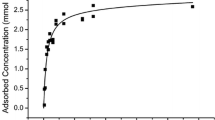
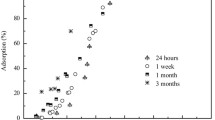
Abstract
We investigated the sorption of Ni to gibbsite of two different surface areas at pH 7.5, in the presence and absence of citrate, over a time period of 180 days. Extended X-ray absorption fine-structure spectroscopy was employed to elucidate the sorption mechanisms at the molecular level. In agreement with former results, Ni-Al layered double hydroxide (LDH) formed in the presence of gibbsite of low surface area. However, gibbsite of high surface area suppressed the formation of the surface precipitate. Instead, two Al atoms neighboring Ni at distances of 2.95–2.98 Å indicated formation of an inner-sphere sorption complex, where each NiO6-octahedron shares edges with two AlO6-octahedra. Focused multiple scattering arising from Al atoms at a distance of 6 Å suggest that sorbed Ni(OH)2(OH2)4 monomers epitaxially extend the hexagonal arrangement of Al atoms in gibbsite. Only after 30 days or more was a small amount of LDH formed. The presence of citrate prevented the formation of LDH, while maintaining the formation of inner-sphere sorption complexes.
Similar content being viewed by others
References
Alcacio, T.E., Hesterberg, D., Chou, J.W., Martin, J.D., Beauchemin, S. and Sayers, D.E. (2001) Molecular scale characteristics of Cu(II) bonding in goethite-humate complexes. Geochimica et Cosmochimica Acta, 65, 1355–1366.
Bargar, J.R., Brown, G.E., Jr. and Parks, G.A. (1998) Surface complexation of Pb(II) at oxide-water interfaces: III. XAFS determination of Pb(II) and Pb(II)-chloro adsorption complexes on goethite and alumina. Geochimica et Cosmochimica Acta, 62, 193–207.
Bargar, J.R., Persson, P. and Brown, G.E., Jr. (1999) Outersphere adsorption of Pb(II)EDTA on goethite. Geochimica et Cosmochimica Acta, 63, 2957–2969
Bruemmer, G.W., Gerth, J. and Tiller, K.G. (1988) Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. Adsorption and diffusion of metals. Journal of Soil Science, 39, 37–52.
Burns, R.G. (1993) Mineralogical Applications of Crystal Field Theory. Cambridge University Press, New York.
Charlet, L. and Manceau, A. (1994) Evidence for the neoformation of clays upon sorption of Co(II) and Ni(II) on silicates. Geochimica et Cosmochimica Acta 58, 2577–2582.
Fitts, J.P., Persson, P., Brown, G.E., Jr. and Parks, G.A. (1999) Structure and bonding of Cu(II)-glutamate complexes at the gamma-Al2O3-water interface. Journal of Colloid and Interface Science, 220, 133–147.
Ford, R.G., Scheinost, A.C., Scheckel, K.G. and Sparks, D.L. (1999) The link between clay mineral weathering and the stabilization of Ni surface precipitates. Environmental Science and Technology, 33, 3140–3144.
Ford, R.G., Scheinost, A.C. and Sparks, D.L. (2001) Frontiers in Metal Sorption/Precipitation Mechanisms on Soil Mineral Surfaces Pp. 41–62 in: Advances in Agronomy, 74, (D.L. Sparks, editor). American Society of Agronomy, Madison, Wisconsin.
Jones, D.L. (1998) Organic acids in the rhizosphere — a critical review. Plant and Soil, 205, 25–44.
Kraemer, S.M., Chiu, V.Q. and Hering, J.G. (1998) Influence of pH on competitive adsorption on the kinetics of ligand-promoted dissolution of aluminum oxide. Environmental Science and Technology, 32, 2876–2882.
Kyle, J.H., Posner, A.M. and Quirk, J.P. (1975) Kinetics of isotopic exchange of phosphate adsorbed on gibbsite. Journal of Soil Science, 26, 32–43.
Lytle, F.W., Greefgor, R.B., Sandstrom, D.R., Marques, E.C., Wong, J., Spiro, C.L., Huffman, G.P. and Huggins, F.E. (1984) Measurement of soft-X-ray absorption spectra with a fluorescence-chamber detector. Nuclear Instruments and Methods in Physics Research Section A — Accelerators, Spectrometers, Detectors and Associated Equipment, 226, 542–548.
Manceau, A., Llorca, S. and Calas, G. (1987) Crystal chemistry of cobalt and nickel in lithiophorite and asbolane from New Caledonia. Geochimica et Cosmochimica Acta, 51, 105–113.
Manceau, A., Schlegel, M., Nagy, K.L. and Charlet, L. (1999) Evidence for the formation of trioctahedral clay upon sorption of Co2+ on quartz. Journal of Colloid and Interface Science, 220, 181–197.
Manceau, A., Schlegel, M.L., Musso, M., Sole, V.A., Gauthier, C., Petit, P.E., Trolard, F. (2000) Crystal chemistry of trace elements in natural and synthetic goethite. Geochimica et Cosmochimica Acta, 64, 3643–3661.
McBride, M.B. (1985) Influence of glycine on Cu2+ adsorption by microcrystalline gibbsite and boehmite. Clays and Clay Minerals, 33, 397–402.
McLaren, R.G., Backes, C.A., Rate, A.W. and Swift, R.S. (1998) Cadmium and cobalt desorption kinetics from soil clays; effect of sorption period. Soil Science Society of America Journal, 62, 332–337.
O’Day, P.A., Brown, G.E., Jr. and Parks, G.A. (1994) X-ray-absorption spectroscopy of cobalt(II) multinuclear surface complexes and surface precipitates on kaolinite. Journal of Colloid and Interface Science, 165, 269–289.
O’Day, P.A, Chisholm-Brause, C.J, Towle, S.N, Parks, G.A. and Brown, G.E., Jr. (1996) X-ray absorption spectroscopy of Co(II) sorption complexes on quartz (alpha-SiO2) and rutile (TiO2). Geochimica et Cosmochimica Acta, 60, 2515–2532.
Ostergren, J.D., Brown, G.E., Jr., Parks, G.A. and Persson, P. (2000a) Inorganic ligand effects on Pb(II) sorption to goethite (α-FeOOH). II. —Sulfate. Journal of Colloid and Interface Science 225, 483–493.
Ostergren, J.D., Trainor, T.P., Bargar, J.R., Brown, G.E., Jr. and Parks, G.A. (2000b) Inorganic ligand effects on Pb(II) sorption to goethite (α-FeOOH) — I. Carbonate. Journal of Colloid and Interface Science 225, 466–482.
Rehr, J. J., Mustre de Leon, J., Zabinsky, S. and Albers, R.C. (1991) Theoretical X-ray absorption fine-structure standards. Journal of the American Chemical Society, 113, 5135–5140.
Ressler, T. (1997) WinXAS: A new software package not only for the analysis of energy-dispersive XAS data. Journal de Physique IV, 7, (C2) 269–270.
Saalfeld, H. and Wedde, M. (1974) Refinement of the crystal structure of gibbsite, Al(OH)3. Zeitschrift für Kristallographie, 139, 129–135.
Scheidegger, A.M., Lamble, G.M. and Sparks, D.L. (1997) Spectroscopic evidence for the formation of mixed-cation hydroxide phases upon metal sorption on clays and aluminum oxides. Journal of Colloid and Interface Science, 186, 118–128.
Scheidegger, A.M., Strawn, D.G., Lamble, G.M. and Sparks, D.L. (1998) The kinetics of mixed Ni-Al hydroxide formation on clay and aluminum oxide minerals: A time-resolved XAFS study. Geochimica et Cosmochimica Acta, 62, 2233–2245.
Scheinost, A.C. and Sparks, D.L. (2000) Formation of layered single- and double-metal hydroxide precipitates at the mineral/water interface: A multiple-scattering XAFS analysis. Journal of Colloid and Interface Science, 223, 167–178.
Scheinost, A.C., Ford, R.G. and Sparks, D.L. (1999) The role of Al in the formation of secondary Ni precipitates on pyrophyllite, gibbsite, talc, and amorphous silica: A DRS study. Geochimica et Cosmochimica Acta, 63, 3193–3203.
Scheinost, A.C., Abend, S., Pandya, K.I. and Sparks, D.L. (2001) Kinetic controls on Cu and Pb sorption by ferrihydrite. Environmental Science and Technology, 35, 1090–1096.
Scheinost, A.C., Kretzschmar, R., Pfister, S. and Roberts, D.R. (2002) Combining selective sequential extractions, X-ray absorption spectroscopy and principal component analysis for quantitative zinc speciation in soil. Environmental Science and Technology, (in press).
Schlegel, M., Manceau, A., Chateigner, D. and Charlet, L. (1999) Sorption of metal ions on clay minerals. I. Polarized EXAFS evidence for the adsorption of Co on the edges of hectorite particles. Journal of Colloid and Interface Science, 215, 140–158.
Schulthess, C.P. and Sparks, D.L. (1986) Back titration technique for proton isotherm modeling of oxide surfaces. Soil Science Society of America Journal, 50, 1406–1411.
Sparks, D.L., Scheidegger, A.M., Strawn, D.G. and Scheckel, K.G. (1999) Kinetics and mechanisms of metal sorption at the mineral-water interface. Pp. 108–113 in: Mineral-Water Interfacial Reactions. Kinetics and Mechanisms (D.L. Sparks and T.J. Grundl, editors). ACS symposium series 715. American Chemical Society, Washington, D.C.
Sutheimer, S.H., Maurice, P.A. and Zhou, Q. (1999) Dissolution of well and poorly crystallized kaolinites: Al speciation and effects of surface characteristics. American Mineraogist, 84, 620–628.
Taylor, R.M. (1984) The rapid formation of crystalline double hydroxy salts and other compounds by controlled hydrolysis. Clay Minerals, 19, 591–603.
Yamaguchi, N.U., Scheinost, A.C. and Sparks, D.L. (2001) Surface-induced nickel hydroxide precipitation in the presence of citrate and salicylate. Soil Science Society of America Journal, 65, 729–736.
Zhou, Z.H., Lin, Y.J., Zhang, H.B., Lin, G.D. and Tsai, K.R. (1997) Syntheses, structures, and spectroscopic properties of nickel(II) citrato complexes, (NH4)2[Ni(Hcit)(H2O)2]22H2O and (NH4)4[Ni(Hcit)2]2H2O. Journal of Coordination Chemistry, 42, 131–141.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, N.U., Scheinost, A.C. & Sparks, D.L. Influence of Gibbsite Surface Area and Citrate on Ni Sorption Mechanisms at pH 7.5. Clays Clay Miner. 50, 784–790 (2002). https://doi.org/10.1346/000986002762090182
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/000986002762090182