Abstract
A severe burn leads to hypermetabolism and catabolism resulting in compromised function and structural changes of essential organs. The release of cytokines has been implicated in this hypermetabolic response. The severity of the hypermetabolic response following burn injury increases with age, as does the mortality rate. Due to the relationship between the hypermetabolic and inflammatory responses, we sought to compare the plasma cytokine profiles following a severe burn in adults and in children. We enrolled 25 adults and 24 children who survived a flame burn covering more than 20% of total body surface area (TBSA). The concentrations of 22 cytokines were measured using the Linco multiplex array system (St. Charles, MO, USA). Large perturbations in the expression of pro- and anti-inflammatory cytokines were seen following thermal injury. During the first week following burn injury, IFN-γ, IL-10, IL-17, IL-4, IL-6, and IL-8 were detected at significantly higher levels in adults compared with children, P < 0.05. Significant differences were measured during the second week post-burn for IL-1β (higher in children) and IL-5 (higher in adults), P < 0.05. IL-18 was more abundant in children compared with adults during the third week post-burn, P < 0.05. Between post-burn d 21 and d 66, IL-1α was detected at higher concentrations in pediatric compared with adult patients, P < 0.05. Only GM-CSF expression was significantly different at all time points; it was detected at lower levels in pediatric patients, P < 0.05. Eotaxin, G-CSF, IL-13, IL-15, IP-10, MCP-1, and MIP-1α were detected at significantly different concentrations in adult compared with pediatric patients at multiple time points, P < 0.05. There were no differences in IL-12, IL-2, IL-7, or TNF levels in adult compared with pediatric burn patients at any of these time points. Following severe flame burns, the cytokine profiles in pediatric patients differ compared with those in adult patients, which may provide insight with respect to the higher morbidity rate in adults. Furthermore, the dramatic discrepancies observed in plasma cytokine detection between children and adults suggest that these two patient populations may benefit from different therapeutic interventions to achieve attenuation of the post-burn inflammatory response.
Similar content being viewed by others
Introduction
The severity of the hypermetabolic response following burns correlates with age and may be a major contributor to the higher morbidity and mortality rates observed in adult burn patients compared with pediatric burn patients (1). We propose that the factor linking age and morbidity may be the inflammatory response. The systemic inflammatory response after a severe burn injury leads to hypermetabolism and thus to protein degradation and catabolism. The hypermetabolic state is characterized by futile protein utilization resulting in induction of a dynamic hypercatabolic state concurrent with altered cytokine expression (2,3). Perturbations in cytokine expression lead to altered immune function and protein metabolism, resulting in compromised function and structural alterations of multiple organ systems (2–6). The expression profile of potential therapeutic targets such as cytokines, may be crucial for determining the best time for intervention. Because the changes in cytokine levels occur prior to the changes in metabolism, it may be possible to modulate the post-burn hypermetabolic response by altering cytokine response, potentially improving morbidity and mortality in patients of advanced age or with large burns. We have published data previously demonstrating that pediatric patients exhibit a hyper-inflammatory response following burn that is characterized by large-scale release of both pro- and anti-inflammatory cytokines during the first wk post burn, followed by a return to normal concentrations over a five-wk period (7). Given the correlation between the hypermetabolic response, inflammatory mediator levels, and the overall inflammatory response, we sought to determine and compare the post-burn plasma cytokine profiles in adults and children following a severe burn. To further elucidate the differences in the pediatric compared with adult response to burn, as well as to identify a potential therapeutic window in adult patients, the aim of this study was to determine temporal cytokine abundance in plasma from severely burned adults, and to compare these profiles to those of pediatric burn patients.
Materials and Methods
Patients
This study was conducted as part of the larger federally-funded Inflammation and the Host Response to Injury Glue Grant. The study was approved by the Institutional Review Boards of the University of Texas Medical Branch (Galveston, TX, USA), Loyola University Medical College (Chicago, IL, USA), University of Texas Southwestern (Dallas, TX, USA), University of Washington Seattle (Seattle, WA, USA), and Massachusetts General Hospital (Boston, MA, USA). Between 2000 and 2007, 306 patients were enrolled and met the following inclusion criteria: 0–89 years of age, admitted to a participating hospital within 96 h after injury, and had burns covering more than 20% of total body surface area (TBSA) requiring at least one surgical intervention. Following admission, patients were treated according to the standard operating procedures for burn care established by the burn patient-oriented research core (published on www.gluegrant.org in September 2004 by the Inflammation and the Host Response to Injury Glue Grant investigators). Prior to participation, written informed consent was obtained from each subject or a family member. Demographics (age, date of burn, date of admission, gender, burn size, and depth of burn) and concomitant injuries (such as inhalation injury, infection, morbidity, and mortality) were prospectively recorded throughout the hospital course. Cytokine analysis was performed on plasma from the first 82 patients enrolled in the program. To compare the adult and pediatric inflammatory response, we selected all surviving patients (25 adults, 24 children) who sustained a flame burn over 20% of TBSA in adults and over 40% of TBSA in children to eliminate burn mechanism as a variable. The remaining 33 patients were excluded from the analysis because there were not enough patients to complete these studies (16 non-survivors, 6 scald burns, and 11 electrical/flash burns).
Sample Handling
Blood was drawn prior to each operative intervention for plasma isolation. Peripheral blood samples were collected and centrifuged at 22° C for 10 min, 400g, within 1 h of being drawn. Samples were frozen at −80° C and stored at the Inflammation and the Host Response to Injury Sample Collection and Coordination Site at the University of Florida College of Medicine, Gainesville, FL, USA.
Plasma Cytokine Measurement
Blood was collected from the burn patients prior to each operative intervention. For this analysis, samples were divided into the following time periods in days post-burn: 0–1 d, 2 d, 3–7 d, 8–14 d, 15–21 d, and 21–66 d. The first wk was divided into multiple time points because of the dramatic differences seen within this time period for pediatric burn patients (7). If a patient had more than one sample per time period, the values were averaged together and these numbers were used for the analysis. The number of total samples per time period post burn was as follows (adult/pediatric): d 0–1 (19/11), d 2 (17/12), d 3–7 (17/11), d 8–14 (14/17), d 15–21 (9/13), and d 22–66 (9/17). More blood was available for the pediatric patients at later time points because they were more severely burned, and, therefore, were in the ICU, returning to the OR much more frequently. Plasma cytokine concentrations were measured using the bead array assay kit from Linco Research (St. Charles, MO, USA), which measures 22 cytokines in a multiplex format. The concentrations of the individual analytes were determined using the MiraiBio software package (Hitachi, San Francisco, CA, USA).
Heart Rate as a Measurement of Hypermetabolism
Resting heart rates were recorded per the Inflammation and the Host Response to Injury protocol.
Statistics
To reduce the impact of biological variation inherent in human plasma samples, outliers greater than two standard deviations above or below the average were removed. For each cytokine/time point, no more than two samples were removed from the analysis. Unpaired Student t tests were used to compare differences in cytokine expression. Data are expressed as percentages or means ± standard error of the mean (SEM), where appropriate. Significance was accepted at P < 0.05.
Results
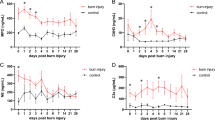
The demographic data for the two cohorts are shown in Table 1. The burn size and time to admission were significantly greater in the pediatric group compared with the adult group (Table 1, P < 0.001). Adult and pediatric heart rates were markedly elevated, implying that the patients were stressed and hypermetabolic throughout acute hospitalization (Figure 1). Although the adults were admitted within 0.5 d of burn, whereas the children were admitted within 1.5 d of the burn to the glue grant clinical sites, many of the children received fluids prior to arriving at the burn unit. We compared resuscitation fluids received 0–24 h and 24–48 h following the burn injury. The amounts of resuscitation fluids, as well as transfusions from the first 48 h following the burn injury for children and adults, were adjusted both for burn size and weight. Within the first 24 h of burn injury, there was no significant difference in the amount of fluids given per kg weight per percent burn for children compared with adults. From 24–48 h following the injury, however, the adults received more fluids than did the children, P < 0.05. The children, however, received more transfusions than did adults during the first 48 h post burn, P < 0.05. When you combine total fluids and transfusions for the first 48 h, however, there is no difference between the amounts that adults and children received.
To determine whether cytokine expression was related to burn size, we matched 11 pediatric and 11 adult patients from within the larger patient pool based on burn size. Comparison of cytokine abundance between these groups revealed that the matched patients exhibited similar patterns to those seen in the larger group, so here we present only the expression values for the larger cohort.
Over the study period, significant increases were measured in plasma GM-CSF at each time point (Figure 2A, P < 0.05). During the first wk post-burn, six cytokines were detected at significantly greater amounts in adult compared with pediatric plasma. When comparing adult with pediatric cytokine levels, IFN-γ, IL-17, and IL-4 were elevated 0–1 d post-burn (Figure 2B–2D, P < 0.05), IL-10 was elevated 0–1 and 2 d post burn (Figure 2E, P < 0.05), and IL-8 was elevated 3–7 d post burn (Figure 2F, P < 0.05). IL-6 was the only cytokine elevated in adult patients at every time point during the first wk (0–1, 2, and 3–7 d post burn) (Figure 2G, P < 0.05). The picture changed after post-burn wk one. IL-1β was increased significantly in pediatric patients during the second post-burn wk (Figure 2H, P < 0.05). Alternatively, IL-5 was expressed at higher levels in adult burn patient plasma during this same period (Figure 2I, P < 0.05). Higher levels of IL-18 were detected in pediatric patients during the third wk post burn (Figure 2J, P < 0.05). IL-1a was higher in the pediatric patients during the 22–66 d post-burn period (Figure 2K, P < 0.05). Eotaxin, G-CSF, IL-13, IL-15, IP-10, MCP-1, and MIP-1a were elevated significantly at specific intervals across the six time periods (Figure 2L–2R, P < 0.05). No differences between pediatric and adult patients were detected in plasma IL-12, IL-2, IL-7, or TNF concentrations (Figure 2S–2V). Cytokine expression in pediatric burn patients as compared with adult burn patients is summarized in Table 2. The data indicate that burned children have a different cytokine profile as compared with adult burn patients.
Cytokine expression (pg/mL) in adult compared with pediatric burn patients. (A) GM-CSF, (B) IFN-γ, (C) IL-17, (D) IL-4, (E) IL-10, (F) IL-8, (G) IL-6, (H) IL-1β, (I) IL-5, (J) IL-18, (K) IL-1α, (L) eotaxin, (M) G-CSF, (N) IL-13, (O) IL-15, (P) IP-10, (Q) MCP-1,(R) MIP-1α, (S) IL-12, (T) IL-2, (U) IL-7, and (V) TNF. Data presented as mean ± SEM. *Significant difference between adult and pediatric values (P < 0.05 by Student t test).
Discussion
Following a severe burn injury, protein degradation and catabolism characteristic of hypermetabolism are exacerbated by the systemic inflammatory response (8,9), resulting in compromised structure and function of muscle, skin, heart, liver, and immune system. These occurrences typically increase the incidence of infection and sepsis, leaving the burn patient vulnerable to multi-organ failure and death (10,11). This circulus vitiosus is difficult to break and current therapies often fall short of adequately reducing inflammation, hypermetabolism, or mortality. Confounders in identifying appropriate therapies are patient age and burn size. Children are better able to survive and to recover from a large burn injury than are adults (1,12). The primary determinants of survival for the adult burn patient are age and burn size. The older the patient and the larger the burn, the greater the risk of dying. We hypothesize that the inflammatory response triggers hypermetabolism resulting in multi-organ failure and subsequent death. Why adults experience higher mortality, however, is unknown. The first step toward elucidating the cause of differential outcomes in pediatric compared with adult patients is to determine whether the inflammatory response differs between these populations. Therefore the aim of the present study was to compare the inflammatory response as measured by plasma cytokine expression profiles in severely burned pediatric and adult patients.
The profiles presented here are representative of the cytokine changes attributed to flame burn injuries in pediatric and adult patients. We found that the concentrations of pro-inflammatory cytokines such as IL-6, IL-8, IL-1α, IL-1β, MCP-1, MIP-1α, IL-15, IL-5, IL-17, IL-18, IP-10, eotaxin, and GM-CSF are significantly different following a severe burn in pediatric compared with adult patients. The same was true for antiinflammatory cytokines such as IL-10, G-CSF, IL-13, IFN-γ, and IL-4. A small subset of cytokines (TNF, IL-2, IL-7, and IL-12) did not differ between pediatric and adult patients. Although the number of patients in this study is small, the results may indicate that adult patients experience a different post-burn inflammatory response than do the children. Dramatic elevations of GM-CSF, IL-10, and IL-6 in the adult patients during the first wk following burn indicate that the patients are undergoing a large inflammatory response that is not indicative of the carefully orchestrated balance of pro- and anti-inflammatory cytokine expression that counterregulate each other that we typically associate with activation of the inflammatory system. Instead, the simultaneous expression of both pro- and anti-inflammatory cytokines indicates that the body may be overcompensating to the burn injury. Concurrent elevation of GM-CSF and IL-10 lends support to this hypothesis as GM-CSF induces the expression of pro-inflammatory cytokines such as TNF, IL-1, and IL-6, while IL-10 inhibits the expression of pro-inflammatory cytokines such as IFN, TNF-β, and IL-2. Many of the cytokines that are modulated in response to burn are involved with activation, or expansion, of sub-populations of immune cells. Differential expression of these cytokines in children and adults may indicate that there is differential activation of the immune response to burn.
Although marked hyperinflammation following a burn injury has been reported (7,8,13), the differences in the magnitude and temporal occurrence of fluctuations of so many plasma cytokine levels between children and adults have not been previously described. Although we wanted to address the hypothesis that hypermetabolism is related to the inflammatory response, the only parameter measured as part of this multi-center trial that gives a rough indication of a patient’s metabolic state is heart rate. Elevated heart rates did persist in these patients throughout the 2-month period of this study, but again this is a crude determinant of hypermetabolism. Many studies have reported the hypermetabolic response to burn; here we show that the response may be different in children as evidenced by their elevated heart rates. Furthermore, we unexpectedly found that although the pediatric patients had larger burns, the adults were in a more hyper-inflammatory state. Even when the patients were matched for burn size, the adult patients still experienced greater inflammation. This finding suggests that adults may be more appropriate candidates for anti-inflammatory therapies following a major burn injury.
These results build upon previous reports that cytokines are elevated early in response to a severe burn and decrease over time. Elevations of IL-6, IL-8, IL-1β, IL-18, and IL-10 were most pronounced during the first wk post burn (3,5,8,13–17). Post-burn TNF expression is somewhat controversial as to whether or not it is temporal (3,5,14) (8,13). We previously published a time course comparing cytokine levels in pediatric burn patients to normal, non-burned children (7). The data that we present here are similar, but not identical, to those findings. We attribute the disparity in detection to use of different multiplex platforms and patient population differences. The specific nature of the antibodies used in the Bio-Rad compared with Linco multiplex platforms are considered proprietary information, and we are therefore unable to determine whether the same epitope, soluble cytokine, or soluble plus bound cytokine are being measured. The current study was limited to patients who experienced a flame burn, whereas the previous study included patients with flame, scald, and electrical injuries.
Because adult patients were admitted to the burn unit on average 1 d sooner than pediatric patients, we were concerned that the adult patients may have been resuscitated more aggressively. Resuscitation volumes were corrected for burn size and body weight. There was no difference in fluids administered per kilogram body weight per percent burn 0–24 h post burn. However, adults received more fluids per kilogram body weight per percent burn than did children 24–48 h post burn. When transfusions during these same time periods were included in the analysis, there was no significant difference between adults and children for volume of fluids and transfusions received during the first 48 h following the injury. If the cytokine profile differences were due to differences in resuscitation parameters, we would expect these differences to resolve within the first wk. We see instead that the differences in cytokine expression continue through 66 d post burn.
How post-burn cytokine expression profiles differ from non-burned cytokine profiles is beyond the scope of this study. While the levels of the measured cytokines vary greatly between adults and children, due to the pleiotropic nature of cytokines, it is not known which mediators are induced directly by the burn injury and which are secondary mediators indicative only of systemic inflammation. Furthermore, it is unknown if these distinctions have significance. Future studies with tightly defined sampling time points as well as with larger numbers of patients allowing for further stratification by age (infants, toddlers, pre-pubertal and pubertal teenagers, young adults, middle aged adults, and the elderly) would allow determination of the biological significance of differences in the cytokine profiles. Caveats aside, here we demonstrate for the first time that adult and pediatric burn patients have significantly different expression patterns of 18 cytokines.
The data presented here indicate that the most effective therapeutic interventions may vary between pediatric and adult burn patients. There is a dearth of effective therapies that modulate post-burn inflammation. Oxandrolone, a popular anti-catabolic agent, has no effect on inflammation (18). Propranolol, used to decrease heart rate and hypermetabolism following burn, has only small effects on the alteration of specific cytokines (19). Growth hormone is an effective agent in children for decreasing post-burn catabolism and also decreases TNF and IL-1β, but not IL-1α, IL-10, or IL-6 (20). The use of growth hormone, however, is contraindicated for use in adult patients because critically ill adult patients had an increased mortality following administration in the ICU (21). Insulin has an anti-inflammatory effect following burn in pediatric patients (22), indicating that further studies in adults may be warranted. Other therapies that have been tried in critically ill patients but failed include specific anti-cytokine therapies including neutralizing monoclonal antibodies, receptors, or antagonists designed to reduce circulating levels of specific cytokines (23). High affinity cytokine traps, first described by Economides et al. (24), are in clinical trials for various cancers and auto-inflammatory diseases, but these have not been tested in critically ill, trauma, or burn patients (www.clinicaltrials.gov). Given the current state of burn therapies, we suggest that further studies with anti-inflammatory agents, such as insulin, given soon after admission to the ICU in both pediatric and adult burn patients should be pursued.
In summary, here we present for the first time in a multi-center trial the differential expression profiles of 18 cytokines in adult compared with pediatric patients. These findings underscore the importance of supporting the notion that children are not just small adults and that therapeutic interventions need to be tested in both populations prior to implementation as standardized protocols.
References
Jeschke MG, et al. (2007) Burn size determines the inflammatory and hypermetabolic response. Crit. Care 11:R90.
Wray CJ, Mammen JM, Hasselgren PO. (2002) Catabolic response to stress and potential benefits of nutrition support. Nutrition 18:971–7.
De Bandt JP, et al. (1994) Cytokine response to burn injury: relationship with protein metabolism. J. Trauma. 36:624–8.
Schwacha MG. (2003) Macrophages and post-burn immune dysfunction. Burns 29:1–14.
Vindenes HA, Ulvestad E, Bjerknes R. (1998) Concentrations of cytokines in plasma of patients with large burns: their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur. J. Surg. 164:647–56.
Herndon DN (ed.) (2002) Total Burn Care. 2nd ed. New York: WB Saunders. 817 pp.
Finnerty CC, et al. (2006) Cytokine expression profile over time in severely burned pediatric patients. Shock 26:13–9.
Yeh FL, Shen HD, Fang RH. (2002) Deficient transforming growth factor beta and interleukin-10 responses contribute to the septic death of burned patients. Burns 28:631–7.
Jeschke MG, et al. (2002) IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol. Med. 8:238–46.
Rennie MJ. (1985) Muscle protein turnover and the wasting due to injury and disease. Br. Med. Bull. 41:257–64.
Arnold J, et al. (1993) Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin. Sci. Lond. 84:655–61.
Pereira CT, et al. (2006) Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. J. Am. Coll. Surg. 202:536–48.
Drost AC, et al. (1993) Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J. Trauma 35:335–9.
Nijsten MW, et al. (1991) Interleukin-6 and its relation to the humoral immune response and clinical parameters in burned patients. Surgery 109:761–7.
Biffl WL, Moore EE, Moore FA, Peterson VM. (1996) Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann. Surg. 224:647–64.
Dehne MG, et al. (2002) Alterations of acute phase reaction and cytokine production in patients following severe burn injury. Burns 28:535–42.
Coban YK, Aral M. (2006) Serum IL-18 is increased at early postburn period in moderately burned patients. Mediators Inflamm. 2006:16492.
Przkora R, et al. (2005) Metabolic and hormonal changes of severely burned children receiving long-term oxandrolone treatment. Ann. Surg. 242:384–9, discussion.
Jeschke MG, Norbury WB, Finnerty CC, Branski LK, Herndon DN. (2007) Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. J. Trauma 62: 676–81.
Spies M, Wolf SE, Barrow RE, Jeschke MG, Herndon DN. (2002) Modulation of types I and II acute phase reactants with insulin-like growth factor-1/binding protein-3 complex in severely burned children. Crit. Care Med. 30:83–8.
Takala J, et al. (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N. Engl. J. Med. 341:785–92.
Jeschke MG, Einspanier R, Klein D, Jauch KW. (2002) Insulin attenuates the systemic inflammatory response to thermal trauma. Mol. Med. 8: 443–50.
Gosain A, Gamelli RL. (2005) Aprimer in cytokines. J. Burn Care Rehabil. 26:7–12.
Economides AN, et al. (2003) Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat. Med. 9:47–52.
Acknowledgments
This study was supported in part by a Large Scale Collaborative Research Grant from the National Institute of General Medical Sciences (U54 GM-62119-04) awarded to Ronald G Tompkins at the Massachusetts General Hospital, Boston, MA, USA, and in part by research grants awarded to David N Herndon at the University of Texas Medical Branch, Galveston, TX, USA, by the National Institute of General Medical Sciences (P50 GM-60338, R01 GM-56687, T32 GM-008256) and by the Shriners Hospitals for Children (8660).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Finnerty, C.C., Jeschke, M.G., Herndon, D.N. et al. Temporal Cytokine Profiles in Severely Burned Patients: A Comparison of Adults and Children. Mol Med 14, 553–560 (2008). https://doi.org/10.2119/2007-00132.Finnerty
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/2007-00132.Finnerty