Abstract
The Resilience is a construct receiving growing attention from the scientific community in geriatrics and gerontology. Older adults show extremely heterogeneous (and often unpredictable) responses to stressors. Such heterogeneity can (at least partly) be explained by differences in resilience (i.e., the capacity of the organism to cope with stressors). The International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force met in Boston (MA,USA) on April 20, 2022 to discuss the biological and clinical significance of resilience in older adults. The identification of persons with low resilience and the prompt intervention in this at-risk population may be critical to develop and implement preventive strategies against adverse events. Unfortunately, to date, it is still challenging to capture resilience, especially due to its dynamic nature encompassing biological, clinical, subjective, and socioeconomic factors. Opportunities to dynamically measure resilience were discussed during the ICFSR Task Force meeting, emphasizing potential biomarkers and areas of intervention. This article reports the results of the meeting and may serve to support future actions in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Resilience is “the ability to recover or optimize function in the face of age-related losses or disease” (1). Indeed, it determines the dynamic propensity of the organism to lose function and subsequently recover after the disruption of homeostasis due to one or more external factors. The study of physical resilience has been indicated as a priority by the US National Institute on Aging (2). In recent years, the construct of resilience has received growing attention, especially during the COVID-19 pandemic (3), which is a global stressor that has been critically implicated in accelerating the aging process (4).
Resilience is multidimensional in nature. In other words, the measurement of resilience may require the longitudinal assessment of multiple factors and cannot be captured by a single generalized test (5, 6). Different measures of resilience have been proposed (4, 6, 7), which tend to distinguish physical and psychological resilience.
The International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force met in Boston (MA, USA) on April 20, 2022 to discuss the clinical and biological significance of resilience. The discussion was specifically focused on how to measure resilience in clinical and research settings. Potential strategies to advance the field were also debated. Preliminary results of ongoing research activities were presented. The present article summarizes the contents of the meeting to further promote the discussion and interactions on the topic.
Resilience in frail older persons: clinical and biological aspects
The person’s resilience depends on his/her physiological and clinical capacities/reserves, allowing him/her to adapt to stressors (8). Environmental factors (including social support) may also be considered a contributor to an individual’s resilience as representing significant determinants of the ability to cope with stressors.
The static assessment of the person’s health is a good, but limited, predictor of his/her capacity to recover after a disruptive event. Whereas capturing the dynamic modifications through longitudinal monitoring allows one to measure the robustness of the biological and homeostatic states. In fact, single health assessments (e.g., static measures of disease burden or frailty) are associated with incident adverse events, but the predictive capacity is improved when multiple assessments are combined in the definition of a health trajectory (9–11).
Unlike young individuals, who have a high level of recovery following stressful events, older persons have a lower and heterogeneous capacity to “bounce back” to pre-stress functional status. In other words, the aging process determines a change of the so-called tipping point (i.e., the threshold defining the homeostatic equilibrium of the organism), consequently varying the capacity to cope with stressors. The identification and assessment of the tipping point may provide a dynamic measure of resilience and inform the design of preventive/therapeutical interventions (9).
Recently, Guion et al. (12) described four distinct trajectories of recovery in nursing home (NH) residents admitted to the emergency department. In particular, the health trajectory was drawn observing the changes of physical performance from the pre-event status to the return to the NH. Those NH residents with the highest level of physical reserves at the baseline showed the best capacity for recovery. On the other hand, those with the worst status at the baseline presented minimal capacity for recovery. However, it is noteworthy that, whereas models like this tend to be quite reliable when observing a population/group, their robustness is substantially lower when applied to the individual level. In fact, the inter-individual variability can significantly affect and deviate the trajectories from expectations. Indeed, there is a need for more than just the assessment of the initial reserves when exploring the inner capacities and reserves of the person.
Chronological age is an essential factor associated with resilience. Applying multi-state modeling approach to estimate 1-year transition probabilities of the New Mexico Aging Process Study data (13), the decline of walking speed and cognitive function among healthy persons was prospectively described over a follow-up of 9 years. The rate of decline experienced by the youngest part of the population (i.e., people aged 60 to 78 years) was relatively small; at the same time, a high capacity for recovery was observed. On the other hand, in persons older than 78 years, a two-fold risk of decline and a halved chance of recovery was estimated after an adverse event.
A small study (14) investigated the effects of two weeks of immobilization followed by four weeks of retraining on muscle function and fiber morphology in a sample of healthy men with similar levels of physical activity, according to age (n=9 older vs. 11 younger individuals). It was reported that older participants were more susceptible to the adverse effects of short-term muscle disuse, in terms of muscle fiber size and rapid force capacity, compared to the younger counterparts. Furthermore, older persons required a longer time for muscle function recovery.
Age-related changes in resilience are likely mediated by biological processes. As suggested by López-Otín and colleagues (15), different strategies are used by organisms to attain biological stability. These strategies are based on homeostatic resilience (i.e., genetic, neural, metabolic, immunological, microbiome-based mechanisms), hormetic regulation (i.e., mitohormesis, healthspan, lifespan), and repairing and regeneration capacities. Indeed, it is crucial to investigate the so-called “hallmarks of aging”, especially given the hypothesis that loss of resilience anticipates the clinical onset of frailty. It would thus be possible that biological tests might predict future health events and guide geroprotective interventions by targeting the hallmarks of aging and modulating the organism’s resilience.
During the Task Force meeting, preliminary results from secondary analyses conducted in the Multidomain Alzheimer Preventive Trial (MAPT) (16) database were presented. The analyses were aimed at investigating the longitudinal relationship of mitochondrial function, regeneration, and inflammation with frailty. Physical frailty was measured according to the phenotypic criteria proposed by Fried and colleagues (17) at the study baseline and every year for two years. Different biomarkers of aging, inflammation, and mitochondrial function (e.g., C-Reactive Protein [CRP], Interleukin-6 [IL-6], Tumor Necrosis Factor Receptor-1 [TNFR-1], Monocyte Chemoattractant Protein-1 [MCP-1], Growth Differentiation Factor-15 [GDF-15], Periostin) were measured. Results showed that those participants who became frail after 12 months had higher levels of TNFR-1 and GDF-15 at the baseline. Of these, participants who reversed to robust after 12 additional months had lower GDF-15 concentrations, a promising biomarker of cellular aging and systemic inflammation (18–20), compared to those who remained frail. The analyses presented limitations (e.g., a small number of frail participants). However, the results were still suggestive of the role that biomarkers of aging may play in the definition of the recovery process.
Kirkland et al. (21) proposed several stressors for potentially assessing the physiological resilience in animal models (i.e., starvation, water deprivation, anesthesia, chemotherapy, trauma, temperature stress, cortisol, circadian rhythm, infection, barbiturates). In daily practice, many other situations can be envisioned as stressful events (e.g., sepsis, infections, vaccinations, immobilization and bed rest, chemotherapy, altered diet, dehydration, surgical stress (22)) to use for studying and measuring resilience. In particular, the recovery from surgical stress may represent a very interesting benchmark to advance in the field, mainly because it is easier to fix in the temporal sequence of the health trajectory. Nevertheless, the main problem in clinical practice is that resilience is diagnosed/seen a posteriori. Consequently, it is complicated to predict whether the older person will recover or not.
The targeting of biological mechanisms with geroscience-based interventions, including pharmacological (i.e., metformin, angiotensin receptor blockers, dasantinib, quercetin), hormonal (i.e., oxytocin), physical (i.e., brain and muscle stimulation) and nutraceutical (i.e., Vitamin D, resveratrol, Omega-3) strategies, represents a promising frontier in the attempt to preserve and improve mobility function in older people (23, 24). In the context of infectious diseases (e.g., COVID-19, influenza, pneumonia), there might also be opportunities to improve the immunological status while acting on the background biology of aging through pharmacological interventions (e.g., low-dose mammalian Target of Rapamycin [mTOR] inhibitors) (25). Chemotherapies may also cause accelerated aging-like states. In this situation, it could be investigated the potential of senotherapeutic drugs that selectively induce apoptosis of senescent cells (i.e., senolytics) or suppress their secretory phenotype (i.e., senomorphics) (26).
Strategies integrating resilience in the daily routine of geriatric medicine are also amenable. The Integrated Care for Older People (ICOPE) initiative proposed by the World Health Organization (WHO) might represent an opportunity in this direction as designed to reshape the care models toward a person-centered approach (27, 28). For example, the Gérontopôle of Toulouse (France) has recently implemented clinical activities based on the ICOPE model (29). Persons included in the program may be asked to be part of research programs devoted to 1) the study of health trajectories and dynamics, and 2) the exploration of possible geroprotectors. In this context, digital medicine and the use of remote monitoring of health parameters open up considerable prospects. The longitudinal analysis of big data in the general population could make it possible to identify in the future subjects with low resilience and implement targeted preventive interventions.
Perspectives on Resilience
Several mechanisms are involved in the aging process, representing the biological substratum of many age-related conditions (e.g., frailty, sarcopenia, cancer, neurodegenerative conditions, immunological disorders, cardiovascular diseases). It has been hypothesized that interventions targeting such background may prevent the onset of typical conditions of old age and represent a potential opportunity to extend human healthspan through the compression of morbidity.
In this context, resilience is an interesting construct to explore the biology of aging because it captures the dynamic system of the organism over time (potentially even before conception; i.e., in utero) (30–32). In other words, an impaired resilience might be considered a marker of accelerated aging, becoming a clinically relevant expression to capture before an overt manifestation (30, 33). At the same time, a compromised resilience may enhance the biological aging, feeding the generation of a vicious cycle.
The measurement of resilience may support the study of the individual’s health trajectory and provide a means for the early identification of those at risk of decline (34). There might indeed be a window for remarkable opportunities to preventively improve the health status by acting on the biology of aging. For example, rapamycin has been shown to extend life span and ameliorate resilience towards age-related conditions (including immune dysfunction) via the inhibition of the mTOR pathway (35–37). During the COVID-19 pandemic, many have hypothesized a role for interventions able to boost or improve resilience in frail older persons at risk of suffering the most severe consequences of the viral infection. Senotherapeutic compounds have also been proposed to support the individual exposed to the pathogenic stress of the severe acute respiratory syndrome due to SARS-CoV-2 infection (38). A potential role of them has also been hypothesized in the long-COVID syndrome (39).
A paradigm for translation is represented by the design of clinical trials to test geroprotective agents. Most funded clinical trials are designed to provide straightforward answers following the so-called “standalone-disease” approach, which investigates and treats a well-defined condition in a sort of isolation from the complexity of the aging process (40). The Targeting Aging with Metformin (TAME) study, a proof-of-concept clinical trial designed to look at the effect of metformin on the incidence of age-related diseases, is an example of how to describe the effects of an intervention on health trajectories before the occurrence of overt diseases (41, 42).
The idea of modeling resilience in animal models to translate results into humans is fascinating. Indeed, resilience can be explored in animals in a relatively simple way and adopting a holistic approach. Interestingly, mice of similar age have shown a broad spectrum of responses after stressors are applied, suggesting the possibility of using them in the study of resilience (21).
The implementation of resilience in the clinical setting requires the development of feasible and meaningful assessment instruments. Preliminary results of a study conducted in genetically heterogeneous female and male mice aimed at exploring if midlife resilience could be predictive of lifespan were anticipated during the Task Force meeting. It was shown that resilient mice presented a longer life expectancy, especially in females. It was also demonstrated that resilience in midlife was associated with better physical, metabolic, and cardiac function at a more advanced age, especially in males. The findings suggest that interventions applied earlier in life or before stressful events (e.g., prehabilitation) might be beneficial.
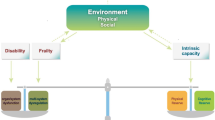
In the Duke University Pepper Center conceptual model of resilience (Figure 1), the pre-stress reserve comprises multiple domains, including psychological, physiological, and cognitive abilities that each person accesses to adapt in the face of a health stressor. At the same time, two different conceptual approaches have been proposed to quantify resilience after a stressor (43). The first one (i.e., the recovery phenotype) is based on observing the individual recovery patterns across health measures over time. This approach is a highly descriptive, multiparametric model that can simultaneously consider multiple outcomes (i.e., latent class trajectory analysis, factor analysis, principal components analysis). Age, comorbidities, and pre-stressor function highly drive the recovery phenotype. The phenotypic way to quantify resilience is useful for prognostic models in clinical practice or classifying outcomes in intervention research. For example, in a recent study, the recovery phenotype approach has been used as a model to study resilience in older adults following a hip fracture (44). In particular, the recovery trajectories for multiple selected outcomes (i.e., daily steps count, time to complete single chair stands, grip strength, gait speed) were described in three different resilience groups (i.e., low, medium, and high resilience). The authors found that the pre-stressor functional status was the strongest predictor of the subsequent recovery.
Another way to quantify the clinical trajectory of recovery is represented by the so-called “expected recovery differential approach”, which is aimed at quantifying how observed outcomes differ from expected outcomes. This approach, based on predictive models performed in large cohorts, accounts for baseline status, stressor-related factors, and environment. It might be particularly appropriate to identify the biological mechanisms underlying resilience (43). The expected recovery differential was recently applied by Parker et al. (45) to identify biomarkers of resilience after hip fracture in older adults. Different biomarkers of aging representing the inflammatory and immunological pathways (i.e., TNFR-1, TNFR-2, soluble vascular adhesion molecule-1 [sVCAM-1], and IL-6), the metabolic and mitochondrial function (i.e., non-esterified fatty acids, lactate, ketones, acylcarnitines, free amino acids, and insulin-like growth factor 1), and the gene expression (i.e., circulating microRNAs [miRNAs]) were evaluated. It was found that the full panel of biomarkers explained 38% of the resilience variance, defined as expected recovery differential. After performing a principal component analysis on the 64 metabolites, four factors were identified. In particular, the most parsimonious set predicting the expected recovery differential (generated by a Least Absolute Shrinkage and Selection Operator [LASSO] regression) explained 27% of resilience variance after hip fracture. These results are in accordance with the hypothesis that those people showing a better recovery after a hip fracture present a lower degree of cellular senescence, inflammation, mitochondrial dysfunction, and muscle impairment (Figure 2) in the days close to the event.
Finally, during the Task Force meeting, the protocol of the PRIME-KNEE study (46) was presented. PRIME-KNEE is a prospective cohort study enrolling 250 patients aged 60 years and older scheduled for elective knee replacement surgery. The primary objective of the PRIME-KNEE study is to validate provocative tests and biomarkers predicting recovery in the perioperative period. For this purpose, participants are assessed for cognitive, physical, and psycho-social reserves during the baseline visit. Moreover, they also undergo a series of provocative tests (i.e., dual-task effect on gait speed, cerebrovascular reactivity assessed by functional near-infrared spectroscopy, peripheral blood mononuclear cells reactivity tests) and blood drawn to assess biomarkers of interest. A follow-up, including in-person visit after six months from surgery and several other measurements (i.e., pain intensity and inference, cognitive change index, 7-day step counts, rehabilitative procedures), is also planned.
Conclusions
Resilience represents a promising area of investigation for research on aging. The biological and clinical understanding of resilience will allow the development of targeted interventions for its improvement. In particular, it will be necessary to 1) define the best way to identify individuals with low resilience and 2) describe the correct methodology for promptly intervening before the onset of adverse events.
The ICFSR Task Force concluded that, given the involvement of multiple organs and systems, measures of resilience should be multidimensional and consider a broad spectrum of outcomes. At present, holistic interventions improving physical, psychological, and cognitive function seem particularly promising to boost resilience. The identification of biomarkers of resilience represents a necessary strategy to improve the understanding of the heterogeneous health trajectories following stressful events.
References
Resnick B, Galik E, Dorsey S, Scheve A, Gutkin S. Reliability and validity testing of the physical resilience measure. The Gerontologist. 2011 Oct;51(5):643–52.
Hadley EC, Kuchel GA, Newman AB, Workshop Speakers and Participants. Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging. J Gerontol A Biol Sci Med Sci. 2017 Jul 1;72(7):980–90.
Amieva H, Avila-Funes JA, Caillot-Ranjeva S, Dartigues JF, Koleck M, Letenneur L, et al. Older People Facing the Crisis of COVID-19: Between Fragility and Resilience. J Frailty Aging. 2021 Apr 1;10(2):184–6.
Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct. J Gerontol Ser A. 2016 Apr 1;71(4):489–95.
Pedone C, Costanzo L, Finamore P, Bandinelli S, Ferrucci L, Antonelli Incalzi R. Defining Resilience in Older People: Does a Subjective Definition of Stressor Work? J Gerontol A Biol Sci Med Sci. 2021 Jul 13;76(8):1480–5.
Merchant RA, Aprahamian I, Woo J, Vellas B, Morley JE. Editorial: Resilience And Successful Aging. J Nutr Health Aging. 2022;26(7):652–6.
Wu C, Lin TZ, Sanders JL. A Simplified Approach for Classifying Physical Resilience among Community-Dwelling Older Adults: The Health, Aging, and Body Composition Study. J Frailty Aging. 2022 Jul 1;11(3):281–5.
Clegg A, Young J, Iliffe S, Rikkert M, Rockwood K. Frailty in elderly people. Lancet. 2013 Feb 7;381(9868):752–62.
Olde Rikkert MGM, Melis RJF. Rerouting Geriatric Medicine by Complementing Static Frailty Measures With Dynamic Resilience Indicators of Recovery Potential. Front Physiol. 2019;10:723.
Hamaker ME, Jonker JM, Rooij SE de, Vos AG, Smorenburg CH, Munster BC van. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol. 2012 Oct 1;13(10):e437–44.
Hubbard JM, Jatoi A. Incorporating biomarkers of frailty and senescence in cancer therapeutic trials. J Gerontol A Biol Sci Med Sci. 2015 Jun;70(6):722–8.
Guion V, Barreto PDS, Rolland Y. Nursing Home Residents’ Functional Trajectories and Mortality After a Transfer to the Emergency Department. J Am Med Dir Assoc [Internet]. 2020 Jul 10 [cited 2020 Jul 11];0(0). Available from: https://www.jamda.com/article/S1525-8610(20)30433-3/abstract
Qualls C, Waters DL, Vellas B, Villareal DT, Garry PJ, Gallini A, et al. Reversible States of Physical and/or Cognitive Dysfunction: A 9-Year Longitudinal Study. J Nutr Health Aging. 2017;21(3):271–5.
Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ørtenblad N, et al. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol Bethesda Md 1985. 2010 Dec;109(6):1628–34.
López-Otín C, Blasco M, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–217.
Vellas B, Carrie I, Gillette-Guyonnet S, Touchon J, Dantoine T, Dartigues J, et al. MAPT study: a multidomain approach for preventing Alzheimer’s disease — Design and baseline data. J Prev Alzheimers Dis. 2014 Jun;1:13–22.
Fried L, Tangen C, Walston J, Newman A, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar 1;56(3):M146–56.
Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021 Oct;17(10):592–607.
Wischhusen J, Melero I, Fridman WH. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front Immunol. 2020 May 19;11:951.
Kim M, Walston JD, Won CW. Associations Between Elevated Growth Differentiation Factor-15 and Sarcopenia Among Community-dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2022 Apr 1;77(4):770–80.
Kirkland JL, Stout MB, Sierra F. Resilience in Aging Mice. J Gerontol A Biol Sci Med Sci. 2016 Nov;71(11):1407–14.
Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating Health Span in Preclinical Models of Aging and Disease: Guidelines, Challenges, and Opportunities for Geroscience. J Gerontol A Biol Sci Med Sci. 2016 Nov;71(11):1395–406.
Anton SD, Cruz-Almeida Y, Singh A, Alpert J, Bensadon B, Cabrera M, et al. Innovations in Geroscience to enhance mobility in older adults. Exp Gerontol. 2020 Dec;142:111123.
Fielding RA. Sarcopenia, Frailty, and Gero-Science: A Decade of Progress and a Bright Future of Discovery. J Frailty Aging. 2021 Apr 1;10(2):82–3.
Wang W, Thomas R, Oh J, Su DM. Thymic Aging May Be Associated with COVID-19 Pathophysiology in the Elderly. Cells. 2021 Mar 12;10(3):628.
Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc. 2017 Oct;65(10):2297–301.
Integrated care for older people (ICOPE): Guidance for person-centred assessment and pathways in primary care. World Health Organization; 2019.
World Health Organization. Integrated care for older people (ICOPE) implementation framework: guidance for systems and services [Internet]. World Health Organization; 2019 [cited 2021 Nov 5]. vi, 41 p. Available from: https://apps.who.int/iris/handle/10665/325669
Tavassoli N, Barreto P de S, Berbon C, Mathieu C, de Kerimel J, Lafont C, et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Health Longev. 2022;3:e394–404.
LeBrasseur NK. Physical Resilience: Opportunities and Challenges in Translation. J Gerontol A Biol Sci Med Sci. 2017 Jul 1;72(7):978–9.
Azzolino D, Spolidoro GCI, Saporiti E, Luchetti C, Agostoni C, Cesari M. Musculoskeletal Changes Across the Lifespan: Nutrition and the Life-Course Approach to Prevention. Front Med. 2021;8:697954.
Sayer AA, Cooper C, Evans JR, Rauf A, Wormald RP, Osmond C, et al. Are rates of ageing determined in utero? Age Ageing. 1998 Sep;27(5):579–83.
Huffman DM, Schafer MJ, LeBrasseur NK. Energetic interventions for healthspan and resiliency with aging. Exp Gerontol. 2016 Dec 15;86:73–83.
LeBrasseur NK, de Cabo R, Fielding R, Ferrucci L, Rodriguez-Manas L, Viña J, et al. Identifying Biomarkers for Biological Age: Geroscience and the ICFSR Task Force. J Frailty Aging. 2021;10(3):196–201.
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014 Dec 24;6(268):268ra179.
Mannick JB, Morris M, Hockey HUP, Roma G, Beibel M, Kulmatycki K, et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018 Jul 11;10(449):eaaq1564.
Bischof E, Siow RC, Zhavoronkov A, Kaeberlein M. The potential of rapalogs to enhance resilience against SARS-CoV-2 infection and reduce the severity of COVID-19. Lancet Healthy Longev. 2021 Feb;2(2):e105–11.
Camell CD, Yousefzadeh MJ, Zhu Y, Prata LGPL, Huggins MA, Pierson M, et al. Senolytics reduce coronavirus-related mortality in old mice. Science. 2021 Jul 16;373(6552):eabe4832.
Lynch SM, Guo G, Gibson DS, Bjourson AJ, Rai TS. Role of Senescence and Aging in SARS-CoV-2 Infection and COVID-19 Disease. Cells. 2021 Nov 30;10(12):3367.
Canevelli M, Bruno G, Vanacore N, de Lena C, Cesari M. Are we really tackling the “evidence-based medicine issue” in Alzheimer’s disease. Eur J Intern Med. 2016;35:e29–30.
Barzilai N, Crandall J, Kritchevsky S, Espeland M. Metformin as a Tool to Target Aging. Cell Metab. 2016 Jun 14;23(6):1060–5.
Justice JN, Niedernhofer L, Robbins PD, Aroda VR, Espeland MA, Kritchevsky SB, et al. Development of Clinical Trials to Extend Healthy Lifespan. Cardiovasc Endocrinol Metab. 2018 Dec;7(4):80–3.
Colón-Emeric C, Pieper CF, Schmader KE, Sloane R, Bloom A, McClain M, et al. Two Approaches to Classifying and Quantifying Physical Resilience in Longitudinal Data. J Gerontol A Biol Sci Med Sci. 2020 Mar 9;75(4):731–8.
Colón-Emeric C, Whitson HE, Pieper CF, Sloane R, Orwig D, Huffman KM, et al. Resiliency Groups Following Hip Fracture in Older Adults. J Am Geriatr Soc. 2019 Dec;67(12):2519–27.
Parker DC, Colón-Emeric C, Huebner JL, Chou CH, Kraus VB, Pieper CF, et al. Biomarkers Associated with Physical Resilience After Hip Fracture. J Gerontol A Biol Sci Med Sci. 2020 Sep 25;75(10):e166–72.
Whitson HE, Crabtree D, Pieper CF, Ha C, Au S, Berger M, et al. A template for physical resilience research in older adults: Methods of the PRIME-KNEE study. J Am Geriatr Soc. 2021 Nov;69(11):3232–41.
Whitson HE, Crabtree D, Pieper CF, Ha C, Au S, Berger M, et al. A template for physical resilience research in older adults: Methods of the PRIME-KNEE study. J Am Geriatr Soc. 2021;69(11):3232–41.
Funding
Open Access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflicts of interest
The Task Force was partially funded by registration fees from industrial participants. These corporations placed no restrictions on this work. Dr. Rooks is employees of Novartis Institutes for BioMedical Research. Dr. Vellas is an investigator in clinical trials sponsored by the Toulouse University Hospital (Inspire Geroscience Program). Dr. Fielding reported grants from National Institutes of Health, grants from USDA Agricultural Research Service, grants, personal fees and other from Axcella Health, Juvicell, Inside Tracker, grants and personal fees from Biophytis, personal fees from Amazentis, Nestlé and Pfizer, outside the submitted work. No conflict of interest declared by the other authors.
Rights and permissions
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Cesari, M., Azzolino, D., LeBrasseur, N.K. et al. Resilience: Biological Basis and Clinical Significance — A Perspective Report from the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force. J Frailty Aging 11, 342–347 (2022). https://doi.org/10.14283/jfa.2022.62
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2022.62