Abstract
Frailty is associated with multiple adverse health outcomes, including mortality. Several methods have been used to characterize frailty, each based on different frailty scales. These include scales based on phenotype, multidomain, and deficit accumulations. Several systematic reviews have examined the association between frailty and mortality; however, it is unclear whether these different frailty scales similarly predict mortality. This umbrella review aims to examine the association between frailty assessed by different frailty scales and all-cause mortality among community-dwelling older adults. A protocol was registered at PROSPERO, and it was conducted following the PRISMA statement. MEDLINE, Embase, PubMed, Cochrane Database of Systematic Reviews, Joanna Briggs Institute (JBI) EBP database, and Web of Science database was searched. Methodological quality was assessed using the JBI critical appraisal checklist and online AMSTAR-2 critical appraisal checklist. For eligible studies, essential information was extracted and synthesized qualitatively. Five systematic reviews were included, with a total of 434,115 participants. Three systematic reviews focused on single frailty scales; one evaluated Fried’s physical frailty phenotype and its modifications; another focused on the deficit accumulation frailty index. The third evaluated the FRAIL (Fatigue, Resistance, Ambulation, Illness, and Loss of weight) scale. The two other systematic reviews determined the association between frailty and mortality using different frailty scales. All of the systematic reviews found that frailty was significantly associated with all-cause mortality. This umbrella review demonstrates that frailty is a significant predictor of all-cause mortality, irrespective of the specific frailty scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
There is increasing attention toward frailty as a clinically meaningful measure of geriatric health (1). Contemporary research has defined frailty’s clinical and physiological characteristics and highlights the vulnerability of frail, older adults to poor health outcomes (2). Accordingly, the number of publications on frailty has increased exponentially over the last few decades (3) as the determination of frailty status is emerging as a significant predictor of outcomes in other fields, including cardiology (4, 5), neurology (6, 7), oncology (8), orthopaedics (9), surgery (10), in addition to geriatrics in general. Consequently, the association between frailty and all-cause mortality has been investigated across different settings and populations.
While the concept of frailty is widely recognized, there is no single explicit criterion to define frailty. In 2013, a consensus statement by six major international scientific societies defined frailty as a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function and increases an individual’s vulnerability for developing disability, dependency, or death (11, 12). In this definition, frailty is viewed as firstly, a clinical entity distinct from disability, sarcopenia, or multimorbidity; secondly, it affects a person’s physical or cognitive domains; and finally, it is considered as a dynamic state, which can improve or deteriorate over time (11). An intermediate or ‘prefrail’ stage has also been recognized (13–15). Several frailty scales have been developed to characterize frailty in older adults, described in three broad categories. The first category includes focused physical scales, which most notably contain the Fried physical frailty phenotype from the Cardiovascular Health Study and adaptations derived from this original scale (13). It consists of five components: unintentional weight loss, muscle weakness, exhaustion or low energy level, slowness or slow gait, and low physical activity.
Persons are frail if three or more of the five criteria are met. The second category of frailty scales is a multidomain scale (16), which describes multidimensional characteristics of frailty containing more than one medical, physical, cognitive, or environmental factor. The third type of scale is a deficit accumulation frailty index (17). It consists of an inventory of various deficits covering multiple domains or body systems and the percentage of deficits calculated. These three types of scales capture different aspects of the frailty syndrome and, therefore, there may be differences in their association with health outcomes. Understanding these differences is important because it could inform how the various measures are best applied. All three categories of frailty assessment scales have some limitations. For example, the phenotype scale does not cover all frailty dimensions, such as cognition or affect (13). The multidomain scale and the deficit accumulation model-based scales are comprehensive but time-consuming. Previously, systematic collection of clinical information was not feasible in many settings, challenging the integration of this scale into regular healthcare practice (18). However, the growing popularity and implementation of electronic health records and automated frailty indexes are increasingly being developed in different countries, e.g., in the USA (19, 20), Australia (21) and various European countries (22). Furthermore, findings from the UK have shown that routine implementation of the electronic frailty index enabled the delivery of evidence-based interventions to improve outcomes in the older population (23).
Few systematic reviews have explored different frailty scales and determined whether frailty assessed by these scales is predictive of all-cause mortality. Furthermore, it remains unclear whether a particular frailty scale is a better predictor of mortality of community-dwelling older adults (13, 24). Therefore, the objective of this umbrella review is to qualitatively synthesize and evaluate the association between frailty determined by different frailty scales and all-cause mortality in community-dwelling older people.
Methods
A protocol was developed, and the review was conducted following the Preferred Reporting Items for Systematic reviews and meta-analyses (PRISMA) statement (25). The protocol was registered at the International Prospective Register of Systematic Reviews or PROSPERO (ID: CRD 42020155407).
Data sources and search strategy
The search strategy aimed to find published systematic reviews and meta-analyses that evaluated the association between frailty and all-cause mortality in community-dwelling older populations. Systematic and comprehensive searches were conducted in electronic databases: MEDLINE, Embase, PubMed, Cochrane Database of Systematic Reviews (CDSR), Joanna Briggs Institute Evidence-Based Practice (JBI EBP) Database, and Web of Science. The search was conducted in October 2019 and updated in July 2020. The search strategy and search terms are provided in Appendix I. Studies conducted on humans and articles published in English were considered eligible for this review, and duplicates were excluded. The searches were independently performed by two authors (ARMSE and CB). Any discrepancies were resolved by discussion.
Inclusion and exclusion criteria
We included systematic reviews and meta-analyses that have reported the association between frailty and mortality among community-dwelling older adults aged 65 years or above using any frailty scales, e.g., Fried physical frailty phenotype or modifications, deficit accumulation frailty index, and multidomain frailty index. We excluded systematic reviews and meta-analyses that included only hospitalized and institutionalized older adults or examined disease-specific outcomes (i.e., falls, fractures, heart failure, etc.) rather than mortality. However, we included two systematic reviews where few studies had participants less than 65 years of age as those were considered in their meta-analyses (26, 27).
Study selection and data extraction
Two reviewers (ARMSE and CB) independently searched titles. They screened abstracts before retrieving the full texts, assessed eligibility for the type of participants, study design, and outcomes. Data were extracted using a standardized form including author and year of publication, location, population characteristic, sample size, the proportion of female participants, age range, frailty scales used, number of deficits used to create the frailty scales, and follow-up period. We also noted the quality and bias assessment, effect sizes, and measure of variance, most commonly hazard ratios with 95% confidence intervals and heterogeneity assessments.
Methodological quality assessment
Manuscripts were assessed for methodological quality before inclusion in the review. The quality assessment of the included five systematic reviews were performed by ARMSE and CB. We used JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses (28) (Table 1) and the online ‘A MeaSurement Tool to Assess systematic Reviews Version 2′ (AMSTAR 2) checklist (29).
Data synthesis and analysis
The studies were combined using qualitative best evidence synthesis, as statistical pooling could not be done due to the high heterogeneity of the included studies’ meta-analyses. We extracted and reported the pooled effect sizes of the outcomes meta-analyzed within the reviews (Table 2).
Results
Search results
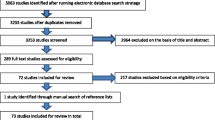
A total of 969 records were identified from the six databases, and after removing the duplicates, 686 were screened for eligibility based on title and abstract. Twenty-three full-text articles were then reviewed for relevance, out of which 18 were excluded because aspects similar to, but not defined explicitly as, frailty were assessed, e.g., gait speed (30, 31), sarcopenia (32), various health indicators (33) or geriatric syndromes (34); outcomes other than mortality were examined, e.g., trauma (35), fractures (9, 36), falls (37), high blood pressure and cardiovascular outcomes (38) or heart failure (4); study population included were from clinical practice (39), nursing home (40) or critical care (41) but not from a community setting; the study involved interventions, e.g., treatment modalities (42); or the article was a systematic review protocol or an umbrella review which evaluated frailty scales for clinical outcomes from community, residential care and hospital settings (43–45). This left five eligible systematic reviews and meta-analyses in the umbrella review (Figure 1). All five reviews were of moderate to high quality, as assessed by the JBI critical appraisal checklist (Table 1) and online AMSTAR-2 checklist. The reviews included 93 studies (some of which were included in multiple systematic reviews), and they assessed a range of outcomes. Of these studies, 77 examined the association between frailty and all-cause mortality over one to sixteen years of follow-up and were the focus of this review. Of the five systematic reviews, one review focused only on studies that used the frailty scale exclusively based on the Fried phenotype and its modifications (11 scales in a total of which four were original and seven modified) (46); one examined the FRAIL scale which is a questionnaire-based phenotype scale with five components, i.e., fatigue, resistance, ambulation, illness, and loss of weight (27); one review included studies assessing the deficit accumulation frailty index, with between 23 and 70 deficit items (26); one review included studies assessing either the Fried phenotype (7 studies) or the deficit accumulation frailty index (8 studies) (47); while the fifth review included 25 different scales of which five were Fried phenotype-based scales, 14 multidomain scales and six were deficit accumulation frailty index containing 23 to 83 deficits (16).
PRISMA 2009 Flow Diagram(50): Frailty Status and All-Cause Mortality in Community-Dwelling Older Individuals: An Umbrella Review
Overall, the participants were predominantly over 65 years of age, with a minimum age for inclusion varying from 50 to 75. However, one study included participants with a minimum age of 15 years (48). The maximum age recorded in one study was 108 years (26). The participants included were community-dwelling individuals from Australia, Canada, China, Israel, Mexico, the United Kingdom (UK), the United States of America (USA), and multiple European countries. Female participants represented 42% to 74% of the sample in most studies (Table 2). Individual study’s frailty outcome was adjusted for a range of two to ten covariates (e.g., age, gender, education, smoking, alcohol intake, socioeconomic conditions) in their analysis.
Overall findings for the association between frailty and all-cause mortality
All five systematic reviews reported a significant association between frailty and an increased risk of mortality; however, the effect size between frailty and mortality varied across the included systematic reviews. For example, the meta-analysis that included 24 studies using three types of scales (i.e., Fried physical frailty phenotype, deficit accumulation frailty index, and multidomain frailty index) estimated an overall hazard ratio of 2.34 (95% CI:1.77, 3.09) between frailty and all-cause mortality (16). The estimated overall relative risk was 1.83 (95% CI: 1.68, 1.98). In their analysis, comparing the non-frail to frail groups, the risk associated with mortality varied depending on the frailty scales used. The Fried physical frailty phenotype was associated with a 2.6-fold increased risk of mortality (HR: 2.58; 95% CI: 1.83, 3.64; I2=89%, P <0.001); the multidomain frailty index with a 2.1-fold increased risk (HR: 2.13; 95% CI: 1.38, 3.29; I2=96%, P <0.001); and the deficit accumulation frailty index a 1.85-fold (HR:1.85; 95%CI: 1.30, 2.63; I2 = not available, P = not available) (16). Similar effect sizes were reported from the systematic review that included only the phenotype-based frailty index and found that frailty was associated with a two-fold increased risk of mortality than robust or non-frail persons (HR: 2.00; 95% CI: 1.73, 2.32) (46). Direct comparison of effect sizes from the other systematic review was not possible, given they considered the association between a one-unit increase in frailty score using the deficit accumulation frailty index and mortality (random effect model: HR:1.04; 95% CI: 1.03, 1.04; fixed effect model: HR: 1.28; 95% CI: 1.26, 1.31 per 0.1 increase in frailty index) (26). Only one systematic review included a questionnaire-based FRAIL scale to assess the relationship between frailty and mortality (27). From the eight studies included in this review, it was found that individuals classified as frail or prefrail, compared to non-frail individuals, had a 3.5-fold and 1.8-fold increased risk of mortality, respectively, over 2.4 years to 4.3 years of follow-up. The predictive value of mortality remained similar across definitions of frailty, ranging from 54% to 70% in the receiver operating characteristic curve areas using a questionnaire-based FRAIL scale (27) and remained around 70% if the Fried physical frailty phenotype or the deficit accumulation frailty index were used (47).
Gender differences
Three of the five reviews examined potential gender differences in the association between frailty and mortality and yielded some conflicting results (26, 46, 47). For example, one review using the Fried physical frailty phenotype and another utilizing the deficit accumulation frailty scale showed that older men with frailty had a higher risk of mortality than older women with frailty (26, 46). However, the third review (47) found mixed results depending on the individual study, with some reporting that men had an increased risk of mortality (49–52). Still, others found that women had an increased risk (51–53). One study reported a dose-response association between a more significant number of deficits and increased mortality in women across all age categories (51). However, this review (47) did not directly compare the risk between gender.
Age
Age did not appear to be an effect modifier of the relationship between frailty assessed using the Fried physical frailty phenotype or deficit accumulation index and mortality. Two of the five systematic reviews examined the association between frailty and mortality according to age groups (26, 46). The pooled estimates showed that the association between the deficit accumulation index and mortality did not vary between those aged below 65 years (HR:1.05; 95% CI: 1.03, 1.07) and those above 65 years (HR:1.04; 95% CI:1.03, 1.05) (26) per unit increase in a frailty index. Likewise, mortality risk was similar for those aged below 80 years (HR:1.62; 95% CI:1.39, 1.89) and above 80 years (HR:1.41; 95% CI:1.17, 1.70) estimated by the Fried physical frailty phenotype (46). Three other systematic reviews did not compare mortality based on age stratification (16, 27, 47).
Follow-up duration
The association between frailty and mortality varied according to follow-up duration. The risk of mortality was the lowest when the follow-up period was less than 12 months (HR:1.33; 95% CI:1.11, 1.60) and was the highest when the follow-up period was between two years to five years (HR: 3.25; 95% CI: 2.14, 4.94) (16). However, another review using the deficit accumulation frailty index found that the risk of mortality was higher when a shorter follow-up time was examined than a more extended follow-up, but effect sizes are not mentioned (26). Likewise, one systematic review compared a follow-up time of 4 years versus 11 years and observed that the strongest association between frailty and mortality was in the shorter follow-up group. However, individual values were not provided (47).
Discussion
This umbrella review synthesized evidence from five large systematic reviews (16, 26, 27, 46, 47) that examined major categories of frailty scales and the association of frailty identified by those scales their association with all-cause mortality in community-dwelling older individuals. A wideranging literature search identified five moderate to high-quality systematic reviews that included 93 primary studies comprising 434,115 participants from different countries. These primary studies used eighty different frailty scales, including Fried physical frailty phenotype, and various modifications of this scale, to multidomain scales. All the systematic reviews found that frailty is a predictor of mortality irrespective of the frailty scale used. These results will inform researchers and clinicians that frailty assessment is vital to predicting mortality.
Though all five systematic reviews reported a significant association between frailty and an increased risk of mortality, the effect size between frailty and mortality varied across the included systematic reviews. That means a person may be frail on one scale but not frail on another scale. Thus, the challenge remains which scale is to be used to predict frailty for researchers and clinicians. Only two of the five systematic reviews included in this umbrella review examined the predictability of frailty scales (27, 47). One review (47) compared the survival estimates based on age and adjusted relative risk using both the Fried physical frailty phenotype and the deficit accumulation frailty index. They found a 50% increased risk of mortality in frail older adults than non-frail older adults using the Fried phenotype. On the other hand, there was about a 15% increase in the risk of mortality per unit increase using the deficit accumulation index in frail older adults compared to those who were not frail (26). The variation in prediction values across the different frailty scales emphasizes the need for standardization across frailty scales for research purposes; however, clinically, it is essential that frailty be assessed and identified early such that appropriate preventive measures can be considered.
The included systematic reviews in this umbrella review examined gender differences (26, 46, 47), the role of age (26, 46) and follow-up duration (16, 26, 47) on frailty and mortality. Nevertheless, heterogeneity due to different population groups, diverse frailty scales and different follow-up periods made it challenging to draw definitive conclusions. However, age did not appear to be an effect modifier of the relationship between frailty assessed using the Fried phenotype or deficit accumulation index and mortality between those aged above or below 65 or those aged above or below 80 years. Gender differences were observed. The association between frailty and mortality also varied according to follow-up duration. These issues require further exploration in future longitudinal studies exploring and comparing different frailty scales’ ability to predict the development of frailty and mortality. Furthermore, most scales primarily focused on frailty’s physical and physiological aspects, although frailty’s social, cognitive and psychological elements are essential and merit future research.
Strengths and limitations
The current umbrella review has multiple strengths. The protocol was registered at PROSPERO, and the PRISMA guidelines were followed in completing this review. The review’s search strategy was robust and reproducible and utilized comprehensive search terms in multiple electronic databases. We evaluated five moderate to high-quality systematic reviews, which examined many participants from different parts of the world. Therefore, the generalizability of the results is high. We included systematic reviews that measured frailty using the commonly available scales, i.e., Fried physical frailty phenotype, multidomain frailty scale (including the questionnaire-based FRAIL scale), and deficit accumulation frailty index, meaning the findings will be relevant more broadly.
However, there are some limitations. This umbrella review did not include intervention studies, or systematic reviews of frail participants from hospitals or nursing homes were excluded. Thus, the findings apply to community-dwelling older individuals only. For researchers, this umbrella review shows that any category of frailty scale has utility for predicting mortality. Finally, this umbrella review focussed on the utility of frailty assessment to predict mortality though it could be considered that delaying mortality is not the only or best objective for the geriatric population. Improving the quality of life before death or extending life free of disability could be considered a critical outcome for assessing risk in frail old persons.
Conclusion
This umbrella review’s findings provide evidence that frailty is associated with mortality risk and highlight the importance of assessing frailty in primary community settings. This review has demonstrated that frailty is a significant predictor of all-cause mortality regardless of the specific frailty scale. For example, frailty assessed using five components that exclude cognition and affect Fried phenotype predicted mortality to a similar extent as did more comprehensive deficit accumulation frailty indices that included 83 items. As such, this implies that researchers and clinicians can use the most appropriate frailty scales given their circumstances, resources, and access to information. Together these findings emphasize that the assessment of frailty status itself may be more important than the choice of which type of scale is used. However, future longitudinal studies exploring the potential predictors for the development of frailty and its association with mortality using different frailty scales to determine the predictability would be beneficial.
Change history
06 June 2021
An Erratum to this paper has been published: https://doi.org/10.14283/jfa.2021.24
Abbreviations
- AMSTAR-2:
-
A MeaSurement Tool to Assess systematic Reviews Version 2
- CDSR:
-
Cochrane Database of Systematic Reviews
- FRAIL:
-
Fatigue, Resistance, Ambulation, Illness, and Loss of weight
- HR:
-
Hazard Ratio
- JBI:
-
Joanna Briggs Institute
- OR:
-
Odds Ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RR:
-
Relative Risk
References
Jang I-Y, Jung H-W, Lee HY, Park H, Lee E, Kim DH. Evaluation of Clinically Meaningful Changes in Measures of Frailty. J Gerontol A Biol Sci Med Sci. 2020;75(6):1143–7.
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001.
Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysisGlobal Incidence of Frailty and Prefrailty Among Community-Dwelling Older AdultsGlobal Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults. JAMA Network Open. 2019;2(8):e198398-e.
Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and Clinical Outcomes in Heart Failure: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2018;19(11):1003–8.e1.
Stewart R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin Chem. 2019;65(1):80–6.
Evans NR, Wall J, To B, Wallis SJ, Romero-Ortuno R, Warburton EA. Clinical frailty independently predicts early mortality after ischaemic stroke. Age and ageing. 2020;49(4):588–91.
Taylor-Rowan M, Cuthbertson G, Keir R, Shaw R, Drozdowska B, Elliott E, et al. The prevalence of frailty among acute stroke patients, and evaluation of method of assessment. Clin Rehabil. 2019;33(10):1688–96.
Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67(5):362–77.
Chen KW, Chang SF, Lin PL. Frailty as a Predictor of Future Fracture in Older Adults: A Systematic Review and Meta-Analysis. Worldviews Evid Based Nurs. 2017;14(4):282–93.
Maxwell CA, Patel MB, Suarez-Rodriguez LC, Miller RS. Frailty and Prognostication in Geriatric Surgery and Trauma. Clin Geriatr Med. 2019;35(1):13–26.
Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–7.
Veronese N. Frailty as Cardiovascular Risk Factor (and Vice Versa). Adv Exp Med Biol. 2020;1216:51–4.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–9.
Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15.
Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1–.e17.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World Journal. 2001;1:323–36.
Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–7.
Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120–5.
Khadka J, Visvanathan R, Theou O, Moldovan M, Amare AT, Lang C, et al. Development and validation of a frailty index based on Australian Aged Care Assessment Program data. Med J Aust. 2020;213(7):321–6.
Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A Frailty Instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatrics. 2010;10(1):57.
Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age and Ageing. 2016;45(3):353–60.
Lunenfeld B, Stratton P. The clinical consequences of an ageing world and preventive strategies. Best Pract Res Clin Obstet Gynaecol 2013;27(5):643–59.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47(2):193–200.
Kojima G. Frailty Defined by FRAIL Scale as a Predictor of Mortality: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2018;19(6):480–3.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews. Int J Evid Based Healthc. 2015;13(3):132–40.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Houles M, Abellan Van Kan G, Rolland Y, Andrieu S, Anthony P, Bauer J, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people. [French]. Cahiers de l’Annee Gerontologique. 2010;2(1):13–23.
Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–9.
Chang SF, Lin PL. Systematic Literature Review and Meta-Analysis of the Association of Sarcopenia With Mortality. Worldviews Evid Based Nurs. 2016;13(2):153–62.
Kusumastuti S, Rozing MP, Lund R, Mortensen EL, Westendorp RGJ. The added value of health indicators to mortality predictions in old age: A systematic review. Eur J Intern Med. 2018;57:7–18.
Kane RL, Shamliyan T, Talley K, Pacala J. The association between geriatric syndromes and survival. J Am Geriatr Soc. 2012;60(5):896–904.
Poulton A, Shaw JF, Nguyen F, Wong C, Lampron J, Tran A, et al. The Association of Frailty With Adverse Outcomes After Multisystem Trauma: A Systematic Review and Meta-analysis. Anesth Analg. 2020;130(6):1482–92.
Xu BY, Yan S, Low LL, Vasanwala FF, Low SG. Predictors of poor functional outcomes and mortality in patients with hip fracture: a systematic review. BMC Musculoskelet Disord. 2019;20(1):568.
Cheng MH, Chang SF. Frailty as a Risk Factor for Falls Among Community Dwelling People: Evidence From a Meta-Analysis. J Nurs Scholarsh. 2017;49(5):529–36.
Zhang XE, Cheng B, Wang Q. Relationship between high blood pressure and cardiovascular outcomes in elderly frail patients: A systematic review and meta-analysis. Geriatr Nurs. 2016;37(5):385–92.
Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37.
Zhang X, Dou Q, Zhang W, Wang C, Xie X, Yang Y, et al. Frailty as a Predictor of All-Cause Mortality Among Older Nursing Home Residents: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2019;20(6):657–63.e4.
Pugh R, John DL, Thorpe C, Subbe C. Frailty measures in the critically ill: are we approaching a critical age? A systematic review. Critical Care. 2014;18(Suppl 1):P50–P.
Van der Elst M, Schoenmakers B, Duppen D, Lambotte D, Fret B, Vaes B, et al. Interventions for frail community-dwelling older adults have no significant effect on adverse outcomes: a systematic review and meta-analysis. BMC Geriatr. 2018;18(1):249.
Moraes MB, Araujo CFM, Avgerinou C, Vidal EIO. Nutritional interventions for the treatment of frailty in older adults: a systematic review protocol. Medicine (Baltimore). 2018;97(52):e13773.
Shears M, McGolrick D, Waters B, Jakab M, Boyd JG, Muscedere J. Frailty measurement and outcomes in interventional studies: protocol for a systematic review of randomised control trials. BMJ Open. 2017;7(12):e018872.
Apostolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, et al. Predicting risk and outcomes for frail older adults: an umbrella review of frailty screening tools. JBI Database Of Systematic Reviews And Implementation Reports. 2017;15(4):1154–208.
Chang SF, Lin PL. Frail phenotype and mortality prediction: a systematic review and meta-analysis of prospective cohort studies. Int J Nurs Stud. 2015;52(8):1362–74.
Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–36.
Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;183(8):E487–94.
Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55(8):1216–23.
Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57(3):492–8.
Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z. Frailty and Mortality Among Chinese at Advanced Ages. J Gerontol B Psychol Sci Soc Sci 2009;64B(2):279–89.
Puts MTE, Lips P, Deeg DJH. Sex Differences in the Risk of Frailty for Mortality Independent of Disability and Chronic Diseases. J Am Geriatr Soc. 2005;53(1):40–7.
Bandeen-Roche K, Xue Q-L, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of Frailty: Characterization in the Women’s Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–6.
Acknowledgments
Not applicable.
Funding
Not funded.
Author information
Authors and Affiliations
Contributions
ARMSE developed the idea, searched the literature, reviewed articles, extracted data, and contributed to writing. CB searched and reviewed the literature. RLW, SEE, ME, and JR reviewed, edited, and contributed to writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
It was not requested being a review of already published literature.
Additional information
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ekram, A.R.M.S., Woods, R.L., Britt, C. et al. The Association Between Frailty and All-Cause Mortality in Community-Dwelling Older Individuals: An Umbrella Review. J Frailty Aging 10, 320–326 (2021). https://doi.org/10.14283/jfa.2021.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2021.20