Abstract
Plasmodium falciparum infection during pregnancy is strongly associated with maternal anaemia and low birth weight, contributing to substantial morbidity and mortality in sub-Saharan Africa. Intermittent preventive treatment in pregnancy with sulfadoxine/pyrimethamine (IPTp-SP) has been one of the most effective approaches to reduce the burden of malaria during pregnancy in Africa. IPTp-SP is based on administering ≥2 treatment doses of sulfadoxine/pyrimethamine to pregnant women at predefined intervals after quickening (around 18–20 weeks). Randomised, controlled trials have demonstrated decreased rates of maternal anaemia and low birth weight with this approach. The WHO currently recommends IPTp-SP in malaria-endemic areas of sub-Saharan Africa. However, implementation has been suboptimal in part because of concerns of potential drug toxicities. This review evaluates the toxicity data of sulfadoxine/pyrimethamine, including severe cutaneous adverse reactions, teratogenicity and alterations in bilirubin metabolism. Weekly sulfadoxine/pyrimethamine prophylaxis is associated with rare but potentially fatal cutaneous reactions. Fortunately, sulfadoxine/pyrimethamine use in IPTp programmes in Africa, with 2–4 treatment doses over 6 months, has been well tolerated in multiple IPTp trials. However, sulfadoxine/pyrimethamine should not be administered concurrently with cotrimoxazole given their redundant mechanisms of action and synergistic worsening of adverse drug reactions. Therefore, HIV-infected pregnant women in malaria endemic areas who are already receiving cotrimoxazole prophylaxis should not also receive IPTp-SP. Although folate antagonist use in the first trimester is associated with neural tube defects, large case-control studies have demonstrated that sulfadoxine/pyrimethamine administered as IPTp (exclusively in the second and third trimesters and after organogenesis) does not result in an increased risk of teratogenesis. Folic acid supplementation is recommended for all pregnant women to reduce the rate of congenital anomalies but high doses of folic acid (5 mg/day) may interfere with the antimalarial efficacy of sulfadoxine/pyrimethamine. However, the recommended standard dose of folic acid supplementation (0.4 mg/day) does not affect antimalarial efficacy and may provide the optimal balance to prevent neural tube defects and maintain the effectiveness of IPTp-SP. No clinical association between sulfadoxine/pyrimethamine use and kernicterus has been reported despite the extensive use of sulfadoxine/pyrimethamine and related compounds to treat maternal malaria and congenital toxoplasmosis in near-term pregnant women and newborns. Although few drugs in pregnancy can be considered completely safe, sulfadoxine/pyrimethamine — when delivered as IPTp — has a favourable safety profile. Improved pharmacovigilance programmes throughout Africa are now needed to confirm its safety as access to IPTp-SP increases. Given the documented benefits of IPTp-SP in malaria endemic areas of Africa, access to this treatment for pregnant women should continue to expand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Plasmodium falciparum malaria remains an enormous global health problem that disproportionately affects young children and pregnant women, particularly in sub-Saharan Africa. There are an estimated 30 million pregnancies per year in malaria-endemic areas.[1]
Although adults in malaria-endemic areas often develop immunity to clinical disease, with pregnancy a new set of antigen targets, in particular chondroitin sulfate A and hyaluronic acid,[2] become exposed, which P. falciparum exploits to sequester in the placenta. Malarial parasites sequester in the vascular space of the placenta resulting in maternal anaemia[3,4] and low birth weight due to both prematurity and intrauterine growth retardation.[5,6] In sub-Saharan Africa, malarial infection is estimated to cause 2–15% of cases of severe maternal anaemia and 8–14% of low birth weight deliveries.[7] These complications from placental malaria infection result in an estimated 10 000 maternal[8] and 100 000–250 000 fetal deaths per year.[1,7,9]
Unfortunately there are few safe and effective therapeutic or prophylactic options available to pregnant women to combat this important cause of morbidity and mortality. Historically, weekly chloroquine prophylaxis was recommended in endemic areas and had a good safety profile.[10] However, widespread resistance has made chloroquine ineffective for the treatment of P. falciparum. A recent cross-sectional study in Africa confirmed a lack of effectiveness of chloroquine chemoprophylaxis in pregnancy.[11]
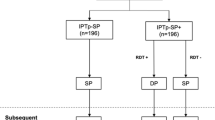
The WHO now recommends intermittent preventive treatment in pregnancy with sulfadoxine/pyrimethamine (IPTp-SP) in addition to insecticide-treated nets and effective case management of symptomatic malaria to reduce the burden of malaria in pregnancy.[12] IPTp-SP is based on the administration of ≥2 full therapeutic doses of sulfadoxine/pyrimethamine to pregnant women at predefined intervals after quickening (the first noted fetal movements that typically occur at 18–20 weeks) linked to routinely scheduled antenatal clinic visits (figure 1).[13] IPTp-SP likely works by intermittently clearing existing asymptomatic parasitaemia (the treatment effect) and by preventing new infections since sulfadoxine/pyrimethamine has a long half-life (the prophylactic effect).[13] IPTp-SP studies from Malawi, Kenya, Mozambique, Mali and Burkina Faso have demonstrated that ≥2 doses of sulfadoxine/pyrimethamine are effective at reducing maternal anaemia,[14–16] placental malaria[14,17–23] and low birth weight,[14,20,21,23,24] as summarised in table I. In randomised, controlled trials, this strategy has been shown to be safe for the mother and fetus as well as superior to weekly chloroquine prophylaxis[14,18] and case management based on treating symptomatic maternal malaria.[17] IPTp-SP has remained effective at preventing malarial complications in semi-immune pregnant women even in areas with high rates of sulfadoxine/pyrimethamine resistance, although a monthly administration regimen may be superior to standard 2-dose IPTp-SP in this setting.[22]
IPTp-SP is now part of the national malaria control strategy of 31 African counties;[25] however, implementation in many of these countries remains low.[26] Unfortunately, despite evidence of safety in clinical trials and a lack of safety problems with programmatic implementation,[27] lingering concerns regarding the safety of sulfadoxine/pyrimethamine in pregnancy have contributed to slow the scale-up of IPTp-SP in many c ountries.[28–31] In this manuscript, we review the safety and toxicity of sulfadoxine and pyrimethamine in pregnancy in relation to their use in IPTp-SP in Africa.
1. Literature Search Methodology
We conducted a literature search using MEDLINE and EMBASE between 1966 and July 2006, cross-referencing the following terms: (i) ‘sulfadoxine’, ‘pyrimethamine’, ‘SP’ or ‘Fansidar’; (ii) ‘sulfonamides’, ‘sulfa drugs’, ‘trimethoprim’, ‘sulfamethoxazole’, ‘TMP’, ‘SMX’, ‘cotrimoxazole’, ‘Bactrim’ or ‘sulfadiazine’; (iii) ‘malaria’ or ‘P. falciparum’; (iv) ‘pregnancy’ or ‘pregnant’; (v) ‘IPT’, ‘IPTp’, ‘IPTp-SP’ or ‘intermittent preventive treatment’; (vi) ‘drug toxicity’, ‘drug hypersensitivity’, ‘drug eruptions’, ‘adverse drug reactions’, ‘side effects’, ‘rash’, ‘severe cutaneous adverse reactions’, ‘SCARs’, ‘kernicterus’ or ‘bilirubin’; (vii) ‘neural tube defects’, ‘teratogenesis’, ‘congenital anomalies’ or ‘birth defects’ ; (viii) ‘folate’ or ‘folic acid’; (ix) ‘HIV or ‘HIV-1’; (x) ‘breastfeeding’, ‘lactation’ or ‘lactating’; and (xi) ‘toxoplasmosis’. We also reviewed references to identify additional published literature, including some articles that predated the earliest available on MEDLINE. Our literature search was not restricted to English language articles.
We reviewed all literature that provided information on the safety and toxicity of sulfadoxine/pyrimethamine in pregnancy. We preferentially considered studies involving the use of sulfadoxine/pyrimethamine in pregnant women in Africa; however, these data were limited. Therefore, we also included studies involving non-pregnant individuals in Africa and pregnant women outside of Africa. Additionally, we incorporated studies involving compounds related to sulfadoxine/pyrimethamine, such as cotrimoxazole (trimethoprim/sulfamethoxazole).
2. Clinical Pharmacology
Sulfadoxine and pyrimethamine competitively inhibit the enzymes dihydropteroate synthase (DHPS) and dihydrofolate reductase (DHFR), respectively.[32] These enzymes catalyse important sequential steps in the generation of folate derivatives. Rapidly dividing cells, such as malarial parasites (also cancer cells and bacteria), depend on folate derivatives as cofactors for the synthesis of nucleotides and amino acids by facilitating the transfer of single carbon units.[33,34] Pyrimethamine is 1000-fold more active against plasmodial DHFR than against the mammalian enzyme.[35] Plasmodial DHFR, unlike the mammalian enzyme, does not upregulate in the face of inhibition, which may explain this selective activity.[36] The combination of DHPS and DHFR inhibition also appears synergistic.[37,38] In both animal and human plasmodium infections, pyrimethamine and sulfadoxine administered together are curative at one-eighth the dose of either used alone.[39,40] In 1971, these observations led to the co-formulation of a fixed combination of sulfadoxine with pyrimethamine under the proprietary name Fansidar®Footnote 1.
Both sulfadoxine and pyrimethamine are well absorbed orally and reach peak plasma concentrations in about 4 hours (range 2–6 hours).[14] Sulfadoxine and pyrimethamine are also highly protein bound (>90% and 87%, respectively) resulting in prolonged clearance with mean elimination half-lives of 169 hours (range 100–230 hours) and 111 hours (range 54–148 hours), respectively.[41–44] Peak plasma concentrations after a single oral dose of 500mg of sulfadoxine and 25mg of pyrimethamine are approximately 50–75 µg/mL and 0.13–0.4 µg/ mL, respectively.[41–43] The recommended dose of sulfadoxine/pyrimethamine for pregnant women receiving IPTp is three tablets or 1500mg of sulfadoxine and 75mg of pyrimethamine. At this treatment dosage, a 50kg pregnant woman would receive 30mg/1.5mg of sulfadoxine/pyrimethamine per kilogram of bodyweight. However, weight-based administration of sulfadoxine/pyrimethamine in children results in substantial inter-individual variation in drug concentrations.[45] In addition, concentrations of sulfadoxine/pyrimethamine are lower than predicted in individuals infected with malaria compared with healthy controls.[46,47] Maternal pharmacokinetics have not been rigorously evaluated but a case series of ten women who were treated for congenital toxoplasmosis during their pregnancy revealed similar drug concentrations as have been observed in non-pregnant individuals.[48] As access to IPTp-SP continues to expand in Africa, a key research topic will be defining the pharmacokinetics of sulfadoxine/pyrimethamine in pregnant African women.
Both sulfadoxine and pyrimethamine are widely distributed in body tissue. They readily cross the placenta[48,49] and are excreted into breast milk.[43] Fetal plasma concentrations of sulfadoxine and pyrimethamine average 97% (range 65–116%) and 66% (range 43–103%) of maternal concentrations, respectively.[48] About 5% of sulfadoxine is acetylated in the plasma;[42] the remaining unacetylated drug is excreted primarily unchanged by the kidneys.[50] Pyrimethamine is metabolised to several uncharacterised by-products in the liver; excretion primarily occurs by the kidneys.[50] Renal insufficiency delays clearance of both drugs.[50]
3. Drug Resistance
P. falciparum resistance mutations to sulfadoxine/pyrimethamine emerged soon after its widespread introduction in Africa.[51] Decreased malarial susceptibility to sulfadoxine/pyrimethamine can decrease the ability of IPTp-SP to clear existing asymptomatic parasitaemia (the treatment effect) and reduce its duration of malarial prophylaxis (the prophylactic effect) by raising the minimum inhibitory concentration.[52] Sulfadoxine/pyrimethamine resistance results from the sequential acquisition of mutations in DHFR and to a lesser degree in DHPS with each mutation conferring an additive reduction in drug susceptibility.[53] The quadruple DHFR mutant genotype containing I164L confers almost complete sulfadoxine/pyrimethamine resistance.[54] Although a triple DHFR mutant genotype (lacking I164L) is frequently encountered in Africa, for unknown reasons the quadruple mutant remains uncommon.[55,56]
The combination of the drug susceptibility of P. falciparum and the degree of an individual’s immunity to malaria determine the clinical efficacy of the drug. One WHO recommended method to measure the clinical efficacy of a drug is the 14-day (or 28-day) parasitological outcome.[57] Parasitological failure is defined by the presence of malarial parasitaemia by microscopy 14 days after treatment and it is often measured in children. Fortunately pregnant women in highly malaria endemic countries have partial malaria immunity and therefore have improved clinical responses to sulfadoxine/pyrimethamine compared with non-immune children.[58]
Only one study has evaluated the effectiveness of IPTp-SP in a setting of diminished sulfadoxine/pyrimethamine clinical effectiveness in children (40% parasitological failure rate at day 14 after treatment).[59] There was no control group but the level of placental malaria was very low (6%) in the 2-dose IPTp-SP group suggesting a beneficial effect.[22] Although this study is encouraging, there was a trend toward improved outcomes with monthly administration compared with standard 2-dose IPTp-SP[22] that did not reach statistical significance but suggests a reduced duration of malarial prophylaxis. IPTp-SP has also not been evaluated in settings with a >50% parasitological failure rate among children or in settings with a high frequency of I164L mutants. Therefore, it will be important to continue to monitor the effectiveness of IPTp-SP as well as develop mechanisms to rapidly test the safety and efficacy of promising new antimalarial agents in pregnancy. As many African countries change first-line therapy for uncomplicated symptomatic malaria from sulfadoxine/pyrimethamine to artemisinin-based combination therapies there is hope that this change may reduce the drug pressure on sulfadoxine/pyrimethamine and preserve its role in IPTp.
4. Severe Adverse Drug Reactions
Doses of sulfadoxine/pyrimethamine used in IPTp regimes are well tolerated. The rates of common minor adverse reactions such as rash, vomiting, diarrhoea, headache and fatigue are generally low and similar to rates observed with placebo.[60] High doses or prolonged therapy with sulfadoxine/pyrimethamine can produce megaloblastic anaemia or general haematological suppression[61–63] by antagonising folate; this complication is usually reversible with folinic acid.[62]
When used for IPTp or malaria treatment in glucose-6-phosphate dehydrogenase-deficient patients, sulfadoxine/pyrimethamine has not been shown to cause haemolysis.[27,64] A survey of the national registers in Sweden and the UK for adverse reactions to sulfa medications from 1968 to 1988 identified 78 serious adverse reactions per 100 000 users of Fansidar®.[65] In this survey, Fansidar® was most commonly used as weekly malaria prophylaxis in international travellers for a mean duration of 8 weeks. The most common serious reactions (as a percentage of total serious reactions) were liver toxicity (25%), cutaneous reactions (21%), fever (17%), respiratory problems (14%), white blood cell dyscrasias (6%) and anaemia (3%).
As with other sulfa-based drugs, the most feared complication of sulfadoxine/pyrimethamine therapy is a hypersensitivity reaction, which can result in a severe cutaneous adverse reaction (SCAR).[66–68] Other severe adverse drug reactions (ADRs), including cholestatic hepatoxicity,[69–71] fulminant hepatic necrosis[72] and hypersensitivity pneumonitis,[73,74] have been reported in non-pregnant adults in the literature but are extremely rare. Indeed, in the survey from Sweden and the UK, there were six reported deaths due to Fansidar® and all were attributed to SCARs.[65] Since SCARs are also the most important complication in the context of IPTp-SP, we concentrate on the frequency of SCARs with sulfadoxine/pyrimethamine.
SCARs associated with sulfadoxine/pyrimethamine have been evaluated with several different study designs. The majority of data are based on large surveillance and case-control studies of travellers from high-income countries who had taken sulfadoxine/pyrimethamine as weekly malaria prophylaxis.[65,66,75–77] Passive surveillance and case-control studies are well suited to detect rare outcomes, such as SCARs, but have limitations. Passive surveillance (relying on unsolicited reporting of SCARs to a central agency) can result in low sensitivity due to under-reporting. Conversely, case-control studies can overestimate the association of a rare event to a drug exposure because of recall bias. Clinical trials and prospective observational cohorts provide the majority of data regarding SCARs in pregnant women in Africa receiving IPTp-SP.[14,15,17,19,22,24] These studies are sensitive but limited by small sample sizes that are underpowered to detect rare complications. Pharmacovigilance is the science and practice of ADR detection, assessment and prevention.[78] Active pharmacovigilance surveillance (employing direct solicitation of SCARs from antenatal clinics and hospitals) provides a cost effective and sensitive method to detect SCARs in women receiving IPTp-SP but has been rarely utilised [27,79]
We preferentially considered studies that evaluated for SCARs in pregnant women in Africa receiving IPTp-SP; however, these data were limited. Therefore, we also included studies involving non-pregnant people. In the section regarding HIV-infected pregnant women, we also incorporated studies involving cotrimoxazole. Cotrimoxazole is closely related to sulfadoxine/pyrimethamine and has been used extensively in HIV-infected people. In each section we present studies involving non-pregnant people first; followed by studies that particularly evaluate for SCARs in pregnant women in Africa receiving IPTp-SP. In the section regarding HIV-infected pregnant women, cotrimoxazole data are presented first; followed by sulfadoxine/pyrimethamine data.
4.1 Severe Cutaneous Adverse Reactions (SCARs)
Routine use of Fansidar® as an antimalarial prophylactic agent is no longer recommended in the US because of severe and even fatal cutaneous adverse reactions including erythema multiforme, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis.[66] The exact incidence of SCARs with Fansidar® has been the subject of debate. Previous evaluations by the US Centers for Disease Control and Prevention (CDC) determined a rate of one SCAR per 5000–8000 US travellers taking Fansidar® as weekly prophylaxis with one fatality per 11 000–25 000.[66] Similar frequencies of SCARs and fatalities have been reported in Swedish and British travellers using Fansidar® for malaria prophylaxis.[75,76] During a cholera epidemic in Mozambique, mass chemoprophylaxis of almost 150 000 people with 2g of sulfadoxine resulted in 22 cases of typical SJS (one per 6773 doses) and three deaths (one per 50 000 doses).[80] However, a study in Switzerland reported only one SCAR per 150 000 travellers with no fatal reactions.[77]
The significance of these discrepancies remains unknown. Some have speculated that genetic differences in sulfonamide acetylation rates might contribute to the susceptibility to SCARs but this theory has not been tested with sulfadoxine, a sulfonamide that is not extensively acetylated.[81] Medication dosage and frequency could also contribute to the rate of SCARs. The recommended dosage of sulfadoxine/pyrimethamine for IPTp is three times the dose used for malarial prophylaxis (1500mg/75mg vs 500mg/25mg) but is taken less frequently (2–4 times over 5 months rather than weekly).
In trials of IPTp-SP in pregnant women, rates of ADRs with sulfadoxine/pyrimethamine have been lower than rates observed with chloroquine[14] and similar to rates observed with placebo.[19] Several clinical trials have attempted to define the risk of SCARs with IPTp-SP. There have been no episodes of SCARs or other serious adverse reactions reported from these African trials (table I) involving >3000 pregnant women given IPTp-Sp.[14,15,17,19,22,24]
Two active pharmacovigilance surveillance systems have been deployed in Africa to estimate the frequency of SCARs with sulfadoxine/pyrimethamine. One large surveillance system for sulfonamide-associated SCARs was deployed at all government hospitals and clinics in Blantyre district, Malawi. Crude rates of SCARs were estimated to be 1.2 per 100 000 exposures to sulfadoxine/pyrimethamine and 1.5 per 100 000 exposures to cotrimoxazole.[79] Although this study did not focus on pregnant women, only one pregnant woman developed a SCAR in >18 months of surveillance despite >30 000 deliveries[79] and with 96% of pregnant women receiving at least one dose of sulfadoxine/pyrimethamine during their pregnancy.[82]
The Ghana National Centre for Pharmacovigilance has also monitored for SCARs associated with sulfadoxine/pyrimethamine by integrating adverse event reporting into its national IPTp-SP programme and by actively soliciting reports.[27] Over 55 000 women received IPTp-SP during 2 years of surveillance and there were only 100 total adverse events of any kind.[27] Although this information is reassuring, there is clearly a need for more data on the rates of SCARs in pregnant women in Africa who receive IPTp-SP. Given the rarity of SCARs, it is essential to strengthen country-wide pharmacovigilance programmes to accurately track these and other adverse reactions.
4.2 SCARs and HIV Infection
In sub-Saharan Africa there are an estimated 13.5 million HIV-infected women of reproductive age.[83] HIV-infected pregnant women have consistently been shown to have higher rates of placental malaria, peripheral parasitaemia, and adverse birth outcomes than HIV-negative pregnant women of the same gravidity.[84] The mechanisms of this increased susceptibility to malaria involve dysfunction of both the cellular[85,86] and humoral immune responses to P. falciparum.[87] HIV-infected pregnant women also have a diminished response to antimalarial treatment[84,88] and administration of sulfadoxine/pyrimethamine (≥3 doses or monthly administration during pregnancy) is required to effectively reduce placental malaria in this population.[17,22]
In addition to being more vulnerable to malarial infection, HIV-infected pregnant women are at greater risk for sulfa-mediated adverse reactions (including SCARs).[89] This increased risk may be due to an impaired capacity to acetylate sulfonamides[90,91] and to scavenge their reactive metabolites,[92] combined with cellular immune dysregulation.[93] Therefore, HIV-infected pregnant women have not only an urgent need to prevent malarial complications in pregnancy but also an increased susceptibility to the adverse effects of treatment.
Cotrimoxazole also inhibits plasmodial DHPS/ DHFR and cotrimoxazole prophylaxis has been demonstrated to reduce mortality in HIV-infected adults[94,95] and children[96] in Africa. Cotrimoxazole prophylaxis of HIV-infected pregnant women with CD4 cell counts <200/mm3 reduced rates of prematurity and neonatal mortality in Zambia.[97] Given this mortality benefit, the WHO recommends that HIV-infected women who meet criteria (CD4 cell count <350/mm3 or WHO stages 3 or 4 disease) should receive cotrimoxazole prophylaxis throughout their pregnancy.[98,99] Although no clinical trials have evaluated the effectiveness of cotrimoxazole prophylaxis at preventing the complications of malaria in pregnancy, several clinical trials of cotrimoxazole prophylaxis in HIV-infected adults and children in Africa have demonstrated its effectiveness at preventing malarial infection.[95,100,101] Cotrimoxazole prophylaxis even reduces the incidence of malaria among HIV-negative people living in the same house with a person taking prophylaxis.[102] Therefore, cotrimoxazole prophylaxis is an option to prevent the complications of malaria in pregnancy for HIV-infected women who meet the WHO criteria. HIV-infected pregnant woman on cotrimoxazole prophylaxis should not also receive IPTp-SP given their redundant mechanisms of action and synergistic worsening of ADRs[79,98,103] However HIV-infected pregnant women who do not meet criteria for cotrimoxazole prophylaxis should continue to receive IPTp-SP.
Several African clinical trials of cotrimoxazole prophylaxis have monitored HIV-infected individuals for ADRs, but none have included pregnant women. In a randomised, placebo-controlled, clinical trial of cotrimoxazole prophylaxis in 771 HIV-infected adults in Côte d’Ivoire recruited from a tuberculosis clinic, there was no difference in the rate of ADRs between the cotrimoxazole and placebo groups over 10 months of follow-up and no patients had to discontinue the study drug because of rash.[94] These findings have been confirmed in three other randomised, controlled trials of cotrimoxazole prophylaxis (two in adults and one in children) as noted in table II.[96,100,104] Several prospective cohorts following HIV-infected adults and children receiving cotrimoxazole prophylaxis have also demonstrated low rates of adverse cutaneous reactions (table II).[105–107] In one prospective cohort in rural Uganda of 423 HIV-infected adults and children receiving cotrimoxazole prophylaxis, only nine patients developed any ADR and three had mucocutaneous involvement during almost 18 months of follow-up.[95] In summary, clinical trials of cotrimoxazole involving >3000 non-pregnant HIV-infected Africans have demonstrated low rates of ADRs. Although these data are encouraging, they highlight the paucity of information on the rates of SCARs in HIV-infected pregnant women receiving cotrimoxazole.
SCARs have been reported with weekly sulfadoxine/pyrimethamine prophylaxis in HIV-infected individuals (the majority being male) in two case reports from the US[108,109] and two case series from Europe.[110,111] In Africa a large surveillance study conducted in Malawi documented rates of SCARs in HIV-infected adults at 4.9 cases per 100 000 exposures to sulfadoxine/pyrimethamine.[79] Although this rate was higher than the rates observed in HIV-negative adults, overall infrequent treatment doses with sulfadoxine/pyrimethamine were associated with a low risk of SCARs.[79]
Several smaller clinical trials have also systematically monitored for adverse reactions to sulfadoxine/pyrimethamine in HIV-infected women during pregnancy. In a clinical trial from Zambia of standard IPTp-SP compared with monthly IPTp-SP and involving 456 pregnant HIV-infected women, there was one fatal cutaneous adverse reaction.[c] In another trial involving 94 HIV-infected pregnant women treated with sulfadoxine/pyrimethamine in western Kenya, three women experienced ADRs but none were SCARs.[17] In a Malawian trial that included 266 HIV-positive pregnant women, <1% of participants reported any ADR including rash, nausea, vomiting or fever; no SCARs were noted and none of these women had to discontinue the study medication.[22] Overall, the ADR rate was similar in HIV-infected and HIV-negative women even though all HIV-infected women also received single-dose nevirapine (for prevention of mother-to-child HIV transmission), which can also cause rash and hepatotoxicity.[22]
In summary, SCARs secondary to sulfadoxine/pyrimethamine and cotrimoxazole may occur more frequently in HIV-infected pregnant women than HIV-negative pregnant women but overall remain a relatively rare occurrence. As HIV-infected pregnant women have more complications from untreated malarial infections, this population should receive >3 doses of IPTp-SP unless there is a known contraindication to these drugs or they are already receiving daily cotrimoxazole prophylaxis. The paucity of population-based data highlights the urgent need to expand pharmacovigilance surveillance programmes for SCARs in these women.
5. Fetal Outcomes
Folic acid supplementation during pregnancy reduces the risk of neural tube defects especially in women with low baseline serum folate levels.[113,114] Given the intrinsic folate antagonism of sulfadoxine/pyrimethamine, the risk for birth defects with IPTp-SP is plausible. We summarise preclinical animal teratogenicity data and the accumulated human experience of fetal outcomes with in utero exposure to sulfadoxine/pyrimethamine and related compounds.
5.1 Teratogenicity — Animal Studies
Although the teratogenicity of sulfadoxine specifically has not been well studied in animal models, the sulfonamide class has been extensively evaluated. At low-doses long-acting sulfonamides do not cause teratogenicity in rats.[115,116] High-doses of sulfonamides (500–1000 mg/kg/day) in rat and mouse models administered from day 9 to 14 induced significant rates of malformation.[117] These cumulative doses are between 100 and 250 times the human dose used for IPTp-SP. The most common malformations were cleft palate, mandibular and tongue abnormalities. Increased rates of fetal resorption were also seen in the rat and mouse models at these doses.[117] Other investigators administering high doses of sulfonamides in pregnant rat and mouse models found cleft palate,[118,119] extra ribs,[120] rib defects,[121] irregular appendicular skeletons,[121] delayed tooth malformations,[116,121] hydroureter[119] and hydronephrosis.[119] Interestingly, rabbits appear to be resistant to sulfonamide teratogenicity.[121]
Pyrimethamine teratogenicity varies by species with rats being more susceptible than others. Low-dose sulfadoxine/pyrimethamine induces folic acid deficiency in rats, which is unusual in humans.[122] In rats pyrimethamine causes predictable dose-dependent teratogenicity.[123] Although an oral dose of 5 mg/kg given to pregnant Wistar rats has no apparent embryotoxicity, an oral dose of 20 mg/kg causes a 75% rate of fetal resorption if given at gestational day 10 (typical gestation is 22 days in the Wistar rat) and a 70% rate of fetal anomalies if given at day 13.[124] For comparison, a 50kg pregnant woman receiving IPTp-SP would receive 1.5 mg/kg of pyrimethamine. Fetal anomalies seen in the Wistar rat include brachygnathia, cleft palate and limb defects. In contrast, administration of between 152–173 mg/kg to golden hamsters had no apparent embryotoxicity and only a massive dose of 229 mg/ kg led to a 40% rate of fetal resorption and 8.7% risk of fetal anomaly.[124] Other studies have shown pyrimethamine administration at 50–100 times the human dose for malaria prophylaxis induced resorption and fetal anomalies (including neural tube defects) in pregnant rats,[123,125] mice,[126] rabbits[126] and pigs.[127] Limb and facial malformations in rats have been found to occur at the site of vascular haemorrhages and malformations.[128] It has been speculated that early macrocytosis of fetal erythrocytes may provoke intravascular thrombosis followed by necrosis and haemorrhage.[129] Simultaneous administration of folinic acid, which unlike folic acid, can bypass DHFR inhibition to replenish folate derivatives, abolishes the teratogenic effects of pyrimethamine.[130]
As sulfadoxine and pyrimethamine are synergistic in their activity against P. falciparum, the combined teratogenic effect was evaluated in pregnant Wistar rats randomised to 14.4 mg/kg doses of sulfadoxine and 0.72 mg/kg doses of pyrimethamine on days 5, 12 and 19 (group A), days 10 and 17 (group B) and days 15 and 22 (group C).[131] Groups A and B produced no litters and fetal resorption was confirmed by histology. Group C produced a normal-sized litter with no malformations. The authors concluded that early administration of sulfadoxine/pyrimethamine induced fetal resorption in rats and the teratogenic potential was 2-fold higher than expected from the drugs individually.[131]
In summary, the teratogenicity data in animals are variable between species, making interpretation difficult. Rats are extremely susceptible to the teratogenic effects of sulfadoxine/pyrimethamine at low concentrations, but other animal species require very high doses to induce fetal anomalies. Although these animal data should help guide our evaluation of teratogenicity risk in humans, they can not be easily extrapolated.
5.2 Teratogenicity — Human Studies
The human experience with pyrimethamine alone in pregnancy is limited. In Germany, a case series of 72 women with toxoplasmosis who were treated with pyrimethamine (followed by treatment with a combination of sulfamerazine and sulfatolamide) in the first trimester found lower rates of abortion, stillbirth and congenital anomalies compared with historical controls of untreated women.[132] In a malaria trial in The Gambia, 518 pregnant women randomised to biweekly prophylaxis with pyrimethamine 25mg and dapsone 100mg had no increase in adverse pregnancy outcomes compared with 531 controls who received placebo.[133] In another malaria trial that randomised 429 Nigerian women to 50 mg/month of pyrimethamine or placebo in the second half of pregnancy, there was no difference in rates of stillbirth or neonatal deaths.[134] An additional study investigating the use of 25 mg/week of pyrimethamine in pregnant Nigerian women also reported no fetal malformations.[135] Between 1964 and 1994, the WHO Collaborating Centre for International Drug Monitoring identified only two cases of congenital anomalies with use of pyrimethamine alone in early pregnancy.[136]
Several high quality case-control studies provide the best quantifiable data on the teratogenic risk of folate antagonists in general. A large case-control study published in 2000 demonstrated an increased risk of cardiovascular defects and oral clefts amongst infants whose mothers were exposed to DHFR inhibitors in the first trimester.[137] This effect was reduced in women who took multivitamin supplements that contained folic acid. No increased risk of congenital anomalies was found in infants whose mothers were exposed to DHFR inhibitors in the second and third trimesters.[137] The same research group showed that folic acid antagonist exposure in the first 2 months of pregnancy was associated with an increased risk of neural tube defects.[138]
A large case-control study in Hungary also demonstrated an association between cotrimoxazole exposure in the first trimester and an increased risk of neural-tube, cleft lip, cardiovascular, and urinary tract defects.[139] A subsequent evaluation by the same authors also demonstrated an association between other sulfonamide use in the second and third months of pregnancy with certain cardiovascular malformations and clubfoot.[140] Additionally, a small case-control study of 197 HIV-infected pregnant women also revealed a higher rate of congenital abnormalities amongst women exposed to both antiretrovirals and folate antagonists during the first trimester.[141] Although only one case patient received sulfadoxine/pyrimethamine in these case-control studies, it is important to note that exposure to folate antagonists in the second and third trimesters (after organogenesis) was not associated with congenital anomalies in any of these studies.
Reports specifically on first trimester exposures to sulfadoxine/pyrimethamine are limited to small case series. In one series of 153 European travellers to East Africa who reported exposures to sulfadoxine/pyrimethamine in the first trimester to a pharmaceutical database, there was a 2.6% rate of spontaneous abortions and 7.8% rate of congenital anomalies.[142] Another travel cohort of 19 women exposed to sulfadoxine/pyrimethamine in the first trimester reported no spontaneous abortions and no congenital anomalies.[142] In comparison, 446 women exposed to mefloquine had a 9.1% rate of spontaneous abortions and 4.8% rate of congenital anomalies.[142] Although these reports had no control group, it is estimated that 9.5% of pregnancies result in clinically recognised spontaneous abortions[143] and an estimated 3% of live births have congenital anomalies[144] in developed countries. Background rates of spontaneous abortions and congenital anomalies have not been established for women living in Africa.
There is substantially more experience with sulfadoxine/pyrimethamine therapy in the second and third trimesters. For example, 34 pregnant women with toxoplasmosis treated with high-dose sulfadoxine/pyrimethamine after the first trimester delivered 32 healthy children, one infant with microcephaly and one infant with an imperforate anus.[145] Three large randomised controlled trials in Africa, comparing IPTp-SP with standard of care involving >3600 pregnant women, revealed no difference in rates of abortions, stillbirths and infant deaths between IPTp-SP and control groups.[14,15,17] Controls received weekly chloroquine,[14] placebo[15] or fever case management with sulfadoxine/pyrimethamine[17] in these three trials. One trial examined all infants at week 1 and 6 for evidence of congenital anomalies. The rate of congenital anomalies was identical (0.3%) in the IPTp-SP and control groups.[17] Finally, in a case-control study in Malawi, there was no association between number of doses of sulfadoxine/pyrimethamine received for IPTp and the rate of congenital anomalies.[24]
Human data on the safety of sulfadoxine/pyrimethamine in pregnancy in the context of IPTp are therefore quite reassuring. Although sulfadoxine/pyrimethamine is not recommended in the first trimester, there are extensive clinical trial data demonstrating its safety in the second and third trimesters when it would be administered for IPTp.
5.3 Folate Supplementation
Daily folic acid supplementation is recommended for all women during pregnancy to reduce the rate of congenital anomalies[146] and maternal anaemia.[147,148] However, folic acid supplementation could diminish the antimalarial activity of sulfadoxine/pyrimethamine and therefore increase susceptibility to developing placental malaria. Many P. falciparum isolates are able to utilise exogenous folate (unlike bacteria),[149,150] and physiological concentrations of folate cause a 1000-fold decrease in sulfadoxine inhibition of P. falciparum in vitro.[151] However, pyrimethamine is only minimally antagonised by folic acid, even at supra-physiological levels.[152] This suggests pyrimethamine may also interfere with folate salvage pathways in P. falciparum[53] by blocking the entry of exogenous folate into erythrocytes[153] in addition to inhibiting DHFR.
Epidemiological data suggest that human folate levels affect the efficacy of sulfadoxine/pyrimethamine.[53] Children treated with sulfadoxine/pyrimethamine and folic acid concurrently have higher rates of parasitological failure than children treated with sulfadoxine/pyrimethamine alone.[154,155] Several large clinical trials have begun to address the impact of folic acid supplementation on IPTp-SP and maternal malaria in Africa. Folic acid supplementation was given to all participants (sulfadoxine/pyrimethamine and placebo arms) of several randomised, clinical trials of IPTp-SP.[14,17,22] Although the dose of folic acid ranged from 0.5mg[22] to 5mg[17] per day, these studies consistently demonstrated that sulfadoxine/pyrimethamine was effective at reducing placental malaria despite folic acid supplementation.
Two recent randomised controlled trials in Africa[156,157] have evaluated the impact of folic acid supplementation on IPTp-SP. Day 14 parasitological outcome was used as the primary endpoint in both trials instead of the traditional outcomes of maternal anaemia, placental malaria, and low birth weight. The Kenyan trial randomised 488 pregnant women with asymptomatic parasitaemia to IPTp-SP and one of 3 regimens: placebo, 0.4 mg/day of folic acid or 5 mg/day of folic acid.[156] Women who received high-dose folic acid (5 mg/day) supplementation had twice the rate of parasitological treatment failure as those receiving placebo. However, women who received 0.4 mg/day of folic acid had similar parasitological cure rates as those receiving placebo.[156] The Gambian trial randomised 1035 pregnant women to receive sulfadoxine/pyrimethamine plus concurrent folic acid or sulfadoxine/pyrimethamine with folic acid given 2 weeks later (after the parasitological outcome was determined).[157] Women received between 0.5 and 1.5mg of folic acid per day depending on their initial haemoglobin level. At 2 weeks, in the subset of women with parasitaemia at enrollment (n = 261), there was no difference in rates of parasitological cure between the women who received concurrent folic acid and the women who delayed folic acid supplementation.[157] Therefore, although high doses of folic acid (5 mg/day) may interfere with the efficacy of sulfadoxine/pyrimethamine, 0.4mg of folic acid supplementation per day (the current WHO recommended dose)[147] may provide an optimal balance to prevent neural tube defects and maintain the effectiveness of IPTp-SP.
6. Bilirubin Metabolism Effects — Kemicterus
Kernicterus (neonatal encephalopathy) is caused by unconjugated bilirubin-induced neurotoxicity. High levels of unconjugated bilirubin in plasma may lead to increased entry of unconjugated bilirubin into CNS cells. However, kernicterus occurs in only a minor fraction of markedly jaundiced newborns and some neonates develop kernicterus despite having ‘physiological’ levels of bilimbin.[158] These differences might be due to genetic differences in ability to actively transport bilirubin out of the CNS.[159] Epidemiological studies suggest factors such as haemolysis and infection may potentiate the development of kernicterus.[158] Sulfonamides are capable of displacing unconjugated bilirubin[61] from albumin, which could theoretically increase a newborn’s risk of kernicterus if taken around the time of labour.
6.1 Kernicterus — Animal Studies
The Gunn strain of rat is deficient in UDP-glucuronyl transferase, which is necessary to conju gate bilirubin. This strain therefore suffers from lifelong non-haemolytic jaundice and a predisposition to kernicterus.[160] Administration of high-dose sulfonamides, including sulfisoxazole[161] and sulfadimethoxine,[162] results in severe kernicterus in Gunn rats. However, in normal newborn rats (non-Gunn strains), sulfonamide administration does not induce kernicterus.[163]
6.2 Kernicterus — Human Studies
Based on the results of an unblinded clinical trial of antibacterial prophylaxis in 193 premature infants, a relationship between sulfonamides and the development of kernicterus was proposed. Premature infants treated with a combination of penicillin and sulfisoxazole had a 63% mortality rate compared with a 27% mortality rate in infants receiving oxytetracycline.[164] On autopsy 42% of the deaths in the penicillin and sulfisoxazole arm had evidence of kernicterus compared with only 4% in the oxytetracycline arm.[164] As a result, sulfonamides became contraindicated in infants aged <2 months. However, in 1984 sulfadoxine/pyrimethamine was demonstrated to be effective therapy for congenital toxoplasmosis in a study of 24 newborns where no episodes of kernicterus were reported.[165] Treatment with pyrimethamine, sulfadiazine and folinic acid for 1 year beginning at birth has become the standard of care for infants with congenital toxoplasmosis. Clinical case series have reported no cases of kernicterus in >800 newborns treated.[166–172]
Long-acting sulfonamides administered to the mother during labour have measurable plasma concentrations in the newborn for several days. After delivery and loss of the placenta’s glucuronyl transferase activity, it has been speculated that the newborn may be susceptible to sulfonamide-induced kernicterus. Several case reports of infants developing severe jaundice after maternal administration of sulfonamides have been published.[173–175] However, a systematic evaluation of 44 infants with perinatal sulfisoxazole exposure compared with 234 controls revealed lower levels of unconjugated bilirubin in the exposed group and identical levels of conjugated bilirubin.[176] There were also no episodes of kernicterus.[176] In third-trimester pregnant women, there is now extensive experience from case series and randomised clinical trials with the use of sulfonamides for rheumatic fever prophylaxis,[177,178] urinary tract infections,[179,180] toxoplasmosis[168–170] and malaria[19,22,24] with no reported episodes of kernicterus. In randomised, controlled trials of IPTp-SP there has also been no association between children who died with jaundice and maternal administration of sulfadoxine/pyrimethamine.[14,15,17]
Although continued monitoring is warranted, extensive evidence exists to assuage concerns that sulfonamides administered late in pregnancy or to term neonates might increase the risk for kernicterus. The experience using sulfadoxine/pyrimethamine in the congenital toxoplasmosis literature is particularly compelling because of the high-doses and prolonged duration of therapy without any cases of kernicterus. Based on these data, concerns regarding kernicterus should not restrict the use of sulfadoxine/pyrimethamine for IPTp in late pregnancy.
6.3 Breastfeeding
Current product information from Roche states that Fansidar® is contraindicated during breastfeeding due to concerns that both sulfadoxine and pyrimethamine are excreted in breast milk at low concentrations.[50] Approximately 3–6% of a single pyrimethamine dose to the mother will be delivered to a breastfeeding infant over 48 hours.[181] Sulfonamides are excreted into breast milk at lower concentrations in general and only 0.45% of a dose of sulfisoxazole was recovered in breast milk over 48 hours.[182] No case reports of possible ADRs related to sulfadoxine/pyrimethamine exposure in breast milk have been reported. The manufacturer’s recommendations against using sulfadoxine/pyrimethamine while breastfeeding is likely due to theoretical concerns regarding kernicterus among infants aged <2 months. However, as stated in the previous section, there is extensive evidence that sulfadoxine/pyrimethamine and related compounds do not induce kernicterus. Concurring with this evidence, the American Academy of Pediatrics considers sulfonamides and pyrimethamine compatible with breastfeeding.[183]
7. Discussion
Malaria in pregnancy remains a major cause of morbidity and mortality in sub-Saharan Africa despite the availability of affordable and effective treatments.[184] IPTp-SP has the potential to significantly reduce both maternal and neonatal morbidity and mortality. Unfortunately, widespread implementation of this public health measure has been slow, in part due to residual concerns regarding the safety of sulfadoxine/pyrimethamine in pregnancy.
Most drugs have never received an extensive safety evaluation in pregnant women because of historical biases to study medications in men[185,186] and pharmaceutical industry concerns regarding liability.[186,187] In addition, the commercial market for many medications in pregnancy is limited, which further deters corporations from testing their product in pregnant women. The lack of high quality clinical studies forces physicians and public health policy makers to rely on extrapolations of available data from animal models, case series and case-control studies. These extrapolations can lead to misguided reservations regarding the safety of sulfadoxine/pyrimethamine by both healthcare providers and pregnant women.[29–31] In the case of sulfadoxine/pyrimethamine, concerns have been raised regarding the risk of maternal SCARs, fetal teratogenicity and alterations in newborn bilirubin metabolism.
Fortunately, several international trials (not funded by the pharmaceutical industry) have provided insight into these problems. The rate of SCARs in Africa has been less than predicted even in studies with enhanced surveillance. This low rate of SCARs may be due to population-level genetic differences or may be a function of the low dose and intermittent administration of sulfadoxine/pyrimethamine in IPTp regimens. As utilisation of IPTp-SP increases there must be enhanced pharmacovigilance to ensure that higher rates of SCARs are not observed. Five sub-Saharan African countries have, in fact, recently introduced national pharmacovigilance programmes in response to changes in their malaria treatment policies.[188] The success of these new pharmacovigilance programmes will depend on their ability to integrate into existing public health programmes (such as national IPTp-SP programmes).[188] Results from these new pharmacovigilance programmes must also be communicated back to local healthcare providers and pregnant women in Africa since community receptiveness is an important determinant for successful IPTp-SP implementation,[29,30]
There is an increasing appreciation of the interactions between HIV and malaria, especially in pregnant women. Although HIV-infected Africans have higher rates of ADRs than HIV-negative individuals, these rates are still low in terms of absolute numbers. Therefore, IPTp-SP can be safely given to HIV-infected pregnant women. However, pregnant women on cotrimoxazole prophylaxis should avoid concurrent sulfadoxine/pyrimethamine treatment because of its redundant mechanism of action and synergistic worsening of ADRs. A priority area of research should be establishing the efficacy of cotrimoxazole prophylaxis in preventing placental malaria and improving fetal outcomes.
Associations between anti-folate use in the first trimester and congenital anomalies have been reported. In the IPTp regimen, sulfadoxine/pyrimethamine is only administered in the second and third trimesters. Although the animal data are ambiguous, large human case-control studies and randomised controlled trials have demonstrated the safety of sulfadoxine/pyrimethamine in the context of IPTp. Furthermore, IPTp-SP remains effective at preventing placental malaria in women taking low-dose (0.4 mg/day) folic acid supplementation. However, countries implementing IPTp-SP with 5mg of folic acid supplementation should review the indications for this high dose.
Finally, sulfadoxine/pyrimethamine use in near-term pregnant women has been hampered by a 1956 clinical trial that demonstrated a high rate of kernicterus in premature infants treated with penicillin and sulfisoxazole. However, recent clinical experience with congenital toxoplasmosis treatment and several large clinical trials using IPTp-SP in Africa have not resulted in any cases of kernicterus. Although the pathogenesis of kernicterus remains unknown, it does not appear to be inducible by sulfonamides administered late in pregnancy or to term neonates. The benefits of IPTp-SP outweigh any theoretical risk of kernicterus and these concerns should not limit its use.
No drug is completely safe. For any public health intervention there must be a careful assessment of the benefits and risks of treatment. With IPTp-SP there is clear and extensive evidence of its benefit and safety to warrant broad implementation as recommended by the WHO. However, continued research will be necessary to evaluate its impact in areas of increasing P. falciparum sulfadoxine/pyrimethamine resistance and HIV co-infection.
8. Conclusion
IPTp-SP has now been effectively used for >10 years in many African countries. On balance, the reviewed data strongly support the safety of sulfadoxine/pyrimethamine in pregnancy. The favourable safety profile of sulfadoxine/pyrimethamine supports its continued use in IPTp regimens as recommended by the WHO.
Notes
1The use of trade names is for product identification purposes only and does not imply endorsement.
References
WHO. Lives at risk: malaria in pregnancy [online]. Available from URL: http://www.who.int/features/2003/04b/en/ [Accessed 2006 Mar 6]
Beeson JG, Brown GV. Plasmodium falciparum-imfected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J Infect Dis 2004 Jan 15; 189(2): 169–79
Shulman CE, Graham WJ, Jilo H, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg 1996 Sep–Oct; 90(5): 535–9
Cot M, le Hesran JY, Miailhes P, et al. Effect of chloroquine prophylaxis during pregnancy on maternal haematocrit. Ann Trop Med Parasitol 1998 Jan; 92(1): 37–43
Steketee RW, Wirima JJ, Hightower AW, et al. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg 1996; 55 (1 Suppl.): 33–41
Sullivan AD, Nyirenda T, Cullinan T, et al. Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. J Infect Dis 1999 Jun; 179(6): 1580–3
Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg 2001 Jan–Feb; 64 (1-2 Suppl.): 28–35
Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg 2001 Jan–Feb; 64 (1–2 Suppl.): 36–44
Marchesini P, Crawley J. Reducing the burden of malaria in pregnancy [online]. Available from URL: http://www.who.int/malaria/rbm/Attachment/20040713/MeraJan2003.pdf [Accessed 2006 Jan 6]
Steketee RW, Wirima JJ, Slutsker L, et al. Malaria treatment and prevention in pregnancy: indications for use and adverse events associated with use of chloroquine or mefloquine. Am J Trop Med Hyg 1996; 55 (1 Suppl.): 50–6
Sirima SB, Sawadogo R, Moran AC, et al. Failure of a chloroquine chemoprophylaxis program to adequately prevent malaria during pregnancy in Koupela District, Burkina Faso. Clin Infect Dis 2003 Jun 1; 36(11): 1374–82
WHO. WHO Expert Committee on Malaria. World Health Organ Tech Rep Ser 2000; 92: i-v, 1–74
World Health Organization. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: WHO Regional Office for Africa, 2004. AFR/ MAL/04/01 [online]. Available from URL: http://www.cdc.gov/malaria/pdf/strategic_framework_mip_04.pdf [Accessed 2006 Jan 6]
Kayentao K, Kodio M, Newman RD, et al. Comparison of intermittent preventive treatment with chemoprophylaxis for the prevention of malaria during pregnancy in Mali. J Infect Dis 2005 Jan 1; 191(1): 109–16
Shulman CE, Dorman EK, Cutts F, et al. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet 1999 Feb 20; 353(9153): 632–6
Njagi JK, Magnussen P, Estambale B, et al. Prevention of anaemia in pregnancy using insecticide-treated bednets and sulfadoxine-pyrimethamine in a highly malarious area of Kenya: a randomized controlled trial. Trans R Soc Trop Med Hyg 2003 May–Jun; 97(3): 277–82
Parise ME, Ayisi JG, Nahlen BL, et al. Efficacy of sulfadoxinepyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. Am J Trop Med Hyg 1998 Nov; 59(5): 813–22
Schultz LJ, Steketee RW, Macheso A, et al. The efficacy of antimalarial regimens containing sulfadoxine-pyrimethamine and/or chloroquine in preventing peripheral and placental Plasmodium falciparum infection among pregnant women in Malawi. Am J Trop Med Hyg 1994 Nov; 51(5): 515–22
Challis K, Osman NB, Cotiro M, et al. Impact of a double dose of sulphadoxine-pyrimethamine to reduce prevalence of pregnancy malaria in southern Mozambique. Trop Med Int Health 2004 Oct; 9(10): 1066–73
van Eijk AM, Ayisi JG, ter Kuile FO, et al. Effectiveness of intermittent preventive treatment with sulphadoxinepyrimethamine for control of malaria in pregnancy in western Kenya: a hospital-based study. Trop Med Int Health 2004 Mar; 9(3): 351–60
Rogerson SJ, Chaluluka E, Kanjala M, et al. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997–99. Trans R Soc Trop Med Hyg 2000 Sep–Oct; 94(5): 549–53
Filler SJ, Kazembe P, Thigpen M, et al. Randomized trial of 2-dose versus monthly sulfadoxine-pyrimethamine intermittent preventive treatment for Malaria in HIV-positive and HIV-negative pregnant women in Malawi. J Infect Dis 2006 Aug 1; 194(3): 286–93
Sirima SB, Cotte AH, Konate A, et al. Malaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso. Am J Trop Med Hyg 2006 Aug; 75(2): 205–11
Verhoeff FH, Brabin BJ, Chimsuku L, et al. An evaluation of the effects of intermittent sulfadoxine-pyrimethamine treatment in pregnancy on parasite clearance and risk of low birthweight in rural Malawi. Ann Trop Med Parasitol 1998 Mar; 92(2): 141–50
WHO/AFRO. Global Antimalarial Drug Database [online]. Available from URL: http://www.who.int/malaria/amdp/amdp_afro.htm [Accessed 2007 Jan 10]
Korenromp E, Miller J, Nahlen B, et al., for World Health Organization (WHO). World Malaria Report 2005 [online]. Available from URL: http://rbm.who.int/wmr2005/index.html [Accessed 2007 Jan 10]
Dodoo A. Safety challenges of preventing malaria during pregnancy. WHO Drug Information 2005; 19(4): 286–7 [online]. Available from URL: http://www.who.int/druginformation/vol19num4_2005/DI19-4.pdf [Accessed 2007 Jan 10]
Nosten F, McGready R, Looareesuwan S, et al. Editorial: Maternal malaria: time for action. Trop Med Int Health 2003 Jun; 8(6): 485–7
Hill J, Kazembe P. Reaching the Abuja target for intermittent preventive treatment of malaria in pregnancy in African women: a review of progress and operational challenges. Trop Med Int Health 2006 Apr; 11(4): 409–18
Mubyazi G, Bloch P, Kamugisha M, et al. Intermittent preventive treatment of malaria during pregnancy: a qualitative study of knowledge, attitudes and practices of district health managers, antenatal care staff and pregnant women in Korogwe District, North-Eastern Tanzania. Malar J 2005; 4: 31
Mbonye AK, Neema S, Magnussen P. Perceptions on use of sulfadoxine-pyrimethamine in pregnancy and the policy implications for malaria control in Uganda. Health Policy 2006 Aug; 77(3): 279–89
Sibley CH, Hyde JE, Sims PF, et al. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol 2001 Dec; 17(12): 582–8
Nzila A, Ward SA, Marsh K, et al. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol 2005 Jun; 21(6): 292–8
Hyde JE. Exploring the folate pathway in Plasmodium falciparum. Acta Trop 2005 Jun; 94(3): 191–206
Ferone R, Burchall JJ, Hitchings GH. Pkismodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol 1969 Jan; 5(1): 49–59
Zhang K, Rathod PK. Divergent regulation of dihydrofolate reductase between malaria parasite and human host. Science 2002 Apr 19; 296(5567): 545–7
Hitchings GH, Burchall JJ. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol Relat Areas Mol Biol 1965; 27: 417–68 497
Watkins WM, Mberu EK, Winstanley PA, et al. The efficacy of antifolate antimalarial combinations in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Parasitol Today 1997 Dec; 13(12): 459–64
Rollo IM. The mode of action of sulphonamides, proguanil and pyrimethamine on Plasmodium gallinaceum. Br J Pharmacol Chemother 1955 Jun; 10(2): 208–14
Hurly MG. Potentiation of pyrimethamine by sulphadiazine in human malaria. Trans R Soc Trop Med Hyg 1959 Sep; 53: 412–3
Weidekamm E, Plozza-Nottebrock H, Forgo I, et al. Plasma concentrations in pyrimethamine and sulfadoxine and evaluation of pharmacokinetic data by computerized curve fitting. Bull World Health Organ 1982; 60(1): 115–22
Edstein MD. Pharmacokinetics of sulfadoxine and pyrimethamine after Fansidar administration in man. Chemotherapy 1987; 33(4): 229–33
PDR guide to drug interactions, side effects, indications, contraindications. Montvale (NJ): Medical Economics, 1997: 2281–2
Barnes KI, Little F, Smith PJ, et al. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin Pharmacol Ther 2006 Dec; 80(6): 582–96
Corvaisier S, Charpiat B, Mounier C, et al. Population pharmacokinetics of pyrimethamine and sulfadoxine in children treated for congenital toxoplasmosis. Antimicrob Agents Chemother 2004 Oct; 48(10): 3794–800
Hellgren U, Kihamia CM, Bergqvist Y, et al. Standard and reduced doses of sulfadoxine-pyrimethamine for treatment of Plasmodium falciparum in Tanzania, with determination of drug concentrations and susceptibility in vitro. Trans R Soc Trop Med Hyg 1990 Jul–Aug; 84(4): 469–72
Winstanley PA, Watkins WM, Newton CR, et al. The disposition of oral and intramuscular pyrimethamine/sulphadoxine in Kenyan children with high parasitaemia but clinically non-severe falciparum malaria. Br J Clin Pharmacol 1992 Feb; 33(2): 143–8
Trenque T, Marx C, Quereux C, et al. Human maternofoetal distribution of pyrimethamine-sulphadoxine. Br J Clin Pharmacol 1998 Feb; 45(2): 179–80
Peytavin G, Leng JJ, Forestier F, et al. Placental transfer of pyrimethamine studied in an ex vivo placental perfusion model. Biol Neonate 2000; 78(2): 83–5
Roche. Fansidar® brand of sulfadoxine and pyrimethamine tablets: complete product information [online]. Available from URL: http://www.rocheusa.com/products/fansidar/ [Accessed 2006 May 7]
Ringwald P, Global Partnership to Roll Back Malaria. Susceptibility of plasmodium falciparum to antimalarial drugs: report on global monitoring, 1996–2004. Geneva: World Health Organization, 2005
White NJ. Intermittent presumptive treatment for malaria. PLoS Med 2005 Jan; 2(1): e3
Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 2005 Mar; 57(1): 117–45
Hankins EG, Warhurst DC, Sibley CH. Novel alleles of the Plasmodium falciparum dhfr highly resistant to pyrimethamine and chlorcycloguanil, but not WR 99210. Mol Biochem Parasitol 2001 Sep 28; 117(1): 91–102
McCollum AM, Poe AC, Hamel M, et al. Antifolate resistance in Plasmodium falciparum: multiple origins and identification of novel dhfr alleles. J Infect Dis 2006 Jul 15; 194(2): 189–97
Nzila A, Ochong E, Nduati E, et al. Why has the dihydrofolate reductase 164 mutation not consistently been found in Africa yet? Trans R Soc Trop Med Hyg 2005 May; 99(5): 341–6
Bioland PB, Ringwald P, Snow RW, World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization, 2003
Kalanda GC, Hill J, Verhoeff FH, et al. Comparative efficacy of chloroquine and sulphadoxine: pyrimethamine in pregnant women and children: a meta-analysis. Trop Med Int Health 2006 May; 11(5): 569–77
Plowe CV, Kublin JG, Dzinjalamala FK, et al. Sustained clinical efficacy of sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Malawi after 10 years as first line treatment: five year prospective study. BMJ 2004 Mar 6; 328(7439): 545
Chandramohan D, Owusu-Agyei S, Carneiro I, et al. Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ 2005 Oct 1; 331(7519): 727–33
Matthews JI, Molitor JT, Hunt KK, et al. Pyrimethamine-induced leukopenia and thrombocytopenia in a patient with malaria and tropical sprue: case report. Mil Med 1973 May; 138(5): 280–3
Goodman LS, Gilman A, Brunton LL, et al. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006
Waxman S, Metz J, Herbert V. Defective DNA synthesis in human megaloblastic bone marrow: effects of homocysteine and methionine. J Clin Invest 1969 Feb; 48(2): 284–9
Khoo KK. The treatment of malaria in glucose-6-phosphate dehydrogenase deficient patients in Sabah. Ann Trop Med Parasitol 1981 Dec; 75(6): 591–5
Bjorkman A, Phillips-Howard PA. Adverse reactions to sulfa drugs: implications for malaria chemotherapy. Bull World Health Organ 1991; 69(3): 297–304
Miller KD, Lobel HO, Satriale RF, et al. Severe cutaneous reactions among American travelers using pyrimethamine-sulfadoxine (Fansidar) for malaria prophylaxis. Am J Trop Med Hyg 1986 May; 35(3): 451–8
Aguemon AR, Houngbe F, Yameogo TM, et al. Toxic epidermal necrolysis. Epidemiologic, clinic and therapeutic aspects at Cotonou University and National Teaching Hospital [in French]. Ann Fr Anesth Reanim 2006 May; 25(5): 505–9
Oduro-Boatey C, Rodrigues O. Stevens-Johnson syndrome in two children in Ghana following anti-malarial treatment. Trop Doct 2005 Apr; 35(2): 118–9
Nair SS, Kaplan JM, Levine LH, et al. Trimethoprimsulfamethoxazole-induced intrahepatic cholestasis. Ann Intern Med 1980 Apr; 92(4): 511–2
Munoz SJ, Martinez-Hernandez A, Maddrey WC. Intrahepatic cholestasis and phospholipidosis associated with the use of trimethoprim-sulfamethoxazole. Hepatology 1990 Aug; 12(2): 342–7
Thies PW, Dull WL. Trimethoprim-sulfamethoxazole-induced cholestatic hepatitis. Inadvertent rechallenge. Arch Intern Med 1984 Aug; 144(8): 1691–2
Zitelli BJ, Alexander J, Taylor S, et al. Fatal hepatic necrosis due to pyrimethamine-sulfadoxine (Fansidar). Ann Intern Med 1987 Mar; 106(3): 393–5
McCormack D, Morgan WK. Fansidar hypersensitivity pneumonitis. Br J Dis Chest 1987 Apr; 81(2): 194–6 498
Svanbom M, Rombo L, Gustafsson L. Unusual pulmonary reaction during short term prophylaxis with pyrimethamine-sulfadoxine (Fansidar). BMJ (Clin Res Ed). 1984 Jun 23; 288(6434): 187
Hellgren U, Rombo L, Berg B, et al. Adverse reactions to sulphadoxine-pyrimethamine in Swedish travellers: implications for prophylaxis. BMJ (Clin Res Ed) 1987 Aug 8; 295(6594): 365–6
Phillips-Howard PA, West LJ. Serious adverse drug reactions to pyrimethamine-sulphadoxine, pyrimethamine-dapsone and to amodiaquine in Britain. J R Soc Med 1990 Feb; 83(2): 82–5
Steffen R, Somaini B. Severe cutaneous adverse reactions to sulfadoxine-pyrimethamine in Switzerland. Lancet 1986 Mar 15; 1(8481): 610
World Health Organization. WHO Collaborating Centre for International Drug Monitoring. The importance of pharmacovigilance. Geneva: World Health Organization Uppsala Monitoring Centre, WHO Collaborating Centre for International Drug Monitoring, 2002
Gimnig JE, MacArthur JR, M’Bang’ombe M, et al. Severe cutaneous reactions to sulfadoxine-pyrimethamine and trimethoprim-sulfamethoxazole in Blantyre District, Malawi. Am J Trop Med Hyg 2006 May; 74(5): 738–43
Hernborg A. Stevens-Johnson syndrome after mass prophylaxis with sulfadoxine for cholera in Mozambique. Lancet 1985 Nov 9; 2(8463): 1072–3
Shear NH, Spielberg SP, Grant DM, et al. Differences in metabolism of sulfonamides predisposing to idiosyncratic toxicity. Ann Intern Med 1986 Aug; 105(2): 179–84
Holtz TH, Kachur SP, Roberts JM, et al. Use of antenatal care services and intermittent preventive treatment for malaria among pregnant women in Blantyre District, Malawi. Trop Med Int Health 2004 Jan; 9(1): 77–82
UNAIDS. AIDS epidemic update: December 2005 [online]. Available from URL: http://www.unaids.org/epi/2005/doc/report_pdf.asp [Accessed 2006 Mar 6]
ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg 2004 Aug; 71 (2 Suppl.): 41–54
Moore JM, Ayisi J, Nahlen BL, et al. Immunity to placental malaria: II. Placental antigen-specific cytokine responses are impaired in human immunodeficiency virus-infected women. J Infect Dis 2000 Sep; 182(3): 960–4
Chaisavaneeyakorn S, Moore JM, Otieno J, et al. Immunity to placental malaria: III. Impairment of interleukin(IL)-12, not IL-18, and interferon-inducible protein-10 responses in the placental intervillous blood of human immunodeficiency virus/malaria-coinfected women. J Infect Dis 2002 Jan 1; 185(1): 127–31
Mount AM, Mwapasa V, Elliott SR, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet 2004 Jun 5; 363(9424): 1860–7
Steketee RW, Wirima JJ, Bioland PB, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 1996; 55 (1 Suppl.): 42–9
WHO. Malaria and HIV interactions and their implications for public health policy [online]. Available from URL: http://www.who.int/malaria/malariandhivaids.html [Accessed 2006 Mar 5]
Lee BL, Wong D, Benowitz NL, et al. Altered patterns of drug metabolism in patients with acquired immunodeficiency syndrome. Clin Pharmacol Ther 1993 May; 53(5): 529–35
Carr A, Gross AS, Hoskins JM, et al. Acetylation phenotype and cutaneous hypersensitivity to trimethoprim-sulphamethoxazole in HIV-infected patients. Aids 1994 Mar; 8(3): 333–7
Carr A, Tindall B, Penny R, et al. In vitro cytotoxicity as a marker of hypersensitivity to sulphamethoxazole in patients with HIV. Clin Exp Immunol 1993 Oct; 94(1): 21–5
Carr A, Vasak E, Munro V, et al. Immunohistological assessment of cutaneous drug hypersensitivity in patients with HIV infection. Clin Exp Immunol 1994 Aug; 97(2): 260–5
Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet 1999 May 1; 353(9163): 1469–75
Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004 Oct 16–22; 364(9443): 1428–34
Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 2004 Nov 20–26; 364(9448): 1865–71
Walter J, Mwiya M, Scott N, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. J Infect Dis 2006 Dec 1; 194(11): 1510–8
WHO. Guidelines for cotrimoxazole prophylaxis for HIV-related infections in children, adolescents and adults in resource limited settings [online]. Available from URL: http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf [Accessed 2006 Mar 5]
UNAIDS/WHO. Provisional WHO/UNAIDS secreteriat recommendations on the widespread use of co-trimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa, 2000 [online]. Available from URL: http://www.unaids.org/publications/documents/care/general/recommendationeng.pdf [Accessed 2006 Mar 6]
Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999 May 1; 353(9163): 1463–8
Thera MA, Sehdev PS, Coulibaly D, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis 2005 Nov 15; 192(10): 1823–9
Malamba SS, Mermin J, Reingold A, et al. Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HlV)-infected persons on the selection of sulfadoxinepyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg 2006 Sep; 75(3): 375–80
Brentlinger PE, Behrens CB, Micek MA. Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa. Lancet Infect Dis 2006 Feb; 6(2): 100–11
Maynart M, Lievre L, Sow PS, et al. Primary prevention with cotrimoxazole for HIV-1-infected adults: results of the pilot study in Dakar, Senegal. J Acquir Immune Defic Syndr 2001 Feb 1; 26(2): 130–6
Watera C, Todd J, Muwonge R, et al. Feasibility and effectiveness of cotrimoxazole prophylaxis for HIV-1-infected adults attending an HIV/AIDS clinic in Uganda. J Acquir Immune Defic Syndr 2006 Jul; 42(3): 373–8 499
Zachariah R, Spielmann MP, Chinji C, et al. Voluntary counselling, HIV testing and adjunctive cotrimoxazole reduces mortality in tuberculosis patients in Thyolo, Malawi. AIDS 2003 May 2; 17(7): 1053–61
Grimwade K, Sturm AW, Nunn AJ, et al. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS 2005 Jan 28; 19(2): 163–8
Raviglione MC, Dinan WA, Pablos-Mendez A, et al. Fatal toxic epidermal necrolysis during prophylaxis with pyrimethamine and sulfadoxine in a human immunodeficiency virus-infected person. Arch Intern Med 1988 Dec; 148(12): 2683–5
Fansidar-associated fatal reaction in an HIV-infected man. MMWR Morb Mortal Wkly Rep 1988 Sep 23; 37(37): 571–2, 7
Teira R, Virosta M, Munoz J, et al. The safety of pyrimethamine and sulfadoxine for the prevention of Pneumocystis carinii pneumonia. Scand J Infect Dis 1997; 29(6): 595–6
Schurmann D, Bergmann F, Albrecht H, et al. Effectiveness of twice-weekly pyrimethamine-sulfadoxine as primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmic encephalitis in patients with advanced HIV infection. Eur J Clin Microbiol Infect Dis 2002 May; 21(5): 353–61
Hamer DH, Mwanakasale V, Chalwe V, et al. Intermittent presumptive therapy of malaria with SP in HIV-seropositive Zambian women: a placebo-controlled, randomized trial [abstract no. 54]. 54th Annual Meeting of the American Society Tropical Medicine and Hygiene; 2005 Dec 11–15; Washington, DC
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991 Jul 20; 338(8760): 131–7
Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999 Nov 11; 341(20): 1485–90
Bohni E, Fust B, Rieder J, et al. Comparative toxicological, chemotherapeutic and pharmacokinetic studies with sulphormethoxine and other sulphonamides in animals and man. Chemotherapy 1969; 14(4): 195–226
Bertazzoli C, Chieli T, Grandi M. Absence of tooth malformation in offspring of rats treated with a long-acting sulphonamide. Experientia 1965 Mar 15; 21(3): 151–2
Kato T, Kitagawa S. Production of congenital anomalies in fetuses of rats and mice with various sulfonamides. Cong Anom 1973; 13(1): 7–15
Suzuki Y, Wakita Y, Kondo S, et al. Effects of sulfamethopyrazine administered to pregnant animals upon the development of their fetuses and neonates. Oyo Yakuri 1973; 7: 1005–19
Wolkowski-Tyl R, Jones-Price C, Kimmel C, et al. Teratologic evaluation of sulfamethazine in CD rats. Teratology 1982; 25: 81A-2A
Kato T, Kitagawa S. Production of congenital skeletal anomalies in the fetuses of pregnant rats and mice treated with various sulfonamides. Cong Anom 1973; 13(1): 17–23
Paget GE, Thorpe E. A teratogenic effect of a sulphonamide in experimental animals. Br J Pharmacol Chemother 1964 Oct; 23: 305–12
Uche-Nwachi EO, Caxton-Martins AE. Sulfadoxinepyrimethamine embryopathy in Wistar rats. Kaibogaku Zasshi 1998 Apr; 73(2): 135–9
Dyban AP, Akimova IM, Svetlova VA. Embryonal development of rats acted upon with 2,4-diamino-5-chlorphenyl-6-ethylpyrimidine [in Russian]. Dokl Akad Nauk SSSR 1965 Aug 21; 163(6): 1514–7
Sullivan GE, Takacs E. Comparative teratogenicity of pyrimethamine in rats and hamsters. Teratology 1971; 4(2): 205–9
Anderson I, Morse LM. The influence of solvent on the teratogenic effect of folic acid antagonist in the rat. Exp Mol Pathol 1966 Apr; 5(2): 134–45
Schvartsman S. Teratogenicity of pyrimethamine. Toxicol Applied Pharmacol 1979; 48: A123
Misawa J, Kanda S, Kokue E, et al. Teratogenic activity of pyrimethamine in Gottingen minipig. Toxicol Lett 1982 Jan; 10(1): 51–4
Tangapregassom AM, Tangapregassom MJ, Horvath C, et al. Vascular anomalies and pyrimethamine-induced malformations in the rat. Teratog Carcinog Mutagen 1985; 5(1): 55–62
Petter C, Bourbon J. Foetal red cell macrocytosis induced by pyrimethamine; its teratogenic role. Experientia 1975 Mar 15; 31(3): 369–70
Kudo G, Tsunematsu K, Shimoda M, et al. Effects of folic acid on pyrimethamine teratogenesis in rats. Adv Exp Med Biol 1993; 338: 469–72
Uche-Nwachi EO. Effect of intramuscular sulfadoxinepyrimethamine on pregnant Wistar rats. Anat Rec 1998 Apr; 250(4): 426–9
Hengst P. Teratogenicity of daraprim (pyrimethamine) in man [in German]. Zentralbl Gynakol 1972 Apr 29; 94(17): 551–5
Greenwood BM, Greenwood AM, Snow RW, et al. The effects of malaria chemoprophylaxis given by traditional birth attendants on the course and outcome of pregnancy. Trans R Soc Trop Med Hyg 1989 Sep-Oct; 83(5): 589–94
Morley D, Woodland M, Cuthbertson WF. Controlled trial of pyrimethamine in pregnant women in an African village. BMJ 1964 Mar 14; 5384: 667–8
Nahlen BL, Akintunde A, Alakija T, et al. Lack of efficacy of pyrimethamine prophylaxis in pregnant Nigerian women. Lancet 1989 Oct 7; 2(8667): 830–4
Phillips-Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Saf 1996 Mar; 14(3): 131–45
Hernandez-Diaz S, Werler MM, Walker AM, et al. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med 2000 Nov 30; 343(22): 1608–14
Hernandez-Diaz S, Werler MM, Walker AM, et al. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol 2001 May 15; 153(10): 961–8
Czeizel AE, Rockenbauer M, Sorensen HT, et al. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reprod Toxicol 2001 Nov-Dec; 15(6): 637–46
Czeizel AE, Puho E, Sorensen HT, et al. Possible association between different congenital abnormalities and use of different sulfonamides during pregnancy. Congenit Anom (Kyoto) 2004 Jun; 44(2): 79–86
Jungmann EM, Mercey D, DeRuiter A, et al. Is first trimester exposure to the combination of antiretroviral therapy and folate antagonists a risk factor for congenital abnormalities? Sex Transm Infect 2001 Dec; 77(6): 441–3
Phillips-Howard PA, Steffen R, Kerr L, et al. Safety of mefloquine and other antimalarial agents in the first trimester of pregnancy. J Travel Med 1998 Sep; 5(3): 121–6
Slama R, Bouyer J, Windham G, et al. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol 2005 May 1; 161(9): 816–23 500
Correa-Villasenor A, Cragan J, Kucik J, et al. The Metropolitan Atlanta Congenital Defects Program: 35 years of birth defects surveillance at the Centers for Disease Control and Prevention. Birth Defects Res A Clin Mol Teratol 2003 Sep; 67(9): 617–24
Barbosa J, Ferreira I. Sulfadoxine-pyrimethamine (Fansidar) in pregnant women with toxoplasma antibody titers. In: Siegenthaler W, Luthy R, editors. The 10th International Congress of Chemotherapy, 1977. Zürich: American Society of Microbiology, 1977: 134–5
Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev 2001 (3): CD001056
WHO. Integrated management of pregnancy and childbirth: pregnancy, childbirth, postpartum and newborn care: a guide for essential practice [online]. Available from URL: http://www.who.int/reproductive-health/publications/pcpnc/pcpnc.pdf [Accessed 2007 Jan 22]
Mahomed K. Iron and folate supplementation in pregnancy. Cochrane Database Syst Rev 2000; (2): CD001135
Asawamahasakda W, Yuthavong Y. The methionine synthesis cycle and salvage of methyltetrahydrofolate from host red cells in the malaria parasite (Plasmodium falciparum). Parasitology 1993 Jul; 107 (Pt 1): 1–10
Krungkrai J, Webster HK, Yuthavong Y. De novo and salvage biosynthesis of pteroylpentaglutamates in the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitai 1989 Jan 1; 32(1): 25–37
Chulay JD, Watkins WM, Sixsmith DG. Synergistic antimalarial activity of pyrimethamine and sulfadoxine against Plasmodium falciparum in vitro. Am J Trop Med Hyg 1984 May; 33(3): 325–30
Watkins WM, Sixsmith DG, Chulay JD, et al. Antagonism of sulfadoxine and pyrimethamine antimalarial activity in vitro by p-aminobenzoic acid, p-aminobenzoylglutamic acid and folic acid. Mol Biochem Parasitai 1985 Jan; 14(1): 55–61
Wang P, Brobey RK, Horii T, et al. Utilization of exogenous folate in the human malaria parasite Plasmodium falciparum and its critical role in antifolate drug synergy. Mol Microbiol 1999 Jun; 32(6): 1254–62
van Hensbroek MB, Morris-Jones S, Meisner S, et al. Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans R Soc Trop Med Hyg 1995 Nov–Dec; 89(6): 672–6
Carter JY, Loolpapit MP, Lema OE, et al. Reduction of the efficacy of antifolate antimalarial therapy by folic acid supplementation. Am J Trop Med Hyg 2005 Jul; 73(1): 166–70
Ouma P, Parise ME, Hamel MJ, et al. A randomized controlled trial of folate supplementation when treating malaria in pregnancy with sulfadoxine-pyrimethamine. PLoS Clin Trials 2006 Oct 20; 1(6): e28
Mbaye A, Richardson K, Balajo B, et al. Lack of inhibition of the anti-malarial action of sulfadoxine-pyrimethamine by folic acid supplementation when used for intermittent preventive treatment in Gambian primigravidae. Am J Trop Med Hyg 2006 Jun; 74(6): 960–4
Ip S, Chung M, Kulig J, et al. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics 2004 Jul; 114(1): el30–53
Ostrow JD, Pascolo L, Shapiro SM, et al. New concepts in bilirubin encephalopathy. Eur J Clin Invest 2003 Nov; 3(11): 988–97
Odell GB. The dissociation of bilirubin from albumin and its clinical implications. J Pediatr 1959 Sep; 55: 268–79
Johnson L, Garcia ML, Figueroa E, et al. Kernicterus in rats lacking glucuronyl transferase: II. Factors which alter bilirubin concentration and frequency of kernicterus. Am J Dis Child 1961 Mar; 101: 322–49
Schutta HS, Johnson L. Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J Pediatr 1969 Dec; 75(6): 1070–9
Blanc WA, Johnson L. Studies on kernicterus; relationship with sulfonamide intoxication, report on kernicterus in rats with glucuronyl transferase deficiency and review of pathogenesis. J Neuropathol Exp Neurol 1959 Jan; 18(1): 165–87; discussion 87–9
Silverman WA, Andersen DH, Blanc WA, et al. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics 1956 Oct; 18(4): 614–25
Maisonneuve H, Faber C, Piens MA, et al. Congenital toxoplasmosis. Tolerability of the sulfadoxine-pyrimethamine combination. 24 cases [in French]. Presse Med 1984 Mar 31; 13(14): 859–62
McLeod R, Mack D, Foss R, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Toxoplasmosis Study Group. Antimicrob Agents Chemother 1992 May; 36(5): 1040–8
Guerina NG, Hsu HW, Meissner HC, et al. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 1994 Jun 30; 330(26): 1858–63
Peyron F, Wallon M, Bernardoux C. Long-term follow-up of patients with congenital ocular toxoplasmosis. N Engl J Med 1996 Apr 11; 334(15): 993–4
Villena I, Aubert D, Leroux B, et al. Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Reims Toxoplasmosis Group. Scand J Infect Dis 1998; 30(3): 295–300
Wallon M, Kodjikian L, Binquet C, et al. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 2004 Jun; 113(6): 1567–72
McLeod R, Boyer K, Karrison T, et al. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-Based, Congenital Toxoplasmosis Study. Clin Infect Dis 2006 May 15; 42(10): 1383–94
Hohlfeld P, Daffos F, Thulliez P, et al. Fetal toxoplasmosis: outcome of pregnancy and infant follow-up after in utero treatment. J Pediatr 1989 Nov; 115 (5 Pt 1): 765–9
Heckel GP. Chemotherapy in pregnancy. JAMA1941; 117(16): 1314–6
Ginzler AM, Cherner C. Toxic manifestations in the newborn infant following placental transmission of sulfanilamide: with a report of 2 cases simulating erythroblastosis fetalis. Am J Obstet Gynecol 1942; 44: 46–55
Dunn PM. The possible relationship between the maternal administration of sulphamethoxypyridazine and hyperbilirubinaemia in the newborn. J Obstet Gynaecol Br Commonw 1964 Feb; 71: 128–31
Kantor HI, Sutherland DA, Leonard JT, et al. Effect on bilirubin metabolism in the newborn of sulfisoxazole administered to the mother. Obstet Gynecol 1961 Apr; 17: 494–500 501
Morgan AD, Wenger NK. Sulfadiazine prophylaxis against rheumatic fever during pregnancy: its safety as regards the infant. J Med Assoc Ga 1965 May; 54: 153–5
Baskin CG, Law S, Wenger NK. Sulfadiazine rheumatic fever prophylaxis during pregnancy: does it increase the risk of kernicterus in the newborn? Cardiology 1980; 65(4): 222–5
Little PJ. The incidence of urinary infection in 5000 pregnant women. Lancet 1966 Oct 29; 2(7470): 925–8
Bailey RR. Single-dose antibacterial treatment for bacteriuria in pregnancy. Drugs 1984 Feb; 27(2): 183–6
Clyde DF, Press J, Shute GT. Transfer of pyrimethamine in human milk. J Trop Med Hyg 1956 Dec; 59(12): 277–84
Kauffman RE, O’Brien C, Gilford P. Sulfisoxazole secretion into human milk. J Pediatr 1980 Nov; 97(5): 839–41
American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001 Sep; 108(3): 776–89
Nahlen BL. Rolling back malaria in pregnancy. N Engl J Med 2000 Aug 31; 343(9): 651–2
Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed Regist 1993 Jul 22; 58(139): 39406–16
Merton V. The exclusion of pregnant, pregnable, and once-pregnable people (a.k.a. women) from biomedical research. Am J Law Med 1993; 19(4): 369–451
Mastroianni AC. HIV, women, and access to clinical trials: tort liability and lessons from DES. Duke J Gend Law Policy 1998 Spring; 5(1): 167–91
Simooya O. The WHO ‘Roll Back Malaria Project’: planning for adverse event monitoring in Africa. Drug Saf 2005; 28(4): 277–86
Acknowledgements
The authors have no conflicts of interest that are directly relevant to the content of this manuscript. No sources of funding were used in the preparation of this review. The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The authors are grateful to Dr Laurence Slutsker for helpful comments on drafts of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, P.J., Thigpen, M.C., Parise, M.E. et al. Safety and Toxicity of Sulfadoxine/Pyrimethamine. Drug-Safety 30, 481–501 (2007). https://doi.org/10.2165/00002018-200730060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200730060-00003