor and AR-C124910XX) were summarized using descriptive statistics. The latter also included geometric mean and coefficient of variation (CV) for all parameters, except for tmax which was summarized using median (range) values. Geometric mean ratio and 95% confidence intervals (Hodges-Lehman) were calculated for AUC and Cmax comparisons between clopidogrel responders and non-responders.
Final-extent IPA was calculated using the formula (PABL — PAT)=PABL, where PABL is the mean pre-dose baseline response, and PAT is the mean PA response at time T. Mean IPA (percentage) was plotted versus time.
The relationship between IPA and ticagrelor, and AR-C124910XX concentrations was assessed using a sigmoid maximal effect model: IPA = (EmaxCγ)/(Cγ + ECγ 50, where Emax = maximum effect, EC50 = concentration producing 50% of maximum effect, γ = sigmoidicity or shape factor and C = plasma concentration.
Results
Patient Disposition and Baseline Characteristics
A total of 155 patients with stable CAD were randomized to ticagrelor treatment: ONSET-OFFSET, n = 57; RESPOND, n = 98.
In ONSET-OFFSET, all patients who were randomized to ticagrelor treatment received at least one dose of ticagrelor, and five of these patients discontinued (one for incorrect enrolment; four due to adverse events [dyspnoea, n = 2; exertional dyspnoea, n = 1; sleep disorders, n = 1]). Pharmacokinetic data were available for all ONSET-OFFSET patients on day 1 (n = 57) and 53 patients at week 6. Pharmacodynamic data were available for up to 53 patients in the intent-to-treat analysis set (n = 54); 3/57 patients were excluded to form the full intent-to-treat set as baseline PA values were not available.
In RESPOND, 41 non-responders and 57 responders received at least one dose of ticagrelor. Overall, seven non-responders discontinued (five for adverse events [ticagrelor treatment: gastrointestinal haemorrhage, n = 1; hypotension, n = 1; ECG T-wave inversion, n = 1; clopidogrel treatment: dyspnoea, n = 1; myalgia, n = 1], one for protocol non-compliance, one for other reasons) and three responders discontinued (one for adverse events [ticagrelor treatment: bradycardia], one developed study-specific discontinuation criteria, one for other reasons). Pharmacokinetic data were available for 80 patients (37 non-responders, 43 responders). Pharmacodynamic data were available for 97 patients (40 non-responders, 57 responders). Exposure-IPA analyses were available for 79 patients (36 non-responders, 43 responders).
Patient demographics were similar between the two trials, and the majority were Caucasian males. Key baseline characteristics were also similar in both trials, and were well balanced between non-responders and responders in RESPOND (table I).
Demographic and key baseline characteristics of patients in ONSET-OFFSET and RESPOND (RESPOND data reproduced from Gurbel et al.,[18] with permission)
Ticagrelor Pharmacokinetics
After a single 180 mg loading dose of ticagrelor, absorption was rapid (figure 2a) and the median tmax for ticagrelor was approximately 2 hours in both ONSET-OFFSET and RESPOND (table II). The ticagrelor Cmax and AUC8 values were broadly comparable between both trials (table II). Formation of AR-C124910XX was also rapid (figure 3a), with a median tmax of 2.0–3.8 hours (table II) in both studies. Overall, exposure (Cmax and AUC8) to the active metabolite was approximately 21% that of the parent compound, after a single loading dose of ticagrelor (table II).
Mean (geometric) plasma concentration-time curves of ticagrelor after (a) a single ticagrelor 180 mg loading dose and (b) the last 90 mg maintenance dose (6 weeks in ONSET-OFFSET; day 14 in RESPOND). n values are the range of patient numbers with quantifiable samples. Note that the x-axes show different time scales. Clop = clopidogrel; Tic = ticagrelor.
Mean (geometric) plasma concentration-time curves of AR-C124910XX after (a) a single ticagrelor 180 mg loading dose and (b) the last 90 mg maintenance dose (6 weeks in ONSET-OFFSET; day 14 in RESPOND). n values are the range of patient numbers with quantifiable samples. Note that the x-axes show different time scales. Clop = clopidogrel; Tic = ticagrelor.
Mean plasma concentration-time profiles of ticagrelor (figure 2b) and AR-C124910XX (figure 3b) at steady state demonstrated that after multiple ticagrelor dosing (90 mg twice daily) [i.e. 6 weeks in ONSET-OFFSET; 14 or 28 days in RESPOND] absorption of ticagrelor and conversion to AR-C124910XX were rapid. These profiles were broadly consistent between the two studies given the large variability of the data. Key pharmacokinetic parameters (Cmax, AUC8, tmax) for ticagrelor were broadly comparable between ONSET-OFFSET and RESPOND (table II). Overall, exposure (AUC8) to the active metabolite after multiple ticagrelor dosing was approximately one-third that of the parent compound. In the ONSET-OFFSET study, the median (range) t1/2 values, calculated at the end of the 6-week maintenance period, were 9.8 (5.6–16.5) and 12.4 (7.3–22.8) hours, respectively.
Effect of Prior Administration of Clopidogrel on Ticagrelor Pharmacokinetics
In the RESPOND trial, pharmacokinetic measurements for the parent and active metabolite after a single ticagrelor loading dose were comparable in patients without prior clopidogrel administration (day 1, period 1, ticagrelor→clopidogrel group) and those who received 14 days of clopidogrel (day 1, period 2, clopidogrel→ticagrelor group) [table III]. Mean plasma concentration-time profiles of ticagrelor (figure 4a) and AR-C124910XX (figure 4b) were unaffected by 14 days of prior exposure to clopidogrel. Similarly, after 14 days of multiple dosing, ticagrelor and AR-C124910XX pharmacokinetic parameters were also unchanged by prior clopidogrel dosing (table IV).
Mean (geometric) plasma concentration-time curves of (a) ticagrelor and (b) AR-C124910XX in patients who received ticagrelor 180 mg (loading dose) initially or 24 hours after receiving 14 days of clopidogrel. n values are the range of patient numbers with quantifiable samples. Data for ticagrelor→clopidogrel (non-responders and responders) from figures 2a and 3a are repeated in figures 4a and 4b, respectively, for ease of comparison. Clop = clopidogrel; Tic = ticagrelor.
Pharmacokinetics of Ticagrelor in Clopidogrel Responders and Non-Responders
Although plasma concentrations of both analytes were highly variable, following a single 180 mg loading dose of ticagrelor, the plasma concentration profiles of the parent compound (figure 2a) and active metabolite (figure 3a) were generally comparable between CAD patients who were responsive and non-responsive to clopidogrel. Geometric mean ratios and the 95% confidence interval indicated that the mean Cmax and AUC8 values for both ticagrelor and AR-C124910XX were either slightly lower (the reason for this observation was that the 180 mg loading dose of ticagrelor was not given systematically) in clopidogrel responders versus non-responders (ticagrelor→ticagrelor group; table II) or comparable in clopidogrel responders (ticagrelor→clopidogrel group; table III) versus non-responders.
Single-dose ticagrelor pharmacokinetics were also unaffected in patients who had no prior exposure to clopidogrel versus those who had clopidogrel for 14 days before ticagrelor (table III, figure 4). This observation was noted in both clopidogrel responders and non-responders (table III, figure 4).
Steady-state pharmacokinetic parameters (i.e. after 2-week maintenance dosing) of ticagrelor were comparable between the clopidogrel responder and non-responder subgroups in RESPOND (tables II and IV). Geometric mean ratios and the 95% confidence interval indicated that the mean Cmax and AUC8 values for AR-C124910XX were either slightly lower in clopidogrel responders (ticagrelor→ticagrelor group; table II) or comparable (ticagrelor→clopidogrel group; table IV) versus non-responders, reflecting the data variability. Ticagrelor and AR-C124910XX pharmacokinetic parameters at steady state were unaffected in clopidogrel responders and non-responders following clopidogrel dosing for 14 days versus no prior exposure to clopidogrel (table IV).
Pharmacodynamics
In both ONSET-OFFSET and RESPOND, a single loading dose of ticagrelor (180 mg) rapidly inhibited PA within 30 minutes of dosing (figure 5a). Final-extent IPA was shown to reach a maximum at 2 hours post-dosing in both studies, and the effect was sustained for the duration evaluated (i.e. 24 and 8 hours post-dosing for ONSET-OFFSET and RESPOND, respectively). The IPA profiles in response to ticagrelor were comparable in CAD patients classified as either responsive or non-responsive to clopidogrel.
Mean (± standard deviation) final extent inhibition of platelet aggregation (induced by 20 μmol/L adenosine diphosphate) time curves of (a) onset of effect after initial loading dose (180 mg) ticagrelor (ITT analysis) and (b) offset of effect after 6 weeks administration of ticagrelor 90 mg twice daily (ITT analysis).[17] n values are the range of patient numbers with IPA data. Data shown in figure 5a were collected at timepoints indicated in the Methods section, but are displaced in the figure to avoid overlap. Note that the x-axes show different time scales. IPA = inhibition of platelet aggregation; ITT = intent-to-treat.
Offset of IPA following ticagrelor administration was assessed in ONSET-OFFSET (figure 5b).[17] Final-extent IPA increased for 2 hours after the final dose of ticagrelor then rapidly declined between 8 and 48 hours post-dosing. IPA continued to decrease thereafter and reached low concentrations by 120 hours post-dosing (figure 5b). For final-extent IPA, the slope of offset (4–72 hours after the last dose) was -1.04 IPA %/h.[17]
Exposure-Inhibition of Platelet Aggregation Relationships
In both ONSET-OFFSET and RESPOND, the exposure-IPA analyses showed that IPA declined with decreasing plasma concentrations of ticagrelor and its active metabolite (table V).
In the ONSET-OFFSET study, pooling the onset (0–24 hours post-first dose) and the offset (0–48 hours post-last dose) data, the model estimated high Emax values (IPA >97%) using either ticagrelor alone or ticagrelor plus AR-C124910XX plasma concentrations (table V). For the offset phase (4–48 hours post-last dose), the model also estimated high Emax values (IPA >100%) with both ticagrelor and ticagrelor plus AR-C124910XX plasma concentrations (table V). Ctrough for ticagrelor in the ONSET-OFFSET study was 305 ng/mL (CV 110%). This concentration was 5.20-fold and 4.99-fold higher than the EC50 estimates for the pooled onset/offset (0–24 hours post-first dose and 0–48 hours post-last dose) and offset (0–48 hours post-last dose) data, respectively (table V). Ctrough for AR-C124910XX in ONSET-OFFSET was 121 ng/mL (CV 65%), which was 7.71-fold higher than the EC50 estimate for the pooled onset/offset (0–24 hours post-first dose and 0–48 hours post-last dose), i.e. 15.7 (standard error 1.8) ng/mL.
Steady-state plasma concentrations in the RESPOND study following ticagrelor dosing at 90 mg twice daily, particularly in clopidogrel responders, were within a narrow range (data not shown), so day 14 data were insufficient for an accurate estimation of Emax model parameters. Therefore, parameters were calculated using day 1 data following a ticagrelor 180 mg loading dose. Data for non-responders were more variable than that for responders. Based on the clopidogrel→ticagrelor day 1, period 2 data, the sigmoid Emax model estimated high Emax values (IPA >96%), which were comparable between non-responders and responders, with ticagrelor or ticagrelor plus AR-C124910XX plasma concentrations (table V). For both responders and non-responders, the EC50 values were low (table V). Although higher EC50 values were noted for non-responders than for responders, the 95% confidence intervals were very wide for non-responders (table V). Mean pre-dose plasma concentrations of ticagrelor on day 14 were 286.9–306.0 ng/mL (non-responders) and 130.5–326.2 ng/mL (responders), which were much higher than the ticagrelor EC50 estimates shown in table V.
Discussion
Both ONSET-OFFSET and RESPOND investigated ticagrelor in stable CAD patients[17,18] at the clinical dose regimen approved in the EU,[5] which was the same regimen that was used in the phase III PLATO study.[7] Given the identical dosing regimen and similar patient demographics, pharmacokinetic and IPA data were compared across both studies to provide an assessment of these parameters in a larger CAD patient population. The sample size provided a more robust evaluation of key ticagrelor pharmacokinetic parameters in CAD patients. Importantly, the patient characteristics were typical of patients previously recruited in large cardiovascular outcome trials.[7,21,22]
Following a single oral ticagrelor 180 mg dose, Cmax, tmax and AUC8 were broadly comparable (i.e. overlapping variation of data) in ONSET-OFFSET and RESPOND. Although AUC8 was not a standard drug-exposure parameter in the ticagrelor development programme, the collection of pharmacokinetic blood samples for longer than 8 hours post-dose in the ONSET-OFFSET and RESPOND studies was not feasible in these patients due to the other multiple demands of the studies and blood volumes required. Despite this limitation, the AUC values reported herein are comparable with previous data following a single ticagrelor dose in healthy subjects (200 mg; mean AUC [standard deviation {SD}]: ticagrelor 8213 [2114] ng · h/mL; AR-C124910XX: 3722 [1668] ng · h/mL),[9] and in patients with stable atherosclerosis (200 mg; mean AUC [CV]: ticagrelor 7581 [35%] ng · h/mL; AR-C124910XX: 1753 [32%] ng · h/mL)[3] or ACS (180 mg; mean AUC [SD]: ticagrelor 6104 [4012] ng · h/mL; AR-C124910XX: 1584 [560] ng · h/mL).[16]
After multiple dosing (ticagrelor 90 mg twice daily), ticagrelor and AR-C124910XX pharmacokinetic parameters were broadly comparable between ONSET-OFFSET (after 6 weeks of dosing) and RESPOND (after 14 days of dosing). Both Cmax and tmax in these studies were also comparable with results reported for healthy volunteers. For example, following ticagrelor 100 mg twice daily for 5 days (n = 13) mean (CV) Cmax and median tmax, respectively, were 626 (46%) ng/mL and 2 hours for ticagrelor, and 219 (49%) ng/mL and 3 hours for AR-C124910XX.[10] Comparable data were also reported following ticagrelor multiple dosing in patients with stable atherosclerosis (100 mg twice daily for 14 days),[3] and ACS patients (90 mg twice daily for 28 days).[16]
In RESPOND, ticagrelor pharmacokinetics were evaluated before and after clopidogrel dosing. Following a single ticagrelor loading dose, key pharmacokinetic parameters and the plasma concentration versus time curves of both ticagrelor and AR-C124910XX were comparable before and after 14 days of clopidogrel dosing. Thus, these data demonstrate that prior exposure to clopidogrel did not affect the absorption and metabolism of ticagrelor. Another aspect of the RESPOND design is the characterization of ticagrelor pharmacokinetics in patients classified as clopidogrel non-responders and responders. Again, the pharmacokinetic profile of ticagrelor and its active metabolite were comparable in these two groups after both single and multiple ticagrelor dosing, and regardless of order of exposure to clopidogrel. Thus, our findings suggest that ticagrelor pharmacokinetics are not significantly associated with the responsiveness to clopidogrel.
Since clopidogrel and ticagrelor were administered sequentially in RESPOND, this allowed some characterization of the potential interaction between the two drugs. In clopidogrel non-responders switching from clopidogrel to ticagrelor, an enhancement of IPA was seen;[18] however, a limitation of RESPOND was that IPA was only evaluated for 8 hours post-dosing so the full extent of this apparent interaction is unclear. Similarly, in the DISPERSE-2 trial greater IPA was seen following ticagrelor administration to ACS patients who had previously received clopidogrel compared with those who were clopidogrel-naïve.[16] These findings are suggestive of a positive pharmacodynamic interaction between ticagrelor and clopidogrel.
In contrast, a healthy volunteer study investigating potential interactions between clopidogrel and cangrelor,[23] a reversible P2Y12 inhibitor structurally similar to ticagrelor,[24] showed that simultaneous administration of cangrelor with clopidogrel resulted in a lower-than-expected sustained platelet inhibition in response to clopidogrel.[23] In contrast, sequential administration of clopidogrel after cangrelor resulted in the sustained platelet inhibition expected with clopidogrel. These pharmacodynamic findings indicate a negative interaction between clopidogrel and cangrelor when these agents are given together.[23] The interaction between ticagrelor and clopidogrel when given together on IPA has not been studied.
The present exploration of exposure-IPA relationships demonstrated that the final-extent IPA during onset correlated with ticagrelor plus AR-C124910XX plasma concentrations in patients with CAD. This association is as expected, given that ticagrelor exerts its antiplatelet activity by binding directly to the P2Y12 receptor.[13] During the offset phase, IPA declined with decreasing plasma concentrations of ticagrelor. However, from the present data, it is not possible to interpret the reasons why IPA was measurable at later timepoints (i.e. from 48 hours post-last dose) when ticagrelor concentrations were not quantifiable in plasma.
High Emax values for final-extent IPA were estimated by the sigmoid Emax model applied to both studies. In ONSET-OFFSET, these values were IPA >97%, indicating that ticagrelor can completely inhibit the final PA response to ADP in patients with stable CAD. Moreover, the model estimated EC50 values for ticagrelor and AR-C124910XX that were only approximately 19% and 13%, respectively, of the trough plasma concentrations of analytes. This finding supports the recommended ticagrelor dosing regimen (180 mg loading dose/90 mg twice daily), illustrating that the plasma concentrations of the parent drug and active metabolite are sufficiently high to achieve and sustain high concentrations of IPA in patients with CAD. This observation is also supported by the findings from RESPOND. High Emax values for ticagrelor, which were comparable between responders (IPA ≥98%) and non-responders (IPA >97%), indicate that ticagrelor can inhibit PA in patients responsive or non-responsive to clopidogrel. Indeed, in RESPOND the antiplatelet effect of ticagrelor was not greatly affected by patient responsiveness to clopidogrel.[18] Ticagrelor at 180 mg loading dose/90 mg twice daily also resulted in sufficiently high plasma concentrations of the parent drug required for IPA in RESPOND, since minimum ticagrelor concentrations were many fold higher than the model estimated ticagrelor EC50 values for both responders and non-responders.
Conclusions
In conclusion, the present subanalyses showed that pharmacokinetics of ticagrelor in patients with stable CAD were comparable to those seen previously. Ticagrelor (180 mg loading dose then 90 mg twice daily) effectively inhibited PA, and trough plasma concentrations of ticagrelor (90 mg twice daily) and AR-C124910XX were sufficient to achieve high IPA in stable CAD patients. Ticagrelor pharmacokinetics were unaffected by prior exposure to clopidogrel and were comparable in patients defined as responsive and non-responsive to clopidogrel. Collectively, these findings provide further evidence that ticagrelor is associated with a faster and superior onset of antiplatelet effects in patients, including those who are clopidogrel non-responders, and that therapy can safely be switched from clopidogrel to ticagrelor.
References
Springthorpe B, Bailey A, Barton P, et al. From ATP to AZD6140: the discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis. Bioorg Med Chem Lett 2007; 17: 6013–8
Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther 2009; 27: 259–74
Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006; 27: 1038–47
Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50: 1844–51
Brilique. Summary of product characteristics 2010 [online]. Available from URL: http://www.ema.europa.eu [Accessed 2011 Dec 19]
Brilinta TM, US full prescribing information, July 2011 [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022433s000lbl.pdf [Accessed 2011 Dec 19]
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–57
Teng R, Butler K. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect in healthy subjects [abstract no. 9]. Can J Clin Pharmacol 2008; 15: e426
Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist in healthy subjects. Eur J Clin Pharmacol 2010; 66: 487–96
Butler K, Teng R. Pharmacokinetics, pharmacodynamics and safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 2010; 70: 65–77
Pereillo J-M, Maftouh M, Andrieu A, et al. Structure and stereochemistry of the active metabolite of clopidogrel. Drug Metab Dispos 2002; 30: 1288–95
Dansette PM, Rosi J, Bertho G, et al. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol 2012; 25(2): 348–56
van Giezen JJJ, Nilsson L, Berntsson P, et al. Ticagrelor binds to human P2Y12 independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. J Thromb Haemst 2009; 7: 1556–65
Teng R, Oliver S, Hayes MA, et al. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos 2010; 38: 1514–21
Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction and differential kinetics. Drug Metab Dispos 2011; 39: 703–10
Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50: 1852–6
Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120: 2577–85
Gurbel PA, Bliden KP, Antonino M, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 2010; 121: 1188–99
Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B 2010; 878: 2299–306
Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003; 107: 2908–13
Weber MA, Bakris GL, Dahlöm B, et al. Baseline characteristics in the avoiding cardiovascular events through combination therapy in patients living with systolic hypertension (ACCOMPLISH) trial: a hypertensive population at high cardiovascular risk. Blood Press 2007; 16: 13–9
Fellström BC, Jardein AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–407
Steinhubl SR, Oh JJ, Oestreich JH, et al. Transitioning patients from cangrelor to clopidogrel: pharmacodynamic evidence of a competitive effect. Thromb Res 2008; 121: 527–34
van Giezen JJ, Humphries RG. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost 2005; 31: 195–204
Acknowledgements
These studies were funded by AstraZeneca. AstraZeneca was involved in the design and conduct of the studies; collection, analysis and interpretation of the data; and the preparation, review and approval of this manuscript.
The authors wish to thank Cathy Whalen (Program Administrator, AstraZeneca LP, Wilmington, DE, USA) for study operations, as well as all of the other staff at the various centres who were involved in the trials. H. Sillén (Bioanalysis Responsible Scientist, AstraZeneca R&D, Mölndal, Sweden), staff at York Bioanalytical Solutions, York, UK, and Quality Data Services, King of Prussia, PA, USA, are thanked for their contribution to the pharmacokinetic sample analyses and data management within these studies. Editorial support was provided by Jackie Phillipson (Medical Writer, Gardiner-Caldwell Communications); this support was funded by AstraZeneca.
S. Husted has received consulting fees from AstraZeneca, Sanofi-Aventis and Eli Lilly, and lecture fees from AstraZeneca, Sanofi-Aventis and Bristol-Myers Squibb.
R. Storey has received consulting fees from AstraZeneca, Eli Lilly, Daiichi Sankyo, Teva, Novartis, The Medicines Company, Eisai, Merck/Schering-Plough, Sanofi-Aventis, Bristol Myers Squibb and Accumetrics, lecture fees from Eli Lilly, Daiichi Sankyo, AstraZeneca, Merck, Iroko and GlaxoSmithKline, and grant support from AstraZeneca, Eli Lilly, Daiichi Sankyo, Merck/Schering-Plough, Dynabyte and Accumetrics. K. Bliden, U.S. Tantry and L. Høimark have no conflicts of interest to declare. K. Butler, C. Wei and R. Teng are employees of AstraZeneca. P.A. Gurbel has received research grants and honoraria from Schering Plough/Merck, Haemoscope, AstraZeneca, Medtronic, Daiichi Sankyo/Lilly, Sanofi-Aventis, Boston-Scientific, Portola, Novartis, Pozen and Bayer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husted, S.E., Storey, R.F., Bliden, K. et al. Pharmacokinetics and Pharmacodynamics of Ticagrelor in Patients with Stable Coronary Artery Disease. Clin Pharmacokinet 51, 397–409 (2012). https://doi.org/10.2165/11599830-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11599830-000000000-00000