Abstract
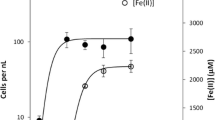
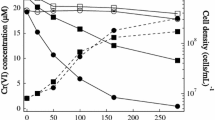
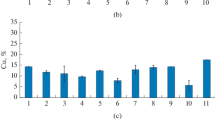
Shewanella putrefaciens CN32 reduces Fe(III) within two illites which have different properties: the Fithian bulk fraction and the <0.2 µm fraction of Muloorina. The Fithian illite contained 4.6% (w/w) total Fe, 81% of which was Fe(III). It was dominated by illite with some jarosite (∼32% of the total Fe(III)) and goethite (11% of the total Fe(III)). The Muloorina illite was pure and contained 9.2% Fe, 93% of which was Fe(III). Illite suspensions were buffered at pH 7 and were inoculated with CN32 cells with lactate as the electron donor. Select treatments included anthraquinone-2,6-disulfonate (AQDS) as an electron shuttle. Bioproduction of Fe(II) was determined by ferrozine analysis. The unreduced and bioreduced solids were characterized by Mössbauer spectroscopy, X-ray diffraction and transmission electron microscopy. The extent of Fe(III) reduction in the bulk Fithian illite was enhanced by the presence of AQDS (73%) with complete reduction of jarosite and goethite and partial reduction of illite. Mössbauer spectroscopy and chemical extraction determined that 21–25% of illite-associated Fe(III) was bioreduced. The extent of bioreduction was less in the absence of AQDS (63%) and only jarosite was completely reduced with partial reduction of goethite and illite. The XRD and TEM data revealed no significant illite dissolution or biogenic minerals, suggesting that illite was reduced in the solid state and biogenic Fe(II) from jarosite and goethite was either released to aqueous solution or adsorbed onto residual solid surfaces. In contrast, only 1% of the structural Fe(III) in Muloorina illite was bioreduced. The difference in the extent and rate of bioreduction between the two illites was probably due to the difference in layer charge and the total structural Fe content between the Fithian illite (0.56 per formula) and Muloorina illite (0.87). There may be other factors contributing to the observed differences, such as expandability, surface area and the arrangements of Fe in the octahedral sheets. The results of this study have important implications for predicting microbe-induced physical and chemical changes of clay minerals in soils and sediments.
Similar content being viewed by others
References
Dong, H. and Peacor, D.R. (1996) TEM observations of coherent stacking relations in smectite, I/S and illite of shales: evidence for MacEwan crystallites and dominance of 2M1 polytypism. Clays and Clay Minerals, 44, 257–275.
Dong, H., Peacor, D.R. and Freed, R.L. (1997) Phase relations among smectite, R1 I/S and illite. American Mineralogist, 82, 379–391.
Dong, H., Fredrickson, J.K., Kennedy, D.W., Zachara, J.M., Kukkadapu, R.K. and Onstott, T.C. (2000) Mineral transformation associated with the microbial reduction of magnetite. Chemical Geology, 169, 299–318.
Dong, H., Kukkadapu, R.K., Fredrickson, J.K., Zachara, J.M., Kennedy, D.W. and Kostandarithes, H.M. (2003) Microbial reduction of structural Fe(III) in illite and goethite. Environmental Science and Technology, 37, 1268–1276.
Eberl, D.D., Drits, V.A. and Środoń, J. (1998) Deducing growth mechanisms for minerals from the shapes of crystal size distribution. American Journal of Science, 298, 449–533.
Ettler, V., Johan, Z. and Hradil, D. (2003) Natural alteration products of sulphide mattes from primary lead smelting. Comptes Rendus Geoscience, 335, 1013–1020.
Fanning, D.S., Keramidas, V.Z. and El-Desoky, M.A. (1989) Micas. Pp. 551–634 in: Minerals in Soil Environments (J.B. Dixon and S.B. Weed, editors). SSSA Book Series, Soil Science Society of America, Madison, Wisconsin.
Fredrickson, J.K., Zachara, J.M., Kennedy, D.W., Dong, H., Onstott, T.C., Hinman, N.W. and Li, S.M. (1998) Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochimica et Cosmochimica Acta, 62, 3239–3257.
Fredrickson, J.K., Zachara, J.M., Kukkadapu, R.K., Gorgy, Y.A., Smith, S.C. and Brown, C.F. (2001) Biotransformation of Ni-substituted hydrous ferric oxide by an Fe(III)-reducing bacterium. Environmental Science and Technology, 35, 703–712.
Greenwood, N.N. and Gibb, T.C. (1971) Mössbauer Spectroscopy. Chapman & Hall, London.
Hower, J., Eslinger, E.V., Hower, M.H. and Perry, E.A. (1976) Mechanism of burial metamorphism of argillaceous sediments. 1. Mineralogical and chemical evidence. Geological Society of America Bulletin, 87, 725–737.
Herbert, R.B. (1997) Properties of goethite and jarosite precipitated from acidic groundwater, Dalarna, Sweden. Clays and Clay Minerals, 45, 261–273.
Jackson, M.L., Lim, C.H. and Zelanzy, L.W. (1986) Oxides, hydroxides, and aluminosilicates. Pp. 101–150 in: Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd edition (A. Klute, editor). American Society of Agronomy-Soil Science Society of America, Madison, Wisconsin.
Kim, J., Dong, H., Seabaugh, J. and Newell, S.W. (2004) Role of microbes in the smectite-to-illite reaction. Science, 303, 830–832.
Kostka, J.E., Stucki, J.W., Nealson, K.H. and Wu, J. (1996) Reduction of structural Fe(III) in smectite by a pure culture of Shewanella putrefaciens strain MR-1. Clays and Clay Minerals, 44, 522–529.
Kostka, J.E., Haefele, E., Viehweger, R. and Stucki, J.W. (1999a) Respiration and dissolution of iron(III)-containing clay minerals by bacteria. Environmental Science and Technology, 33, 3127–3133.
Kostka, J.E., Wu, J., Nealson, K.H. and Stucki, J.W. (1999b) The impact of structural Fe(III) reduction by bacteria on the surface chemistry of smectite clay minerals. Geochimica et Cosmochimica Acta, 63, 3705–3713.
Kukkadapu, R.K., Zachara, J.M., Smith, S.C., Fredrickson, J.K. and Liu, C.X. (2001) Dissimilatory bacterial reduction of Al-substituted goethite in subsurface sediments. Geochimica Cosmochimica Acta, 65, 2913–2924.
Kukkadapu, R.K., Zachara, J.M., Fredrickson, J.K. and Kennedy, D.W. (2004) Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe(III)-reducing bacterium: formation of carbonate green rust in the presence of phosphate. Geochimica et Cosmochimica Acta, 68, 2799–2814.
Lovely, D.R. (2000) Environmental Microbe-Metal Interactions. ASM Press, Washington, D.C.
Murad, E. and Cashion, J. (2004) Iron oxides and oxyhydroxides. Pp. 159–188 in: Mössbauer Spectroscopy of Environmental Materials and their Industrial Applications (E. Murad and J. Cashion, editors). Springer, Boston, USA.
Nealson, K.H. and Little, B. (1997) Breathing manganese and iron: solid-state respiration. Advances in Applied Microbiology, 45, 213–238.
Norrish, K. and Pickering, J.G. (1983) Clay minerals. Pp. 281–308 in: Soils, an Australian Viewpoint. Division of Soils, Commonwealth Scientific and Industrial Research Organization, Australia Imprint, Melbourne — CSIRO, Academic Press, London.
O’Reilly, S.E., Bickmore, B.R. and Furukawa, Y. (2004) Dissolution of microbial reduced nontronite in a flow-through system. Pp. 86 in: 41st Annual Meeting of the Clay Minerals Society, Program and Abstracts.
Peacor, D.R. (1992) Diagenesis and low-grade metamorphism of shales and slates. Pp. 335–380 in: Minerals and Reactions at the Atomic Scale: Transmission Electron Microscopy (P.R. Buseck, editor). Reviews in Mineralogy, 27, Mineralogical Society of America, Washington, D.C.
Phillips, E.J.P. and Lovely, D.R. (1987) Determination of Fe(III) and Fe(II) in oxalate extracts of sediments. Soil Society of America Journal, 51, 938–941.
Rancourt, D.G. and Ping, J.Y. (1991) Voigt-based method for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy. Nuclear Instruments and Methods Physics Research, 41, 891–893.
Roden, E.E. and Zachara, J.M. (1996) Microbial reduction of crystalline Fe(III) oxides: Influence of oxide surface area and potential for cell growth. Environmental Science and Technology, 30, 1618–1628.
Stucki, J.W., Komadel, P. and Wilkinson, H.T. (1987) Microbial reduction of structural iron(III) in smectites. Soil Science Society of America Journal, 51, 1663–1665.
Taneja, S.P. and Jones, C.H.W, (1984) Mössbauer studies of iron-bearing minerals in coal and coal ash. Fuel, 63, 695–701.
van der Zee, C., Roberts, D.R., Rancourt, D.G. and Slomp, C.P. (2003) Nanogoethite is the dominant reactive oxyhydroxide phase in lake and marine sediments. Geology, 31, 993–996.
Zachara, J.M., Fredrickson, J.K., Li, S.M., Kennedy, D.W., Smith, S.C. and Gassman, P.L. (1998) Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. American Mineralogist, 83, 1426–1443.
Zachara, J.M., Fredrickson, J.K., Smith, S.C. and Gassman, P.L. (2001) Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochimica et Cosmochimica Acta, 65, 75–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seabaugh, J.L., Dong, H., Kukkadapu, R.K. et al. Microbial reduction of Fe(III) in the Fithian and Muloorina illites: Contrasting extents and rates of bioreduction. Clays Clay Miner. 54, 67–79 (2006). https://doi.org/10.1346/CCMN.2006.0540109
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2006.0540109