Abstract
While cardiac functional recovery is attenuated in the elderly following cardiac surgery with obligatory global myocardial ischemia/reperfusion (I/R), the underlying mechanism remains incompletely understood. We observed previously that human and mouse myocardium releases heat shock protein (HSP) 27 during global I/R. Extracellular HSP27 induces myocardial inflammatory response and plays a role in postischemic cardiac dysfunction in adult mouse hearts. This study was to determine the role of extracellular HSP27 and Toll-like receptor 4 (TLR4) in the attenuated functional recovery in aging mouse hearts following global I/R. Hearts isolated from aging (18–24 months) and adult (4–6 months) mice were subjected to ex vivo global I/R. Augmented release of HSP27 in aging hearts was associated with greater production of cytokines (TNF-α and IL-1β) and worse functional recovery. Anti-HSP27 suppressed the inflammatory response and markedly improved functional recovery in aging hearts. Perfusion of recombinant HSP27 to aging hearts resulted in greater cytokine production and more severe contractile depression in comparison to adult hearts. TLR4 deficiency abolished cytokine production and functional injury in aging hearts exposed to recombinant HSP27. Interestingly, aging hearts had higher TLR4 protein levels and displayed enhanced TLR4-medi-ated NF-κB activation following HSP27 stimulation or I/R. Extracellular HSP27 and TLR4 jointly enhance the inflammatory response and hamper functional recovery following I/R in aging hearts. The enhanced inflammatory response to global I/R and attenuated postischemic functional recovery in aging hearts are due, at least in part, to augmented myocardial release of HSP27 and elevated myocardial TLR4 levels.
Similar content being viewed by others
Introduction
Cardiac surgery is frequently performed in elderly patients for treatment of ischemic heart disease and calcific aortic valve disease. Myocardial injury and associated perioperative morbidities, including stroke and renal failure, are more common in elderly patients following cardiac surgery performed with the aid of cardiopulmonary bypass that obligates global myocardial ischemia and reperfusion (I/R) (1–3). In animal models of global myocardial I/R, cardiac functional recovery is reduced in aging hearts (4–6). While previous work on animal models suggests that a multifactorial mechanism may be involved in the exaggerated cardiac dysfunction in aging hearts (7,8), the mechanism responsible for the worsened outcome in the elderly after myocardial global I/R is incompletely understood (9). Further investigations are needed to improve the understanding of the underlying mechanism and to identify therapeutic targets for improving postischemic functional recovery in aging hearts.
Cardiac surgery with global myocardial I/R induces an inflammatory response in the myocardium characterized by the production and release of proinflammatory mediators (10–15). Pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), are known to contribute to cardiac dysfunction caused by global I/R (16–18). Further, worsened myocardial injury after I/R in aging hearts is associated with enhanced myocardial production of proinflammatory mediators (19), the mechanism by which aging exaggerates the myocardial inflammatory response to I/R remains unclear.
Toll-like receptors (TLRs), functioning as “danger detectors” in the cellular response to stress and injury, can be activated by danger-associated molecular patterns (DAMPs) (20). Both TLR2 and TLR4 have been found to play a role in the myocardial inflammatory response to regional I/R (21,22) and global I/R (16,23). Our previous studies found that TLR4, particularly myocardial tissue TLR4, plays a major role in mediating myocardial expression of proinflammatory mediators in response to global I/R (16,24). Furthermore, cardiodepressant cytokines TNF-α and IL-1β link TLR4 to postischemic cardiac dysfunction (16).
A number of endogenous proteins, including heat shock proteins (HSPs), high mobility group box 1 (HMGB1) and cardiac myosin, can function as DAMPs to activate myocardial TLR4 when they are released or secreted into the extracellular spaces (25–28). In this regard, increased circulating levels of HSP70 have been reported in patients after cardiac surgery with cardiopulmonary bypass (29). We recently observed that human hearts release HSP27 (termed HSP25 in rodents) following global I/R, and this small stress protein can induce an inflammatory response in cardiac microvascular endothelial cells (ECs) through a mechanism involving TLR4 (30). It is possible that exaggerated activation of TLRs by DAMPs is involved in the mechanism underlying myocardial inflammatory response and cardiac dysfunction caused by global myocardial I/R in aging hearts.
We hypothesize that aging enhances myocardial DAMP release and/or TLR4 function to augment the myocardial inflammatory response and thereby to hamper cardiac functional recovery after global myocardial I/R. The present study was undertaken to determine: 1) the effect of aging on myocardial release of HSP27 following global I/R; 2) the role of TLR4 and extracellular HSP27 in myocardial production of proinflammatory cytokines and cardiac functional injury in aging hearts; and 3) whether aging has an impact on myocardial TLR4 levels and/or activity.
Materials and Methods
Chemicals and Reagents
Recombinant HSP27 (98% homology to rodent HSP25), a low-endotoxin preparation, was purchased from Assay Designs. This preparation contains a trace amount of endotoxin, less than 2.0 pg/µg protein, as measured by Limulus assay. The highest concentration of recombinant HSP27 used in the isolated heart experiments was 2.0 µg of protein/mL. Thus, endotoxin concentrations in all experiments were lower than 4.0 pg/mL. We have confirmed that heat-denature of HSP27 in this preparation abolishes its effect on cytokine production in hearts (30).
A rabbit polyclonal antibody against HSP27 (recognizing rodent HSP25) was purchased from Assay Designs. An assay kit for NF-κB p65 DNA-binding activity was purchased from Active Motif. A rabbit polyclonal antibody against mouse TLR4 was purchased from Santa Cruz Biotechnology. Cytokine enzyme-linked immunosorbent assay (ELISA) kits were purchased from R & D Systems. Other reagents were purchased from Sigma Chemical Co.
Animals and Isolated Heart Perfusion
Male C3H/HeJ (TLR4-deficient, expressing dysfunctional TLR4) were purchased from Jackson Laboratory, and male C3H/HeN (TLR4-competent, wild-type) mice were purchased from Charles River Laboratories. Two age groups of mice, 4–6 months (adult) and 18–24 months (aging), were used. This investigation was approved by the Animal Care and Research Committee of the University of Colorado Denver, and it conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996). Mice were anesthetized and anticoagulated with pentobarbital (60 mg/kg, Abbot Laboratories) and heparin (1000 units/kg, Elkins-Sinn) before heart isolation. Isolated hearts were perfused with Krebs-Henseleit solution, pH 7.4, using the isovolumetric Langendorff technique as described previously (16,31). For global myocardial I/R experiments, hearts were equilibrated by perfusion of the Krebs-Henseleit solution for 20 min, then subjected to 20 min of normothermic global ischemia and 60 min reperfusion. An ultrathin latex balloon was inserted into the left ventricle, and the balloon volume was adjusted to achieve left ventricular end-diastolic pressure of 8–12 mmHg during the initial equilibration. Pacing wires were fixed to the right atrium and all hearts were paced at 450 beats/min. Left ventricular developed pressure (LVDP) was recorded continuously during the experiment with a computerized pressure amplifier/digitizer. Myocardial tissue was collected at the end of reperfusion to assess cytokine levels.
For anti-HSP27 experiments, hearts were perfused with anti-HSP27 (1.0 µg/mL in coronary circulation) or isotype-matching non-immune IgG (1.0 µg/mL in coronary circulation) for 10 min before ischemia and for 20 min after initiation of reperfusion. For HSP27 perfusion experiments, hearts were perfused with recombinant HSP27 (0–2.0 µg/mL in coronary circulation) for 30 min followed by washout for 60 min.
Endothelial Cell Isolation and Treatment
Mouse cardiac microvascular ECs were isolated using a previously reported method (32). Briefly, beating hearts were immersed in ice-cold calcium-free phosphate-buffered saline (PBS), and dipped into 70% ethanol to devitalize epicardial mesothelial cells and endocardial endothelial cells. Ventricular tissue was minced into fine pieces, and digested in nominally calcium-free Hank’s balanced salt solution (HBSS) supplemented with collagenase II (1.0 mg/mL), glucose (2.0 mg/mL), taurine (2.5 mg/mL), bovine serum albumin (BSA, 0.1%), and MgCl2 (1.4 mmol/L). Then, the tissue was digested in a solution containing 0.125% trypsin, 0.1 mmol/L EDTA and 2.0 mg/mL glucose dissolved in HBSS. Cells were separated from tissue debris and remaining myocytes by spinning at 27 × g for 5 min. The supernatant was centrifuged at 155 × g rpm (4°C) for 8 min to collect endothelial cells. Cells from 3–4 hearts were pooled and resuspended in 10 mL Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal cattle serum, penicillin (50 U/mL) and streptomycin (50 mg/mL). Cells were seeded in 24-well plates and cultured at 37°C for 2 h. Nonattached cells were removed. Experiments were performed using cultures of 90% confluence.
ECs isolated from adult and aging WT hearts were subjected to hypoxia (37°C, 1 h). Control cells were incubated in a normoxic condition. At the end of the experiment, culture media was collected for analysis of extracellular HSP27, and cells are stained with specific antibody to localize HSP27.
Elisa
HSP27 in coronary effluent and cell culture media, as well as TNF-α and IL-1β in myocardial homogenate, were analyzed using ELISA kits as described previously (16,30,33). Recombinant proteins were used to construct standard curves. Absorbance of standards and samples was determined spectrophotometrically at 450 nm using a microplate reader (Bio-Rad). Results were plotted against the standard curve.
Immunoblotting
Myocardial levels of TLR4 protein were determined by immunoblotting as described previously (16) using a rabbit polyclonal antibody against mouse TLR4 (1:1000 dilution).
Immunofluorescence Staining
Immunofluorescence staining of HSP27 was performed to examine HSP27 distribution in cardiac microvascular ECs. Cells were treated with a mixture of 30% acetone and 70% methanol for 5 min, washed with PBS and fixed with 4% paraformaldehyde. Then cells were incubated with a rabbit polyclonal antibody against HSP27, followed by Cy3-tagged goat anti-rabbit IgG (imaged on the red channel). Nuclei were stained with bis-benzimide (DAPI, imaged on the blue channel) and glycoproteins on cell surfaces with Alexa 488-tagged wheat germ agglutinin (imaged on the green channel). Microscopy was performed with a Leica DMRXA digital microscope (Leica Mikroskopie and System GmbH).
NF-κB Activity Assay
NF-κB p65 DNA-binding activity in myocardial homogenate was analyzed using a transcription factor assay kit as described previously (31). This quantitative assay is based on the specific binding of the active form of NF-κB to a consensus oligonucleotide attached to the plate.
Statistics
Data are expressed as mean ± standard error (SE). Comparisons between groups were performed using StatView software (Abacus Concepts) with one-way analysis of variance (ANOVA) with the post hoc Fisher test. A difference was considered significant at P < 0.05. Significant differences were confirmed with Mann-Whitney non-parametric test.
Results
Augmented Release of HSP27 Contributes to the Mechanism of Enhanced Cytokine Response and Attenuated Functional Recovery in Aging Hearts
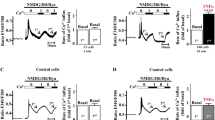
We recently observed that human and mouse myocardium releases HSP27 during early reperfusion following global I/R (30). Further, extracellular HSP27 up-regulates cytokine production in murine cardiac microvascular ECs and human coronary artery ECs (30). In the present study, we examined the impact of aging on cardiac release of HSP27 during I/R. As shown in Figure 1A, HSP27 release was detected immediately after the initiation of reperfusion, and the levels of HSP27 in coronary effluent were high during the first 5 min of reperfusion and declined thereafter. Compared to adult hearts, aging hearts released higher levels of HSP27 at all time points examined (Figure 1A).
Aging hearts release higher levels of HSP27. A) Hearts from adult (4–6 months) and aging (18–24 months) mice were subjected to global ischemia/reperfusion (I/R: I 20 min/R 60 min). Coronary effluent is collected before ischemia and at 0–5 min or 55–60 min of reperfusion, and heat shock protein 27 (HSP27) was analyzed. HSP27 release occurred primarily in early reperfusion, and aging hearts released higher levels of HSP27. Data are expressed as mean ± SE. n = 8; aP < 0.05 versus baseline; bP < 0.05 versus time-matched adult I/R. B) Cardiac microvascular endothelial cells isolated from adult and aging mice were subjected hypoxia (95% nitrogen and 5% oxygen) for 1 h. A representative image shows that HSP27 distribution is changed from a diffused pattern to a granular pattern following hypoxia and that HSP27 is localized on the cell membranes. ELISA data show that cells from aging hearts release a greater amount of HSP27 during hypoxia. Data are expressed as mean ± SE. n = 5; aP < 0.05 versus normoxia; bP < 0.05 versus adult hypoxia.
In vitro experiments revealed that cardiac microvascular ECs secrete HSP27 in response to hypoxia. The representative image in Figure 1B shows that HSP27 distribution is changed from a diffused pattern to a granular pattern when cells are subjected to hypoxia and that HSP27 is localized on the cell membranes. ELISA assay revealed that cells from aging mice secrete a greater amount of HSP27 during hypoxia (Figure 1B).
To determine the role of extracellular HSP27 in the attenuated postischemic functional recovery in aging hearts, we assessed the effect of polyclonal antibody against HSP27 on LVDP during I/R. Anti-HSP27 markedly improved cardiac functional recovery in aging hearts following ischemia and diminished the age-related difference in cardiac functional recovery (Figure 2A). However, cardiac functional recovery in the IgG + I/R group was not different from that of I/R alone (not shown). Anti-HSP27 also reduced myocardial levels of TNF-α and IL-1β in both adult and aging hearts. Noticeably, it had a greater effect on aging hearts, markedly reducing the age-related differences in myocardial cytokine levels (Figure 2B). These results support the notion that augmented myocardial release of HSP27 in aging hearts enhances the inflammatory response and interferes with the recovery of contractile function after global I/R.
Extracellular HSP27 mediates postischemic inflammatory response and functional injury in aging hearts. Hearts from adult and aging mice were subjected to global ischemia/reperfusion (I/R: I 20 min/R 60 min). Hearts were treated with a polyclonal antibody against heat shock protein 27 (HSP27 Ab, 1.0 µg/mL) or control IgG (IgG, 1.0 µg/mL) for 10 min before ischemia and for 20 min after initiation of reperfusion. A) Anti-HSP27 improved cardiac functional recovery in both adult and aging hearts, but had a greater effect in aging hearts. Data are expressed as mean ± SE. n = 7; aP < 0.05 versus adult IgG + I/R; bP < 0.05 versus aging IgG + I/R. B) Anti-HSP27 reduced myocardial cytokine levels in adult and aging, and the effect was greater in aging hearts. Data are expressed as mean ± SE. n = 7; aP < 0.05 versus age-matched control; bP < 0.05 VS. adult I/R or IgG + I/R; cP < 0.05 versus age-matched IgG + I/R.
Extracellular HSP27 Has a Greater Impact on Aging Hearts
To confirm the effect of extracellular HSP27 on myocardial cytokine production and cardiac contractile function, we perfused recombinant HSP27 to isolated mouse hearts. Perfusion of HSP27 caused greater depression of cardiac contractility in aging hearts (Figures 3A, B). It is noteworthy that TLR4 deficiency abrogated the effect of HSP27 perfusion on cardiac contractility in both adult and aging hearts (Figure 3B). Perfusion of HSP27 also induced greater production of TNF-α and IL-1β, and TLR4 deficiency abolished the effect of HSP27 on myocardial cytokine levels (Figure 3C). Thus, extracellular HSP27 is capable of inducing myocardial inflammatory response and cardiac contractile depression, and aging hearts have elevated reactivity to extracellular HSP27 in terms of cytokine production and contractile depression. TLR4 deficiency diminishes the reactivity to extracellular HSP27 in aging hearts.
Extracellular HSP27 exerts more profound proinflammatory and injurious effects on aging hearts through TLR4. Hearts isolated from TLR4-competent (WT) and TLR4-deficient (TLR4d) mice were perfused with recombinant HSP27 (1.0 or 2.0 µg/mL) for 30 min followed by 60 min washout. A) HSP27 dose-dependently depressed LVDP in both adult and aging WT hearts, but had a greater effect on aging hearts. Data are expressed as mean ± SE. n = 6 in each group; aP < 0.05 versus baseline (as 100%); bP < 0.05 versus dose-matched adult, cP < 0.05 versus age-matched hearts treated with a lower dose of HSP27. B) TLR4 deficiency essentially abolished the effect of HSP27 (2.0 µg/mL) on LVDP in both adult and aging hearts. Data are expressed as mean ± SE. n = 6 in each group; aP < 0.05 versus baseline (as 100%); bP < 0.05 versus time-matched adult WT. C) HSP27 dose-dependently upregulated myocardial production of cytokines in WT hearts, and aging WT hearts exhibited a greater response. TLR4 deficiency abolished the effect of HSP27 on myocardial cytokine production in both adult and aging hearts. Data are expressed as mean ± SE. n = 6 in each group; aP < 0.05 versus age/genotype-matched control; bP < 0.05 versus dose-matched adult, cP < 0.05 versus age-matched hearts treated with a lower dose of HSP27.
Elevated TLR4 Levels in Aging Hearts Result in Enhanced NF-κB Activation in Response to Extracellular HSP27 and I/R
To understand whether increased response to extracellular HSP27 in aging hearts is due to altered myocardial TLR4 levels, we analyzed myocardial TLR4 protein in adult and aging hearts. Interestingly, aging hearts had higher levels of TLR4 protein in comparison to adult hearts (Figure 4A). To examine whether elevated TLR4 levels in aging hearts result in enhanced TLR4 signaling, we analyzed myocardial NF-κB DNA-binding activity following perfusion of HSP27 or I/R in adult and aging hearts. As shown in Figures 4B and 4C, both HSP27 and I/R induced greater NF-κB activation in aging hearts. TLR4 deficiency abrogated NF-κB activation in both adult and aging hearts following exposure to HSP27 or I/R. Thus, extracellular HSP27 and I/R induce myocardial NF-κB activation through TLR4. Elevated TLR4 levels in aging hearts results in enhanced NF-κB response to extracellular HSP27 or I/R.
Increased myocardial TLR4 levels in aging hearts is associated with enhanced TLR4-mediated NF-κB activation. A) Left ventricular tissue was collected from hearts of untreated 5 adult and 4 aging mice. A representative immunoblot shows higher myocardial TLR4 protein levels in aging hearts. B) Hearts isolated from adult and aging TLR4-competent (WT) and TLR4-deficient (TLR4d) mice were perfused with recombinant HSP27 (1.0 or 2.0 µg/mL) for 30 min followed by 20 min washout. Aging WT hearts exhibited greater myocardial NF-κB activation in comparison to adult WT hearts. TLR4 deficiency abrogated the effect of HSP27 on myocardial NF-κB DNA-binding activity in both adult and aging hearts. Data are expressed as mean ± SE. n = 6 in each group, aP < 0.05 versus age/genotype-matched control, bP < 0.05 versus adult WT treated with the same dose of HSP27; cP < 0.05 versus age-matched WT treated with a lower dose of HSP27. C) Hearts isolated from adult and aging TLR4-competent (WT) and TLR4-deficient (TLR4d) mice were subjected to 20 min of ischemia followed by 20 min of reperfusion. Aging WT hearts exhibited greater myocardial NF-κB activation following I/R in comparison to adult WT hearts. TLR4 deficiency greatly reduced myocardial NF-κB DNA-binding activity in aging hearts and abrogated the age-related difference in postischemic NF-κB activity. Data are expressed as mean ± SE. n = 6 in each group, aP < 0.05 versus age/genotype-matched control, bP < 0.05 versus adult WT I/R; cP < 0.05 versus aging WT I/R.
Discussion
It has been reported that global myocardial I/R induces an inflammatory response in human myocardium (10–14,34), and proinflammatory cytokines appear to play a role in cardiac complications associated with surgeries that obligate global myocardial I/R (17,18). Further, patients over age 65 suffer greater morbidity and mortality than younger cohorts following coronary artery bypass grafting or heart valve surgery. While higher circulating levels of IL-6 and IL-8 were found after coronary artery bypass grafting in patients over age 65 than in younger patients (15,35), the mechanism underlying the enhanced myocardial inflammatory response remains to be determined. Furthermore, global myocardial I/R has a more significant impact on cardiac function in elder patients undergoing cardiac surgery (36) and in aging animal hearts (4–6). The underlying mechanism is not fully understood.
In the present study, we determined the role of extracellular HSP27 and TLR4 in myocardial inflammatory response and cardiac functional injury caused by global I/R in aging hearts. The results show that augmented release of HSP27 in aging hearts is responsible for greater production of cytokines and attenuated functional recovery. TLR4 is involved in mediating the proinflammatory and injurious effects of extracellular HSP27 in both adult and aging hearts. However, TLR4 has a greater role in aging hearts due to an elevation of this innate immunoreceptor levels in the myocardium. It appears that targeting myocardial TLR4 is a potential therapeutic approach for suppression of myocardial inflammatory response and improvement of cardiac functional recovery in aging hearts subjected to global I/R.
Extracellular HSP27 Plays a Role in the Enhanced Inflammatory Response and Attenuated Functional Recovery in Aging Hearts
A number of cell types are capable of secreting HSP (37,38). In a recent study, we observed that human and mouse myocardium releases HSP27 in response to ischemia, and extracellular HSP27 evokes an inflammatory response in cardiac vascular ECs (30). We observed augmented release of HSP27 in aging murine hearts and cardiac microvascular ECs from aging hearts. It appears that hypoxia associated with ischemia is a factor that causes HSP27 release from the myocardium as hypoxia alone is sufficient to cause the release of this small HSP. While the cells that release HSP27 during I/R in the intact heart with heterogeneity of cell types remain unclear from the present study, the results show that cardiac microvascular cells are capable of releasing HSP27 in response to hypoxia. It is possible that secretion via exosomes is involved as HSP27 migrates to cell membranes in a form of granular structure. In this regard, secretion of HSPs via exosomes has been reported in several cell types (37). Future studies are needed to examine what cell types are involved in myocardial release of HSP27 in the intact heart and to determine the cellular mechanism underlying HSP27 release by cells.
Interestingly, augmented release of HSP27 in aging hearts is associated with enhanced myocardial inflammatory response and attenuated cardiac functional recovery following global I/R. Two lines of evidence suggest an important role of extracellular HSP27 in the mechanism underlying the enhanced myocardial inflammatory response and attenuated cardiac functional recovery in aging hearts. First, anti-HSP27 has a greater effect in the suppression of myocardial cytokine production and in the improvement of cardiac functional recovery in aging hearts subjected to global I/R. In addition, perfusion of recombinant HSP27 has a more profound effect in upregulation of myocardial cytokine production and depression of cardiac contractility in aging hearts.
A number of studies provide evidence that proinflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-18, contribute to the mechanisms of cardiac dysfunction caused by global myocardial I/R and other injurious insults (16,39–43). We previously found that knockout of TNF-α or IL-1β improves cardiac functional recovery in a mouse heart model of global I/R (16). In addition, we observed that TLR4 deficiency or knockout reduces myocardial production of TNF-α and IL-1β, and protects the heart against postischemic dysfunction in the same model (16). Thus, proinflammatory cytokines, particularly TNF-α and IL-1β, are cardiodepressants and play a mechanistic role in hampering cardiac functional recovery following global myocardial I/R. The results of the present study show that enhanced myocardial production of TNF-α and IL-1β in aging hearts correlates with attenuated postischemic cardiac functional recovery. Suppression of myocardial cytokine production with anti-HSP27 improves cardiac functional recovery in aging hearts. Conversely, upregulated myocardial production of proinflammatory cytokines by perfusion of recombinant HSP27 reduces cardiac contractility in aging hearts. Thus, extracellular HSP27 has proinflammatory and injurious effects in the myocardium. The worsened postischemic cardiac functional recovery in aging hearts following global I/R is due to, at least partly, the enhanced myocardial cytokine production mediated by extracellular HSP27.
Several studies demonstrate that HSP27 has a protective role in the cardiovascular system. In this regard, overexpression of HSP27 protects cardiac myocytes against ischemic injury (44) and cardiac-specific expression of HSP27 protects against endotoxin-induced cardiac dysfunction (45). However, HSP27 exerts a protective effect when located in the cell. Intracellular HSP27 is known to activate the phosphatidylinositol 3-kinase/Akt pathway (46), and this survival pathway plays an important role in mediating the cardioprotective effect of intracellular HSP27 (45). Recent data indicate that HSP27 is also found in the extracellular space and the abundance of HSP27 in circulation is an emerging biomarker for ischemic events (47). Here we demonstrated that extracellular HSP27 is proinflammatory and detrimental, particularly in aging hearts undergoing I/R. It is likely that extracellular HSP27 functions as a DAMP to exaggerate myocardial injury through upregulation inflammatory responses.
TLR4 Plays an Important Role in Mediating the Effect of Extracellular HSP27 in Aging Hearts
In the present study, we evaluated the role of TLR4 in myocardial inflammatory response and functional injury caused by extracellular HSP27 in aging hearts. Our results show that TLR4 deficiency reduces the effects of extracellular HSP27 on myocardial production of proinflammatory cytokines and cardiac contractility. Interestingly, the hyperresponsiveness to extracellular HSP27 in aging hearts is abrogated by TLR4 deficiency. Therefore, TLR4 is responsible for the aging-related hyper-responsiveness to extracellular HSP27.
The role of TLR4 in myocardial I/R injury is well recognized (48). However, the mechanism by which I/R activates TLRs remains elusive. Several endogenous factors including HSP60 (26,49), HSP70 (25,50), constitutive heat shock cognate protein HSC70 (24,31), cold-inducible RNA-binding protein (51), HMGB1 (27,52) and cardiac myosin (28) have been reported to activate TLR4 in cell culture systems or in tissues. Whereas intracellular HSP27 activates the phosphatidylinositol 3-kinase/Akt pathway to exert a protective effect against myocardial injury (45), extracellular HSP27 appears to be an endogenous TLR4 activator in the myocardium and plays a role in the enhanced myocardial inflammatory response to I/R in aging hearts. The findings are consistent with our recent observation that extracellular HSP27 activates NF-κB and evokes an inflammatory response in cardiac microvascular ECs through a mechanism involving TLR4 (30). The hyper-responsiveness of aging hearts to extracellular HSP27 indicates that the level of this innate immunoreceptor in the myocardium or its signaling efficiency may be altered by aging.
Elevated Levels of Myocardial TLR4 Contributes to the Hyper-Responsiveness to Extracellular HSP27 and I/R in Aging Hearts
To date, the effects of aging on myocardial TLR4 expression and signaling are unclear. We found that myocardial levels of TLR4 protein are increased in aging hearts. Moreover, aging hearts exhibit greater TLR4-dependent NF-κB activation in response to HSP27 or I/R. This finding may explain the hyper-responsiveness of aging hearts to extracellular HSP27 and I/R.
Several studies have sought to determine impact of aging on TLR4 expression. Elevated expression of TLR4 in skeletal muscle has been reported in old, healthy people (53). More interestingly, senescence accelerated-prone (SAMP8) mice display elevated expression of TLR4 in the heart (54). However, the studies on TLR4 expression in peripheral monocytes or macrophages have resulted in inconsistent findings (55–57). Nevertheless, it appears that activation of selected proinflammatory signaling pathways and production of proinflammatory cytokines in response to injurious insults are paradoxically enhanced in aging subjects while innate immune responses are attenuated (58).
Our findings suggest that an elevated level of myocardial TLR4 is a contributing factor in aging-related worsened recovery of cardiac function following global I/R. In this regard, elevated expression of TLR4 in monocytes and atrial muscle has been correlated to cardiac dysfunction in patients undergoing coronary artery bypass surgery (59). However, the factors that influence myocardial TLR4 levels in aging hearts remain unknown from the present study. In mouse lungs, oxidants have been found to increase tissue TLR4 mRNA levels through mRNA stabilization (60). A similar mechanism may be operative in aging hearts as antioxidant capacity is attenuated and oxidant generation is enhanced in aging hearts (8). It should be noted that TLR4 signaling, as measured by NF-κB activation, is markedly enhanced in aging hearts while myocardial TLR4 levels are moderately increased. It appears that other mechanisms also contribute to the enhanced TLR4 signaling in aging hearts. Future studies are needed to identify the factors that upregulate TLR4 expression and/or signaling in the aging heart.
Conclusion
The results of the present study show that: 1) attenuated functional recovery after global I/R in aging mouse hearts is associated with augmented release of HSP27 and enhanced myocardial cytokine response; 2) extracellular HSP27 induces myocardial inflammatory response and depresses cardiac contractility through TLR4; 3) aging hearts have increased TLR4 levels and display enhanced TLR4 signaling in response to a stimulation, 4) TLR4 occupies a major role in the enhanced inflammatory response and greater contractile depression induced by extracellular HSP27 in aging hearts. It appears that aging augments myocardial release of HSP27 during global I/R and alters TLR4-mediated myocardial immunoresponse, resulting in exaggerated production of cardiodepressant cytokines and attenuated cardiac functional recovery (Figure 5). Targeted regulation of myocardial TLR4 signaling may have therapeutic potential for improving postischemic cardiac functional recovery in aging hearts.
Schematic diagram depicting the mechanism underlying worsened recovery of cardiac function in aging hearts following global I/R. Myocardial I/R causes the release of HSP27, and extracellular HSP27 is one of the factors that activate myocardial TLR4. Aging exaggerates myocardial inflammatory responses to I/R through enhancing HSP27 release and elevating myocardial TLR4 levels, resulting in attenuated recovery of contractile function.
Disclosure
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Filsoufi F, et al. (2007) Results and predictors of early and late outcomes of coronary artery bypass graft surgery in octogenarians. J. Cardiothorac. Vasc. Anesth. 21:784–92.
Kolh P, Kerzmann A, Honore C, Comte L, Limet R. (2007) Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur. J. Cardiothorac. Surg. 31:600–6.
Ngaage DL, Cowen ME, Griffin S, Guvendik L, Cale AR. (2008) Early neurological complications after coronary artery bypass grafting and valve surgery in octogenarians. Eur. J. Cardiothorac. Surg. 33:653–9.
Liu P, Xu B, Cavalieri TA, Hock CE. (2002) Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc. Res. 56:443–53.
McCully JD, et al. (2006) Age-and gender-related differences in ischemia/reperfusion injury and cardioprotection: effects of diazoxide. Ann. Thorac. Surg. 82:117–23.
Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. (2005) Age-related changes in ischemic tolerance in male and female mouse hearts. J. Mol. Cell. Cardiol. 38:245–56.
Kostyak JC, Hunter JC, Korzick DH. (2006) Acute PKCS inhibition limits ischaemia-reperfusion injury in the aged rat heart: Role of GSK-3β. Cardiovasc. Res. 70:325–34.
Liu P, Xu B, Cavalieri TA, Hock CE. (2004) Attenuation of antioxidative capacity enhances reperfusion injury in aged rat myocardium after MI/R. Am. J. Physiol. Heart Circ. Physiol. 287:H2719–27.
Hannan EL, et al. (2006) Risk stratification of in-hospital mortality for coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 47:661–8.
Bronicki RA, Hall M. (2016) Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr. Crit. Care Med. 17:S272–8.
Meldrum DR, et al. (1998) Human myocardial tissue TNFa expression following acute global ischemia in vivo. J. Mol. Cell. Cardiol. 30:1683–9.
Narayan P, et al. (2011) On-pump coronary surgery with and without cardioplegic arrest: comparison of inflammation, myocardial, cerebral and renal injury and early and late health outcome in a single-centre randomised controlled trial. Eur. J. Cardiothorac. Surg. 39:675–83.
Stoppe C, et al. (2015) Interaction of MIF family proteins in myocardial ischemia/reperfusion damage and their influence on clinical outcome of cardiac surgery patients. Antioxid. Redox. Signal. 23:865–79.
Wei M, et al. (2001) Inflammatory cytokines and soluble receptors after coronary artery bypass grafting. Cytokine. 15:223–8.
Wei M, et al. (2003) Imbalance of pro-and anti-inflammatory cytokine responses in elderly patients after coronary artery bypass grafting. Aging Clin. Exp. Res. 15:469–74.
Cha J, et al. (2008) Cytokines link toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann. Thorac. Surg. 85:1678–85.
Suleiman MS, Zacharowski K, Angelini G. (2008) Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br. J. Pharmacol. 153:21–33.
Tomasdottir H, et al. (2003) Tumor necrosis factor gene polymorphism is associated with enhanced systemic inflammatory response and increased cardiopulmonary morbidity after cardiac surgery. Anesth. Analg. 97:944–9.
Yabluchanskiy A, et al. (2016) Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J. Gerontol. A. Biol. Sci. Med. Sci. 71:475–83.
Miyake K. (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 19:3–10.
Chong AJ, et al. (2004) Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J. Thorac. Cardiovasc. Surg. 128:170–9.
Shimamoto A, et al. (2006) Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 114:I–270–4.
Kaczorowski DJ, et al. (2007) Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 84:1279–87.
Ao L, Zou N, Cleveland JC, Fullerton DA, Meng X. (2009) Myocardial TLR4 is a determinant of neutrophil infiltration after global myocardial ischemia: mediating KC and MCP-1 expression induced by extracellular HSC70. Am. J. of Physiol. Heart Circ. Physiol. 297:H21–8.
Asea A, et al. (2002) Novel signal transduction pathway utilized by extracellular HSP70 role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028–34.
Li Y, et al. (2011) Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J. Biol. Chem. 286:31308–19.
Tsung A, et al. (2007) HMGB1 release induced by liver ischemia involves Toll-like receptor 4-dependent reactive oxygen species production and calcium-mediated signaling. J. Exp. Med. 204:2913–23.
Zhang P, Cox CJ, Alvarez KM, Cunningham MW. (2009) Cutting edge: cardiac myosin activates innate immune responses through TLRs. J. Immunol. 183:27–31.
Dybdahl B, et al. (2002) Inflammatory response after open heart surgery release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 105:685–90.
Jin C, et al. (2014) Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol. Med. 20:280–9.
Zou N, et al. (2008) Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 294:H2805–13.
Li J-M, Mullen AM, Shah AM. (2001) Phenotypic properties and characteristics of superoxide production by mouse coronary microvascular endothelial cells. J. Mol. Cell. Cardiol. 33:1119–31.
Ao L, Song Y, Fullerton DA, Dinarello CA, Meng X. (2007) The interaction between myocardial depressant factors in endotoxemic cardiac dysfunction: role of TNF-α in TLR4-mediated ICAM-1 expression. Cytokine. 38:124–9.
Valen G, Paulsson G, Vaage J. (2001) Induction of inflammatory mediators during reperfusion of the human heart. Ann. Thorac. Surg. 71:226–32.
Howell KW, et al. (2016) Interleukin 6 production during cardiac surgery correlates with increasing age. J. Surg. Res. 201:76–81.
Lüss H, et al. (2002) Biochemical mechanisms of hibernation and stunning in the human heart. Cardiovasc. Res. 56:411–21.
Lancaster GI, Febbraio MA. (2005) Exosome-dependent trafficking of HSP70 A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280:23349–55.
Vega VL, et al. (2008) Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 180:4299–307.
Cain BS, et al. (1999) Tumor necrosis factor-alpha and interleukin-1 beta synergistically depress human myocardial function. Crit. Care Med. 27:1309–18.
Meng X, et al. (2005) Signaling for myocardial depression in hemorrhagic shock: roles of Tolllike receptor 4 and p55 TNF-α receptor. Am. J. Physiol. Regu. Integr. Comp. Physiol. 288:R600–6.
Pathan N, et al. (2011) Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit. Care Med. 39:1692–711.
Raeburn CD, et al. (2002) Neutralization of IL-18 attenuates lipopolysaccharide-induced myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 283:H650–7.
Wang M, et al. (2009) IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Proc. Natl. Acad. Sci. U.S.A. 106:17499–504.
Vander Heide RS. (2002) Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am. J. of Physiol. Heart Circ. Physiol. 282:H935–41.
You W, et al. (2009) Cardiac-specific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock. 32:108–17.
Havasi A, et al. (2008) Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J. Biol. Chem. 283:12305–13.
Batulan Z, et al. (2016) Extracellular release and signaling by heat shock protein 27: role in modifying vascular inflammation. Front. Immunol. 7:285.
Kay E, Scotland RS, Whiteford JR. (2014) Tolllike receptors: Role in inflammation and therapeutic potential. Biofactors. 40:284–94.
Ohashi K, Burkart V, Flohé S, Kolb. (200) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–61.
Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. (2011) Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ. J. 75:2445–52.
Qiang X, et al. (2013) Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 19:1489–95.
Kaczorowski DJ, et al. (2009) Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 87:1455.
Ghosh S, et al. (2015) Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J. Gerontol. A Biol. Sci. Med. Sci. 70:232–46.
Karuppagounder V, et al. (2016) Modulation of macrophage polarization and HMGB1-TLR2/TLR4 cascade plays a crucial role for cardiac remodeling in senescence-accelerated prone mice. PLoS One. 11:e0152922.
Boehmer ED, Goral J, Faunce DE, Kovacs EJ. (2004) Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 75:342–9.
Metcalf TU, et al. (2015) Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell. 14:421–32.
Van Duin D, Shaw AC. (2007) Toll-like receptors in older adults. J. Am. Geriatr. Soc. 55:1438–44.
Montgomery RR, Shaw AC. (2015) Paradoxical changes in innate immunity in aging: recent progress and new directions. J. Leukoc. Biol. 98:937–43.
Avlas O, et al. (2015) TLR4 expression is associated with left ventricular dysfunction in patients undergoing coronary artery bypass surgery. PLoS One. 10:e0120175.
Fan J, et al. (2002) Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J. Immunol. 168:5252–9.
Acknowledgments
This study was supported in part by National Institutes on Aging Grant AG039545.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (http://creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Ao, L., Zhai, Y., Jin, C. et al. Attenuated Recovery of Contractile Function in Aging Hearts Following Global Ischemia/Reperfusion: Role of Extracellular HSP27 and TLR4. Mol Med 22, 863–872 (2016). https://doi.org/10.2119/molmed.2016.00204
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2016.00204