Abstract
Background and Objectives: Existing methods for the prediction of human clearance of therapeutic proteins involve the use of allometry approaches. In general, these approaches have concentrated on the role of body weight, with only occasional attention given to more specific physiological parameters. The objective of this study was to develop a mechanism-based model of hepatic clearance (CLH), which combines a single-species scaling approach with liver physiology, for predicting CLH of selected glycoprotein derivate therapeutics, and to compare the outcome of this novel method with those of two empirical methods obtained from the literature — namely, the single-exponent theory and multiple-species allometry. Thus, this study was designed as an explanatory study to verify if the addition of physiological information is of benefit for extrapolating clearance of selected therapeutic proteins from one species to another.
Methods: Five glycoprotein derivate therapeutics that are known to be principally eliminated by asia-loglycoprotein receptors (ASGPRs) under in vivo conditions were selected. It was assumed that the interspecies differences in CLH reported for these compounds are reflected by the interspecies differences in the abundance of these receptors. Therefore, key scaling factors related to these differences were integrated into one model. Fourteen extrapolation (prediction) scenarios across species were used in this study while comparing the single-species model, based on physiology, with the single-exponent theory. In addition, the physiological model was compared with multiple-species allometry for three proteins.
Results: In general, the novel physiological model is superior to the derived allometric methods. Overall, the physiological model produced a predicted CLH value with levels of accuracy of 100% within 3-fold, 100% within 2-fold and about 82% within 1.5-fold, compared with the observed values, whereas the levels of accuracy decreased to 93%, 77% and 53%, respectively, for allometry. The proposed physiological model is also superior to allometry on the basis of the root mean square error and absolute average fold error values.
Conclusions: It has been demonstrated that interspecies differences in the abundance of ASGPRs principally govern interspecies variations in CLH of compounds that are principally eliminated by ASGPRs. Overall, the proposed physiological model is an additional tool, which should facilitate investigation and prediction of human CLH of specific glycoproteins solely on the basis of clearance data determined in a single preclinical species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Early prediction of human pharmacokinetics of therapeutic proteins is playing an increasingly important role in drug development. A review of protein clearance has shown that excellent allometric relationships (clearance versus body weight) can exist between animals and humans.[1–8] Some authors have recently revisited the allometric scaling principles of clearance of 34 therapeutic proteins, and found that the optimal exponent was estimated to be about 0.8.[6] Recently, different methods for projecting human clearance from animal pharmacokinetic data were examined for 13 therapeutic monoclonal antibodies exhibiting linear pharmacokinetics over the tested dose ranges.[7] A better correlation between the observed human clearance and the estimated human clearance was obtained by using pharmacokinetic data from cynomolgus monkeys, with an optimal scaling exponent of 0.85, than by using other scaling approaches. Therefore, the single species-scaling approach using a fixed exponent (the single-exponent theory) affords a viable alternative to predict human plasma hepatic clearance (CLH) of several therapeutic proteins, especially in the absence of nonlinear pharmacokinetics and species-specific clearance mechanisms.[1–7] Conversely, in studying the allometric scaling of clearance of coagulation factors and tissue-type plasminogen activators, Mahmood[8] observed a wider range of exponents (0.67–0.91), indicating that the use of a fixed-exponent approach could be inappropriate in other cases. Therefore, Mahmood[8] demonstrated that single-species scaling could be useful, but the predicted clearance may not be as accurate as that predicted from scaling of three or more species. If, for some unforeseen reason, one has to use the single-exponent theory (about 0.8) or multiple-species allometry, then extreme caution is needed in the interpretation of data, as the predicted clearance may be in error.[8] Therefore, there is still scope to challenge these two empirical approaches (the single-exponent theory and multiple-species allometry).
Accumulation of information on the role of physiology in the extrapolation procedure should be encouraged. Standard allometry is normally based on the body weight, which limits the investigation of specific physiological determinants that could also influence the prediction of clearance. Alternative approaches to the use of a body weight value for allometry should include those that are more closely based on liver physiology, since this organ expresses several receptors that are involved in CLH of several therapeutic proteins under in vivo conditions. Therefore, the involvement of this aspect in the prediction of CLH of therapeutic proteins has been considered likely. The clearances of high-molecular-weight compounds from plasma are usually due to cellular uptake by endocytosis, especially in the liver and the kidneys, and/or to irreversible binding of these compounds to plasma proteins. Numerous studies have suggested that low-density lipoprotein receptor-related proteins (LRPs), asialoglycoprotein receptors (ASGPRs) and mannose receptors (MRs) present on the liver cell surface might play important roles as clearance mechanisms for several therapeutic proteins under in vivo conditions.[9–18] In particular, high-mannose oligosaccharides have clearance governed by MRs, whereas LRPs — an oligosaccharide-independent mechanism — are members of the low-density lipoprotein (LDL) receptor family, which also play a role in endocytosis. ASGPRs depend on removal pathways that involve glycan recognition. Hepatic ASGPRs are lectins, which bind glycoproteins from which a sialic acid has been removed to expose terminal galactose residues. These receptors remove the target glycoproteins from the circulation.[12–18] Because of its specificity, predominant expression on hepatocytes and high capacity for receptor-mediated endocytosis, the ASGPR has been validated as a target for CLH of several therapeutic proteins such as erythropoietin, tissue plasminogen activators, interferon and various hormones.[3,8,19–27]
Literature data suggest that the amino acid composition of ASGPRs is closely related between species. For example, there is 80% amino acid homology between the major human (H1) and rat (RHL1) ASGPR subunits.[26] In addition, a review of protein clearance has shown that excellent allometric relationships can exist between animals and humans for substrates of ASGPRs.[1] These observations should indicate conservation of the function/affinity of the clearance mechanism across species. Therefore, it could be assumed that the variation in the affinity between compounds in a single species is greater than the variation in the affinity between species for a single compound. The mechanism of CLH must be assessed prior to any scaling. In this case, if the primary receptors that are related to the hepatic uptake of a therapeutic protein are related to ASGPRs, it could consequently also be assumed that the interspecies differences in clearance reported for a glycoprotein that is eliminated by ASGPRs are reflected by the interspecies differences in the abundance of these receptors. In this context, the number of ASGPRs per gram of liver (or per kilogram of body weight) in several preclinical species could easily be estimated from the literature.[13–18] Moreover, significant interspecies differences in the abundance of ASGPRs have been reported. Whether or not this may explain the interspecies differences in clearance is still not known for compounds that are mainly eliminated by ASGPRs. Therefore, such a novel principle of scaling should also be challenged.
The objective of this study was to develop a mechanism-based model of CLH, which combines a single-species scaling approach with liver physiology, for predicting CLH of selected glycoprotein derivate therapeutics that are principally eliminated by ASGPRs, and to compare the outcome of this novel method with those of two methods obtained from the literature — namely, the single-exponent theory and multiple-species allometry. Thus, this study was designed as an explanatory study to verify if the addition of physiological information is of benefit for extrapolating clearance of selected therapeutic proteins from one species to another.
Methods
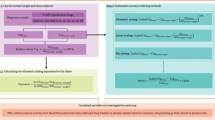
This study was performed in two steps. The first step consisted of development of the single-species model of CLH, based on liver physiology, and the second step consisted of comparison of this novel model with more empirical models that have been reported in the literature (namely, the single-exponent theory and multiple-species allometry).
The Dataset
Compounds were considered only if their in vivo CLH is principally mediated by ASGPRs (according to mechanistic experimental studies published in the literature) and if the necessary preclinical and clinical data were available. In other words, if the primary receptors related to the hepatic uptake of a carbohydrate protein seemed to be a different type of receptor (e.g. LRPs, MRs), that protein was not further analysed in this study. Therefore, the contribution of LRPs and MRs should be small. Five glycoprotein derivate therapeutics that are known to be principally eliminated by ASGPRs under in vivo conditions were selected on the basis of a review of the literature.[3,8,19–27] Recombinant human erythropoietin (rhEPO) is a glycoprotein hormone, which controls erythropoiesis, or red blood cell production. It is a cytokine for erythrocyte precursors in the bone marrow.[3,19,20] Lanoteplase, reteplase (BM 06022) and pamiteplase are recombinant plasminogen activators which, when administered as a single-bolus intravenous injection, display thrombolytic activity.[8,21–25] Finally, recombinant neutrophil inhibitory factor (rNIF) [UK 279276] is a glycoprotein which has also been investigated as an intravenous treatment for stroke.[26,27] Since the pharmacokinetics of rNIF can be nonlinear in dogs,[27] the clearance value studied at the lowest dose was considered for this compound. These proteins have clearance data in more than one preclinical species and in humans. The corresponding average value of clearance determined in vivo is presented for each species and compound in table I.
Modelling Assumptions
A non-saturable, high-affinity/high-capacity clearance process is assumed to be present in all animal species, as well as in humans, for each compound studied, and it is believed to involve hepatic uptake mediated by ASGPRs. Consequently, it was considered that the sole factor governing the interspecies variation in CLH under in vivo conditions is the interspecies difference in the content of ASGPRs, as previously assumed.
Development of the Physiological Model
Physiological Modelling of Hepatic Clearance (CLH)
Development of the physiological model of CLH was performed by combining the value of clearance observed in one species with key scaling factors obtained from the literature, which are related to interspecies differences in the abundance of ASGPRs. Since the initial literature data expressed the abundance of ASGPRs in terms of total receptors per cell of liver, extrapolation factors were essential to convert the total receptors per cell of liver to total receptors per gram of liver, and subsequently to total receptors per kilogram of body weight (equations 1 and 2):
where CL is the in vivo clearance referring to plasma kinetics (either observed or predicted, measured in mL/min/kg) in animals and humans; ISFtarget species/index species is the global interspecies scaling factor (i.e. total receptors per unit kilogram of body weight in the target species relative to the index species); AF is the specific factor for abundance, which corresponds to physiological differences in the abundance of ASGPRs in the liver (i.e. total receptors per cell of liver in the target species relative to the index species); HF is the specific factor for hepatocellularity, which corresponds to physiological differences in cellularity (i.e. the number of cells per gram of liver in the target species relative to the index species); and LF is the specific factor considering the differences in the fractional content of the liver (i.e. grams of liver per kilogram of body weight in the target species relative to the index species). Table II presents details of the ISF values resulting from each species that was studied, whereas table III presents the global ISFtarget species/index species values according to the extrapolation scenarios in figure 1.
Estimation of the Abundance Factor
The total number of ASGPRs per cell of liver in each species was obtained from experimental studies published in the literature.[13–18] Briefly, in vitro binding studies of a ligand of ASGPR, asialofetuin, were performed at 37°C with animal and human hepatocytes to estimate the numbers of these receptors.[13–18] These studies gave the numbers of cell surface occupied receptors, assuming that one asialofetuin molecule binds to one receptor molecule. Using fresh hepatocytes, the total numbers of ASGPRs were estimated to be 2.44 × 105 receptors/cell in mice, 5.21 × 104 receptors/cell in dogs and 1.4 × 105 receptors/cell in humans.[13–18] For rats, there is more than one value reported in the literature (1.8 × 105, 2.1 × 105 and 4.08 × 105 receptors/cell). For the purpose of this study, the intermediate value was used (2.1 × 105 receptors/cell) [table II].
Estimation of the Hepatocellularity Factor
Hepatocellularity, expressed as 106 cells per gram of liver, is assumed to be 135, 117, 215 and 100 in mice, rats, dogs and humans, respectively (table II).[28,29] In humans, this value ranged from 74 to 139.[28,29] Again, the intermediate value (100) was used in this study.
Estimation of the Liver Factor
The fractional content of the liver (grams of liver per kilogram of body weight) is equal to 88, 37, 32 and 26 in mice, rats, dogs and humans, respectively (table II).[30]
Allometric Modelling of CLH
Single-Species Extrapolation Based on the Single-Exponent Theory
Single-species-based allometry (the single-exponent theory) has been reported in several studies.[6–8] Equation 3 shows this approach:
where CL referring to plasma kinetics is expressed as mL/min and body weight (BW) is expressed in kilograms.
Multiple-Species Extrapolation
For multiple-species allometry, a power function was applied (equation 4):
Using log-log transformed data, equation 4 transforms into a linear function (equation 5):[1]
where m is the slope and log(b) is the intercept resulting from the correlations between CL referring to plasma kinetics (mL/min) [y axis] and BW (kilograms) [x axis] that have been observed in the preclinical species.
Comparative Assessment Between the Physiological Modeland Allometry
Single-Species Model Based on Physiology versus the Single-Exponent Theory
Investigated Extrapolation Scenarios
Fourteen extrapolation (prediction) scenarios across species were used in this study for five therapeutic proteins, to assess the single-species models of CLH (this study and the single-exponent theory), as depicted in figure 1.
Single-Species Model Based on Physiology versus Multiple-SpeciesAllometry
A complete preclinical and clinical dataset was available for three proteins: rhEPO, reteplase and pamiteplase (table I). Consequently, multiple-species allometry was applied to these compounds and compared with the proposed physiological model for prediction of human CLH.
Input Parameters
The input data used in the studied models are shown in tables I–III.
Statistical Analyses
The prediction accuracy was assessed by comparing predicted and observed values of CLH, using specific statistical parameters.[31] Specific fold errors of deviation between the predicted and observed values (% predictions within fold errors of ≤1.5, ≤2 and ≤3) were calculated, as well as the coefficient of correlation (r) values. Furthermore, absolute average fold errors (AAFEs) and root mean square errors (RMSEs) were used as a measure of spread and to rank precision, respectively.[31] Finally, plots of predicted versus observed CLH values (mL/min/kg) were also created.
Results
Comparative Assessment Between the Physiological Modeland Allometry
Single-Species Model Based on Physiology versus the Single-Exponent Theory
In most cases, the novel physiological model was superior to allometry derived from the single-exponent theory for the 14 extrapolation scenarios that were investigated for five therapeutic proteins. In this study, the physiological model predicted CLH with levels of accuracy of 100% within 3-fold, 100% within 2-fold and about 79% within 1.5-fold, compared with the observed value, whereas the levels of accuracy decreased to 93%, 86% and 57%, respectively, for allometry. The proposed physiological model was also superior to allometry on the basis of the RMSE and AAFE values (table IV). Both approaches resulted in a high degree of correlation between the predicted and experimental values, but with a slightly higher correlation for the single-exponent theory (r = 0.97) than for the physiological model (r = 0.94). This was also reflected graphically (figure 2). Note that use of an exponent of 0.75 in equation 3 (the single-exponent theory) compared with 0.8 (in this study) did not provide a superior level of accuracy, in general (data not shown).
Plot of predicted vs observed CLH in the single-species models: (a) physiological model of this study (n = 14; r = 0.94); (b) empirical model [single-exponent theory] (n = 14; r = 0.97). The CLH values are provided in table IV. CL H = hepatic clearance.
An additional finding was that the scaling of CLH in vivo was relatively proportional to the interspecies differences in liver abundance of ASGPRs (the ISF). In general, the greater the number of ASGPRs in a species (ISF values in table II), the greater the CLH (per kilogram of body weight) and vice versa (table I). For example, CLH in vivo for humans and dogs was nearly the same (per kilogram of body weight) for all compounds for which these data were available (rhEPO, reteplase and pamiteplase) [table I]. The ratio of the ISF (per kilogram of body weight) between these two species was unity (table III), which means that the scaling factor was unity.
Single-Species Model Based on Physiology versus Multiple-SpeciesAllometry
Multiple-species allometry provided a high degree of correlation and exponent values ranging from 0.60 to 0.89. With allometry, CLH was underpredicted in comparison with the observed CLH by about a 2-fold error for two compounds out of three (rhEPO and pamiteplase) [table V]. For the other compound (reteplase), CLH was adequately predicted. Again, the proposed physiological model is superior to multiple-species allometry because CLH was more accurately predicted for two out of the three compounds that were studied (rhEPO and pamiteplase). For reteplase, the two approaches provided a similar level of accuracy (table V).
Discussion
The present study had the merits of (i) proposing a novel physiological approach for the prediction of CLH of therapeutic proteins; (ii) exploring the validity of this approach by using five therapeutic proteins and, in particular, several extrapolation scenarios across diverse species (14 scenarios); (iii) comparing the physiological approach with standard allometry for the current dataset of proteins; (iv) offering a first attempt to explore further mechanisms, by associating interspecies differences in the abundance of ASGPRs with interspecies differences in CLH values; (v) accumulating information on the role of liver physiology in the extrapolation procedure; and (vi) suggesting ways of applying this approach in drug development. These aspects are discussed further below.
The main route of elimination greatly influences the predictability of intravenous clearance. Therefore, it is difficult to estimate clearance of therapeutic proteins when preparing first-in-man trials, because multiple clearance mechanisms are potentially involved in the clearance of these drugs.[32] In this study, only compounds that are known to be eliminated by ASGPRs (according to mechanistic studies reported in the literature) were investigated. Considering the relative success of the proposed single-species extrapolation model, it could be assumed that the model can only be applied in these circumstances. In other words, if it is not known which metabolic pathway is involved, then the model presented here cannot be applied to predict human clearance from a single preclinical species. Because there is still room for improvement, a full quantitative structure-activity relationship (QSAR)/in silico method remains to be established in a more quantitative way to identify the main metabolic pathway for therapeutic proteins, and the first attempts to develop QSARs for therapeutic proteins[33,34] may help to further drive future work. Coupled with QSAR/in silico methods predicting the metabolic pathway of a therapeutic protein, the proposed single-species method should be applicable in drug development while ASGPRs are identified. Similarly, if the main mechanism of clearance could be determined in one or more preclinical species in experimental studies (in vitro and/or in vivo), and if the mechanism is related to ASGPRs, this process should allow CLH prediction before entering into human studies. Alternatively, the proposed physiological model could also be used to support mechanistic studies of clearance processes.[32] Consequently, the proposed model is an additional tool, which provides a meaningful testing tier to investigate whether a studied molecule is cleared by these receptors or not. In this case, inaccurate predictions of CLH would have been expected in this study if the CLH were strongly determined by receptors other than ASGPRs and/or some selected structural elements of these therapeutic proteins. Conversely, accurate predictions of CLH in vivo for 14 extrapolation scenarios across species suggest some biological underpinning related to the abundance of ASGPRs in each species. Therefore, the results of this study enforce the role of ASGPRs in the mechanism of clearance of the five glycoproteins that were studied, which had originally been suggested by other authors.[3,19–27]
The principle of the proposed model is also applicable to other receptors such as LRPs and MRs, and to species such as monkeys, while the essential physiological input data on the abundance of these receptors and/or the QSAR method become available in the literature. The high usage of monkeys in drug development, irrespective of the high cost, is perhaps not surprising. In this study, such a physiological model for monkeys has not been challenged, principally because the essential input data on the abundance of ASGPRs cannot be found in the literature.
A proof of concept for the proposed physiological model is that the interspecies differences in CLH in vivo follow the interspecies differences in abundance of ASGPRs (i.e. the ISF values). For example, CLH in vivo in humans and dogs is nearly the same, per kilogram of body weight (table I). Consequently, the abundance of ASGPRs per kilogram of body weight (the ISF) is also similar in these species, as previously mentioned (table II). An important finding is that standard empirical allometry did not perform better than the physiological model developed in this study. This has been verified using several statistical parameters and extrapolation scenarios. However, since the difference in predictivity between the prediction methods is sometimes small, it could be that both the proposed physiological model and allometry depend on body weight. In other words, it could be that the resulting exponent is comparable within these approaches if both the scaling factors (ISFs) and CL values are somehow related to the body weight. In this case, it seems that the values of the scaling factor used for ASGPRs (i.e. the ISFs) also correlate with the body weight, which could be a rational explanation. The resulting exponent and coefficient of correlation were 0.74 and 0.99, respectively, for the current dataset (data not shown). These compare well to multiple-species allometry following correlation of CLH with the body weight (exponent ranges from 0.69 to 0.89, and coefficient of correlation ranges from 0.96 to 0.99) [table V]. The single-exponent theory presented a single exponent of about 0.8.[6] In essence, the proposed single-species model highlights the role of additional physiological determinants of the liver (e.g. the abundance of ASGPRs) that appear to be related to the body weight, which appears to influence the clearance of the selected therapeutic proteins. Scientifically, this is of great importance, since a more in-depth analysis of the role of physiology on CLH has been conducted in this study.
The values of the resulting exponent are comparable but not all identical. Consequently, a single (unique) value could not be identified across the studied methods and compounds. This is not in total accordance with the single-exponent theory.[6] In this context, Mahmood[8] also observed relatively different exponent values across compounds in multiple-species allometry. However, it could be that the variability in the observed exponents depends on which datasets of clearance values are used,[3] or it could be that for specific compounds, the clearance mechanisms are not conserved across species.
The concept of single-species scaling was first introduced for small molecules, and later for some therapeutic proteins, as mentioned.[6–8,35] In this study, we have concisely challenged this concept with compound examples. Irrespective of the number of compounds that were studied, use of multiple species did not demonstrate a significant gain in accuracy compared with the single-species concept for the selected compounds that are cleared by ASGPRs (tables IV and V). This is in accordance with the literature.[6–8,35,36] It is considered important from the perspective of prediction of clearance in drug discovery, since clearance data in only one species could potentially be used to predict clearance in another species (e.g. humans).
Although the findings of the present study were established in a dataset using five glycoproteins, based on the availability of preclinical and clinical data, it still needs to be considered limited. However, it is worth mentioning that several (14) extrapolation scenarios across species were challenged. Nevertheless, it is difficult to be fully confident in the ability to compare data analysis across compounds, and this may influence confidence in the reliability of the conclusions presented here. Consideration of more compounds may change the conclusion of this study. However, while it is acknowledged that this could be important, it appears that the current physiological model performed relatively well compared with allometry. On a separate note, throughout this study, analysis of the current dataset of drugs was limited to prediction of the mean, without consideration of sensitivity, variability and uncertainty. Although these issues are beyond the scope of the present study, analysis of sensitivity, variability and uncertainty would apply not only to the current physiological model but also to the conventional approaches that are used for allometry. In the present study, all approaches were compared by using the same datasets, at least limiting the variability of the datasets. Once the issues of sensitivity, variability and uncertainty have been addressed, it will be feasible to assess predictions, and the risks associated with uncertain predictions, more objectively.
It is well accepted that interspecies scaling works best for compounds that follow linear pharmacokinetics.[1–8] Therefore, use of the current physiological model of clearance should be less successful for compounds that do not follow this rule. In other words, as the clearance process predicted for ASGPRs is assumed to be linear with time and dose, compounds that follow nonlinear pharmacokinetics could not be considered by the present approach. In this context, rNIF has linear pharmacokinetics in rats but not in dogs.[27] Two mediated elimination pathways occur in dogs — namely, ASGPR and CD11b (single-pass type-1 α). For this reason, the clearance determined at the lowest dose was investigated particularly for the rat-to-dog extrapolation scenario, which was successfully predicted. Similarly, alteration of bone marrow function results in a significant change in rhEPO pharmacokinetics, indicating that bone marrow can also be a route of rhEPO elimination for low doses and may contribute to its non-linear pharmacokinetics via receptor-mediated endocytosis.[3] Despite the non-linear pharmacokinetic behaviour of rhEPO, CLH was quite well correlated with the body weight,[3] as well as being accurately predicted (in this study) [tables IV and V] at the doses for which clearance data were reported in healthy subjects.[19]
In predicting CLH, it is possible that interspecies differences in binding to plasma proteins have no impact on the therapeutic proteins that were studied. This is particularly true if the magnitude of binding of these macromolecules is relatively similar in each species. In the absence of definitive data in that domain, this assumption cannot be verified. Therefore, further clinical studies are necessary to also examine the impact of plasma protein binding on the scaling of clearance of the current compounds.[24]
Conclusions
A singles-species model, using liver physiology, has been developed for prediction of CLH in animals and humans. According to the current findings, interspecies scaling of clearance of glycoprotein derivate therapeutics that are eliminated by ASGPRs seems to be governed by interspecies differences in the abundance of these receptors. Therefore, the physiological model is appropriate only for glycoprotein derivate therapeutics that are principally eliminated by ASGPRs. Superior or quite similar levels of accuracy were obtained with this model compared with standard allometry. Overall, the proposed physiological model is an additional tool, which should facilitate investigation and prediction of human clearance of specific glycoproteins solely on the basis of clearance data determined in a single preclinical species.
References
Mordenti J, Chen SA, Moore JA, et al. Interspecies scaling of clearance and volume of distribution data for five therapeutic proteins. Pharm Res 1991; 8: 1351–9
Mahmood I. Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci 2004; 93: 177–85
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos 2007; 35: 1672–8
Ling J, Zhou H, Jiao Q, et al. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol 2009; 49: 1382–402
Mahmood I. Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci 2009; 98: 3850–61
Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Drug Metab Dispos 2010; 31: 253–63
Deng R, Iyer S, Theil FP, et al. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs 2011; 3: 61–6
Mahmood I. Pharmacokinetic allometric scaling of coagulation factors and tissue-type plasminogen activators. Haemophilia 2009; 15: 1109–17
Seydel W, Stang E, Roos N, et al. Endocytosis of the recombinant tissue plasminogen activator alteplase by hepatic endothelial cells. Arzneimittelforschung 1991; 41: 182–6
Strickland DK, Medved L. Low-density lipoprotein receptor-related protein (LRP)-mediated clearance of activated blood coagulation co-factors and proteases: clearance mechanism or regulation? J Thromb Haemost 2006; 14: 84–6
Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol 2001; 23: 53–67
Otter M, Barrett-Bergshoeff MM, Rijkens DC. Binding of tissue-type plasminogen activator by the mannose receptor. J Biol Chem 1991; 266: 13931–5
Pardridge WM, van Herle AJ, Naruse RT, et al. In vivo quantification of receptor-mediated uptake of asialoglycoproteins by rat liver. J Biol Chem 1983; 258: 990–4
Abe M, Lai J, Kortylewicz ZP, et al. Radiolabeled constructs for evaluation of the asialoglycoprotein receptor status and hepatic functional reserves. Bioconjugate Chem 2003; 14: 997–1006
Eisenberg C, Seta N, Appel M, et al. Asialoglycoprotein receptor in human isolated hepatocytes from normal liver and its apparent increase in liver with histological alterations. J Hepatol 1991; 13: 305–9
Kuhlenschmidt MS, Hoffmann WE, Rippy MK. Glucocorticoid hepatology: effect of receptor-mediated endocytosis of asialoglycoproteins. Biochem Med Metab Biol 1991; 46: 152–68
Dodeur M, Coumoul S, Scarmato P, et al. Asialoorosomucoid degradation by normal and diabetic rat hepatocytes. Eur J Biochem 1984; 140: 577–81
Slama A, Zinbi H, Feger J, et al. Comparative determination of the asialoglycoprotein receptor by ligand and antibody binding in hepatocytes from normal and diabetic rats. Biol Cell 1988; 63: 367–9
Bleuel H, Hoffmann R, Kaufmann B, et al. Kinetics of subcutaneous versus intravenous epoetin-beta in dogs, rats and mice. Pharmacology 1996; 52: 329–38
Marmont AM. Erythropoietin: biochemical characteristics. Biological effects, indications and results of use in hematology. Tumori 1997; 83: S3–15
Kostis JB, Dockens RC, Thadani U, et al. Comparison of pharmacokinetics of lanoteplase and alteplase during acute myocardial infection. Clin Pharmacokinet 2002; 41: 445–52
Komoriya K, Kato Y, Hayashi Y, et al. Characterization of the hepatic disposition of lanoteplase, a rationally designed variant of tissue plasminogen activator, in rodents. Drug Metab Dispos 2007; 35: 469–75
Oikawa K, Kaminura H, Watanabe T, et al. Pharmacokinetic properties of a novel tissue-type plasminogen activator pamiteplase after single intravenous administration to rats, dogs and monkeys. Thromb Res 2001; 101: 493–500
Oikawa K, Watanabe T, Miyamoto I, et al. Determination of pharmacokinetics and protein binding of a novel tissue-type plasminogen activator, pamiteplase in human plasma. Xenobiotica 2000; 10: 993–1003
Martin U, Kohler G, Sponer G, et al. Pharmacokinetics of the novel recombinant activator BM 06.022 in rats, dogs, and non-human primates. Fibrinolysis 1992; 6: 39–43
Webster R, Phipps J, Hyland R, et al. Evaluation of the role of the asialoglycoprotein receptor in the clearance of UK-279,276 (recombinant neutrophil inhibitory factor). Xenobiotica 2003; 33: 946–56
Webster R, Edington A, Phipps J, et al. Pharmacokinetics and clearance processes of UK-279,276 (rNIF) in rat and dog: comparison with human data. Xenobiotica 2006; 36: 341–9
Sohlenius-Sternbeck AK. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol In Vitro 2006; 20: 1582–6
Barter ZE, Bayliss MK, Beaune PH, et al. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Current Drug Metab 2007; 8: 33–45
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993; 10: 1093–5
Poulin P, Theil FP. Development of a novel method for predicting human volume of distribution at steady-state of basic drugs and comparative assessment with existing methods. J Pharm Sci 2009; 98: 4941–61
Appa RS, Theill C, Hansen L, et al. Investigating clearance mechanisms for recombinant activated factor VII in a perfused liver model. Thromb Haemost 2010; 104: 243–51
Yang ZR, Chou KC. Correlation of metabolic pathways with the primary structure in acetylated proteins. Open Bioinforma J 2008; 2: 90–6
Ramadugu KS, Chung YH, Ernesto J, et al. In silico prediction of the 3D structure of trimeric asialoglycoprotein receptor bound to triantennary oligosaccharide. J Am Chem Soc 2010; 132: 9087–95
Tang H, Hussain A, Leal M, et al. Interspecies prediction of human drug clearance based on scaling data from one or two animal species. Drug Metab Dispos 2007; 35: 1886–93
Ring BJ, Chien JY, Adkison KK, et al. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 3: comparative assessment of prediction methods of human clearance. J Pharm Sci. Epub 2011 May 3
Acknowledgements
The author acknowledges Dr Frank-Peter Theil (Genentech, South San Francisco, CA, USA) for kindly providing useful suggestions and discussion. The author also acknowledges Dr Sami Haddad (University of Montréal, Montréal, Québec, Canada) for his kind assistance in the conduct of this study. No funding was provided to conduct this study and prepare the manuscript; consequently, it represents only the view of the author. The author has no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poulin, P. A Single-Species Approach Considering Additional Physiological Information for Prediction of Hepatic Clearance of Glycoprotein Derivate Therapeutics. Clin Pharmacokinet 50, 665–674 (2011). https://doi.org/10.2165/11592610-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11592610-000000000-00000