Abstract
The mechanism of formation of nanocrystalline gadolinium orthoferrite (GdFeO3) during the heat treatment of gadolinium and iron(III) hydroxides synthesized by ultrasound-assisted co-precipitation was studied. The obtained samples were investigated by energy-dispersive X-ray spectroscopy, simultaneous thermal analysis via coupled differential scanning calorimetry and thermogravimetric analysis (DSC–TGA), powder X-ray diffraction (PXRD) and Fourier-transform infrared (FTIR) spectroscopy. The DSC–TGA results confirm that the formation of GdFeO3 occurs after the complete decomposition of gadolinium oxycarbonate derivatives. The PXRD results indicate that GdFeO3 is formed as a result of the reaction of amorphous iron(III) oxide (am-Fe2O3) at temperatures of 675–700 °C with amorphous gadolinium oxide (am-Gd2O3) (primary carbonate-independent pathway) and at temperatures of 725–775 °C with cubic gadolinium oxide (c-Gd2O3) transformed from the hexagonal gadolinium oxide (h-Gd2O3) which results from the decomposition of gadolinium oxycarbonate derivatives at temperatures of 675–725 °C (secondary carbonate-dependent pathway). The FTIR results are consistent with the assumption that gadolinium oxycarbonate derivatives decompose with the formation of h-Gd2O3 in the last-mentioned temperature range. The enthalpy of the reaction of formation of nanocrystalline GdFeO3 defined from the DSC–TGA data is equal to − 16.89 ± 0.36 kJ mol−1. The activation energy for the formation of nanocrystalline GdFeO3 obtained from the DSC data is equal to 1193.62 ± 112.05 kJ mol−1, 1202.27 ± 112.06 kJ mol−1 and 1151.08 ± 106.53 kJ mol−1 according to the Kissinger, Augis–Bennett/Boswell and Flynn–Wall–Ozawa methods, respectively. Also, based on the DSC data, the true onset temperature of the formation of nanocrystalline GdFeO3 was found to be ~ 756 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare-earth orthoferrites (REFeO3, where RE is a rare-earth element: Sc, Y, La–Lu) are a class of chemical compounds with an orthorhombic perovskite structure that have been actively studied due to their magnetic, optical and electrical properties [1,2,3,4,5,6,7]. One of these compounds is gadolinium orthoferrite (GdFeO3) which has been proposed for various applications such as catalysis [8], photocatalysis [9], electrocatalysis [10], luminescence [11], gas sensing [12] and as a contrast agent for magnetic resonance imaging (MRI) [13,14,15,16]. In the synthesis of GdFeO3 nanocrystals, various methods have been employed, including co-precipitation [17], hydrothermal method [18], solution combustion [19], sol–gel [20], microwave method [21], sonochemical method [22] and using heterobimetallic [23] and metal–organic [24] precursors. The co-precipitation method, which we preferred in our previous works [25,26,27,28,29,30], makes it possible to synthesize nanocrystalline GdFeO3 with minimal foreign chemical impurities since the co-precipitated gadolinium and iron(III) hydroxides are dehydrated during heat treatment with the formation of the corresponding oxides, which then form the compound of interest. However, due to the active sorption of carbon oxide (CO2) from the ambient air into the reagent solutions during their preparation or onto the surface of co-precipitated hydroxides during their washing and drying, gadolinium carbonate can be formed. Such partial carbonatization of co-precipitated hydroxides may lead to sample inhomogeneity in terms of phase composition, and as a result, to the formation of another phase along with GdFeO3 after heat treatment [25, 26, 30]. Therefore, it is of scientific and practical interest to study and understand the role of carbonates in the formation mechanism of nanocrystalline GdFeO3 by co-precipitation.
In the literature, several publications have investigated the mechanism of formation of GdFeO3 nanocrystals by the co-precipitation method [31,32,33]. Prakash et al. [31] investigated the formation of nanocrystalline GdFeO3 by sintering a synthesized sample of co-precipitated hydroxides at temperatures of 600–1200 °C. They found that gadolinium oxide (Gd2O3) was present in the sintered products up to a temperature of 1000 °C, above which single-phase GdFeO3 nanocrystals were obtained; therefore, they consider the temperature of 1200 °C to be optimal for sintering. The authors reported the presence of carbonates in the synthesized sample, but they did not indicate their role in the formation of GdFeO3. Tugova and Karpov [32] studied the mechanism of formation of nanocrystalline GdFeO3 upon the calcination of co-precipitated hydroxides at temperatures of 500–900 °C. They established that the products heat treated at temperatures of 500–600 °C contain gadolinium dioxymonocarbonate (Gd2O2CO3) which decomposes at temperatures of 600–700 °C to form cubic Gd2O3, and starting from a temperature of 700 °C, GdFeO3 is formed. Based on these results, they concluded that the formation of GdFeO3 nanocrystals occurs through two mechanisms: the first mechanism is related to the simultaneous dehydration of co-precipitated hydroxides, while the other is associated with both dehydration and decarbonation, which leads to an increase in the temperature of GdFeO3 synthesis. The work of Popkov et al. [33] confirmed the results of the previous one [32] that the formation of GdFeO3 nanocrystals proceeds via two routes: from the co-precipitated hydroxides along the main route and from the products of partial carbonatization along the side one.
In this paper, we continue the work of the aforementioned colleagues, focus on simultaneous thermal analysis of the precursor and study in detail the mechanism of formation of GdFeO3 nanocrystals synthesized employing the ultrasound-assisted co-precipitation method, which apparently, maintains sample homogeneity by enhancing diffusion and mixing processes [30]. The content of carbonates in the products of heat treatment of the synthesized sample of co-precipitated hydroxides at different temperatures is estimated. In addition, the true onset temperature of the formation of nanocrystalline GdFeO3 is found, and the enthalpy and the activation energy for this formation reaction are also defined.
Materials and methods
Synthesis of samples
In this work, gadolinium and iron(III) hydroxides were synthesized by direct co-precipitation accompanied by ultrasonication. After that, samples of the obtained co-precipitated hydroxides were heated at different temperatures in the air.
An aqueous solution of stoichiometric amounts of gadolinium nitrate hexahydrate “Gd(NO3)3·6H2O” (puriss., 99.9%) and iron(III) nitrate nonahydrate “Fe(NO3)3·9H2O” (pur., 98.00%) with a concentration of 0.01 mol L−1 for each salt was used as a source of gadolinium and iron(III) ions. An aqueous solution of sodium hydroxide (NaOH) (p.a.) with a concentration of 1 mol L−1 was used as a precipitating medium. Gadolinium and iron(III) nitrates react with sodium hydroxide to form water-soluble sodium nitrate (NaNO3) and a precipitate of gadolinium hydroxide “Gd(OH)3” and iron(III) hydroxide “Fe(OH)3” (exchange reaction). The equation for this reaction can be written as follows:
Gd(NO3)3(aq) + Fe(NO3)3(aq) + 6NaOH(aq) = Gd(OH)3(s) + Fe(OH)3(s) + 6NaNO3(aq).
Co-precipitation of gadolinium and iron(III) hydroxides
For the synthesis of co-precipitated hydroxides, 100 mL of 1 mol L−1 sodium hydroxide solution was added dropwise to 100 mL of 0.01 mol L−1 gadolinium and iron(III) nitrates solution, which was constantly stirred with a magnetic stirrer and irradiated with ultrasound by immersing the ultrasonic horn of a ‘‘Venpan type UD-20 automatic’’ ultrasonic disintegrator into it throughout the entire co-precipitation process. The synthesis was repeated two more times, each time with 100 mL of each reagent solution. Then, the resulting precipitates with the supernatants from the three syntheses were mixed using a magnetic stirrer. After settling, most of the supernatant was decanted, and the co-precipitated hydroxides were separated from the remainder by centrifugation. Thereafter, the obtained precipitate was washed three times with distilled water to remove residual sodium hydroxide and sodium nitrate. The washed co-precipitated hydroxides were separated from water by centrifugation and then dried at a temperature of 45 °C.
Heat treatment of co-precipitated hydroxides
Samples of the synthesized co-precipitated hydroxides with a mass of about 50 mg were heated at different temperatures in the range of 650–850 °C with an interval of 25 °C (650, 675, 700, 725, 750, 775, 800, 825 and 850 °C) for 4 h in the air.
Characterization of co-precipitated hydroxides
Energy-dispersive X-ray spectroscopy
The content of gadolinium and iron in the synthesized sample of co-precipitated hydroxides was determined by energy-dispersive X-ray spectroscopy (EDXS) using a “TESCAN VEGA3” scanning electron microscope (TESCAN ORSAY HOLDING, Brno-Kohoutovice, Czech Republic) with an accelerating voltage of 30.0 kV coupled with an “x-act” silicon drift detector (Oxford Instruments, Abingdon, Oxfordshire, UK).
Differential scanning calorimetry and thermogravimetric analysis
The synthesized sample of co-precipitated hydroxides was investigated by differential scanning calorimetry and thermogravimetric analysis (DSC–TGA) using a “NETZSCH STA 449 F3” simultaneous thermal analyzer (NETZSCH-Gerätebau GmbH, Selb, Germany) up to 900 °C at three different heating rates (10, 20 and 30 °C min−1) in an inert atmosphere of argon. The sample masses were 11.160, 11.568 and 11.183 mg at heating rates of 10, 20 and 30 °C min−1, respectively. The DSC and TGA curves of the reference substances (potassium chromate “K2CrO4” and barium carbonate “BaCO3”) were obtained using the same simultaneous thermal analyzer at the same three heating rates. The sample mass of K2CrO4 was 15.677 mg at a heating rate of 10 °C min−1 and 16.130 mg at heating rates of 20 and 30 °C min−1, while the sample mass of BaCO3 was 11.933 mg at all heating rates. All measurements were carried out in platinum crucibles (DSC/TG pan Pt). The processing of the DSC–TGA data was carried out using the “NETZSCH Proteus Thermal Analysis” software.
Characterization of heat-treated products
Powder X-ray diffraction
The powder X-ray diffraction (PXRD) patterns were recorded using a ‘‘Rigaku SmartLab 3’’ diffractometer (Rigaku Corporation, Tokyo, Japan) in the Bragg angle range of 15–61° at a scan speed of 0.2 deg min−1 and a step width of 0.01°. The processing of the PXRD data was carried out using the ‘‘SmartLab Studio II’’ software. The mass fractions of the crystalline phases were estimated by the Rietveld refinement method using the crystal structure data of the Inorganic Crystal Structure Database (ICSD) structures with the identifiers of ICSD 27278, ICSD 162247 and ICSD 150677 for orthorhombic GdFeO3, hexagonal Gd2O3 and cubic Gd2O3, correspondently. The relative mass fractions of the X-ray amorphous phases in the products that underwent heat treatment at temperatures up to 775 °C were estimated by normalizing the obtained diffractograms of these products, determining the integrated intensity of the amorphous halo on the normalized diffraction patterns in the Bragg angle range of ~ 15.2–27.2° and recalculating the resulting values as percentages of the maximum. The estimated results of mass fractions of the X-ray amorphous and crystalline phases in each heat-treated product were combined by subtracting the integrated intensity of the amorphous halo from 100% and recalculating the mass fractions of crystalline phases as fractions of the obtained value.
Fourier-transform infrared spectroscopy
The Fourier-transform infrared (FTIR) spectra in the wavenumber range of 400–4000 cm−1 were obtained by Kubelka–Munk transformation of diffuse reflectance data acquired using a “Bruker INVENIO-S” FTIR spectrometer (Bruker Optics, Ettlingen, Germany) with a “PIKE” diffuse reflectance attachment (PIKE Technologies, Madison, WI, USA). The relative mass percentages of gadolinium oxycarbonate derivatives in the products that underwent heat treatment at temperatures up to 800 °C were estimated by normalizing the obtained FTIR spectra of these products, determining the integrated intensity of the asymmetric and symmetric stretching vibrations of carbonate groups (CO 2−3 ) on the normalized FTIR spectra in the wavenumber range of ~ 1260–1625 cm−1 and recalculating the resulting values as percentages of the maximum.
Results and discussion
Energy-dispersive X-ray spectroscopy
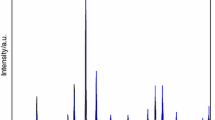
According to the EDX spectrum presented in Fig. 1, the synthesized sample of co-precipitated hydroxides is practically chemically pure. The carbon detected in the investigated sample is related to the presence of carbonate groups, the appearance of which, as we mentioned earlier, is a consequence of the active sorption of CO2 from the ambient air into the reagent solutions during their preparation or onto the surface of co-precipitated hydroxides during their washing and drying. Based on the EDXS results, the atomic ratio of gadolinium to iron (Gd:Fe) is (49.92 ± 0.03)%:(50.08 ± 0.03)%; therefore, the average Gd:Fe atomic ratio is about 1.000:1.003, which is very close to the atomic ratio of 1:1 in GdFeO3, i.e., the content of gadolinium and iron in the synthesized sample corresponds to the stoichiometry of the compound of interest. It is worth noting that the Gd:Fe atomic ratio is preserved during the synthesis since neither gadolinium nor iron is included in the composition of compounds that are volatile during the heat treatment of co-precipitated hydroxides.
Differential scanning calorimetry and thermogravimetric analysis
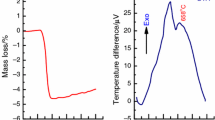
As was shown earlier [25, 27, 29, 33], upon heat treatment, gadolinium and iron(III) hydroxides undergo a series of physicochemical processes before forming GdFeO3. The DSC and TGA curves of the synthesized sample of co-precipitated hydroxides at different heating rates (10–30 °C min−1) are presented in Fig. 2a and b, respectively. The first derivatives of the TGA curves (the DTG curves) are presented in supplementary Fig. S1. On the obtained DSC curves (Fig. 2a), 7 thermal effects can be detected: 6 endothermic (effects indicated by downward pointing arrows 1–6) and one exothermic (effect indicated by upward pointing arrow 8), whereas on the presented DTG curves (Fig. S1), 7 endothermic effects are observed (effects 1–7). These effects are associated with the evaporation of the physically adsorbed water on the sample surface (endothermic effect 1: ~ 53–63 °C by DSC, ~ 51–58 °C by DTG); the dehydration of iron(III) hydroxide to form iron(III) oxide (endothermic effect 2: ~ 126–163 °C by DSC, ~ 132–156 °C by DTG); the dehydration of gadolinium hydroxide in three stages: firstly to form gadolinium oxide sesquihydrate (endothermic effect 3: ~ 226–247 °C by DSC, ~ 223–246 °C by DTG), secondly to form gadolinium oxyhydroxide (endothermic effect 4: ~ 294–319 °C by DSC, ~ 299–322 °C by DTG), and thirdly, to gadolinium oxide (endothermic effect 6: ~ 414–452 °C by DSC, ~ 420–442 °C by DTG); the first stage of decomposition of gadolinium carbonate to form an oxycarbonate derivative (endothermic effect 5: ~ 371–384 °C by DSC, ~ 369–392 °C by DTG); the last stage of decomposition of gadolinium oxycarbonate derivative to form gadolinium oxide (endothermic effect 7: ~ 756–758 °C by DTG), and finally, the formation of gadolinium orthoferrite from gadolinium and iron(III) oxides (exothermic effect 8: ~ 764–772 °C by DSC). All these results are summarized in Table 1. As can be seen from Table 1, the temperatures of the maximum thermal and mass loss effects generally tend to increase with increasing heating rate. According to the TGA curves (Fig. 2b), the total mass loss is about 21.69%, 21.75% and 20.58% at a heating rate of 10, 20 and 30 °C min−1, correspondently. These mass losses are related to the release of water (effects 1–4 and 6) and CO2 (effects 5 and 7). The results obtained are in good agreement with the previous ones [32, 33], which confirm that the formation of GdFeO3 occurs after the mass loss is completed, i.e., after the complete decomposition of gadolinium oxycarbonate derivatives.
DSC (a) and TGA (b) curves of the co-precipitated hydroxides at different heating rates. The inset in (b) shows the exothermic effect of GdFeO3 formation (t is the temperature, tmax is the temperature of the maximum thermal effect). The arrows pointing down indicate endothermic effects (effects 1–6), while the arrows pointing up indicate exothermic effects (effect 8)
Enthalpy of the reaction of formation of nanocrystalline GdFeO3
To found the enthalpy of formation of nanocrystalline GdFeO3 based on the DSC–TGA results, two reference substances were used: K2CrO4 and BaCO3. Potassium chromate and barium carbonate undergo certain physicochemical transformations at temperatures below and above the formation temperature of GdFeO3, respectively. According to the data used to calibrate the thermal analyzer, at a heating rate of 10 °C min−1, K2CrO4 transforms from orthorhombic to hexagonal form in an endothermic process [34] at a temperature of 668.0 °C with an enthalpy of 35.000 J g−1, while BaCO3 melts in an endothermic process at a temperature of 808.0 °C with an enthalpy of 94.900 J g−1.
At each heating rate (β), the onset temperature (tonset) and peak area (A) of the endothermic effects of the reference substances and the exothermic effect of GdFeO3 formation were determined from the DSC curves, whereas the values of sample mass at each onset temperature (monset) were determined from the TGA curves. Peak areas in µV °C were calculated from their values in µV s given by the software using the heating rate according to Eq. 1.
Then, the “specific” peak areas (A*) were calculated by dividing the obtained peak areas by the corresponding sample masses at onset temperature as shown in Eq. 2.
After that, the known enthalpy values associated with the endothermic effects of the reference substances were divided by the corresponding “specific” peak areas as shown in Eq. 3.
Then, the resulting values of ΔH/A* were plotted as a function of tonset at each heating rate (Fig. 3), where each dependence is a straight line drawn between two reference points: one for K2CrO4 and the other for BaCO3.
Based on the equations of the straight lines obtained, the values of ΔH/A* for GdFeO3 at each heating rate were calculated using the corresponding onset temperatures of the exothermic effect of GdFeO3 formation. All these data are summarized in Table 2.
Thereafter, multiplying the resulting values of ΔH/A* for GdFeO3 at each heating rate by the corresponding “specific” peak areas calculated earlier by Eq. 2, the enthalpy of the reaction of formation of nanocrystalline GdFeO3 can be estimated (Eq. 4).
According to Eq. 4, the enthalpy of the reaction of formation of nanocrystalline GdFeO3 is in the range from about − 63.36 to − 66.54 J g−1 or from about − 16.54 to − 17.37 kJ mol−1. In other words, it is approximately equal to − 64.67 ± 1.36 J g−1 or − 16.89 ± 0.36 kJ mol−1. This value is much less than the enthalpy of formation of GdFeO3 at 298.15 K (− 1351.432 ± 8.368 kJ mol−1 [35]), which can be explained by the fact that the standard enthalpy of formation corresponds to the formation of GdFeO3 from its constituent elements (Gd, Fe and O) in their standard states, while in this work, the enthalpy of formation of GdFeO3 from gadolinium and iron(III) oxides was estimated. Furthermore, the calculated value does not correspond to bulk GdFeO3, but to nanosized GdFeO3; therefore, it should be smaller.
Activation energy for the formation of nanocrystalline GdFeO3
To estimate the activation energy for the formation of nanocrystalline GdFeO3 based on the DSC data, three kinetic methods were applied: the Kissinger method [36], the Augis–Bennett/Boswell method [37, 38] and the Flynn–Wall–Ozawa (FWO) method [39, 40]. These non-isothermal kinetic methods depend on the relationship between the heating rate and the absolute temperature at which the reaction reaches its maximum value [41]. The mathematical formulas for these methods can be written as shown in Eqs. 5–7.
Kissinger method
Augis–Bennett/Boswell method
Flynn–Wall–Ozawa method
In these equations, β is the heating rate, Tmax is the absolute temperature at which the reaction reaches its maximum value, i.e., the peak of the thermal effect (in our work, Tmax is the absolute temperature of the maximum exothermic effect of GdFeO3 formation on the corresponding DSC curve), Ea is the activation energy, A is the pre-exponential factor and R is the molar gas constant.
Based on the Kissinger method (Eq. 5), plotting ln(β/T 2max ) against 1/Tmax results in a straight line with a slope of − Ea/R and a y-intercept of ln(AR/Ea). Similarly, in accordance with the Augis–Bennett/Boswell method (Eq. 6), plotting ln(β/Tmax) versus 1/Tmax gives a straight line with a slope of − Ea/R, whereas according to the FWO method (Eq. 7), plotting of ln β against 1/Tmax gives a straight line with a slope of − 1.052Ea/R. Figure 4 shows these straight lines obtained using the previously determined temperatures of the maximum exothermic effect of GdFeO3 formation on the DSC curves at different heating rates (Table 1, effect 8). In the first two methods, the activation energy for the formation of nanocrystalline GdFeO3 can be calculated by multiplying the resulting slopes by − R, while in the third method, the obtained slope should be multiplied by − R and divided by 1.052. The DSC data used and the calculation results are summarized in Table 3.
According to the calculation results, the activation energy for the formation of nanocrystalline GdFeO3 is approximately equal to 1193.62 ± 112.05 kJ mol−1, 1202.27 ± 112.06 kJ mol−1 and 1151.08 ± 106.53 kJ mol−1 based on the Kissinger, Augis–Bennett/Boswell and FWO methods, correspondently, which are quite close. The relative standard error of the estimated values ranges from about 9.25 to 9.39%. This high value of activation energy is due to the high temperature required for the complete decomposition of gadolinium oxycarbonate derivatives before the formation of GdFeO3, as discussed earlier (See Section “Differential scanning calorimetry and thermogravimetric analysis”). In accordance with the Kissinger method, the pre-exponential factor has an average value of about 1.62 × 1060 min−1.
True onset temperature of the formation of nanocrystalline GdFeO3
The values of the onset temperature of the exothermic effect of GdFeO3 formation previously determined from the DSC curves (Table 2) are found to be about 759, 765 and 768 °C at a heating rate of 10, 20 and 30 °C min−1, respectively. Therefore, the temperature at which nanocrystalline GdFeO3 begins to form increases with increasing heating rate. To found the true onset temperature of the formation of nanocrystalline GdFeO3, i.e., at a heating rate of 0 °C min−1, the dependence of tonset on β was plotted, and as a result, a straight line was obtained (Fig. 5). By extrapolating the resulting straight line to the y-axis (where β = 0 °C min−1), the true onset temperature of the formation of nanocrystalline GdFeO3 was found to be about 756 °C, which is quite close to the temperature of 750 °C used in our previous works [25,26,27,28,29,30].
Powder X-ray diffraction
The diffractograms of the products of heat treatment of the co-precipitated gadolinium and iron(III) hydroxides in the temperature range of 650–850 °C for 4 h are presented in Fig. 6. According to these diffraction patterns, the heat-treated product obtained at a temperature of 650 °C is X-ray amorphous, whereas the products that underwent heat treatment at a temperature of 800 °C and higher are pure-phase crystalline samples and consist of orthorhombic gadolinium orthoferrite (o-GdFeO3) (ICSD 27278, space group 62: Pbnm). On the other hand, the X-ray phase analysis of the heat-treated products obtained in the temperature range of 675–775 °C shows that, in addition to o-GdFeO3, they also contain other crystalline phases that, apparently, can be associated with hexagonal gadolinium oxide (h-Gd2O3) (ICSD 162247, space group 164: P-3m1) and cubic gadolinium oxide (c-Gd2O3) (ICSD 150677, space group 206: Ia-3). Along with these three crystalline phases, the last-mentioned products also contain X-ray amorphous phases, as seen from the amorphous halo in the Bragg angle range of ~ 15.2–27.2°. These phases include amorphous iron(III) oxide (am-Fe2O3), amorphous gadolinium oxide (am-Gd2O3) and/or amorphous gadolinium oxycarbonate derivatives “am-Gd2O3-x(CO3)x”. The estimated results of mass fractions of the X-ray amorphous and crystalline phases in all heat-treated products are presented in Fig. 7.
In accordance with Fig. 7, with an increase in the treatment temperature from 650 to 700 °C, the mass fraction of X-ray amorphous phases sharply decreases (from 100% at 650 to ~ 31% at 700 °C), while the mass fraction of o-GdFeO3 sharply increases (from 0 at 650 to ~ 61% at 700 °C). This can be explained by the formation of o-GdFeO3 as a result of the reaction of am-Fe2O3 and am-Gd2O3 at temperatures of 675–700 °C. This primary pathway of GdFeO3 formation is independent of the decomposition of gadolinium oxycarbonate derivatives.
On the other hand, the mass fractions of h-Gd2O3 and c-Gd2O3 progressively increase with an increase in the treatment temperature up to 725 °C (reaching a maximum of ~ 13% for h-Gd2O3 and ~ 1.5% for c-Gd2O3), above which they begin to decrease gradually and completely disappear at a temperature of 800 °C. It is well known that under normal conditions, gadolinium oxide may exist in cubic (C-type, space group 206: Ia-3) or monoclinic (B-type, space group 12: C2/m) forms, whereas the hexagonal form (A-type, space group 164: P-3m1), at ambient pressure, is only stable at very high temperatures (more than 2443 K/ ~ 2170 °C) [42, 43]. However, the formation of h-Gd2O3 in the products heat treated in the temperature range of 675–775 °C is most likely related to the presence of gadolinium oxycarbonate derivatives, which, if they were crystalline, could have a hexagonal form that is more stable than other crystalline forms, e.g., the hexagonal form of Gd2O2CO3 is more stable than its tetragonal and monoclinic forms [44]. Based on this assumption and the results obtained, in the temperature range of 675–725 °C, gadolinium oxycarbonate derivatives progressively decompose with the formation of CO2 and h-Gd2O3, which then transforms to the form stable in the synthesis conditions, c-Gd2O3, which, at temperatures of 725–775 °C, reacts with am-Fe2O3 to form o-GdFeO3. This is the secondary pathway of GdFeO3 formation that depends on the decomposition of gadolinium oxycarbonate derivatives. It should be noted that the formation of o-GdFeO3 as a result of the reaction of am-Fe2O3 and h-Gd2O3, i.e., without the transformation of h-Gd2O3 to c-Gd2O3, may be possible, but there is no clear evidence for this, especially since the mass fraction of c-Gd2O3 is less than the mass fraction of h-Gd2O3 in all heat-treated products containing both of these phases, which indicates that the GdFeO3 formation along this pathway occurs with the participation of c-Gd2O3 consumed. Besides, since the last stage of decomposition of gadolinium oxycarbonate derivatives proceeds at a temperature of ~ 756–758, as follows from the DTG curves (Table 1, effect 7), h-Gd2O3 can be formed even above the temperature of 725 °C; however, we cannot observe this on the diffractograms of the products heat treated at temperatures of 750–775 °C, most likely because above the temperature of 725 °C, h-Gd2O3 transforms to c-Gd2O3 which then reacts with am-Fe2O3 faster than h-Gd2O3 is produced from the decomposition of gadolinium oxycarbonates derivatives. Similarly, the GdFeO3 formation along this pathway can begin below the temperature of 725 °C, but in this case, the reaction of c-Gd2O3 (and perhaps h-Gd2O3) with am-Fe2O3 is slower than the production of h-Gd2O3 from the decomposition of gadolinium oxycarbonate derivatives. The obtained results are in good agreement with the results of DSC, according to which the exothermic effect of GdFeO3 formation is observed at a temperature of ~ 764–772 °C (Table 1, effect 8).
Figure 8 illustrates the proposed mechanism for the formation of nanocrystalline GdFeO3 synthesized, i.e., by ultrasound-assisted co-precipitation, as follows from the DSC–TGA and PXRD data.
Fourier-transform infrared spectroscopy
The FTIR spectra of the products of heat treatment of the synthesized sample of co-precipitated gadolinium and iron(III) hydroxides in the temperature range of 650–850 °C for 4 h are presented in Fig. 9. The wavenumbers of the absorption bands that correspond to the main vibrations in the obtained FTIR spectra of the products that underwent heat treatment in the temperature range of 650–775 °C and their assignment are summarized in Table 4.
The broad absorption band in the region of high wavenumbers centered at ~ 3256–3385 cm−1 (band 1) is a consequence of the overlapping of asymmetric and symmetric stretching vibrations of water “ν(H2O)”, which is physically adsorbed on the surface of the samples during their storage and/or preparation for investigation [28]. The absorption band of the bending vibration of physically adsorbed water “δ(H2O)” is observed at ~ 1636–1637 cm−1 (band 2). The bands observed at ~ 1507–1508 cm−1 (band 3) and at ~ 1391–1398 cm−1 (band 4) can be attributed to the asymmetric “νas(CO32−)” and symmetric “νs(CO32−)” stretching vibrations of carbonate groups, respectively, whereas the bands observed at ~ 1045–1088 cm−1 (band 5), at ~ 849–859 cm−1 (band 6) and at ~ 664–670 cm−1 (band 7) may be assigned to the symmetric stretching vibration of the C–O bond “νs(C–O)”, the out-of-plane bending “π(CO32−)” and rocking “ρ(CO32−)” vibrations of carbonate groups, correspondently [45]. The band observed at ~ 564–570 cm−1 (band 8) can be attributed to the stretching vibrations of the Gd–O bond “ν(Gd–O)” and the Fe–O bond “ν(Fe–O)” in the Gd–O–Fe and Fe–O–Fe systems [46], while the band observed at ~ 435–444 cm−1 (band 9) may be assigned to the O–Fe–O bending vibration “δ(O–Fe–O)” in octahedral FeO6 groups [31]. The last-mentioned absorption band (band 9) is practically absent in the FTIR spectrum of the product heat treated at a temperature of 650 °C, which is X-ray amorphous as followed from the PXRD results (see Section “Powder X-ray diffraction”).
As can be seen from Fig. 9, the intensity of the asymmetric and symmetric stretching vibrations of carbonate groups (Table 4, bands 3 and 4) on the normalized FTIR spectra in the wavenumber range of ~ 1260–1625 cm−1 gradually decreases with an increase in heating temperature to become practically negligible at a temperature of 800 °C. The relative mass percentages of gadolinium oxycarbonate derivatives in the products that underwent heat treatment at temperatures up to 800 °C, estimated based on the integrated intensity of these vibrations, are presented in Fig. 10. The mass fractions of h-Gd2O3 in these heat-treated products, previously estimated using the PXRD data, were recalculated as percentages of the maximum and presented in the same figure for comparison.
Relative mass percentages of gadolinium oxycarbonate derivatives in the products heat treated at temperatures up to 800 °C according to the FTIR data (purple) and relative mass fractions of h-Gd2O3 in these products as followed from the PXRD results (orange). “Relative (rel.)” here means that the values are calculated relative to the maximum
The results presented in Fig. 10 are in good agreement with our assumption that gadolinium oxycarbonate derivatives progressively decompose with the formation of h-Gd2O3 up to the temperature of 725 °C, above which the mass fraction of h-Gd2O3 begins to decrease due to the formation of o-GdFeO3 along with the secondary pathway, i.e., with the participation of c-Gd2O3 (see Section “Powder X-ray diffraction”). Above the temperature of 725 °C, gadolinium oxycarbonate derivatives continue to decompose gradually, but, as we discussed earlier, h-Gd2O3 then transforms to c-Gd2O3 which reacts with am-Fe2O3 faster than h-Gd2O3 is produced; therefore, we observe not an increase but a decrease in the mass fraction of h-Gd2O3. At a temperature of 800 °C, h-Gd2O3 completely disappears and gadolinium oxycarbonate derivatives become practically negligible.
Carbonate content in gadolinium oxycarbonate derivatives
To estimate the carbonate content in gadolinium oxycarbonate derivatives, i.e., to estimate the values of x in the formula Gd2O3-x(CO3)x at different temperatures, we used the TGA data at temperatures of 700, 725 and 750 °C at different heating rates (10–30 °C min−1) as well as the PXRD data for the products that underwent heat treatment at these temperatures, which include only am-Fe2O3 and am-Gd2O3-x(CO3)x as amorphous phases. As discussed earlier, at temperatures of 675–700 °C, am-Fe2O3 reacts with am-Gd2O3 to form o-GdFeO3 along the primary pathway (see Section “Powder X-ray diffraction”); therefore, the product heat treated at a temperature of 675 °C still contains am-Gd2O3, the presence of which complicates the calculation (the unnormalized diffraction pattern of this product shown in Fig. S2 confirms that, at a treatment temperature of 675 °C, o-GdFeO3 is not fully crystallized in comparison with the products heat treated at higher temperatures). As for the product heat treated at a temperature of 775 °C, the relative mass percentage of gadolinium oxycarbonate derivatives is rather small (Fig. 10), which may also affect the calculation results.
According to the EDXS results, the average Gd:Fe atomic ratio is about 1.000:1.003, which is maintained during heat treatment (see Section “Energy-dispersive X-ray spectroscopy”); therefore, the average Gd:Fe molar ratio can be considered equal to 1:1 in all heat-treated products, which means that the amounts in moles (n) of gadolinium and iron are equal (Eq. 8).
The amounts in moles of gadolinium and iron in the selected heat-treated products can be determined from Eqs. 9 and 10, respectively.
Substituting Eqs. 9 and 10 in Eq. 8 and rearranging, we can determine the amount in moles of Gd2O3-x(CO3)x (Eq. 11).oxycarbonate derivatives, one mole
Since the amount of a substance in moles is equal to its mass (m) divided by its molar mass (M), Eq. 11 can be written as shown in Eq. 12.
The molar mass of gadolinium oxycarbonate derivatives can be determined as a function of x from Eq. 13.
Dividing both sides of Eq. 12 by the mass of the sample at a temperature of t (mt) and considering that the mass of a phase divided by the sample mass at a certain temperature is equal to the mass fraction of that phase (w) at that temperature, Eq. 12 is converted to Eq. 14.
The mass fraction of am-Fe2O3 in the selected samples can be determined from the estimated mass fraction of amorphous phases (wamorph) by subtracting the mass fraction of the other amorphous phase, Gd2O3-x(CO3)x, as shown in Eq. 15.
Substituting Eq. 15 in Eq. 14 and rearranging, we can determine the mass fraction of gadolinium oxycarbonate derivatives in the investigated samples as a function of x (Eq. 16).
In accordance with the decomposition equation for gadolinium oxycarbonate derivatives, one mole of Gd2O3-x(CO3)x decomposes with the release of x mole of CO2, and a mass of m(Gd2O3-x(CO3)x) decomposes with the release of a mass of m(CO2); therefore, using chemical arithmetic, we can write Eq. 17.
Since the mass of Gd2O3-x(CO3)x at a certain temperature, as mentioned above, is equal to its mass fraction multiplied by the sample mass at that temperature, Eq. 17 can be written as shown in Eq. 18.
Substituting Eq. 16 in Eq. 18, rearranging and solving for x, we obtain the required equation (Eq. 19).
Using Eq. 19, the carbonate content in gadolinium oxycarbonate derivatives in the investigated samples can be estimated. At different heating rates (10–30 °C min−1), the sample mass at a temperature of t (mt), where t = 700, 725 and 750 °C, was determined from the TGA curves (Fig. 2b). In addition, the mass of CO2 included in the sample at a temperature of t “m(CO2)”, i.e., its mass that will be released upon the complete decomposition of gadolinium oxycarbonate derivatives in this sample, was also determined using the TGA data as the difference between the sample mass at each temperature (mt) and the sample mass at the end (completion) of mass loss on the TGA curve (mend). The mass fractions of the amorphous phases (wamorph), h-Gd2O3 “w(h-Gd2O3)” and c-Gd2O3 “w(c-Gd2O3)” at each investigated temperature were previously estimated based on the PXRD data (Fig. 7). The TGA and PXRD data used and the calculation results are summarized in Table 5. The dependences of x in Gd2O3-x(CO3)x on temperature at different heating rates are presented in Fig. 11.
Dependences of x in the formula of gadolinium oxycarbonate derivatives “Gd2O3-x(CO3)x” on temperature (t) at different heating rates. The inset shows the extrapolation of these dependences to the temperature range in which the obtained DSC and DTG curves showed the first stage of gadolinium carbonate decomposition with the formation of an oxycarbonate derivative. R2 is the coefficient of determination
As follows from Fig. 11, the obtained dependences can be described by linear equations with coefficients of determination in the range of 0.9363–0.9999. The extrapolation of these dependences to the temperature of 775 °C shows that x at this temperature is estimated at ~ 0.00–0.22 with an average value of ~ 0.12, which is in good agreement with the PXRD and FTIR results. On the other hand, extrapolating the resulting dependences to the temperature range in which the obtained DSC and DTG curves showed the first stage of gadolinium carbonate decomposition with the formation of an oxycarbonate derivative (Table 1, effect 5), the x value in this derivative may be estimated at ~ 1.56–2.33 with an average value of ~ 1.91. This result is very close to the literature data [33], according to which, at a temperature of ~ 363–370 °C, gadolinium carbonate “Gd2(CO3)3” decomposes to gadolinium monooxydicarbonate “Gd2O(CO3)2” with the release of CO2.
Conclusions
In this work, a complex and detailed experimental investigation of the mechanism of formation of GdFeO3 nanocrystals was performed, which shows that the co-precipitated gadolinium and iron(III) hydroxides upon heat treatment undergo a series of physicochemical processes including the dehydration to the corresponding oxides and the decomposition of gadolinium oxycarbonate derivatives prior to the formation of GdFeO3. The PXRD results indicated that the GdFeO3 formation occurs via two pathways: a primary carbonate-independent pathway involving am-Gd2O3 and a secondary carbonate-dependent pathway involving h-Gd2O3 and c-Gd2O3. The FTIR results showed that gadolinium oxycarbonate derivatives gradually decompose with increasing heating temperature to become practically negligible at a temperature of 800 °C. The resulting linear equations for the dependence of x in Gd2O3-x(CO3)x on temperature predict that gadolinium carbonate at a temperature of ~ 266–426 °C decomposes to Gd2O(CO3)2 which, at a temperature of ~ 553–601 °C, decomposes to Gd2O2CO3, which, in turn, decomposes to Gd2O3 at a temperature of ~ 776–839 °C.
References
Bamzai KK, Bhat M. Electrical and magnetic properties of some rare earth orthoferrites (RFeO3 where R = Y, Ho, Er) systems. Integr Ferroelectr. 2014;158:108–22.
Zhou Z, Guo L, Yang H, Liu Q, Ye F. Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J Alloys Compd. 2014;583:21–31.
Ramu N, Muralidharan R, Meera K, Jeong YH. Tailoring the magnetic and magnetoelectric properties of rare earth orthoferrites for room temperature applications. RSC Adv. 2016;6:72295–9.
Nakhaei M, Sanavi KD. Study on structural, magnetic and electrical properties of ReFeO3 (Re= La, Pr, Nd, Sm & Gd) orthoferrites. Physica B Condens Matter. 2021;612: 412899.
Warshi MK, Mishra V, Sagdeo A, Mishra V, Kumar R, Sagdeo PR. Structural, optical and electronic properties of RFeO3. Ceram Int. 2018;44:8344–9.
Wang Z-Q, Lan Y-S, Zeng Z-Y, Chen X-R, Chen Q-F. Magnetic structures and optical properties of rare-earth orthoferrites RFeO3 (R = Ho, Er, Tm and Lu). Solid State Commun. 2019;288:10–7.
Sultan K, Samad R, Islam SAU, Habib MZ, Ikram M. Effect of rare earth ions (R = Pr, Eu and Ho) on the structural and electrical properties of orthoferrites. J Electron Mater. 2019;48:6003–7.
Yafarova LV, Chislova IV, Zvereva IA, Kryuchkova TA, Kost VV, Sheshko TF. Sol–gel synthesis and investigation of catalysts on the basis of perovskite-type oxides GdMO3 (M = Fe, Co). J Solgel Sci Technol. 2019;92:264–72. https://doi.org/10.1007/s10971-019-05013-3.
Niu X, Li H, Liu G. Preparation, characterization and photocatalytic properties of REFeO3 (RE=Sm, Eu, Gd). J Mol Catal A Chem. 2005;232:89–93.
Li L, Wang X, Lan Y, Gu W, Zhang S. Synthesis, photocatalytic and electrocatalytic activities of wormlike GdFeO3 nanoparticles by a glycol-assisted sol-gel process. Ind Eng Chem Res. 2013;52:9130–6. https://doi.org/10.1021/ie400940g.
Zhang Y, Zheng A, Yang X, He H, Fan Y, Yao C. Cubic GdFeO3 particle by a simple hydrothermal synthesis route and its photoluminescence and magnetic properties. CrystEngComm. 2012;14:8432.
Niu X, Du W, Du W. Preparation, characterization and gas-sensing properties of rare earth mixed oxides. Sens Actuators B Chem. 2004;99:399–404.
Söderlind F, Fortin MA, Petoral RM Jr, Klasson A, Veres T, Engström M, et al. Colloidal synthesis and characterization of ultrasmall perovskite GdFeO3 nanocrystals. Nanotechnology. 2008;19: 085608.
Pinho SLC, Amaral JS, Wattiaux A, Duttine M, Delville M-H, Geraldes CFGC. Synthesis and characterization of rare-earth orthoferrite LnFeO3 nanoparticles for bioimaging. Eur J Inorg Chem. 2018;2018:3570–8. https://doi.org/10.1002/ejic.201800468.
Athar T, Vishwakarma SK, Bardia A, Khan AA. Super paramagnetic iron oxide and gadolinium (FeGdO3) nanopowder synthesized by hydrolytic approach passes high level of biocompatibility and MRI-based dual contrast property for competent molecular imaging and therapeutic interventions. Biomed Phys Eng Express. 2016;2:025010. https://doi.org/10.1088/2057-1976/2/2/025010.
Deka S, Saxena V, Hasan A, Chandra P, Pandey LM. Synthesis, characterization and in vitro analysis of α-Fe2O3–GdFeO3 biphasic materials as therapeutic agent for magnetic hyperthermia applications. Mater Sci Eng C. 2018;92:932–41.
Abiev RSh, Almjasheva OV, Popkov VI, Proskurina OV. Microreactor synthesis of nanosized particles: the role of micromixing, aggregation, and separation processes in heterogeneous nucleation. Chem Eng Res Des. 2022;178:73–94.
Mariyappan V, Keerthi M, Chen S-M, Jeyapragasam T. Nanostructured perovskite type gadolinium orthoferrite decorated RGO nanocomposite for the detection of nitrofurantoin in human urine and river water samples. J Colloid Interface Sci. 2021;600:537–49.
Ateia EE, Hussein B, Singh C, Okasha N. Study of physical properties of Co substituted GdFeO3 orthoferrites and evaluation of their antibacterial activity. J Inorg Organomet Polym Mater. 2020;30:4320–8.
Santhosh BS, Yashas SR, Kumara Swamy N, Shivaraju HP. Application of non-hierarchical gadolinium ortho-ferrite nanostructure for LED-driven photocatalytic mineralization of doxycycline hydrochloride. J Mater Sci: Mater Electron. 2022;33:11676–86.
Tang P, Hu Y, Lin T, Jiang Z, Tang C. Preparation of nanocrystalline GdFeO3 by microwave method and its visible-light photocatalytic activity. Integr Ferroelectr. 2014;153:73–8. https://doi.org/10.1080/10584587.2014.902720.
Sivakumar M, Gedanken A, Bhattacharya D, Brukental I, Yeshurun Y, Zhong W, et al. Sonochemical synthesis of nanocrystalline rare earth orthoferrites using Fe(CO)5 precursor. Chem Mater. 2004;16:3623–32. https://doi.org/10.1021/cm049345x.
Mathur S, Shen H, Lecerf N, Kjekshus A, Fjellvåg H, Goya GF. Nanocrystalline orthoferrite GdFeO3 from a novel heterobimetallic precursor. Adv Mater. 2002;14:1405–9.
Lone IH, Khan H, Jain AK, Ahmed J, Ramanujachary KV, Ahmad T. Metal-organic precursor synthesis, structural characterization, and multiferroic properties of GdFeO3 nanoparticles. ACS Omega. 2022;7:33908–15.
Albadi Y, Martinson KD, Shvidchenko AV, Buryanenko IV, Semenov VG, Popkov VI. Synthesis of GdFeO3 nanoparticles via low-temperature reverse co-precipitation: the effect of strong agglomeration on the magnetic behavior. Nanosyst Phys Chem Math. 2020;11:252–9.
Albadi Y, Sirotkin AA, Semenov VG, Abiev RS, Popkov VI. Synthesis of superparamagnetic GdFeO3 nanoparticles using a free impinging-jets microreactor. Russ Chem Bull. 2020;69:1290–5. https://doi.org/10.1007/s11172-020-2900-x.
Popkov VI, Albadi Y. The effect of co-precipitation temperature on the crystallite size and aggregation/agglomeration of GdFeO3 nanoparticles. Nanosyst Phys Chem Math. 2021;12:224–31.
Albadi Y, Ivanova MS, Grunin LY, Martinson KD, Chebanenko MI, Izotova SG, et al. The influence of co-precipitation technique on the structure, morphology and dual-modal proton relaxivity of GdFeO3 nanoparticles. Inorganics (Basel). 2021;9:39.
Albadi Y, Abiev RS, Sirotkin AA, Martinson KD, Chebanenko MI, Nevedomskiy VN, et al. Physicochemical and hydrodynamic aspects of GdFeO3 production using a free impinging-jets method. Chem. Eng. Process.: Process Intensif. 2021;166: 108473.
Albadi Y, Ivanova MS, Grunin LY, Makarin RA, Komlev AS, Chebanenko MI, et al. Ultrasound-assisted co-precipitation synthesis of GdFeO3 nanoparticles: structure, magnetic and MRI contrast properties. Phys Chem Chem Phys. 2022;24:29014–23.
Prakash BJ, Rudramadevi BH, Buddhudu S. Analysis of ferroelectric, dielectric and magnetic properties of GdFeO3 nanoparticles. Ferroelectr Lett Sect. 2014;41:110–22. https://doi.org/10.1080/07315171.2014.956020.
Tugova EA, Karpov ON. Nanocrystalline perovskite-like oxides formation in Ln2O3–Fe2O3–H2O (Ln = La, Gd) systems. Nanosyst Phys Chem Math. 2014;5:854–60.
Popkov VI, Tugova EA, Bachina AK, Almyasheva OV. The formation of nanocrystalline orthoferrites of rare-earth elements XFeO3 (X = Y, La, Gd) via heat treatment of coprecipitated hydroxides. Russ J Gen Chem. 2017;87:2516–24. https://doi.org/10.1134/S1070363217110020.
Natarajan M, Secco EA. Anisotropic conductivity and phase transformation studies in potassium chromate crystals. Can J Chem. 1974;52:2436–8.
Glushko VP, editor. Thermal constants of substances. handbook in 10 Issues. Issue 8. Part 1. Tables of accepted values. Moscow: USSR Academy of Sciences. All-Union Institute of Scientific and Technical Information. Institute for High Temperatures; 1978.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Boswell PG. On the calculation of activation energies using a modified Kissinger method. J Therm Anal. 1980;18:353–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci B. 1966;4:323–8.
Zhang X. Applications of kinetic methods in thermal analysis: a review. Eng Sci. 2021;14:1–13.
Zinkevich M. Thermodynamics of rare earth sesquioxides. Prog Mater Sci. 2007;52:597–647.
Zhang FX, Lang M, Wang JW, Becker U, Ewing RC. Structural phase transitions of cubic Gd2O3 at high pressures. Phys Rev B. 2008;78: 064114.
Ge W, Li Z, Lei Z, Chen T, Fu Z, Peng R, et al. Synthesis of hexagonal phase Gd2O2CO3: Yb3+, Er3+upconversion nanoparticles via SiO2 coating and Nd3+ doping. CrystEngComm. 2015;17:5702–9.
Gaspar RDL, Mazali IO, Sigoli FA. Particle size tailoring and luminescence of europium(III)-doped gadolinium oxide obtained by the modified homogeneous precipitation method: Dielectric constant and counter anion effects. Colloids Surf A Physicochem Eng Asp. 2010;367:155–60.
Sai Vandana C, Hemalatha Rudramadevi B. Effect of Cu2+ substitution on the structural, magnetic and electrical properties of gadolinium orthoferrite. Mater Res Express. 2018;5(4): 046101. https://doi.org/10.1088/2053-1591/aab7a8
Acknowledgements
The study was partially performed using the equipment of the Engineering Center of Saint Petersburg State Institute of Technology.
Author information
Authors and Affiliations
Contributions
Y. Albadi and V. I. Popkov contributed to conceptualization, methodology, validation and formal analysis; Y. Albadi and A. K. Bachina did investigation; A. K. Bachina and V. I. Popkov helped in resources; Y. Albadi did data curation, writing—original draft, visualization and writing—review & editing; V. I. Popkov contributed to writing—review & editing, supervision, project administration and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albadi, Y., Bachina, A.K. & Popkov, V.I. Physicochemical processes and thermochemical parameters of GdFeO3 formation from amorphous hydroxides: decisive role of carbonate impurities. J Therm Anal Calorim 148, 13281–13295 (2023). https://doi.org/10.1007/s10973-023-12647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12647-7