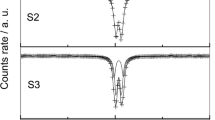
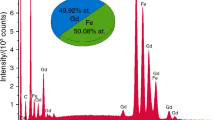
Abstract
Aluminum substitution is a common phenomenon in environmental iron oxides and oxyhydroxides, affecting the color, magnetic character, surface features, etc. Several methods for preparing Al-substituted iron oxyhydroxides can be found in the literature, resulting in samples with particular properties. In the present study, the synthesis of aluminum-substituted goethites, AlxFe1-xOOH with 0 ⩽ x ⩽ 0.15, by homogeneous precipitation and the transformation to aluminum-substituted hematites, (AlxFe1-x)O3, are presented. The goethite samples were produced at 90°C from solutions of urea and iron and aluminum nitrates in the presence of ammonium sulfate (GU series). Although attempts were made to incorporate up to 33 mole% of Al into the goethite, only ~15 mole% was found to be within the structure, due to the final pH, ~7, of the synthesis. Another feature of these goethites was a lateral alignment of the tabular particles. By heating batches of the GU samples at 400°C and 800°C, two series of Al-hematites were obtained, denoted here as the HX400 and HX800 samples, respectively. X-ray diffraction, thermal analysis, Karl-Fischer titration, transmission electron microscopy, and Mössbauer spectroscopy were used to characterize the samples. The X-ray patterns showed the samples to be pure iron phases, with particle sizes of ~10 nm for the GU and HX400 samples, and of ~70 nm for the HX800 samples. An inversion in the intensities of the (104) and (110) diffraction peaks of hematite was observed to be dependent on the aluminum substitution and was explained by small particle sizes, shape anisotropy, and the presence of nanopores. The cell parameters of both GU and HX samples showed a small decrease with increasing aluminum substitution up to x ≈ 0.15. The amount of adsorbed sulfate, presumably as an aluminum hydroxy sulfate gel, increased with aluminum substitution in all GU and HX samples, reaching a maximum of ~6.5 wt.% for the highest substitution. Heating at 100°C did not remove all of the adsorbed water, and significantly higher temperatures were required to achieve complete removal. Mossbauer spectra at 295 K and 80 K are typical for small-particle goethite and hematite, and revealed that Al-for-Fe substitution in all samples seems to be limited to ~15 mol.%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Aquino, A.J.A., Tunega, D., Haberhauer, G., Gerzabek, M.H., and Lischka, H. (2008) Acid-base properties of a goethite surface model: A theoretical view. Geochimica et Cosmochimica Acta, 72, 3587–3602.
Bakardjieva, S., Štengl, V., Šubrt, J., and Većerníková, E. (2005) Characteristic of hydrous iron (III) oxides prepared by homogeneous precipitation of iron (III) sulphate with urea. Solid State Sciences, 7, 367–374.
Barrero, C.A., Vandenberghe, R.E., and De Grave, E. (1999) The effect of Al-content and crystallinity on the magnetic properties of goethites. Hyperfine Interactions, 122, 39–46.
Barrero, C.A., Betancur, J.D., Greneche, J.M., Goya, G.F., and Berquó, T.S. (2006) Magnetism in non-stoichiometric goethite of varying total water content and surface area. Geophysical Journal International, 164, 331–339.
Betancur, J.D., Barrero, C.A., Greneche, J.M., and Goya, G.F. (2004) The effect of water content on the magnetic and structural properties of goethite. Journal of Alloys and Compounds, 369, 247–251.
Boily, J.F. and Felmy, A.R. (2008) On the protonation of oxoand hydroxo-groups of the goethite (α-FeOOH) surface: A FTIR spectroscopic investigation of surface O-H stretching vibrations. Geochimica et Cosmochimica Acta, 72, 3338–3357.
Burton, A.W., Ong, K., Rea, T., and Chan, I.Y. (2009) On the estimation of average crystallite size of zeolites from the Scherrer equation: a critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous and Mesoporous Materials, 117, 75–90.
Cornell, R.M. and Schwertmann, U. (1996) The Iron Oxides. VCH Publishers, Weinheim, Germany.
da Costa, G.M., Novack, K.M., Elias, M.M.C., and da Cunha, C.C.R.F. (2013) Quantification of moisture contents in iron and manganese ores. ISIJ International, 53, 1732–1738.
Dang, M.Z., Rancourt, D.G., Dutrizac, J.E., Lamarche, G., and Provencher, R. (1998) Interplay of surface conditions, particle size, stoichiometry, cell parameters, and magnetism in synthetic hematite-like materials. Hyperfine Interactions, 117, 271–319.
De Grave, E. and Bowen, L.H. (1982) Mössbauer study of aluminum-substituted hematites. Journal of Magnetism and Magnetic Materials, 27, 98–108.
De Grave, E., Bowen, L.H., Vochten, R., and Vandenberghe, R.E. (1988) The effect of crystallinity and Al substitution on the magnetic structure and Morin transition in hematite. Journal of Magnetism and Magnetic Materials, 72, 141–151.
Duvigneaud, P.H. and Derie, R. (1980) Shape effects on crystallite size distributions in synthetic hematites from X-ray line-profile analysis. Journal of Solid State Chemistry, 34, 323–333.
Ford, R.G. and Bertsch, P.M. (1999) Distinguishing between surface and bulk dehydration-dehydroxylation reactions in synthetic goethites by high-resolution thermogravimetric analysis. Clays and Clay Minerals, 47, 329–337.
Frandsen, C., Legg, B.A., Comolli, L.R., Zhang, H., Gilbert, B., Johnson, E., and Banfield, J.F. (2014) Aggregationinduced growth and transformation of ß-FeOOH nanorods to micron-sized α-Fe2O3 spindles. CrystEngComm, 16, 1451–1458.
Frost, R.L., Ding, Z., and Ruan, H.D. (2003) Thermal analysis of goethite: relevance to Australian indigenous art. Journal of Thermal Analysis and Calorimetry, 71, 783–797.
Fysh, S.A. and Clark, P.E. (1982a) Aluminous goethite: a Moössbauer study. Physics and Chemistry of Minerals, 8, 180–187.
Fysh, S.A. and Clark, P.E. (1982b) Aluminous hematite: a Mössbauer study. Physics and Chemistry of Minerals, 8, 257–267.
Ghose, S.K., Waychunas, G.A., Trainor, T.P., and Eng, P.J. (2010) Hydrated goethite (α-FeOOH) (100) interface structure: Ordered water and surface functional groups. Geochimica et Cosmochimica Acta, 74, 1943–1953.
Gialanella, S., Girardi, F., Ischia, G., Lonardelli, I., Mattarelli, M., and Montagna, M. (2010) On the goethite to hematite phase transformation. Journal of Thermal Analysis and Calorimetry, 102, 867–873.
Jiang, J.Z., Ståhl, K., Nielsen, K., and da Costa, G.M. (2000) Anisotropic x-ray line broadening in goethite-derived haematite. Journal of Physics: Condensed Matter, 12, 4893–4898.
Langford, J.I. and Wilson, A.J.C. (1978) Scherrer after sixty years: a survey and some new results in the determinations of crystallite size. Journal of Applied Crystallography, 11, 102–113.
Leetma, K., Gomez, M.A., Becze, L., Guo, F., and Demopoulos, G.P. (2014) Comparative molecular characterization of aluminum hydroxy-gels derived from chloride and sulphate salts. Journal of Chemical Technology and Biotechnology, 89, 206–213.
Legg, B.A., Zhu, M., Comolli, L.R., Gilbert, B., and Banfield, J.F. (2014) Determination of the three-dimensional structure of ferrihydrite nanoparticle aggregates. Langmuir, 30, 9931–9940.
Li, D., Nielsen, M.H., Lee, J.R.I., Frandsen, C., Banfield, J.F., and De Yoreo, J.J. (2012) Direction-specific interactions control crystal growth by oriented attachment. Science, 336, 1014–1018.
Lima de Faria, J. (1963) Dehydration of goethite and diaspore. Zeitschrift für Kristallographie, 119, 176–203.
Löffler, L. and Mader, W. (2006) Anisotropic X-ray peak broadening and twin formation in hematite derived from natural and synthetic goethite. Journal of the European Ceramic Society, 26, 131–139.
Murad, E. and Johnston, J.H. (1987) Iron oxides and oxyhydroxides. Pp. 507–582 in: Mössbauer Spectroscopy Applied to Inorganic Chemistry. Vol. 1 (G.J. Long, editor). Plenum Press, New York.
Naono, H. and Fujiwara, R. (1980) Micropore formation due to thermal decomposition of acicular microcrystals of α-FeOOH. Journal of Colloidal and Interface Science, 73, 406–415.
Penn, R.L. and Banfield, J.F. (1998) Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science, 281, 969–971.
Pomiès, M.P., Morin, G., and Vignaud, C. (1998) XRD study of the goethite-hematite transformation: application to the identification of heated prehistoric pigments. European Journal of Solid State Inorganic Chemistry, 35, 9–25.
Qin, H., Tan, X., Huang, W., Jiang, J., and Jiang, H. (2015) Application of urea precipitation method in preparation of advanced ceramic powders. Ceramics International, 41, 11598–11604.
Ruan, H.D. and Gilkes, R.J. (1995) Dehydroxylation of aluminous goethite: unit cell dimensions, crystal size and surface area. Clays and Clay Minerals, 43, 196–211.
Ruan, H.D., Frost, R.L., and Kloprogge, J.T. (2001) The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochimica Acta Part A, 57, 2575–2586.
Ruan, H.D., Frost, R.L., Kloprogge, J.T., and Duong, L. (2002) Infrared spectroscopy of goethite dehydroxylation. II. Effect of aluminum substitution on the behavior of hydroxyl units. Spectrochimica Acta Part A, 58, 479–491.
Schwertmann, U. (1984) The double dehydroxylation peak of goethite. Thermochimica Acta, 78, 39–46.
Schwertmann, U. and Cornell, R.M. (1991) Iron Oxides in the Laboratory. VCH Publishers, Weinheim, Germany.
Schwertmann, U., Cambier, P., and Murad, E. (1985) Properties of goethite of varying crystallinity. Clays and Clay Minerals, 33, 369–378.
Schwertmann, U., Fitzpatrick, R.W., Taylor, R.M., and Lewis, D.G. (1979) The influence of aluminum on iron oxides. Part II. Preparation and properties of A-substituted hematites. Clays and Clay Minerals, 27,105–112.
Vandenberghe, R.E., De Grave, E., and de Bakker, P.M.A. (1994) On the methodology of the analysis of Mössbauer spectra. Hyperfine Interactions, 83, 29–49.
Vandenberghe, R.E., Van San, E., De Grave, E., and da Costa, G.M. (2001) About the Morin transition in hematite in relation with particle size and aluminum substitution. Czech Journal of Physics, 51, 663–675.
Wells, M.A., Gilkes, R.J., and Anand. R.R. (1989) The formation of corundum and aluminous hematite by the thermal dehydroxylation of aluminous goethite. Clay Minerals, 24, 513–530.
Willard, H.H. and Tang, N.K. (1937) A study of the precipitation of aluminum basic sulfate by urea. Journal of the American Chemical Society, 59, 1190–1196.
Zhang, H. and Banfield, J.F. (2014) Interatomic Coulombic interactions as the driving force for oriented attachment. CrystEngComm, 16, 1568–1578.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Da Costa, G.M., De Grave, E. Coprecipitation of Aluminum Goethite and Amorphous Al-Hydroxy-Sulfate Using Urea and Characterization of the Thermal Decomposition Products. Clays Clay Miner. 64, 283–298 (2016). https://doi.org/10.1346/CCMN.2016.0640304
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.0640304