Abstract
Barium titanate (BTO) is a ferroelectric perovskite used in electronics and energy storage systems because of its high dielectric constant. Decreasing the BTO particle size was shown to increase the dielectric constant of the perovskite, which is an intriguing but contested result. We investigated this result by fabricating silicone-matrix nanocomposite specimens containing BTO particles of decreasing diameter. Furthermore, density functional theory modeling was used to understand the interactions at the BTO particle surface. Combining results from experiments and modeling indicated that polymer type, particle surface interactions, and particle surface structure can influence the dielectric properties of polymer-matrix nanocomposites containing BTO.
Graphical abstract

The dielectric properties, surfaces, and interfaces of silicone-matrix nanocomposites containing barium titanate particles were investigated through experimental measurements of fabricated specimens, finite element modeling of the nanocomposites, and density functional theory simulations of surface interactions on barium titanate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barium titanate (BaTiO3 or BTO) is a ferroelectric perovskite with a tetragonal lattice structure at room temperature that exhibits exceptional electrical polarizability.[1] Studies into BTO have been motivated by its potential to significantly impact the field of energy storage.[1,2,3] For example, multilayer ceramic capacitors used in voltage regulators and power supplies are composed of hundreds of dielectric layers, which makes miniscule BTO nanoparticles with enhanced dielectric properties highly desirable in these applications.[1,2,3] BTO is also an environmentally favorable alternative to energy storage materials that contain lead.[2,4] Therefore, research into the structure–properties relationships of BTO has been motivated by the various energy storage applications of the perovskite material.
The dielectric constant of BTO impacts its ability to store energy, but the relationships between the structure and dielectric constant of BTO are still not fully understood.[2] Several studies have suggested that the dielectric constant of BTO can be affected by the size and structure of the perovskite. For instance, BTO particles suspended in propylene carbonate[5] were reported to exhibit a peak dielectric constant of 15,000 at a particle diameter of 70 nm, which then decreased to the bulk dielectric constant of BTO as particle size increased. In contrast, studies on sintered BTO specimens showed that the dielectric constant of BTO decreased as grain sizes became smaller.[6,7,8] The detrimental effect of decreasing grain size on the dielectric constant of sintered BTO was attributed to (1) the room temperature tetragonal ferroelectric structure of BTO transforming to a cubic paraelectric structure below a critical grain size of 200 nm[6,8,9] and (2) small-grained specimens containing a larger number of disordered boundary regions that have lower dielectric properties.[8] However, sintered specimens have different interfacial characteristics than particles in a suspension, which has inspired alternative methods of investigating the dielectric constants of BTO by incorporating the perovskite into thermosetting and thermoplastic polymers.[10,11,12,13] An increase in the dielectric constant of BTO at smaller particle diameters was not revealed in these studies, but challenges in fabricating polymers filled with BTO have limited the information gained from BTO-filled polymer-matrix nanocomposites[10,11,13]. For example, epoxy,[12] low density polyethylene (LDPE),[10] and acrylonitrile butadiene styrene (ABS)[11] have been used to create BTO-polymer nanocomposites. Challenges with using epoxy, LDPE, and ABS have included difficulties with incorporating small diameter (≤ 100 nm) BTO nanoparticles into the polymers, as well as issues with achieving high levels of BTO loading (≥ 30 vol%) in the matrices. Thus, elastomer-matrix nanocomposites containing BTO could potentially address the challenges of thermoset and thermoplastic matrices and reveal the impact of BTO particle size on the dielectric constant of the perovskite.
Experiments aimed at determining the dielectric constants of BTO in polymers could be complicated by compounds that are present in the nanocomposites. This is because both the dielectric properties of BTO nanoparticles and the process of fabricating BTO nanocomposites can be influenced by the surface layer of the particles.[8,14] Density functional theory (DFT) can give precise atomic-scale simulations of BTO surfaces, which enables a detailed understanding of how the crystal lattice is affected by a particle’s environment. For example, previous DFT studies have shown that the surfactant tert-butyl phosphonic acid (tBuPA) binds more strongly than water to the TiO2-terminated surface of BTO.[15] DFT has also been used to simulate BaO-terminated BTO surfaces, and studies have focused on the binding strengths of water, tBuPA, and hydrogen gas,[14] as well as the binding and dissociation of water at different levels of surface coverage.[16] However, previous investigations have not considered defects on the surface of the BTO lattice that can have an effect on reactivity. Additional DFT studies on the surfaces and interfaces of BTO are currently needed to gain a better understanding of the structure–property relationships of BTO-filled polymer-matrix nanocomposites. Advancing the understanding of surface interactions of BTO in polymers could lead to new methods of (1) uniformly dispersing inorganic fillers in polymers and (2) achieving higher volume loadings of ferroelectric nanoparticles in polymer-matrix nanocomposites, which will enable the development of improved materials with enhanced permittivity for electronic devices and energy storage systems.[17,18]
Here, we present the results of experimental studies aimed at investigating the relationships between the particle diameter and dielectric constant of BTO nanoparticles incorporated into an elastomer. Furthermore, we report DFT simulations that provide insight into the surface interactions between BTO, surface defects, water molecules, hydrogen, and hydroxyl radicals. The DFT results elucidate how water and dissociated radicals that may be present during nanocomposite fabrication can react with the BaO-terminated surfaces of BTO. Therefore, this report (1) provides new experimental and computational knowledge on processes that can affect the structure of elastomers containing BTO and (2) advances our understanding of the relationship between the particle size and dielectric constant of BTO. Combining the experimental and computational results indicated that polymer type, particle surface interactions, and particle surface structure can influence the dielectric properties of polymer-matrix nanocomposites containing BTO.
Materials and methods
Experimental materials
BTO powders that were synthesized through a hydrothermal process[11] were provided by Sakai Chemical Industry Co., Ltd. BTO particles with diameters of 100 nm (BT-01), 200 nm (BT-02), 300 nm (BT-03), 400 nm (BT-04), and 500 nm (BT-05) were used in this study. The surfactant tBuPA was purchased from Sigma-Aldrich. The polydimethylsiloxane (PDMS) used to fabricate BTO-filled elastomer-matrix nanocomposites was Dow Sylgard 184.
Specimen fabrication process
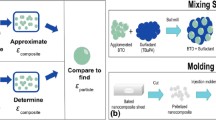
Nanocomposites composed of PDMS and tBuPA-coated BTO particles of varying diameters were fabricated using a nine-step process. First, tBuPA was added to BTO in a mixing container. The amounts of tBuPA and BTO used in this study were determined using the equations provided in section S1 of the Supplementary Information (SI). Second, tBuPA and BTO were ball milled for a total of four hours in a Retsch PM100 at 150 rpm using 0.8 mm yttria-stabilized zirconia as grinding media. Third, the grinding balls were filtered out after the ball milling process to obtain a powder consisting of tBuPA-coated BTO particles and the filtered powder was deposited into a separate container. Fourth, an amount of PDMS that was needed to fabricate specimens with a BTO loading of 30 vol% was deposited into the planetary mixing container. Fifth, the PDMS curing agent was added to the planetary mixing container, which was then subjected to planetary mixing for 90 s using a Mazerustar KK-250S planetary mixer. Sixth, ball-milled tBuPA-coated BTO powder was poured into the mixture of PDMS and curing agent, which was then subjected to planetary mixing. Seventh, the uniform mixture of tBuPA-coated BTO, PDMS, and curing agent were poured into flat-bottomed plastic jars and degassed at 700 mmHg. Eighth, the plastic jars containing the nanocomposite mixtures were placed in planetary mixing containers and subjected to planetary mixing. As shown in Fig. S1, the purpose of the eighth step is to flatten and smoothen the top surface of the mixture within the jars to form disk-shaped specimens. Ninth, the flattened mixtures were cured at room temperature for 72 h, which resulted in disk-shaped nanocomposite specimens. A transmission electron microscope image of a cured BTO-PDMS specimen is shown in Fig. 1(a). This nine-step process was used to create nanocomposites containing BT-01, BT-02, BT-03, BT-04, and BT-05.
(a) Transmission electron microscope image of BT-04 in a PDMS matrix. Scale bar is 1 µm. (b) A typical BTO-PDMS nanocomposite with gold electrodes sputtered onto each side of the specimen. (c) Process of determining nanocomposite and particle dielectric constants using capacitance measurements of fabricated specimens and COMSOL.
Nanocomposite characterization
After curing, the BTO loadings of the fabricated specimens were determined using a Sartorius density determination kit affixed to a Sartorius balance, which utilizes the Archimedes method.[19,20] A Quorum Technologies Q150T sputter coater was then used to deposit gold film with a thickness of 100 nm on both sides of each specimen to create a parallel plate capacitor [Fig. 1(b)]. Capacitance measurements of the gold-sputtered nanocomposites were taken using a capacitance meter operating at 800 Hz and the custom-built capacitance measurement device shown in Fig. S2. All capacitance measurements were taken inside a Faraday cage to minimize external perturbations, and the dielectric constant and permittivity of each specimen were calculated using the equations shown in section S3 of the SI. In addition to measuring the capacitances of fabricated nanocomposites, pure PDMS specimens were also fabricated and measured to compare the properties of pure PDMS to the nanocomposites. The thickness and sputtered diameter of each specimen were each measured three times and averaged to calculate dielectric constant.
Finite element modeling of BTO in PDMS
The process that was used to determine the dielectric constants of BTO is shown in Fig. 1(c). COMSOL Multiphysics version 6.0 and its capabilities in electrostatics modeling were used to perform finite element (FE) modeling of the BTO-PDMS nanocomposites to determine the dielectric constants of BTO particles. As shown in Fig. S3, the model consists of a PDMS matrix that is shaped as a rectangular solid. Thin gold electrodes with a thickness that are each 1% of the vertical height of the rectangular solid were placed on the top and bottom faces of the PDMS matrix. The inner surface of the top electrode was held at a 10 V difference between the inner surface of the bottom electrode. Within the PDMS matrix are ellipsoidal particles representing BTO, which have shorter semiaxes equal to the radius of the particles and the longer semiaxis equal to two times the radius. The number of particles and size of the rectangular solid were chosen to match experimentally determined BTO loadings. A tetrahedral mesh was applied to all entities in the model. To maintain speed, repeatability, and reproducibility in the simulations, element sizes were chosen with predefined options in COMSOL. The mesh size chosen for the FE modeling was “fine.” Dielectric constants of nanocomposites were obtained in COMSOL using a volumetric internal energy calculation that was previously reported by Kaufman et al.[12] Dielectric constants of the BTO particles were then iteratively adjusted in COMSOL until the FE values for nanocomposite dielectric constants matched the experimental nanocomposite dielectric constants. Both the (1) modeling of ellipsoidal BTO particles in PDMS rectangular solids used in this study and (2) extraction of BTO particle dielectric constants using COMSOL and experimental data have been used to successfully determine the dielectric constants of BTO in polymers.[10,11,12]
DFT methods
DFT calculations were performed using SeqQuest,[21] a local basis DFT code with the slab functionality. The Perdew-Burke-Ernzerhof (PBE) functional was chosen, as it generally provides good results for surface binding calculations and enables direct comparison to previous work.[15] Electronic structures were described using norm-conserving pseudopotentials and Gaussian basis sets. The energy of a slab of BTO, periodic in two dimensions and finite in the third, was minimized by allowing the geometry of the slab to relax via an accelerated steepest descent method. Calculations were considered converged and self-consistent when elements of the Hamiltonian matrix changed by no more than 0.0005 Ry (0.0068 eV).[21] Slabs were 6 atoms by 6 atoms in the periodic dimensions and 5 atoms deep in the non-periodic direction, which minimized computational resource use while allowing enough space that water and other small ligands would not significantly repel each other. A reference slab without ligands was relaxed to determine the baseline atom positions, and it adopted a cubic phase. Subsequent simulations with ligands used this reference for the initial atom positions and froze the lowest two layers of atoms in place, which did not allow them to relax and prevented a ferroelectric response. The small dipole of a ligand binding to the BTO surface can create a large ferroelectric polarization response in the slab, which can overwhelm the change in energy due to ligand binding, so suppression of this response was desired to study the surface–ligand interactions. Previous research has shown that five layers of atoms, with the lowest two held in place, accurately determine binding energy and suppress ferroelectric response without excessive calculation.[15] Binding energies were calculated using the following equation:
The energy of a free water molecule was determined by relaxing water under the above conditions, except that the simulation was finite (not periodic) in all three dimensions.
Results and discussion
Dielectric constants of BTO-PDMS specimens
Measurements of the dielectric constants of BTO-PDMS specimens revealed that decreasing the size of BTO in PDMS did not significantly affect the dielectric constants of the nanocomposites. As shown in Fig. 2, specimens containing BTO with diameters of 300 nm and 400 nm were found to have slightly higher average nanocomposite dielectric constants relative to specimens containing BTO with diameters of 100 nm, 200 nm, and 500 nm. However, the average dielectric constants were all within the error bars of the 100 nm specimen. Based on these experimental results alone, the similar dielectric properties of PDMS containing comparable loadings of BTO with different diameters indicate that the dielectric constant of BTO does not change with particle size. However, comparing the results of previous experimental and FE studies on BTO-polymer nanocomposites[10,11,22] to the data shown in Fig. 2 and the COMSOL results shown in Fig. S4 indicates that the PDMS matrix could be preventing the properties of BTO from being accurately determined. The dielectric constants of BTO nanoparticles shown in Fig. S4 were obtained using FE models that utilized the composite dielectric constants shown in Fig. 2. Significant variations in the data in Fig. S4 were observed between samples of the same particle size at 100 and 200 nm, but the dielectric constant of BTO was observed to increase as particle size decreased from 500 to 300 nm. Despite the large error bars in the data, the dielectric constants of BTO in PDMS shown in Fig. S4 were much lower than the BTO dielectric constants that were reported in previous studies on BTO in LDPE[10] and ABS.[11] Similar BTO powders and filler loadings were used in these previous studies with thermoplastic matrices, which indicates that different polymer types can influence the measured BTO dielectric constant. Indeed, the BTO-polymer interface has been shown to have a considerable effect on the resulting dielectric properties of a nanocomposite.[23] A possible explanation for the discrepancy between the BTO dielectric constants obtained from BTO-LDPE, BTO-ABS, and BTO-PDMS specimens is that the elastomer matrix could be inhibiting the ferroelectric properties of the BTO filler. Specifically, a large contrast between composite and particle dielectric constant could lead to a highly inhomogeneous electric field.[23] An incompatibility between the polymer matrix and filler could also impede the formation of a homogenous composite.[23] Thus, the experimental and FE results in this study indicate that (1) PDMS could be preventing an accurate measurement of BTO dielectric constants and/or (2) the BTO dielectric constant does not drastically increase as particle size is reduced.
DFT simulations
DFT simulations produced the results shown in Tables S1, S2, S3, S4, S5 of the SI. To begin our analysis on surface interactions of BTO, we tested a single water molecule at the BaO-terminated surface without defects. Coordinate locations for the atoms in water were determined using the bond angle and lengths of a relaxed water molecule simulation. Additionally, a distance of 2.25 Å was chosen as the distance between the surface and water molecules, as this distance was close enough for molecules to be pulled toward the surface if desired and far enough to avoid repelling forces from the surface. Within these constraints, various rotations and placements of the water molecules were tested. These were allowed to simulate until finding a relaxed energy state. After simulation, the binding energy of the water was calculated as described in Eq. 1. Negative results of a large magnitude from these calculations were identified as favorable surface interactions.
Seven simulations were conducted with the defect-free BTO slab and various water positions. Of these simulations, the most favorable binding energy was −0.829 eV, which resulted from the water molecule being initially positioned with all three atoms in the same horizontal plane and one hydrogen atom centered over an oxygen atom on the slab surface. During the energy relaxation, the water molecule rotated upwards while maintaining a 1.29 Å distance between the water hydrogen and BTO surface oxygen [Fig. 3(a)], which suggests a favorability for the dipole interaction between the BTO surface oxygen and nearby water. Among the seven defect-free simulations, two other simulations had binding energies close to −0.829 eV, as seen in Table S3. These simulations relaxed similarly, having one hydrogen atom of the water molecule positioned toward a surface oxygen, while the rest of the molecule was positioned further away from the surface. The remaining simulations resulted in binding energies that were an order of magnitude lower, as shown in Table S3. In these simulations, the oxygen in the water molecule was positioned over the surface barium and did not relax to give a clear hydrogen–oxygen interaction. These results indicate that the hydrogen–oxygen interaction between the water molecule and the BTO surface without defects is necessary for water molecules to bind to the surface. Therefore, the binding of water to the surface is generally favorable and is dominated by the dipole interactions between surface oxygens and hydrogens in the water.
DFT simulations were then carried out to study the interactions between a water molecule and a BTO surface with varying vacancy defects. The goal of these simulations was to determine how vacancy defects alter binding behaviors. Water molecules were oriented and simulated on surfaces with a barium vacancy defect as well as an oxygen vacancy defect. To calculate the binding energy of these water molecule orientations, slabs with defects were allowed to relax in a similar manner as defect-free slabs and calculations were performed identically to the defect-free slab cases. For the placement of a water molecule in slabs with defects, bond angles and lengths of water were also found from the relaxation of a single water molecule. With the slabs with defects, however, the distance between a water molecule and the surface was changed from the simulations of the defect-free slabs. A defective slab allowed the water molecule to be placed closer to the surface of the slab, as close as the plane of the surface itself, without being too close as to be repelled by away from the surface. Three cases were simulated: (1) a water molecule at the surface of the slab, (2) a water molecule 1.12 Å from the surface, and (3) a water molecule 2.25 Å from the surface. Multiple water molecule orientations were simulated within these different distances.
When the water molecule was simulated with the barium defect, the most favorable binding energy resulted from the simulation with the water molecule placed such that the hydrogen atoms bent down toward the surface and the oxygen was 1.12 Å from the surface [Fig. 3(b)]. Additionally, the water molecule was placed slightly offset from the barium defect location. During the simulation, the hydrogens rotated slightly so that they were pointing toward the BTO surface oxygens. The resulting binding energy from this orientation was −0.780 eV, which indicates that the binding energy is not significantly affected by the defect. These results show that the hydrogen–oxygen interaction is still imperative for favorable binding positions.
Simulating the water molecule on the surface with the oxygen defect led to a less favorable binding energy. The most favorable binding was achieved when the water molecule was initially positioned similarly to the simulation most favorable for the barium defect. The water molecule was placed with the hydrogen atoms bent down toward the surface with the oxygen 1.12 Å from the surface. Additionally, the water molecule was placed slightly offset from the oxygen defect location. The water molecule rotated completely such that its final relaxed position had one hydrogen pointing toward an oxygen atom in the second layer of the BTO slab. This final relaxed energy was calculated to be −0.611 eV. The collective binding energies for water and a BTO slab with oxygen defects were less favorable than those with barium defects. The plotted positions of this simulation [Fig. 3(c)] showed that locating the water molecule over an oxygen defect impeded the hydrogen–oxygen dipole necessary for strong binding, which is why it was the lowest binding energy of the three surface conditions. However, due to the presence of other oxygen atoms nearby, this did not significantly affect the binding ability of the water to the surface.
Since the defective surface produced similar binding energies as a defect-free surface, we may draw the same conclusions as those for the defect-free surface. Understanding the behavior of the water molecule on a BTO surface containing defects is important as it is not realistic to have a BTO lattice without defects.[24] Thus, a water molecule on the BaO-terminated surface will bind based on the ability of the water molecule to be placed in a way that is favorable for the hydrogen–oxygen interaction. These results indicate that defects in the surface structure of a BTO particle are unlikely to affect the macro-scale favorability of water adsorption on the surface.
To complement calculations of the binding energy of water to the surface, the dissociation of water into hydrogen and hydroxyl radicals on a defect-free surface was investigated. First, the interaction of lone hydrogen atoms with a defect-free surface was simulated by placing hydrogen atoms above a variety of surface oxygen atoms and allowing them to relax to their lowest energy positions [Fig. 3(d)]. The system had a spin state of 1 to reflect the radical nature of the hydrogen. The hydrogen atoms were found to bind strongly to surface oxygen atoms, oriented directly above a surface oxygen with the O–H bond being perpendicular to the surface. The bond length of O–H was found to be 0.97 Å in all cases, consistent with covalent bonding,[25] and the binding energy was −1.94 eV to −2.08 eV. Likewise, hydroxyl radicals were placed above the surface of a defect-free slab in several orientations and relaxed to their lowest energy orientations. The system was run with a spin of 1 to simulate a hydroxyl radical and multiple distinct low-energy orientations of the hydroxyl relative to the surface were observed.
The energy required for water to dissociate into hydrogen and hydroxyl radicals far apart on the BTO surface was calculated using the following equation:
These dissociation energies ranged from + 1.80 to + 1.98 eV, as well as an anomalous high energy result of + 2.38 eV when the oxygen of the hydroxyl was very far from the surface. The positive dissociation energies indicate that the splitting of water into radicals far apart on the BaO surface is not energetically favorable. While dissociation of water has been calculated to be favorable with sparse coverage on a BaO surface,[16] these results pertain to hydrogen and hydroxyl fragments that remain physically near one another. The positive dissociation energies suggest that without the interaction between hydrogen and hydroxyl fragments, the dissociation of water is not thermodynamically favored.
The climbing image nudged elastic band (NEB) method[26] was used to calculate the spatial and energetic path for a hydrogen atom to transfer between surface oxygen atoms. The relaxed positions of lone hydrogen atoms above lattice oxygen atoms, as described in the investigation of water dissociation, were used as the stable endpoints for the NEB calculations. In one case, the lattice oxygens atoms were directly adjacent (4.03 Å apart). In the other case, the oxygen atoms were diagonally opposed across a single unit cell (5.70 Å apart) with a barium atom between them (Fig. 4). From these endpoints, the NEB method allowed the determination of the energy and atomic positions of intermediates along both reaction pathways.
(a) Minimum energetic pathway for a hydrogen atom to move between adjacent oxygen atoms (orange diamonds) and diagonally spaced oxygen atoms (blue circles). The diagonal path is shown to have a higher activation energy and a more pronounced plateau in the middle of the path. Reaction coordinate is a normalized function of the distances that atoms travel over the course of the reaction. (b) Spatial path showing starting, ending, and intermediate positions of hydrogen and lattice atoms along the adjacent pathway.
The activation energy for each hydrogen movement pathway was calculated as the difference between the maximum energy and the initial energy, which was found to be −1.83 eV for the adjacent path and −1.96 eV for the diagonal path. These activation energies are close to the binding energy of a single hydrogen atom to any surface oxygen (−1.94 eV to −2.08 eV). This suggests that breaking the O–H bond is the main energetic barrier for this hydrogen movement. However, the slight lowering of the adjacent path activation energy suggests that the hydrogen does not fully break away from the surface, and that the surface is able to somewhat stabilize the path. However, the activation energy is much lower than what would be needed to break an O–H bond, such as in water, in the gas phase.[27]
The water dissociation and hydrogen movement results indicate that if a water molecule binds to the surface, it may be unfavorable for a hydrogen atom to move away from the corresponding hydroxyl group, and that there is a considerable energy barrier for it to move around the surface at all. This phenomenon could impact the kinetics of hydrogen evolution, water binding, and water dissociation on the surface of BTO. These results may be of interest for work using BTO as a catalyst for splitting water to hydrogen. In this application, the strong binding of adsorbed hydrogen atoms to lattice oxygen atoms, and the resulting energetic barrier for hydrogen to move across the surface, may slow the kinetics of two hydrogen atoms encountering one another on the surface and forming H2 gas. This result suggests that BaO-terminated surfaces may have rather poor catalytic properties for this reaction.
Conclusions
This study advances our knowledge of the structure–property relationships and surface interactions in nanocomposites of PDMS and BTO. The experimental data obtained from fabricated BTO-PDMS nanocomposites indicate that the dielectric constant of BTO may not be changing as its size decreases. However, attempts to extract the dielectric constants of BTO nanoparticles by combining measured nanocomposite dielectric constants with FE modeling have generated results that indicate that PDMS may be preventing the dielectric constants of BTO particles from being determined. Additionally, DFT simulations suggest that water binding on the BaO surface is slightly weakened by Ba or O vacancy defects, and that hydrogen atoms are impeded in their movement across the surface by strong O–H bonds.
The data presented in this manuscript justify further research into BTO and elastomers containing the perovskite material. Future DFT studies should focus on the impact of Ba and O vacancy defects on the dissociation of water, as the effect of these defects on the surface interactions of water and other ligands remains largely unexplored and these sites may have novel reactivity. A better understanding of how and where water dissociates and binds to the BTO surface could indicate how to improve surface functionalization by identifying which ligands can most effectively displace adsorbed water. Consequently, enhanced surface functionalization could improve nanocomposite formation through reduced agglomeration with less intensive processing. BTO could also be incorporated into other elastomers using the methods described in this work to determine if PDMS does indeed impact the measurement of BTO dielectric constants. BTO loadings greater than 30 vol% are achievable in elastomers using the techniques presented herein, and BTO-elastomer specimens with higher BTO content could provide further insight into the properties of BTO. Furthermore, commercially available BTO nanoparticles with diameters below 100 nm could be incorporated into elastomers using the methods described in this report, which could further reveal relationships between the particle size and dielectric constant of BTO.
Integrating tBuPA-coated BTO nanoparticles into elastomers can also enable new applications for BTO-filled nanocomposites in electronics and energy storage. For example, BTO-elastomer nanocomposites that leverage the high dielectric constant of BTO with the elasticity of elastomers can be used as energy storage materials for wearable and flexible electronics.[28] Ceramic-polymer composites have also been proposed as a promising option for embedded capacitors, which would increase packing and electrical efficiency and decrease cost when incorporated into a printed wiring board.[29] Additionally, the needs for pulsed power capacitors include high power density and the ability to supply high currents. Pulsed power capacitors can be used in applications such as X-ray equipment, defibrillators, high power microwave systems, and large science experiments such as the Sandia National Laboratories Z Pulsed Power Facility.[18] Capacitors for these applications require high energy densities (~ 1 J/cm3 or greater), high breakdown strength, and high permittivity.[30] Polymer-inorganic nanocomposites are a viable route to achieving these requirements.[18] This work addresses increasing the permittivity in polymer composite dielectric materials through increased volume loading and dispersion of the ferroelectric nanoparticles. Ultimately, future research to gain a better understanding of the dielectric and surface properties of BTO could result in enhancements to the performance of the perovskite in power electronics, piezoelectric devices, capacitors, and flexible electronics applications.
Data availability
Further data are available from the authors upon reasonable request.
References
S.A. Paniagua, Y. Kim, K. Henry, R. Kumar, J.W. Perry, S.R. Marder, Surface-initiated polymerization from barium titanate nanoparticles for hybrid dielectric capacitors. ACS Appl. Mater. Interfaces 6, 3477 (2014). https://doi.org/10.1021/am4056276
P. Sharma, P. Kumar, R.S. Kundu, J.K. Juneja, N. Ahlawat, R. Punia, Structural and dielectric properties of substituted barium titanate ceramics for capacitor applications. Ceram. Int. 41, 13425 (2015). https://doi.org/10.1016/j.ceramint.2015.07.131
W.-B. Li, D. Zhou, L.-X. Pang, R. Xu, H.-H. Guo, Novel barium titanate based capacitors with high energy density and fast discharge performance. J. Mater. Chem. A 5, 19607 (2017). https://doi.org/10.1039/C7TA05392D
I.H. Mutlu, P. Colkesen, B. Ulug, A. Tumbul, Lead-free ferroelectric BaTİO3 (BTO) thin films produced by the green process. Kuwait J. Sci. 50, 539 (2023). https://doi.org/10.1016/j.kjs.2023.06.001
S. Wada, H. Yasuno, T. Hoshina, S.-M. Nam, H. Kakemoto, T. Tsurumi, Preparation of nm-sized barium titanate fine particles and their powder dielectric properties. Jpn. J. Appl. Phys. 42, 6188 (2003). https://doi.org/10.1143/JJAP.42.6188
S.M. Aygün, J.F. Ihlefeld, W.J. Borland, J.-P. Maria, Permittivity scaling in Ba1−xSrxTiO3 thin films and ceramics. J. Appl. Phys. (2011). https://doi.org/10.1063/1.3514127
Q. Li, T. Ju, R. Li, S. Wang, Y. Yang, H. Ishida, Y.W. Harn, J. Chen, B. Hirt, A. Sehirlioglu, Z. Lin, L. Zhu, Investigation into the crystal structure-dielectric property correlation in barium titanate nanocrystals of different sizes. Nanoscale 15, 7829 (2023). https://doi.org/10.1039/d3nr00350g
M.H. Frey, Z. Xu, P. Han, D.A. Payne, The role of interfaces on an apparent grain size effect on the dielectric properties for ferroelectric barium titanate ceramics. Ferroelectrics 206, 337 (1998). https://doi.org/10.1080/00150199808009168
Y. Tan, J. Zhang, Y. Wu, C. Wang, V. Koval, B. Shi, H. Ye, R. McKinnon, G. Viola, H. Yan, Unfolding grain size effects in barium titanate ferroelectric ceramics. Sci. Rep. 5, 9953 (2015). https://doi.org/10.1038/srep09953
D. Brito, G. Quirarte, J. Morgan, E. Rackoff, M. Fernandez, D. Ganjam, A. Dato, T.C. Monson, Determining the dielectric constant of injection-molded polymer-matrix nanocomposites filled with barium titanate. MRS Commun. 10, 587 (2020). https://doi.org/10.1557/mrc.2020.69
E. Cooper, E. De Anda, E. Flitz, H. Kim, N. Casañas, L. Johnson, Z. Kedzierski, J. Domrzalski, A. Dato, T. Monson, Investigating the dielectric constant of barium titanate in a polymer-matrix nanocomposite. MRS Adv. 7, 799 (2022). https://doi.org/10.1557/s43580-022-00319-x
J.L. Kaufman, S.H. Tan, K. Lau, A. Shah, R.G. Gambee, C. Gage, L. MacIntosh, A. Dato, P.N. Saeta, R.C. Haskell, T.C. Monson, Permittivity effects of particle agglomeration in ferroelectric ceramic-epoxy composites using finite element modeling. AIP Adv. (2018). https://doi.org/10.1063/1.5053442
Q. Zhao, W. Li, Z. Hou, Q. Yang, G. Zhang, Effect of BaTiO3 size on dielectric performance of ultraviolet-cured epoxy acrylic composites with BaTiO3. J. Mater. Eng. Perform. (2023). https://doi.org/10.1007/s11665-023-08540-x
J.N. Domrzalski, T.E. Stevens, R.M. Van Ginhoven, K.J. Fritzsching, B.J. Walder, E.M. Johnson, R.E. Lewis, E.C. Vreeland, C.J. Pearce, D.A. Vargas, E.N. Coker, E.J. Martinez, J.K. Grey, T.C. Monson, Surface functionalized barium titanate nanoparticles: a combined experimental and computational study. ECS J. Solid State Sci. Technol. (2022). https://doi.org/10.1149/2162-8777/ac6f7d
J. Marvin, J. Nicholson, C. Turek, E. Iwasa, N. Pangrekar, W.C. Fowler, R. Van Ginhoven, T.C. Monson, Analyzing barium titanate TiO2 surface interactions with tert-butylphosphonic acid using density functional theory. MRS Commun. 13, 1209 (2023). https://doi.org/10.1557/s43579-023-00425-3
X. Li, B. Wang, T.-Y. Zhang, Y. Su, Water adsorption and dissociation on BaTiO3 single-crystal surfaces. J. Phys. Chem. C 118, 15910 (2014). https://doi.org/10.1021/jp5051386
M.N. Tchoul, S.P. Fillery, H. Koerner, L.F. Drummy, F.T. Oyerokun, P.A. Mirau, M.F. Durstock, R.A. Vaia, Assemblies of titanium dioxide-polystyrene hybrid nanoparticles for dielectric applications. Chem. Mater. 22, 1749 (2010). https://doi.org/10.1021/cm903182n
J. Borchardt, J. Alexander, K. Slenes, R. De La Fuente: Ceramic-polymer composite for high energy density capacitors, in 2007 16th IEEE International Pulsed Power Conference (IEEE2008), pp. 294 https://doi.org/10.1109/PPPS.2007.4651842
K.V. Mahesh, S. Balanand, R. Raimond, A. Peer Mohamed, S. Ananthakumar, Polyaryletherketone polymer nanocomposite engineered with nanolaminated Ti3SiC2 ceramic fillers. Mater. Design 63, 360 (2014). https://doi.org/10.1016/j.matdes.2014.06.034
A.A. Thanki, R.K. Goyal, Study on effect of cubic- and tetragonal phased BaTiO3 on the electrical and thermal properties of polymeric nanocomposites. Mater. Chem. Phys. 183, 447 (2016). https://doi.org/10.1016/j.matchemphys.2016.08.052
P.A. Schultz, Quantum Electronic STructure. Sandia National Laboratories, https://dft.sandia.gov/quantum-electronic-structure/. Accessed 21 May 2024
G. Ferro, D. Ganjam, M. Gibson, K. Partington, A. Trikha, M. Wu, J. Domrzalski, A. Dato, T. Monson, Investigating the dielectric properties of barium titanate nanocomposites using transmission electron microscopy image processing. MRS Adv. 6, 631 (2021). https://doi.org/10.1557/s43580-021-00095-0
P. Barber, S. Balasubramanian, Y. Anguchamy, S. Gong, A. Wibowo, H. Gao, H. Ploehn, H.C. Zur Loye, Polymer composite and nanocomposite dielectric materials for pulse power energy storage. Materials 2, 1697 (2009). https://doi.org/10.3390/ma2041697
S. Wada, T. Suzuki, T. Noma, Role of lattice defects in the size effect of barium titanate fine particles. J. Ceram. Soc. Jpn. 104, 383 (1996). https://doi.org/10.2109/jcersj.104.383
A.R. Hoy, P.R. Bunker, A precise solution of the rotation bending Schrödinger equation for a triatomic molecule with application to the water molecule. J. Mol. Spectrosc. 74, 1 (1979). https://doi.org/10.1016/0022-2852(79)90019-5
G. Henkelman, B.P. Uberuaga, H. Jónsson, A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901 (2000). https://doi.org/10.1063/1.1329672
D. Lefaivre, P. Marmet, Electroionization of D2O and H2O and study of fragments H+ and OH+. Can. J. Phys. 56, 1549 (1978). https://doi.org/10.1139/p78-208
A. Arogbonlo, C. Usma, A.Z. Kouzani, I. Gibson, Design and fabrication of a capacitance based wearable pressure sensor using E-textiles. Proc. Tech. 20, 270 (2015). https://doi.org/10.1016/j.protcy.2015.07.043
M.T. Sebastian, H. Jantunen, Polymer-ceramic composites of 0–3 connectivity for circuits in electronics: a review. Int. J. Appl. Ceram. Tec. 7, 415 (2010). https://doi.org/10.1111/j.1744-7402.2009.02482.x
W. Huebner, S. Zhang, B. Gilmore, M.L. Krogh, B. Schultz, R. Pate, L. Rinehart, J. Lundstrom: High breakdown strength, multilayer ceramics for compact pulsed power applications, in Digest of Technical Papers. 12th IEEE International Pulsed Power Conference. (Cat. No. 99CH36358) (IEEE1999), pp. 1242 https://doi.org/10.1109/PPC.1999.823749
Acknowledgments
Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia LLC, a wholly owned subsidiary of Honeywell International Inc. for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government. The authors thank Dr. Susan Heidger of the Air Force Research Laboratory/High Power Microwave Electromagnetic Microwave Division for significant support of this work. The authors thank Sakai Chemical Industry Co., Ltd. for providing the BTO samples used in this work. The authors are also grateful to the Harvey Mudd College Chemistry Department for its support. Transmission electron microscopy imaging was performed at the Central Facility for Advanced Microscopy and Microanalysis at UC Riverside.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia LLC, a wholly owned subsidiary of Honeywell International Inc. for the U.S. Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. This work was in part supported by the Air Force Research Laboratory, Directed Energy Directorate, High Power Microwave Division (AFRL/RDH). This study was also supported by the National Science Foundation under Grant No. 1943599. DFT studies used Bridges-2 at Pittsburgh Supercomputing Center through allocation CHE230101 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296.
Author information
Authors and Affiliations
Contributions
H.F., J.S., and V.B. fabricated elastomer-matrix nanocomposites containing barium titanate. H.F., J.S., and J.G. performed capacitance and density measurements of nanocomposites. J.G. simulated the nanocomposites using finite element modeling. Density functional theory simulations were conducted by A.P., M.G., and K.N. The manuscript was written by A.P., H.F., J.S., M.G., J.G., A.D., T.M., and R.V.G. The project was supervised by A.D., T.M., and R.V.G.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pritchard, A., Fuentes, H., Santosa, J. et al. Understanding surfaces and interfaces in nanocomposites of silicone and barium titanate through experiments and modeling. MRS Communications (2024). https://doi.org/10.1557/s43579-024-00638-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43579-024-00638-0