Abstract
Solid-state batteries (SSBs) are expected to play an important role in vehicle electrification within the next decade. Recent advances in materials, interfacial design, and manufacturing have rapidly advanced SSB technologies toward commercialization. Many of these advances have been made possible in part by advanced characterization methods, which elucidated materials properties, interfacial behaviors, and degradation modes and their underlying causes. These insights have informed efforts to advance manufacturing and synthesis approaches and achieve improved performance. To reach widespread adoption, challenges associated with rate capability, reducing or eliminating the need for stack pressure, and high-throughput manufacturing must be addressed. This article summarizes recent progress, current understanding, ongoing challenges, and future needs to accelerate the development of next-generation, high-performance SSBs for electric vehicle applications. In doing so, the importance of scalable and sustainable processing of battery components is emphasized as critical to the maturation and commercial success of SSB technology.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background/introduction
As demand for cleaner and more efficient transportation grows, and electric vehicles (EVs) transition from early-adopters to the mainstream, the limitations of traditional lithium-ion batteries (LIBs) have become increasingly apparent. Although LIBs continue to make incremental improvements to energy density, charging rate, and low-temperature performance, significant challenges remain. Further improvements are needed to make EVs a viable/attractive option for an ever-wider range of customers and use-cases, particularly those in cold climates, rural areas, or requiring hauling/towing heavy loads.
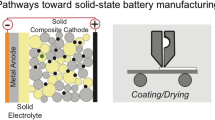
Solid-state batteries (SSBs), characterized by their use of solid electrolytes (SEs) instead of volatile/flammable liquids (Figure 1), could revolutionize the EV landscape. SSBs offer significantly enhanced energy densities if they utilize high-specific-capacity electrodes, including Li metal or alloys. Replacing volatile/flammable liquids may also improve safety and thermal stability, decreasing the need for thermal management systems that are expensive and add peripheral mass. Therefore, SSBs stand at the forefront of addressing the critical demands of higher energy density, long cycle life, and fast-charging capabilities for the next generation of battery technology.
Multiple entities ranging from small startups and academic research groups to multinational corporations are working feverishly to demonstrate the viability of SSBs and scale-up manufacturing in a cost-effective manner that is compatible with the price constraints of the consumer vehicle market. Recent advancements in the field have been propelled by a synergistic combination of cutting-edge characterization tools and innovative synthesis and processing methods, pushing closer to commercially viable high energy density (>400 Wh/kg), fast-charging SSBs (Figure 2). Interestingly, there are a wide range of different materials and approaches being developed in parallel, without a clear winner. One of the approaches that has been adopted by several companies is a hybrid solid-state battery, where a dense solid separator is used, but the cathode interface is aided by the use of a liquid or gel (Figure 1b).
As SSBs have inched closer to commercialization, practical requirements and constraints have come into better focus. In addition to offering substantial improvements to energy density and safety, SSBs must operate stably with minimal external stack pressure, charge at least as quickly as current LIBs, and be manufacturable at large scale with high yield and low cost. All of these requirements must be met for SSBs to achieve significant market penetration for EV applications.
This article will summarize key recent progress toward these goals and offer a forward-looking analysis of remaining challenges. In particular, we will focus on advances in material and cell manufacturing along with advanced characterization approaches that have yielded insights into cell behaviors and materials properties.
Over the past ~15 years, a range of SEs have been discovered that possess high enough ionic conductivity (>0.5 mS/cm at room temperature) to make them potentially useful for large-scale SSBs.1 Among the most promising current materials are the garnets (oxides), favored for their wider electrochemical stability window, sulfides, favored for their higher conductivity and low-temperature processability, and polymers/composites potentially offering a more direct pathway to scalability (Figure 3).
Ongoing work continues to develop new SEs, including high-entropy materials,2 halides,3 and more. While the search continues for new materials that possess even higher ionic conductivity, wider electrochemical stability windows, facile processing, and more earth-abundant constituents, the primary focus of the research community and industry has shifted toward enabling practical devices. For a more comprehensive overview of previous work, the reader may be interested in previous review articles that have covered various aspects related to solid-state batteries, including sulfide,4 halide,5 oxide,6 antiperovskite,7 and inorganic-polymer composite8 electrolytes, the remaining challenges,1 mechanics,9 thin-film electrolyte processing,10 and interface stability.11
Current understanding, recent progress, and remaining challenges
The remaining challenges vary somewhat across different electrolyte and electrode chemistries, but in general, fabricating and maintaining stable electrode/electrolyte interfaces during cycling has been the key factor limiting performance (Figure 4). Unlike LIBs, in which the liquid electrolyte can flow and wet any exposed surface area, SSBs rely on intimate solid/solid interfaces to facilitate ion and electron transport. This is made more challenging by volume changes and (electro)chemical reactions that can degrade contact with time and cycling.
Overcoming these challenges is critical for the performance and practicality of SSBs in EV applications. The landscape of energy-storage technologies is undergoing a transformative shift, focusing not only on the enhancement of the performance metrics such as energy density, safety, and long cycle life, but are also increasingly aligned with principles of sustainability. The synthesis of novel solid electrolytes and electrode materials is undergoing progress, leveraging eco-friendly processes, abundant resources, and energy-efficient methods. Concurrently, advancements in cell manufacturing are revolutionizing the production landscape, making it more scalable, efficient, and environmentally responsible. We will anchor our discussion on the key requirements, opportunities, and challenges related to each major SSB component. We will then focus on how recent cutting-edge developments are converging toward the creation of SSBs that promise not only to redefine energy-storage solutions, but to do so with a minimized environmental footprint.
Anodes and anode interfaces
To maximize energy density, the anode is often in the metallic form of the working ion (e.g., Li metal). Considering the challenges and costs associated with manipulation, utilizing free standing Li foils might not be practical. Furthermore, achieving scalable integration of metallic Li that maintains high chemical purity and accommodates the highly dynamic nature of the anode/separator interface is challenging (Figure 4). This interface experiences time-varying fluxes of charge and mass, overpotentials, and mechanical stresses. It has become increasingly clear that understanding the interfacial behaviors of SSBs requires consideration of the couplings between these phenomena.9,12,13 Much of the recent progress in materials synthesis and manufacturing of anodes and anode interfaces has been focused at overcoming Li penetration and void formation, two ways that interface evolution can cause cell failure.
Li penetration
Li filament growth causing short-circuit during fast charging is nearly ubiquitous across all SE systems.14,15,16 In many cases, avoiding Li penetration and short-circuit is the primary factor limiting the maximum charging rate of SSBs. As a result, a great deal of research effort has been expended to understand the underlying causes of this phenomena, and to suppress it.
As an aside, the authors would like to note that some literature still refers to the Li filaments that grow through solid electrolytes as “dendrites,” despite the fact that they do not fit with the definition of a dendrite as a branching fractal-shaped structure that branches along certain crystallographic directions.17 Although it is possible that in some instances, the term “dendrites” could be fitting, it is not universally the case, as in many cases the filaments do not branch at all.14,18 Here, we will use the language “Li penetration” and “Li filaments” to describe the phenomenon and the structures, respectively.
Over the past few years, new experimental and modeling approaches have elucidated the nuances and underlying causes of the behavior. The ubiquity of Li penetration across polycrystalline, monocrystalline, and glassy materials confirms that previous works suggesting that grain boundaries were the culprit could not be the entire story.15 Subsequently, operando video microscopy was used to show that the propagation of Li filaments in ceramic SEs could have multiple morphology types.14 This helped to explain the apparently conflicting results in literature by showing that the phenomena could have multiple origins depending on the system.
It has also been shown that by modulating externally applied mechanical stress in a garnet SE,19 and through operando synchrotron x-ray tomography in an argyrodite SE,18 at least one propagation mode of Li filaments is driven by a mechanical crack-opening mechanism. By leveraging state-of-the-art characterization approaches, the community has made significant strides toward understanding the evolution of mechanical stresses that leads to filament propagation. Prior works often lacked the spatial/temporal resolution, framework, or ability to take measurements in representative materials/cells. The use of machine learning approaches to aid in segmentation and interpretation of data has begun to enhance advanced characterization approaches,20 but there are many possible applications that remain to be explored. Combining these studies with careful measurements of mechanical21,22,23 and electrochemical properties24,25 has informed modeling efforts that continue to provide valuable insights into system behaviors and guide rational design for improved performance.
Although the mechanisms that drive propagation are increasingly well understood, the initial nucleation of these features remains a topic of debate. The importance of flaws/defects in the SE and/or Li metal in nucleation is well established; however, the nature of these flaws and how they result in a crack/filament remains to be conclusively established. Several works have suggested that grain boundaries could have smaller bandgaps and lead to nonnegligible electronic conductivity in small regions.26,27,28,29,30,31,32 As a result, Li0 could plate out at these sites, initiating a Li filament that can then propagate as described above. Whether or not this occurs in all cases is unclear, but will be important to establish moving forward.
Many of the efforts to achieve low interface resistance, improved mechanical properties, fewer defects, and high SE relative densities described below are driven by the need to suppress Li penetration to enable fast charging, and substantial progress has been made in this area. Looking ahead, if the initiation of Li filaments can be suppressed/eliminated by engineering the grain boundaries or pores/defects in the surface region, this could be a powerful approach to increasing rate performance and making SSBs more robust under a wide range of operating conditions.
Void formation
It is vital that discharging does not cause interface degradation which impacts cell lifetime or subsequent safe charging rates. The formation of voids during stripping (discharge) of the Li/SE interface has become an important area of focus for the community in recent years.33 In addition to causing cell polarization by reducing interfacial contact area, the inhomogeneous contact can subsequently cause current focusing and accelerate the onset of Li penetration.34,35
It is therefore vital to understand and prevent void formation, keeping in mind the practical limitations for stack pressure and temperature for EV applications. It is generally agreed upon that EV batteries must operate below 1 MPa of external pressure (ideally closer to 0.1 MPa), and as close to ambient temperature as possible.1,12 Many of the best-performing SSBs published have operated well outside of these practical constraints. Although it is valuable as a proof of concept, the community must remain focused on scalable approaches to manufacture and operate SSBs under scalable/realistic conditions.
Several characterization approaches have been leveraged to build understanding of void formation. These include operando acoustic transmission,24 synchrotron x-ray tomography with machine learning,20 and solid-state nuclear magnetic resonance (NMR),24 which enabled detection and visualization of voids at the buried Li/SE interface. Coupled with electrochemical measurements such as complex impedance, the results have enabled rapid identification of the conditions under which voids form and grow. This understanding has guided the design of approaches to mitigate the negative impacts of void formation. For example, it was shown that a combination of elevated temperature and stack pressure can be used to heal the Li/SE interface (enabling Li creep) and return it to a pristine condition prior to subsequent charging.34 In another case, a flexible carbon felt was used to modulate stack pressure and enable more uniform plating and stripping.36 These add to previous approaches of wetting layers and surface treatments that increase the lithiophilicity of the SE, promote nucleation, and enhance adhesion by decreasing interfacial energy.37,38
Several approaches have emerged to simplify the manufacturing process of anode materials while maintaining high energy density and good performance, including:
Anode-free manufacturing
Anode-free SSBs are promising due to the potential of unlocking maximum energy density (by eliminating excess Li), improved safety, and ease of manufacturing. The battery is fabricated in the discharged state with a bare current collector replacing the anode. The Li anode forms during the first charge cycle by electroplating Li from the cathode (Figure 4). This approach has been demonstrated in liquid-based39,40,41 and solid-based systems,42 with up to 80% capacity retention over 90 cycles in liquid-based systems39 and near 100% Coulombic efficiencies for 50 cycles at a C/10 rate and a capacity of 0.8 mAh/cm2, for the solid-based systems.42
The performance of anode-free cells is constrained by the efficiency of lithium plating and stripping and how uniformly Li is plated and reversibly stripped during cycling. The main challenges are the heterogeneous Li nucleation and growth at the Li/SE interface that can stem from the adhesion strength between the current collector and the SE, as well as the deformation of the current collector at low stack pressures (<1 MPa). With increased stack pressures, factors such as the evenness of pressure distribution,14 misalignment of the assembly fixtures with the cell components, and local discrepancies in electrolyte thickness or surface roughness can influence the locations of lithium deposition. These factors can alter the local pressure exerted and evolve over time with the growth of lithium deposits, affecting the electrochemical performance and stability of the cell.43 This is exacerbated in anode-free cells due to the lack of a compliant Li-metal layer that can help distribute applied stack pressure more evenly across the cell. Creative approaches to alleviate the need for stack pressure and to maintain intimate interfacial contact are needed.
These findings underscore the interplay between manufacturing processes, cell assembly, and electrochemical behavior that must be well understood for scaling efforts. Additionally, the effect of surface chemistry inhomogeneity between the current collector and the SE, which could cause uneven transport and adhesive strength between them, represents a less investigated area that warrants exploration.
Seeded lithium deposition and alloy interlayers
To circumvent the need for freestanding Li foils and thereby minimize excess lithium in SSBs, one approach involves altering the SE/current collector interface with metal clusters, metal layers, or alloy interlayers. This aims to either eliminate/reduce the nucleation overpotential of Li plating and guide Li nucleation, enabling stable cycling and improved Coulombic efficiency. Instead, an alloying nucleation overpotential above 0 V is observed before plateauing.44
This approach has been demonstrated in SSBs with the use of Ag, Au, Pt, Al, and45,46 Al-In clusters or interlayers,47,48,49 where all facilitate reversible lithium plating and stripping. Cui et al. reported the dynamic Li plating behaviors on 10 metallic substrates. In the metal layers where Li is soluble and has good lattice compatibility with Li (In, Ag, Au, Pd, and Al), the formation of Li-metal alloys enables the layers to have more affinity for Li, thus lowering the overpotential for Li nucleation and providing uniform and abundant Li deposition sites for Li plating. Good lattice compatibility and high affinity for Li promote in-plane isotropic Li growth rather than out-of-plane anisotropic Li growth, as observed in Cu, Ti, Ni, Bi, and Cr.50 It is important to note that only an alloy layer with fast lithium-diffusion properties would allow for long-cycle battery life.25
An alternative approach that has been pursued is the incorporation of indium into aluminum foils (Figure 5), which are used as anode materials. This method enhances both the rate behavior and the reversibility at relevant areal capacities (2–5 mAh/cm2).46 The improvement is attributed to the formation of a LiIn network distributed throughout the aluminum matrix. Such a network is thought to promote rapid diffusion of lithium ions, thus increasing the interfacial area available for the lithium-aluminum reaction.
Emerging anode manufacturing methods. (a–c) Anode-free manufacturing. Reprinted with permission from Reference 42. © 2020 Springer Nature. (d, e) Alloy interlayers. Reprinted with permission from Reference 46. © 2023 Springer Nature. (a) Potential response upon Li anode formation at constant cathodic current, plating Li onto a current collector (CC). (b) Potential response upon plating and stripping 5 mAh/cm2 of Li onto a CC. (c) Cross-sectional scanning electron microscopy (SEM) and energy-dispersive x-ray spectroscopy (EDS) between solid electrolyte and CC as assembled and after plating 5 mAh/cm2. (d) Cryo focused ion beam (FIB)-SEM image and EDS map of pristine Al-In alloy foil used as an interlayer between current collector and solid electrolyte. (e) Areal capacity and Coulombic efficiency (CE) with cycling.
Although the incorporation of metal clusters or interlayers favorably decreases or eliminates the nucleation overpotential necessary for Li plating, the SE chemical stability against the in situ plated Li metal needs to be considered. If detrimental surface layers are expected to form at the Li/SE interface as Li metal is plated, the addition of a composite layer with alloy-forming metals and carbon has proven effective to prevent chemical decomposition of the SE. For example, the introduction of a thin composite anode layer on the current collector, which comprises Ag nanoparticles and carbon black with poly(vinylidene fluoride) binder, effectively regulated Li deposition. As Ag is soluble in Li and reduces the nucleation energy for the formation of Li,51,52 it assists the uniform deposition of Li on the current collector, and thereby improving the performance of the SSB. Carbon acted as a physical barrier between the sulfide electrolyte and Li metal, improving durability of the SE due to chemical reactions and was suggested to prevent Li metal penetration through the SE. This approach allowed the fabrication of a pouch cell (0.6 Ah) with energy density >900 W/l with stable Coulombic efficiency over 99.8% over 1000 cycles.47
The scalability of adding metal clusters or interlayers in solid-state batteries (SSBs) hinges on factors such as deposition techniques, costs, and compatibility with current manufacturing workflows. Techniques such as physical and chemical vapor deposition offer precise control and uniformity but are limited by their need for high vacuum, slow throughput, and high costs, especially when using expensive metals such as gold and platinum. These challenges make such methods less practical for large-scale production compared to composite interlayers. For instance, Lee et al.47 demonstrated the scalability of Ag-C composite layers using a more feasible screen printing method, which aligns better with existing production lines and scales efficiently from small to large outputs. Nonetheless, the financial and environmental costs associated with precious metals and solvent drying in such processes must also be considered.
Electrolyte/separator
A SE must satisfy several criteria to ensure cell’s optimal performance, safety, and durability. Key specifications among these are high ionic conductivity and negligible electronic conductivity, essential for efficient ionic transport between electrodes and, thus, crucial for achieving relevant charge and discharge rates. Ideally, the SE is thin (1–20 µm)53 to shorten the ion travel path between the anode and cathode, facilitating ion transport and lowering the ohmic drop of the cell, enhancing rate performance and power density. Moreover, a thinner electrolyte contributes to an overall higher energy density due to the decreased weight/thickness. However, achieving optimal thicknesses without compromising the electrolyte’s structural integrity and mechanical strength is a significant challenge, as the material will experience repeated stresses upon active materials’ volume changes during cycling.
The SE needs to have a wide electrochemical window, or protected by a coating such that the conduction band minimum (CBM) lies higher in energy than the chemical potential of the anode material. In contrast, the valence-band minimum (VBM) should ideally be lower in energy than the chemical potential of the cathode material. The proper alignment of the CBM and VBM will prevent electron transfer from the electrode to the electrolyte or vice versa, mitigating the risk of electrochemical reactions at the electrode/electrolyte interface that could compromise the battery’s performance and lifespan. Chemical compatibility (stability) with both anode and cathode materials is imperative to prevent adverse interface reactions that degrade battery performance over time.
Solid-state cathode and cathode interfaces
To ensure high energy density, the cathode will be the thickest component in the SSB (Figure 1), where the optimal thickness balances the need for high-energy capacity with effective, uniform ion and electron transport. Establishing close contact among the various constituents of the composite cathode, such as the active material, conductive additives, and particles of solid electrolyte, is vital for reducing interface resistance within the cathode. As a result, a dense microstructure is preferred, enabling an increase in active material content within the same spatial confines, thereby directly increasing the energy density of the battery.
Achieving intimate contact between the different components of the composite cathode (e.g., active material, conductive additives, and solid-electrolyte particles) is crucial for minimizing the resistance at interfaces within the cathode, facilitating efficient charge transfer, and enhancing the electrode’s overall performance. Moreover, it helps ensure facile ionic and electronic transport. Consequently, a dense microstructure is desired, leading to higher loadings of active material within the same volume, directly contributing to an increase in the energy density of the battery.
Because electrochemical reactions require ions and electrons simultaneously, both must be supplied at the same rate. Thus, the ionic and electronic conductivity ratio should be close to unity across the composite cathode. Balancing the partial ionic and electronic transport for the specific chemistries of active material and solid electrolyte across the composite’s thickness can lead to full cathode active material utilization.54
The composite cathode must accommodate volume changes during the ion insertion and extraction processes without significant structural degradation, highlighting the importance of the ductility of the composite. This property is essential for maintaining the mechanical integrity of the electrode, ensuring long cycle life, and preventing capacity fade over time. Additionally, composite cathode materials that exhibit stability in air would simplify manufacturing processes and enhance the safety of the battery. Air stability reduces the risk of degradation or reactions with atmospheric moisture and oxygen, which can compromise the component’s electrochemical performance and operational safety. Incorporating a pre-intercalated composite cathode is required in batteries where the anode is formed in situ (anode-free).
Advanced densification
Advanced densification methods are crucial for optimizing the structural integrity and electrochemical performance of SSB components. These techniques aim to improve the connectivity and density of the solid electrolyte and electrode materials, thereby enhancing ion transport, reducing internal resistance, and increasing the mechanical strength of the electrochemical cells. As SSBs move closer to commercialization, the role of scalable processing/manufacturing methods become increasingly central. Each method discussed here offers unique advantages in terms of energy efficiency, processing times, and the ability to achieve high material densities, promising to unlock new levels of performance previously unattainable with traditional manufacturing approaches.
Ultrafast high-temperature sintering (Figure 6) has been proposed as a process capable of sintering a wide range of ceramic materials within seconds by radiative heating under an inert atmosphere. Temperatures up to 3000°C can be achieved in 10 s while maintaining the composition of volatile elements such as lithium due to minimal exposure at high temperatures. Successful processing of Ta-doped Li6.5La3Zr1.5Ta0.5O12 (LLZTO) was achieved with relative densities up to 97% and relative small grain size (8.5 ± 2 µm).55 Even though this method shows great promise in terms of manufacturing time, it was only proven on bulk ceramics (pellets), so more exploration toward thin membranes (~20 µm) is necessary.
Emerging manufacturing processes for solid electrolytes and solid-state cathodes. (a) Ultrafast high-temperature sintering (UHS) of ceramics. Reprinted with permission from Reference 55. © 2020 AAAS. (b) Rapid-induction sinter forging. (c) Cold sintering of a LiFePO4 (LFP) cathode with carbon nanofillers (CNF) using LiOH solution, low magnification (left), high magnification (right). Reprinted with permission from Reference 58. © 2018 Elsevier. (d) Co-extrusion of hybrid electrolytes with macroscale interfaces. Reprinted with permission from Reference 68. © 2019 American Chemical Society. (e) Schematic of aerosol deposition method. Reprinted with permission from Reference 69. © 2020 Wiley-VCH. (f) Selective laser sintering additive manufacturing (Reference 78).
Rapid-induction sinter forging has been introduced as a novel method for the roll-to-roll continuous manufacturing of thin films. In this technique, a precursor powder can be placed on a substrate and concurrently subjected to heat and pressure in a direction parallel to the film’s thickness.56 The simultaneous application of heat and pressure can lead to higher relative densities of the resulting components compared to sintering methods, similar to hot-pressing. Additionally, the absence of lateral restrictions in the sinter forging area, which is perpendicular to the direction of pressing, reduces residual shear stresses and defects. This method facilitates the continuous and scalable manufacturing of thin films with high throughput that is compatible with roll-to-roll manufacturing. Areas of exploration within this fabrication method include optimal processing conditions (temperature, pressure, heating, and cooling rates) for a wide range of chemistries.
Cold sintering offers a low-temperature alternative (room temperature to 300°C) for producing ceramic components for SSBs, using less energy than traditional methods. This method uses a transient liquid phase and uniaxial pressure to compact powder into dense monoliths well below their melting points, often with just a ~5% volume of the liquid phase.57,58 The lower temperatures create a new opportunity spectrum to design grain boundaries and create new types of composites among material combinations that previously had incompatible processing windows, especially for solid-state composite cathodes and may enable a more seamless integration with SEs. Although promising for energy-efficient and more environmentally friendly manufacturing, a deeper understanding of cold sintering’s fundamentals, material compatibility, and process mechanisms is critical for its integration into SSB production lines. This includes in-depth studies on solvent-particle interactions and optimization of sintering parameters for improved densification and ionic conductivity.59
Layer-by-layer manufacturing
Tape casting is a mature technology currently used in lithium-ion battery manufacturing that has been implemented by various studies as a promising method for the fabrication of all-SSBs due to its ability to form thin ceramic bodies.60,61 This technique has been used to process composite cathodes and solid electrolytes for all-SSBs, demonstrating good cycle performance and specific capacity in cathodes, but decreased ionic conductivity in sulfide- and halide-based SEs.62,63,64 Moreover, multilayer tape casting has been demonstrated to achieve high areal capacity and withstand high current densities with the use of an ionic liquid electrolyte to reduce interfacial resistances between solid electrolyte and cathode.65 Challenges encountered in this process encompass selecting suitable binder and solvent systems, managing the variability in particle size distribution, and ensuring uniform thickness during final densification. Furthermore, creating thin, freestanding films requires meticulous attention to changes in surface composition, physical shrinkage, adherence to surfaces, warping, and breakage.62,67 Also, the cost associated with solvent recovery and toxicity of the solvents used needs to be taken into consideration.
The adoption of extrusion techniques for producing components of SSBs, particularly composite electrolytes, is noteworthy for its solvent-free approach. This method involves blending solid-electrolyte particles with polymer binders at high temperatures to create a viscous paste. The paste then undergoes a film-forming process through die casting with a flat film die and is subsequently rolled between two chilled rollers to induce quenching. This extrusion process yields a thin membrane that can either be directly co-extruded with an electrode or laminated onto the electrode after forming.66,67,68 Co-extrusion offers the benefits of lower production costs by simultaneously fabricating the electrolyte and electrode and potentially enhancing the interfacial contact between them. In addition, it reduces the need for multiple processing steps typically required in other manufacturing methods to integrate both SE and solid-state cathodes, simplifying the production process and reducing potential defects and impurities that could be introduced during multiple processing steps. The scalability and environmentally friendly characteristics, stemming from the lack of toxic solvents, position this approach as a promising route for the mass production of SSBs incorporating polymer components. However, the interplay between processing conditions, mechanical strength, ionic conductivity, and cycling stability need to be thoroughly understood, and alternative binders are needed.
Both tape casting and co-extrusion are compatible with roll-to-roll processes, thus, they promise a smooth integration with existing manufacturing infrastructure used in conventional lithium-ion batteries.
Aerosol deposition, a method for producing SSB components without high-temperature processing, accelerates fine particles onto a substrate to create compact layers. This process enables the fabrication of thin solid electrolytes (around 5 μm) and the integration of various SE in solid-state cathodes, offering benefits in design flexibility and energy density.69 However, it is still an emerging technology, with current speeds limited to about 100 mm3/min,70 leading to low production rates and high costs. Enhancements such as using wider nozzles and conveyor systems are expected to increase deposition rates.71 Overcoming engineering challenges such as scaling up, depositing on nonplanar surfaces, and improving efficiency are critical for commercial success, alongside optimizing process efficiency and material recirculation to reduce costs.70
Additive manufacturing could offer a transformative approach to composite cathode design, enabling precise control over geometry and composition. This precision improves the tunability of ionic and electronic tortuosity, crucial for thick cathodes. Furthermore, the integration of SEs with cathodes could be achieved by utilizing the thick solid-state cathode as a structural support. This approach would allow for the direct processing of the SE onto the cathode, circumventing the need to manage fragile films that are ≤20 µm in thickness and prone to fracturing. In addition, the ability to create functional gradients, such as variations in composition or structure, could optimize the properties and mitigate stresses that evolve at the interface. Recent studies have shown the success of aerosol jet printing for creating solid polymer composite electrolytes on LiFePO4 cathodes72 and direct ink writing for Li7La3Zr2O12,73 LiFePO4, and Li4Ti5O12 electrodes.74 However, ink-based methods require extensive rheological adjustments for printability and additional steps to remove binders to prevent their degradation during cycling.75 The development of slurries faces challenges in creating precisely controlled inks, loading sufficient active material, and requiring extensive post-processing to remove solvents and sinter particles.76 Selective laser sintering (SLS) offers a binder-free alternative that has successfully fabricated Li1+xAlxTi2−x(PO4)3 ceramic electrolytes and NCA cathodes with relative densities up to 96% density, but with the presence of cracks.77,78 SLS will require the optimization of cooling rates, deposition layer timing, and feedback mechanisms to advance this approach. The development of standards for powder reutilization, quality control, and certification will be crucial to promote sustainable additive manufacturing in battery production.
Sustainable manufacturing
As the demand of SSBs grows, so does the need for sustainable processing methods that mitigate environmental impact and promote eco-friendly manufacturing practices. Sustainable processing of battery components not only aligns with global environmental objectives, but also ensures the longevity and viability of the battery industry. In this context, developing and implementing green manufacturing techniques for SSB components is crucial. Two approaches that stand out in the quest for sustainability include solvent-free processing methods due to a significant reduction of emissions and waste. These methods eliminate environmental and health hazards associated with solvent-based processes and enhance the quality and performance of electrodes by eliminating unwanted reactions from solvent residues upon cycling.
The integration of recycled materials into the SSB manufacturing process would be a substantial move toward sustainability. Innovative recycling and upcycling processes are needed, where materials from spent batteries are reclaimed and repurposed for new battery production. This approach will not only decrease the reliance on virgin materials but also significantly reduce the environmental footprint of battery production. By closing the loop on material usage, the battery industry can minimize waste and contribute to a more sustainable future.
Embracing more sustainable processing methods can help ensure that advancements in energy storage go hand in hand with environmental protection and the efficient use of resources.
Cell assembly
Conventional lithium-ion batteries connect all cell stacks in parallel, enhancing total capacity by connecting all anode and cathode current collector foils together. A distinct advantage offered by solid-state batteries is the potential for a bipolar stacking configuration (Figure 7). In such a setup, the anode of one cell and the cathode of the next cell share the same bipolar current collector, creating a series connection where the current is drawn exclusively from the stack’s outermost layers. This arrangement minimizes the need for welding joints of current collector foils and tabs, resulting in more efficient use of the available packaging space.
While the bipolar design could drive an increase in energy density, power density, and voltage compared to conventional lithium-ion batteries, there are several technical hurdles that need to be overcome, including complex manufacturing processes for producing asymmetric electrode sheets (anode or cathode on each side), risk of internal short-circuits among unit cells, control of accurate and reliable cell stacking and potential for corrosion due to large potential gradients (i.e., highly reductive and highly oxidative).1,69,79 To achieve an effective construction and operation of bipolar SSBs, technological progress and critical evaluation in cell configuration, material design, and manufacturing will be required.
Ensuring the integrity of the atmosphere throughout the SSB manufacturing process will be essential, requiring diligent monitoring of humidity and periodic evaluations of seals to avoid air and moisture exposure. Leveraging cutting-edge sensing and monitoring technologies will allow for the real-time assessment of battery component quality and assembly integrity. This approach could significantly reduce the incidence of defects and improve the overall reliability of SSBs by enabling adjustments during the manufacturing process. Additionally, integrating robotics and artificial intelligence (AI) into SSB production processes could bring a higher level of precision and efficiency.
The lack of a traditional anode in anode-free batteries calls for reassessment of standard packaging methods to meet the specific needs and overcome the challenges of anode-free designs. This includes creating specialized current collectors and packaging materials capable of handling mechanical stresses without failure. Anode-free cells undergo significant volume changes during lithium plating and stripping in charge/discharge cycles, necessitating packaging that can flexibly support these fluctuations to preserve the cell’s structural integrity and electrical connectivity. Solutions may include flexible or expandable materials that adjust to volume changes while maintaining the cell’s airtight seal. Innovative packaging solutions are essential for leveraging the advantages of anode-free batteries, ensuring their safety, reliability, and manufacturability through integrated cell engineering.
Future outlook
In summary, the solid-state battery community faces several critical challenges that demand innovative solutions for the technology to realize its full potential:
-
1.
Increase charge rates: A primary focus should be on improving the charging rate, as faster charging is a key driver for the widespread adoption of electric vehicles and portable electronics. Advances in electrode materials, electrolyte design, and interface engineering are essential to enhance ionic conductivity and reduce resistance, thereby enabling faster charging without compromising safety or cycle life.
-
2.
Enable low-temperature charging: SSBs that can charge rapidly at subfreezing temperatures are another crucial aspect that requires attention. SSBs must demonstrate reliable performance in a range of environmental conditions, especially in colder climates. This would offer a significant advantage over current LIBs. Developing electrolyte formulations and electrode materials that maintain high conductivity at lower temperatures is imperative for ensuring the viability of SSBs in diverse operating environments.
-
3.
Prioritize and verify safety: To achieve the promise of SSBs as safer alternatives to conventional lithium-ion batteries, the community must work toward minimizing or eliminating the use of gels and liquids in the cell design. Solid electrolytes may offer intrinsic safety advantages, but the community must not ignore possible safety implications of the incorporation of liquids and gels to enhance interface performance. Even in the absence of liquid electrolytes, further investigation of SSB safety is needed to avoid possible negative publicity that could sway public opinion and slow EV adoption. Research should focus on developing standardized testing protocols to evaluate and compare the safety profiles of various solid-state battery technologies. Comparing different solid-electrolyte materials, such as sulfides and oxides, and evaluating the impacts of hybrid approaches will help identify the most suitable options in terms of safety, stability, and performance.
-
4.
Dive deeper into coupled phenomena: Understanding electrochemomechanical coupling is crucial for optimizing the mechanical stability of SSBs during cycling. This requires a comprehensive interdisciplinary investigation of the interplay between chemical reactions, ion transport, and mechanical stresses within the battery components. A deeper understanding will lead to the development of mechanically robust materials and interfaces that can withstand the rigors of repeated charging and discharging cycles. Self-healing or actuated healing approaches that can restore the integrity of electrolytes or interfaces will be valuable to enable materials and cells that are less susceptible to manufacturing defects, localized damage, and various types of abuse that are likely in real-world applications.
-
5.
Improve understanding of composite cathode behavior and interfaces: Although great progress has been made on the anode side, the integration of all-solid-state cathodes remains very challenging. This is a result of transport limitations, chemical/electrochemical/mechanical stability, and high interface impedances. Future work should focus on understanding and overcoming these limitations, to enable all-SSBs that do not rely on liquids or gels to maintain intimate contact and low impedance in composite cathodes.
-
6.
Relax/eliminate stack pressure requirements: Reducing stack pressure requirements and achieving uniform stack pressure are essential for manufacturing scalability and cost-effectiveness. The community should focus on materials and designs that allow for lower stack pressures while maintaining efficient ionic transport and mechanical integrity. This will facilitate the development of solid-state batteries that are not only high-performing, but also economically viable for mass production.
-
7.
Pursue scalable and sustainable processing: Scalable and sustainable manufacturing processes are crucial to enable the mass production of environmentally friendly SSBs while minimizing resource depletion and ecological impact, ensuring the long-term viability of the industry. Advancing solvent-free processing methods will help eliminate hazardous emissions and reduce waste. Optimizing energy consumption throughout the manufacturing process and developing efficient recycling and upcycling methods are essential to minimize reliance on virgin materials. Finally, the community should focus on cross-disciplinary efforts to foster innovations in abundant and less environmentally harmful materials to support long-term sustainability goals.
In conclusion, addressing these challenges requires a collaborative effort from researchers, engineers, and industry stakeholders. Continuing innovation will be aided by application of the ongoing advances in materials characterization synthesis and manufacturing. By focusing on these key areas, the solid-state battery community can pave the way for a future where these advanced energy-storage devices contribute significantly to sustainable and efficient energy solutions.
References
J. Janek, W.G. Zeier, Nat. Energy 8, 230 (2023)
S. Li, J. Lin, M. Schaller, S. Indris, X. Zhang, T. Brezesinski, C.W. Nan, S. Wang, F. Strauss, Angew. Chem. Int. Ed. 62, e202314155 (2023). https://doi.org/10.1002/ANIE.202314155
K. Tuo, C. Sun, S. Liu, Electrochem. Energy Rev. 6, 17 (2023)
D. Ren, L. Lu, R. Hua, G. Zhu, X. Liu, Y. Mao, X. Rui, S. Wang, B. Zhao, H. Cui, M. Yang, H. Shen, C.Z. Zhao, L. Wang, X. He, S. Liu, Y. Hou, T. Tan, P. Wang, Y. Nitta, M. Ouyang, eTransportation 18, 100272 (2023)
C. Wang, J. Liang, J.T. Kim, X. Sun, Sci. Adv. 8, 9516 (2022)
P. Jiang, G. Du, J. Cao, X. Zhang, C. Zou, Y. Liu, X. Lu, Energy Technol. 11, 2201288 (2023). https://doi.org/10.1002/ente.202201288
W. Xia, Y. Zhao, F. Zhao, K. Adair, R. Zhao, S. Li, R. Zou, Y. Zhao, X. Sun, Chem. Rev. 122, 3763 (2022)
L.-Z. Fan, H. He, C.-W. Nan, Nat. Rev. Mater. 6, 1003 (2021). https://doi.org/10.1038/s41578-021-00320-0.
S. Kalnaus, N.J. Dudney, A.S. Westover, E. Herbert, S. Hackney, Science. 381, eabg5998 (2023). https://doi.org/10.1126/SCIENCE.ABG5998
M. Balaish, J. Carlos Gonzalez-Rosillo, K. Joong Kim, Y. Zhu, Z.D. Hood, J.L.M. Rupp, Nat. Energy 6, 227 (2021). https://doi.org/10.1038/s41560-020-00759-5
Y. Xiao, Y. Wang, S.H. Bo, J.C. Kim, L.J. Miara, G. Ceder, Nat. Rev. Mater. 5, 105 (2019)
M.J. Wang, E. Kazyak, N.P. Dasgupta, J. Sakamoto, Joule 5, 1371 (2021)
Y. Tang, L. Zhang, J. Chen, H. Sun, T. Yang, Q. Liu, Q. Huang, T. Zhu, J. Huang, Energy Environ. Sci. 14(2), 602 (2021). https://doi.org/10.1039/d0ee02525a
E. Kazyak, R. Garcia-Mendez, W.S. LePage, A. Sharafi, A.L. Davis, A.J. Sanchez, K.-H. Chen, C. Haslam, J. Sakamoto, N.P. Dasgupta, Matter 2, 1025 (2020)
L. Porz, T. Swamy, B.W. Sheldon, D. Rettenwander, T. Frömling, H.L. Thaman, S. Berendts, R. Uecker, W.C. Carter, Y.M. Chiang, Adv. Energy Mater. 7, 1701003 (2017)
Y. Qi, C. Ban, S.J. Harris, Joule 4, 2599 (2020)
G. Wranglén, Electrochim. Acta 2(1–3), 130 (1960)
Z. Ning, D.S. Jolly, G. Li, R. De Meyere, S.D. Pu, Y. Chen, J. Kasemchainan, J. Ihli, C. Gong, B. Liu, D.L.R. Melvin, A. Bonnin, O. Magdysyuk, P. Adamson, G.O. Hartley, C.W. Monroe, T.J. Marrow, P.G. Bruce, Nat. Mater. 20, 1121 (2021)
C.D. Fincher, C.E. Athanasiou, C. Gilgenbach, M. Wang, B.W. Sheldon, W. Craig Carter, Y.-M. Chiang, Joule 6(12), 2794 (2022). https://doi.org/10.1016/j.joule.2022.10.011.
M.B. Dixit, A. Verma, W. Zaman, X. Zhong, P. Kenesei, J.S. Park, J. Almer, P.P. Mukherjee, K.B. Hatzell, ACS Appl. Energy Mater. 3, 9534 (2020)
W.S. LePage, Y. Chen, E. Kazyak, K.-H. Chen, A.J. Sanchez, A. Poli, E.M. Arruda, M.D. Thouless, N.P. Dasgupta, J. Electrochem. Soc. 166, A89 (2019)
A. Masias, N. Felten, R. Garcia-Mendez, J. Wolfenstine, J. Sakamoto, J. Mater. Sci. 54, 2585 (2019)
C. Xu, Z. Ahmad, A. Aryanfar, V. Viswanathan, J.R. Greer, Proc. Natl. Acad. Sci. U.S.A. 114, 57 (2017)
W. Chang, R. May, M. Wang, G. Thorsteinsson, J. Sakamoto, L. Marbella, D. Steingart, Nat. Commun. 12, 6369 (2021). https://doi.org/10.1038/s41467-021-26632-x
T. Krauskopf, R. Dippel, H. Hartmann, K. Peppler, B. Mogwitz, F.H. Richter, W.G. Zeier, J. Janek, Joule 3, 2030 (2019)
F. Han, A.S. Westover, J. Yue, X. Fan, F. Wang, M. Chi, D.N. Leonard, N.J. Dudney, H. Wang, C. Wang, Nat. Energy 4, 187 (2019)
H.K. Tian, Z. Liu, Y. Ji, L.Q. Chen, Y. Qi, Chem. Mater. 31, 7351 (2019)
O. Cojocaru-Mirédin, J. Schmieg, M. Müller, A. Weber, E. Ivers-Tiffée, D. Gerthsen, J. Power Sources 539, 231417 (2022). https://doi.org/10.1016/j.jpowsour.2022.231417
P. Barai, K. Higa, A.T. Ngo, L.A. Curtiss, V. Srinivasan, J. Electrochem. Soc. 166, A1752 (2019)
B.S. Vishnugopi, M.B. Dixit, F. Hao, B. Shyam, J.B. Cook, K.B. Hatzell, P.P. Mukherjee, Adv. Energy Mater. 12, 2102825 (2022). https://doi.org/10.1002/aenm.202102825
B. Gao, R. Jalem, H.K. Tian, Y. Tateyama, Adv. Energy Mater. 12, 2102151 (2022). https://doi.org/10.1002/aenm.202102151
M. Feng, J. Pan, Y. Qi, J. Phys. Chem. C 125, 15821 (2021)
W. Zaman, L. Zhao, T. Martin, X. Zhang, Z. Wang, Q.J. Wang, S. Harris, K.B. Hatzell, ACS Appl. Mater. Interfaces 15, 37401 (2023)
K. Lee, E. Kazyak, M.J. Wang, N.P. Dasgupta, J. Sakamoto, Joule 6, 2547 (2022)
J.A. Lewis, S.E. Sandoval, Y. Liu, D.L. Nelson, S.G. Yoon, R. Wang, Y. Zhao, M. Tian, P. Shevchenko, E. Martínez-Pañeda, M.T. McDowell, Adv. Energy Mater. 13(12), 2204186 (2023). https://doi.org/10.1002/AENM.202204186
D. Cao, T. Ji, Z. Wei, W. Liang, R. Bai, K.S. Burch, M. Geiwitz, H. Zhu, Nano Lett. 23, 9392 (2023)
S. Lee, K. Lee, S. Kim, K. Yoon, S. Han, M.H. Lee, Y. Ko, J.H. Noh, W. Kim, K. Kang, Sci. Adv. 8, eabq0153 (2022)
X. He, X. Ji, B. Zhang, N.D. Rodrigo, S. Hou, K. Gaskell, T. Deng, H. Wan, S. Liu, J. Xu, B. Nan, B.L. Lucht, C. Wang, ACS Energy Lett. 7, 131 (2022)
R. Weber, M. Genovese, A.J. Louli, S. Hames, C. Martin, I.G. Hill, J.R. Dahn, Nat. Energy 4, 683 (2019)
A.A. Assegie, J.H. Cheng, L.M. Kuo, W.N. Su, B.J. Hwang, Nanoscale 10, 6125 (2018)
J. Qian, B.D. Adams, J. Zheng, X. Wu, W.A. Henderson, J. Wang, M.E. Bowden, S. Xu, J. Hu, J.-G. Zhang, Adv. Funct. Mater. 26, 7097 (2016)
M.J. Wang, E. Carmona, A. Gupta, P. Albertus, J. Sakamoto, Nat. Commun. 11, 5201 (2020)
E. Kazyak, M.J. Wang, K. Lee, S. Yadavalli, A.J. Sanchez, M.D. Thouless, J. Sakamoto, N.P. Dasgupta, Matter 5, 3912 (2022)
R.A. Huggins, J. Power Sources 81, 13 (1999)
S.E. Sandoval, J.A. Lewis, B.S. Vishnugopi, D. Lars Nelson, M.M. Schneider, F.J. Quintero Cortes, C.M. Matthews, J. Watt, M. Tian, P. Shevchenko, P.P. Mukherjee, M.T. McDowell, Joule 7(9), 2054 (2023). https://doi.org/10.1016/j.joule.2023.07.022
Y. Liu, C. Wang, S.G. Geun, S.Y. Han, J.A. Lewis, D. Prakash, E.J. Klein, T. Chen, D.H. Kang, D. Majumdar, R. Gopalaswamy, M.T. McDowell, Nat. Commun. 14, 3975 (2023). https://doi.org/10.1038/s41467-023-39685-x
Y.-G. Lee, S. Fujiki, C. Jung, N. Suzuki, N. Yashiro, R. Omoda, D.-S. Ko, T. Shiratsuchi, T. Sugimoto, S. Ryu, J.H. Ku, T. Watanabe, Y. Park, Y. Aihara, D. Im, I.T. Han, Nat. Energy 5, 299 (2020). https://doi.org/10.1038/s41560-020-0575-z
C. Haslam, J. Sakamoto, J. Electrochem. Soc. 170, 040524 (2023)
A. Muller, L. Paravicini, J. Morzy, M. Krause, J. Casella, N. Osenciat, M.H. Futscher, Y.E. Romanyuk, ACS Appl. Mater. Interfaces 16, 695 (2024)
C. Cui, H. Yang, C. Zeng, S. Gui, J. Liang, P. Xiao, S. Wang, G. Huang, M. Hu, T. Zhai, H. Li, Sci. Adv. 8, eadd2000 (2022)
C.-M. Park, H. Jung, H.-J. Sohn, Electrochem. Solid-State Lett. 12, A171 (2009)
C. Yang, Y. Yao, S. He, H. Xie, E. Hitz, L. Hu, Adv. Mater. 29, 1702714 (2017)
P. Albertus, V. Anandan, C. Ban, N. Balsara, I. Belharouak, J. Buettner-Garrett, Z. Chen, C. Daniel, M. Doeff, N.J. Dudney, B. Dunn, S.J. Harris, S. Herle, E. Herbert, S. Kalnaus, J.A. Libera, D. Lu, S. Martin, B.D. McCloskey, M.T. McDowell, Y.S. Meng, J. Nanda, J. Sakamoto, E. Self, S. Tepavcevic, E. Wachsman, C. Wang, A.S. Westover, J. Xiao, T. Yersak, ACS Energy Lett. 6(4), 1399 (2021)
T.A. Hendriks, M.A. Lange, E.M. Kiens, C. Baeumer, W.G. Zeier, Batter. Supercaps 6, e202200544 (2023)
C. Wang, W. Ping, Q. Bai, H. Cui, R. Hensleigh, R. Wang, A. H. Brozena, Z. Xu, J. Dai, Y. Pei, C. Zheng, G. Pastel, J. Gao, X. Wang, H. Wang, J.-C. Zhao, B. Yang, X. (Rayne) Zheng, J. Luo, Y. Mo, B. Dunn, L. Hu, Science 368, 521 (2020).
J. Sakamoto, M.J. Wang, N. Taylor, Rapid-induction sinter forge for roll-to-roll continuous manufacturing of thin films, US Patent application 17/163,726 (2021)
J.-H. Seo, K. Verlinde, J. Guo, D.S. Baba Heidary, R. Rajagopalan, T.E. Mallouk, C.A. Randall, Scr. Mater. 146, 267 (2018)
S.S. Berbano, J. Guo, H. Guo, M.T. Lanagan, C.A. Randall, J. Am. Ceram. Soc. 100(5), 2123 (2017)
J. Guo, R. Floyd, S. Lowum, J.P. Maria, T. Herrison de Beauvoir, J.H. Seo, C.A. Randall, Annu. Rev. Mater. Res. 49, 275 (2019)
H. He, W. Weppner, Ionics (Kiel) 7, 469 (2001)
K. Kerman, A. Luntz, V. Viswanathan, Y.-M. Chiang, Z. Chen, J. Electrochem. Soc. 164, A1731 (2017)
T. Ates, M. Keller, J. Kulisch, T. Adermann, S. Passerini, Energy Storage Mater. 17, 204 (2019)
D.H.S. Tan, A. Banerjee, Z. Deng, E.A. Wu, H. Nguyen, J.-M. Doux, X. Wang, J. Cheng, S.P. Ong, Y.S. Meng, Z. Chen, ACS Appl. Energy Mater. 2, 6542 (2019)
Y.-T. Chen, M.A.T. Marple, D.H.S. Tan, S.-Y. Ham, B. Sayahpour, W.-K. Li, H. Yang, J.B. Lee, H.J. Hah, E.A. Wu, J.-M. Doux, J. Jang, P. Ridley, A. Cronk, G. Deysher, Z. Chen, Y.S. Meng, J. Mater. Chem. A 10, 7155 (2020)
G.T. Hitz, D.W. McOwen, L. Zhang, Z. Ma, Z. Fu, Y. Wen, Y. Gong, J. Dai, T.R. Hamann, L. Hu, E.D. Wachsman, Mater. Today 22, 50 (2019)
P.-A. Lavoie, R. Laliberté, J. Dubé, C. Gagnon, Co-extrusion manufacturing process of thin film electrochemical cell for lithium polymer batteries and apparatus therefor, US Patent 7,700,019 (2003)
L.G. Johnson, D.K. Johnson, Solid-state batteries and methods of fabrication thereof, European Patent EP3168914A1 (2013)
M.B. Dixit, W. Zaman, Y. Bootwala, Y. Zheng, M.C. Hatzell, K.B. Hatzell, ACS Appl. Mater. Interfaces 11, 45087 (2019)
J. Schnell, H. Knörzer, A.J. Imbsweiler, G. Reinhart, Energy Technol. 8, 1901237 (2020)
D. Hanft, J. Exner, M. Schubert, T. Stocker, P. Fuierer, R. Moos, J. Ceram. Sci. Technol. 6, 147 (2015)
H. Bae, J. Choi, G.M. Choi, Solid State Ionics 236, 16 (2013)
L.J. Deiner, T. Jenkins, T. Howell, M. Rottmayer, Adv. Eng. Mater. 21, 1900952 (2019)
D.W. McOwen, S. Xu, Y. Gong, Y. Wen, G.L. Godbey, J.E. Gritton, T.R. Hamann, J. Dai, G.T. Hitz, L. Hu, E.D. Wachsman, Adv. Mater. 30, 1707132 (2018)
M. Cheng, Y. Jiang, W. Yao, Y. Yuan, R. Deivanayagam, T. Foroozan, Z. Huang, B. Song, R. Rojaee, T. Shokuhfar, Y. Pan, J. Lu, R. Shahbazian-Yassar, Adv. Mater. 30, 1800615 (2018)
E. Ferraris, J. Vleugels, Y. Guo, D. Bourell, J.P. Kruth, B. Lauwers, CIRP Ann. 65, 761 (2016)
F. Zhang, M. Wei, V.V. Viswanathan, B. Swart, Y. Shao, G. Wu, C. Zhou, Nano Energy 40, 418 (2017)
K.A. Acord, A.D. Dupuy, X. Wang, A.L. Vyatskikh, O.K. Donaldson, T.J. Rupert, J.J. Wu, Q.N. Chen, J.M. Schoenung, J. Mater. Sci. 36, 4565 (2021)
K.A. Acord, A.D. Dupuy, U.S. Bertoli, B. Zheng, W.C. West, N. Chen, A.A. Shapiro, J.M. Schoenung, J. Mater. Process. Technol. (2021). https://doi.org/10.1016/j.jmatprotec.2020.116827
K.N. Jung, H.-S. Shin, M.-S. Park, J.-W. Lee, ChemElectroChem 6, 3842 (2019)
Funding
On behalf of all authors, the corresponding authors state that no funding was received for this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the idea for the article, conducted literature review, writing, created figures, wrote, and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kazyak, E., García-Méndez, R. Recent progress and challenges for manufacturing and operating solid-state batteries for electric vehicles. MRS Bulletin 49, 717–729 (2024). https://doi.org/10.1557/s43577-024-00740-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-024-00740-7