Abstract
This state of the art presents an overview on the effects of calcined clay inclusion on the fresh properties of concrete under the framework of RILEM TC-282 CCL. Progress in recent literature was reviewed to determine the effects of calcined clay, particularly metakaolin and lower grade kaolinite clays, on fresh concrete properties and how to control them using admixtures, particle packing, and mixture proportioning. A summary of recent studies on the use of superplasticizers in modified (or combined form) to improve compatibility have shown promising outcomes to control the rheological properties of calcined clay binders. Superplasticizer demand required to achieve workable concrete increases with increasing dosage of calcined clay and increases substantially for concrete produced with calcined clay at water-to-cementitious material ratios below 0.40. A comparative analysis of data from several literature shows that the addition of calcined clay could reduce setting time when used without superplasticizers. Addition of superplasticizers could help to control and increase the setting time significantly. Calcined clay can be used to make concrete with similar workability and setting times as concrete containing Portland cement through the use of polycarboxylate-based superplasticizers. However, more studies in future should focus on retention of workability by suitable methodologies for various construction activities. Care should be exercised to avoid long setting times with high dosages of superplasticizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Calcined clays were one of the early supplementary cementitious materials (SCMs) used to substitute Portland cement (PC) concrete and were initially used to reduce the heat of hydration and improve durability [1,2,3,4,5,6]. Pozzolanic activity of calcined clays has been demonstrated since the early 19th century by Louis Vicat, and, its incorporation in PC-based materials was under investigation from mid 28th century [7, 8]. However, the high fineness and water demand were major obstacles to its use as a mineral admixture in concrete initially when there was limited usage of water-reducing admixtures (WRA). Calcined clay is experiencing a renaissance recently because of the widespread geographic availability of these clays [9, 10], favourable economics for manufacture [11], potential for large reductions in concrete greenhouse gas footprint [12], and suitable fresh state, mechanical and durability properties for use in reinforced concrete systems [13,14,15,16]. In the past, a significant portion of the calcined clay used in concrete was primarily metakaolin (MK), which is a purified and highly reactive form of kaolinite-based calcined clay. MK is typically limited to 8–20% cement replacement levels because of concern over workability and cost [17, 18]. Raw clay sources used to make MK are refined and must have a high percentage of kaolinite clay [19]. The availability of such high purity kaolin clay deposits is limited worldwide. Moreover, several industries such as paper, pollution control, coating, and pottery need such high purity kaolin clay materials, which leads to higher demand and cost of MK as a cement substitute. For example, the commercial cost of pure kaolin clay can be twice that of PC, which restricts the use of these materials in construction projects due to economic viability. The additional cost comes from processing required to remove impurities such as quartz sand, iron-based compounds, and other mineral impurities to whiten the clay color and increase the kaolinite content. Pure forms of MK has been typically used only in high-performance concrete (HPC) as a premium SCM, at low water-cementitious material ratio (w/b), or in instances where white color is needed for architectural reasons [9].
As an alternative to purer forms of MK, lower-grade calcined clay made from clays with moderate kaolin contents, as low as 40% kaolinite content in raw clay, are explored in the past decade. These lower grade clay sources can also make suitable pozzolanic material with little processing despite the complex chemical and mineralogical composition of clays [20,21,22]. Such forms of kaolin clays are well-distributed worldwide in large quantities for potential use as cement substitutes. Calcined clay is made by heat treatment, often referred to as calcination, of natural clay source at moderately-high temperatures, typically between 700 and 850 °C [9, 23]. Calcination is usually followed by grinding to reduce particle size suitable for use in cementitious materials. The chemical and physical properties of calcined clay can vary depending on the clay type, clay content, firing process, grinding, and grinding aids used [23,24,25,26]. More recently, it has been found that calcined clay and fine limestone powder can be used together as cement substitute to produce a blended binder known as limestone calcined clay cement (LC3) [9, 27, 28]. All components in the ternary limestone-calcined clay-clinker system react synergistically to provide enhanced strength and durability to concrete [29, 30]. Powdered limestone is considered to be an ideal component for increasing clinker substitution level [31], specifically with alumina rich—SCMs like calcined clays due to improved chemical reaction.
The role of calcined clay on workability, setting and fresh properties has been a subject of concern for the research community and concrete producers [32,33,34]. In the last decade, considerable work has been performed to assess the effects of calcined clay on fresh concrete properties, including rheology, stability, setting, and workability retention. These efforts have led to the development of solutions to tailor the fresh properties of concrete containing calcined clay, allowing it to be used successfully in most concrete applications. Furthermore, the increasing complexity of clay type, its variation, composite ternary formulations composed of calcined clay has produced some new challenges in controlling the behavior of fresh concrete, which is pointed out in this review. This paper reviews advances in the use of chemical admixture used to control the properties of concrete made with calcined clay, the effects of calcined clay on fresh properties of concrete, how to use chemical admixtures to control them, and incorporating calcined clays in mixture design.
2 Admixture use with calcined clays
Polycarboxylate ether (PCE) polymers have been used in concrete construction since the late eighties. There has been a noticeable improvement over former technologies such as lignosulphonate or sulphonated naphthalene formaldehyde (SNF) condensates over the years. The intrinsic dispersing ability of PCE is noticeably higher and helps to achieve the target workability while reducing the water content in concrete beyond what was previously possible, opening the way to high performance and self-consolidating concrete [35, 36].
All PCE molecules share a common branched structure which is known by various names such as ‘bottle-brush’ [37,38,39] or ‘comb-copolymer’ [40,41,42]. A basic description of PCE structures would be a main anionic chain made of acrylic or methacrylic acid from which protrude side-grafts usually made of methoxy-terminated poly(ethylene glycol) (PEG) polymers. They are usually obtained either by esterification of the main chain acids by the terminal alcohols of the methoxy-PEG, or by free-radical copolymerization of the unsaturated carboxylic acid with a PEG-ester of the same acid. Over the years, some variants were produced, with the insertion of maleic acid or acrylamidomethyl propane sulphonic acid into the main chain. However, only subtle changes were brought to graft chemistry mainly for the sake of polymerization efficiency or cost-reduction with PEG-tethered isoprenyl, vinyl or methallyl alcohols [43].
2.1 PCE incompatibility with calcined clays
The interaction between clays and PCE dispersants has been extensively studied due to the observed loss of dispersion efficiency, leading to higher demands of those admixtures content. Such problems usually arise when the aggregates, especially sands, are poorly or not washed and contain natural clays [44, 45]. In some instance, PCE molecule was found to be incorporated with interlayer space of aluminosilicate, as shown in [45]. Cases in which dehydroxylation is not complete could experience some of these same incompatibility issues.
Many studies agree on the primary mechanism of PCE consumption, namely the intercalation of macromolecules in the interlayer space of phyllosilicates [46,47,48,49,50], also named as chelation mechanism. Even if small amounts of surface adsorption could be measured, it appears that intercalated PCE induces a deformation of the clay sheet-like structure, easily observed by X-ray diffraction through increasing the interlayer thickness. This indicates that the clay platelet stacked structure deforms to accommodate large molecules. A comprehensive review on the subject is reported in Ma’s article [51].
Most authors observed that only the side grafts of the PCE macromolecules, namely poly(ethylene oxide) (PEO) chains, produce an intercalation effect. However, a study reported in [46] demonstrated that this intercalation induces a decrease of the interlayer water amount, according to a dehydration-like mechanism. Another study by Lu et al. [52] proposed that the units of the PEG chains interact with interlayer cations in a crown-ether-like fashion, replacing the hydration water of the said ions (Fig. 1).
Sketch of the crown-ether-like configuration of PEG units needed for sodium interaction [52]
It was shown that intercalation is more pronounced for longer PEO chains than for the shorter ones [48, 49], which led to the design of very short side-graft PCE molecules to improve clay tolerance [50, 53]. Other modifications involved the insertion of amide groups [54] or substitution of PEO by β-cyclodextrin [55], leading to mixed results in mitigating the intercalation, the total polymer consumption and the cementitious material workability. No intercalation of PCE was reported for kaolinite and illite; it is argued that their structure naturally leads to such behavior [51]. The kaolinite interlayer space is devoid of any cation, which eliminates the basic PEO chelation mechanism described previously. This leads to the conclusion that most PCE interactions with kaolinite or illite would merely involve surface adsorption. It was indeed shown that PCE molecules are efficient dispersants of kaolinite suspensions [56].
2.2 Compatibility of PCE with calcined clays
Calcination is reported to have different effects based on the mineralogy of clay. First, it was shown that 800 °C calcination induces a decrease in the specific surface of montmorillonite and illite while kaolinite seems to be far less sensitive [57, 58]. It is then expected that surface adsorption would be noticeably decreased after calcination for the first two, while kaolinite will retain much of its adsorption capacity.
Most compatibility studies seem to be devoted to kaolinite-rich calcined clay, pure calcined kaolin and more recent studies have a focus on lower grade calcined clay and calcined clay-limestone combination. A comprehensive study with commercially available PCE polymers was conducted by Nair et al. [59], comparing OPC, a 30% fly ash-blended cement and LC3, a blend of Portland clinker gypsum, calcined clay and limestone. The extensive experimental program involved paste and concrete testing, in both fresh and hardened state. It was observed that PCEs needed to be added at a higher dosage than required for PC or the fly ash-blended cement for equivalent performance. Interestingly, the correlation of optimum dosage in concrete versus paste followed a peculiar trend, with almost no change in required dosage for concrete when the water-binder ratio (w/b) was above 0.40 and a sharp increase below 0.40 shown in Fig. 2.
Optimum dosage in concrete versus the superplasticizer saturation dosage in paste made of limestone calcined clay , used with permission from Nair et al. [59]. sp/c is the superplasticizer-to-cement ratio obtained using Marsh cone tests after which the flow does not increase based on flow time versus superplasticizer dosage curve. w/b: water-binder ratio
Original polymer structures were also explored for compatibility with calcined clay-limestone blends, as described in [34]. They consisted of very sparsely (less than 1%) grafted acrylic acid-acrylamidomethyl propane sulfonic acid (AA-AMPS) copolymer main chain, obtained by free radical terpolymerization. The side chains were roughly 23 units long, and the molecular weight was typically five to ten times as high as typical PCE weights. This resulted in polymers with a very high ionic character, yielding a robust calcium-chelating property and a dispersing effect that uses electrostatic repulsion, as shown by zeta potential measurements in Fig. 3.
Zeta potential in a synthetic pore solution of a OPC and b calcined clay with PCE dosage, used with permission from Akhlaghi et al. [34]
Adsorption featured sharper initial slopes and pronounced saturation plateaus on limestone and calcined clay when compared to PC, as shown in Fig. 4. The expression of the results in mg adsorbed by the unit weight of mineral misled the authors on the relative capacities to accommodate polymers at the surface: the specific surface being 5 times higher for limestone and calcined clay compared to PC, their saturation occurs at lower levels per unit surface area. The main finding of that study is that much polymer remains in solution to fuel further adsorption over time. Hence good workability retention was achieved [34].
PCE adsorption by material type in synthetic pore solution, used with permission from Akhlaghi et al. [34]
A study devoted to different chemistries of grafts was performed by Schmid and Plank [43]. They used free radical polymerization with macromonomers bearing different unsaturated functions to assess their relative influence on the dispersion of blends of OPC with calcined kaolinite. Namely, the functions involved were ω-methoxy PEG methacrylate (MPEG), isoprenyl PEG (IPEG) and α-methallyl-ω-methoxy PEG (HPEG). IPEGs are usually produced by ethoxylation of isoprenol and should probably not bear an ω-methoxy group shown on Fig. 5. The HPEG-based polymers are commercially available products. The polymers were synthesized with variable molar amounts a and b and variable graft ethylene glycol units. The comonomer used with MPEG was methacrylic acid for better reaction conversion, while acrylic acid was used with the other two shown in (Fig. 5).
Macromonomers used in the study of Schmid etal., used with permission from Svhmid and Plank [43]
A natural clay was calcined from a source containing low amounts of kaolinite (23%), with illite–smectite (32%) and quartz (20%) present as the other main components in the material. After calcination at 750 °C for 30 min, 60% of amorphous phases were achieved with a specific surface four times as high as PC. The behavior of all polymers was then assessed in combination with increasing amounts of calcined clay in a blend with a PC. The required dosages for a targeted paste flowability and adsorption isotherm were measured. All the dispersants used in the study required dosages that increased with the proportion of calcined clay in the blend. The IPEG-based polymers required the highest dosages, while the HPEG-based commercial polymers demonstrated to be the most efficient. Whenever applicable (i.e. on IPEG and MPEG based polymers), the grafting ratio had a mild influence on the dosage required except on the pure calcined clay, for which the lowest grafting ratio provided the best efficiency in terms of dosage needed to disperse the solid particles [43].
Adsorption was only investigated with MPEG-based polymers and showed a similar trend on calcined clay cement and just PC; the higher the grafting ratio and the graft length, the lower the adsorption. Interestingly, adsorption onto calcined clay features a more pronounced plateau than onto PC for all structures. Nevertheless, some adsorbed amounts seemed similar for calcined clay and PC; hence adsorption seems barely responsible for the differences in fluidity, which they attribute to a different mixing protocol in the two tests. However, the authors may have been misled by comparing their results in terms of polymer mass adsorbed per unit weight of the material, neglecting the four-fold higher specific surface of calcined clay, as measured by the BET method. When considering this, adsorption by unit surface area of calcined clay was lower than for PC, providing a more convincing explanation for the fluidity difference [43].
2.3 Other admixtures
A few studies are available on other admixtures, and most of them are about retarders, usually associated with a polymer dispersant of some sort and never alone. For instance, in Nair et al. [59], a designated ‘lignosulphonate-based’ retarder helped recover some workability retention. Since lignosulphonates are usually classified in the plasticizer category and retard mainly when they contain sugars (see e.g. [60, 61]), it is inferred here that a special unrefined grade was used and that sugars are also suitable retarders for calcined-clay systems. More recently, a combination of PCE with layered double hydroxide (LDH) has been proven to improved fluidity retention in calcined clay blended cement [62]. The PCE molecules in LDH were gradually exchanged with sulfate ions to produce improved flow retention in the cementitious systems containing calcined clay. More such combined formulations needs to be explored in future studies.
3 Rheology
The rheological properties of cementitious materials are a key consideration when designing the formulation of cementitious products. Assessment of rheological properties can be undertaken using different test methods and give information on the different physical properties of paste, including yield stress, viscosity, thixotropy, flowability and viscoelasticity. Cement paste rheological properties are influenced by many different factors. These parameters include particle size distribution, solid volume fraction or packing density, particle shape and texture, and interparticle forces [63]. Interparticle forces can occur from surface charge [64, 65]. C–S–H nucleation and build up during hydration can also increase rheological parameters [65]. Chemical admixtures are often used to control interparticle forces as previously described.
Calcined clay particles can change the cement paste parameters that govern the rheological properties and warrants further discussion. [66,67,68].
3.1 Controlling rheology with chemical admixtures
The work of Vance et al. [68] showed that including fine particles of limestone significantly decrease the plastic viscosity of cement paste. However, a decrease in the limestone particle size to 0.7–3.0 μm, was found to cause an increase in the plastic viscosity and yield stress of pastes. The sole use of limestone can lead to the segregation of PC pastes (when mixed with a PCE superplasticizer) [68]. The inclusion of calcined clay materials can reduce this segregation and, when used in conjunction with limestone, reduce the workability of the paste. By mixing a PC-limestone blend with MK, the yield stress decreases with the increase in limestone content compared to that of PC and MK due to the packing effect of fine limestone and the electrostatic effects between MK and limestone particles [69].
Superplasticizers are required to control workability and setting times when calcined clays are used as SCMs [14, 59, 70]. Ferreiro et al. [70]showed by comparing 1:1 and 2:1 clays that the water demand is significantly lower for 2:1 clays. That study also showed that delayed addition of superplasticizer, a few minutes after the water, improved workability. The use of WRAs increases the workability of concrete containing calcined clay-based cementitious materials. Comparison between slump and concrete rheology results were reported by Paiva et al. [71] and showed that yield stress and viscosity decreased as slump increased with MK. The results also showed that WRA dosage increased significantly as MK dosage increased [71]. The use of these materials is governed by ASTM C494 [72], with specific consideration given to Type A and Type F admixtures [73]. Zaribaf and Kurtis [73] have assessed various WRAs on ternary blends of PC-limestone-MK, with up to 30% replacement, using polycarboxylate ethers (PCE), lignosulfonates, naphthalene and polymelamine sulfonate based admixtures. For high MK content ternary blends, PCEs were the most efficient to increase the workability. Moreover, both powdered and liquid based PCE were effective, which could allow to produce pre-mixed MK/PCE blends. The work of Nazario-Santos et al. [69] has shown that keeping the superplasticizer content below 0.5 wt% is ideal for controlling the workability of all MK blended cements.
The inclusion of PCE-based superplasticisers can significantly affect the rheological properties of cement paste [74]. The authors reported a significant reduction in yield stress when PCE was added to a calcined clay paste. This reduction in viscosity was attributed to better dispersion of the calcined clay particles within the cement paste. However, the type of clay was reported to impact the behavior of calcined clay-based paste significantly. Calcined phyllosilicates have shown shear thickening behavior, whilst MK has shown higher yield stress without any associated shear thickening behavior [74].
3.2 Yield stress and viscosity
The inclusion of MK within different cementitious systems was found to drastically increase both plastic viscosity and yield stress of paste [66,67,68, 75]. In addition, the incorporation of MK increased both water demand and Vebe time and systematically decreased slump and compacting factor [76, 77].
Calcined clay particle size and presence of impurities have been found to have a considerable effect on the resulting cementitious system rheological properties. A study by Lorentz et al. [63] showed that the yield stress and the viscosity could be related to the material particle size and zeta potential. However, these issues were overcome through the use of additional (excess) chemical admixtures. Calcined clay surface area is a function of the calcination process, mainly temperature and grinding. The particle size distribution increases as the calcination temperature increase up to 800 °C. Sintering likely starts to occur at calcination temperatures above 800 °C, increasing the particle size. The workability and reactivity of the clays are then dependent on the surface area after grinding. The finer the particle size distribution, the higher the pozzolanic reactivity, and the higher the water demand [20].
Sonebi et al. [66] researched the rheological parameters of cement grouts with MK content ranging from 6 to 20% of PC. The results indicated that for a given dosage of viscosity-modifying agent (VMA) and superplasticizer (SP), an increase in MK content caused a significant increase in the yield stress, plastic viscosity, cohesion plate and flow time, and a reduction in mini-slump. Cassagnabère et al. [78] investigated the slump, flow, and apparent viscosity at different shear rates of cement/MK-based mortars. The results showed that both slump and viscosity are strongly dependent on the morphology of MK. A decrease in roundness and increase in the angularity of particles caused a decrease in slump and an increase in viscosity values. The influence of the size and shape of particles on viscosity is dependent on shear intensity. The nature and content of impurities such as quartz, illite, or uncalcined kaolinite are the main parameters that affected the morphology of particles, the water demand, and flow properties (viscosity values) of the MK mortars.
Vance et al. [68] demonstrated that increasing the MK content in the paste significantly increased both yield stress and plastic viscosity. For example, when the MK content increased from 0 to 10%, the plastic viscosity doubled, and the yield stress increased by 1.75 times (Fig. 6). This observation is attributed to MK's very high surface area and the tendency of MK fines to agglomerate. Agglomeration of particles causes water to become trapped, preventing it from lubricating particles and causing significant elevations in the measured yield stress and plastic viscosity.
Influence of fly ash and MK on the rheological properties of binary pastes from Vance et al., used with permission from Vance et al. [68]
Paiva et al. [71] investigated the effects of MK on concrete workability. With the increase in MK content, both water content and admixture content had to be increased to keep the workability at a constant slump of 90 ± 10 mm. Janotka et al. [67] reported that mortars with MK fit the Herschel–Bulkley model much better than the Bingham mode, with the yield stress increasing as the MK content increased. MK with the highest pozzolanic activity and highest specific surface induced the highest increase in yield stress.
3.3 Thixotropic and dilatant effects
Thixotropic behavior within cement paste is often described as the need to apply strong shear to a cement paste in order to bring the paste back to its reference state [79]. Cement pastes containing MK and a superplasticizer have been shown to display an increase in the thixotropic behavior with an increase in MK content [80, 81]. The increasing dilatancy of the cement paste has been attributed to interaction? (or jamming) of the angular, plate like MK particles [80]. Calcined clays thixotropic effects have been shown to be caused by negatively charged calcined clay surfaces as measured by zeta potential and layered particles. This increases particle flocculation, reducing water availability. This mechanism is different than seen in Portland cement pastes, where thixotropy is caused by C–S–H nucleation and buildup [64, 82]. In the context of an extrusion-based 3D-printing study [83], the quick and intense structural build-up was observed in the cementitious material produced with calcined clays from two different origins, being more pronounced for clays with higher kaolin content. However, looking at the initial slump flow diameter results, for the same water content, the mixture that presented higher thixotropy also showed lower flow, pointing to the water adsorptivity of calcined clays as the main factor for the behavior of the mixture.
3.4 Pumpability and extrudability
Calcined clays can be used to produce shotcrete characterized by low dust generation and reduced rebound, therefore suitable for the application of thin layers on dry substrates [84]. It was also referred that shotcrete with calcined clay led to a denser concrete with lower water penetration depth.
A lab-scale extrusion test method based on a ram extruder was proposed in a recent study [85] to efficiently observe the extruded filaments of the fresh mixture and quantify the required extrusion pressures at different ages. Mixtures with calcined clays showed good shape stability before their initial setting. Although pointing to the content of MK as the main factor, the large variation of particles fineness could also impact on the studied rheological properties. Namely, using finer calcined clay may increase the extrusion shear strength of fresh mixtures, which may bring difficulties for extruding and reduce the printability window/open time. However, the buildability and structural build-up behavior of mix designs for 3D concrete printing could be enhanced by using finer calcined clays [86, 87].
4 Mixture cohesion
Limited work has been reported on the segregation resistance of concrete mixes produced using calcined clay. Caldarone et al. [76] reported that although the slump of concrete mixes reduces with the addition of MK, the superplasticizer dosage required is 25–35% lower than that required for similar doses of silica fume. In addition, mixes with MK were less sticky because of the lower superplasticizer dosage required than mixes with silica fume and could have a better surface finishability. This study also reported that although the mixes with lower w/b seemed to have poor workability when prepared using MK, good compaction was achieved upon vibration.
Bai et al. [77] reported that the addition of MK to mixes with fly ash reduces the sensitivity of the mixes to superplasticizers and the tendency of the mixes to bleed upon water-reducing admixture overdosing. This was attributed to the blocking of the capillary channels by MK particles. It has also been reported that despite the higher surface area of MK particles, the increase in compactability and VeBe time was relatively small. This has been attributed to the ease of flow of the mixes under vibration due to the thixotropic nature of clay suspensions and improved packing of calcined clay particles between the cement particles. Paiva et al. [71] reported that the agglomeration of MK particles has a significant influence on the workability of concrete and that superplasticisers can effectively break these agglomerates. Perlot et al. [88] reported that MK increases the cohesion and robustness of self-compacting concrete mixes as bleeding and segregation were reported to be reduced. This was attributed to the high thixotropic nature of the mixes containing MK, as reported by Vejmelková et al. [89].
The influence of the limestone and calcined clay combination on stability has also been investigated. Perlot et al. [88] found the combination of limestone and calcined clay to be highly suitable for self-consolidating concrete industrial production as they were found to reduce mixing sequence time and improve cohesion. While there is some consensus on the influence of MK on cohesion, the influence of limestone is not clear. While Larsen and Naruts [90] reported an improvement in the cohesion of concrete mixes containing limestone, Muzenda et al. [75] reported that the presence of limestone in the blend binders reduces the cohesion of the mixes in the tack test. However, it may be considered that limestone has long been used as an additive to improve cohesion in self-compacting concrete mixes. Vardhan et al. [91] reported a lower admixture dosage requirement for the production of self-compacting concrete with 45% cement replacement by a blend of limestone and calcined clay than a similar replacement level by fly ash. This has been attributed to the higher robustness of the mix with limestone and calcined clay. Another study by Nair et al. [59] reported bleeding in mixes containing calcined clay and limestone when admixture dosages were increased to achieve initial workability levels similar to mixes with PC. However, this was controlled by modifying the fine aggregate contents in the concete mixture as reported in that study.
5 Plastic shrinkage
Very limited and conflicting findings on the influence of MK on early-age shrinkage and cracking have been reported in the literature. Branch et al. [92] reported that the shrinkage strain in mixes containing MK is higher than mixes containing fly ash or blast-furnace slag. This could partly be explained by the lower bleeding capacity and rate that has been reported for mixes with calcined clays [93]. Still, due to the faster strength development with MK (compared to fly ash and slag concretes), cracking was not observed in the restrained ring test. However, Niknezhad et al. [94] reported a reduction in free shrinkage, restrained shrinkage stress and crack width in concretes containing MK. Much more work is needed in this area to understand the bleeding and plastic shrinkage of concrete containing calcined clay.
6 Setting
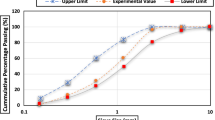
Setting is the time duration required for the concrete/mortar/pastes to lose its workability and start to harden. Setting time of cement pastes is typically measured by the Vicat needle test. The result of this test is mainly dependent on the consistency of the cement paste, so in this test cement paste mixtures are made at a standard consistency. Figure 7 plots the increase in the standard consistency reported in the literature for a blended binder containing calcined clays [6, 95,96,97,98,99]. An increase in cement replacement by calcined clay increases paste standard consistency, requiring a higher w/b in the setting test, leading to increased setting time at lower dosages, as shown in Fig. 8. Use of MK increases the standard consistency more than calcined clay because of the higher kaolinite content in MK and its accompanying increase in in surface area.
Figure 8 shows the change in the initial and final standard setting time (measurement based on standard consistency) for data compiled from the literature at different replacement levels of calcined clay[6, 95,96,97,98,99]. Lower-grade calcined clay and MK are demarcated in the analysis. It can be seen that the data points are scattered, although MK is more likely to have a lower setting time compared to lower grade and alternative calcined clay. On the other hand, Fig. 9 compiles data taken from the literature on setting time at the same w/b ratio with and without the addition of a superplasticizer (SP). It should be noted that the classification between MK and calcined clay was not shown in this figure. Comparing the data with and without SP, it is clear that the replacement with calcined clays reduces the setting time and large increases in setting time are typically caused by the admixture [100]. As superplasticizers are generally added to improve workability with the addition of calcined clay, its retarding effect may, in practice lead to an increase in setting time.
7 Mix design
As discussed in the previous sections, calcined clay can significantly influence the fresh properties of concrete and affect its placement onsite. Concrete mix design must therefore be adjusted to account for this influence. For concrete mixes with similar water content and superplasticizer (SP) dosage, the slump was found to decrease with increasing MK replacement level [16, 110]. This increased water-demand can be compensated by using a higher water content or increasing the water reducer dosage. While studies have shown that polycarboxylate based admixtures are the most effective, calcined clay based concrete can be more sensitive to the dosage of this type of admixture, with some studies indicating the possibility of bleeding upon over-dosing [59]. The effect of water content on SP demand to produce a similar slump was investigated by Nair et al. [59]. Concrete mixes with water content below 160 kg/m3 required a significant increase in the superplasticizer dosage to produce a similar initial slump. To separate the effect of water content from the influence of calcined clay, some studies considered concrete mixes with similar water content (per m3 of concrete) to focus on superplasticizer requirement [111,112,113,114]. The studies reported that an increase in calcined clay content required an increase in SP% to produce a similar target slump.
In cement paste, the relative increase in admixture dosage required to maintain the same flow with the addition of calcined clays was higher than that required in concrete due to the influence of aggregates [32]. Additionally, since fine aggregate is also known to contribute to the stickiness of mortar and concrete, reducing fine aggregate content has been suggested to compensate for the influence of calcined clays on water demand and cohesion. A similar reduction in fine aggregate content was adopted for concretes with MK together with a reduction in w/b [112] (See Table 1). Table 1 lists typical concrete mix designs with calcined clays. In all cases, SP dosage increased when the PC replacement level increased or the w/b was reduced. Güneyisi et al. [115] used a SNF-based water reducer. Most of the other studies adopted PCE-based superplasticizer.
It is possible to interfere with the concrete workability characteristics by controlling paste content and aggregate proportions [116]. In calcined clay systems, this method can be suitable to produce desirable slump characteristics in concrete systems by tailoring mixture proportioning including controlling paste and fine aggregate content along with SP dosage. In some cases, SP dosage above 1–2% is reported for concrete containing calcined clay to produce a suitable initial slump, as shown in Table 1. Hence, it is important to develop a rational mixture proportioning approach to address potential slump issues in concrete with calcined clay. The tradeoff between increasing superplasticizer dosage and effectiveness of mixture proportioning needs to be explored further to facilitate widespread industry adoption of calcined clay-based binders for diverse concrete applications. Table 2 presents the influence of mixture proportioning by accommodating binder content to improve both slump and slump retention characteristics in calcined clay systems. The Q value denotes the distribution coefficient as per the modified Andreassen model. The value typically varies from 0.21 to 0.37 depending on slump or workability requirements of the concrete mixture [117].
Concrete mixtures designs need to be developed considering the influence of calcined clay on the rate of strength development. The high superplasticizer content typically used with calcined clay can significantly affect the early age hydration and alter strength development rates. Despite the higher superplasticizer addition, the early age strength of calcined clay-based concretes can be significantly improved by 7 days [30, 119]. Both mix design procedure and quality control should be adjusted in accordance with the time-dependent strength development observed for these concretes.
Self-compacting concrete (SCC) mixtures with calcined clays have been shown to be more viscous than other concretes, requiring higher superplasticizer dosage. An increase in V-funnel time was reported for self-compacting concrete containing MK [33] and calcined clay-limestone combinations [32]. However, compared to other cements, the higher cohesion requires the fine aggregate content to be reduced, partially compensating for the increased admixture demand. Also, the use of calcined clay was found to reduce the segregation tendency in SCC concrete mixes without the use of viscosity modifying admixtures.
8 Research needs/recommendations for future research
Several areas of concrete fresh property performance when calcined clay is used have been identified as areas in need of further research. They can be summarized as follows:
-
PCE consumption of concrete containing calcined clay is high when compared to PC applications. A better understanding of the physical chemistry, the ionic conditions of the pore solution and the microscopic phenomena at the origins of workability loss (agglomeration, thixotropy, yield stress increase) may lead to more efficient molecules, thus a lower admixture cost.
-
The response of calcined-clay-blended cements to admixtures other than dispersants is not well documented. Research is needed in air entrainers, and a more chemically precise study of retarders seems necessary to obtain an improved compromise in terms of workability retention and early age strength. In addition, research on the mechanism of slump loss in concrete is needed, including if thixotropic or yield stress growth is responsible.
-
Calcined-clay can accelerate concrete setting, mixture proportioning should be adjusted based upon the application to achieve suitable setting times. Although initial studies have shown that lignosulphonates, in combination with PCEs, can act as retarders, a more detailed study is required on the subject
-
Information on the effects of calcined clay on concrete pumpability is still needed.
-
Systematic work on the effects of calcined clay on bleeding is needed. This should include a fundamental understanding of the interaction of calcined clays and water, including agglomeration.
9 Conclusions
Calcined clay has great potential as a supplementary cementitious material to improve concrete properties. One of the biggest concerns concrete users have with calcined clay-based concrete is the control of fresh state properties. Increases in calcined clay particle size and surface area, internal porosity in sintered particles, and presence of incompletely calcined 2:1 clays or mineral impurities have been found to decrease slump, and increase the yield stress, viscosity, and thixotropy of cementitious systems. Common PCE structures may be considered efficient enough to provide PC-calcined clay blends to control initial fresh state properties. However, new variants of PCE in modified forms were found to produce improved retention behavior. This could future pathways to obtain sufficient control on concrete workability for diverse contrustion applications. It was shown that polymer structure has the same influence as on straight PC systems; hence an adsorption-electrosteric repulsion dual mechanism is still involved. As a result, polymer efficiencies seem to rank in the same order as on PC. However, most studies show that the polymer consumption is higher than for PC systems, most probably a result of the much (several times) higher specific surface and faster reactivity of calcined clays. There remains a need to optimise dispersant efficiency to reduce dosages, hence cost, and future research should be carried out in this direction.
Calcined clay systems have produced conflicting changes in setting time compared to straight PC systems as measured by the Vicat needle test. However, this is primary due to higher w/b than control mixtures to achieve a standard consistency. When measured on mortar or concrete, calcined clay without any superplasticizers can reduce setting time. Superplasticizer could be used to control setting bahviour of calcined clay and calcined clay-limestone binders.
Calcined clay mixtures can be proportioned for a range of strength and workability classes. Superplasticizer demand required to achieve workable concrete increases with increasing dosage of calcined clay and increases substantially for w/b below 0.40. In order to improve workability, users can either (1) increase SP dosage, (2) use a retarder, (3) increase the paste content, (4) design particle size distribution in the mixture to improve packing, or (5) adjust the mixing and hauling procedure. In addition, calcined clay can significantly increase mixture segregation resistance and reduce bleeding in SCC.
Availability of data and material
All data cited in this review paper is available in the referenced sources.
Abbreviations
- PC:
-
Portland cement
- MK:
-
Metakaolin
- LC3 :
-
Cement with limestone and calcined clay
- w/b:
-
Water-binder ratio or water-cementitious materials ratio
- PCE:
-
Polycarboxylate ether
- WRA:
-
Water-reducing admixtures
- SNF:
-
Sulphonated naphthalene formaldehyde
- HPC:
-
High performance concrete
- LC2 :
-
Limestone and calcined clay admixture
- LDH:
-
Layered double hydroxide
References
Detwiler R, Bhatty J, Barger G, Hansen E (2001) Durability of concrete containing calcined clay. Concr Int 23:43–47
Khatib JM, Wild S (1996) Pore size distribution of metakaolin paste. Cem Concr Res 26:1545–1553. https://doi.org/10.1016/0008-8846(96)00147-0
Badogiannis E, Tsivilis S (2009) Exploitation of poor greek kaolins: durability of metakaolin concrete. Cem Concr Compos 31:128–133. https://doi.org/10.1016/j.cemconcomp.2008.11.001
Pera J (2001) Metakaolin and calcined clays. Cem Concr Compos 23(6):iii. https://doi.org/10.1016/S0958-9465(00)00098-6
Poon CS, Lam L, Kou SC et al (2001) Rate of pozzolanic reaction of metakaolin in high-performance cement pastes. Cem Concr Res 31:1301–1306. https://doi.org/10.1016/S0008-8846(01)00581-6
Badogiannis E, Kakali G, Dimopoulou G et al (2005) Metakaolin as a main cement constituent. Exploitation of poor Greek kaolins. Cem Concr Compos 27:197–203. https://doi.org/10.1016/j.cemconcomp.2004.02.007
Santarelli L (1948) Pozzolane e cementi pozzolanici (Pozzolanas and pozzolana cements) Laboratorio chimico centrale della S.A. 'Italcementi,' ROme, pp 22
de Coutinho AS (1958) Pozzolans, concrete with pozzolans and pozzolanic cements
Scrivener K, Martirena F, Bishnoi S, Maity S (2018) Calcined clay limestone cements (LC3). Cem Concr Res 114:49–56. https://doi.org/10.1016/j.cemconres.2017.08.017
Riding KA, Zayed A (2020) What’s old is new again: a vision and path forward for calcined clay use in the USA. In: Proceedings of the 3rd International conference on calcined clays for sustainable concrete. Bishnoi S (ed), Springer, pp 785–792
Desai P, Kalathingal A (2020) Fresh and hardened properties of pastes and concretes with LC3 and its economic viability: Indian ready mix industry perspective. In: Proceedings of the 3rd international conference on calcined clays for sustainable concrete. Bishnoi S (ed), Springer, pp 821–832
Scrivener KL, John VM, Gartner EM (2018) Eco-efficient cements: potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res 114:2–26. https://doi.org/10.1016/j.cemconres.2018.03.015
Nguyen QD, Afroz S, Castel A (2020) Influence of clay calcination method on the mechanical properties and chloride diffusion resistance of limestone calcined clay cement (LC3) concrete. J Mar Sci Eng 8:1–14. https://doi.org/10.3390/JMSE8050301
Sabir B, Wild S, Bai J (2001) Metakaolin and calcined clays as pozzolans for concrete: a review. Cem Concr Compos 23:441–454. https://doi.org/10.1016/S0958-9465(00)00092-5
Gruber KA, Ramlochan T, Boddy A et al (2001) Increasing concrete durability with high-reactivity metakaolin. Cem Concr Compos 23:479–484. https://doi.org/10.1016/S0958-9465(00)00097-4
Brooks JJ, Megat Johari MA (2001) Effect of metakaolin on creep and shrinkage of concrete. Cem Concr Compos 23:495–502. https://doi.org/10.1016/S0958-9465(00)00095-0
Siddique R, Klaus J (2009) Influence of metakaolin on the properties of mortar and concrete: a review. Appl Clay Sci 43:392–400. https://doi.org/10.1016/j.clay.2008.11.007
Nazário Santos F, Gomes R, de Sousa S, José Faria Bombard A et al (2017) Rheological study of cement paste with metakaolin and/or limestone filler using mixture design of experiments. Constr Build Mater 143:92–103. https://doi.org/10.1016/j.conbuildmat.2017.03.001
ACI 232.1 (2012) Report on the use of raw or processed natural pozzolans in concrete. Farmington Hills, MI
Alujas A, Fernández R, Quintana R et al (2015) Pozzolanic reactivity of low grade kaolinitic clays: influence of calcination temperature and impact of calcination products on OPC hydration. Appl Clay Sci 108:94–101. https://doi.org/10.1016/j.clay.2015.01.028
Avet F, Snellings R, Alujas Diaz A et al (2016) Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem Concr Res 85:1–11. https://doi.org/10.1016/j.cemconres.2016.02.015
Barger GS, Hansen ER, Wood MR et al (2001) Production and use of calcined natural pozzolans in concrete. Cem Concr Aggregates 23:73–80. https://doi.org/10.1520/cca10478j
Fernandez R, Martirena F, Scrivener KL (2011) The origin of the pozzolanic activity of calcined clay minerals: a comparison between kaolinite, illite and montmorillonite. Cem Concr Res 41:113–122. https://doi.org/10.1016/j.cemconres.2010.09.013
Tironi A, Trezza MA, Scian AN, Irassar EF (2012) Kaolinitic calcined clays: factors affecting its performance as pozzolans. Constr Build Mater 28:276–281. https://doi.org/10.1016/j.conbuildmat.2011.08.064
Kaminskas R, Kubiliute R, Prialgauskaite B (2020) Smectite clay waste as an additive for Portland cement. Cem Concr Compos 113:103710. https://doi.org/10.1016/j.cemconcomp.2020.103710
Toledo Filho RD, Gonçalves JP, Americano BB, Fairbairn EMR (2007) Potential for use of crushed waste calcined-clay brick as a supplementary cementitious material in Brazil. Cem Concr Res 37:1357–1365. https://doi.org/10.1016/j.cemconres.2007.06.005
Scrivener KL (2014) Options for the future of cements. Indian Concr J 88:11–21
Antoni M, Rossen J, Martirena F, Scrivener K (2012) Cement substitution by a combination of metakaolin and limestone. Cem Concr Res 42:1579–1589
Avet F, Scrivener K (2018) Investigation of the calcined kaolinite content on the hydration of limestone calcined clay cement (LC3). Cem Concr Res 107:124–135
Dhandapani Y, Sakthivel T, Santhanam M et al (2018) Mechanical properties and durability performance of concretes with limestone calcined clay cement (LC3). Cem Concr Res 107:136–151. https://doi.org/10.1016/j.cemconres.2018.02.005
Dhandapani Y, Santhanam M, Kaladharan G, Ramanathan S (2021) Towards ternary binders involving limestone additions—A review. Cem Concr Res 143:106396. https://doi.org/10.1016/j.cemconres.2021.106396
Bishnoi S, Emmanuel AC, Harshvardhan (2020) Field and laboratory experience on the efficient and durable mixture design of concretes using limestone calcined clay cement. Indian Concr J 94:46–52
Barkat A, Kenai S, Menadi B et al (2019) Effects of local metakaolin addition on rheological and mechanical performance of self-compacting limestone cement concrete. J Adhes Sci Technol 33:963–985. https://doi.org/10.1080/01694243.2019.1571737
Akhlaghi O, Aytas T, Tatli B et al (2017) Modified poly(carboxylate ether)-based superplasticizer for enhanced flowability of calcined clay-limestone-gypsum blended Portland cement. Cem Concr Res 101:114–122. https://doi.org/10.1016/j.cemconres.2017.08.028
Sakai E, Yamada K, Ohta A (2003) Molecular structure and dispersion-adsorption mechanisms of comb-type superplasticizers used in Japan. J Adv Concr Technol 1:16–25. https://doi.org/10.3151/jact.1.16
Yamada K, Ogawa S, Hanehara S (2001) Controlling of the adsorption and dispersing force of polycarboxylate-type superplasticizer by sulfate ion concentration in aqueous phase. Cem Concr Res. https://doi.org/10.1016/S0008-8846(00)00503-2
Iruthayaraj J (2008) Poly (Ethylene Oxide) based bottle-brush polymers and their interaction with the anionic surfactant sodium dodecyl sulphate solution and interfacial properties. Royal Technical University, Stockholm
Claesson PM, Makuska R, Varga I et al (2010) Bottle-brush polymers: adsorption at surfaces and interactions with surfactants. Adv Colloid Interface Sci 155:50–57
Hsu H-P, Paul W, Binder K (2011) Structure of bottle brush polymers on surfaces: weak versus strong adsorption. J Phys Chem B 115:14116–14126. https://doi.org/10.1021/jp204006z
Chiang WS, Fratini E, Ridi F et al (2013) Microstructural changes of globules in calcium-silicate-hydrate gels with and without additives determined by small-angle neutron and X-ray scattering. J Colloid Interface Sci 398:67–73. https://doi.org/10.1016/j.jcis.2013.01.065
Ilg M, Plank J (2019) Synthesis and properties of a polycarboxylate superplasticizer with a jellyfish-like structure comprising hyperbranched polyglycerols. Ind Eng Chem Res 58:12913–12926. https://doi.org/10.1021/acs.iecr.9b02077
Ridi F, Fratini E, Luciani P et al (2012) Tricalcium silicate hydration reaction in the presence of comb-shaped superplasticizers: boundary nucleation and growth model applied to polymer-modified pastes. J Phys Chem C 116:10887–10895. https://doi.org/10.1021/jp209156n
Schmid M, Plank J (2020) Dispersing performance of different kinds of polycarboxylate (PCE) superplasticizers in cement blended with a calcined clay. Constr Build Mater 258:119576. https://doi.org/10.1016/j.conbuildmat.2020.119576
Nehdi ML (2014) Clay in cement-based materials: critical overview of state-of-the-art. Constr Build Mater 51:372–382. https://doi.org/10.1016/j.conbuildmat.2013.10.059
Lei L, Plank J (2014) Synthesis and properties of a vinyl ether-based polycarboxylate superplasticizer for concrete possessing clay tolerance. Ind Eng Chem Res 53:1048–1055. https://doi.org/10.1021/ie4035913
Ait-Akbour R, Boustingorry P, Leroux F et al (2015) Adsorption of PolyCarboxylate Poly(ethylene glycol) (PCP) esters on montmorillonite (Mmt): effect of exchangeable cations (Na+, Mg 2+ and Ca 2+ ) and PCP molecular structure. J Colloid Interface Sci 437:227–234. https://doi.org/10.1016/j.jcis.2014.09.027
Ait-Akbour R, Taviot-Guého C, Leroux F, et al (2015) Interaction of montmorillonite with poly(ethylene glycol) and poly(methacrylic acid) polymers. Consequences on the influence of clays on superplasticizer efficiency. In: American concrete institute, ACI Special Publication
Ng S (2012) Interactions of polycarboxylate based superplasticizers with montmorillonite clay in portland cement and with calcium aluminate cement. Technische Universität München
Ng S, Plank J (2012) Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem Concr Res 42:847–854. https://doi.org/10.1016/j.cemconres.2012.03.005
Lei L, Plank J (2014) A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem Concr Res 60:1–10. https://doi.org/10.1016/j.cemconres.2014.02.009
Ma Y, Shi C, Lei L et al (2020) Research progress on polycarboxylate based superplasticizers with tolerance to clays - a review. Constr Build Mater 255:119386. https://doi.org/10.1016/j.conbuildmat.2020.119386
Lu Y, Kong ST, Deiseroth HJ, Mormann W (2008) Structural requirements for the intercalation of polyether polyols into sodium-montmorillonite: the role of oxyethylene sequences. Macromol Mater Eng 293:900–906. https://doi.org/10.1002/mame.200800155
Lei L, Plank J (2012) A concept for a polycarboxylate superplasticizer possessing enhanced clay tolerance. Cem Concr Res 42:1299–1306. https://doi.org/10.1016/j.cemconres.2012.07.001
Sun C, Zhou H, Li X, et al (2015) The clay-tolerance of amide-modified polycarboxylate superplasticizer and its performance with clay-bearing aggregates. In: International conference on Materials, Environmental And Biological Engineering (MEBE 2015). pp 237–241
Xu H, Sun S, Wei J et al (2015) β-Cyclodextrin as pendant groups of a polycarboxylate superplasticizer for enhancing clay tolerance. Ind Eng Chem Res 54:9081–9088. https://doi.org/10.1021/acs.iecr.5b02578
Li Y, Zhang Y, Zheng J et al (2013) Dispersion and rheological properties of concentrated kaolin suspensions with polycarboxylate copolymers bearing comb-like side chains. J Eur Ceram Soc. https://doi.org/10.1016/j.jeurceramsoc.2013.07.009
Pefferkorn E, Nabzar L, Varoqui R (1987) Polyacrylamide Na-kaolinite interactions: effect of electrolyte concentration on polymer adsorption. Colloid Polym Sci 265:889–896. https://doi.org/10.1007/BF01421817
Fernandez Lopez R (2009) Calcined clayey soils as a potential replacement for cement in developing countries. 4302:Ph.D Thesis, EPFL. https://doi.org/10.5075/epfl-thesis-4302
Nair N, Mohammed Haneefa K, Santhanam M, Gettu R (2020) A study on fresh properties of limestone calcined clay blended cementitious systems. Constr Build Mater 254:119326. https://doi.org/10.1016/j.conbuildmat.2020.119326
Mario Collepardi (2005) Chemical admixtures today. In: Proceedings of second international symposium on concrete tecnology for sustainable development with emphasis on infrastructure. pp 527–541
Perche F, Houst YF, Bowen P, Hofmann H (2003) Adsorption of lignosulfonates and polycarboxylates depletion and electroacoustic methods. 7th Int conf superplast other chem admixtures concr suppl pap pp 1–15
Ran L, Lei L, Tongbo S, Plank J (2020) Approaches to achieve fluidity retention in low-carbon calcined clay blended cements. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.127770
Lorentz B, Zhu H, Mapa D, et al (2020) Effect of clay mineralogy, particle size, and chemical admixtures on the rheological properties of CCIL and CCI/II systems. In: Proceedings of the 3rd international conference on calcined clays for sustainable concrete. pp 211–218
Hou P, Muzenda TR, Li Q et al (2021) Mechanisms dominating thixotropy in limestone calcined clay cement (LC3). Cem Concr Res 140:106316. https://doi.org/10.1016/J.CEMCONRES.2020.106316
Ferron R, Gregori A, Sun Z, Shah SP (2007) Rheological method to evaluate structural buildup in self-consolidating concrete cement pastes. ACI Mater J 104:242–250
Sonebi M, Lachemi M, Hossain KMA (2013) Optimisation of rheological parameters and mechanical properties of superplasticised cement grouts containing metakaolin and viscosity modifying admixture. Constr Build Mater 38:126–138. https://doi.org/10.1016/j.conbuildmat.2012.07.102
Janotka I, Puertas F, Palacios M et al (2010) Metakaolin sand-blended-cement pastes: rheology, hydration process and mechanical properties. Constr Build Mater 24:791–802. https://doi.org/10.1016/j.conbuildmat.2009.10.028
Vance K, Kumar A, Sant G, Neithalath N (2013) The rheological properties of ternary binders containing Portland cement, limestone, and metakaolin or fly ash. Cem Concr Res 52:196–207. https://doi.org/10.1016/j.cemconres.2013.07.007
Nazário Santos F, Gomes R, de Sousa S, José Faria Bombard A, Lopes Vieira S (2017) Rheological study of cement paste with metakaolin and/or limestone filler using mixture design of experiments. Constr Build Mater 143:92–103. https://doi.org/10.1016/j.conbuildmat.2017.03.001
Ferreiro S, Herfort D, Damtoft JSS (2017) Effect of raw clay type, fineness, water-to-cement ratio and fly ash addition on workability and strength performance of calcined clay—limestone Portland cements. Cem Concr Res 101:1–12. https://doi.org/10.1016/j.cemconres.2017.08.003
Paiva H, Velosa A, Cachim P, Ferreira VM (2012) Effect of metakaolin dispersion on the fresh and hardened state properties of concrete. Cem Concr Res 42:607–612. https://doi.org/10.1016/j.cemconres.2012.01.005
ASTM C494 (2015) Standard Specification for Chemical Admixtures for Concrete. ASTM Int. https://doi.org/10.1520/C0494
Zaribaf BH, Kurtis KE (2018) Admixture compatibility in metakaolin–Portland-limestone cement blends. Mater Struct Constr 51:1–13. https://doi.org/10.1617/s11527-018-1154-7
Sposito R, Beuntner N, Thienel K-C (2019) Rheology, setting and hydration of calcined clay blended cements in interaction with PCE. Mag Concr Res. https://doi.org/10.1680/jmacr.19.00488
Muzenda TR, Hou P, Kawashima S et al (2020) The role of limestone and calcined clay on the rheological properties of LC3. Cem Concr Compos 107:103516. https://doi.org/10.1016/j.cemconcomp.2020.103516
Caldarone M, Gruber KA, Burg RG (1994) High reactivity metakaolin (HRM) a new generation mineral admixture. Concr Int 16:37–40
Bai J, Wild S, Sabir BB, Kinuthia JM (1999) Workability of concrete incorporating pulverized fuel ash and metakaolin. Mag Concr Res 51:207–216
Cassagnabère F, Diederich P, Mouret M et al (2013) Impact of metakaolin characteristics on the rheological properties of mortar in the fresh state. Cem Concr Compos 37:95–107. https://doi.org/10.1016/j.cemconcomp.2012.12.001
Roussel N, Ovarlez G, Garrault S, Brumaud C (2012) The origins of thixotropy of fresh cement pastes. Cem Concr Res 42:148–157. https://doi.org/10.1016/j.cemconres.2011.09.004
Curcio F, DeAngelis BA (1998) Dilatant behavior of superplasticized cement pastescontaining metakaolin. Cem Concr Res 28:629–634
Cyr M, Legrand C, Mouret M (2000) Study of the shear thickening effect of superplasticizers on the rheological behaviour of cement pastes containing or not mineral additives. Cem Concr Res 30:1477–1483. https://doi.org/10.1016/S0008-8846(00)00330-6
Ferron RD, Shah S, Fuente E, Negro C (2013) Aggregation and breakage kinetics of fresh cement paste. Cem Concr Res 50:1–10. https://doi.org/10.1016/J.CEMCONRES.2013.03.002
Beigh MAB, Nerella VN, Schröfl C, Mechtcherine V (2020) Studying the rheological behavior of limestone calcined clay cement (LC3) mixtures in the context of extrusion-based 3d-printing. In: Proceedings of the 3rd international conference on calcined clays for sustainable concrete. pp 229–236
Thienel C, Beuntner N, Chucholowski C, Scherb S (2018) Performance of calcined clays in mineral construction materials. In: Ibausil-20. Internationale Baustofftagung. p 18
Chen Y, Chaves Figueiredo S, Yalçinkaya Ç, et al (2019) The effect of viscosity-modifying admixture on the extrudability of limestone and calcined clay-based cementitious material for extrusion-based 3D concrete printing. Mater (Basel, Switzerland) 12(9):1374
Chen Y, Romero Rodriguez C, Li Z et al (2020) Effect of different grade levels of calcined clays on fresh and hardened properties of ternary-blended cementitious materials for 3D printing. Cem Concr Compos. https://doi.org/10.1016/j.cemconcomp.2020.103708
Chen Y, Chaves Figueiredo S, Li Z et al (2020) Improving printability of limestone-calcined clay-based cementitious materials by using viscosity-modifying admixture. Cem Concr Res 132:106040. https://doi.org/10.1016/j.cemconres.2020.106040
Perlot C, Rougeau P, Dehaudt S (2013) Slurry of metakaolin combined with limestone addition for self-compacted concrete. Application for precast industry. Cem Concr Compos 44:50–57. https://doi.org/10.1016/j.cemconcomp.2013.07.003
Vejmelková E, Keppert M, Grzeszczyk S et al (2011) Properties of self-compacting concrete mixtures containing metakaolin and blast furnace slag. Constr Build Mater 25:1325–1331. https://doi.org/10.1016/j.conbuildmat.2010.09.012
Larsen LO, Naruts VV (2016) Self-compacting concrete with limestone powder for transport infrastructure. Mag Civ Eng 68:76–85. https://doi.org/10.5862/MCE.68.8
Harshvardhan, Emmanuel AC, Bishnoi S (2020) Assessment of sorptivity and porosity characteristics of self-compacting concrete from blended cements using calcined clay and Fly Ash at various replacement levels. In: Proceedings of the 3rd international conference on calcined clays for sustainable concrete. Bishnoi S (ed), Springer, pp 691–699
Branch J, Hannant DJ, Mulheron M (2002) Factors affecting the plastic shrinkage cracking of high-strength concrete. Mag Concr Res 54:347–354. https://doi.org/10.1680/macr.2002.54.5.347
Cordoba GP, Zito SV, Sposito R et al (2020) Concretes with calcined clay and calcined shale: workability, mechanical, and transport properties. J Mater Civ Eng 32:1–11. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003296
Niknezhad D, Kamali-Bernard S, Garand C (2015) Influence of mineral admixtures (metakaolin, slag, fly ash) on the plastic, free, and restrained shrinkage of SCCs. Concreep 10:1157–1166
Amer AA, El-Hoseny S (2017) Properties and performance of metakaolin pozzolanic cement pastes. J Therm Anal Calorim 129:33–44. https://doi.org/10.1007/s10973-017-6087-9
Amin N-ul (2010) Use of clay as a pozzolona in high strength Portland cement and its thermal activation Chinese J. Geochemistry 29(143):145. https://doi.org/10.1007/s11631-010-0143-5
Özcan F, Kaymak H (2018) Utilization of metakaolin and calcite: working reversely in workability aspect - as mineral admixture in self-compacting concrete. Adv Civ Eng. https://doi.org/10.1155/2018/4072838
Rahhal V, Talero R (2014) Very early age detection of ettringite from pozzolan origin. Constr Build Mater 53:674–679. https://doi.org/10.1016/j.conbuildmat.2013.10.082
Shah V, Parashar A, Mishra G et al (2020) Influence of cement replacement by limestone calcined clay pozzolan on the engineering properties of mortar and concrete. Adv Cem Res 32:101–111. https://doi.org/10.1680/jadcr.18.00073
Marchetti G, Rahhal VF, Irassar EF (2017) Influence of packing density and water film thickness on early-age properties of cement pastes with limestone filler and metakaolin. Mater Struct 50:111. https://doi.org/10.1617/s11527-016-0979-1
Brooks JJ, Johari MAM, Mazloom M (2000) Cement & concrete composites effect of admixtures on the setting times of high-strength. Cem Concr Compos 22:293–301. https://doi.org/10.1016/S0958-9465(00)00025-1
Elinwa AU (2006) Experimental characterization of Portland cement-calcined soldier-ant mound clay cement mortar and concrete. Constr Build Mater 20:754–760. https://doi.org/10.1016/j.conbuildmat.2005.01.053
Govindarajan D, Gopalakrishnan R, Rao PS (2008) Electron paramagnetic resonance study on metakaolin-admixtured cement paste at different hydrated periods. Radiat Eff Defects Solids 163:795–804. https://doi.org/10.1080/10420150701692315
Güneyisi E, Gesoğlu M, Gu E et al (2008) Properties of self-compacting mortars with binary and ternary cementitious blends of fly ash and metakaolin. Mater Struct 41:1519–1531. https://doi.org/10.1617/s11527-007-9345-7
Justice JM, Kurtis KE (2007) Influence of metakaolin surface area on properties of cement-based materials. J Mater Civ Eng 19:762–771. https://doi.org/10.1061/(ASCE)0899-1561(2007)19:9(762)
Khaleel OR, Abdul Razak H (2012) The effect of powder type on the setting time and self compactability of mortar. Constr Build Mater 36:20–26. https://doi.org/10.1016/j.conbuildmat.2012.04.079
Mwiti MJ, Karanja TJ, Muthengia WJ (2018) Properties of activated blended cement containing high content of calcined clay. Heliyon 4:e00742. https://doi.org/10.1016/j.heliyon.2018.e00742
Niknezhad D, Kamali-Bernard S, Mesbah HA (2017) Self-compacting concretes with supplementary cementitious materials: shrinkage and cracking tendency. J Mater Civ Eng 29:04017033. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001852
Wang B, Ma H, Li M, Han Y (2013) Effect of metakaolin on the physical properties and setting time of high performance concrete. Key Eng Mater 539:195–199. https://doi.org/10.4028/www.scientific.net/KEM.539.195
Vu DD, Stroeven P, Bui VB (2001) Strength and durability aspects of calcined kaolin-blended Portland cement mortar and concrete. Cem Concr Compos 23:471–478. https://doi.org/10.1016/S0958-9465(00)00091-3
EFNARC (2002) Specification and guidelines for self-compacting concrete. Rep from EFNARC 44:32
Bakera AT, Alexander MG (2019) Use of metakaolin as a supplementary cementitious material in concrete, with a focus on durability properties. RILEM Tech Lett 4:89–102
Gesoǧlu M, Güneyisi E, Özturan T, Mermerdaş K (2014) Permeability properties of concretes with high reactivity metakaolin and calcined impure kaolin. Mater Struct 47:709–728
Mermerdaş K, Gesoǧlu M, Güneyisi E, Özturan T (2012) Strength development of concretes incorporated with metakaolin and different types of calcined kaolins. Constr Build Mater 37:766–774. https://doi.org/10.1016/j.conbuildmat.2012.07.077
Güneyisi E, Gesoğlu M, Mermerdaş K (2010) Strength deterioration of plain and metakaolin concretes in aggressive sulfate environments. J Mater Civ Eng 22:403–407. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000034
de Larrard F (1999) Concrete mixture proportioning: a scientific approach (Modern Concrete Technology Series)
Kumar SV, Santhanam M (2003) Particle packing theories and their application in concrete mixture proportioning: a review. Indian Concr J 77:1324–1331
Vaasudevaa B (2020) Assessment of slump retention characteristics in high performance concrete (Master Thesis, IIT Madras)
Avet F, Sofia L, Scrivener K (2019) Concrete performance of limestone calcined clay cement (LC3) compared with conventional cements. Adv Civ Eng Mater 8:20190052. https://doi.org/10.1520/acem20190052
Acknowledgements
TC Membership: Chair: Fernando Martirena-Hernandez, Cuba; Deputy Chair: Manu Santhanam, India; Regular Members: Eduardo Irassar, Argentina; Arnaud Castel, David Law, Sumaiya Afroz, Taehwin Kim, Vinh Dao, Australia; Jan Elsen, Ruben Snellings, Belgium; Silvia Vieira, Brazil; Arezki Tagnit-Hamou, William Wilson, Canada; Kequan Yu, Tongbo Sui, Zengfeng Zhao, China; Oscar Oswaldo Vásquez, Colombia; Adrian Alujas, Roger Samuel Roger, Cuba; Joergen Skibsted, Mariana Canut, Sergio Ferreiro Garzón, Wolfgang Kunther, Denmark; Fabrizio Moro, François Avet, Gabriel Pham, Gilles Escadeillas, Pascal Dion, Pascal Boustingorry, Victor Poussardin, France; Alisa Machner, Elsa Qoku, Frank Dehn, Karl-Christian Theinel, Matthias Maier, Mohsen Ben Haha, Germany; Luis Velasquez, Guatemala; Anuj Parashar, Sri Kalyana Rama Jyosyula, Ravindra Gettu, Shashank Bishnoi, Talakokula Visalakshi, Tushar Bansal, Yuvaraj Dhandapani, India; Laith Al-Jaberi, Iraq; Luca Valentini, Italy; Joseph Mwiti Marangu, Kenya; Sol Moi Park, Korea; J Ivan Escalante-Garcia, Mexico; Hassan Ez-Zaki, Morroco; Roman Jaskulski, Poland; Angela Maria Nunes, Karyne Ferreira do Santos, Manuel Vieira, Portugal; Guoqing Geng, Singapore; Franco Zunino, Karen Scrivener, Switzerland; Alastair Marsh, Daniel Geddes, Hoda Beltagui, Wenzhong Zhu, Fragkoulis Kanavaris, John Provis, Shiju Joseph, Susan Bernal Lopez, Theodore Hanein, UK; Claire White, Katelyn O’Quinn, Kyle Riding, Maria C.G. Juenger, USA
Funding
No funding was provided for this work. This work was performed by a volunteer working subgroup of RILEM committee 282-CCL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review paper conception and design. The first draft of the manuscript was written by YD, SJ, DAG, ZZ, PB, SB, MV, and KA. Riding and was edited by FM, AC, FK, and KA. Riding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None. This paper was prepared by a working subgroup of RILEM committee 282-CCL.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper has been prepared by RILEM TC 282-CCL. The paper has been reviewed and approved by all members of the TC.
TC Membership: Chair: Fernando Martirena-Hernandez, Cuba; Deputy Chair: Manu Santhanam, India; Regular Members: Eduardo Irassar, Argentina; Arnaud Castel, David Law, Sumaiya Afroz, Taehwin Kim, Vinh Dao, Australia; Jan Elsen, Ruben Snellings, Belgium; Silvia Vieira, Brazil; Arezki Tagnit-Hamou, William Wilson, Canada; Kequan Yu, Tongbo Sui, Zengfeng Zhao, China; Oscar Oswaldo Vásquez, Colombia; Adrian Alujas, Roger Samuel Roger, Cuba; Joergen Skibsted, Mariana Canut, Sergio Ferreiro Garzón, Wolfgang Kunther, Denmark; Fabrizio Moro, François Avet, Gabriel Pham, Gilles Escadeillas, Pascal Dion, Pascal Boustingorry, Victor Poussardin, France; Alisa Machner, Elsa Qoku, Frank Dehn, Karl-Christian Theinel, Matthias Maier, Mohsen Ben Haha, Germany; Luis Velasquez, Guatemala; Anuj Parashar, Sri Kalyana Rama Jyosyula, Ravindra Gettu, Shashank Bishnoi, Talakokula Visalakshi, Tushar Bansal, Yuvaraj Dhandapani, India; Laith Al-Jaberi, Iraq; Luca Valentini, Italy; Joseph Mwiti Marangu, Kenya; Sol Moi Park, Korea; J Ivan Escalante-Garcia, Mexico; Hassan Ez-Zaki, Morroco; Roman Jaskulski, Poland; Angela Maria Nunes, Karyne Ferreira do Santos, Manuel Vieira, Portugal; Guoqing Geng, Singapore; Franco Zunino, Karen Scrivener, Switzerland; Alastair Marsh, Daniel Geddes, Hoda Beltagui, Wenzhong Zhu, Fragkoulis Kanavaris, John Provis, Shiju Joseph, Susan Bernal Lopez, Theodore Hanein, UK; Claire White, Katelyn O’Quinn, Kyle Riding, Maria C.G. Juenger, USA.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhandapani, Y., Joseph, S., Geddes, D.A. et al. Fresh properties of concrete containing calcined clays: a review by RILEM TC-282 CCL. Mater Struct 55, 151 (2022). https://doi.org/10.1617/s11527-022-01971-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-01971-3