Abstract
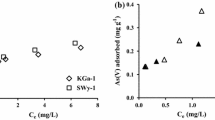
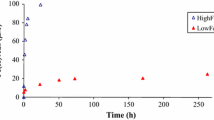
Siderophores are low molecular weight organic ligands synthesized by aerobic microorganisms to acquire Fe. In addition to Fe(III), siderophores may complex other metals such as Pb and Cd. This study compared the effects of the trihydroxamate siderophores desferrioxamine-B (DFO-B), desferrioxamine-D1 (DFO-D1), desferrioxamine-E (DFO-E), and the monohydroxamate siderophore-like ligand acetohydroxamic acid (aHA) on Pb and Cd (except for DFO-E) adsorption to kaolinite (KGa-1b) at pH 4.5 to 9, in 0.1 M NaClO4, at 22°C, in the dark. At pH >6, all of the studied ligands decreased Pb adsorption to kaolinite: aHA by 5–40% and DFO-B, DFO-D1 and DFO-E by 30–75%; the greater effects were at higher pH. The studied ligands decreased Cd adsorption to kaolinite at pH >8: aHA by 5–20% and the trihydroxamates by as much as 80%. We also observed enhancement of Pb adsorption in the presence of DFO-B at pH ≈5–6.0, probably due to adsorption of the doubly positively charged H3Pb (DFO-B)2+ complex, although spectroscopic evidence is needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Anderegg, G., L’Eplattenier, F. and Schwarzenbach, G. (1963) Hydroxamatkomplexe II. Die Anwendung der pH-methode. Helvetica Chimica Acta, 46, 1400–1408.
Borgias, B., Hugi, A.D. and Raymond, K.N. (1989) Isomerization and solution structures of desferrioxamine B complexes of Al3+ and Ga3+. Inorganic Chemistry, 28, 3538–3545.
Cervini-Silva, J. and Sposito, G. (2002) Steady-state dissolution kinetics of aluminum-goethite in the presence of desferrioxamine-B and oxalate ligands. Environmental Science and Technology, 36, 337–342.
Crumbliss, A. (1991) Aqueous solution equilibrium and kinetic studies of iron siderophore and model siderophore complexes. Pp. 177–233 in: Handbook of Microbial Iron Chelates (G. Winkelmann, editor). CRC Press, Boca Raton, Florida.
Evers, A., Hancock, R.D., Martell, A.E. and Motekaitis, R.J. (1989) Metal ion recognition in ligands with negatively charged oxygen donor groups. Complexation of Fe(III), Ga(III), In(III), Al(III) and other highly charged metal ions. Inorganic Chemistry, 29, 2189–2195.
Fortin, D., Davis, B. and Beveridge, T.J. (1996) Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiology Ecology, 21, 11–24.
Hernlem, B.J., Vane, L.M. and Sayles, G.D. (1996) Stability constants for complexes of the siderophores desferrioxamine B with selected heavy metal cations. Inorganica Chimica Acta, 244, 179–184.
Hersman, L., Lloyd, T. and Sposito, G. (1995) Siderophore-promoted dissolution of hematite. Geochimica et Cosmochimica Acta, 59, 3327–3330.
Holmén, B.A. and Casey, W.H. (1996) Hydroxamate ligands, surface chemistry, and the mechanism of ligand promoted dissolution of goethite [α-FeOOH(s)]. Geochimica et Cosmochimica Acta, 60, 4403–4416.
Holmén, B.A., Tejedor-Tejedor, M.I. and Casey, W.H. (1997) Hydroxamate complexes in solution at the goethite-water interface: a cylindrical internal reflection Fourier transform infrared spectroscopy study. Langmuir, 13, 2197–2206.
Kalinowski, B.E., Liermann, L.J., Brantley, S.L., Barnes, A. and Pantano, C.G. (2000) X-ray photoelectron evidence for bacteria-enhanced dissolution of hornblende. Geochimica et Cosmochimica Acta, 64, 1331–1343.
Konetschny-Rapp, S., Jung, G., Raymond, K.N., Meiews, J. and Zahner, H. (1992) Solution thermodynamics of the ferric complexes of new desferrioxamine siderophores obtained by directed fermentation. Journal of the American Chemical Society, 114, 2224–2230.
Kraemer, S.M. (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquatic Sciences, 66, 3–18.
Kraemer, S.M., Cheah, S.-F., Zapf, R., Xu, J., Raymond, K.N. and Sposito, G. (1999) Effect of hydroxamate siderophores on Fe release and Pb(II) adsorption by goethite. Geochimica et Cosmochimica Acta, 63, 3003–3008.
Kraemer, S.M., Xu, J., Raymond, K.N. and Sposito, G. (2002) Adsorption of Pb(II) and Eu(III) by oxide minerals in the presence of natural and synthetic hydroxamate siderophores. Environmental Science and Technology, 36, 1287–1291.
Liermann, L.J., Kalinowski, B.E., Brantley, S.L. and Ferry, J.G. (2000) Role of bacterial siderophores in dissolution of hornblende. Geochimica et Cosmochimica Acta, 64, 587–602.
Martell, A.E. and Smith, R.E. (2001) NIST Stability Constants of Metal Complexes Database 46, version 6.0, US Department of Commerce, Gaithersburg, Maryland.
Meiwes, J., Fiedler, H.P., Zahner, H., Koneschny-Rapp, S. and Jung, G. (1990) Production of desferrioxamine-E and new analogs by direct fermentation and feeding fermentation. Applied Microbiology and Biotechnology, 32, 505–510.
Neilands, J.B. (1981) Microbial iron compounds. Annual Reviews Biochemistry, 50, 715–732.
Neubauer, U., Nowack, B., Furrer, G. and Schulin, R. (2000) Heavy metal sorption on clay minerals affected by the siderophore desferrioxamine B. Environmental Science and Technology, 34, 2749–2755.
Parkhurst, D.L. and Appelo, C.A.J. (1999) User’s guide to PHREEQC (version 2) - a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report 99–4259.
Rosenberg, D.R. and Maurice, P.A. (2003) Siderophore adsorption to and dissolution of kaolinite at pH 3 to 7 at 22°C. Geochimica et Cosmochimica Acta, 67, 223–229.
Ruggiero, C.E., Matonic, J.H., Reilly, S.D. and Neu, M.P. (2002) Dissolution of plutonium(IV) hydroxide by desferrioxamine siderophores and simple organic chelators. Inorganic Chemistry, 41, 3593–3595.
Sutheimer, S.H., Maurice, P.A. and Zhou, Q. (1999) Dissolution of well and poorly crystallized kaolinites: Al speciation and effects of surface characteristics. American Mineralogist, 84, 620–628.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hepinstall, S.E., Turner, B.F. & Maurice, P.A. Effects of Siderophores on Pb and Cd Adsorption to Kaolinite. Clays Clay Miner. 53, 557–563 (2005). https://doi.org/10.1346/CCMN.2005.0530601
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2005.0530601