Abstract
Background: The renin system is an attractive target for antihypertensive therapy in patients with diabetes mellitus. However, diabetes is associated with changes in gastrointestinal, renal and hepatic function that may affect the absorption and disposition of oral drugs. This study compared the pharmacokinetics and pharmacodynamics of the orally active direct renin inhibitor, aliskiren, in healthy volunteers and patients with type 2 diabetes.
Methods: This was an open-label study conducted in 30 patients with type 2 diabetes and 30 healthy volunteers matched for age, bodyweight and race. Following a 10-hour fast, all participants received a single oral dose of aliskiren 300mg. Blood samples were taken at frequent intervals for 96 hours post-dose for determination of plasma concentrations of aliskiren (using a high-performance liquid chromatography-tandem mass spectroscopy method). Plasma renin activity (PRA) and renin concentration (RC) were also measured for 24 hours after dosing.
Results: Aliskiren exhibited similar pharmacokinetics in patients with type 2 diabetes and healthy volunteers. Exposure to aliskiren was slightly higher in patients with type 2 diabetes compared with healthy volunteers (mean area under the plasma concentration-time curve from 0 to 24 hours 1859 vs 1642 ng · h/mL; maximum observed plasma drug concentration 394 vs 348 ng/mL), while apparent clearance corrected for bioavailability was slightly lower (205 vs 234 L/h) and elimination half-life slightly longer (44 vs 39.9 hours), but there were no statistically significant differences for any pharmacokinetic parameters. There was no significant correlation between glycaemic control (% glycosylated haemoglobin) and any of the measured pharmacokinetic parameters in patients with type 2 diabetes. Aliskiren caused sustained suppression of PRA for at least 24 hours after dosing despite increasing RC; there were no major differences in the pharmacodynamic effects of aliskiren between patients with type 2 diabetes and healthy volunteers. Aliskiren was well tolerated in both patient groups, with no clinically significant changes in laboratory values and a low risk of adverse events.
Conclusion: Aliskiren showed a similar pharmacokinetic profile in healthy volunteers and patients with type 2 diabetes, and administration of a single oral 300mg dose of aliskiren was well tolerated by both patients and healthy volunteers. The pharmacodynamic effects of aliskiren were also similar in healthy volunteers and diabetic patients, with sustained inhibition of renin system activity observed for at least 24 hours after dosing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The renin system plays a crucial role in the regulation of blood pressure (BP) and the development of hypertension, acting through the peptide hormone angiotensin (Ang) II.[1,2] Increased tissue renin system activity may also be a major factor in the development of organ damage in diabetes mellitus.[3,4] Nearly three-quarters of all patients with diabetes have hypertension,[5] and antihypertensive agents that target the renin system, such as ACE inhibitors and angiotensin receptor antagonists (ARAs), are therefore recommended for the treatment of patients with hypertension and diabetes.[6] Clinical trials have shown that treatment with ACE inhibitors or ARAs to inhibit the renin system slows the progression of diabetic renal disease.[7–9] However, the absolute rate of decline in glomerular filtration rate (GFR) in the ARA trials remained 4- to 5-fold greater than the expected loss because of aging.[10] Thus, there remains scope for new therapies to provide greater BP reduction and associated clinical benefits in patients with diabetes and hypertension.
Aliskiren is a novel, orally effective direct renin inhibitor that inhibits the renin system at its point of activation.[11] Orally administered aliskiren displays linear pharmacokinetics over the dose range 75–600mg in healthy volunteers.[12] Aliskiren is excreted primarily unchanged in the faeces, with metabolism and renal excretion playing only a minor role, and shows only moderate protein binding (47–51%).[13] In healthy volunteers, oral doses of aliskiren were well tolerated and provided dose-dependent and sustained suppression of plasma renin activity (PRA) and inhibition of the production of Ang I and Ang II.[14] Clinical studies in patients with hypertension have demonstrated that aliskiren significantly reduces both systolic blood pressure (SBP) and diastolic blood pressure (DBP) in a dose-dependent manner, with a safety and tolerability profile similar to placebo and ARAs.[15,16] Aliskiren exhibited an elimination half-life from plasma of 25–30 hours in healthy volunteers, indicating that the drug is suitable for once-daily oral administration.[14,17]
The presence of diabetes is associated with changes in renal and hepatic function and plasma protein binding.[18] Moreover, approximately 30% of patients with type 2 diabetes also commonly exhibit alterations in gastrointestinal tract function (including impaired motility and delayed gastric emptying), caused by hyperglycaemia and autonomic neuropathy.[19,20] Although changes in renal and hepatic function would not be expected to influence the disposition of aliskiren, changes in gastric emptying may alter the rate and extent of absorption of orally administered drugs. Therefore, the primary aim of this study was to determine whether the pharmacokinetics of aliskiren, following administration of a single 300mg oral dose, differ between patients with type 2 diabetes and healthy volunteers. The safety, tolerability and pharmacodynamics of aliskiren were also evaluated.
Methods
Study Participants
Healthy volunteers and patients with type 2 diabetes between 30 and 75 years of age were enrolled in this study. All participants were non-smokers and otherwise in good health as determined by medical history, physical examination, ECG, blood tests and urinalysis. Eligible healthy volunteers were required to have a DBP of 50–90mm Hg and SBP of 90–140mm Hg. Patients with type 2 diabetes were enrolled at screening if they exhibited a DBP of 50–100mm Hg and SBP of 100–160mm Hg, had been diagnosed with diabetes for at least 1 year, had a fasting blood glucose level of 126–270 mg/dL (7–15 mmol/L), a body mass index (BMI) of 20–35 kg/m2 and a glycosylated haemoglobin (HbA1c) level of 7–13%. Upon meeting the eligibility criteria, each diabetic patient was paired with a healthy volunteer matched according to age (±5 years), bodyweight (±10kg) and race.
Exclusion criteria for patients with diabetes included a history of type 1 diabetes, diabetes secondary to pancreatic injury or conditions such as Cushing’s syndrome or acute metabolic diabetic complications (such as ketoacidosis or hyperosmolar state), or chronic insulin treatment (>4 weeks of treatment in the absence of an intercurrent illness) in the 6 months prior to the study. Patients with diabetes were also excluded if they had a history of gastrointestinal surgery (e.g. partial bowel or gastric resections), or if they showed evidence of severe or untreated symptomatic autonomic neuropathy or gastroparesis.
This study was reviewed and approved by local ethical review boards, carried out in accordance with Good Clinical Practice, and adhered to the principles of the Declaration of Helsinki of the World Medical Association. All participants provided written informed consent.
Study Design
This was an open-label, single-centre, single-dose study designed to assess the pharmacokinetics, safety and tolerability, and pharmacodynamics of aliskiren in healthy volunteers and patients with type 2 diabetes.
Following a 21-day screening period, healthy volunteers (n = 30) and patients with type 2 diabetes (n = 30) were enrolled in the study and underwent a baseline evaluation at day −1. On day 1, a single oral dose of aliskiren 300mg was administered to all participants between 7:30am and 9:00am after a 10-hour fast. Over the following 96 hours, blood samples were taken at frequent intervals for assessment of pharmacokinetic and pharmacodynamic parameters. Participants were domiciled in the study centre for at least 12 hours prior to dosing and at least 48 hours after dosing. All participants underwent a study completion evaluation at 96 hours post-dose.
Study participants were not permitted to engage in strenuous physical exercise for 7 days before dosing or take alcohol (ethanol) from 72 hours before dosing until after the study completion evaluation. Intake of xanthine-containing food or beverages was discontinued 48 hours before dosing and was not permitted during the domiciled period. Patients with diabetes continued to take antidiabetic medication at a constant dosage throughout the study; all other medications (except paracetamol [acetaminophen] and drugs required to treat adverse events) were prohibited from 14 days before dosing until the study completion evaluation.
Pharmacokinetic Assessments
Blood samples were taken pre-dose and at frequent intervals over a period of 96 hours following the administration of aliskiren (0.25, 0.5, 1.0, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 36, 48, 72 and 96 hours post-dosing) and plasma aliskiren concentrations were measured by a high-performance liquid chromatography-tandem mass spectroscopy (HPLC-MS/MS) method. Briefly, the assay consisted of solid-phase extraction of plasma samples on Oasis MCX cartridges (Waters Corporation, Milford, MA, USA) using an automated system. After the conditioning steps (200µL of methanol, then 200µL of buffer at pH 12), 200µL of alkalinised sample was transferred to the well. The sample was washed with 400µL of methanol/water (25 : 75, v/v). After the elution step (300µL of acetonitrile/water [90 : 10, v/v] containing 1% acetic acid), the extract was partially evaporated (concentration by approximately 2-fold) and then diluted with 200µL of 1% acetic acid in water. Reversed-phase HPLC was performed using a MetaSil Basic 5µm column (Metachem, Palo Alto, CA, USA) with gradient elution from 10 mmol/L aqueous ammonium acetate/acetonitrile (75 : 25, v/v) to 10 mmol/L aqueous ammonium acetate/acetonitrile (40 : 60, v/v) over 0.4 minutes. Detection was performed in MS/MS using electro spray ionisation (ESI) with an API 3000 mass spectrometer (Applied Biosystems, Foster City, CA, USA). A derivative of aliskiren (gem-dimethyl d6-aliskiren) was used as an internal standard. The lower limit of quantification (LLQ) of this assay for plasma samples was approximately 0.5 ng/mL.
Pharmacokinetic parameters calculated for aliskiren included area under the plasma concentration-time curve from 0 to 24 hours (AUC24) and extrapolated to infinity (AUC∞), maximum observed plasma concentration (Cmax), time to reach Cmax (tmax), elimination half-life (t1/2) and apparent clearance corrected for bioavailability (CL/F), and were determined by non-compartmental analysis using WinNonlin Enterprise (version 4.1, Pharsight Corporation, Mountain View, CA, USA).
Safety and Tolerability Assessments
Safety and tolerability assessments were conducted in all subjects, and included the monitoring and recording of all adverse events and concomitant medications or significant non-drug therapies. The duration, severity (mild, moderate, severe), relationship to the study drug and outcome were recorded for all adverse events, whether volunteered by the subject, discovered by investigator questioning, or detected through physical examination, laboratory test or other means. Evaluation of haematology, blood chemistries and urinalysis was performed at baseline, at 24 hours post-dose and at the end of the study. ECG recordings were taken at baseline, pre-dose and at 4, 12 and 24 hours post-dose.
The GFR was estimated for individual subjects from baseline demographics and serum measurements using the Modification of Diet in Renal Disease (MDRD) equation (equation 1).[21] This equation has been validated as an accurate predictor of GFR in patients with diabetes.[22]
where SCr is serum creatinine, SUN is serum urea nitrogen, gender factor = 0.762 if subject is female or 1 if male, and race factor = 1.18 if subject is Black or 1 otherwise.
Pharmacodynamic Assessments
PRA and renin concentration (RC) were measured in plasma samples taken at baseline and at 1, 2, 4, 6, 12 and 24 hours post-dose. RC was measured by immunochemiluminescence using the Nichols Direct Renin assay (Nichols Institute, San Clemente, CA, USA). The LLQ for this assay was 0.8 mU/L, and intra-assay precision was 3.7–7.2%. PRA was measured using a radioimmunoassay method by Specialty Labs (Valencia, CA, USA); the LLQ for this assay was 0.5 ng/mL/h.
Statistical Methods
Log-transformed pharmacokinetic parameters (AUC24, AUC∞ and Cmax) were compared between the two groups using a linear model with group as a fixed factor and matched pair as a random factor. Point estimates and the corresponding 90% confidence intervals (CIs) for the ratio of geometric means (for patients with diabetes compared with healthy volunteers) were calculated for AUC24, AUC∞ and Cmax. Aliskiren AUC and Cmax exhibited a coefficient of variation (CV) of between 0.50 and 0.70; with a selected sample size of at least 28 subjects per group, the present study, therefore, had at least 83% power to detect a treatment difference of 40% between mean AUC and Cmax values, assuming a CV of 0.50.
Comparisons of log-transformed CL/F, Cmax/AUC∞ and t1/2 values between groups were made similar to AUC and Cmax. tmax values were compared using a nonparametric test on the differences of the matched pairs (Wilcoxon signed-rank test). Correlations between baseline HbA1c values and pharmacokinetic parameters were assessed by linear regression. For pharmacodynamic parameters, PRA and RC were analysed using log-transformed data; geometric mean ratios and 90% CIs were calculated for these data when comparing post-dose means across groups or post-dose versus pre-dose values within a group. Descriptive statistics were provided for changes in supine BP.
Results
Baseline and Demographic Characteristics of Study Participants
A total of 60 participants were enrolled in this study (30 healthy volunteers and 30 patients with type 2 diabetes). Baseline and demographic data for the two study groups are summarised in table I; with the exception of HbA1c levels and concomitant treatment with antidiabetic medications, there were no significant differences in baseline characteristics between the two groups. The most common antidiabetic medications were sulphonylureas, metformin and sulphonylurea/metformin combinations. Three of the patients with type 2 diabetes were hypertensive (BP >130/80mm Hg) at baseline; one of these patients was receiving antihypertensive drug treatment (with irbesartan/hydrochlorothiazide). All subjects completed the study and were included in the pharmacokinetic, safety and pharmacodynamic evaluations.
Pharmacokinetic Parameters
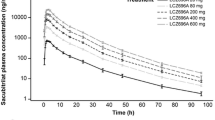
Following the administration of a single 300mg oral dose the plasma concentration-time profiles for aliskiren showed no marked differences between healthy volunteers and patients with type 2 diabetes (figure 1). Pharmacokinetic parameters for aliskiren are summarised in table II. Exposure to aliskiren was slightly, but not significantly, higher in patients with type 2 diabetes compared with healthy volunteers, whether assessed by AUC24 (ratio of geometric means 1.15 [90% CI 0.89, 1.49]; p = 0.347), AUC∞ (ratio of geometric means 1.16 [90% CI 0.89, 1.50]; p = 0.349) or Cmax (ratio of geometric means 1.14 [90% CI 0.85, 1.51]; p = 0.455).
Mean t1/2 values for aliskiren were comparable between healthy volunteers (40 hours) and patients with type 2 diabetes (44 hours). Although tmax, Cmax/AUC∞ and CL/F were all slightly lower in patients with type 2 diabetes compared with healthy volunteers (table II), there were no significant differences between groups.
There was no evidence of a significant correlation between glycaemic control (baseline % HbA1c) in the patients with diabetes and any of the aliskiren pharmacokinetic parameters. Figure 2 shows that there was no significant correlation in the patients with type 2 diabetes between HbA1c and AUC24 (r = −0.07; p = 0.706), Cmax (r = −0.08; p = 0.684) or t1/2 (r = −0.26; p = 0.163). There was also no significant correlation between HbA1c and tmax (r = 0.23; p = 0.215) or CL/F (r = 0.15; p = 0.421).
Correlation between baseline glycosylated haemoglobin (HbA1c) and (a) area under the plasma concentration-time curve from 0 to 24 hours (AUC24), (b) maximum observed concentration (Cmax) and (c) elimination half-life (t1/2) in patients with type 2 diabetes mellitus. Figure shows mean HbA1c values and respective pharmacokinetic parameters for each subject with type 2 diabetes. Correlation coefficients (r values) and respective p-values were determined by linear regression; no statistically significant correlations were observed (p > 0.05 for all parameters).
Patients with type 2 diabetes and healthy volunteers were not matched for sex in this study, but overall there were no notable differences between men and women in mean ± SD AUC24 (men 1602 ± 911 vs women 1889 ± 1191 ng · h/mL), Cmax (317 ± 190 vs 421 ± 311 ng/mL), CL/F (240 ± 153 vs 201 ± 118 L/h) or t1/2 (42.8 ± 8.6 vs 41.1 ± 11.2 hours).
It was also notable that the majority of subjects enrolled in this study were in the ‘other’ category for race, which denotes not Caucasian, Black or Oriental. However, there were no notable differences between the pooled group of Caucasian/Black subjects and subjects of other racial origin for mean ± SD AUC24 (Caucasian/Black 1790 ± 1112 vs other 1731 ± 1055 ng · h/mL), Cmax (427 ± 350 vs 343 ± 205 ng/mL), CL/F (200 ± 99 vs 230 ± 152 L/h) or t1/2 (42.4 ± 9.8 vs 41.7 ± 10.2 hours).
Pharmacodynamic Parameters
Administration of a single oral dose of aliskiren 300mg led to small reductions in mean supine SBP and DBP that were apparent within 2 hours of dosing in healthy volunteers and patients with type 2 diabetes (figure 3). The reductions in BP persisted for at least 24 hours after dosing, but BP values had returned to pre-treatment levels by 96 hours post-dose. In the three diabetic patients who were hypertensive (BP >130/80mm Hg) at baseline, BP was lowered to normotensive levels at 24 hours after the administration of aliskiren in each case (from 138/85 to 107/69mm Hg, 139/83 to 112/67mm Hg and 148/80 to 129/65mm Hg). Aliskiren caused no notable changes in pulse rate in healthy volunteers or patients with diabetes.
Supine blood pressure following administration of a single 300mg oral dose of aliskiren. Supine systolic and diastolic blood pressure values are presented for healthy volunteers (n = 30) and patients with type 2 diabetes mellitus (n = 30). Data are presented as the mean ± standard error of the mean.
RC and PRA were measured for 24 hours following the administration of a single dose of aliskiren 300mg. Geometric mean RC pre-dose was higher (p < 0.05) in patients with type 2 diabetes (geometric mean 16.7 mU/L) compared with healthy volunteers (10.1 mU/L). Aliskiren caused a significant increase in geometric mean RC (figure 4 a) that peaked at approximately 6 hours post-dose in both groups (an increase of 653% and 471% in healthy volunteers and diabetic patients, respectively). RC values were significantly increased from pre-dose levels (p < 0.001) throughout the 24 hours after dosing, but there were no major differences in RC levels for change from baseline between healthy volunteers and patients with diabetes at any timepoint.
Geometric mean PRA was similar pre-dose in patients with type 2 diabetes compared with healthy volunteers (1.03 vs 1.07 ng/mL/h). Aliskiren significantly reduced PRA (p < 0.001) to below the LLQ of the assay (0.5 ng/mL/h) in both groups (figure 4 b), with PRA suppression being achieved at 1 hour post-dose and sustained for at least 24 hours (p < 0.001 vs baseline at all post-dose timepoints in both groups).
Safety and Tolerability
Aliskiren was well tolerated in healthy volunteers and patients with type 2 diabetes. There were no clinically significant changes in laboratory values, vital signs (except for the small reductions in BP as noted earlier) or ECG recordings following administration of aliskiren, and no study discontinuations. A total of 17 adverse events were reported by 7 subjects during the study: 13 adverse events in 5 healthy volunteers and 4 adverse events in 2 patients with diabetes. The most frequently reported adverse events were headache and diarrhoea (3 events each). All adverse events reported were mild in severity.
Discussion
This study shows that the pharmacokinetics of the novel direct renin inhibitor aliskiren following administration of a single 300mg oral dose are similar in patients with type 2 diabetes and age-, bodyweight- and race-matched healthy volunteers. Patients with type 2 diabetes exhibited only small and non-significant increases in Cmax and AUC compared with healthy volunteers, and there was no correlation between glycaemic control (HbA1c) and the pharmacokinetics of aliskiren.
The lack of significant differences in aliskiren pharmacokinetic parameters between patients with type 2 diabetes and healthy volunteers indicates that the presence of diabetes had no notable effect on the rate (tmax, Cmax/AUC∞) or extent (AUC∞, Cmax) of absorption of aliskiren, or on the elimination (CL/F, t1/2) of the drug. Recent evidence suggests that hyperglycaemia resulting from poor glycaemic control may contribute to disturbances in gastric function in patients with diabetes.[23] However, the present study found no significant correlations between glycaemic control (baseline HbA1c) and any aliskiren pharmacokinetic parameters. Other physiological changes associated with type 2 diabetes (such as alterations in renal and hepatic function and plasma protein binding)[18] would not be expected to alter the pharmacokinetics of aliskiren, because aliskiren exhibits only moderate protein binding and is eliminated primarily unchanged in the faeces, with renal clearance (<10% of total clearance) and metabolism playing only a minor role.[14,24]
It is notable that the t1/2 of aliskiren observed in this study (40 and 44 hours in healthy volunteers and patients with type 2 diabetes, respectively) was somewhat longer than the value of 24 hours reported for healthy males by Nussberger et al.[14] and the 25–30 hours observed by Azizi et al.[17] This most likely reflects the longer sampling period employed in the present study (96 hours) compared with studies by Nussberger et al.[14] and Azizi et al.[17] (both 48 hours). Although aliskiren t1/2 values showed considerable inter-subject variability in the present study, recent studies in healthy volunteers using a sampling period of 72 or 96 hours post-dose (during which time the majority of the aliskiren AUC is captured) suggest that the t1/2 for aliskiren in the therapeutically relevant concentration range is 30–40 hours.[12,25] This is consistent with the fact that steady state is achieved within 5–7 days of once-daily administration, and with the approximately 2-fold accumulation of aliskiren at steady state compared with administration of a single dose.[25]
Aliskiren treatment resulted in substantial reductions in PRA and increases in RC in healthy volunteers and patients with diabetes, consistent with the known pharmacology of aliskiren.[26] The rise in RC occurs as a result of the loss of the normal Ang II-mediated feedback inhibition of renin secretion by the kidneys, and is therefore an indicator of renin system suppression.[27,28] Despite the increase in RC, aliskiren effectively reduced PRA to below the LLQ of the assay within 1 hour of dosing in healthy volunteers and patients with diabetes, and PRA remained suppressed for at least 24 hours. The effects of aliskiren on PRA and RC were essentially similar in both groups.
Administration of a single 300mg oral dose of aliskiren was well tolerated both by healthy volunteers and by patients with type 2 diabetes. There were no notable changes in laboratory values, vital signs (other than small reductions in BP) or ECG measurements following treatment with aliskiren, and the rate of adverse events was low. Of the most frequently reported adverse events, headache is commonly observed following the administration of a BP-lowering drug to normotensive subjects, while the three reports of diarrhoea all came from one subject.
It is important to note the limitations of this study. As is the case in most pharmacokinetic profiling studies, aliskiren was administered to subjects in the fasted state. This study would therefore not have detected any influence of meals, or concomitant medication intake with meals, on potential diabetic abnormalities in gastric emptying or bowel function. Moreover, although a single-dose study design is useful for the comparison of pharmacokinetic parameters between patient groups, it does not reflect the once-daily, multiple-dose treatment regimen that will be used for aliskiren in the long-term treatment of hypertension, and so provides limited information as to the safety and tolerability of aliskiren. Nevertheless, there are unlikely to be notable differences in the safety of aliskiren between single- or multiple-dose administration. Indeed, a recent study in healthy subjects showed only modest increases in exposure to aliskiren of less than 2-fold at steady state compared with single-dose administration, and no difference in tolerability between the two dosing regimens.[25]
Conclusion
The results of this study demonstrate that aliskiren exhibits comparable pharmacokinetics and pharmacodynamics in healthy volunteers and patients with type 2 diabetes and is well tolerated in both groups.
References
Lavoie JL, Sigmund CD. Minireview: overview of the reninangiotensin system: an endocrine and paracrine system. Endocrinology 2003; 144: 2179–83
Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension 2001; 37: 1047–52
Hanes DS, Nahar A, Weir MR. The tissue renin-angiotensinaldosterone system in diabetes mellitus. Curr Hypertens Rep 2004; 6: 98–105
Giacchetti G, Sechi LA, Rilli S, et al. The renin-angiotensinaldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab 2005; 16: 120–6
Selby JV, Peng T, Karter AJ, et al. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care 2004; 10: 163–70
Bakris GL. Protecting renal function in the hypertensive patient: clinical guidelines. Am J Hypertens 2005; 18: 112S–9S
Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456–62
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–60
Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–9
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39: S1–266
Wood JM, Maibaum J, Rahuel J, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun 2003; 308: 698–705
Vaidyanathan S, Limoges D, Yeh C-M, et al. Aliskiren, an orally effective renin inhibitor, shows dose linear pharmacokinetics in healthy volunteers. Clin Pharmacol Ther 2006; 79: 64 (PIII-23)
Data on file, Novartis Pharmaceuticals Corporation, 2005
Nussberger J, Wuerzner G, Jensen C, et al. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril. Hypertension 2002; 39: E1–8
Gradman AH, Schmieder RE, Lins RL, et al. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005; 111: 1012–8
Stanton A, Jensen C, Nussberger J, et al. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension 2003; 42: 1137–43
Azizi M, Menard J, Bissery A, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsarian on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 2004; 15: 3126–33
Gwilt PR, Nahhas RR, Tracewell WG. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet 1991; 20: 477–90
Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127: 1592–622
Horowitz M, O’Donovan D, Jones KL, et al. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med 2002; 19: 177–94
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–70
Rigalleau V, Lasseur C, Perlemoine C, et al. Estimation of glomerular filtration rate in diabetic subjects: Cockcroft formula or Modification of Diet in Renal Disease study equation? Diabetes Care 2005; 28: 838–43
Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol 2002; 97: 604–11
Vaidyanathan S, Jin Y, Schiller H, et al. Aliskiren, a novel oral renin inhibitor, has no interaction with cytochrome P450 isoenzymes in vitro. Basic Res Pharmacol Toxicol 2005; 97 Suppl. 1: 239
Vaidyanathan S, Jermany J, Yeh C-M, et al. Aliskiren, a novel orally effective renin inhibitor, exhibits similar pharmacokinetics and pharmacodynamics in Japanese and Caucasian subjects. Br J Clin Pharmacol 2006; 26: In press
Azizi M, Webb R, Nussberger J, et al. Renin inhibition with aliskiren: where are we now, and where are we going? J Hypertens 2006; 24: 243–56
Vander AJ, Geelhoed GW. Inhibition of renin secretion by angiotensin. II. Proc Soc Exp Biol Med 1965; 120: 399–403
Bing J. Rapid marked increase in plasma renin in rats treated with inhibitors of the renin system: effects of 1-sar-8-ala-angiotensin II and of a synthetic converting enzyme inhibitor (nonapeptide, SQ 20.881) on normal and adrenalectomized rats. Acta Pathol Microbiol Scand [A] 1973; 81: 376–8
Acknowledgements
This study was supported by Novartis Pharmaceuticals. The sponsor was responsible for the design and conduct of the study, and the collection and management of the data. All authors are employees of Novartis and are thus eligible for Novartis stock and stock options. The authors were responsible for the analysis and interpretation of the data and the preparation of the manuscript. The authors acknowledge the editorial assistance of Dr Richard White.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, C., Vaidyanathan, S., Yeh, CM. et al. Aliskiren Exhibits Similar Pharmacokinetics in Healthy Volunteers and Patients with Type 2 Diabetes Mellitus. Clin Pharmacokinet 45, 1125–1134 (2006). https://doi.org/10.2165/00003088-200645110-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645110-00006